Abstract
This overview focuses on bioconjugates of water-soluble polymers with low molecular weight drugs and proteins. After a short discussion of the origins of the field, the state-of-the-art is reviewed. Then research directions needed for the acceleration of the translation of nanomedicines into the clinic are outlined. Two most important directions, synthesis of backbone degradable polymer carriers and drug-free macromolecular therapeutics, a new paradigm in drug delivery, are discussed in detail. Finally, the future perspectives of the field are briefly discussed.
Keywords: Nanomedicine, bioconjugates, cancer, degradable spacers, long-circulating polymeric drug carriers, N-(2-hydroxypropy)methacrylamide), drug-free macromolecular therapeutics
1. Introduction
Hydrophilic polymers are widely used in medicine both in soluble and insoluble (hydrogel) forms. Water-soluble polymers have been used in the clinics and/or clinical trials for the modification of proteins, modification of liposomes, surface modification of biomaterials, and as carriers of drugs, genes, and oligonucleotides.
Neutral, synthetic water-soluble polymers are inert in the organism. Moieties incorporated into the macromolecular structure that complement cell surface receptors or antigens on a subset of cells render the macromolecule biorecognizable [1-4]. For efficiency, targetable polymer – drug conjugates should be biorecognizable at two levels: at the plasma membrane, eliciting selective recognition and internalization by a subset of target cells [5,6], and intracellularly, where lysosomal enzymes induce the release of drug from the carrier [3,7-11]. The latter is a prerequisite for transport of the drug across the lysosomal membrane into the cytoplasm and translocation into the organelle decisive for biological activity. Designs are being evaluated, where the drug, after being released from the carrier, is channeled into a specific subcellular compartment (e.g., mitochondria [12,13] or nucleus [14,15]) to maximize its activity. The design principles or polymer-drug conjugates have been identified and summarized [1]; this bodes well for the future design and synthesis of more effective conjugates.
This overview focuses on water-soluble polymers and their drug and protein conjugates. The discussion focuses mainly on N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer conjugates. However, the design principles are generally valid for other hydrophilic water-soluble polymeric carriers. Modification of vesicular carriers, liposomes and nanoparticles with water-soluble polymers are mentioned to highlight the advantages of polymer modification. The achievements and state-of-the art in polymer bioconjugates are reviewed, followed by a brief discussion of research needed to speed up the translation of the conjugates into the clinic. Finally, new approaches are discussed and the future of the field briefly outlined.
2. Origins
The conjugation of drugs to synthetic and natural macromolecules was initiated nearly sixty years ago – for reviews of early work see refs. [16,17]. Jatzkewitz used a dipeptide (GL) spacer to attach a drug (mescaline) to polyvinylpyrrolidone in the early fifties [18] and Ushakov's group synthesized numerous water-soluble polymer – drug conjugates in the sixties and seventies, focusing on conjugates of polyvinylpyrrolidone and various antibiotics [19-21]. Mathé et al. pioneered conjugation of drugs to immunoglobulins, setting the stage for targeted delivery [22]. DeDuve discovered that many enzymes are localized in the lysosomal compartment of the cell and the lysosomotropism of macromolecules [23], important phenomena for the design of polymer-drug conjugates. Finally, Ringsdorf presented a clear concept of the use of polymers as targetable drug carriers [4].
Detailed studies of the biocompatibility of soluble [24-28] and crosslinked [29-35] hydrophilic polymers in author's laboratory lead to application of hydrogels in the clinic [36] and to the selection of HPMA polymers and copolymers as biocompatible drug carriers [37,38]. Comprehensive studies of the enzyme-catalyzed cleavage of oligopeptide sequences in hybrid HPMA copolymers [3,7-11,39-44] resulted in the choice of the GFLG oligopeptide spacer as a drug attachment/release site [7]; it has been used in numerous studies worldwide.
The early research on HPMA copolymer-drug conjugates, interdisciplinary collaborations with John Lloyd, Ruth Duncan, Blanka Říhová and others have been described in detail in a previous review [2]. Milestones in HPMA (co)polymer research are summarized in Table 1 of ref. [2].
HPMA copolymer-based macromolecular therapeutics have been evaluated in clinical trials for therapeutic validation since early 1990s. These include HPMA copolymer-doxorubicin (DOX) [45-47], HPMA copolymer-DOX-galactosamine [48], HPMA copolymer-camptothecin [49], HPMA copolymer-paclitaxel [50], and HPMA copolymer-platinates [51]. Results have proven the advantages of the concept of binding low molecular weight drugs to polymer carriers, biocompatibility of the conjugates, and the decrease of side effects. The latter resulted in a higher maximum tolerated dose (MTD) of polymer conjugates (expressed in drug equivalent) when compared to free drugs.
3. State-of-the-art
3.1 Targeted vs. non-targeted conjugates
The impact of a targeting moiety on the activity of polymer-drug conjugates is an intensely discussed topic. The answer will depend on the type of tumor and the structure of conjugate. I shall present three examples that differ in the need for a targeting moiety:
a) Targeting might not be needed
In the treatment of solid tumors the enhanced permeability and retention (EPR) effect [52,53] and manipulation of molecular weight [54,55] may be sufficient for the design of effective conjugates. Interestingly, in solid tumor animal models, targeted conjugates demonstrated an enhancement of efficacy when compared to non-targeted ones [56,57]; however, part of the enhancement may be attributed to the increased molecular weight of antibody targeted conjugates. An example of the new design of efficient non-targeted polymer-drug conjugates is discussed in section 4.1.
b) Targeting might be beneficial, but more data need to be acquired
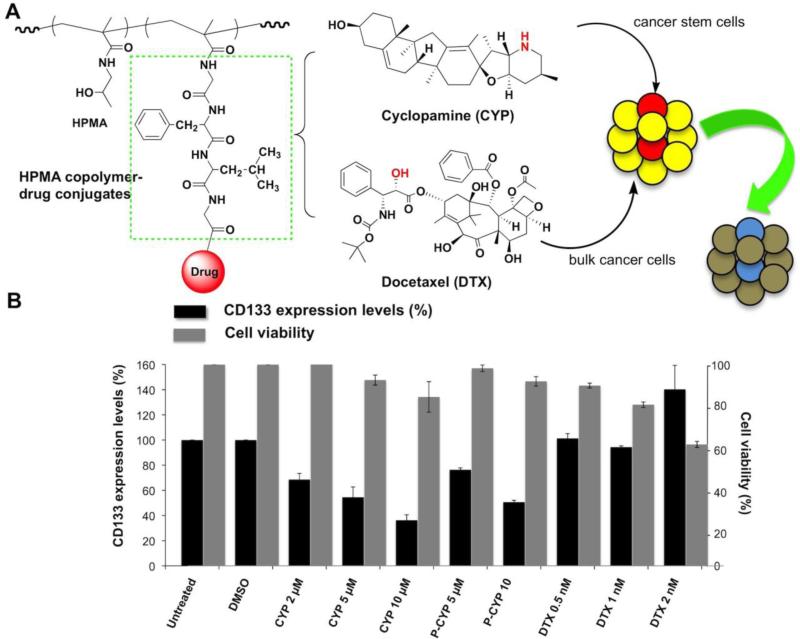
The second example relates to the development of prostate cancer stem cell (CSC) therapies. This involves conjugates whose selectivity is based on the cell phenotype, a rationale where the effect of targeting may be important, but not enough information is available. CSCs are undifferentiated cells with the ability to self-renew and differentiate to the phenotypically diverse tumor cell population; consequently, they are capable to generate a continuously growing tumor [58-60]. A challenge for the elimination of CSCs is their inherent resistance to therapies, which target differentiated target cells. Thus, we proposed, for the treatment of prostate cancer, a combination of two polymeric drugs, one targeting CSCs, the other differentiated cells [61]. As the CSC selective conjugate we designed an HPMA copolymer-cyclopamine conjugate (P-CYP). Cyclopamine (CYP), a natural steroidal alkaloid, inhibits the Hedgehog (Hh) pathway by directly binding to a membrane receptor Smoothened (SMO), suppressing SMO and its downstream activities, eventually leading to apoptotic cell death [62,63]. It is known that the blockade of Hh pathway led to down-regulation of stem cell self-renewal gene expressions, along with complete and long-term prostate cancer regression without recurrence [64]. We used docetaxel (DTX) and its HPMA copolymer conjugate to evaluate whether prostate cancer cells respond differently to various therapeutics (DTX is the traditional first line chemotherapeutic). Indeed, using a prostate cancer epithelial cell line RC-92a/hTERT, the selectivity of CYP and its HPMA copolymer conjugate (P-CYP) toward a subset of CD133 positive cells was observed (Figure 1) [61]. Apparently, using conjugates targeted by antiCD133 antibodies (or fragments) should enhance the efficacy. This hypothesis, however, needs to be validated.
Figure 1.
A) The design of the combination HPMA copolymer-based macromolecular therapeutics for improving the treatment of prostate cancer, by targeting both bulk cancer cells and prostate cancer stem cells (CSC)s. B) Summary of changes of CD133+ prostate CSCs and whole cell viabilities following in vitro exposure of RC-92a/hTERT prostate cancer cells to HPMA copolymer-cyclopamine conjugate (P-CYP), free cyclopamine (CYP) or docetaxel (DTX). Black columns: CD133 expression level (%); gray columns: cell viability (%). The data are presented as mean±SD of the experiments done in triplicate. *, p < 0.05; **, p < 0.01. Vehicle (DMSO) treated and untreated cells were used as controls. Adapted with from [61].
c) Targeting is beneficial
In the treatment of blood cancers, such as Non-Hodgkin's lymphoma, the advantages of targeted conjugates are obvious. An example of such conjugates are the drug-free macromolecular therapeutics (discussed in section 4.2); their activity depends on two consequent recognition events: recognition of the Fab’ fragment by the CD20 receptor followed by recognition of two coiled-coil forming peptide sequences [65,66].
3.2 Overcoming multidrug resistance
The acquired resistance of malignant tumors to therapeutics is one of the major causes of cancer therapy failure [67]. Membrane transporters from the ATP-Binding Casette (ABC) transport proteins families (P-glycoprotein, multidrug resistance-associated proteins and others) reduce the intracellular drug concentration. The elucidation of the function of P-glycoprotein [68], other ATP-driven efflux pumps [69,70], as well as other mechanisms of multidrug resistance [71] have had a major impact on the understanding of multidrug resistance in human tumors. The exclusion of nanomedicines, including polymer-drug conjugates, from the cytoplasm of the cell, through intracellular trafficking in membrane-limited organelles, renders the efflux pumps less efficient [70]. Subcellular trafficking along the endocytic pathway from the plasma membrane to the perinuclear region changes the gradient of distribution of drugs inside cells [72,73]. The concentration gradient of free drugs is directed from the plasma membrane to the perinuclear region (in the direction of diffusion); in contrast, polymer-bound drugs, released from the carrier in the lysosomal compartment located in the perinuclear region, have a concentration gradient in the opposite direction. Consequently, the interaction/recognition of the released drug by the P-glycoprotein efflux pump is minimized [72]. Quantitative determination of intracellular DOX concentration following exposure of human ovarian carcinoma cells to free and HPMA copolymer-bound DOX showed an enhanced intracellular accumulation of HPMA copolymer-bound DOX [74]. Efficient bypassing of multidrug resistance was detected for other drug delivery systems internalized by endocytosis, namely lipid/polymer particle assemblies [75] and multicomponent delivery systems [76]. Importantly, in contrast to free DOX, HPMA copolymer-DOX conjugates did not induce multidrug resistance de novo both after acute and chronic exposure of A2780 human ovarian carcinoma cells [74,77]. An alternative approach is to use an anti-P-glycoprotein targeting antibody and a photosensitizer that is plasma membrane active [78]. The potential of polymer-drug conjugates to overcome multidrug resistance was validated in animal experiments [79,80].
3.3 Combination therapy using polymer-bound drugs
The majority of cancers are being treated by combination of drugs. First combination therapy using polymer bound drugs focused on a mixture of HPMA copolymer – DOX conjugate and HPMA copolymer – photosensitizer (meso chlorin e6 N-aminoethylamide) conjugate [81]. On two cancer models, Neuro 2A neuroblastoma [81] and human ovarian carcinoma OVCAR-3 xenografts in nude mice [82-84] it was shown that combination therapy produced cures that could not be obtained with either chemotherapy or photodynamic therapy alone. Incorporation of anti-CD47 antibodies [56] or Fab’ fragments [57] to these conjugates further increased the therapeutic efficacy.
From the synthetic and scale-up point of view it is preferable to use a mixture of two conjugates, each containing one drug. However, Vicent et al. have shown that for some drug combinations binding two drugs to the same macromolecule results in higher efficacy when compared to a mixture of two polymer drugs [85]. There are probably numerous reasons for the (rare) advantage of two drugs on one macromolecule when compared to a mixture of two conjugates containing one drug each. One may hypothesize that two drugs of similar physicochemical properties (e.g., hydrophobicity) would have similar efficacies in both scenarios due to similar body and subcellular distributions. However, when a combination of drugs with different physicochemical properties (e.g., hydrophobic and hydrophilic or neutral and charged) needs to be used, then the efficacy of a mixture of two conjugates may be different from a conjugate containing one drug each due to differences in biodistribution and subcellular pharmacokinetics.
Thus it is important to evaluate the combination system thoroughly before deciding which pathway to choose. A suitable start is to use the combination index (CI) analysis to quantify the synergism, antagonism, and additive effects of binary combinations of free and polymer-bound drugs [86,87].
Interestingly, two biologically active compounds bound to one macromolecule may possess more than two biological activities. A new macromolecular therapeutics for the treatment of bone neoplasms was designed by conjugating aminobisphosphonate alendronate (ALN), and the potent anti-angiogenic agent TNP-470 with HPMA copolymers. In this conjugate, ALN has two functions – bone-targeting moiety and antiangiogenic activity. The bi-specific HPMA copolymer conjugate reduced vascular hyperpermeability and remarkably inhibited human osteosarcoma growth in mice warranting its use on osteosarcomas and bone metastases [88,89].
3.4 Non-cancerous diseases
3.4.1 Musculoskeletal diseases
HPMA copolymer – drug conjugates may be used also for the treatment of diseases other than cancer [90]. Bone-targeted HPMA copolymer conjugated with a bone anabolic agent (prostaglandin E1; PGE1) were designed for the treatment of osteoporosis and other musculoskeletal diseases [91-98]. The biorecognition of the conjugates by the skeleton was mediated by an octapeptide of D-aspartic acid (D-Asp8) or alendronate [92,95].
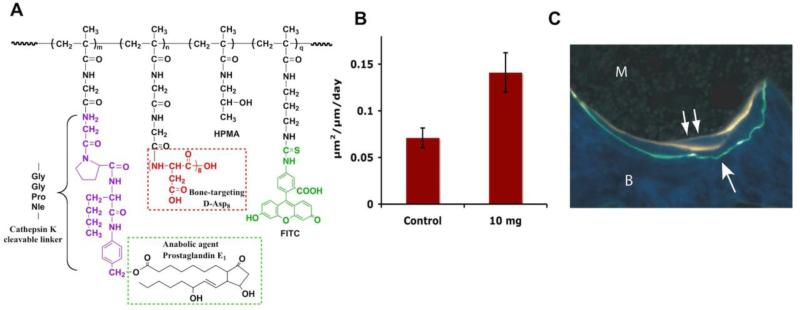
This system has the potential to deliver the bone anabolic agent, PGE1, specifically to the hard tissues after systemic administration. Once bound to bone, the PGE1 will be preferentially released at the sites of higher turnover rate (greater osteoclasts activity) via cathepsin K (osteoclast specific) catalyzed hydrolysis of a specific peptide spacer and subsequent 1,6-elimination [91,98]. When given in anabolic dosing range, the released PGE1 will activate corresponding EP receptors on bone cells surface to achieve net bone formation. The main features of the design are HPMA copolymer backbone containing cathepsin K cleavable oligopeptide side-chains (Gly-Gly-Pro-Nle) terminating in either D-Asp8 or in p-aminobenzyloxycarbonyl-1-prostaglandin E1, a PGE1 prodrug [98].
Remarkably, preferential deposition of D-Asp8-targeted conjugate to the bone resorption sites was observed in ovariectomized rats; in contrast the ALN targeted conjugates did not show a preferential bone-binding site. This strongly supports the higher turnover sites/drug-release hypothesis [95]. In vivo experiments on ovariectomized rats have proven the concept. Following a single i.v. administration of the HPMA copolymer-D-Asp8-PGE1 conjugate to aged, ovariectomized rats, bone formation rates were substantially greater than controls when measured 28 days later (Figure 2) [96].
Figure 2.
Treatment of ovariectomized rats with FITC labeled HPMA copolymer-D-Asp8-PGE1 conjugate. A) The structure of HPMA copolymer-PGE1 conjugate; B) Bone formation rate in ovariectomized rats (age > 20 months; n = 4) four weeks after one intravenous injection of 10 mg/kg of P-D-Asp8-PGE1-FITC conjugate; and C) At 4 weeks after the administration of a single injection of the HPMA copolymer-D-Asp8-PGE1, the conjugate can be seen buried in the bone (arrow) with the new bone formation occurring in the same region (double arrows), as indicated by tetracycline labels. Magnification 250x. Adapted from [97].
Obviously, a similar concept can be used for targeting bone cancer metastases. Alendronate-targeted HPMA copolymer-TNP470 conjugates were successful on animal models of bone cancer [88,89].
3.4.2 Inflammatory and infectious diseases
Wang et al. have shown that macromolecular therapeutics preferentially accumulate in inflammatory tissues [99] in general and inflammatory arthritis in particular [91,100,101]. They termed the novel targeting mechanism “ELVIS” (Extravasation through Leaky Vasculature and the subsequent Inflammatory cell-mediated Sequestration) [102,103]. This concept suggests high potential of polymer-drug conjugates in the treatment of inflammatory disease.
Successful designs were published by Ghandehari's lab on receptor-mediated antileishmanial agent – HPMA copolymer conjugates. They used NPC1161 (8-[(4-amino-1-methylbutyl)amino]-5-[3,4-dichlorophenoxy]-6-methoxy-4-methylquinoline) [104] or amphotericin B as drugs [105] and N-acylated mannosamine as targeting moiety. Similar results were reported by Nicoletti et al. [106].
3.5 Modification of proteins and vesicular carriers with water-soluble polymers
The concept to modify proteins with water-soluble polymers was initiated in the late 70s. Davis and coworkers have shown that attachment of semitelechelic (ST) poly(ethylene glycol) (PEG) to therapeutic proteins results in an increase of their resistance to proteolysis, reduction of their antigenicity, and prolongation of intravascular half-life [107]. Currently modification of proteins, liposomes, and nanoparticles with ST polymers is a widely used method [108-113].
3.5.1 Poly(ethylene glycol)
PEG is the most frequently used polymer; it is commercially available and PEG-modified proteins and liposomes were approved by the FDA. The single functional group on ST-PEG provides the opportunity to conjugate or graft the macromolecule to other species or surfaces. The extent of protein property changes depends on the degree of PEG substitution and PEG molecular weight and architecture (linear or branched) [111-113]. Numerous proteins modified with PEG have been FDA approved for clinical use, including: adenosine deaminase [114], asparaginase [115], interferons α2a [116] and α2b [117], G-CSF (granulocyte colony-stimulating factor) [118], anti-TNFα Fab’ [119], and uricase [120]. Similarly, PEG was used for the modification of (stealth) liposomes that possess longer circulation half-lives due to decreased recognition by macrophages of the reticuloendothelial system [121]. Similar results were obtained following modification of nanoparticles with PEG [122]. Thus, PEG possesses a distinguished record of clinical successes.
3.5.2 Accelerated blood clearance, vacuolation, and elimination of PEG conjugates
Wide clinical applications of PEG modified proteins and vesicles benefited numerous patients. However, the following issues may need attention in the near future. Repeating administration of PEGylated liposomes results in decreased circulation time [123-130]. Apparently, anti-PEG IgM antibodies are induced by the first dose. After second injection, IgM selectively binds to the surface of PEGylated liposomes, leading to complement activation. Response depends on physicochemical properties of injected liposomes as a first dose, time interval between injection, lipid dose, and drug encapsulation [131,132]. Similar results were observed with PEG modified nanoparticles [130,133]. Rapid clearance of PEG-asparaginase has been reported for up to one-third of patients treated with acute lymphoblastic leukemia (ALL), potentially rendering their treatment ineffective [129]. Similarly, in phase I clinical trials of PEG-uricase 5 out of 13 patients developed low titer IgM and IgG antibodies directed against PEG [124].
Since doxorubicin and mitotoxantrone loaded liposomes appear not to induce the accelerated blood clearance (ABC) phenomenon, not enough attention was devoted to this problem. However, Ma et al. [123] have shown that topotecan loaded liposomes induce the ABC phenomenon. They propose that the difference is due to the impact of drugs on the cell cycle. Non-cell cycle specific drugs (doxorubicin and mitoxantrone) prevent the ABC phenomenon by the reticuloendothelial system (RES) and B cell blockade, whereas cell cycle specific drug (topotecan) may induce ABC [123]. This hypothesis is worth pursuing in further experiments.
Additional biocompatibility issues with PEG [124] are vacuolation, i.e., formation of cytoplasmic vacuoles in cortical tubular epithelial cells [125] and unclear routes of elimination from the organism [136], since the hydrodynamic volume of PEGs used for modification of vesicular carriers and proteins is above the renal threshold.
3.5.3 Alternate approaches
Alternate approaches should focus on the use of water-soluble polymers of different structures. Several structures have been evaluated including polyoxazolines [137,138], poly(N-vinyl-2-pyrrolidone) (PVP) [133], polyacryloylmorpholine [133], poly(N,N-dimethylacrylamide) [133], and polyHPMA [108,139,140]. Repeated administration of nanoparticles coated with PVP did not produce the ABC phenomenon and antibodies against PVP were not detected [133]. Poly(HPMA) exhibits similar properties as poly(ethylene glycol) when used for modification of enzymes or vesicular carriers. Modification of nanospheres, based on a copolymer of methyl methacrylate, maleic anhydride, and methacrylic acid, with ST-polyHPMA resulted in decreased protein adsorption in vitro and increased intravascular half-life, as well as decreased accumulation in the liver, after intravenous administration into rats. The higher the molecular weight of ST-polyHPMA, the more pronounced the changes in these properties [140]. These data seem to indicate the influence of the hydrodynamic thickness of the coating layer on the process of opsonization and capture by Kupffer cells of the liver and macrophages of the spleen [140].
Similarly, carboxyl and amino group modification of chymotrypsin with ST-polyHPMA-CONHNH2 and ST-polyHPMA-COOSu (N-hydroxysuccinimide ester) produced conjugates [139] with comparable properties to PEG-modified chymotrypsin [109].
Another option is to modify proteins via multipoint attachments; this also results in enhanced intravascular half-life. For example, cobra venom acetylcholinesterase was modified with activated polyHPMA. The secondary OH groups of poly(HPMA) (Mw 25-30 kDa) were activated with 4-nitrophenyl chloroformate in dimethylformamide followed by attachment of acetylcholinesterase in borate buffer. The poly(HPMA)-modified acetylcholinesterase demonstrated a 70-fold prolongation of enzyme activity in blood after intravenous injection into mice when compared to unmodified enzyme. In addition, the thermoinactivation rate of the polyHPMA-acetylcholinesterase conjugate was 74 times smaller that that of native enzyme [141]. A similar concept is the lateral modification of polyelectrolyte complexes of polycations (poly-L-lysine or polyethyleneimine) with DNA by multivalent HPMA copolymers. The intravascular half-life of the unmodified complex (<5 min in mice) could be extended by the multivalent attachment of polyHPMA to > 90 minutes [142].
4. Suggestions for design improvement to enhance the speed of translation
The advantages of polymer-drug conjugates over free drugs have been well documented [1,2, 134,143-145]. In clinical trials, the biocompatibility of synthetic polymer carriers (HPMA copolymer) has been proven as well as the decrease of non-specific side effects when compared to low molecular weight drugs [45,146]. However, the translation of laboratory research into the clinics has been slow.
The results of numerous studies up to date provide leads for the research directions needed that could speed-up the translation. To this end, the following paths should be explored [2,147]: design of long-circulating conjugates to enhance the accumulation in solid tumors due to the EPR effect; relationship between the structure of conjugates and the mechanism of internalization and subcellular trafficking; optimization of the structure, architecture [148-150], and conformation [151] of multifunctional and multivalent conjugates; mechanism of action specific for macromolecular therapeutics; and further studies on combination therapy with polymer-bound drugs. Last but not least, new design paradigms based on totally new concepts should be pursued. It appears, however, that two design strategies are at present the most important to pursue: design of long-circulating conjugates and new design paradigms. Let's discuss both.
4.1 Design, synthesis and evaluation of long-circulating, backbone degradable HPMA copolymer-conjugates
It is well established that high molecular weight (long-circulating) polymer conjugates accumulate efficiently in solid tumor tissue due to the enhanced permeability and retention (EPR) effect [53]. However, the molecular weight and the intravascular half-life of the HPMA copolymer conjugates evaluated in clinical trials have been suboptimal [147]. To achieve substantial accumulation of the polymer-drug conjugate in solid tumor (due to the EPR effect) a sustained concentration gradient is needed. The concentration depends on the administered dose and the circulation time depends on the molecular weight of the carrier. However, higher molecular weight drug carriers with a nondegradable backbone deposit and accumulate in various organs, impairing biocompatibility. Previous attempts to design and synthesize long-circulating conjugates produced branched, partially crosslinked copolymers with enzymatically degradable sequences [152]. The synthetic process and the polymer structure were difficult to control; consequently, the reproducibility was poor. Nevertheless, the results proved that higher molecular weight of carrier transfers into higher accumulation in the tumor tissue with concomitant enhancement of efficacy [153].
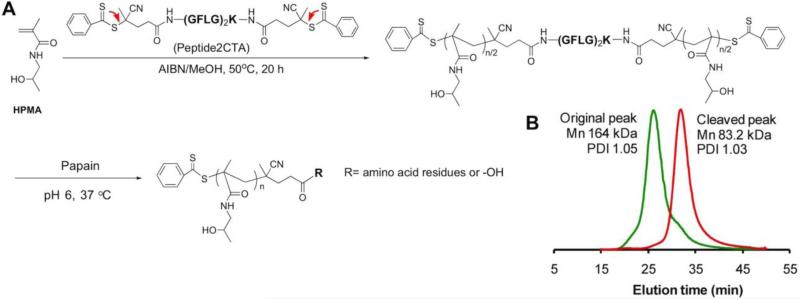
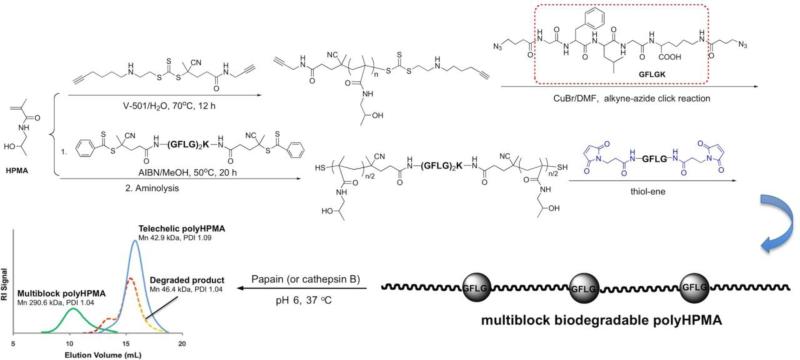
To this end we designed new, second-generation anticancer nanomedicines based on high molecular weight HPMA copolymer - drug carriers containing enzymatically degradable bonds in the main chain (polymer backbone) [154-156]. The proposed new design permits tailor-made synthesis of well-defined backbone degradable HPMA copolymers. The synthetic process consists of two main steps: first, the synthesis of a telechelic HPMA copolymer by reversible addition-fragmentation chain transfer (RAFT) polymerization, followed in the second step by chain extension using alkyne-azide [154,155] or thiol-ene [156] click reactions. In addition, we synthesized a new RAFT CTA, Nα,Nε-bis(4-cyano-4-(phenylcarbonothioylthio)pentanoyl glycylphenylalanylleucylglycyl)lysine (Peptide2CTA), containing an enzymatically degradable (GFLG) spacer; the enzymatically degradable oligopeptide sequence was capped at both ends with 4-cyano-4-(phenylcarbonothioylthio)pentanoate. During RAFT polymerization the HPMA monomers incorporate at both dithiobenzoate groups of the Peptide2CTA with identical efficiency. When the final polymer was incubated with papain, a thiol proteinase with similar specificity as lysosomal proteinases, the molecular weight decreased to half of the original value. Thus it is possible to prepare a degradable diblock copolymer of narrow molecular weight distribution in one step, eliminating the chain extension reaction (Figure 3) [156].
Figure 3.
Desing of a RAFT chain transfer agent that contains two active sites connected via an enzyme sensitive sequence (Peptide2CTA) permits the synthesis of a biodegradable diblock copolymer in one step. (A) Polymerization of HPMA in methanol mediated by Peptide2CTA and 2,2’azobisisobutyronitrile (AIBN). [HPMA]0/[CTA]0 = 2000, [HPMA]0 = 1 M; [CTA]0/[AIBN]0 = 2.5. (B) Following incubation of the polyHPMA with papain, the molecular weight decreased to half of the original polymer, indicating that the monomers inserted with equal efficacy at both dithiobenzoate sites. Adapted from [156].
Multiblock polyHPMAs with Mw as high as 300 kDa and containing degradable GFLG sequences were obtained by chain extension followed by fractionation using size exclusion chromatography (SEC). The exposure of the multiblock HPMA copolymer to model enzyme papain or lysosomal cathepsin B (pH 6, 37 °C) resulted in complete degradation of GFLG segments and decrease of the molecular weight of the carrier to the initial one (Figure 4) [156]. These data support our hypothesis and bode well for the success of the proposed design of backbone degradable HPMA copolymers composed of alternating segments of HPMA copolymer, with molecular weight below the renal threshold, and lysosomally degradable GFLG containing oligopeptides.
Figure 4.
Rationale of the design of new long-circulating anticancer nanomedicines based on a degradable multiblock polymeric carrier. First, a semitelechelic polymer is synthesized by RAFT polymerization, followed by chain extension via azide-alkyne or thiol-ene click reaction [154-156].
The enhanced activity of 2nd generation of conjugates has been proven in vivo. Long-circulating backbone degradable HPMA copolymer conjugates with doxorubicin [157], paclitaxel [158], or gemcitabine [unpublished data] demonstrated higher efficacy in suppressing the growth of human ovarian carcinoma xenografts in nude mice than 1st generation conjugates (non-degradable, Mw below the renal threshold). Similarly, bone-targeted long-circulating conjugates containing prostaglandin E1 had higher accumulation on bone tissue and greater indices of bone formation in an ovariectomized rat osteoporosis model [unpublished data].
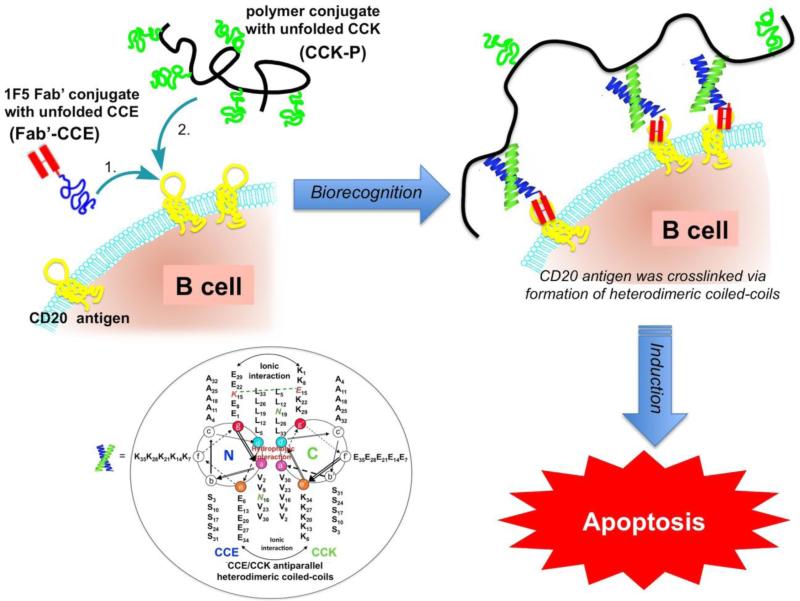
4.2 New paradigm: Drug-free macromolecular therapeutics
We recently designed, synthesized and evaluated a new concept for the treatment of B cell Non-Hodgkin lymphoma (NHL). It is based on the biorecognition of coiled-coil peptides at the cell surface and crosslinking of CD20 receptors without the involvement of low molecular weight drugs [65,66].
As with any well-designed drug delivery system, the biological target is of great importance. CD20 is one of the most reliable cell surface markers of B lymphocytes [159,160]. CD20 is expressed on most NHL malignant cells as well as on normal B cells. However, it is not expressed on stem cells and mature plasma cells. Consequently, normal numbers of B cells can be restored after treatment [161]. Clinical success was reached when Rituximab, a human-murine antibody (Ab) chimera, received FDA approval for the treatment of NHL [162-165]. Although treatments for NHLs have made great improvements, refractive malignancies still occur that are nonresponsive to current therapies in about half of all patients, indicating that improved treatment strategies are needed [166].
The CD20 is a non-internalizing receptor; its crosslinking by multifunctional conjugates, such as HPMA copolymers containing multiple (anti-CD20 1F5 antibody) Fab’ fragments [167-169], dextran – antibody (Rituximab) conjugates [170], and Rituximab dimers [171] results in apoptosis.
The design of drug-free macromolecular therapeutics is based on the biological rationale mentioned above and on our previous work on a hybrid self-assembled system in which a pair of oppositely charged pentaheptad (35 amino acid) peptides (CCE and CCK) was designed and attached to HPMA copolymer backbone, respectively. Individually, CCE and CCK are random coils, but their equimolar mixture formed antiparallel coiled-coil heterodimers and served as physical crosslinkers [172]. HPMA graft copolymers, CCE-P and CCK-P (P is the HPMA copolymer backbone), self-assembled into hybrid hydrogels with a high degree of biorecognition [173,174].
We hypothesized that this unique biorecognition of CCK and CCE peptide motifs could be applied to a living system and mediate a biological process. This would provide a bridge between the designs of biomaterials and macromolecular therapeutics. Indeed, the biorecognition of CCE/CCK peptide motifs at the cellular surface is able to initiate apoptosis. Exposure of Raji B cells to an anti-CD20 Fab’-CCE conjugate decorated the cell surface with CCE (CD20 is a non-internalizing receptor) through antigen-antibody fragment recognition. Further exposure of the decorated cells to CCK-P (grafted with multiple copies of CCK) resulted in the formation of CCE/CCK coiled-coil heterodimers at the cell surface. This second biorecognition induced the crosslinking of CD20 receptors and triggered the apoptosis of Raji B cells in vitro [65] and in a Non-Hodgkin lymphoma animal model in vivo [66]. This is a new concept, where the biological activity of drug-free macromolecular therapeutics is based on the biorecognition of peptide motifs (Figure 5) [174].
Figure 5.
Design of drug-free macromolecular therapeutics. Induction of apoptosis in human Burkitt's non-Hodgkin's lymphoma (NHL) Raji B cells by crosslinking of its CD20 antigens mediated by antiparallel coiled-coil heterodimer formation at the cell surface. Fab’-CCE is a conjugate of the Fab’ fragment of the 1F5 antibody and the CCE peptide (YGGEVSALEKEVSALEKKNSALEKEVSALEKEVSALEK); CCK-Polymer is a HPMA copolymer containing 9 grafts of the CCK peptide (CYGGKVSALKEKVSALKEEVSANKEKVSALKEKVSALKE) per macromolecule. Inset: Helical wheel representation of CCE/CCK coiled-coil heterodimers. The view is shown looking down the superhelical axis from the N-terminus of CCE and from the C-terminus of CCK. CC denotes coiled-coil peptides, E and K denote peptides in which most of e and g positions are occupied by either glutamic acid or lysine, respectively. Adapted from [65,66].
5. Conclusions and future prospects
The advantages of macromolecular therapeutics over low molecular weight drugs have been recognized in preclinical evaluation on numerous cancer models and in clinical trials [175-177]. Nevertheless, the translation into the clinic is the major challenge of the field [178].
The field of water-soluble polymer-drug conjugates is at crossroads. Scientifically, the design principles for bioconjugates are well defined; the challenge is to combine the efficient design of the conjugates with the understanding of the biological features of cancer, including heterogeneity of cancer cells, tumor microenvironment, and metastasis [60,61].
The progress will occur on several levels, including: A) Continuous progress of our knowledge resulting in the design of bioconjugates with higher activities. Some examples of these strategies were described above, such as design of conjugates for the treatment of musculoskeletal diseases, combination therapy using polymer-bound drugs, and backbone-degradable long-circulating conjugates. Additional topics include new targeting strategies by identification of targeting peptides by combinatorial chemistry and other techniques [179-180], analysis of interplay of individual factors in multivalent conjugates on the final properties [151], identification of signaling pathways that are specific for macromolecular therapeutics [181], design of conjugates targeting stem cells [60,61], immunomodulating activities of macromolecular therapeutics [182], design of polymer conjugates as anti-angiogenic agents [183], and conjugates capable of subcellular targeting [12-15,184,185]. B) The remarkable progress in imaging techniques that permits non-invasive monitoring of the fate of conjugates will undoubtedly contribute to a more rational design of polymer therapeutics and theranostics [186-194]. C) Qualitative change in the approach to design and treatment. This includes manipulation of tumor microenvironment and new, non-conventional approaches to research. An example of approaches that could improve efficacy of nanomedicines via modification of the cancer environment is the technique of Provenzano et al. [195]. They improved the access to pancreatic ductal adenocarcinoma by remodeling the stroma by administration of PEG-modified recombinant human hyaluronidase. Combined enzyme and cytotoxic therapy resulted in the decrease of interstitial fluid pressure and better access of the drug (gemcitabine) to the tumor [195]. Similar approaches would benefit macromolecular therapeutics. Examples of non-conventional approaches to research are the design of genetically engineered polymers capable to store and propagate information [196,197] and application of design principles from biomaterials to nanomedicines to create a cytotoxic system, drug-free macromolecular therapeutics, where the low molecular weight drug is not needed [65,66].
Finally, I strongly believe that the interdisciplinary approach to the science and applications of polymer-drug conjugates will result in their translation into the clinic within this decade.
Acknowledgements
The research in the author's laboratory was supported in part by the National Institutes of Health (recently NIH grants CA51578, CA132831, GM69847, EB5288, GM95606, and CA156933), Department of Defense grant W81XWH-04-1-0900, and the University of Utah Research Foundation. I thank all past and present coworkers and numerous collaborators. I am truly indebted to all of them; their scientific contributions are reflected in the references.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kopeček J, Kopečková P. Design of polymer-drug conjugates. In: Kratz F, Senter P, Steinhagen H, editors. Drug Delivery in Oncology. Chapter 17. Vol. 2. Wiley-VCH; Weinheim, Germany: 2012. pp. 485–512. [Google Scholar]
- 2.Kopeček J, Kopečková P. HPMA copolymers: Origins, early developments, present, and future. Adv. Drug Delivery Rev. 2010;62:122–149. doi: 10.1016/j.addr.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopeček J. Controlled degradability of polymers – a key to drug delivery systems. Biomaterials. 1984;5:19–25. doi: 10.1016/0142-9612(84)90062-0. [DOI] [PubMed] [Google Scholar]
- 4.Ringsdorf H. Structure and properties of pharmacologically active polymers. J. Polym. Sci., Polym. Symp. 1975;51:135–153. [Google Scholar]
- 5.Říhová B, Kopeček J. Biological properties of targetable poly[N-(2-hydroxypropyl)methacrylamide] - antibody conjugates. J. Controlled Release. 1985;2:289–310. [Google Scholar]
- 6.Duncan R, Kopeček J, Rejmanová P, Lloyd JB. Targeting of N-(2-hydroxypropyl)methacrylamide copolymers to liver by incorporation of galactose residues. Biochim. Biophys. Acta. 1983;755:518–521. doi: 10.1016/0304-4165(83)90258-1. [DOI] [PubMed] [Google Scholar]
- 7.Rejmanová P, Pohl J, Baudyš M, Kostka V, Kopeček J. Polymers containing enzymatically degradable bonds. 8. Degradation of oligopeptide sequences in N-(2-hydroxypropyl)methacrylamide copolymers by bovine spleen cathepsin B. Makromol. Chem. 1983;184:2009–2020. [Google Scholar]
- 8.Šubr V, Kopeček J, Pohl J, Baudyš M, Kostka V. Cleavage of oligopeptide side-chains in N-(2-hydroxypropyl)methacrylamide copolymers by mixtures of lysosomal enzymes. J. Controlled Release. 1988;8:133–140. [Google Scholar]
- 9.Kopeček J. Biodegradation of polymers for biomedical use. In: Benoit H, Rempp P, editors. IUPAC Macromolecules. Pergamon; Oxford: 1982. pp. 305–320. [Google Scholar]
- 10.Kopeček J, Rejmanová P. Enzymatically degradable bonds in synthetic polymers. In: Bruck SD, editor. Controlled Drug Delivery. I. CRC Press; Boca Raton, Florida: 1983. pp. 81–124. [Google Scholar]
- 11.Kopeček J, Rejmanovå R, Duncan R, Lloyd JB. Controlled release of drug model from N-(2-hydroxypropyl)methacrylamide copolymers. Ann N.Y. Acad. Sci. 1985;446:93–104. doi: 10.1111/j.1749-6632.1985.tb18393.x. [DOI] [PubMed] [Google Scholar]
- 12.Callahan J, Kopeček J. Semitelechelic HPMA copolymers functionalized with triphenylphosphonium as drug carriers for membrane transduction and mitochondrial localization. Biomacromolecules. 2006;7:2347–2356. doi: 10.1021/bm060336m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuchelkar V, Kopečková P, Kopeček J. Novel HPMA copolymer-bound constructs for combined tumor and mitochondrial targeting. Molecular Pharmaceutics. 2008;5:776–786. doi: 10.1021/mp800019g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callahan J, Kopečková P, Kopeček J. Intracellular trafficking and subcellular distribution of a large array of HPMA copolymers. Biomacromolecules. 2009;10:1704–1714. doi: 10.1021/bm801514x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuchelkar V. Ph.D. Dissertation. Department of Bioengineering, University of Utah; 2008. Strategies to enhance the photodynamic effect of N-(2-hydroxypropyl)methacrylamide copolymer bound mesochlorin e6. [Google Scholar]
- 16.Kopeček J. Soluble biomedical polymers. Polim. Med. 1977;7:191–221. [PubMed] [Google Scholar]
- 17.Kopeček J. Soluble polymers in medicine. In: Williams DF, editor. Systemic Aspects of Biocompatibility. II. CRC Press; Boca Raton, Florida: 1981. pp. 159–180. [Google Scholar]
- 18.Jatzkewitz H. Peptamin (glycyl-L-leucyl-mescaline) bound to blood plasma expander (polyvinylpyrrolidone) as a new depot form of a biologically active primary amine (mescaline) Z. Naturforsch. 1955;10b:27–31. [Google Scholar]
- 19.Givetal NI, Ushakov SN, Panarin EF, Popova GO. Experimantal studies on penicillin polymer derivatives (in Russian) Antibiotiki. 1965;10:701–706. [PubMed] [Google Scholar]
- 20.Shumikina KI, Panarin EF, Ushakov SN. Experimental study of polymer salts of penicillins (in Russian) Antibiotiki. 1966;11:767–770. [PubMed] [Google Scholar]
- 21.Panarin EF, Ushakov SN. Synthesis of polymer salts and amidopenicillines (in Russian) Khim. Pharm. Zhur. 1968;2:28–31. [Google Scholar]
- 22.Mathé G, Loc TB, Bernard J. Effect sur la leucémie L1210 de la souris d'une combinaison par diazotation d'a méthoptérine et de γglobulines de hamsters porteurs de cette leucémie par hétérogreffe. Compte-rendus del'Académie des Sciences. 1958;3:1626–1628. [PubMed] [Google Scholar]
- 23.De Duve C, De Barsy T, Poole B, Trouet A, Tulkens P, van Hoof F. Lysosomotropic agents. Biochem. Pharmacol. 1974;23:2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- 24.Kopeček J, Šprincl L, Lím D. New types of synthetic infusion solutions. I. Investigation of the effect of solutions of some hydrophilic polymers on blood. J. Biomed. Mater. Res. 1973;7:179–191. doi: 10.1002/jbm.820070206. [DOI] [PubMed] [Google Scholar]
- 25.Paluska E, Činátl J, Korčáková L, Štěrba O, Kopeček J, Hrubá A, Nezvalová J, Staněk R. Immunosuppressive effect of a synthetic polymer - poly[N-(2-hydroxypropyl)methacrylamide] (Duxon) Folia Biologica. 1980;26:304–311. [PubMed] [Google Scholar]
- 26.Korčáková L, Paluska E, Hašková V, Kopeček J. A simple test for immunogenicity of colloidal infusion solutions - the draining lymph node activation. Z. Immun. Forsch. 1976;151:219–223. [PubMed] [Google Scholar]
- 27.Šprincl L, Exner J, Šterba O, Kopeček J. New types of synthetic infusion solutions. III. Elimination and retention of poly[N-(2-hydroxypropyl)methacrylamide] in a test organism. J. Biomed. Mater. Res. 1976;10:953–963. doi: 10.1002/jbm.820100612. [DOI] [PubMed] [Google Scholar]
- 28.Paluska E, Hrubá A, Štěrba O, Kopeček J. Effect of a synthetic poly[N-(2-hydroxypropyl)methacrylamide] (Duxon) on haemopoiesis and graft versus host reaction. Folia biologica. 1986;32:91–102. [PubMed] [Google Scholar]
- 29.Kopeček J, Šprincl L. Relationship between the structure and biocompatibility of hydrophilic gels. Polim. Med. 1974;4:109–117. [PubMed] [Google Scholar]
- 30.Šprincl L, Vacík J, Kopeček J, Lím D. Biological tolerance of poly(N-substituted methacrylamides) J. Biomed. Mater. Res. 1971;5:197–205. doi: 10.1002/jbm.820050307. [DOI] [PubMed] [Google Scholar]
- 31.Kopeček J, Šprincl L, Bažilová H, Vacík J. Biological tolerance of poly(N-substituted acrylamides) J. Biomed. Mater. Res. 1973;7:111–121. doi: 10.1002/jbm.820070109. [DOI] [PubMed] [Google Scholar]
- 32.Šprincl L, Kopeček J, Lím D. Effect of porosity of heterogeneous poly(glycol monomethacrylate) gels on the healing-in of test implants. J. Biomed. Mater. Res. 1971;5:447–458. doi: 10.1002/jbm.820050503. [DOI] [PubMed] [Google Scholar]
- 33.Šprincl L, Vacík J, Kopeček J, Lím D. Biological tolerance of ionogenic hydrophilic gels. J. Biomed. Mater. Res. 1973;7:123–136. doi: 10.1002/jbm.820070110. [DOI] [PubMed] [Google Scholar]
- 34.Ulbrich K, Šprincl L, Kopeček J. Biocompatibility of poly(2,4-pentadiene-1ol) J. Biomed. Mater. Res. 1974;8:155–161. doi: 10.1002/jbm.820080205. [DOI] [PubMed] [Google Scholar]
- 35.Šprincl L, Kopeček J, Lím D. Effect of the structure of poly(glycol monomethacrylate) gels on the calcification of implants. Calc. Tiss. Res. 1973;13:63–72. doi: 10.1007/BF02015397. [DOI] [PubMed] [Google Scholar]
- 36.Voldřich Z, Tománek Z, Vacík J, Kopeček J. Long-term experience with the poly(glycol monomethacrylate) gel in plastic operations of the nose. J. Biomed. Mater. Res. 1975;9:675–685. doi: 10.1002/jbm.820090612. [DOI] [PubMed] [Google Scholar]
- 37.Kopeček J, Bažilová H. Poly[N-(2-hydroxypropyl)methacrylamide]. I. Radical polymerization and copolymerization. Europ. Polym. J. 1973;9:7–14. [Google Scholar]
- 38.Bohdanecký M, Bažilová H, Kopeček J. Poly[N-(2-hydroxypropyl)methacrylamide]. II. Hydrodynamic properties of diluted polymer solutions. Europ. Polym. J. 1974;10:405–410. [Google Scholar]
- 39.Kopeček J, Rejmanová P, Chytrý V. Polymers containing enzymatically degradable bonds 1. Chymotrypsin catalyzed hydrolysis of p-nitroanilides of phenylalanine and tyrosine attached to side-chains of copolymers of N-(2-hydroxypropyl)methacrylamide. Makromol. Chem. 1981;182:799–809. [Google Scholar]
- 40.Kopeček J, Rejmanová P. Reactive copolymers of N-(2-hydroxypropyl)methacrylamide with N-methacryloylated derivatives of L-leucine and L-phenylalanine. II. Reaction with the polymeric amine and stability of crosslinks towards chymotrypsin in vitro. J. Polym. Sci. Polym. Symp. 1979;66:15–32. [Google Scholar]
- 41.Ulbrich K, Strohalm J, Kopeček J. Polymers containing enzymatically degradable bonds. 3. Poly[N-(2-hydroxypropyl)methacrylamide] chains connected by oligopeptide sequences cleavable by trypsin. Makromol. Chem. 1981;182:1917–1928. [Google Scholar]
- 42.Ulbrich K, Zacharieva EI, Obereigner B, Kopeček J. Polymers containing enzymatically degradable bonds. 5. Hydrophilic polymers degradable by papain. Biomaterials. 1980;1:199–204. doi: 10.1016/0142-9612(80)90017-4. [DOI] [PubMed] [Google Scholar]
- 43.Kopeček J, Cífková I, Rejmanová P, Strohalm J, Obereigner B, Ulbrich K. Polymers containing enzymatically degradable bonds. 4. Preliminary experiments in vivo. Makromol. Chem. 1981;182:2941–2949. [Google Scholar]
- 44.Duncan R, Cable HC, Lloyd JB, Rejmanová P, Kopeček J. Polymers containing enzymatically degradable bonds. 7. Design of oligopeptide side-chains in poly[N-(2-hydroxypropyl)methacrylamide] copolymers to promote efficient degradation by lysosomal enzymes. Makromol. Chem. 1983;184:1997–2008. [Google Scholar]
- 45.Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, Thomson AH, Murray LS, Hilditch TE, Murray T, Burtles S, Fraier D, Frigerio E, Cassidy J, on behalf of the Cancer Research Campaign Phase I/II Committee Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Clin. Cancer Res. 1999;5:83–94. [PubMed] [Google Scholar]
- 46.Thomson AH, Vassey PA, Murray LS, Cassidy J, Fraier D, Frigerio E, Twelves C. Population pharmacokinetics in phase I drug development: a phase I study of PK1 in patients with solid tumors. Brit. J. Cancer. 1999;81:99–107. doi: 10.1038/sj.bjc.6690657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seymour LW, Ferry DR, Kerr DJ, Rea D, Whitlock M, Ponyer R, Boivin C, Hesslewood S, Twelves C, Blackie R, Schätzlein A, Jodrell D, Bissett D, Calvert H, Lind M, Robbins A, Burtless S, Duncan R, Cassidy J. Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal carcinoma. Int. J. Oncol. 2009;34:1629–1636. doi: 10.3892/ijo_00000293. [DOI] [PubMed] [Google Scholar]
- 48.Seymour LW, Ferry DR, Anderson D, Hesslewood S, Julyan PJ, Poyner R, Doran J, Young AM, Burtles S, Kerr DJ. Hepatic drug targeting: phase I evaluation of polymer-bound doxorubicin. J. Clin. Oncol. 2002;20:1668–1676. doi: 10.1200/JCO.2002.20.6.1668. [DOI] [PubMed] [Google Scholar]
- 49.Shoemaker NE, van Kesteren C, Rosing H, Jansen S, Swart M, Lieverst J, Fraier D, Breda M, Pellizzoni C, Spinelli R, Grazia Porro M, Beijnen JH, Schellens JHM, ten Bokkel Huinink WW. A phase I and pharmacokinetic study of MAG-CPT, a water-soluble polymer conjugate of camptothecin. Brit. J. Cancer. 2002;87:608–614. doi: 10.1038/sj.bjc.6600516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meerum Terwogt JM, ten Bokkel Huinink WW, Schellens JH, Schot M, Mandjes IA, Zurlo MG, Rocchetti M, Rosing H, Koopman FJ, Beijnen JH. Phase I clinical and pharmacokinetic study of PNU166945, a novel water-soluble polymer-conjugated prodrug of paclitaxel. Anticancer Drugs. 2001;12:315–323. doi: 10.1097/00001813-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Rademaker-Lakhai JM, Terret C, Howell SB, Baud CM, De Boer RF, Pluim D, Beijnen JH, Schellens JH, Droz JP. A phase I and pharmacological study of the platinum polymer AP5280 given as an intravenous infusion once every 3 weeks in patients with solid tumors. Clin. Cancer Res. 2004;10:3386–3395. doi: 10.1158/1078-0432.CCR-03-0315. [DOI] [PubMed] [Google Scholar]
- 52.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer therapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 53.Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjugate Chem. 2010;21:797–802. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 54.Seymour LW, Duncan R, Strohalm J, Kopeček J. Effect of molecular weight of N-(2-hydroxypropyl)methacrylamide copolymers on body distribution and rate of excretion after subcutaneous, intraperitoneal and intravenous administration. J. Biomed. Mater. Res. 1987;21:1341–1358. doi: 10.1002/jbm.820211106. [DOI] [PubMed] [Google Scholar]
- 55.Noguchi Y, Wu J, Duncan R, Strohalm J, Ulbrich K, Akaike T, Maeda H. Early phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues. Jpn. J. Cancer Res. 1998;89:307–314. doi: 10.1111/j.1349-7006.1998.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shiah J-G, Sun Y, Kopečková P, Peterson CM, Straight RC, Kopeček J. Combination chemotherapy and photodynamic therapy of targetable N-(2-hydroxypropyl)methacrylamide copolymer – doxorubicin/mesochlorin e6 – OV-TL16 antibody immunoconjugates. J. Controlled Release. 2001;74:249–253. doi: 10.1016/s0168-3659(01)00325-x. [DOI] [PubMed] [Google Scholar]
- 57.Lu Z-R, Shiah J-G, Kopečková P, Kopeček J. Polymerizable Fab’ antibody fragment targeted photodynamic cancer therapy in nude mice. STP Pharma Sci. 2003;13:69–75. [Google Scholar]
- 58.Clevers H. The cancer stem cell: premises, promises and challenges. Nature Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 59.Dick JE. Looking ahead in cancer stem cell research. Nature Biotechnol. 2009;27:44–46. doi: 10.1038/nbt0109-44. [DOI] [PubMed] [Google Scholar]
- 60.Zhou Y, Kopeček J. Biological rationale for the design of polymeric anti-cancer nanomedicines. J. Drug Targeting. 2012 doi: 10.3109/1061186X.2012.723213. doi: 10.3109/1061186X.2012.723213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou Y, Yang J, Kopeček J. Selective inhibitory effect of HPMA copolymer-cyclopamine conjugate on prostate cancer stem cells. Biomaterials. 2012;33:1863–1872. doi: 10.1016/j.biomaterials.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Altaba AR, Sánchez P, Dahmane N. Hedgehog signaling in cancer formation and maintenance. Nature Rev. Cancer. 2003;3:903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 63.Mimeault M, Johansson SL, Henichart JP, Depreux P, Batra SK. Cytotoxic effects induced by Docetaxel, Gefitinib, and Cyclopamine on side population and nonside population cell fractions from human invasive prostate cancer cells. Mol. Cancer Ther. 2010;9:617–630. doi: 10.1158/1535-7163.MCT-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signaling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 65.Wu K, Liu J, Johnson RN, Yang J, Kopeček J. Drug-free macromolecular therapeutics: Induction of apoptosis by coiled-coil-mediated cross-linking of antigens on the cell surface. Angew. Chem. Int. Ed. 2010;49:1451–1455. doi: 10.1002/anie.200906232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu KG, Yang J, Liu J, Kopeček J. Coiled-coil based drug-free macromolecular therapeutics: In vivo efficacy. J. Controlled Release. 2012;157:126–131. doi: 10.1016/j.jconrel.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Persidis A. Cancer multidrug resistance. Nature Biotechnol. 1999;17:94–95. doi: 10.1038/5289. [DOI] [PubMed] [Google Scholar]
- 68.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loe DW, Decley RG, Cole SPC. Biology of the multidrug resistance associated protein, MRP. Eur. J. Cancer. 1996;32A:945–957. doi: 10.1016/0959-8049(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 70.Minko T. HPMA copolymers for modulating cellular signaling and overcoming multidrug resistance. Adv. Drug Delivery Rev. 2010;62:192–202. doi: 10.1016/j.addr.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 71.Tew KD. Gluthatione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–4320. [PubMed] [Google Scholar]
- 72.Omelyanenko V, Kopečková P, Gentry C, Kopeček J. Targetable HPMA copolymer – adriamycin conjugates. Recognition, internalization, and subcellular fate. J. Controlled Release. 1998;53:25–37. doi: 10.1016/s0168-3659(97)00235-6. [DOI] [PubMed] [Google Scholar]
- 73.Omelyanenko V, Gentry C, Kopečková P, Kopeček J. HPMA copolymer - anticancer drug - OV-TL16 antibody conjugates. 2. Processing in epithelial ovarian carcinoma cells in vitro. Int. J. Cancer. 1998;75:600–608. doi: 10.1002/(sici)1097-0215(19980209)75:4<600::aid-ijc18>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 74.Minko T, Kopečková P, Kopeček J. Comparison of the anticancer effect of free and HPMA copolymer-bound adriamycin in human ovarian carcinoma cells. Pharmaceutical Res. 1999;16:986–996. doi: 10.1023/a:1018959029186. [DOI] [PubMed] [Google Scholar]
- 75.Li B, Zu H, Yao M, Xie M, Shen H, Shen S, Wang X, Jin Y. Bypassing multidrug resistance in human breast cancer cells with lipid/polymer particle assemblies. Int. J. Nanomedicine. 2012;7:187–197. doi: 10.2147/IJN.S27864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pakunlu RI, Wang Y, Tsao W, Pozharov V, Cook TJ, Minko T. Enhancement of the efficacy of chemotherapy for lung cancer by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense: novel multicomponent delivery system. Cancer Res. 2004;64:6214–6224. doi: 10.1158/0008-5472.CAN-04-0001. [DOI] [PubMed] [Google Scholar]
- 77.Minko T, Kopečková P, Kopeček J. Chronic exposure to HPMA copolymer-bound adriamycin does not induce multidrug resistance in a human ovarian carcinoma cell line. J. Controlled Release. 1999;59:133–148. doi: 10.1016/s0168-3659(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 78.Fowers KD, Kopeček J. Targeting of multidrug-resistant human ovarian carcinoma cells with anti-P-glycoprotein antibody conjugates. Macromol. Biosci. 2012;12:502–514. doi: 10.1002/mabi.201100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Minko T, Kopečková P, Kopeček J. Efficacy of chemotherapeutic action of HPMA copolymer-bound doxorubicin in a solid tumor model of ovarian carcinoma. Int. J. Cancer. 2000;86:108–117. doi: 10.1002/(sici)1097-0215(20000401)86:1<108::aid-ijc17>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 80.Batrakova EV, Li S, Brynskikh AM, Sharma AK, Li Y, Boska M, Gong N, Mosley RL, Alakhov VY, Gendelman HE, Kabanov AV. Effects of Pluronic and doxorubicin on drug uptake, cellular metabolism, apoptosis and tumor inhibition in animal models of MDR cancers. J. Controlled Release. 2010;143:290–301. doi: 10.1016/j.jconrel.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krinick NL, Sun Y, Joyner D, Spikes JD, Straight RC, Kopeček J. A polymeric drug delivery system for the simultaneous delivery of drugs activatable by enzymes and/or light. J. Biomat. Sci. Polym. Ed. 1994;5:303–324. doi: 10.1163/156856294x00040. [DOI] [PubMed] [Google Scholar]
- 82.Peterson CM, Lu JM, Sun Y, Peterson CA, Shiah J-G, Straight RC, Kopeček J. Combination chemotherapy and photodynamic therapy with N-(2-hydroxypropyl)methacrylamide copolymer-bound anticancer drugs inhibit human ovarian carcinoma heterotransplanted in nude mice. Cancer Res. 1996;56:3980–3985. [PubMed] [Google Scholar]
- 83.Shiah J-G, Sun Y, Peterson CM, Kopeček J. Biodistribution of free and N-(2-hydroxypropyl)methacrylamide copolymer-bound meso chlorin e6 and adriamycin in nude mice bearing human ovarian carcinoma OVCAR-3 xenografts. J. Controlled Release. 1999;61:145–157. doi: 10.1016/s0168-3659(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 84.Shiah J-G, Sun Y, Peterson CM, Straight RC, Kopeček J. Antitumor activity of HPMA copolymer-meso chlorin e6 and adriamycin conjugates in combination treatments. Clin. Cancer Res. 2000;6:1008–1015. [PubMed] [Google Scholar]
- 85.Vicent MJ, Greco F, Nicholson RI, Paul A, Griffiths PC, Duncan R. Polymer therapeutics designed for a combination therapy of hormone-dependent cancer. Angew. Chem. Int. Ed. 2005;44:4061–4066. doi: 10.1002/anie.200462960. [DOI] [PubMed] [Google Scholar]
- 86.Hongrapipat J, Kopečková P, Prakongpan S, Kopeček J. Enhanced antitumor activity of combinations of free and HPMA copolymer-bound drugs. Int. J. Pharmaceutics. 2008;351:259–270. doi: 10.1016/j.ijpharm.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hongrapipat J, Kopečková P, Liu J, Prakongpan S, Kopeček J. Combination chemotherapy and photodynamic therapy with Fab’ fragment targeted HPMA copolymer conjugates in human ovarian carcinoma cells. Mol. Pharmaceutics. 2008;5:696–709. doi: 10.1021/mp800006e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Segal E, Pan H, Ofek P, Ugadawa T, Kopečková P, Kopeček J, Satchi-Fainaro R. Targeting angiogenesis-dependent calcified neoplasms using combined polymer therapeutics. PLoS ONE. 2009;4(4):e5233. doi: 10.1371/journal.pone.0005233. doi:10.1371/journal.pone.0005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Segal E, Pan H, Benayoun L, Kopečková P, Shaked Y, Kopeček J, Satchi-Fainaro R. Enhanced antitumor activity and safety profile of targeted nano-scaled HPMA copolymer – alendronate – TNP470 conjugate in the treatment of bone malignancies. Biomaterials. 2011;32:4450–4463. doi: 10.1016/j.biomaterials.2011.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Low SA, Kopeček J. Targeting polymer therapeutics to bone. Adv. Drug Delivery Rev. 2012;64:1189–1204. doi: 10.1016/j.addr.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang D, Miller SC, Sima M, Parker D, Buswell H, Goodrich KC, Kopečková P, Kopeček J. The arthrotropism of macromolecules in adjuvant-induced arthritis rat model: a preliminary study. Pharmaceutical Res. 2004;21:1741–1749. doi: 10.1023/b:pham.0000045232.18134.e9. [DOI] [PubMed] [Google Scholar]
- 92.Wang D, Miller S, Sima M, Kopečková P, Kopeček J. Synthesis and evaluation of water-soluble polymeric bone-targeted drug delivery systems. Bioconjugate Chem. 2003;14:853–859. doi: 10.1021/bc034090j. [DOI] [PubMed] [Google Scholar]
- 93.Pan H, Kopečková P, Wang D, Yang J, Miller S, Kopeček J. Water-soluble HPMA copolymer–prostaglandin conjugates containing a cathepsin K sensitive spacer. J. Drug Targeting. 2006;14:425–435. doi: 10.1080/10611860600834219. [DOI] [PubMed] [Google Scholar]
- 94.Wang D, Sima M, Mosley RL, Davda JP, Tietze N, Miller SC, Gwilt PR, Kopečková P, Kopeček J. Pharmacokinetic and biodistribution studies of bone-targeting drug delivery system based on N-(2-hydroxypropyl)methacrylamide) copolymers. Mol. Pharmaceutics. 2006;3:717–725. doi: 10.1021/mp0600539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang D, Miller SC, Shlyakhtenko LS, Portillo AM, Liu X-M, Papngkorn K, Kopečková P, Lyubchenko Y, Higuchi WI, Kopeček J. Osteotropic peptide that differentiates functional domains of the skeleton. Bioconjugate Chem. 2007;18:1375–1378. doi: 10.1021/bc7002132. [DOI] [PubMed] [Google Scholar]
- 96.Pan HZ, Sima M, Kopečková P, Wu K, Gao SQ, Liu J, Wang D, Miller SC, Kopeček J. Biodistribution and pharmacokinetic studies of bone-targeting N-(2-hydroxypropyl)methacrylamide copolymer-alendronate conjugates. Mol. Pharmaceutics. 2008;5:548–558. doi: 10.1021/mp800003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miller SC, Pan H, Wang D, Bowman BM, Kopečková P, Kopeček J. Feasibility of using a bone-targeted, macromolecular delivery system coupled with prostaglandin E1 to promote bone formation in aged, estrogen-deficient rats. Pharmaceutical Res. 2008;25:2889–2895. doi: 10.1007/s11095-008-9706-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pan H, Liu J, Dong Y, Sima M, Kopečková P, Brandi ML, Kopeček J. Release of prostaglandin E1 from N-(2-hydroxypropyl)methacrylamide copolymer conjugates by bone cells. Macromol. Biosci. 2008;8:599–605. doi: 10.1002/mabi.200700338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu X-M, Miller SC, Wang D. Beyond oncology – Application of HPMA copolymers in non-cancerous diseases. Adv. Drug Delivery Rev. 2010;62:258–271. doi: 10.1016/j.addr.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yuan F, Quan L-D, Cui L, Goldring SR, Wang D. Development of macromolecular prodrug for rheumatoid arthritis. Adv. Drug Delivery Rev. 2012 doi: 10.1016/j.addr.2012.03.006. doi: 10.1016/j.addr.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu XM, Quan LD, Tian J, Alnouti Y, Fu K, Thiele GM, Wang D. Synthesis and evaluation of a well-defined HPMA copolymer-dexamethasone conjugate for effective treatment of rheumatoid arthritis. Pharmaceutical Res. 2008;25:2910–2919. doi: 10.1007/s11095-008-9683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang D, Goldring SR. The bone, the joints and the balm of gilead. Mol. Pharmaceutics. 2011;8:991–993. doi: 10.1021/mp200328t. [DOI] [PubMed] [Google Scholar]
- 103.Yuan F, Quan LD, Cui L, Goldring SR, Wang D. Development of macromolecular drugs for rheumatoid arthritis. Adv. Drug Delivery Rev. 2012;64:1205–1219. doi: 10.1016/j.addr.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nan A, Nanayakkara NP, Walker LA, Yardley V, Croft SL, Ghandehari H. N-(2-Hydroxypropyl)methacrylamide (HPMA) copolymers for targeted delivery of 8-aminoquinoline antileishmanial drugs. J. Controlled Release. 2001;77:233–243. doi: 10.1016/s0168-3659(01)00514-4. [DOI] [PubMed] [Google Scholar]
- 105.Nan A, Croft SL, Yaedley V, Ghandehari H. Targetable water-soluble polymer-drug conjugates for the treatment of visceral leishmaniasis. J. Controlled Release. 2004;94:115–127. doi: 10.1016/j.jconrel.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 106.Nicoletti S, Seifert K, Gilbert IH. N-(2-Hydroxypropyl)methacrylamide-amphotericin B (HPMA-AmB) copolymer conjugates as antileishmanial agents. Int. J. Antimicrob. Agents. 2009;33:441–448. doi: 10.1016/j.ijantimicag.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulation time of bovine liver catalase. J. Biol. Chem. 1977;252:3582–3586. [PubMed] [Google Scholar]
- 108.Lu Z-R, Kopečková P, Kopeček J. Semitelechelic Poly[N-(2-hydroxypropyl)methacrylamide] for biomedical applications. In: Ottenbrite RM, Kim SW, editors. Polymeric Drugs & Delivery Systems. Technomics Publishing Co.; Lancaster, PA: 2001. pp. 1–14. [Google Scholar]
- 109.Chiu H-C, Zalipsky S, Kopečková P, Kopeček J. Enzymatic activity of chymotrypsin and its poly(ethylene glycol) conjugates toward low and high molecular weight substrates. Bioconjugate Chem. 1993;4:290–295. doi: 10.1021/bc00022a007. [DOI] [PubMed] [Google Scholar]
- 110.Lu Z-R, Kopečková P, Wu Z, Kopeček J. Functionalized semitelechelic poly[N-(2-hydroxypropyl)methacrylamide] for protein modification. Bioconjugate Chem. 1998;9:793–804. doi: 10.1021/bc980058r. [DOI] [PubMed] [Google Scholar]
- 111.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nature Rev. Drug Disc. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 112.Chen C, Constantinou A, Deonarain M. Modulating antibody pharmacokinetics using hydrophilic polymers. Expert Opin. Drug Deliv. 2011;8:1221–1236. doi: 10.1517/17425247.2011.602399. [DOI] [PubMed] [Google Scholar]
- 113.Pasut G, Veronese FM. State-of-the-art in PEGylation: The great versatility achieved after forty years of research. J. Controlled Release. 2012;161:461–472. doi: 10.1016/j.jconrel.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 114.Levy V, Hershfield MS, Fernandez-Mejia C, Polmar SH, Scrudiery D, Berger M, Sorensen RU. Adenosine deaminase deficiency with late onset or recurrent infections: response to treatment with polyethylene glycol modified adenosine deaminase. J. Pediatr. 1988;113:312–317. doi: 10.1016/s0022-3476(88)80271-3. [DOI] [PubMed] [Google Scholar]
- 115.Graham LM. PEGASPARAGINASE: a review of clinical studies. Adv. Drug Deliv. Rev. 2003;55:1293–1302. doi: 10.1016/s0169-409x(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 116.Reddy KR, Modi MW, Pedder S. Use of peginterferon α2a (40KD) (Pegasys®) for the treatment of hepatitis C. Adv. Drug Deliv. Rev. 2002;54:571–586. doi: 10.1016/s0169-409x(02)00028-5. [DOI] [PubMed] [Google Scholar]
- 117.Wang YS, Youngster S, Grace M, Bausch J, Bordens R, Wyss DF. Structural and biological characterization of pegylated recombinant interferon α2b and its therapeutic implications. Adv. Drug Deliv. Rev. 2002;54:547–570. doi: 10.1016/s0169-409x(02)00027-3. [DOI] [PubMed] [Google Scholar]
- 118.Kinstler O, Moulinex G, Treheit M, Ladd D, Gegg C. Mono-N-terminal poly(ethylene glycol)-protein conjugates. Adv. Drug Deliv. Rev. 2002;54:477–485. doi: 10.1016/s0169-409x(02)00023-6. [DOI] [PubMed] [Google Scholar]
- 119.Nesbitt AM, Stephens S, Chartash EK. Certolizumab pegol: a PEGylated antitumor necrosis factor alpha biological agent. In: Veronese FM, editor. PEGylated Protein Drugs: Basic Science and Clinical Applications. Birkhäuser Verlag; Basel, Switzerland: 2009. pp. 229–254. [Google Scholar]
- 120.Sherman MR, Saifer MG, Perez-Ruiz F. PEG-uricase in the management of treatment-resistant gout and hyperuricemia. Adv. Drug Deliv. Rev. 2008;60:59–68. doi: 10.1016/j.addr.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 121.Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyas O, Henderson R, Berry G, Gabizon A. Pegylated liposomal doxorubicin (doxil): reduced cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann. Oncol. 2000;11:1029–1033. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- 122.Peracchia MT, Fattal E, Desmaele D, Besnard M, Noël JP, Gomis JM, Appel M, d'Angelo J, Couvreur P. Stealth PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J. Controlled Release. 1999;60:121–128. doi: 10.1016/s0168-3659(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 123.Ma Y, Yang Q, Wang L, Zhou X, Zhao Y, Deng Y. Repeated injections of PEGylated liposomal topotecan induces accelerated blood clearance phenomenon in rats. Eur. J. Pharmaceutical Sci. 2012;45:539–545. doi: 10.1016/j.ejps.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 124.Sundy JS, Ganson NJ, Kelly SJ, Scarlett EJ, Rehrig CD, Huang W, Hershfield MS. Pharmacokinetics and pharmacodynamics of intravenous PEGylated recombinant mammalian urate oxidase in partients with refractory goat. Arthritis Rheum. 2007;56:1021–1028. doi: 10.1002/art.22403. [DOI] [PubMed] [Google Scholar]
- 125.Wang XY, Ishida T, Ichihara M, Kiwada H. Influence of the physicochemical properties of liposomes on the accelerated blood clearance phenomenon in rats. J. Controlled Release. 2005;104:91–102. doi: 10.1016/j.jconrel.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 126.Ishida T, Harada M, Wang XY, Ichihara M, Irimura K, Kiwada H. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: Effects of lipid dose and PEG surface density and chain length of the first dose liposomes. J. Controlled Release. 2005;105:305–317. doi: 10.1016/j.jconrel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 127.Ishida T, Ichihara M, Wang XY, Yamamoto K, Kimura J, Majima E, Kiwada H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Controlled Release. 2006;112:15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 128.Koide H, Asai T, Hatanaka K, Akai S, Ishii T, Kenjo E, Ishida T, Kiwada H, Tsukada H, Oku N. T-cell independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int. J. Pharmaceutics. 2010;392:218–223. doi: 10.1016/j.ijpharm.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 129.Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, Garatty G. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–111. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 130.Kaminskas LM, McLeod VM, Porter CJH, Boyd BJ. Differences in colloidal structure of PEGylated nanomaterials dictate the likelihood of accelerated blood clearance. J. Pharmaceutical Sci. 2011;100:5069–5077. doi: 10.1002/jps.22682. [DOI] [PubMed] [Google Scholar]
- 131.Ishida T, Kiwada H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PRGylated liposomes. Int. J. Pharmaceutics. 2008;354:56–62. doi: 10.1016/j.ijpharm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 132.Koide H, Asai T, Hatanaka K, Akai S, Ishii T, Kenjo E, Ishida T, Kiwada H, Tsukada H, Oku N. T-cell independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int. J. Pharmaceutics. 2010;392:218–223. doi: 10.1016/j.ijpharm.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 133.Ishigara T, Maeda T, Sakamoto H, Takasaki N, Shigyo M, Ishida T, Kiwada H, Mizushima Y, Mizuahima T. Evasion of nanoparticle accelerated blood clearance phenomenon by coating of nanoparticles with various hydrophilic polymers. Biomacromolecules. 2010;11:2700–2706. doi: 10.1021/bm100754e. [DOI] [PubMed] [Google Scholar]
- 134.Markovsky E, Baabur-Cohen H, Eldar-Boock A, Omer L, Tiram G, Ferber S, Ofek P, Polyak D, Scomparin A, Satchi-Fainaro R. Administration, distribution, metabolism, and elimination of polymer therapeutics. J. Controlled Release. 2012;161:446–460. doi: 10.1016/j.jconrel.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 135.Bendele A, Seely J, Richey C, Sennelo G, Shopp G. Renal tubular vacuolation in animals treated with polyethylene-glycol-conjugated proteins. Toxicol. Sci. 1998;42:152–157. doi: 10.1006/toxs.1997.2396. [DOI] [PubMed] [Google Scholar]
- 136.Webster R, Didier E, Harris P, Siegel N, Stadler J, Tilbury L, Smith D. Pegylated proteins: evaluation of their safety in the absence of definitive metabolism studies. Drug Metab. Dispos. 2007;35:9–16. doi: 10.1124/dmd.106.012419. [DOI] [PubMed] [Google Scholar]
- 137.Viegas TX, Bentley MD, Harris JM, Fang Z, Yoon K, Dizman B, Weimer R, Mero A, Pasut G, Veronese FM. Polyoxazolines: Chemistry, properties, and applications in drug delivery. Bioconjugate Chem. 2011;22:976–986. doi: 10.1021/bc200049d. [DOI] [PubMed] [Google Scholar]
- 138.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 139.Lu Z-R, Kopečková P, Wu Z, Kopeček J. Functionalized semitelechelic poly[N-(2-hydroxypropyl)methacrylamide] for protein modification. Bioconjugate Chem. 1998;9:793–804. doi: 10.1021/bc980058r. [DOI] [PubMed] [Google Scholar]
- 140.Kamei S, Kopeček J. Prolonged blood circulation in rats of nanospheres surface-modified with semitelechelic poly[N-(2-hydroxypropyl)methacrylamide] Pharmaceutical Res. 1995;12:663–668. doi: 10.1023/a:1016247206531. [DOI] [PubMed] [Google Scholar]
- 141.Lääne A, Aaviksaar A, Haga M, Chytrý V, Kopeček J. Preparation of polymer-modified enzymes of prolonged circulation times. Poly[N-(2-hydroxypropyl)methacrylamide] bound acetylcholinesterase. Makromol. Chem. Suppl. 1985;9:35–42. [Google Scholar]
- 142.Oupický D, Ogris M, Howard KA, Dash PR, Ulbrich K, Seymour LW. Importance of lateral and steric stabilization of polyelectrolyte gene delivery vectors for extended systemic circulation. Mol. Ther. 2002;5:463–472. doi: 10.1006/mthe.2002.0568. [DOI] [PubMed] [Google Scholar]
- 143.Ulbrich K, Šubr V. Structural and chemical aspects of HPMA copolymers as drug carriers. Adv. Drug Delivery Rev. 2010;62:150–166. doi: 10.1016/j.addr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 144.Říhová B, Kovář M. Immunogenicity and immunomodulatory properties of HPMA-based polymers. Adv. Drug Delivery Rev. 2010;62:184–191. doi: 10.1016/j.addr.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 145.Duncan R. Polymer therapeutics as nanomedicines: new perspectives. Curr. Opin. Biotechnol. 2011;22:492–501. doi: 10.1016/j.copbio.2011.05.507. [DOI] [PubMed] [Google Scholar]
- 146.Paz-Ares L, Ross H, O'Brien M, Riviere A, Gatzemeier U, Von Pawel J, Kaukel E, Freitag L, Digel W, Bischoff H, Garcia-Campello R, Iannotti N, Reiterer P, Bover J, Prendiville J, Eisenfeld AJ, Oldham FB, Bandstra B, Singer JW, Bonomi P. Phase III trial comparing paclitaxel poliglumex vs. docetaxel in the second-line treatment of non-small-cell lung cancer. Brit. J. Cancer. 2008;98:1608–1613. doi: 10.1038/sj.bjc.6604372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kopeček J. Biomaterials and drug delivery – past, present, and future. Mol. Pharmaceutics. 2010;7:922–925. doi: 10.1021/mp1001813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fox ME, Szoka FC, Fréchet JMJ. Soluble polymer carriers for the treatment of cancer: The importance of molecular architecture. Acc. Chem. Res. 2009;42:1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Etrych T, Šubr V, Strohalm J, Šírová M, Říhová B, Ulbrich K. HPMA copolymer-doxorubicin conjugates: The effects of molecular weight and architecture on biodistribution and in vivo activity. J. Controlled Release. 2012 doi: 10.1016/j.jconrel.2012.06.029. doi:10.1016/j.jconrel.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 150.Etrych T, Strohalm J, Chytil P, Říhová B, Ulbrich K. Novel star HPMA-based polymer conjugates for passive targeting to solid tumors. J. Drug Targeting. 2011;19:874–889. doi: 10.3109/1061186X.2011.622402. [DOI] [PubMed] [Google Scholar]
- 151.Ding H, Kopečková P, Kopeček J. Self-association properties of HPMA copolymers containing an amphipatic heptapeptide. J. Drug Targeting. 2007;15:465–474. doi: 10.1080/10611860701500016. [DOI] [PubMed] [Google Scholar]
- 152.Dvořák M, Kopečková P, Kopeček J. High-molecular weight HPMA copolymer – adriamycin conjugates. J. Controlled Release. 1999;60:321–332. doi: 10.1016/s0168-3659(99)00087-5. [DOI] [PubMed] [Google Scholar]
- 153.Shiah J-G, Dvořák M, Kopečková P, Sun Y, Peterson CM, Kopeček J. Biodistribution and antitumor efficacy of long-circulating N-(2-hydroxypropyl)-methacrylamide copolymer-doxorubicin conjugates in nude mice. Eur. J. Cancer. 2001;37:131–139. doi: 10.1016/s0959-8049(00)00374-9. [DOI] [PubMed] [Google Scholar]
- 154.Yang J, Luo K, Pan H, Kopečková P, Kopeček J. Synthesis of biodegradable multiblock copolymers by click coupling of RAFT-generated heterotelechelic polyHPMA conjugates. Reactive Functional Polym. 2011;71:294–302. doi: 10.1016/j.reactfunctpolym.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luo K, Yang J, Kopečková P, Kopeček J. Biodegradable multiblock N-(2-hydroxypropyl)methacrylamide copolymers via reversible addition-fragmentation chain transfer polymerization and click chemistry. Macromolecules. 2011;44:2481–2488. doi: 10.1021/ma102574e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pan H, Yang J, Kopečková P, Kopeček J. Backbone degradable multiblock N-(2-hydroxypropyl)methacrylamide copolymer conjugates via reversible addition-fragmentation chain transfer polymerization and thiol-ene coupling reaction. Biomacromolecules. 2011;12:247–252. doi: 10.1021/bm101254e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pan H, Sima M, Yang J, Kopeček J. Synthesis of long-circulating backbone degradable HPMA copolymer-doxorubicin conjugates and evaluation of molecular weight dependent antitumor efficacy. Macromol. Biosci. doi: 10.1002/mabi.201200353. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang R, Luo K, Yang J, Sima M, Sun Y, Janát-Amsbury MM, Kopeček J. Synthesis and evaluation of a backbone biodegradable multiblock HPMA copolymer nanocarrier for the systemic delivery of paclitaxel. J. Controlled Release. doi: 10.1016/j.jconrel.2012.12.009. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tedder TF, McIntyre G, Schlossman SF. Heterogeneity in the B1 (CD20) cell surface molecule expressed by human B-lymphocytes. Mol. Immunol. 1998;25:1321–1330. doi: 10.1016/0161-5890(88)90047-8. [DOI] [PubMed] [Google Scholar]
- 160.Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol. Today. 1994;15:450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 161.Anderson KC, Bates MP, Slaugenhoupt BL, Pinkus GS, Schlossman SF, Nadler LM. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood. 1984;63:1424–1433. [PubMed] [Google Scholar]
- 162.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin's lymphoma. N. Engl. J. Med. 2008;359:613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 163.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JF, Raab R, Newman RA, Hanna N, Anderson DR. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- 164.McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 165.McLaughlin P, White CA, Grillo-López AJ, Maloney DG. Clinical status and optimal use of rituximab for B-cell lymphomas. Oncology (Williston Park) 1998;12:1763–1769. discussion 1769-1770, 1775-1777. [PubMed] [Google Scholar]
- 166.Riaz W, Hernandez-Ilizaliturri FJ, Czuczman MS. Strategies to enhance rituximab anti-tumor activity in the treatment of CD20-positive B-cell neoplasms. Immunol. Res. 2010;46:192–205. doi: 10.1007/s12026-009-8121-x. [DOI] [PubMed] [Google Scholar]
- 167.Johnson RN, Kopečková P, Kopeček J. Synthesis and evaluation of multivalent branched HPMA copolymer-Fab’ conjugates targeted to the B-cell antigen. Bioconjugate Chem. 2009;20:129–137. doi: 10.1021/bc800351m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Johnson RN, Kopečková P, Kopeček J. Biological activity of anti-CD20 multivalent HPMA copolymer-Fab’ conjugates. Biomacromolecules. 2012;13:727–735. doi: 10.1021/bm201656k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chu T-W, Yang J, Kopeček J. Anti-CD20 multivalent HPMA copolymer-Fab’ conjugates for the direct induction of apoptosis. Biomaterials. 2012;33:7174–7181. doi: 10.1016/j.biomaterials.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang N, Khawli LA, Hu P, Epstein AL. Generation of rituximab polymer may cause hyper-cross-linking-induced apoptosis in non-Hodgkin's lymphomas. Clin. Cancer Res. 2005;11:5971–5980. doi: 10.1158/1078-0432.CCR-05-0554. [DOI] [PubMed] [Google Scholar]
- 171.Ghetie MA, Bright H, Vitetta ES. Homodimers but not monomers of Rituxan (chimeric anti-CD20) induce apoptosis in human B-lymphoma cells and synergize with a chemotherapeutic agent and an immunotoxin. Blood. 2001;97:1392–1398. doi: 10.1182/blood.v97.5.1392. [DOI] [PubMed] [Google Scholar]
- 172.Yang J, Xu C, Wang C, Kopeček J. Refolding hydrogels self-assembled from HPMA graft copolymers by antiparallel coiled-coil formation. Biomacromolecules. 2006;7:1187–1195. doi: 10.1021/bm051002k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang J, Wu K, Koňák Č, Kopeček J. Dynamic light scattering study of the self-assembly of HPMA hybrid graft copolymers. Biomacromolecules. 2008;9:510–517. doi: 10.1021/bm701001f. [DOI] [PubMed] [Google Scholar]
- 174.Kopeček J, Yang J. Smart self-assembled hybrid hydrogel biomaterials. Angew. Chem. Int. Ed. 2012;51:7396–7417. doi: 10.1002/anie.201201040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li C, Wallace S. Polymer-drug conjugates: Recent development in clinical oncology. Adv. Drug Delivery Rev. 2008;60:886–898. doi: 10.1016/j.addr.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Canal F, Sanchis J, Vincent MJ. Polymer-drug conjugates as nano-sized medicines. Curr. Opin. Biotechnol. 2011;22:894–900. doi: 10.1016/j.copbio.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 177.Říhová B, Kubáčková K. Clinical implications of N-(2-hydroxypropyl)methacrylamide copolymers. Curr. Pharm. Biotechnol. 2003;4:311–322. doi: 10.2174/1389201033489711. [DOI] [PubMed] [Google Scholar]
- 178.Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol. Pharmaceutics. 2011;8:2101–2141. doi: 10.1021/mp200394t. [DOI] [PubMed] [Google Scholar]
- 179.Ding H, Prodinger WM, Kopeček J. Two-step fluorescence screening of CD21-binding peptides with one-bead one-compound library and investigation of binding of HPMA copolymer – peptide conjugates. Biomacromolecules. 2006;7:3037–3046. doi: 10.1021/bm060508f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kopansky E, Shamay Y, David A. Peptide-directed HPMA copolymer-doxorubicin conjugates as targeted therapeutics for colorectal cancer. J. Drug Targeting. 2011;19:933–943. doi: 10.3109/1061186X.2011.632011. [DOI] [PubMed] [Google Scholar]
- 181.Nishiyama A, Nori A, Malugin A, Kasuya Y, Kopečková P, Kopeček J. Free and N-(2-hydroxypropyl)methacrylamide copolymer-bound geldanamycin derivative induce different stress responses in A2780 human ovarian carcinoma cells. Cancer Res. 2003;63:7876–7882. [PubMed] [Google Scholar]
- 182.Říhová B, Kovář L, Kovář M, Hovorka O. Cytotoxicity and immunostimulation: double attack on cancer cells with polymeric therapeutics. Trends Biotechnol. 2009;27:11–17. doi: 10.1016/j.tibtech.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 183.Segal E, Satchi-Fainaro R. Design and development of polymer conjugates as anti-angiogenic agents. Adv. Drug Delivery Rev. 2009;61:1159–1176. doi: 10.1016/j.addr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 184.D'Souza GG, Weissig V. Subcellular targeting: a new frontier for drug-loaded pharmaceutical nanocarriers and the concept of magic bullet. Expert Opin. Drug Deliv. 2009;6:1135–1148. doi: 10.1517/17425240903236101. [DOI] [PubMed] [Google Scholar]
- 185.Nori A, Kopeček J. Intracellular targeting of polymer-bound drugs for cancer chemotherapy. Adv. Drug Delivery Rev. 2005;57:609–636. doi: 10.1016/j.addr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 186.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nature Rev. Drug Disc. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 187.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lu Z-R. Molecular imaging of HPMA copolymers: visualizing drug delivery in cell, mouse and man. Adv. Drug Delivery Rev. 2010;62:246–257. doi: 10.1016/j.addr.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 189.Schluep T, Hwang J, Hildebrandt IJ, Czernin J, Choi CHJ, Alalbi CA, Mack BC, Davis ME. Pharmacokinetics and tumor dynamics of the nanoparticle IT-101 from PET-imaging and tumor histological measurements. Proc. Natl. Acad. Sci. U.S.A. 2009;106:11394–11399. doi: 10.1073/pnas.0905487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lu Z-R, Wu X. Polydisulfide based biodegradable macromolecular magnetic resonance imaging contrast agents. Isr. J. Chem. 2010;50:220–232. doi: 10.1002/ijch.201000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticels. Adv. Drug Delivery Rev. 2010;62:1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic nanomedicine. Acc. Chem. Res. 2011;44:1029–1038. doi: 10.1021/ar200019c. [DOI] [PubMed] [Google Scholar]
- 193.Chen H, Kim S, Li L, Wang S, Park K, Cheng J-X. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Förster resonance energy transfer imaging. Proc. Natl. Acad. Sci. U.S.A. 2008;105:6596–6601. doi: 10.1073/pnas.0707046105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu Y, Chang E, Liu H, Jiang H, Gambhir SS, Cheng Z. Proof-of-concept study of monitoring cancer drug therapy with Cerenkov luminescence imaging. J. Nucl. Med. 2012;53:312–317. doi: 10.2967/jnumed.111.094623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pinheiro VB, Taylor AI, Cozens C, Abramov M, Renders M, Zhang S, Chaput JC, Wengel J, Peak-Chew S-Y, McLaughlin SH, Herdewijn P, Holliger P. Synthetic genetic polymers capable of heredity and evolution. Science. 2012;336:341–344. doi: 10.1126/science.1217622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Joyce GF. Toward an alternative biology. Science. 2012;336:307–308. doi: 10.1126/science.1221724. [DOI] [PubMed] [Google Scholar]