Abstract
The NF-κB signaling pathway is critical in myeloma cell proliferation, inhibition of apoptosis, and emergence of therapy resistance. The chemotherapeutic drugs, dexamethasone (Dex) and bortezomib (BTZ), are widely used in clinical protocols for multiple myeloma (MM) and inhibit the NF-κB signaling pathway by distinct mechanisms. This study evaluates the efficacy of combination therapy with Dex and BTZ and investigates the mechanistic underpinning of endogenous and therapy-induced NF-κB activation in MM. Human myeloma cells and bone marrow stromal cells (BMSCs) were used in monocultures and co-cultures to determine the cytotoxic effects of Dex and/or BTZ. Our results show that combined treatment of Dex with BTZ enhanced direct apoptosis of drug-sensitive and drug-resistant myeloma cells. In the presence of BMSCs, Dex plus BTZ combination inhibited ionizing radiation (IR)-induced interleukin (IL)-6 secretion from BMSCs and induced myeloma cytotoxicity. Mechanistically, Dex treatment increased IκBα protein and mRNA expression and compensated for BTZ-induced IκBα degradation. Dex plus BTZ combination inhibited basal and therapy-induced NF-κB activity with cytotoxicity in myeloma cells resistant to BTZ. Furthermore, combination therapy down-regulated the NF-κB targeted gene expression of IL-6 and manganese superoxide dismutase (MnSOD), which can induce chemo- and radio-resistance in MM. This study provides mechanistic rationale for combining the NF-κB-targeting drugs Dex and BTZ in myeloma therapy and supports potential combinations of these drugs with radiotherapy and additional chemotherapeutic drugs, for clinical benefit in MM.
Introduction
Multiple myeloma (MM), a malignant disease of plasma cells, exhibits a very high frequency of resistance to anti-neoplastic drugs [1]. It is estimated that, in the United States, approximately 21,700 new cases of MM will be diagnosed during 2012 and over 10,000 individuals will die of the disease [2]. The current five-year survival rate for patients with MM is 40% and, to date, MM remains incurable. The standard treatment, high dose chemotherapy with stem cell transplantation, has improved the response rate in patients with MM but has a number of associated toxicities [3]. The glucocorticoid analog dexamethasone (Dex) and the proteasome-inhibiting drug bortezomib (BTZ; also called PS-341 or Velcade) are among the most effective and widely used treatments for MM [3, 4]. The combination of Dex with BTZ along with other drugs such as thalidomide, doxorubicin, cisplatin, cyclophosphamide, and etoposide has resulted in improvements in both response rates and long-term outcomes [5].
The nuclear factor (NF)-κB signaling pathway is chronically active in myeloma cells via microenvironment-dependent interactions and by abnormalities in genes encoding for regulators and effectors of NF-κB signaling [6]. Also, NF-κB signaling in stromal cells that constitute the cellular microenvironment can lead to production of myeloma growth factors such as IL-6 [7]. Indeed, the NF-κB pathway has long been an attractive target for myeloma therapy as chemotherapeutic drugs thought to act largely by inhibiting NF-κB signaling (such as Dex, BTZ, thalidomide, lenalidomide, arsenic trioxide, and curcumin) have shown potent cytotoxic activity in several myeloma cell lines and primary patient samples [8]. Aberrant NF-kB activation has been associated with the emergence of resistance to anti-cancer drugs and radiation in MM [9–11].
Dex and BTZ have been shown to target NF-κB activity by distinct mechanism(s). Dex, a glucocorticoid analog, inhibits NF-κB activity by “transactivation” via transcription of IκB and also by “transrepression” via a reduction in transcription of the NF-κB genes [12]. The molecular mechanism(s) of BTZ anti-tumor activity in MM has been extensively studied and has been shown to be rendered, in part, by blocking both canonical and non-canonical NF-κB signaling by inhibiting degradation of IκB proteins [6]. Previously, we have demonstrated that stress-inducing agents such as ionizing radiation (IR) enhance formation of the NF-κB-IκB complex [13]. In addition, we have reported that NF-κB-regulated expression of IL-6 by stromal cells promotes resistance to oxidative stress-inducing therapies (Dex and IR) by inducing manganese superoxide dismutase (MnSOD) production in myeloma cells [10]. Finally, our published results indicate that Dex [9] and BTZ [14] can selectively and independently radiosensitize myeloma cells in vitro and in vivo by inhibiting basal and IR-induced NF-κB activation.
The present study was designed to investigate whether Dex and BTZ combination treatment can inhibit NF-κB activation leading to increased myeloma cell cytotoxicity. Biochemical studies utilizing Dex combined with BTZ demonstrated that combination treatment increased IκBα expression and inhibited constitutive and therapy-induced NF-κB activation in a myeloma cell line that did not demonstrate increased cytotoxicity in response to BTZ treatment alone. Furthermore, Dex and BTZ combination therapy down-regulated NF-κB driven gene expression of IL-6 and MnSOD that can induce chemo- and radio-resistance in MM. The work presented here indicates that combination therapy with Dex and BTZ can overcome resistance developed towards either therapeutic agent alone and, therefore, is viable as treatment option that can be potentially combined with radiotherapy and additional chemotherapeutic drugs, to improve the prognosis of myeloma patients.
Materials and methods
Cell lines, primary cells, and tissue culture
Myeloma cell line RPMI-8226 (8226, CCL-155) and BMSCs (HS-5, CRL-11882) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Myeloma cell lines MM.1S and ANBL-6 were a generous gift from Dr. Steve Rosen (Northwestern University, Chicago) and Dr. Diane Jelinek (Mayo Clinic, Rochester), respectively. The BTZ resistant 8226 cell line (8226BR) was established by culturing 8226 cells under gradual BTZ selection to reach a final concentration of 10 nM BTZ. HBME-1, a human BM endothelial cell line, was a generous gift from Dr. Kenneth Pienta (University of Michigan, Ann Arbor). All cell lines were grown in RPMI complete medium in a humidity-controlled incubator (37°C, 5% CO2) as described previously [9, 10].
Co-cultures of MM.1S cells stably expressing firefly luciferase (Luc) protein with HS-5 BMSCs were established as described previously [9]. Primary BMSCs were established from discarded de-identified bone marrow samples obtained from the University of Iowa Hospitals and Clinics. Briefly, BM mononuclear cells were separated by Ficoll-Hypaque (Stem Cell Technologies Inc., Vancouver, BC, Canada) density sedimentation and incubated in complete medium; after 24h non-adherent cells were removed. The cultures were then propagated until they reached sub-confluence (about 2 weeks) after which they were utilized for experiments.
MTT cell viability assay
This assay was performed as described previously [13]. Briefly, cells were treated with Dex (1 µM, Sigma-Aldrich, St. Louis, MO) and/or BTZ (10 nM, LC labs, Woburn, MA); the MTT assay was performed at 24h using a commercially available kit from ATCC. In specific experiments, the glutathione precursor N-acetylcysteine (NAC, 10 mM, Sigma Aldrich) was added 1h before Dex and/or BTZ treatments. All treatments were performed in triplicate and the mean ± SD was determined.
Measurement of apoptosis
Apoptosis induced by Dex and/or BTZ treatment was measured at 24h using a caspase-3 fluorescence assay (Cayman Chemical, Ann Arbor, MI) as described previously [9, 10]. Caspase-3 activity was expressed as units per milligram of total protein.
IL-6 ELISA
HS-5 cells and primary BMSCs were treated with Dex (1 µM) and/or BTZ (10 nM) followed by IR (6 Gy, delivered as a single dose using Cs-137 source, dose rate of 0.83 Gy/min). Culture supernatant was collected at 24h and used for IL-measurements using a commercially available ELISA kit (eBiosciences, San Diego,CA) as described before [9].
Luciferase assays
For viability studies in co-cultures, myeloma (MM.1S-Luc) and BMSCs (HS-5) cells were co-cultured, pre-treated for 6h with IL-6 (50 ng/ml, R&D Systems, Minneapolis, MN) followed by Dex (1 µM) and/or BTZ (10 nM) treatments. Alternatively, co-cultures were treated with Dex and/or BTZ followed by IR (6 Gy). Cell lysate was prepared at 24 h and the luciferase assay (Promega Inc, Madison, WI) was performed as described previously [9].
Western blot analysis
Protein immunoblotting was performed according to standard protocols as described previously [9, 10, 13]. Cells were treated with Dex and/or BTZ for the indicated times and equal amounts of protein were electrophoresed in a 10% or 12.5% reducing SDS-PAGE gel. Proteins were transferred to PVDF membranes, non-specific binding was blocked with 5% skim milk in TBST buffer (4mM Tris base, 10mM NaCl, pH 7.5, 0.1% Tween-20), and incubated overnight at 4°C with primary antibodies against IκBα, phospho-IκBα, or tubulin (all antibodies were from Cell Signaling Technology, Danvers, MA) and then incubated with secondary antibody for 1h at RT. Blots were developed by enhanced chemiluminescence assay (Thermo Scientific, Waltham, MA). Bands were visualized by autoradiography and then analyzed using Image J 1.38x software (http://rsbweb.nih.gov/ij/index.html). For illustrations, Adobe Photoshop CS4 was used to convert figures to grayscale, crop to an appropriate size, and then were contrast corrected using Photoshop’s “Auto Contrast” tool.
Quantitative PCR (qPCR)
Gene expression of IκBα, SOD2, and IL-6 was examined using qPCR as described previously [10]. Briefly, total RNA was extracted at 12h from untreated, Dex (1 µM) and/or BTZ (10 nM) treated myeloma cells that were exposed to either 6 Gy of IR or exogenous IL-6 (50 ng/ml for 2h). cDNA was synthesized using the iScript kit (BioRad, Hercules, CA) and qPCR was carried out in a 7500 real-time PCR system (Applied Biosystems, Carlsbad, CA). Gene-specific primers for IκBα (NM_020529) were: forward 5'-ACCTGGTGTCACTCCTGTTGAAGT-3' and reverse 5'-ACTCTCTGGCAGCATCTGAAGGTT-3'; and for IL-6 (NM_000600.3) were: forward 5′-TAGCCGCCCCACACAGACAG-3′ and reverse 5′-GGCTGGCATTTGTGGTTGGG-3′. qPCR primers for SOD2 and 18S RNA were the same as reported previously [10]. The fold difference was calculated using the formula 2−Δ(ΔCT) and was plotted as the mean (n = 3).
NF-κB assay
Myeloma cells were treated with Dex and/or BTZ followed by either sham or 6 Gy IR. Nuclear extracts were prepared at 2h and used to quantify the transcriptional activity of NF-κB (TransAM NF-κB assay kit, Active Motif, Carlsbad, CA) as described previously [13]. The absorbance of the untreated cells was set equal to one and the fold activation/repression in NF-κB activity was calculated after normalizing the samples for cellular protein content.
Statistical analysis
GraphPad Prizm 4.0 software (GraphPad Software, San Diego, CA) was used for data handling, analysis, and presentation. Statistical significance was determined using two-tailed unpaired t-test with a confidence interval of 95%.
Results
Dex and BTZ combination therapy increased apoptotic cell death of drug-resistant myeloma cells
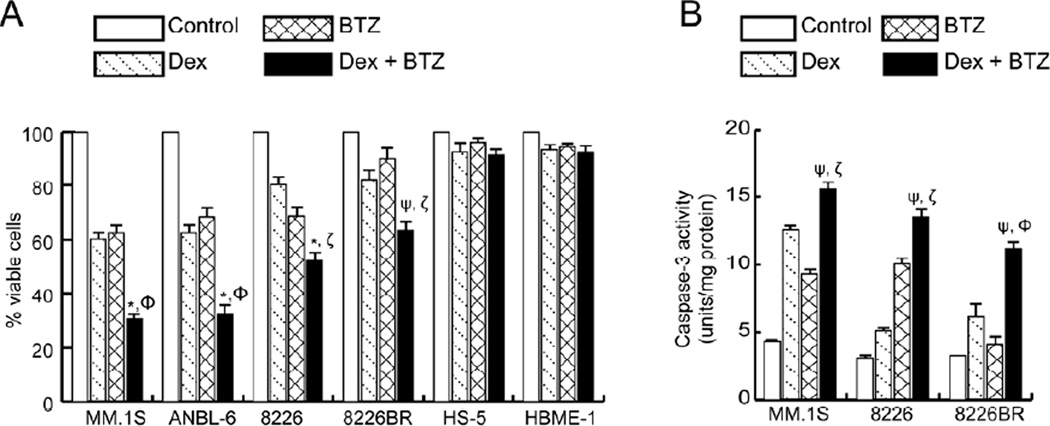
The cytotoxicity of Dex and BTZ alone, and in combination, was assessed in a panel of human myeloma cell lines that are differentially sensitive to Dex and/or BTZ; as a control, bone marrow accessory cell lines (HS-5 and HBME-1, Fig. 1A) were also tested. A MTT cell viability assay performed after 24h of treatment with Dex resulted in an approximately 40% reduction in cell viability for MM.1S and ANBL-6 cells; these myeloma cell lines have been reported to be sensitive to Dex-induced cell death [15, 16]. For 8226 cells (both a wild type and a BTZ-resistant variant), Dex treatment resulted in approximately 20% cytotoxicity; our previously published results indicate that the 8226 cell line is relatively resistant to Dex treatment [9]. Exposure to BTZ (24h) resulted in approximately 35% cytotoxicity in all myeloma cell lines except for 8226BR cells which were adapted to grow in BTZ (10 nM). Combined treatment of Dex and BTZ increased the killing of all myeloma cells relative to single agents (Fig. 1A). For bone marrow accessory cells, combined treatment with Dex and BTZ did not increase the toxicity of these drugs when compared to single agent treatments.
Figure 1.
Cytotoxic effects of Dex (1 µM) and BTZ (10 nM) in single and combined treatments assessed at 24h by (A) MTT assay in myeloma and bone marrow accessory cells and (B) by caspase 3 activity in MM.1S, 8226, and 8226BR cells. Values presented are the mean of triplicate readings ± SD and are representative of 3 independent experiments. *P<0.005 and ψP<0.05 compared with Dex, ΦP<0.005 and ζP<0.05 compared with BTZ.
Since Dex [9] and BTZ [13] induce apoptotic cell death in myeloma cells, we assessed whether the concerted inhibition of myeloma cell viability observed with Dex and BTZ was associated with increased apoptosis. For both MM.1S and 8226 cells, combined treatment of Dex and BTZ showed increased caspase 3 activity, as compared to single agents (Fig. 1B). Furthermore, in 8226BR cells, caspase 3 activity was marginally increased with Dex treatment which was further increased with BTZ co-treatment (Fig. 1B). Taken together, Figure 1 indicates that the Dex plus BTZ drug combination renders selective and increased killing of both drug-sensitive and -resistant myeloma cell lines.
Combination therapy with Dex and BTZ inhibits IR-induced IL-6 secretion from BMSCs and attenuates IL-6-induced chemo- and radio-resistance in myeloma cells
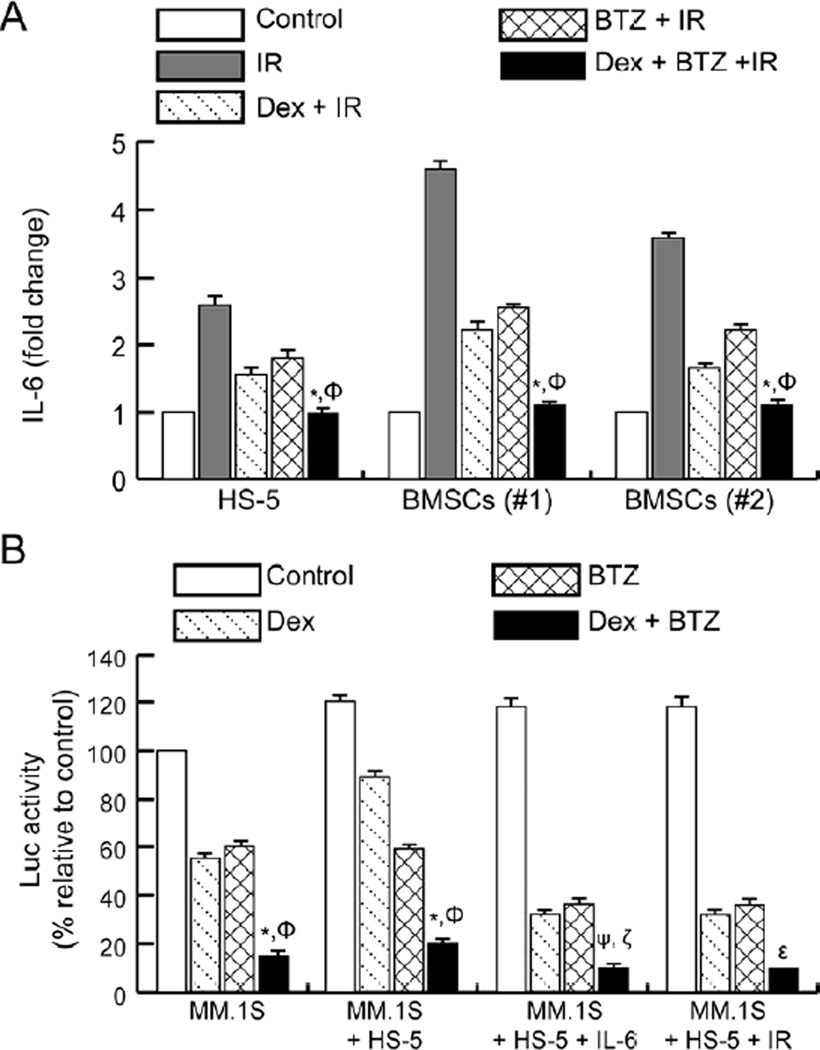
In MM, the in vivo microenvironment of tumor cells is associated with increased levels of IL-6 that are secreted predominantly by BMSCs [11]. We have recently reported that oxidative stress-inducing agents such as IR trigger IL-6 secretion from BMSCs [9, 10] and that Dex-treatment can inhibit IR-induced IL-6 secretion from BMSCs [9]. We next determined whether the combination of Dex and BTZ was more effective than Dex treatment alone in inhibiting IR-induced IL-6 synthesis using a BMSCs cell line (HS-5) and two primary human BMSCs. Exposure to IR (6 Gy) resulted in an approximately 2.3-fold increase in IL-6 levels from HS-5 cells while primary BMSCs showed approximately 3.5–4.5-fold increases in IL-6 levels (Fig. 2A). Treatment with Dex or BTZ inhibited IR-induced IL-6 secretion from BMSCs while the combined treatment of Dex and BTZ further attenuated IR-induced IL-6 secretion from BMSCs (Fig. 2A).
Figure 2.
Combining Dex and BTZ inhibits IR-induced IL-6 release from BMSCs and exhibits increased anti-myeloma activity in presence of BMSCs. (A) BMSCs (established cell line and primary samples) were pre-treated with Dex (1 µM) and/or BTZ (10 nM) for 6h followed by IR (6 Gy) and IL-6 was measured in the culture medium at 24h post-IR. Results are presented as the fold-change in IL-6 concentration relative to un-irradiated controls. *P<0.05 compared with Dex + IR and ΦP<0.05 compared with BTZ + IR. (B) MM.1S-Luc cells were either cultured alone or co-cultured with HS-5 and exposed to exogenous IL-6 (50 ng/ml for 2h) or given IR (6 Gy) followed by treatment with Dex (1 µM) and/or BTZ (10 nM) for 24h after which Luc activity was measured. The Luc activity in untreated control cells was set to 100% and relative changes were determined. The results shown are representative of 3 independent experiments. *P<0.05 and ΦP<0.05 compared with Dex and BTZ respectively, ψP<0.005 and ζP<0.05 compared with Dex + IL-6 and BTZ + IL-6 respectively, and εP<0.05 compared with Dex + IR or BTZ + IR.
The role of IL-6 as the primary cytokine associated with emergence of myeloma cell resistance to Dex [17] and IR has been established [10]. Studies were extended to include direct co-cultures of MM.1S myeloma (stably expressing luciferase) and HS-5 cells [9]; luc activity was measured to determine whether the combination of Dex with BTZ could result in increased myeloma cell killing. Again, combined treatment with Dex and BTZ showed more effective myeloma cell killing than either agent alone (Fig. 2B). Since combination therapy decreased IL-6 release from BMSCs (Fig. 2A), the role of IL-6 on myeloma cell survival, proliferation, and emergence of therapy resistance was assessed by pre-treating the co-cultures with exogenous IL-6 (50 ng/ml, 6 h) followed by Dex and/or BTZ addition and measurement of myeloma viability by luc assay at 24 h. Addition of exogenous IL-6 induced Dex resistance in MM.1S myeloma cells, as also seen in direct co-culture relative to MM.1S cells alone; this effect was abrogated by co-treatment with BTZ (Fig. 2B).
We have shown previously that IR-induced IL-6 synthesis form BMSCs plays a significant role in the emergence of radio-resistance in myeloma cells [10]. We next examined the anti-myeloma effect of Dex plus BTZ after IR. Irradiation resulted in increased survival and proliferation of myeloma cells that was blunted with co-treatment with Dex or BTZ (Fig. 2B) (we have previously reported Dex [9] and BTZ-mediated selective radio-sensitization of myeloma cells in in vitro and in vivo studies [13, 14]). Noticeably, the combination of Dex and BTZ with IR resulted in significantly lower survival of myeloma cells compared to single drug therapy with IR. Taken together, the combination of Dex and BTZ showed increased anti-myeloma activity towards myeloma cells both in monocultures (Fig. 1A) and in the presence of BMSCs (Fig. 2B). Mechanistically, the combination of Dex and BTZ inhibited stroma-induced chemo- and radio-resistance in myeloma cells by direct myeloma cell cytotoxicity and by the blunting of IL-6 survival pathways.
Dex co-treatment inhibits BTZ-induced IκBα degradation in myeloma cells
In MM, the NF-κB signaling pathway is constitutively active, and inhibition of NF-κB activity has been associated with the clinical efficacy of several anti-myeloma chemotherapeutic drugs including Dex and BTZ [6]. Activation of NF-κB depends on the signal-induced phosphorylation of IκBα, IκBα ubiquitination, and subsequent degradation by the proteasome; this process results in the nuclear translocation of NF-κB where it regulates gene transcription. Dex treatment has been shown to up-regulate IκBα mRNA and protein expression and result in inhibition in NF-κB activity [18]. BTZ treatment has also been shown to result in the accumulation of phosphorylated IκBα and the cytoplasmic sequestration and inhibition of NF-κB activity [19]. However, more recently, a heterogeneous effect of BTZ on NF-κB activity was reported in myeloma cell lines [20]. In a few myeloma cell lines, treatment with BTZ resulted in IκBα down-regulation [20] and was found to be associated with calpain-dependent proteolysis of IκBα [21].
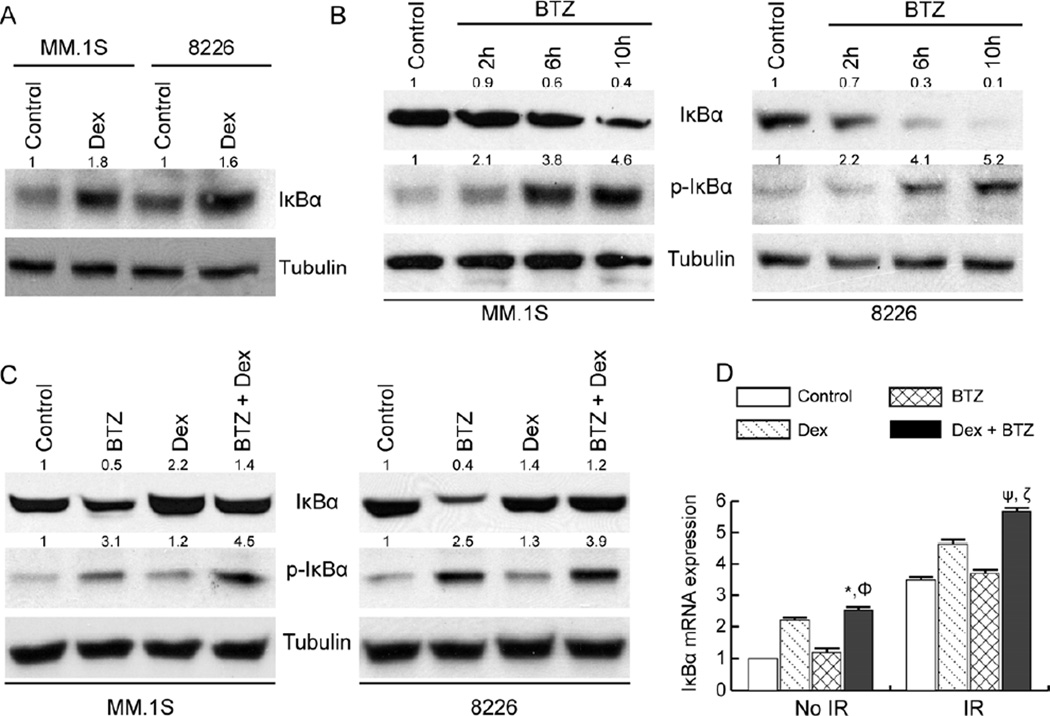
Since Dex and BTZ target NF-κB activity by distinct mechanisms, we performed molecular IκBα inhibition studies using Dex and BTZ combination therapy. MM.1S and 8226 cells were selected based on their differential sensitivity towards Dex and BTZ [9, 20] and their dependence on the canonical NF-κB pathway [20]. In both cell lines, Dex exposure increased IκBα protein levels at 24h as determined by immunoblotting (Fig. 3A); Dex treatment did not significantly alter the phosphorylated IκBα (p-IκBα) levels in MM.1S and 8226 cells (Fig. 3C). Compared to MM.1S cells, 8226 cells showed relatively lower steady-state levels of IκBα protein; in the time kinetics study, BTZ treatment resulted in a rapid time-dependent decrease in IκBα protein levels in 8226 cells since by 6h most of the IκBα protein was degraded (Fig. 3B). BTZ treatment induced a less severe and slower IκBα degradation kinetics in MM.1S cells (Fig. 3B). For both MM.1S and 8226 cells, BTZ treatment resulted in increased p-IκBα levels by 6h that was sustained till 10h (Fig. 3B). Taken together, the results in Figures 3A and 3B suggest that unlike MM.1S cells, 8226 cells may have constitutive NF-κB activation (due to relatively lower levels of endogenous IκBα protein), Dex up-regulates IκBα expression, and BTZ treatment could result in a differential modulation of IκBα levels and hence NF-κB activation in myeloma cells.
Figure 3.
The effect of Dex and BTZ on IκBα protein and mRNA level in myeloma cells. Western blot analysis of whole cell lysates for (A) IκBα protein after Dex treatment (1 µM for 24h), (B) IκBα and p-IκBα after BTZ treatment (10 nM) at the indicated time points, and (C) IκBα and p-IκBα after Dex and/or BTZ treatment at 24h. Tubulin levels were used as loading control. Band intensities relative to respective tubulin controls, are shown above each blot. (D) qPCR analysis of IκBα mRNA in 8226 cells at 12h after treatment with Dex (1 µM) and/or BTZ (10 nM). The results are presented as the fold change in mRNA expression relative to 18S mRNA and are normalized to control, un-irradiated cells (mean of two independent experiments). *P<0.05 and ΦP<0.001 compared with Dex and BTZ respectively, ψP<0.05 and ζP<0.01 compared with Dex + IR and BTZ + IR, respectively.
We next determined how Dex and BTZ, in combination, would alter IκBα protein expression relative to each drug alone. In both MM.1S and 8226 cells, combined treatment of Dex with BTZ attenuated BTZ-mediated decreases in IκBα levels (Fig. 3C). In both myeloma cell lines, BTZ treatment increased p-IκBα levels while combination treatment led to p-IκBα levels that were significantly higher than untreated or Dex-treated cells (Fig. 3C). This result shows that compared to single drug treatments, combination treatment with Dex and BTZ results in increased expression of IκBα and p-IκBα and may result in effective inhibition of NF-κB signaling in myeloma cell lines showing reduced sensitivity to cytotoxic effects of BTZ or Dex alone.
We have reported that reactive oxygen species (ROS) production by anti-myeloma agents (such as IR and Dex) is associated with NF-κB activation [10]. We next determined how IκBα mRNA levels were modulated by Dex and/or BTZ under normal and IR-induced oxidative stress conditions. Studies were done in 8226 cells as BTZ treatment showed an unexpected NF-κB activation in this cell type (Fig. 3B and [20]). Since Dex primarily inhibits NF-κB activity by transcription of IκBα [12] and NF-κB is known to control the expression of IκBα thereby inhibiting inducible NF-κB activity [22], we measured IκBα mRNA levels by qPCR. In non-irradiated cells, treatment with Dex increased IκBα mRNA levels while combining Dex with BTZ showed a further increase in IκBα mRNA levels relative to either drug alone (Fig. 3D). Treatment with IR resulted in an approximately 3.5-fold increase in IκBα mRNA levels that was further enhanced by co-treatment with Dex and BTZ (Fig. 3D). Taken together, the effect of combined treatment of Dex and BTZ on IκBα protein and mRNA expression suggest that combined treatment with Dex and BTZ could potentially inhibit therapy-induced NF-κB activation in myeloma cells.
Co-treatment with Dex inhibits both constitutive and BTZ therapy-induced NF-κB signaling in myeloma cells
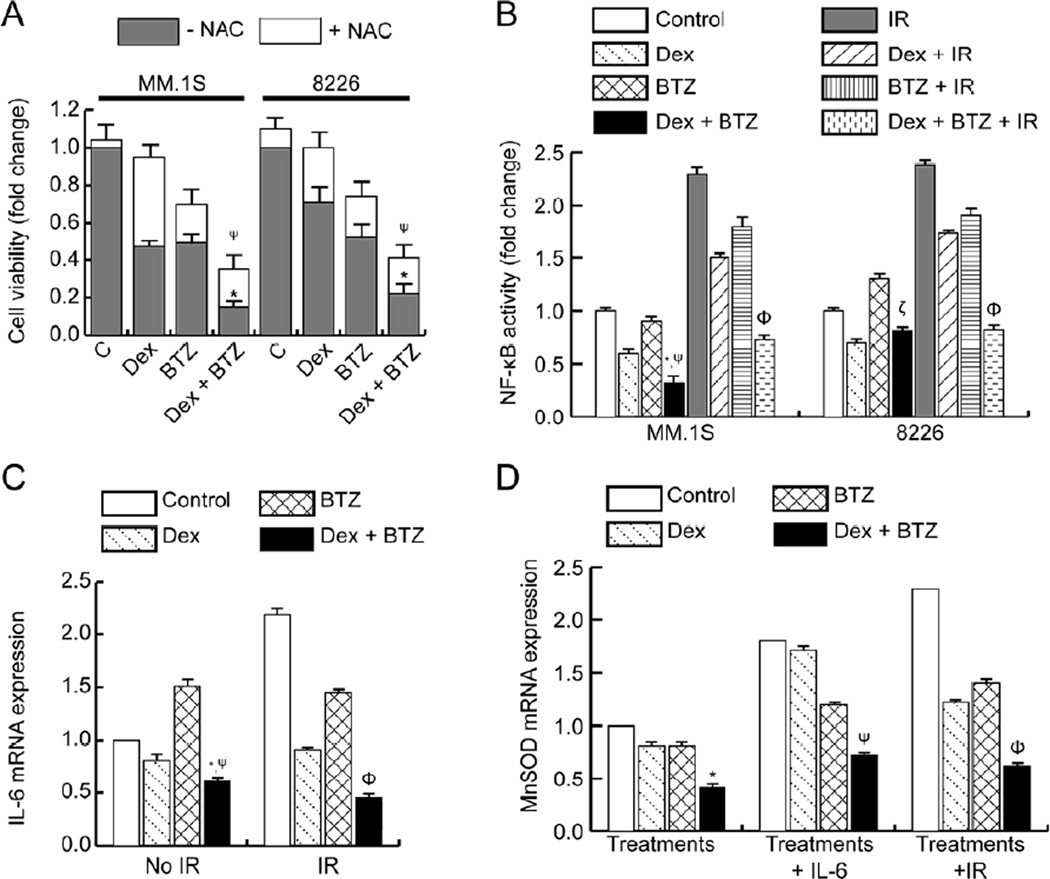
Antioxidants have been shown to reduce the anti-myeloma activity of BTZ [23] and we have reported the role of ROS in Dex-induced radio-sensitization of myeloma cells [9]. Also, a direct correlation between IR and NF-κB activation in myeloma cells was recently reported by us [10]. Since NF-κB is a redox-regulated transcription factor [24] and certain chemotherapeutic drugs (such as Dex and BTZ) and believed to exert anti-myeloma effects by ROS-mediated apoptosis [25], we evaluated the role of N-acetylcysteine (NAC) on cytotoxicity of Dex and/or BTZ. In both MM.1S and 8226 cells, NAC treatment completely reversed Dex-induced cell death as also reported in myeloma and lymphoma cells [9, 26] and partially reversed the BTZ-induced cytotoxicity (Figure 4A). Also, treatment with NAC partially rescued myeloma cell death induced by Dex plus BTZ drug combination (Figure 4A) suggesting that combination drug treatment is inducing myeloma cell death by both ROS-dependent and ROS-independent pathways.
Figure 4.
The effect of Dex and BTZ treatments on the NF-κB pathway in myeloma cells. (A) Myeloma cells were treated with Dex and/or BTZ either in absence or presence of NAC and cytotoxic effects were assessed at 24h by MTT assay. (B) Nuclear translocation and DNA binding activity of NF-κB/p65 were analyzed 2h after Dex and/or BTZ treatment. Data are shown as fold change relative to untreated control and are representative of three independent experiments. *P<0.05 and ψP<0.05 compared with Dex and BTZ respectively, ΦP<0.05 compared to Dex + IR or BTZ + IR, ζP<0.01 compared to BTZ. (C) qPCR analysis of IL-6 mRNA at 12h in 8226 cells that were unirradiated or given IR, in addition to treatment with Dex and/or BTZ. *P<0.05 and ψP<0.01 compared with Dex and BTZ respectively, ΦP<0.05 compared to Dex + IR or BTZ + IR. (D) qPCR analysis of MnSODmRNA at 12h in MM.1S cells exposed to exogenous IL-6 (50 ng/ml for 2h) or given IR (6 Gy) followed by treatment with Dex and/or BTZ. *P<0.05 compared with Dex or BTZ, and ψP<0.05 compared with Dex + IL-6, ΦP<0.05 compared to Dex + IR or BTZ + IR.
Next, the effect of Dex and/or BTZ treatments on activation and nuclear translocation of NF-κB/p65 was determined by assaying for NF-κB DNA-binding activity in nuclear lysates as described previously [13]. For both MM.1S and 8226 cells, Dex treatment inhibited basal NF-κB activity while BTZ treatment induced either modest inhibition or a slight increase in NF-κB activity in MM.1S and 8226 cells, respectively (Fig. 4B). Combined treatment with Dex and BTZ resulted in a further decrease in NF-κB activity in MM.1S cells and attenuated BTZ-induced activation of NF-κB signaling in 8226 cells (Fig. 4B). Our previously published work demonstrated that BTZ can inhibit IR-induced NF-κB activation in myeloma cell lines [13]. We next asked whether combination therapy could also inhibit IR-induced NF-κB activation. For both MM.1S and 8226 cells, IR resulted in significant NF-κB activation as also reported previously [13] and was blocked in a concerted fashion after combined treatment with Dex and BTZ (Fig. 4B).
Activated NF-κB has been shown to regulate a number of genes that are associated with myeloma cell survival, proliferation, and emergence of therapy resistance [6]. We next determined whether combining Dex and BTZ resulted in down-regulation of NF-κB targeted gene expression of IL-6. In 8226 cells, basal levels of IL-6 mRNA were decreased after Dex treatment alone, increased after BTZ treatment alone, and further decreased by combining Dex plus BTZ (Fig. 4C). IR-induced IL-6 mRNA expression was also inhibited by Dex plus BTZ therapy (Fig. 4C). Thus, combination therapy can potentially block both irradiated stroma- (Fig. 2A) and myeloma- (Fig 4C) induced IL-6 synthesis by inhibiting NF-κB activation; these events would result in both increased killing as well as a decreased emergence of therapy resistance in myeloma cells.
We have recently shown that IL-6 and oxidative stress-inducing anti-myeloma agents (such as IR and Dex) can activate NF-κB followed by increased SOD2 gene expression resulting in emergence of resistance to these agents [10]. We next investigated whether Dex plus BTZ combination therapy could inhibit MnSOD mRNA expression resulting from exposure to exogenous IL-6 or IR and thus potentially sensitize myeloma cells to chemo-radiotherapy. Combination therapy inhibited endogenous MnSOD mRNA expression in MM.1S cells (Fig. 4D). Treatment with Dex plus BTZ significantly attenuated the IL-6 mediated up-regulation on MnSOD expression (Fig. 4D). Furthermore, the combination of Dex with BTZ effectively inhibited IR-induced up-regulation of MnSOD expression (Fig. 4D). Taken together, results of Figure 4B-4D demonstrate that the combination of Dex and BTZ is effective in 1) inhibiting BTZ-induced NF-κB activation, 2) inhibiting IL-6 expression which is an established pro-proliferative and chemo-resistance inducing cytokine in myeloma cells, and 3) inhibiting MnSOD expression that induces chemo-and radio-resistance in myeloma cells.
Discussion
Utilization of novel chemotherapeutic agents combined with a better understanding of myeloma cytogenetics has contributed to improvement in clinical response rates for MM [27, 28]. Yet MM remains incurable as the disease ultimately acquires resistance to frontline therapies [3]. Myeloma patients show clinical responses to steroid-based therapies; as such, Dex remains a central component in both MM monotherapy as well as part of combinatorial therapy [25]. However, prolonged usage of glucocorticoids is associated with adverse events such as hyperglycemia, osteoporosis, and increased susceptibility to infections [29]. Furthermore, the emergence of glucocorticoid-resistant MM disease results from expression of aberrant glucocorticoid receptors and over-expression of IL-6 in the BM microenvironment [30]. BTZ is a relatively new chemotherapeutic drug that has shown excellent clinical efficacy in both single- and multi-drug regimens in MM [5]. However, dose-limiting toxicities and the development of resistance limit the long-term usage of BTZ [31, 32].
Therefore, to improve response rates with manageable toxicity and to minimize of therapy resistance, a continued investigation of combination treatments with a mechanistic understanding of the clinically active drugs available for MM therapy is essential. Clinical regimens incorporating BTZ and Dex along with cytotoxic agents such as thalidomide and cyclophosphamide are being utilized for treatment of newly diagnosed MM [28]. Patients with refractory MM that are treated with combination of Dex with BTZ have shown good clinical responses [33]. Since both Dex and BTZ are clinically active anti-myeloma drugs, the present study was designed to gain a mechanistic understanding of whether the combination chemotherapy could have effective anti-myeloma activity in divergent myeloma cell lines.
MM disease progression and emergence of therapy resistance is closely associated with NF-κB signaling [34]. In myeloma cells, NF-κB is activated by pathway mutations [6] and by pro-inflammatory cytokines present in the tumor microenvironment [35]. Adhesion of myeloma cells to bone marrow stromal cells has been shown to result in NF-κB mediated transcription and synthesis of IL-6 from stromal cells, which plays an important role in myeloma cell proliferation and emergence of resistance to Dex [11]. The NF-κB pathway has been shown to be associated with osteoclast maturation, thus NF-κB inhibition is of therapeutic benefit in targeting MM osteolytic lesions [36]. Furthermore, we have previously shown that irradiation results in unwanted NF-κB activation in myeloma cells and that combining IR independently with BTZ [13, 14] or Dex [9] results in both constitutive and IR-induced NF-κB activation and radio-sensitization of myeloma cells. Thus, a mechanistic understanding of NF-κB inhibition would provide a rationale to combine clinically active drugs such as Dex and BTZ to not only improve clinical response rates but also to inhibit and potentially overcome drug resistance in MM.
The anti-myeloma activity of both Dex and BTZ is associated with their ability to inhibit the NF-κB signaling pathway. Dex-induced apoptosis of myeloma cells is associated with NF-κB inhibition by transcription of IκB and NF-κB genes [12]. The proteasome inhibitor, BTZ, has been shown to result in cytoplasmic accumulation of phosphorylated IκBα and inhibition of NF-κB activity [19]. Conversely, BTZ treatment has also recently been shown to result in activation of the canonical NF-κB pathway by down-regulating IκBα [20] via calpain-mediated proteolysis [21]. We therefore investigated the combination of NF-κB targeted therapy in drug-sensitive and -resistant myeloma cell lines. In the current study, a direct comparison of MM.1S was not made with their Dex-resistant counterpart (MM.1R) since MM.1R cells lack a functional α-isoform of the glucocorticoid receptors. We have instead used the 8226 cells that express GRα and show resistance to Dex due to increased expression of GRα and modulations in CD23, CD38, CD44 and CD58 expressions [37]. Also, MM.1S and 8226 exhibit different responses to BTZ due to selective NF-κB pathway utilization; in MM.1S both the canonical and non-canonical NF-κB pathways are active while the canonical pathway is predominantly active in 8226 cells [20]. Compared to MM.1S cells, 8226 cells showed lower steady state levels of IκB suggesting increased endogenous NF-κB activity in 8226 cells; a similar correlation between IκB protein and constitutive NF-κB activation has been reported in Hodgkin/Reed-Sternberg cells [38]. Here, we found that BTZ treatment resulted in rapid degradation of IκB which is likely to be responsible for NF-κB activation; however, combining BTZ treatment with Dex resulted in a net inhibition of NF-κB, results in myeloma cell cytotoxicity (Fig. 5). Also, combining Dex and BTZ was found to be not cytotoxic to bone marrow accessory cell lines (HS-5 and HBME-1, Fig. 1). In this study we have not determined the effect of Dex and/or BTZ on NF-κB activity in HS-5 since these drug concentrations did not exhibit cytotoxicity towards bone marrow accessory cells lines when used in alone or in combination with IR [9, 13].
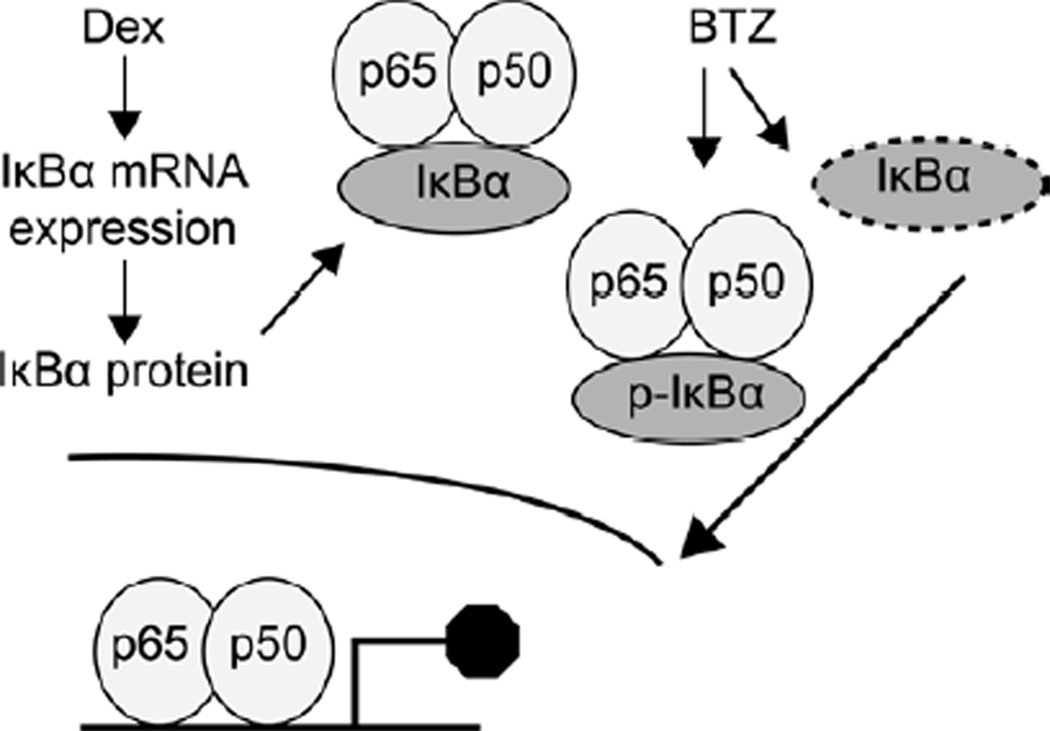
Figure 5.
A model of Dex and BTZ molecular inhibition of the NF-κB pathway in myeloma cells. Dex treatment up-regulates IκBα protein expression while BTZ treatment can have a heterogeneous effect on NF-κB activity. Combination of Dex with BTZ results in an overall inhibition of NF-κB activation and downstream gene expression in myeloma cells.
In MM patients, elevated IL-6 serum levels correlate with poor prognosis; furthermore, IL-6 has been shown to be one of the primary cytokines supporting myeloma cell growth, survival, and drug resistance [7]. IL-6 can activate the protein tyrosine phosphatase, SHP2, and related adhesion focal tyrosine kinase (RAFTK) as well as induce Dex-resistance in myeloma cells [17]. BTZ has been shown to inhibit IL-6-induced survival signaling pathways in myeloma cells by inhibiting extracellular signal-regulated kinases, by inhibiting activation of STAT molecules (signal transducers and activators of transcription) and Akt, and by down-regulating signal transducer gp130 [39]. In our recent study, we elucidated the role of IL-6 mediated NF-κB activation in the emergence of therapy resistance in MM [10]. We have previously shown that bone marrow stromal cell lines (HS-5 and SR-4986) secrete IL-6 [9, 10], and treatment with Dex marginally attenuates IL-6 secretion from SR-4987 cells [9]. In the present study we have determined the effect of Dex and BTZ on IL-6 release from irradiated stromal cells mainly because (a) under in vitro conditions, the basal level of IL-6 secreted by untreated BMSCs is usually low and close to the detection limit of the available ELISA assays, and (b) IR results is a robust increase in IL-6 secretion from BMSCs [9, 10]. This study demonstrates that combined treatment with Dex and BTZ attenuates paracrine IL-6 secretion from irradiated stromal cells and thus contributes to myeloma cell killing and inhibition of therapy resistance. Since Dex treatment effectively attenuates IL-6 secretion from irradiated stroma [9] and BTZ has been shown to be effective in blunting IL-6-mediated survival signaling in myeloma cells, we can speculate that combined treatment with Dex and BTZ may effectively eradicate myeloma cells in their native bone marrow microenvironment in patients undergoing oxidative stress-inducing therapy. It remains to be determined if the use of antioxidants would affect the myeloma cell death induced by ROS-inducing chemotherapy drugs and radiation therapy. In a recent study addition of antioxidant (NAC or vitamin C) after the treatment with BTZ did not compromise the anti-myeloma activity of BTZ but protected the nerve Schwann cells; use of antioxidants may hold a clinical benefit by reducing BTZ-induced peripheral neuropathy [40].
In summary, NF-κB signaling stimulates transcription of proteins that promote myeloma cell survival, inhibit apoptosis, and induce chemo- and radio-resistance. The present study provides insight into the molecular mechanism of the anti-myeloma activity of BTZ and Dex via the NF-κB signaling axis for potential clinical benefit in MM.
Acknowledgements
The authors thank the Radiation and Free Radical Research Core Facility (The University of Iowa, P30 CA086862) for their services. We thank Chetana Davis and Dr. Soumen Bera for technical assistance with experiments. We thank Cedar Ridge Medical Writing for their editorial services and Gareth Smith for editing illustrations. This work was supported by National Institutes of Health Grants [CA127958 (AG), T32CA078586 (KS)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tricot G. New insights into role of microenvironment in multiple myeloma. Lancet. 2000;355:248–250. doi: 10.1016/S0140-6736(00)00019-2. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KC, Pazdur R, Farrell AT. Development of effective new treatments for multiple myeloma. J Clin Oncol. 2005;23:7207–7211. doi: 10.1200/JCO.2005.02.4950. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor P, Ramakrishnan V, Rajkumar SV. Bortezomib combination therapy in multiple myeloma. Semin Hematol. 2012;49:228–242. doi: 10.1053/j.seminhematol.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilmore TD. Multiple myeloma: lusting for NF-kappaB. Cancer Cell. 2007;12:95–97. doi: 10.1016/j.ccr.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Hideshima T, Podar K, Chauhan D, Anderson KC. Cytokines and signal transduction. Best Pract Res Clin Haematol. 2005;18:509–524. doi: 10.1016/j.beha.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist. 2007;12:664–689. doi: 10.1634/theoncologist.12-6-664. [DOI] [PubMed] [Google Scholar]
- 9.Bera S, Greiner S, Choudhury A, et al. Dexamethasone-induced oxidative stress enhances myeloma cell radiosensitization while sparing normal bone marrow hematopoiesis. Neoplasia. 2010;12:980–992. doi: 10.1593/neo.101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown CO, Salem K, Wagner BA, et al. Interleukin-6 counteracts therapy-induced cellular oxidative stress in multiple myeloma by up-regulating manganese superoxide dismutase. Biochem J. 2012;444:515–527. doi: 10.1042/BJ20112019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauhan D, Uchiyama H, Akbarali Y, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- 12.Sharma S, Lichtenstein A. Dexamethasone-induced apoptotic mechanisms in myeloma cells investigated by analysis of mutant glucocorticoid receptors. Blood. 2008;112:1338–1345. doi: 10.1182/blood-2007-11-124156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goel A, Dispenzieri A, Greipp PR, Witzig TE, Mesa RA, Russell SJ. PS-341-mediated selective targeting of multiple myeloma cells by synergistic increase in ionizing radiation-induced apoptosis. Exp Hematol. 2005;33:784–795. doi: 10.1016/j.exphem.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Goel A, Dispenzieri A, Geyer SM, Greiner S, Peng KW, Russell SJ. Synergistic activity of the proteasome inhibitor PS-341 with non-myeloablative 153-Sm-EDTMP skeletally targeted radiotherapy in an orthotopic model of multiple myeloma. Blood. 2006;107:4063–4070. doi: 10.1182/blood-2005-09-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenstein S, Krett NL, Kurosawa Y, et al. Characterization of the MM-1 human multiple myeloma (MM) cell lines: a model system to elucidate the characteristics, behavior, and signaling of steroid-sensitive and -resistant MM cells. Exp Hematol. 2003;31:271–282. doi: 10.1016/s0301-472x(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 16.Hardin J, MacLeod S, Grigorieva I, et al. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994;84:3063–3070. [PubMed] [Google Scholar]
- 17.Chauhan D, Pandey P, Hideshima T, et al. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J Biol Chem. 2000;275:27845–27850. doi: 10.1074/jbc.M003428200. [DOI] [PubMed] [Google Scholar]
- 18.Chauhan D, Auclair D, Robinson EK, et al. Identification of genes regulated by dexamethasone in multiple myeloma cells using oligonucleotide arrays. Oncogene. 2002;21:1346–1358. doi: 10.1038/sj.onc.1205205. [DOI] [PubMed] [Google Scholar]
- 19.Hideshima T, Chauhan D, Richardson P, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277:16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 20.Hideshima T, Ikeda H, Chauhan D, et al. Bortezomib induces canonical nuclear factorkappaB activation in multiple myeloma cells. Blood. 2009;114:1046–1052. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Chen S, Yue P, et al. Proteasome inhibitor PS-341 (bortezomib) induces calpaindependent IkappaB(alpha) degradation. J Biol Chem. 2010;285:16096–16104. doi: 10.1074/jbc.M109.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 23.Perrone G, Hideshima T, Ikeda H, et al. Ascorbic acid inhibits antitumor activity of bortezomib in vivo. Leukemia. 2009;23:1679–1686. doi: 10.1038/leu.2009.83. [DOI] [PubMed] [Google Scholar]
- 24.Flohe L, Brigelius-Flohe R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kappa B activation. Free Radic Biol Med. 1997;22:1115–1126. doi: 10.1016/s0891-5849(96)00501-1. [DOI] [PubMed] [Google Scholar]
- 25.Goel A, Spitz DR, Weiner GJ. Manipulation of cellular redox parameters for improving therapeutic responses in B-cell lymphoma and multiple myeloma. J Cell Biochem. 2012;113:419–425. doi: 10.1002/jcb.23387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tome ME, Jaramillo MC, Briehl MM. Hydrogen peroxide signaling is required for glucocorticoid-induced apoptosis in lymphoma cells. Free Radic Biol Med. 2011;51:2048–2059. doi: 10.1016/j.freeradbiomed.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corre J, Avet-Loiseau H. The impact of genomics on the management of myeloma. J Natl Compr Canc Netw. 2011;9:1200–1206. doi: 10.6004/jnccn.2011.0097. [DOI] [PubMed] [Google Scholar]
- 28.Palumbo A, Rajkumar SV. Treatment of newly diagnosed myeloma. Leukemia. 2009;23:449–456. doi: 10.1038/leu.2008.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 30.Frankfurt O, Rosen ST. Mechanisms of glucocorticoid-induced apoptosis in hematologic malignancies: updates. Curr Opin Oncol. 2004;16:553–563. doi: 10.1097/01.cco.0000142072.22226.09. [DOI] [PubMed] [Google Scholar]
- 31.Lonial S, Waller EK, Richardson PG, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106:3777–3784. doi: 10.1182/blood-2005-03-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144:895–903. doi: 10.1111/j.1365-2141.2008.07573.x. [DOI] [PubMed] [Google Scholar]
- 33.Yuan ZG, Jin J, Huang XJ, et al. Different dose combinations of bortezomib and dexamethasone in the treatment of relapsed or refractory myeloma: an open-label, observational, multi-center study in China. Chin Med J (Engl) 2011;124:2969–2974. [PubMed] [Google Scholar]
- 34.Gilmore TD, Garbati MR. Inhibition of NF-kappaB signaling as a strategy in disease therapy. Curr Top Microbiol Immunol. 2011;349:245–263. doi: 10.1007/82_2010_105. [DOI] [PubMed] [Google Scholar]
- 35.Hideshima T, Chauhan D, Schlossman R, Richardson P, Anderson KC. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene. 2001;20:4519–4527. doi: 10.1038/sj.onc.1204623. [DOI] [PubMed] [Google Scholar]
- 36.Giuliani N, Rizzoli V, Roodman GD. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood. 2006;108:3992–3996. doi: 10.1182/blood-2006-05-026112. [DOI] [PubMed] [Google Scholar]
- 37.Genty V, Dine G, Dufer J. Phenotypical alterations induced by glucocorticoids resistance in RPMI 8226 human myeloma cells. Leuk Res. 2004;28:307–313. doi: 10.1016/j.leukres.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Krappmann D, Emmerich F, Kordes U, Scharschmidt E, Dorken B, Scheidereit C. Molecular mechanisms of constitutive NF-kappaB/Rel activation in Hodgkin/Reed-Sternberg cells. Oncogene. 1999;18:943–953. doi: 10.1038/sj.onc.1202351. [DOI] [PubMed] [Google Scholar]
- 39.Hideshima T, Chauhan D, Hayashi T, et al. Proteasome inhibitor PS-341 abrogates IL-6 triggered signaling cascades via caspase-dependent downregulation of gp130 in multiple myeloma. Oncogene. 2003;22:8386–8393. doi: 10.1038/sj.onc.1207170. [DOI] [PubMed] [Google Scholar]
- 40.Nakano A, Abe M, Oda A, et al. Delayed treatment with vitamin C and N-acetyl-Lcysteine protects Schwann cells without compromising the anti-myeloma activity of bortezomib. Int J Hematol. 93:727–735. doi: 10.1007/s12185-011-0850-7. [DOI] [PubMed] [Google Scholar]