Abstract
The overproduction of reactive oxygen species and resulting damage are central to the pathology of many diseases. The study of the temporal and spatial accumulation of reactive oxygen species has been limited due to the lack of specific probes and techniques capable of continuous measurement. We demonstrate the use of a miniaturized electrochemical cytochrome C (Cyt C) biosensor for real-time measurements and quantitative assessment of superoxide production and inactivation by natural and engineered antioxidants in acutely prepared brain slices from mice. During control conditions, superoxide radicals produced from the hippocampal region of the brain in 400 μm thick sections were well within the range of detection of the electrode. Exposure of the slices to ischemic conditions increased the superoxide production two fold and measurements from the slices were stable over a 3–4 hour period. The stilbene derivative and anion channel inhibitor, 4,4′-diisothiocyano-2,2′-disulfonic stilbene (DIDS), markedly reduced the extracellular superoxide signal under control conditions suggesting that a transmembrane flux of superoxide into the extracellular space may occur as part of normal redox signaling. The specificity of the electrode for superoxide released by cells in the hippocampus was verified by the exogenous addition of superoxide dismutase (SOD) which decreased the superoxide signal in a dose-dependent manner. Similar results were seen with the addition of the SOD-mimetic, cerium oxide nanoparticles (nanoceria) where the superoxide anion radical scavenging activity of nanoceria with an average diameter of 15 nm was equivalent to 527 U of SOD for each 1 μg/ml of nanoceria added. This study demonstrates the potential of electrochemical biosensors for studying real-time dynamics of reactive oxygen species in a biological model and the utility of these measurements in defining the relative contribution of superoxide to oxidative injury.
Keywords: superoxide mobility, cerebral ischemia, electrochemical, cytochrome C microbiosensor, cerium oxide nanoparticles, nanoceria
Introduction
Reactive oxygen and nitrogen species (ROS and RNS) including superoxide, nitric oxide (NO), hydrogen peroxide (H2O2), hydroxyl and peroxynitrite radicals are potent oxidizing and nitrating agents that are produced under a variety of physiological and pathophysiological conditions. Physiological levels of these species appear to be involved in myriad of physiological processes. For example endogenously generated oxidants act as second messengers, transcriptional regulators, and modulators of ion channels and enzyme activity [1, 2]. The principle source of superoxide production during physiological conditions is thought to be the mitochondria, where the rate of superoxide production in vitro is estimated to be 0.15–2 % of the total cellular oxygen consumption [3, 4]. Other potential sources of superoxide production include xanthine oxidases (XOD) and the nicotinamide adenine dinucleotide phosphate (NADPH) family of oxidases. Of these, the latter appears to be unique in that superoxide production is thought to play an important role in normal cellular redox signaling [5, 6]. The activity of both XOD and NADPH oxidases can be markedly increased following tissue injury or disease states [7, 8].
Overproduction of ROS and RNS has been associated with development of a wide variety of neurodegenerative diseases as a result of their high chemical reactivity and potential for inducing oxidative damage to proteins, cells and tissues [9–11]. Methods for monitoring ROS and RNS levels in intact, living tissues is a critical first step in unraveling their physiological roles in both healthy and disease states. Unfortunately, continuous in situ monitoring of these species in biological systems has been very challenging due to their high reactivity, low concentrations and short half-lives. Moreover, the study of kinetics in cell free systems is difficult due to the many interrelated coupled redox reactions that change dynamically over time.
Cerebral ischemia is a leading cause of death and long-term disability. During ischemia, blood flow to the tissue is inadequate to sustain the metabolism of the tissue, resulting in a progressive decline in mitochondrial function and uncoupling of the electron transport chain [12]. Consequently, many of the biological cascades involved in ischemic cell death have been related with overproduction of ROS species, including superoxide, which contributes to oxidative damage [13–16]. Moreover, XOD and NADPH oxidases have been implicated as the principle contributors of superoxide generation leading to tissue damage following ischemia-reperfusion injury [17, 18]. In support of this hypothesis, inhibition of NADPH oxidase decreases ROS levels, preserves integrity of the blood-brain barrier and neuronal function [17].
Although many biochemical processes contribute to the oxidative load generated by superoxide under normal and pathological conditions, the damaging effects of superoxide are thought to be restricted to the cells generating this free radical. The trans-cellular movement of superoxide across biological membranes is not thought to contribute to any appreciable extent except in erythrocytes [19]. Unlike H2O2, which freely diffuses across membranes, superoxide is relatively impermeable owing to its low water solubility in the charged state. Although the neutral, protonated form of superoxide (pKa 4.9) could traverse biological membranes, its low intracellular concentration provides little driving force for diffusion into adjacent cellular compartments. Moreover, the identification and localization of the superoxide dismutase (SOD) family of isozymes in the mitochondria (SOD 2), cytosol (SOD 1), and more recently the extracellular matrix (SOD 3), suggests that vectorially produced superoxide resides and reacts within defined cellular compartments. This notion has been challenged by the findings that superoxide may cross mitochondrial and plasma membrane through voltage dependent anion channels (VDAC). Biochemical evidence for VDAC distribution in the plasma membrane arose from the finding that VDAC1 is present in caveloae, a specialized domain of the plasma membrane involved in endocytosis [20]. Multiple functions have been ascribed to VDAC including purine nucleotide transport (ATP and ADP), anion-channel-like activity and transmembrane redox regulation arising from VDAC’s NADH reductase activity [21–24].
Evidence that superoxide may selectively cross through anion channels was originally proposed by Lynch and Fridovich in XOD loaded lipid vesicles [19] and later by Mao and Poznansky [25] in erythrocyte ghost membranes and in human amniotic cells [26]. Han et al [27] showed that ~55% of the mitochondrial generated superoxide exited across the outer mitochondrial membrane through 4,4′-diisothiocyano-2,2′-disulfonic stilbene (DIDS) sensitive anion channels in endosomes isolated from isolated mitochondria from the heart. More recently, a DIDS-sensitive superoxide flux was reported across the plasma membranes of both epithelial-derived endosomes [28] and endothelial cells. Despite the mounting evidence for the existence of superoxide permeable channels, little is known regarding the mobility of this free radical and extent to which this superoxide contributes to the extracellular oxidant load. Understanding this relationship can potentially reshape our views on how the trans-cellular movement of free radicals can influence oxidative damage in adjacent cells and tissues.
In general, measurement of ROS in living organisms has been a significant analytical challenge. Most ROS are highly reactive and short lived and therefore difficult to detect in complex biological matrices. Additionally, ROS often are produced and/or neutralized in subcellular compartments, which requires detection methods directed to specific subcellular localization. There are few methods that measure superoxide anion directly (i.e. electron paramagnetic resonance) and most techniques utilize indirect absorbance or fluorescence measurements [29] or oxidation products most of which are relatively non-specific and have limited temporal or spatial resolution. Thus goals of the current study were three-fold. First, using an electrochemical Cyt C biosensor we wished to quantitatively monitor in real-time, superoxide levels in living brain tissue. Second, using an in vitro ischemic brain slice model, we wished to demonstrate proof-of–concept of electrode specificity using several superoxide scavengers including superoxide dismutase and cerium dioxide nanoparticles. Lastly, we wished to evaluate the role of VDAC in the transmembrane flux of superoxide in brain slices during control and ischemic conditions.
Materials and Methods
Reagents and stock solutions
XOD from bovine milk (EC 1.17.3.2), Cyt C from horse heart, SOD, hypoxanthine (HX), 11-mercapto-1-undecanol (MU), 3-Mercapto,1-propionic acid (MPA), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), N-hydroxysuccinimide (NHS) and nanoceria (cerium oxide (CeO2) nanoparticles with 15 nm average diameter determined by Field Emission Scanning Electron Microscopy and Dynamic Light Scattering) were purchased from Sigma (St Louis, MO) and used as received. Sodium phosphate (monobasic), sodium hydroxide, sodium phosphate (dibasic, anhydrous), potassium chloride, EDTA and ethyl alcohol were purchased from Fisher Scientific. Sulfuric acid (95.4%) and ethanol were purchased from J.T Baker (Phillipsburg, NJ). DIDS was purchased from Sigma–Aldrich, dissolved in DMSO and used at a final concentration of 500 μM. All reagents were of analytical grade and were used without further purification. All solutions were prepared using distilled, deionized water (Millipore, Direct-Q system) with a resistivity of 18.2 MΩcm.
Instrumentation
Cyclic voltammetry (CV) and amperometric experiments were carried out with a CHI Electrochemical Analyzer (CHI Inc., TX, USA). All experiments were carried out using a three electrode system with a conventional cell equipped with a Ag/AgCl electrode (Ag/AgCl/3M NaCl) as reference electrode, a platinum wire (BAS, MW-1032) as counter electrode and a Cyt C functionalized gold wire microelectrode with a protruding tip of 1.5 mm long and a diameter of 0.25 mm.
Fabrication of the Cyt C biosensor
Gold wires with a diameter of 0.25 mm were cleaned electrochemically in 0.1 M H2SO4 by cycling the potential between 0 and +1.4 V at a scan rate of 0.1 Vs−1 until the characteristic cyclic voltammogram for gold was obtained. The cleaned Au wire electrodes were then rinsed with water and ethanol. An electrodeposited layer of gold nanoparticles was formed by applying a potential of −0.2 V for 60 sec to the electrode immersed in a HAuCl4 solution at a concentration of 0.01 M. Immediately after the gold deposition, the electrodes were thoroughly washed with water and ethanol and incubated for 96 hours at +4°C in an ethanolic solution containing a mixture of carboxyl and hydroxyl-terminated thiols (1.25mM of 3-Mercapto propionicacid [MPA] and 3.75 mM of 11-Mercapto Undecanol [MU]). The thiol modified electrodes were rinsed with ethanol and water to remove any unattached thiol molecules. To facilitate Cyt C immobilization, the carboxyl groups of the surface thiols were first activated with EDC and NHS. This was performed by incubating the electrodes in an aqueous solution containing 200 mM EDC and 50 mM NHS for 30 min. Cyt C was covalently immobilized by incubating the thiol modified gold wire electrode in a Cyt C solution at a concentration of 5×10−6 M for 2 hours. Finally the Cyt C modified electrodes were thoroughly rinsed with 0.1 M sodium phosphate buffer (PBS) solution to remove any unattached or weakly adsorbed Cyt C. The electrodes were stored at +4°C in a 0.1 M PBS solution containing 100 μM EDTA at pH 7.5 solution until use.
Electrochemical monitoring of the superoxide radical in mice brain slices using the biosensor
CD-1 mice of either sex were used for these experiments. All animals used in this study were housed in the St. Lawrence University’s vivarium, fed ad libitum and kept in a normal light/dark cycle. All procedures were approved by the St. Lawrence University Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult (2–4 month old) mice, which have more mature, fully developed central nervous system [30] and retain greater regional connectivity and physiological interactions [31], were sacrificed via rapid decapitation, their brains were quickly removed and placed in a chilled, choline-based slicing solution containing (in mM), 24 choline bicarbonate, 135 choline chloride, 1 kynurenic acid, 0.5 CaCl2, 1.4 Na2PO4, 5 glucose, 1 KCl, 20 MgCl2(315 mOsm) [32]. Transverse hippocampal slices of 400 μm thickwere cut along a rostral to caudal axis (−1.2 to −2.8 mm Bregma) using a Leica VT1200 vibratome (Leica Microsystems; Wetzlar, Germany) and allowed to recover for 1 hour in control artificial cerebral spinal fluid (aCSF) containing (in mM), 124 NaCl, 3 KCl, 2.4 CaCl2, 1.3 MgSO4, 1.24 KH2PO4, 26 NaHCO3, 5 glucose and bubbled with 5% CO2, 95% O2 gas (pH 7.4,300 mOsm). The culture medium contained 50% minimum essential medium (Hyclone Scientific; Logan, Utah), 25% horse serum, 25% Hanks balanced salt solution (supplemented with 28 mM glucose, 20 mM HEPES and 4 mM NaHCO3), 50 U/mL penicillin and 50 μg/mL streptomycin, pH 7.2 [30]. Solution osmolarity was measured using a vapor pressure osmometer and corrected to 295 mOsm (Wescor, Inc.; Logan, Utah). Hippocampal slices were placed in a culture dish and maintained in a humidified incubator (Eppendorf Company, New Brunswick, Galaxy 14S; Cambridge, UK) at 37°C with 5% CO2 during the experiments.
Amperometric measurements of enzymatically generated superoxide were performed in a conventional electrochemical cell, under constant stirring in air saturated 0.1 M Na-PBS with 100 μM EDTA at pH 7.5, or in culture medium, at an applied potential of +0.15 V. The current-time amperometric curves used to calibrate the Cyt C biosensor were generated upon addition of various XOD concentrations ranging from 2.5 to 80 mU/mL) to the air saturated PBS or culture medium containing 100 μM HX. All electrochemical experiments were performed at 37°C.
Electrochemical measurements for endogenously produced superoxide in hippocampal brain slices were performed by placing the recording electrode midline along the dorsal-ventral axis of the slice. The sections used for the recording were located −2.06 mm from Bregma and included the hippocampus, dentate gyrus, thalamus and portions of the hypothalamus. All the electrochemical recordings of superoxide in brain slices with the three-electrode system were performed in the incubator. During control conditions, the O2 and CO2 concentrations (~ 17% O2/5% CO2) reached steady state in approximately 30 minutes after the door was closed. Control recordings under normoxic and normocapnic conditions lasted approximately 180 minutes at which time, the response of the electrode to accumulated superoxide had reached a steady-state. Electrochemical experiments with brain slices for superoxide monitoring under ischemic conditions were carried out in the same incubator at 37°C by switching gas conditions to 84% N2, 15% CO2 and 1% O2. In experiments that required the addition of nanoceria or the addition of DIDS, these materials were delivered directly to the culture well plate in the incubator remotely with a syringe actuator connected by polyethylene tubing.
Results
Biosensor calibration and characterization
Cyt C immobilization and electrochemical characterization
The detection principle of the superoxide anion radical is based on the redox reaction between the Cyt C, immobilized onto the surface of a gold wire electrode and the generated superoxide [11, 33]. A new combination of self-assembled monolayer of mixed thiols of 3-mercaptopropionic acid (MPA) and 11-mercaptoundecanol (MU) was used in this work to covalently immobilize Cyt C onto the gold wire electrodes, modified with gold nanoparticles, through thiols chemistry. The immobilized Cyt C is reduced by superoxide and the reduced Cyt C is regenerated electrochemically at the electrode surface. The concentration of superoxide was quantified by constant potential amperometry with the electrode poised at the potential of 0.15 V vs. Ag/AgCl, which corresponds to the oxidation potential of Cyt C. The electrogenerated current is proportional to the concentration of the superoxide at the electrode surface. A schematic diagram of the reaction process at the gold electrode is shown in Figure 1. The immobilization of the Cyt C onto the gold electrode surface was confirmed by measuring the oxidation and reduction peaks of Cyt C using CV. The peak current (Ip) for both the cathodic and anodic current were linearly proportional to scan rate, suggesting that the electrode reaction was typical of a surface controlled process (Supplementary Information, S1). Surface coverage (Γ) of the electrode with Cyt C, calculated using the Laviron equation [34, 35] by integrating the reduction peak shows a surface concentration of 3.17 × 10−11 mol.cm−2 redox active Cyt C. The low surface coverage indicates the electrochemical behavior of a thin layer in which the redox process at the electrode surface is surface-confined and diffusionless.
Figure 1.

Experimental set-up for real-time monitoring of superoxide in brain slices. Right panel shows a schematic representation of the Cyt C immobilized onto the gold wire electrode through the self-assembly of mixed thiols monolayers, and the redox process of Cyt C in the presence of superoxide
Biosensor calibration
Biosensor calibration and optimization was carried out in standard solutions of superoxide generated by the HX/XOD system. XOD catalyzes the oxidation of HX in the presence of molecular oxygen, to uric acid and H2O2, with the production of superoxide, as an intermediate in this reaction [36]. The calibration curve obtained with the enzymatically generated superoxide was used to quantify the superoxide levels produced in brain slices. Since superoxide can undergo spontaneous dismutation to H2O2, the steady-state sensor signal was quantified by taking into account both generation and dismutation of the enzymatically generated superoxide [37–41]. Biosensor calibration was performed using an optimum HX concentration of 100 μM. The amperometric responses of the Cyt C biosensor generated upon addition of variable amounts of XOD ranging from 2.5 to 80 mU/mL to the reaction cell containing HX was measured and correlated with the theoretically estimated superoxide concentration. The calibration curve of the biosensor in culture medium showed in Figure 2 demonstrates the dependence of the sensor signal as a function of XOD activity as well as the theoretically estimated steady-state superoxide concentration. The sensor signal was proportional to the square root of the XOD concentration for enzymatic activities upto 80 mU/mL (Figure 3). There was little difference between the biosensor response to superoxide generated in PBS and the culture medium. The linear range for the detection of superoxide from the calibration curves were 0–1.23 μM and 0–1.32 μM, with sensitivities of 11.78 × 102 AM−1m−2 and 14.45 × 102 AM−1m−2 in PBS and culture medium respectively. The sensitivity of the Cyt C electrode is superior to that reported in literature with other sensor configurations including a mixed long-chain thiols based Cyt C electrode (2.76 × 102 AM−1m−2) [41] and a mixed short-chain thiols Cyt C electrode (0.56 × 102 AM−1m−2) [37].
Figure 2.
Typical amperometric current-time response of the Cyt C biosensor upon addition of various concentrations of XOD in the presence of 100 μM HX. Measurements are performed in culture medium at an applied potential of 0.15 V. The current difference between the baseline and the maximum value reached at the plateau at 70 sec was used to build the calibration curve of the biosensor. The decrease in the current after reaching the plateau can be due to the disproportionation of the enzymatically produced superoxide radicals into H2O2.
Figure 3.
Biosensor calibration using in situ enzymatically generated superoxide by the HX and XOD system. (A) Calibration and linear range of the biosensor in culture medium. (B) Dependence of the experimental sensor response on the theoretically estimated superoxide concentration in culture medium (C) Dependence of the experimental sensor signal (O) and the theoretically estimated steady-state superoxide concentration (x) on XOD activity in 0.1M 0.1 M Na-PBS containing 100 μM EDTA at pH 7.5. The HX concentration was 100 μM and XOD concentrations were 2.5, 5, 10, 20, 30, 40, 50, 60, 70 and 80 mU/mL.
The sensor response to superoxide was observed in less than 1 sec after XOD injection. A steady-state limiting current value was reached in 4–5 sec. The detection limit of the biosensor, calculated according to the 3σs/R′ criteria (R′ is the slope of the linear calibration curve and σs is the standard deviation of the amperometric signal of the blank solution) were 4.1 nM and 2.3 nM in PBS and culture medium respectively. The biosensor demonstrated good functionality and high sensitivity in the culture medium. The enhanced signal may be due to a higher stability of the superoxide radical in the culture medium whereas in PBS superoxide tends to decompose spontaneously at faster rates into H2O2 [42]. The biosensors were stable up to 7 days with no change in response when stored in PBS at +4°C. Cyclic voltammograms upon repeated scans showed stable redox peaks for 200 consecutive cycles, which demonstrates the stability of the biosensor (Supplementary Information, S2).
Biosensor selectivity
Electrochemically active uric acid (UA) is formed as a bi-product of the HX/XOD superoxide generation system used to calibrate the biosensor. Due to the short half-life of the superoxide radical, we studied the response selectivity against uric acid and the specificity of the sensor towards the superoxide radical. In the optimized configuration of mixed thiols, the biosensor showed no response when concentrations of uric acid of up to 50 μM where added into the medium (Supplementary Information, S3). In addition, the sensor showed no response to H2O2 which can be explained by the low applied potential of 0.15 V. To further confirm that the signal is solely associated with the produced superoxide radicals, SOD was added to inactivate the superoxide radicals. When a concentration of 10 U/ml SOD was added to a solution containing 1.04 μM superoxide (theoretical concentration), the electrochemical response was suppressed entirely until it reached the baseline signal, indicating that the superoxide radicals generated were completely inactivated by SOD (Figure 4). The results of these studies demonstrate that the signal is specific to superoxide radicals with no interference from the electrochemically active reaction by-products. The observed selectivity indicates that the modification of the electrode surface with the layer of densely packed thiols is effective at blocking diffusion of interfering species to the electrochemically active surface [41]. The thiol layer also reduces protein adsorption, ensuring good functionality of the sensor in the culture medium.
Figure 4.
In situ generation of superoxide anion radicals by addition of 100 μM HX and 50 mU/mL XOD to the reaction cell (arrows indicates the injection time) and then corresponding amperometric response upon addition of XOD. Also shown is the current drop upon addition of 10 U/mL SOD, which suppresses the enzymatically generated superoxide anion radicals.
Real-time measurement of superoxide in hippocampal brain slice using the Cyt C biosensor
The biosensor allows continuous monitoring of superoxide radicals in the extracellular matrix with high temporal resolution, high sensitivity and selectivity. This was achieved by placing the electrode on the rostral surface of brain slice without penetrating the tissue. The biosensor was fabricated with an L-shape configuration to provide a horizontal surface on which to position the slice for the duration of the experiment. Figure 5 shows the amperometric response of the Cyt C biosensor in contact with the brain slice. After the sensor reached a baseline response, the brain slice was placed onto the surface of the electrode and an immediate increase in the current was recorded. The current response stabilized and reached a maximum value in ~20–30 min after placing the slice onto the sensor surface. The sensor response corresponding to the superoxide produced by the brain slice was calculated by measuring the current difference between the maximum response reached after placing the slice onto the electrode and the background signal. The background signal was measured in the same dish in identical experimental conditions after removing the slice from the electrode surface. For quantitative analysis, the superoxide concentration in brain slices was calculated using the calibration curve obtained with the HX/XOD system in the culture medium. Under normal physiological conditions, the superoxide concentration in the extracellular matrix quantified by the biosensor was 1.95 μM. To demonstrate that the measured superoxide is originating from the brain tissue, a control experiment was conducted under the same experimental conditions but by placing the brain slice in the recording dish ~1 cm away from the electrochemical active surface. When the electrode was not in direct contact with the brain tissue, the sensor did not show any amperometric response (Figure 5; inset). This indicates that only superoxide levels that are generated when the biological tissue is in direct contact with the electrode are measured.
Figure 5.
Real-time amperometric response of the Cyt C biosensor in contact with the hippocampal brain slice. The increase in the current indicates release of extracellular superoxide radicals from the brain slice in normal physiological conditions. The inset shows a control experiment with the brain slice placed in the measurement cell but far away from the electrode surface.
To further demonstrate the specificity of the signal towards the superoxide radicals originating from the extracellular space of the brain slice, we used the addition of SOD exogenously to facilitate the conversion of superoxide to hydrogen peroxide (Figure 6). Each sequential addition of 500 U/mL SOD in the reaction cell decreased the amperometric current by approximately 14% which suggests that the superoxide radicals were inactivated by the added SOD. Three sequential additions of 500 U/mL SOD reduced the electrode signal near to baseline levels suggesting that the exogenously added SOD resulted in the dismutation of the superoxide produced. The residual current in Figure 6 can be attributed to the capacitive current associated with the brain slice. After removing the slice from the electrode surface, the current reached a background value that is slightly higher than the initial baseline current recorded at the beginning of the experiment. This finding is likely the result of several factors including nonspecific adsorption of proteins, the presence of residual tissue detritus on the electrode surface, or mechanical damage of the Cyt C layer produced when placing and removing the brain slice to/from the electrode surface. To account for these effects, the superoxide concentration was estimated using the residual current measured after removing the slice from the electrode surface as baseline value.
Figure 6.
Specificity of the biosensor response towards superoxide released by the hippocampal brain slice in normal physiological conditions. With each addition of 500 U/ml SOD there is a decrease in the amperometric response indicating specific inactivation of the produced superoxide radicals. The arrows indicate the time of SOD additions and the exposure of the brain to/from the electrode surface.
Evaluation of transmembrane flux of superoxide in brain slices through VDAC channels
To examine the potential role of the transmembrane flux of superoxide into the extracellular space, DIDS (0.5 mM final concentration for each addition; 2 mM total) was added to the culture media during the recordings. Previous studies have shown that superoxide can penetrate a variety of biological membranes through the VDAC, which are inhibited with DIDS [27, 28]. The mechanism by which DIDS inhibits anion channels is not known, although the inhibition appears to occur in the extracellular space and is reversible in some systems. During control conditions, the addition of DIDS to the culture media resulted in a decrease in the superoxide signal (Figure 7) with each subsequent addition. Since it was not possible to stir the solution during the experiment while recording, it is likely the DIDS concentration was not uniform throughout the solution. In separate experiments, the addition of DIDS alone had no effect on sensor output.
Figure 7.
Effect of four consecutive additions of DIDS (0.5 mM final concentration in the reaction cell) on the amperometric response of the Cyt C biosensor. The arrows indicate the time of DIDS additions.
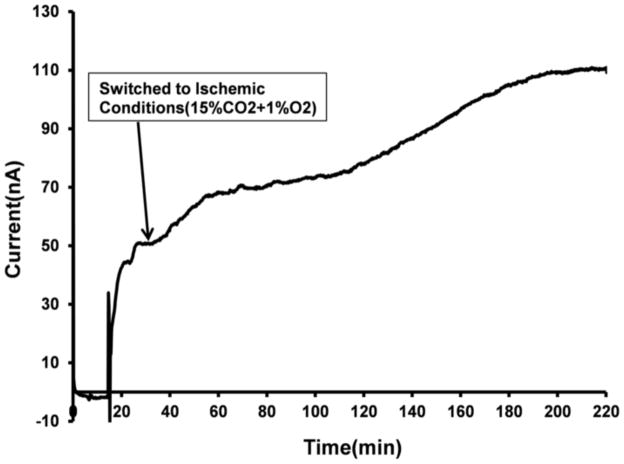
Real-time continuous monitoring of superoxide dynamics during ischemia
Immediately following the transition to hypoxic/hypercapnic gas mixtures in the incubator, superoxide levels began to increase and reached a steady-state after ~180 min (Figure 8). Compared to the normoxic superoxide levels, peak superoxide levels increased approximately 2-fold during ischemia. Based on our calculations this resulted in an average increase in superoxide from 1.95 μM to 4.1 μM. In contrast to our findings during normoxia, the sequential addition of DIDS had no effect on the extracellular levels of superoxide.
Figure 8.
Typical current-time response of Cyt C biosensor showing continuous monitoring of superoxide in mouse hippocampal brain slice exposed to simulated ischemic conditions ischemic conditions. The arrows indicate the time when the brain slice was placed onto the electrode surface, and the onset of the ischemic insult, by exposing the slice to hypoglycemic, acidic and hypoxic conditions (glucose was lowered to 2 mM and the cell was bubbled with 15 % CO2 and 1% oxygen).
Assessing antioxidant activity of cerium oxide nanoparticles in ischemic brain slices
In the present study, we used the Cyt C biosensor to quantitatively assess the time-course inactivation of extracellular superoxide by nanoceria. Previous work from our lab showed that nanoceria is capable of scavenging a variety of free radicals including nitric oxide, superoxide and peroxynitrite in brain slices [32] and the catalytic activity of nanoceria reduces superoxide to H2O2. The addition of nanoceria with an average particle size of 15 nm induced an immediate decrease in the superoxide signal during control conditions, confirming the neutralizing activity of these nanoparticles against superoxide (Figure 9). Consecutive addition of 1 μg/mL nanoceria decreased the superoxide levels by 14.5±4.5% (n=5) per addition. Following two consecutive nanoparticle additions, the superoxide levels remained stable for the remainder of the experiment. In contrast, the addition of the same amount of nanoceria during ischemia decreased the superoxide signal by 8.1±2.2% (n=3) per addition. Moreover, following two sequential nanoparticle additions, the superoxide signal decreased progressively towards baseline for the remainder of the experiment. Section S4 in the Supplementary Information shows the decrease in the extracellular superoxide concentration for both SOD and nanoceria. Based on these findings, a 1 μg/mL nanoceria concentration induced a decrease in the superoxide level under normoxic conditions equivalent to that of 527 U SOD activity units, as calculated from the amperometric responses of the biosensor in brain slices.
Figure 9.
Effect of nanoceria (successive additions of 1 mg/mL to the reaction cell) on the superoxide level quantified by the Cyt C biosensor under (A) physiological and (B) simulated ischemic conditions.
Discussion
This study demonstrates the potential of electrochemical microbiosensors for studying the release, accumulation and mobility of the superoxide radical, and potentially of other oxidative stress markers in brain tissue with high spatial and temporal resolution and can facilitate our fundamental understanding of the neurophysiology of oxidative stress related diseases.
Current methods for monitoring the superoxide anion radical in hippocampal brain slices include colorimetric and fluorescent assays that measure intracellular superoxide [43]. A drawback of these methods is the difficulty of obtaining a continuous, quantitative, real-time measurement of superoxide production in biological tissue. Most often researchers interested in assessing oxidative damage rely on downstream markers such as lipid and protein oxidation [44–46]. Due to their small sizes (micron to submicron diameter) electrochemical sensors can provide real-time measurements of ROS and RNS species [47]. The microbiosensor used in this work consists of a gold wire electrode modified with mixed layers of thiols and immobilized Cyt C as a bio-recognition element [33]. Electrochemical sensors for the detection of superoxide have been reported in literature [48] but these have seldom been used to measure real-time production of superoxide in intact biological tissues. Most studies have relied on standard solutions of enzymatically produced superoxide, with few examples using cell cultures. Rarely have the studies demonstrated the applicability of similar devices to measure extracellular superoxide in biological tissues [49–51]. There are no reports on the use of electrochemical microbiosensors for studying superoxide mobility across membranes and their application for studying oxidant and antioxidant mechanisms of natural or synthetic antioxidants in the brain. Our ability to measure superoxide in such a small volume of tissue in this study suggests that these electrodes should be equally sensitive in vivo.
To explore the potential of this technology, we examined the nature of the superoxide signal in the extracellular space of the brain slice. There is increasing evidence that free radicals play a central role in redox signaling both in health and disease [9]. Madesh et al. first suggested that extracellular superoxide produced by plasmalemmal NADPH oxidase or superoxide crossing the plasma membrane through a VDAC-like channel modified cell surface proteins to mediate cell signaling involved in programmed cell death [52]. Recent proteomic studies have confirmed the presence of VDAC in the plasma membrane of human cells [53–55] including cultured septal and hippocampal neuronal cells and in membrane preparations from human brain cortex [56]. Although preliminary, this study is the first report of DIDS eliminating the superoxide signal in intact brain tissue. The DIDS-sensitive accumulation of superoxide during control conditions suggests that the transmembrane flux of superoxide occurred through a VDAC-like channel and not simply an artifact of compromised cell membranes associated with the sectioning process. The VDAC is a highly conserved protein both in terms of its structural and functional features across species [57]. It has been hypothesized that VDAC sub-serves a variety of diverse roles including cellular ATP release, maxi-anion channel, volume control, a component of the GABAA receptor, a plasma membrane NADH oxido-reductase and a modulator of programmed cell death. Our observation that extracellular superoxide concentrations were unaffected by DIDS during ischemia suggests that VDAC may either become insensitive to the effects of DIDS or close during the ischemic period. Consistent, with this latter notion, previous studies have shown that NADH levels increase during the period of ischemia [58–60] and elevated cytosolic NADH concentrations will promote the closure of VDAC and apoptotic signals [53, 61, 62]. The fact that extracellular superoxide is elevated during the ischemic period even with closure of VDAC suggests that there are alternate pathways for transmembrane flux of superoxide or there may be activation of plasmalemmal NADPH oxidase. Additional experiments will be needed to explore these questions but this study hallmarks the importance of this technology in the study of the biological effects of free radicals.
Previous work from our lab [63–65] and others [66–75] have demonstrated the ability of ceria to neutralize a variety of biologically relevant free radicals. The 3+/4+ valence of nanoceria allows the particle to participate in reversible redox reactions similar to endogenous redox enzymes in cells. Given its high redox potential (1.55 V), ceria can accept electrons from hydroxyl, superoxide and peroxynitrite in the +4 state and donate electrons to H2O2 in the +3 state. Our data acquired with the electrochemical Cyt C microsensor shows that the catalytic activity for ceria is remarkably high with respect to superoxide dismutase; 1 μg of ceria was equivalent to ~527 U of SOD in neutralizing superoxide. The progressive decrease in superoxide accumulation after repeated administration of ceria during ischemia is likely due to the cumulative uptake and intracellular scavenging of superoxide by the nanoparticles. We have previously shown that particles of nanoceria are endocytosed within 1 hour where they localize to mitochondria, neurofilaments and lipid/myelin membranes [63]. Given its regenerative, catalytic nature, nanoceria has become attractive as a potential pharmacological agent and potent antioxidant [66–74]. This being said it has become increasingly clear that the contradictory biological effects of nanoceria (see Yokel and others) [76–79] reported in the literature stem from differences in the physical (i.e. size, zeta potential) and chemical characteristics (presence of dopants or stabilizers) of the particles. Modest changes in the synthetic identity of the particles can confer very different biological outcomes. We have recently explored the therapeutic potential of nanoceria in murine model of multiple sclerosis [80] using both custom synthesized and commercially available nanoceria. We found that the biological effects and deposition of the particles in the brain were dependent on multiple physical/chemical characteristics. Exploring the factors that contribute to the biological action of nanoceria will be an important area of research, as the therapeutic potential of these compounds will be developed in the future.
Conclusions
This work demonstrates the ability of electrochemical biosensors to study real-time dynamics of reactive oxygen species in a biological model. The electrochemical measurements provided evidence of trans-membrane mobility and extracellular superoxide release in normal physiological and simulated ischemic conditions in a brain slice model of ischemia. Pharmacological manipulations of VDAC channels, which facilitate transport of mitochondrial superoxide to the extracellular space, demonstrate the mobility of superoxide measured electrochemically. The dynamics of superoxide overproduction during ischemic brain injury was quantified over time and the specificity of signal was validated using SOD as a model endogenous antioxidant. The potential of this technology for studying the time course inactivation and providing quantitative assessment of the superoxide scavenging capacity by antioxidant compounds has been demonstrated with an emerging engineered antioxidant, nanoceria, which holds potential for treatment and therapy of oxidative diseases. In summary, this study demonstrates the potential of electrochemical sensors for providing quantitative assessment of real-time changes of extracellular ROS and studying fundamental mechanisms to understand ROS-mediated processes in normal physiological and pathophysiological conditions involved in disease progression and therapy.
Supplementary Material
Highlights.
Superoxide levels were examined in a mouse hippocampal brain slice model of ischemia.
Electrochemical measurements provide evidence for extracellular superoxide release.
Specificity of the response was demonstrated via addition of superoxide dismutase.
Nanoceria may be a promising therapeutic agent for the treatment of ischemic injury.
Acknowledgments
This work was supported by NSF # 0954919, NIH #R21NS078738-01 and USAR W911NF-11-1-0304 to SA. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free radical biology & medicine. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Paulsen CE, Carroll KS. Orchestrating Redox Signaling Networks through Regulatory Cysteine Switches. Acs Chemical Biology. 2010;5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson BH. The role of manganese superoxide dismutase in health and disease. J Inherit Metab Dis. 1998;21:598–603. doi: 10.1023/a:1005427323835. [DOI] [PubMed] [Google Scholar]
- 4.Dickinson BC, Peltier J, Stone D, Schaffer DV, Chang CJ. Nox2 redox signaling maintains essential cell populations in the brain. Nat Chem Biol. 2011;7:106–112. doi: 10.1038/nchembio.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daiber A, Frein D, Namgaladze D, Ullrich V. Oxidation and nitrosation in the nitrogen monoxide/superoxide system. Journal of Biological Chemistry. 2002;277:11882–11888. doi: 10.1074/jbc.M111988200. [DOI] [PubMed] [Google Scholar]
- 6.Daiber A, Bachschmid M. Enzyme inhibition by peroxynitrite-mediated tyrosine nitration and thiol oxidation. Curr Enzyme Inhibit. 2007;3:103–117. [Google Scholar]
- 7.Kuroda J, Ago T, Matsushima S, Zhai PY, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Archiv-European Journal of Physiology. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 9.Zweier JL, Talukder MAH. The role of oxidants and free radicals in reperfusion injury. Cardiovascular Research. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Salvemini D, Cuzzocrea S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radical Bio Med. 2002;33:1173–1185. doi: 10.1016/s0891-5849(02)00961-9. [DOI] [PubMed] [Google Scholar]
- 11.Arnold S, Feng ZQ, Kakiuchi T, Knoll W, Niki K. Investigation of the electrode reaction of cytochrome c through mixed self-assembled monolayers of alkanethiols on gold(111) surfaces. Journal of Electroanalytical Chemistry. 1997;438:91–97. [Google Scholar]
- 12.Siemionow M, Arslan E. Ischemia/reperfusion injury: a review in relation to free tissue transfers. Microsurgery. 2004;24:468–475. doi: 10.1002/micr.20060. [DOI] [PubMed] [Google Scholar]
- 13.Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends in Molecular Medicine. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuda S, Umeda M, Uchida H, Kato H, Araki T. Alterations of oxidative stress markers and apoptosis markers in the striatum after transient focal cerebral ischemia in rats. Journal of Neural Transmission. 2009;116:395–404. doi: 10.1007/s00702-009-0194-0. [DOI] [PubMed] [Google Scholar]
- 15.Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A. 1990;87:5144–5147. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Floyd RA, Carney JM. Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann Neurol. 1992;32(Suppl):S22–27. doi: 10.1002/ana.410320706. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono T, Tsuruta R, Fujita M, Aki HS, Kutsuna S, Kawamura Y, Wakatsuki J, Aoki T, Kobayashi C, Kasaoka S, Maruyama I, Yuasa M, Maekawa T. Xanthine oxidase is one of the major sources of superoxide anion radicals in blood after reperfusion in rats with forebrain ischemia/reperfusion. Brain Research. 2009;1305:158–167. doi: 10.1016/j.brainres.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 19.Lynch RE, Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978;253:4697–4699. [PubMed] [Google Scholar]
- 20.Lisanti MP, Scherer PE, Tang Z, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994;4:231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 21.Baker MA, Lane DJR, Ly JD, De Pinto V, Lawen A. VDAC1 is a transplasma membrane NADH-ferricyanide reductase. Journal of Biological Chemistry. 2004;279:4811–4819. doi: 10.1074/jbc.M311020200. [DOI] [PubMed] [Google Scholar]
- 22.Darbandi-Tonkabon R, Hastings WR, Zeng CM, Akk G, Manion BD, Bracamontes JR, Steinbach JH, Mennerick SJ, Covey DF, Evers AS. Photoaffinity Labeling with a neuroactive steroid analogue - 6-AZI-Pregnanolone labels voltage-dependent anion channel-1 in rat brain. Journal of Biological Chemistry. 2003;278:13196–13206. doi: 10.1074/jbc.M213168200. [DOI] [PubMed] [Google Scholar]
- 23.Blatz AL, Magleby KL. Single voltage-dependent chloride-selective channels of large conductance in cultured rat muscle. Biophys J. 1983;43:237–241. doi: 10.1016/S0006-3495(83)84344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahamonde MI, Fernandez-Fernandez JM, Guix FX, Vazquez E, Valverde MA. Plasma membrane voltage-dependent anion channel mediates antiestrogen-activated maxi Cl− currents in C1300 neuroblastoma cells. The Journal of biological chemistry. 2003;278:33284–33289. doi: 10.1074/jbc.M302814200. [DOI] [PubMed] [Google Scholar]
- 25.Mao GD, Poznansky MJ. Electron spin resonance study on the permeability of superoxide radicals in lipid bilayers and biological membranes. FEBS Letters. 1992;305:233–236. doi: 10.1016/0014-5793(92)80675-7. [DOI] [PubMed] [Google Scholar]
- 26.Ikebuchi Y, Masumoto N, Tasaka K, Koike K, Kasahara K, Miyake A, Tanizawa O. Superoxide anion increases intracellular pH, intracellular free calcium, and arachidonate release in human amnion cells. Journal of Biological Chemistry. 1991;266:13233–13237. [PubMed] [Google Scholar]
- 27.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent Anion Channels Control the Release of the Superoxide Anion from Mitochondria to Cytosol. Journal of Biological Chemistry. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 28.Mumbengegwi DR, Li Q, Li C, Bear CE, Engelhardt JF. Evidence for a Superoxide Permeability Pathway in Endosomal Membranes. Molecular and Cellular Biology. 2008;28:3700–3712. doi: 10.1128/MCB.02038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalyanaraman B, Darley-Usmar V, Davies KJA, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radical Bio Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Njagi J, Erlichman JS, Aston JW, Leiter JC, Andreescu S. A sensitive electrochemical sensor based on chitosan and electropolymerized Meldola blue for monitoring NO in brain slices. Sensors and Actuators B-Chemical. 2010;143:673–680. [Google Scholar]
- 31.Sullivan BL, Leu D, Taylor DM, Fahlman CS, Bickler PE. Isoflurane prevents delayed cell death in an organotypic slice culture model of cerebral ischemia. Anesthesiology. 2002;96:189–195. doi: 10.1097/00000542-200201000-00033. [DOI] [PubMed] [Google Scholar]
- 32.Erlichman Joseph S, AH, Damon Tracey L, 1, Hart Michael, Kurascz Jennifer, Li Aihua, Leiter James C. Inhibition of Monocarboxylate Transporter 2 in the Retrotrapezoid Nucleus in Rats: A Test of the Astrocyte–Neuron Lactate-Shuttle Hypothesis. Journal of Neuroscience. 2008;28:4888–4896. doi: 10.1523/JNEUROSCI.5430-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato Y, Mizutani F. Electrochemical responses of cytochrome c on a gold electrode modified with mixed monolayers of 3-mercaptopropionic acid and n-alkanethiol. Journal of Electroanalytical Chemistry. 1997;438:99–104. [Google Scholar]
- 34.Laviron E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 1974;52:355–393. [Google Scholar]
- 35.Laviron E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 1979;101:19–28. [Google Scholar]
- 36.Fujita M, Tsuruta R, Kasaoka S, Fujimoto K, Tanaka R, Oda Y, Nanba M, Igarashi M, Yuasa M, Yoshikawa T, Maekawa T. In vivo real-time measurement of superoxide anion radical with a novel electrochemical sensor. Free radical biology & medicine. 2009;47:1039–1048. doi: 10.1016/j.freeradbiomed.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Tammeveski K, Tenno TT, Mashirin AA, Hillhouse EW, Manning P, McNeil CJ. Superoxide electrode based on covalently immobilized cytochrome c: Modelling studies. Free Radical Bio Med. 1998;25:973–978. doi: 10.1016/s0891-5849(98)00182-8. [DOI] [PubMed] [Google Scholar]
- 38.McCord JM, Fridovich I. The Reduction of Cytochrome c by Milk Xanthine Oxidase. Journal of Biological Chemistry. 1968;243:5753–5760. [PubMed] [Google Scholar]
- 39.Behar D, Czapski G, Rabani J, Dorfman LM, Schwarz HA. Acid dissociation constant and decay kinetics of the perhydroxyl radical. The Journal of Physical Chemistry. 1970;74:3209–3213. [Google Scholar]
- 40.Chen XJ, West AC, Cropek DM, Banta S. Detection of the superoxide radical anion using various alkanethiol monolayers and immobilized cytochrome c. Anal Chem. 2008;80:9622–9629. doi: 10.1021/ac800796b. [DOI] [PubMed] [Google Scholar]
- 41.Ge B, Lisdat F. Superoxide sensor based on cytochrome c immobilized on mixed-thiol SAM with a new calibration method. Analytica Chimica Acta. 2002;454:53–64. [Google Scholar]
- 42.Gobi KV, Mizutani F. Efficient mediatorless superoxide sensors using cytochrome c-modified electrodes: surface nano-organization for selectivity and controlled peroxidase activity. Journal of Electroanalytical Chemistry. 2000;484:172–181. [Google Scholar]
- 43.Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. The Journal of biological chemistry. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 45.Schopfer FJ, Batthyany C, Baker PR, Bonacci G, Cole MP, Rudolph V, Groeger AL, Rudolph TK, Nadtochiy S, Brookes PS, Freeman BA. Detection and quantification of protein adduction by electrophilic fatty acids: mitochondrial generation of fatty acid nitroalkene derivatives. Free radical biology & medicine. 2009;46:1250–1259. doi: 10.1016/j.freeradbiomed.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kochanek PM, Berger RP, Bayir H, Wagner AK, Jenkins LW, Clark RS. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Current opinion in critical care. 2008;14:135–141. doi: 10.1097/MCC.0b013e3282f57564. [DOI] [PubMed] [Google Scholar]
- 47.Wilson GS, Johnson MA. In-vivo electrochemistry: What can we learn about living systems? Chemical Reviews. 2008;108:2462–2481. doi: 10.1021/cr068082i. [DOI] [PubMed] [Google Scholar]
- 48.Mesároš Š, Vaňková Ž, Grunfeld S, Mesárošová A, Malinski T. Preparation and optimization of superoxide microbiosensor. Analytica Chimica Acta. 1998;358:27–33. [Google Scholar]
- 49.Buttemeyer R, Philipp AW, Mall JW, Ge BX, Scheller FW, Lisdat F. In vivo measurement of oxygen-derived free radicals during reperfusion injury. Microsurgery. 2002;22:108–113. doi: 10.1002/micr.21733. [DOI] [PubMed] [Google Scholar]
- 50.Fabian RH, Dewitt DS, Kent TA. In-Vivo Detection of Superoxide Anion Production by the Brain Using a Cytochrome-C Electrode. J Cerebr Blood F Met. 1995;15:242–247. doi: 10.1038/jcbfm.1995.30. [DOI] [PubMed] [Google Scholar]
- 51.Scheller W, Jin W, Ehrentreich-Forster E, Ge B, Lisdat F, Buttemeier R, Wollenberger U, Scheller FW. Cytochrome C based superoxide sensor for in vivo application. Electroanal. 1999;11:703–706. [Google Scholar]
- 52.Madesh M, Hawkins BJ, Milovanova T, Bhanumathy CD, Joseph SK, Ramachandrarao SP, Sharma K, Kurosaki T, Fisher AB. Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J Cell Biol. 2005;170:1079–1090. doi: 10.1083/jcb.200505022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lisanti MP, Scherer PE, Vidugiriene J, Tang ZL, Hermanowskivosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of Caveolin-Rich Membrane Domains Isolated from an Endothelial-Rich Source - Implications for Human-Disease. Journal of Cell Biology. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stockwin LH, Blonder J, Bumke MA, Lucas DA, Chan KC, Conrads TP, Issaq HJ, Veenstra TD, Newton DL, Rybak SM. Proteomic analysis of plasma membrane from hypoxia-adapted malignant melanoma. J Proteome Res. 2006;5:2996–3007. doi: 10.1021/pr0601739. [DOI] [PubMed] [Google Scholar]
- 55.Schindler J, Lewandrowski U, Sickmann A, Friauf E, Nothwang HG. Proteomic analysis of brain plasma membranes isolated by affinity two-phase partitioning. Mol Cell Proteomics. 2006;5:390–400. doi: 10.1074/mcp.T500017-MCP200. [DOI] [PubMed] [Google Scholar]
- 56.Ramirez CM, Gonzalez M, Diaz M, Alonso R, Ferrer I, Santpere G, Puig B, Meyer G, Marin R. VDAC and ERalpha interaction in caveolae from human cortex is altered in Alzheimer’s disease. Mol Cell Neurosci. 2009;42:172–183. doi: 10.1016/j.mcn.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Shoshan-Barmatz V, Israelson A, Brdiczka D, Sheu SS. The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death. Curr Pharm Des. 2006;12:2249–2270. doi: 10.2174/138161206777585111. [DOI] [PubMed] [Google Scholar]
- 58.Perez-Pinzon MA, Mumford PL, Rosenthal M, Sick TJ. Antioxidants, mitochondrial hyperoxidation and electrical recovery after anoxia in hippocampal slices. Brain Res. 1997;754:163–170. doi: 10.1016/s0006-8993(97)00066-8. [DOI] [PubMed] [Google Scholar]
- 59.Perez-Pinzon MA, Mumford PL, Carranza V, Sick TJ. Calcium influx from the extracellular space promotes NADH hyperoxidation and electrical dysfunction after anoxia in hippocampal slices. J Cereb Blood Flow Metab. 1998;18:215–221. doi: 10.1097/00004647-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 60.Foster KA, Margraf RR, Turner DA. NADH hyperoxidation correlates with enhanced susceptibility of aged rats to hypoxia. Neurobiol Aging. 2008;29:598–613. doi: 10.1016/j.neurobiolaging.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rostovtseva TK, Bezrukov SM. VDAC regulation: role of cytosolic proteins and mitochondrial lipids. J Bioenerg Biomembr. 2008;40:163–170. doi: 10.1007/s10863-008-9145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zizi M, Forte M, Blachly-Dyson E, Colombini M. NADH regulates the gating of VDAC, the mitochondrial outer membrane channel. The Journal of biological chemistry. 1994;269:1614–1616. [PubMed] [Google Scholar]
- 63.Estevez AY, Pritchard S, Harper K, Aston JW, Lynch A, Lucky JJ, Ludington JS, Chatani P, Mosenthal WP, Leiter JC, Andreescu S, Erlichman JS. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free radical biology & medicine. 2011;51:1155–1163. doi: 10.1016/j.freeradbiomed.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Estevez A, Erlichman JS. Cerium Oxide Nanoparticles for the Treatment of Neurological Oxidative Stress Diseases. Oxidative Stress: Diagnostics, Prevention, and Therapy. :255–288. [Google Scholar]
- 65.Andreescu S, Ornatska M, Erlichman JS, Estevez A, Leiter JC. Fine Particles in Medicine and Pharmacy. Vol. 2011. Springer Science and Business Media, LLC; 2011. Biomedical Applications of Metal Oxide Nanoparticles. [Google Scholar]
- 66.Chen J, Patil S, Seal S, McGinnis JF. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol. 2006;1:142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 67.Das M, Patil S, Bhargava N, Kang JF, Riedel LM, Seal S, Hickman JJ. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28:1918–1925. doi: 10.1016/j.biomaterials.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D’Angelo B, Santucci S, Benedetti E, Di Loreto S, Phani RA, Falone S, Amicarelli F, Ceru MP, Cimini A. Cerium Oxide Nanoparticles Trigger Neuronal Survival in a Human Alzheimer Disease Model By Modulating BDNF Pathway. Curr Nanosci. 2009;5:167–176. [Google Scholar]
- 69.Hirst SM, Karakoti A, Singh S, Self W, Tyler R, Seal S, Reilly CM. Bio-distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Environmental toxicology. 2011 doi: 10.1002/tox.20704. [DOI] [PubMed] [Google Scholar]
- 70.Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM. Anti-inflammatory Properties of Cerium Oxide Nanoparticles. Small. 2009;5:2848–2856. doi: 10.1002/smll.200901048. [DOI] [PubMed] [Google Scholar]
- 71.Celardo I, Pedersen JZ, Traversa E, Ghibelli L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale. 2011;3:1411–1420. doi: 10.1039/c0nr00875c. [DOI] [PubMed] [Google Scholar]
- 72.Colon J, Hsieh N, Ferguson A, Kupelian P, Seal S, Jenkins DW, Baker CH. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomed-Nanotechnol. 2010;6:698–705. doi: 10.1016/j.nano.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 73.Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovascular Research. 2007;73:549–559. doi: 10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tarnuzzer RW, Colon J, Patil S, Seal S. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano letters. 2005;5:2573–2577. doi: 10.1021/nl052024f. [DOI] [PubMed] [Google Scholar]
- 75.Niu JL, Wang KK, Kolattukudy PE. Cerium Oxide Nanoparticles Inhibits Oxidative Stress and Nuclear Factor-kappa B Activation in H9c2 Cardiomyocytes Exposed to Cigarette Smoke Extract. J Pharmacol Exp Ther. 2011;338:53–61. doi: 10.1124/jpet.111.179978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hardas SS, Butterfield DA, Sultana R, Tseng MT, Dan M, Florence RL, Unrine JM, Graham UM, Wu P, Grulke EA, Yokel RA. Brain distribution and toxicological evaluation of a systemically delivered engineered nanoscale ceria. Toxicological sciences: an official journal of the Society of Toxicology. 2010;116:562–576. doi: 10.1093/toxsci/kfq137. [DOI] [PubMed] [Google Scholar]
- 77.Yokel RA, Florence RL, Unrine JM, Tseng MT, Graham UM, Wu P, Grulke EA, Sultana R, Hardas SS, Butterfield DA. Biodistribution and oxidative stress effects of a systemically-introduced commercial ceria engineered nanomaterial. Nanotoxicology. 2009;3:234–248. [Google Scholar]
- 78.Park EJ, Choi J, Park YK, Park K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology. 2008;245:90–100. doi: 10.1016/j.tox.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 79.Auffan M, Rose J, Orsiere T, De Meo M, Thill A, Zeyons O, Proux O, Masion A, Chaurand P, Spalla O, Botta A, Wiesner MR, Bottero JY. CeO2 nanoparticles induce DNA damage towards human dermal fibroblasts in vitro. Nanotoxicology. 2009;3:161–U115. [Google Scholar]
- 80.DeCoteau WE, Estevez AY, Leo-Nyquist S, Heckman K, KRJSE Ceria Nanoparticles Reduce Disease Severity in a Mouse Model of Multiple Sclerosis. TechConnect World Abstracts. 2011 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.