Abstract
Targeting of drugs and their carrier systems by using receptor-mediated endocytotic pathways was in its nascent stages 25 years ago. In the intervening years, an explosion of knowledge focused on design and synthesis of nanoparticulate delivery systems as well as elucidation of the cellular complexity of what was previously-termed receptor-mediated endocytosis has now created a situation when it has become possible to design and test the feasibility of delivery of highly specific nanoparticle drug carriers to specific cells and tissue. This review outlines the mechanisms governing the major modes of receptor-mediated endocytosis used in drug delivery and highlights recent approaches using these as targets for in vivo drug delivery of nanoparticles. The review also discusses some of the inherent complexity associated with the simple shift from a ligand-drug conjugate versus a ligand-nanoparticle conjugate, in terms of ligand valency and its relationship to the mode of receptor-mediated internalization.
Keywords: Endocytosis, Drug Delivery, Cellular Targeting, Multivalent Targeting
1. Introduction
The concept of the use of receptor targeting for selective drug binding and internalization has been attractive since early advances in cell biology began to reveal built-in internalization pathways that enabled selective uptake of essential constituents from the external environment. The exploitation of these endogenous uptake pathways for drug delivery began to be tested in the 1980s and 1990s, in particular with findings on the properties of tumor cells that enabled identification of receptors that were overexpressed in rapidly proliferating cells. Some of the earliest approaches utilized the transferrin receptor (TfR), a receptor binding the glycoprotein, transferrin (Tf), which sequesters free iron in the serum, and facilitates its entry into cells. TfR is selectively enriched in proliferating tumor cells [1], an observation which was subsequently used to direct selective internalization of a host of transferrin-toxin and transferrin-DNA conjugates, directly or encapsulated in liposomes or other structures, in a large body of work summarized in the excellent review by Widera et al. [2]. TfR remains a major drug delivery target, both in tumors as well as for enabling transcytosis of conjugates across barrier epithelial and endothelial cells. Another early target, the low density lipoprotein receptor (LDLR) has been of continuous interest since the early demonstration of LDL-mediated targeting of liposomes to leukemic lymphocytes in 1985 [3]. However, the repertoire of other prospective receptor targets for internalization of conjugates has significantly increased since this early work.
Interest in receptor targeting for selective uptake and internalization of drugs has expanded even further with the advent of new macromolecular drugs including DNA, peptides and proteins, because of the limitations in their ability to access vesicular or cytosolic targets. The availability of sophisticated nanotechnology approaches to encapsulate drugs, providing controlled release capacity as well as protection of macromolecules from degradation prior to reaching the site of action, has provided an additional level of complexity, since the physical properties of the particle as well as the surface composition are altered with the addition of targeting moieties to influence uptake, sometimes in unpredictable ways.
Our objective in this review article is to highlight recent advances in drug delivery that combine receptor-mediated endocytotic targeting strategies with nanomaterials, with a focus on applications that have shown promise of efficacy beyond simple cell culture into organismal models and which are poised for future translation for use in human medicine. Indeed, the use of a nanomaterial scaffold prompts concerns regarding ligand spacing and valency, which are discussed from the perspective of how they affect the internalization process. In this rapidly evolving field, other recent and excellent reviews have also provided comprehensive analyses of the importance of diverse cell biological endocytotic pathways to drug uptake and internalization [4] and on the interaction of diverse nanomaterials with cells and their preference for internalization through different endocytotic pathways [5], so we do not focus on these areas.
2. Cellular internalization pathways
It is widely accepted that endocytosis is the predominant route of uptake of macromolecules, whether they are soluble cargo or membrane proteins, into cells. In drug delivery applications, the endocytotic pathway has always held significant promise for the targeted delivery and uptake of therapeutic macromolecules into cells. However, in the past 25 years, progress in exploiting the endocytotic pathway for drug delivery has come in fits and starts, likely due to the complexity of this process as well as the complexity of the diseases that are targeted by this approach, and how these diseases may in turn alter endocytotic trafficking. This first section will review the fundamentals of endocytosis and post-endocytotic trafficking.
In 1987, the extent to which the function and mechanism of endocytosis was understood is illustrated in a review by Pearse and Crowther [6]:
“If all kinds of molecules were to enter coated vesicles on a random basis, the components of the different compartments would rapidly intermix. However, on the plasma membrane at least, selected membrane components are concentrated in coated pits, while other molecules characteristic of the parent membrane are excluded. Thus, the formation of this structure controls which proteins are transferred to another compartment and which remain behind.”
Moreover, the critical major protein components of the currently best-understood endocytotic pathway, the clathrin-mediated endocytotic pathway, were thus rather amorphously described:
“We use the term “100-kd-50-kd proteins” to denote all the accessory polypeptides. A “cage” is a polyhedral structure of complete or incomplete clathrin triskelions, whereas a “coat” is a clathrin assembly that also contains 100-kd-50-kd accessory proteins.”
Even the pioneering work of Brown and Goldstein on the LDLR, the best-characterized endocytosing receptor at the time, was still at a nascent stage [7]. Progress in the investigation of endocytosis over the past 25 years has resulted in the characterization of a number of distinct endocytotic pathways, some of which are illustrated in Figure 1. However, the commonalities observed among the different types of endocytotic pathways have established the following paradigm for endocytosis [8–10]. The formation of an endocytotic membrane vesicle or tubule is mediated by cytosolic coat proteins, which are nucleated and polymerized at specific sites on the plasma membrane to drive subsequently the deformation of the plasma membrane, ultimately to form an endocytotic vesicle. Simultaneously, coat proteins serve as “selection devices” that determine what cargoes are to incorporate into a nascent endocytotic vesicle, such as a receptor bound to its ligand. The culmination of these processes results in the cargo being sequestered within a coated membrane vesicle or tubule.
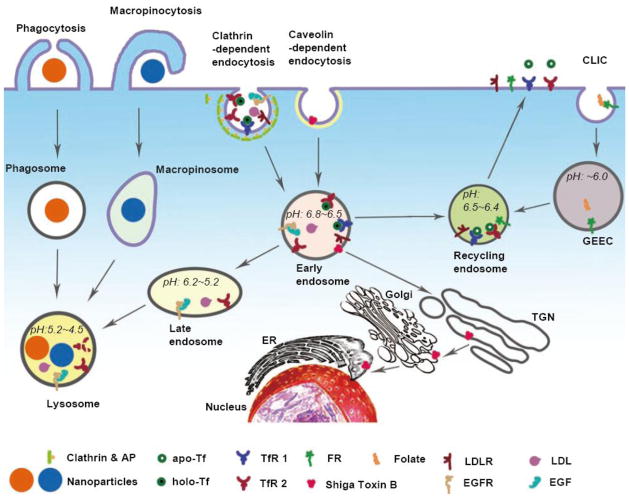
Figure 1. Distinct pathways for cargo internalization by cells.
The internalization pathways discussed in this article are illustrated in this figure: Phagocytosis involves surface receptors and engulfs large particles through envelopment by the plasma membrane. Macropinocytosis generates large macropinosomes containing extracellular fluid and soluble protein. Clathrin-dependent endocytosis involves the assembly of clathrin and adaptor proteins on a region of the plasma membrane in which particular receptors are clustered to form a nascent vesicle destined for internalization. Caveolin-dependent endocytosis involves the assembly of caveolin coats on regions of the plasma membrane rich in particular lipid rafts to form a nascent vesicle destined for internalization. CLIC is involved in the endocytosis of GPI-anchored proteins, and it is also a major contributor to fluid and bulk membrane uptake by cells. However, not all CLIC cargo have formally been shown to traffic to GEECs.
On the other hand, despite the paradigmatic commonalities, multiple distinct endocytotic pathways have been characterized over the past 25 years on the basis of a combination of the following criteria:
Distinct endocytotic pathways are ultrastructurally distinct; that is, different endocytotic vesicles “look” distinct when visualized by electron microscopy.
Distinct endocytotic pathways are pharmacologically distinct; they are inhibited by different classes of drugs.
Biochemical studies show that coat protein composition is distinct for the different endocytotic pathways, and, as a corollary, gene mining has revealed novel genes encoding distinct coat proteins or genes encoding coat proteins that are related to those that are already known, thus representing potential subsets of previously characterized endocytotic pathways.
The cargo (membrane protein, or receptor plus ligand) incorporated into nascent endocytotic vesicles of distinct endocytotic pathways is distinct, and the post-endocytotic trafficking of the endocytosed cargo may differ, depending upon the combination of cargo and coat proteins involved in the recognition of specific cargoes. These cargoes can then be used as markers for their respective endocytotic and post-endocytotic pathways. However, it is not unusual for a specific cargo to be found within multiple endocytotic pathways, as mentioned below. In addition, small ras-like GTPases, the families of ras-like proteins from brain (Rabs) and ADP-ribosylation factor (Arf) proteins are involved in the biogenesis of organelles and the regulation of post-endocytotic trafficking of receptors [11], and are also used as markers of specific post-endocytotic organelles, as mentioned below.
For a summary of all endocytotic pathways characterized to date, the reader is referred to the following excellent reviews [8–13]. This review will henceforth focus on those receptors, endocytotic pathways, and post-endocytotic pathways that have been most effectively harnessed for drug targeting and uptake. An overview of these pathways is shown in Figure 1.
2.1. Clathrin-mediated endocytosis and post-endocytotic trafficking
Clathrin-mediated endocytosis, historically referred to as “receptor-mediated endocytosis,” is the best characterized endocytotic pathway, and is the pathway upon which the paradigm for the mechanistic basis of all endocytotic pathways is based [9]. Fundamental components of the clathrin coat protein complex, known as adaptor proteins, are recruited to the cytosolic face of the plasma membrane where they bind to the cargo membrane protein to be endocytosed. The recruitment appears to be regulated by local changes in lipid components. The cargo to be endocytosed encompasses a large spectrum of plasma membrane proteins, such as nutrient receptors, membrane transporters, cell adhesion proteins, and signaling receptors. The clathrin-mediated pathway is also co-opted by bacteria and viruses to enter their target cells, suggesting that this pathway can internalize a wide variety of cargoes and can also accommodate significant variety in the sizes and shapes of endocytosed cargo [9, 14]. The adaptors also engage clathrin, the major coat protein that ultrastructurally defines this endocytotic pathway. Both clathrin and adaptors recruit accessory proteins to the site. This process is iterative, resulting in the localized polymerization of a coat complex that will drive the deformation of the membrane into an endocytotic vesicle and will define the shape of the nascent vesicle or tubule that encompasses and concentrates the cargo. These protein-protein interactions may also catalyze the interaction of the nascent endocytotic vesicle with the actin cytoskeleton that helps result in its fission from the plasma membrane.
Upon formation of a coated vesicle, the lumen begins to acidify, due to the primary active transport of H+ by the vacuolar H+-ATPase incorporated into the coated vesicle. The coated vesicle is then uncoated by members of the heat shock protein family, thereby allowing the uncoated vesicle to fuse with the early endosome, the first major organelle along the post-endocytotic pathway. Intra-organellar acidification continues within the early endosome, dropping the luminal pH to 6.8-6.5. From the early endosome, membrane cargoes are sorted to one of a number of major post-endocytotic pathways: the recycling pathway, the degradative pathway, the retrograde pathway, or the transcytotic pathway. The early endosome is also the site at which the lowered pH causes the dissociation of some receptors from their ligands and begins the activation of hydrolytic enzymes. The small GTPases Rab5 and Rab4 regulate trafficking through the early endosome.
Cargoes sent to the degradative pathway, such as the epidermal growth factor receptor (EGFR) bound to its ligand, EGF, will be trafficked through a series of increasingly acidic organelles, the late endosome/multivesicular body (pH 6.2-5.2) and the lysosome (pH 5.2-4.5). The ever-decreasing luminal pH of these organelles will provide the environment to effect the continued dissociation of ligands from their receptors and the continued activation of luminal acid-dependent hydrolases. The major classes of lysosomal acidic hydrolases are proteases, lipases, phosphatases, sulfatases, and glycosidases. These hydrolases are involved in the degradation of endocytosed cargo, such as receptor ligands and possibly the receptors themselves. This pathway effectively represents a terminal pathway for endocytosed cargo.
Many drug delivery strategies have employed the pH change within endosomes and post-endosomal compartments like late endosomes and lysosomes as a trigger to activate conformational changes encompassed in delivery vehicles that trigger the leakage of endocytosed cargo to cytosol from these acidic compartments, by harnessing “endosomolysis”. Examples of agents sensitive to such pH changes include polymers [15–17], pH sensitive nanogels [18, 19], as well as proteins critical to pathogen infection such as the hemagglutinin glycoprotein from influenza [20], listeriolysin [21], melittin [22], and many other viral proteins. These and many others have been creatively employed in a host of drug delivery strategies over the past several decades, with varying success. Likewise, the hydrolytic constituents of the lysosomes in particular have been exploited as triggers for release of active drugs from prodrugs.
Cargoes sent to the recycling pathway may transiently reside in a distinct endosomal compartment, the recycling endosome, whose luminal pH is approximately 6.5-6.4, prior to their reappearance at the plasma membrane. Depending upon the receptor, the rate at which recycling occurs may be very rapid, such as with the transferrin receptor (TfR), or more slowly, as with certain G-protein coupled receptors (GPCR) [23]. Rab11 and arf6 are major regulators of sorting at the recycling endosome. Along the way, with the TfR as a prototypical example, the receptor’s ligand will have dissociated from the receptor, typically in the early endosome, and the receptors are recycled to the plasma membrane as empty receptors.
Certain membrane proteins are trafficked along the retrograde pathway to the trans-Golgi network (TGN), ultimately to be recycled to the secretory pathway, where they are involved in secretory vesicle formation or in the biogenesis of lysosomes. Proteins following such a pathway would include proteins that contribute to the contents of secretory vesicles or lysosomes, such as the mannose-6 phosphate receptor, or proteins involved in vesicle fusion with the plasma membrane, the receptors for soluble N-ethylmaleimide sensitive factor attachment proteins (SNARE). In addition, some bacterial toxins are retrogradely sorted to the TGN, such as Shiga toxin B (StxB) and cholera toxin B (CtxB), and require further retrograde transport to the endoplasmic reticulum (ER), where they are then exported into the cytosol to exert their toxicity on cytosolic targets [24, 25]. For some endocytosed cargo, the final destination along the retrograde pathway will even be the nucleus, although it is not clear that this final destination occurs under normal conditions. In cancer cells, the EGFR together with the transmembrane mucin, MUC1, have been shown to be retrogradely trafficked to the ER, exported to the cytosol, and imported into the nucleus for a role in transactivation [26].
Polarized cells, in particular epithelial and endothelial cells, possess another post-endocytotic trafficking pathway, the transcytotic pathway. Macromolecular ligands or cell surface proteins are ferried intact across the cell, in an apical-to-basolateral or basolateral-to-apical (for endothelial cells, luminal-to-abluminal) direction, via multiple vesicular intermediates that traverse multiple endosomal compartments. Select transmembrane receptors internalized via clathrin-dependent endocytosis and lipid rafts have been identified as cargo for the transcytotic pathway [27–30]. There have been many excellent reviews on the cell biology of transcytosis, so it will not be covered in significant detail here [2, 28, 31–38].
However, it is instructive to describe briefly the best-characterized basolateral-to-apical transcytosing receptor, the polymeric immunoglobulin receptor (pIgR) and the best-characterized apical-to-basolateral transcytosing receptor, the neonatal Fc receptor (FcRn), both of which initiate their transcytotic journeys through clathrin-dependent endocytosis. Studies centered around these receptors as markers of transcytotic pathways have resulted in the mechanistic characterization of the regulation of transcytosis across epithelia and endothelia [28, 32]. The pIgR mediates the basolateral-to-apical transcytosis of polymeric immunoglobulins, such as dimeric IgA or pentameric IgM, in the defense of mucosal surfaces.
The pIgR is delivered to the basolateral domain from the TGN. At the basolateral membrane, it may bind to its ligand, and irrespective of ligand occupancy, it is endocytosed in a clathrin-dependent fashion. The receptor is then sorted through the basolateral early endosome, the common recycling endosome, to the apical recycling endosome, and finally trafficked to the apical membrane. Once at the apical membrane, the extracellular ligand-binding domain of the pIgR is cleaved, thereby releasing the polymeric immunoglobulin, complexed with the cleaved receptor fragment, into the external secretions. If the pIgR did not bind to ligand at the basolateral membrane, the empty receptor is still cleaved, and the receptor fragment is released into the external secretions. The pIgR can also recycle at the basolateral and apical membranes [28].
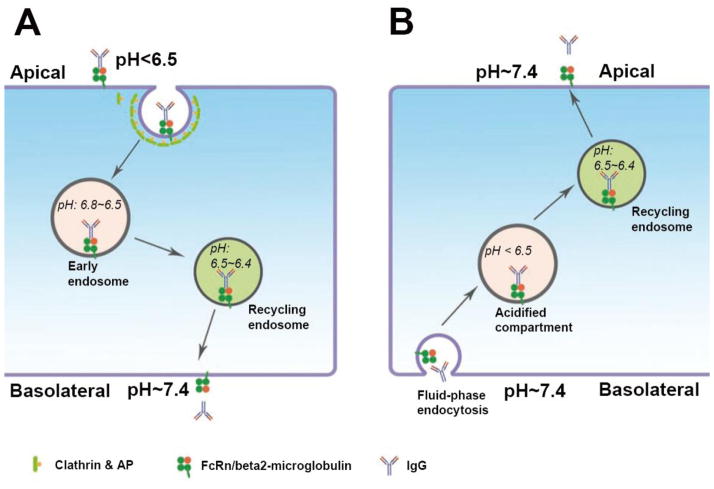
In contrast, the FcRn can mediate bidirectional transcytosis of its ligand, immunoglobulin G (IgG) [32] (Figure 2). The functional receptor is a heterodimer comprised of FcRn and beta2-microglobulin. The basis for transcytotic transport is the pH-sensitive binding of FcRn to IgG, with a high affinity interaction occurring at pH < 6.5, but not at the physiological pH of 7.4. Thus, FcRn at the apical membrane of an epithelial cell can bind to IgG present in acidic luminal fluids, be transcytosed to the blood side, and release its cargo. This process occurs in enterocytes in the neonatal absorption of IgG from mother’s milk in rodents [39, 40]. The alternate route is for the IgG to be endocytosed by fluid-phase uptake, then transported to acidic endosomes, in which the FcRn resides, and to bind to FcRn. The FcRn-IgG complex can then be transcytosed to the side contralateral to that which endocytosed the IgG. This process occurs in humans, in the fetal absorption of maternal serum IgG by transcytosis across the syncytiotrophoblast cells of the placenta [41, 42]. Some of the organelles through which the FcRn is sorted in its apical-to-basolateral transcytosis appear to be the same as those traversed by the pIgR in its basolateral-to-apical transcytosis [43], while there also appears to be differential regulation of each receptor through their respective pathways, and even differential regulation of the trafficking of the FcRn through either of its polarized transcytotic pathways [44].
Figure 2. Bi-directional transcytosis of FcRn receptor in polarized epithelial cells.
A) The pH-sensitive binding of FcRn to IgG occurs at pH < 6.5, but not at the physiological pH of 7.4. FcRn in the apical membrane of an epithelial cell can bind to IgG present in acidic luminal fluids for subsequent internalization and release of IgG at the basolateral membrane. B) Without a pH gradient across the polarized cells, e.g. alveolar epithelial cells or placental syncytiotrophoblast cells, FcRn is capable of transporting IgG bidirectionally. IgG can be endocytosed by fluid-phase uptake. Endocytosed IgG is then transported to an acidified endosomal compartment, in which the FcRn resides, and then binds to FcRn. The FcRn-IgG complex can then be transcytosed to the side contralateral to that which endocytosed the IgG.
However, the designation of FcRn as a “neonatal” receptor is a misnomer, as there is significant expression of the FcRn in adult tissues with demonstrable physiological significance in the adult animal. The FcRn is expressed in the adult in vascular endothelium, and is highly expressed in endothelial cells of the central nervous system and the choroid plexus. It is also expressed in the adult gut, kidneys, airways, and professional antigen-presenting cells, such as monocytes, macrophages, and dendritic cells. The proposed functions of FcRn at these sites are intriguing. For example, in the gut and in human intestinal epithelial cell lines, it has been shown that enterocytes can transcytose IgG in the blood-to-lumen direction, thus providing a pathway for secretion of IgG into mucosal fluids [45, 46]. FcRn-dependent immune protection against mucosal infections by IgG transcytosed into uterine/vaginal fluids [47] and gastric juice [48] has also been demonstrated. Moreover, in the gut, the apical-to-basolateral transcytosis of IgG-antigen complexes could then deliver these complexes to mucosal dendritic cells [46]. A vigorous FcRn-dependent, lumen-to-blood, transcytotic pathway exists in airway epithelium [49, 50], as well as in the brain-to-blood direction [51, 52].
Surprisingly, in addition to the IgG transport function of FcRn, studies from FcRn-deficient mice have shown that FcRn appears to prolong the half-life of IgG in the circulation and extends the half-life of serum albumin by currently uncharacterized mechanisms [53, 54].
Thus, the role of FcRn in the transport of IgG and its role in the metabolism of IgG and albumin have significant pharmaceutical relevance and impact with respect to the delivery and pharmacokinetics of Fc-fusion products and therapeutic monoclonal antibodies. Overall, the characterization of transcytotic receptors such as the pIgR and FcRn also provides for the potential development of therapies utilizing these pathways for macromolecular transport across cellular barriers.
A recent development in clathrin-dependent endocytosis has been the discovery and characterization of so-called “alternate” clathrin adaptors that work independently of, or in conjunction with, the canonical AP-2 clathrin adaptor [10]. Some of these adaptors are highlighted in the sections below on specific cell surface receptors. These alternate adaptors contribute to a higher degree of selectivity in the regulation of the endocytosis of these receptors and highlight the potential for selective pharmacological manipulation of specific, clathrin-dependent endocytotic pathways. Indeed, a recent chemical biology approach for clathrin-dependent endocytosis has identified and enabled characterization of a drug “selective” for the inhibition of the internalization of Tf, EGF, and human immunodeficiency virus, but not of StxB [55].
To complicate matters further, cell surface receptors can be routed to different internalization pathways depending upon prevailing cellular conditions. For example, the EGFR will be internalized by clathrin-dependent endocytosis, caveolae-dependent endocytosis, or macropinocytosis, depending upon the concentration of EGF [8, 56]. A similar situation occurs with the transforming growth factor (TGF) receptor. If the TGF-receptor is internalized through clathrin-dependent endocytosis, it is recycled to the plasma membrane. However, if it is internalized through caveolae-dependent endocytosis, it is sorted into the degradative pathway [56].
It is clear that even early endosomes fed from clathrin-dependent endocytosis are distinct, relative to the eventual fates of the receptor-cargo complex. There is a dynamic, highly mobile population that matures rapidly toward late endosomes; these endosomes contain cargo such as LDL and EGF. There is also a more slowly maturing population occupied by recycling cargo, such as Tf. The fates of the receptor-ligand complexes may depend upon the adaptors that are initially recruited for the endocytosis of the specific cell surface receptor [57].
In summary, the endocytotic and post-endocytotic pathways are highly dynamic platforms to regulate the internalization and processing of internalized cargo. In addition, the pathways of internalization and processing of cargo can differ by cell types, even within the same general pathway, that will be dependent upon the differential expression of endocytotic protein complexes in each cell type. In addition, these pathways can differ according to the physiological or pathological status of the target cell, which, as illustrated below, may be used to develop targeted drug delivery strategies. In the next several sections, we illustrate the trafficking dynamics of three different receptor pathways, each of which harnesses the clathrin-mediated endocytotic pathways in a different manner.
2.1.1. Transferrin receptor (TfR) trafficking
There are two isoforms of the TfR: TfR1 and TfR2 [58], which have different trafficking mechanisms (Figure 1). TfR1 is the canonical recycling receptor that is typically described. At the plasma membrane, TfR1 binds to its ligand, Fe3+-loaded transferrin (holo-Tf). The receptor-ligand complex undergoes clathrin-mediated endocytosis, which is highly dependent upon its interaction with the AP-2 clathrin adaptor, and is delivered to early endosomes. In the lowered pH environment, the Fe3+ dissociates from holo-Tf, forming apo-Tf, and the Fe3+ is exported from the endosome to the cytosol by membrane transporters. However, at the pH of the early endosome, the apo-Tf remains bound to TfR1. The TfR1-apo-Tf complex is trafficked to recycling endosomes, and ultimately back to the plasma membrane. At the pH of the extracellular fluid, the apo-Tf has a low affinity for TfR1 and dissociates. The apo-Tf is available to bind to Fe3+ again, and the TfR is available to bind to another holo-Tf. However, ligand binding is not obligatory for internalization of TfR1.
The endocytosis of TfR1 may be modulated by accessory interacting proteins. The familial hemochromatosis protein (HFE) is a transmembrane protein and negative regulator of TfR internalization [59, 60]. HFE is highly expressed in liver and intestine. Particular mutations in HFE will result in the loss of inhibition of TfR endocytosis, resulting in cellular iron overload. Another TfR1-interacting protein, TfR trafficking protein (TTP), is a cytosolic protein that regulates TfR1 endocytosis [61]. Its depletion inhibits TfR1 endocytosis, and, interestingly, its overexpression also inhibits TfR1 endocytosis, suggesting that the stoichiometry of TfR1 and TTP must be balanced for the optimal endocytosis of TfR1.
In the presence of holo-Tf, the endocytosis of TfR2 appears to be identical to that of TfR1. However, in the absence of holo-Tf, TfR2 is internalized and rapidly degraded via the lysosomal pathway [62]. The switch from a recycling pathway to a degradative one involves an interaction of TfR2 with a cytosolic accessory protein, phosphofurin acidic cluster sorting protein (PACS1), which does not interact with TfR1. Thus, the post-endocytotic processing of any cargo internalized by TfR may differ, depending upon the TfR isoform to which it is bound, which is in turn related to tissue expression of the different TfR subtypes and whether they are influenced by disease.
Tf (and likely the TfR itself), has been demonstrated to undergo transcytosis in gastrointestinal epithelium [63] as well as across the blood-brain barrier [64], and this transcytotic pathway has been exploited for the trans-epithelial and trans-endothelial delivery of therapeutic agents across these barriers.
2.1.2. Low density lipoprotein receptor (LDLR) trafficking
LDL receptor is a single-chain transmembrane glycoprotein responsible for binding and endocytosis of LDL, and it is the founding member of the LDLR superfamily. This receptor family includes megalin [65, 66], also known as LDLR-related protein 2 (LRP2), a multipurpose endocytotic receptor, and the vitellogenin receptor [67], involved in the endocytosis of yolk proteins. At the plasma membrane, LDLR binds to LDL, and the complex is internalized through the clathrin-dependent pathway. However, it differs from the internalization of the TfR; in addition to the AP-2 clathrin adaptor, LDLR endocytosis is dependent upon adaptor proteins from the Dab2/Numb/ARH family of proteins, which bind to the cytoplasmic domain of the LDLR (and related receptors), as well as to AP-2 clathrin adaptors and clathrin [68–70]. Once endocytosed, the LDLR is trafficked into early endosomes, where lower pH decreases the affinity of LDLR for LDL. LDL dissociates and is trafficked to late endosomes and finally to lysosomes, where the LDL is hydrolyzed into peptides, amino acids, cholesterol, and other lipid products. These are all then exported from the lysosome for utilization by the cell. The empty LDLR is recycled to the plasma membrane to mediate another round of LDL binding and internalization. The multiple ligands that bind to the LDL-related receptor, megalin, also dissociate in the lowered pH environment of endosomes, and the receptor is recycled [65].
2.1.3. Epidermal growth factor receptor (EGFR) trafficking
The EGFR is a single-chain transmembrane receptor responsible for binding EGF and activating multiple intracellular pathways, such as the well-characterized mitogenic pathway. While the endocytosis of the TfR and the LDLR is constitutive, the endocytosis of the EGFR is dependent upon ligand binding, thus linking the down-regulation of the signaling complex to ligand binding. Upon ligand binding, the activation steps of the EGFR include dimerization and autophosphorylation of tyrosine residues. In addition, the EGFR is a receptor that may be sorted through multiple endocytotic pathways, possibly depending upon the ligand concentration in the extracellular milieu. Evidence suggests that at low concentrations of ligand, the EGFR is internalized through clathrin-dependent endocytosis, and at high ligand concentrations, a clathrin-independent, raft/caveolar-dependent endocytotic pathway is also used [71]. In these cells, sorting through the clathrin-dependent pathway is primarily a recycling pathway, while sorting through the raft/caveolar pathway leads to degradation of EGFR. However, there are other observations suggesting to the contrary: the EGFR may enter both pathways irrespective of ligand concentration, and even some data that suggest that the internalization of EGFR is entirely clathrin-dependent [72]. These differences may result from use of different cell types for analysis as well as differences in experimental approaches.
A combination of signaling and endocytotic adaptors associates at early steps with the activated EGFR. One of the proteins recruited to this early complex is c-cbl, an ubiquitin ligase that catalyzes the ubiquitination of the cytoplasmic domain of the EGFR. There is general agreement that EGFR ubiquitination regulates the endocytosis and postendocytotic trafficking of the EGFR. Ubiquitination of the EGFR at the plasma membrane allows for its interaction with and internalization by clathrin adaptors that possess ubiquitin-interacting motifs. Once at the early endosome, if EGF dissociates from the EGFR, the EGFR is de-ubiquitinated and rapidly recycled. There is evidence that ligand-bound EGFR may also rapidly recycle [73]. If the EGFR remains ubiquitinated or is re-ubiquitinated in early or late endosomes, then a distinct coat protein complex, the endosomal sorting complex required for transport (ESCRT) complex, interacts with the EGF-EGFR complex, to generate multivesicular bodies containing the EGF-EGFR complex. The multivesicular bodies ultimately fuse with lysosomes to effect the degradation of EGF and the EGFR, although the EGFR can also slowly recycle from the late endosome as well [73, 74].
Stimulation of the EGFR by high EGF concentrations also mobilizes other newly characterized endocytotic pathways, macropinocytosis or circular dorsal ruffles (described below), through which the EGFR may be internalized.
2.2. Macropinocytosis and circular dorsal ruffles
Stimulation of cells by growth factors such as EGF or platelet-derived growth factor also induces macropinocytosis and circular dorsal ruffles, two other modes of clathrin- and caveolin-independent endocytosis [75–78]. The fundamental features and effectors of macropinocytosis are being actively characterized. Macropinocytosis occurs at the periphery of adherent cells, at areas active in the formation of membrane ruffles involving dynamic actin cytoskeletal rearrangements. Occasionally these ruffles are then thought to fold back upon themselves to envelop fluid and plasma membrane domains to form endocytotic structures known as macropinosomes, with sizes ranging from 0.2 to 10 μm in diameter. Thus macropinocytosis represents a high volume endocytotic pathway for membrane and fluid internalization.
Macropinocytosis was once thought to be a somewhat artifactual phenomenon observed only in cultured cells, but growing evidence suggests that these large volume uptake mechanisms may exist in cells in vivo [75, 77, 78]. In addition, multiple pathogens, from protozoa to viruses, have evolved clever mechanisms to enter cells via macropinocytosis [77, 78]. Moreover, macropinocytosis has generated interest relative to drug delivery applications [79], with reports of macropinocytosis being the preferential pathway for the internalization of arginine-rich cell-penetrating peptides [80, 81], although this activity may be cell-specific [82]. Nonetheless, there is a very intriguing report on a particular arginine-rich peptide conjugated to a pro-apoptotic peptide that binds to leukemia- and lymphoma-derived cell lines and is internalized through macropinocytosis, resulting in the selective killing of these cells [83]. Given the apparent diversity of cargoes, from fluid to particles, internalized by macropinocytosis, and its capacity for high-volume uptake, this pathway will likely continue to attract attention in the drug delivery field.
Circular dorsal ruffles form on the free dorsal surface of cells, in an apparent wave-like manner [75, 84, 85]. High concentrations of EGF activate circular dorsal ruffles, with EGFR becoming concentrated in these ruffles, and EGFR then being internalized by tubular endocytotic membranes. Despite their similarities, macropinocytosis and circular dorsal ruffles appear to be distinct, as the proteins that regulate each of these pathways are different [75]. Currently, the physiological and potential pharmaceutical relevance of circular dorsal ruffles is unknown.
2.3. Clathrin-independent endocytosis—caveolar endocytosis
Caveolae are flask-shaped, 60–80 nm-diameter invaginations of the plasma membrane and are clearly structurally and functionally distinct from clathrin-coated endocytotic structures. A major structural protein of caveolae is the integral membrane protein caveolin, of which there are 3 isoforms, caveolin-1, -2, and -3. Caveolin-1 and -2 are widely expressed, but caveolin-3 is expressed exclusively in muscle.
Caveolin adopts a hairpin structure within cellular membranes, in which its amino- and carboxy-termini are cytoplasmic, and the hairpin turn is possibly exposed extracellularly. Biochemically, caveolins oligomerize, are associated with lipid rafts, and become incorporated into so-called detergent resistant membranes, although there are also non-caveolin detergent resistant membranes. Interestingly, neurons and leukocytes are cells that do not express caveolin nor do they form caveolae. Conversely, caveolin appears to be necessary and perhaps sufficient to form caveolae. In cells that normally do not express caveolin and do not form caveolae, if caveolin-1 is ectopically expressed in these cells, caveolae will then form [86]. However, recent work suggests that another family of proteins (cavins) that associate with the cytoplasmic side of caveolae, are important in the formation of caveolae [87]. In addition, knockout organisms for cavin phenocopy knockouts for caveolin [88, 89].
While a number of signaling protein complexes have been found to associate with and are regulated by caveolae [90], there are only a few cargoes that have been demonstrated to be internalized via caveolae. Most are exogenous pathogens or fragments of pathogens, such as simian virus 40 (SV40), StxB and CtxB. These exogenous cargoes may be internalized via caveolae by virtue of their binding to specific glycosphingolipids that interact with caveolin1 and are therefore concentrated in caveolae. Endogenous cargo internalized by caveolae include sphingolipids and glycosylphosphatidylinositol (GPI)-anchored membrane proteins (GPI-AP), such as CD59, a mediator of the complement reaction [91–93], although these cargoes are not necessarily restricted to internalization by caveolae (see Section 2.4). In addition, unlike the case with clathrin-coated membranes, in cells in which caveolae have been observed in real time, only a small percentage of caveolae are dynamic and appear to be involved in endocytosis, which is also dependent upon the presence of membrane cholesterol.
The cargoes endocytosed by caveolae are delivered to either early endosomes or specific caveolin-1-containing endosomes, known as caveosomes [94]. Caveosomes are distinct from early and recycling endosomes and do not appear to contain material endocytosed by clathrin-dependent endocytosis, such as Tf. In addition, the intra-organellar pH of caveosomes is neutral, in contrast to the acidic pHs of early and late endosomes. However, recent data suggest that caveosomes are organelles that are artifactually generated by the overexpression of caveolin-1 in cells and are actually modified late endosomes [95]. Thus, caveolar cargo, such as SV40, is trafficked through typical early and late endosomes prior to being retrogradely sorted to the endoplasmic reticulum.
Endothelial cells, in particular, those lining the blood-brain barrier or lung alveoli, appear to possess numerous caveolae, which are thought to mediate transcytosis from the blood side to the abluminal side. Nitrated albumin [96] and albumin complexed to myeloperoxidase [97], and native albumin [98] have been observed to transcytose across endothelia via caveolae, likely in a receptor-mediated fashion. Perhaps one of the most convincing demonstrations of caveolae-mediated transcytosis is observed in an intravital microscopic study of the transcytosis of antibodies against aminopeptidase P, a protein enriched in caveolae, across lung endothelial cells [99]. These results suggest that the rate of caveolae-mediated trafficking may depend upon cell type.
2.4. Clathrin independent carriers (CLIC) and GPI-anchored protein-enriched early endosomal compartment (GEEC) pathway
As its name implies, this pathway was uncovered after clathrin- and caveolin-dependent pathways were inhibited [100]. Endocytotic CLIC membranes are fairly prevalent, with a tubular or ring-like in morphology, but no ultrastructurally apparent “coat” [8]. Recent work has shown that CLICs are a high-volume internalization pathway in fibroblasts, internalizing three times the volume internalized by the clathrin-dependent pathway [101, 102]. Thus, CLICs are a major contributor to fluid and bulk membrane uptake by cells, although it is not clear that all cargoes endocytosed by CLICs traffic to GEECs. And, as is the case with caveolar – dependent endocytosis, only a few cargoes have been identified for CLIC/GEECs. Notable among these cargoes are fluid-phase markers and GPI-APs, such as the folate receptor (FR)-α, and CtxB [101, 102], the latter of which, as mentioned above, is also a cargo for caveolae. The FR was originally thought to be a cargo for caveolae, but later was shown to enter caveolae only after cross-linking [103]. Further evidence that the CLIC/GEEC pathway is distinct from clathrin-dependent endocytosis is that the FR sorted into GEEC does not colocalize with TfR, which is a marker of early endosomes fed from the clathrin-dependent pathway [104]. In addition, inhibition of the small GTPase Arf1 inhibits the uptake of the FR into GEEC, but does not affect the uptake of TfR. Moreover, the luminal pH of GEEC is close to 6, which is more acidic than typical early endosomes (~6.5) [105]. Finally, the delivery of the FR from GEEC into recycling endosomes is regulated by the small GTPase cdc42 [106]. Thus, traffic along the CLIC/GEEC pathway appears to be regulated by small GTPases distinct from the family of GTPases that play a predominant role in the regulation of post-endocytotic traffic in the clathrin-dependent pathway, the rab family of GTPases.
Recent additional cargoes characterized for CLIC are: major histocompatibility complex class I protein [107]; the cell adhesion molecules CD44 [102, 108] and ICAM1 [108]; the GPI-APs CD55 [108], CD59 [107], and Thy-1 (CD90) [101]; the heavy chain of amino acid transporters CD98 [101]; CD147 (emmprin) [108]; and, the glucose transporter Glut1. Thus, intriguingly, CLICs may regulate cell adhesion and cell migration [101]. However, it is not clear whether these cargoes are post-endocytotically sorted to GEECs. In addition, it is not clear whether this variety of cargoes reflects the diversity of subtypes of CLIC pathways and/or differences in post-endocytotic sorting in different cell types, particularly to GEECs. Cargo internalized via the CLIC pathway, if not sorted to the GEEC, may be alternatively sorted to recycling endosomes or lysosomes, a process that may also differ among different cell types [8].
GPI-APs are also transcytosed in a basolateral-to-apical direction in cultured MDCK cells [27] and in polarized hepatic cells [109]. The transcytotic pathway for GPI-APs appears to be distinct from cargo entering the basolateral-to-apical transcytotic pathway via clathrin-mediated endocytosis, such as for the pIgR. GPI-APs together with basolateral proteins are delivered to the basolateral membrane from the trans-Golgi network. The GPI-APs are rapidly endocytosed into caveolae-like structures, along with fluid phase markers, while basolateral proteins are not. GPI-APs are then transcytosed to the apical membrane via clathrin-free transport carriers [27].
2.5. Alterations in the internalization and trafficking of membrane receptors upon cross-linking
There are clear examples of alterations in the internalization and post-endocytotic trafficking of receptors upon cross-linking of those receptors by exogenous agents such as anti-receptor antibodies. The example of the FR was mentioned in Section 2.4., and an example of EGFR trafficking being altered by the therapeutic monoclonal antibody Herceptin® is described in Section 4.1. On the other hand, pertuzumab, a monoclonal antibody that inhibits the dimerization of the EGFR with other HER receptors, enhances the degradative down-regulation of the EGFR [110]. Similarly, antibodies against the nicotinic acetylcholine receptor, when added, will induce the internalization of the receptor [111]. Transcytosing receptors can also be influenced by ligand valency. For the FcRn, ligand valency affects the efficiency of its transcytosis and the fraction of occupied receptors that gets diverted to lysosomes for degradation [112]. Thus, multivalency, described in Section 5, will likewise potentially impact the post-endocytotic trafficking of the target proteins, which may then enhance or detract from the therapeutic objective of the product.
3. Cellular targeting strategies applied to nanoparticles
The concept of efficient and targeted delivery of drug carriers to or into the target tissue or organ has historically attracted attention, especially for potent drugs with toxicity concerns. From the earliest studies exploiting selective enrichment of TfR on proliferating tumor cells to target Tf-toxin conjugates, rapid progress in cell biology, genomics and proteomics has now expanded this approach for drug delivery in a variety of circumstances; for instance, target cells can be distinguished from non-target cells by screening for specific biomarkers on the plasma membrane. Delivery of drug carriers to target cells by recognition of surface biomarkers is a promising approach with clear advantages. First, cellular specificity may reduce toxicity and keep adverse reactions to the minimum. Second, introduction of the cellular specificity may overcome the limitations of some previous therapies. For instance, implant of radioactive isotopes or passive drug delivery strategies based on the EPR effect are only applicable to solid tumors above a certain size [113]; whereas, the cellular level specificity provides the possibility of treating diseases in a variety of tumor and non-tumor tissue that are distinguished by their unique cellular properties as opposed to the tissue’s physical properties.
In this section, we discuss new advances in drug delivery strategies focused on targeting nanoparticles to examples of biomarkers on the cell surface which enable their internalization, with emphasis on their in-vivo applications. These approaches are categorized by the examples of the diverse biological targets used for tissue internalization, and illustrative examples for each category are summarized in Figure 3. These are by no means comprehensive, as other excellent work has focused on use of additional receptor targets; however these targets largely have more information available on their receptor-mediated endocytotic uptake pathways as described in Section 2. It should likewise be mentioned that the literature has shown that the addition of targeting moieties may not influence the general biodistribution of nanoparticles in terms of their tissue deposition, but may have its greatest potential in enhancing internalization to appropriate target cells within tissues [114–116]..
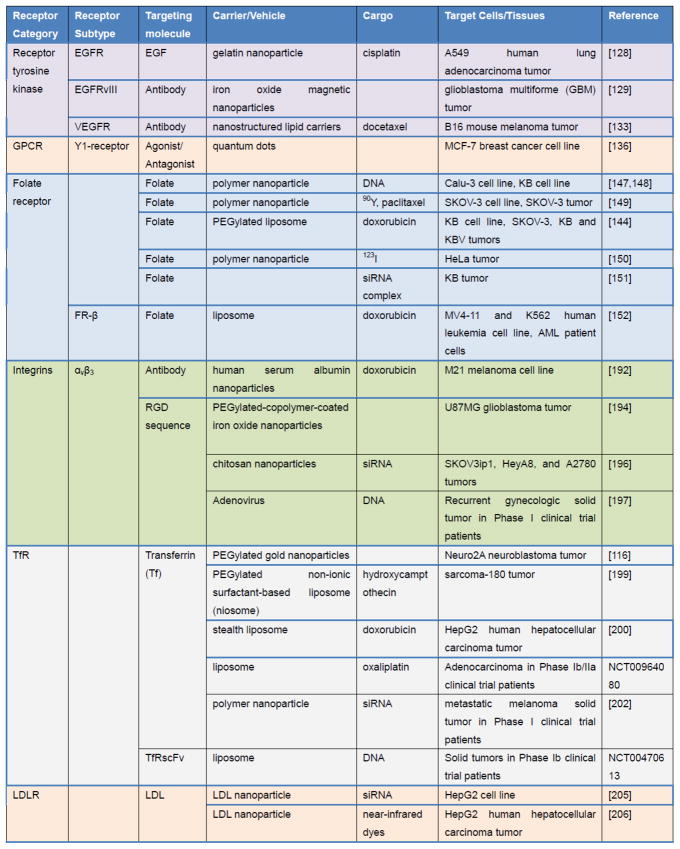
Figure 3. Examples of cellular targeting strategies.
In this table, the term “tumor” indicates tumors induced by injected cells in nude mice.
3.1. Nanotechnology
Nanotechnology has the potential to add remarkable value to therapeutic agents by providing the means by which drugs may be encapsulated to achieve a number of endpoints, including but not limited to controlled release, tissue targeting, penetration of barrier tissues and protection of the encapsulated drug. Additional opportunities lie in the ability of nanotechnology to combine therapeutics spatially for co-delivery, as well as to combine therapeutic and imaging agents for “theranostic” imaging and treatment. Moreover, nanoparticle formulations offer the potential to protect encapsulated or conjugated macromolecular drugs such as siRNA or proteins from environmental degradation. A variety of nanoparticles, ranging between 1–100 nm, are commonly exploited for drug delivery. These structures include liposomes, micelles formed from polymers, protein-based nanoparticles and hybrid structures with mixed properties.
To date, the most successful clinical applications of nanoparticles for drug delivery include liposomal formulations such as AmBisome, PEG/liposome encapsulated doxorubicin such as Doxil® and other liposomal and PEGylated proteins, antibodies or small molecule drugs [117]. Use of nanoparticles largely allows these agents to achieve sustained plasma levels while encapsulated, with the liposomal size preventing the rapid clearance of drug by the kidneys that occurs with the free agent [118]. Additional benefits of the nanoparticle carrier are relevant to the apparent trapping of carriers containing drug in the interstitium of solid tumors, due to the so-called enhanced permeability and retention (EPR) effect.
The approved limited use of nanoparticles in the clinic thus far does not match the potential utility of these diverse agents, since their size is ideally suited to intracellular uptake through the endocytotic mechanisms described above. However, nanoparticle interaction with cells and the cellular internalization machinery is quite complex and it has been challenging to identify unifying principles for particle internalization via specific pathways across cell types. Nanoparticle shape, surface charge and other physical properties affect their endocytosis in ways that are not yet completely understood. This area has recently been comprehensively reviewed [5]. It has also been documented that particle charge and size significantly impact the ability of nanoparticles to traverse physiological tissue barriers such as the mucus layer [119] as well as their in vivo distribution [120, 121]. Encapsulation of therapeutic agents into nanoparticles with a particular affinity for an internalization pathway is potentially highly efficient mode of membrane targeting. However, most cells are not highly specialized to recognize particles alone as targets for selective uptake, with the exception of professional scavenging cells such as reticuloendothelial cells and macrophages [122, 123].
Addition of targeting ligands with specificity for receptors, either protein, carbohydrate or lipid based, to nanoparticles is an ideal strategy for targeting of nanoparticles to particular cells and/or for reaching specific membrane encapsulated compartments in cells. Membrane compartments are a desirable target for several reasons—a number of therapeutic targets for some of society’s most challenging diseases (e.g., Alzheimer’s disease) are located to such compartments. Second, the gradual acidification of compartments ranging from pH 6.5 in peripheral endosomes to pH 4.5 in lysosomes offers an opportunity to activate a variety of pH sensitive triggers as discussed above; these include induction of conformational changes in pH sensitive peptides [124, 125] sufficient for membrane disruption and pH-triggered release of drugs from prodrug formulations. Finally, use of constitutive recycling mechanisms can sustain plasma levels of peptide drugs such as insulin by transiently storing and re-releasing drug, exemplified by previous work on transferrin [126]. Targeting of nanoparticles is also a strategy for accessing and reaching peripheral non-tumor tissue that may have more limited blood flow and may not be as amenable to targeting by physical methods.
3.2. Epidermal growth factor receptor (EGFR) nanoparticle targeting
Receptor tyrosine protein kinases phosphorylate tyrosine amino acid residues, and trigger downstream signal transduction. This protein superfamily includes EGFR, vascular endothelial growth factor receptor (VEGFR), insulin receptor and others. These proteins internalize largely but not exclusively via clathrin mediated endocytotic pathway although complexities associated with concentration-dependent diversion to other pathways has been suggested as discussed in Section 2.1.3. The amplification, over-expression or mutation of members of the EGFR family have been demonstrated to play important roles in the pathogenesis and progression of certain aggressive types of cancer [127]. Therefore, the members of the EGFR family are ideal targets for delivering drugs to these cells. A straightforward approach for targeting EGFRs is to conjugate drug carriers with EGF. Using this approach, the use of biotinylated-EGF-modified gelatin nanoparticle carriers enhanced the aerosol delivery efficiency of cisplatin to plasma and lungs via inhalation in mice; it also significantly reduced the size of A549 cell-induced tumors [128]. However, EGF-EGFR targeting simultaneously introduces exogenous EGF with biological activity into the internal environment, and this constitutes a potential risk for clinical applications. Using antibodies to target EGFR can overcome this problem. Iron oxide magnetic nanoparticles conjugated with an antibody targeting EGFRvIII decreased the survival and EGFR phosphorylation levels in GBM cells in-vitro, but showed no toxicity in human astrocytes [129]. Their convection-enhanced delivery led to initial distribution of these nanoparticles within or adjacent to intracranial human xenograft tumors in nude mice, and a significant increase in animal survival [129].
Interestingly, the trafficking mechanism of antibodies bound to EGFR has been found to be different from that of EGF. Trastuzumab, also known as Herceptin®, is a monoclonal antibody that specifically binds to EGFR. In SKBr3 breast cancer cells, internalized Trastuzumab was highly colocalized with the recycling marker Tf, and was degraded quite slowly [130]; however, internalized EGF was sorted differently from Tf [131] and was more rapidly degraded [132].
VEGFR is overexpressed on the surface of a variety of tumor cells and on tumor neovasculature in situ, and is therefore considered a drug delivery target [133]. Docetaxel-loaded nanostructured lipid carriers conjugated with antibodies against VEGFR-2 exhibited superior cytotoxicity against cancer cell lines compared with non-targeted analogs. They showed better tolerance and antitumor efficacy in a mouse model bearing B16 melanoma compared with non-targeted analogs, which was attributed to the increased accumulation of drug in both tumor and tumor vasculature [133].
3.3. G-protein coupled receptor (GPCR) nanoparticle targeting
GPCRs represent the largest family of receptors, and serve as the targets of over 40% of the drugs approved by the FDA; however only a small number of GPCRs have been explored for targeting purposes [134, 135]. The ligands for GPCRs involve broad categories, including peptides, neurotransmitters, amino acids, hormones, lipids and chemokines [135]. GPCRs have two major classes of ligands: agonists that lead to receptor internalization upon binding and antagonists that do not [136]. Internalization is largely due to clathrin-mediated endocytotic pathways although there is considerable complexity and diversity to the pathways [137]. The polymorphisms and genetic mutants of GPCRs in humans may also increase the complexity of developing drug delivery strategies targeting GPCRs [138].
Theoretically, GPCRs could serve as gateways for nanoparticle binding and cellular uptake because of their high tissue specificity [136, 139]. Intriguing work targeting GPCRs was carried out on MCF-7 breast cancer cells expressing the human Y1-receptor, using quantum dots conjugated with agonist-type or antagonist-type peptide ligands [136]. Both agonist- and antagonist-modified quantum dots had excellent binding specificity, but exhibited a tremendous difference in cellular uptake: specifically, only agonist-modified quantum dots were internalized. Interestingly, these modified quantum dots bound to several receptor molecules simultaneously, and such multi-ligand binding characteristics can lead to significantly elevated affinities by 5 orders of magnitude compared with free ligand. These important discoveries may pave the way for in-vivo application of drug carriers targeting GPCRs in the near future.
3.4. Folate receptor (FR) nanoparticle targeting
Overexpression of FR occurs in many human malignancies, especially aggressively growing cancers, and is associated with poor disease prognosis [140–142]. The FR has two glycosyl phosphatidylinositol (GPI)-anchored isoforms: α and β. FR-α is frequently over-expressed in epithelial cancers, whereas FR-β expression is found in myeloid leukemia and activated macrophages associated with chronic inflammatory diseases [143]. Based on the natural high affinity of folate for the folate receptor, drug carriers conjugated with folate may also bind tightly to the folate receptor and trigger cellular uptake via endocytosis [144]. As a GPI-AP, folate receptor internalization is thought to use clathrin-independent CLIC-GEEC internalization pathways [104, 145, 146].
Compared with antibodies, small molecules like folate are much easier to be manufactured at an industrial scale, which is an apparent merit of folate targeting for clinical application. For instance, an amphiphilic peptide conjugated to folate can be mixed with polymers to form nanoparticles that are capable of encapsulating DNA for gene delivery [147, 148]. The uptake of folate-conjugated nanoparticles by cell lines overexpressing folate receptors can be inhibited by competition with free folate, indicating the specificity of folate-targeting [147, 148].
Folate targeting has been extensively studied for its application in in vivo delivery of drug carriers [142, 144, 149]. Folate-targeted nanoparticle delivery of paclitaxel and the radio-therapeutic yttrium-90 (90Y), showed significantly higher potency than the non-targeted analogs in in vivo efficacy studies using an ovarian peritoneal metastasis mouse model [149]. PEGylated liposomal doxorubicin infused with folate showed better activity relative to free doxorubicin in tumor models induced by human nasopharyngeal epidermoid carcinoma cell line, KB, and its vincristine-resistant derivative (KBV), both of which overexpress the folate receptor [144].
Lu et al. [150] used poly-lactic acid (PLA)-derived polymers to prepare folate-functionalized nanoparticles, which were labeled with 123I to investigate their in vivo distribution. In BALB/c nude mice injected with folate receptor-positive HeLa cells, intravenously injected nanoparticles generated strong signals in the lung, liver, kidney, and in the xenograft tumors. The time-dependent signal intensity in the tumors suggested specific folate-targeting to tumors by drug-loaded nanoparticles; whereas the nanoparticles without folate showed high nonspecific accumulation in the liver [150]. In contrast, nano-sized siRNA complexes bearing folate did not significantly accumulate in tumors formed from KB cells, but exhibited a moderate silencing effect in vivo on the mitotic kinesin Eg5 gene [151].
Interestingly, the application of folate targeting is not limited to solid tumors, but also leukemia cells[143]. FR-β is expressed in approximately 70% of the cases of acute myelogenous leukemia (AML) blast cells [152]. FR-β-targeted liposomal doxorubicin showed higher in-vitro toxicity than its non-targeted analog in MV4–11 (human acute myelocytic leukemia) and K562 (human erythromyeloblastoid leukemia) cell lines. It also showed stronger inhibition in a colony formation assay with MV4–11, K562 cells and AML patient cells than its non-targeted analog [152]. This suggests the potential clinical application of folate targeting of liposomal nanoparticles in treating AML.
3.5. Integrin nanoparticle targeting
Integrins are heterodimeric transmembrane receptors that mediate the interactions between endothelial cells and the extracellular matrix [153]. They are involved in a large number of fundamental intracellular processes, such as cell-cell and cell-matrix adhesion, differentiation, stress responses and apoptosis [154]. This diverse family of receptors consists of at least 18α and 8β subunits that can dimerize in more than 24 different combinations to yield surface receptors capable of recognizing one or more extracellular matrix (ECM) components [155]. The resulting crosstalk with growth factors and cytokines regulates intracellular signaling that is essential in controlling cell survival, proliferation, and migration, whereas interaction of integrins with ECM provides the traction necessary for cell motility and invasion. Their internalization during cell migration and focal adhesion disassembly is thought to largely proceed by clathrin-mediated endocytosis [156, 157] although additional evidence suggests that different integrin subtypes may use other endocytotic mechanisms such as caveolar endocytosis under some conditions [158].
In solid tumors, integrins serve as regulators of the migration and invasion of tumor cells [159]. Expression of the integrins αvβ3, αvβ5, α5β1, α6β4, α4β1 and αvβ6 is correlated with disease progression in various tumor types [159]. In addition, 9 out of 24 integrin heterodimers have been implicated in blood vessel formation: α1β1 [160–162], α2β1 [160–162], α4β1 [163], α5β1 [164–168], [169], [170–175], [170, 171, 173], αvβ1αvβ3αvβ5αvβ8 [171, 176–178] and α6β4 [179, 180]. Among these, αvβ3 receptors are overexpressed in endothelial cells undergoing angiogenesis, although they are not typically found on quiescent cells [181, 182]. This renders them attractive antitumor targets, since antagonists of this receptor that effectively compete with its natural ligand cause apoptosis in proliferating vessels [182].
The physical role of αvβ3 integrin is thought to involve matrix remodeling and degradation during endothelial cell migration. Matrix metalloproteinase-2 (MMP-2) is known to bind αvβ3 integrin in its active form, therefore allowing migrating endothelial cells to actively remodel the extracellular matrix [183]. Degradation of the native collagen by MMP-2 exposes otherwise unavailable αvβ3 integrin binding sites, facilitating endothelial cell invasion of the extracellular matrix. This model of endothelial cell migration is consistent with well-studied models of fibroblast migration wherein fibronectin receptors serve as “molecular feet” that bind their substrate at the leading edge of the cell and remain fixed as the cell moves forward.
A search for high affinity ligands for integrin receptors resulted in a number of peptidic [184, 185] or small molecule ligands [186]. Of particular interest to angiogenic therapies are the cyclic RGD peptides that have shown high affinity and specificity for αv integrins. These ligands have been shown to selectively target tumor vasculature when injected into tumor bearing mice [187]. The specificity exhibited by peptides containing an RGD motif or small molecules resulting from combinatorial screen is remarkable given the relatively small molecular interface they present for binding to integrin receptors. Given that integrins are typically concentrated on the apical surface of migrating cells, a targeting approach that utilizes integrin-binding ligands is plausible. From a design perspective, such ligands need to be covalently linked to nanoparticles that serve as drug delivery vehicles. In addition to acting as more efficient delivery agents, it is also possible that such multivalent nanocontainers would be capable of interfering with the endocytotic cycle of αvβ3 integrin or the angiogenic process itself.
A synergy of integrin-mediated signaling with growth factor response was suggested over a decade ago. Ruoslahti et al. has found that engagement of the αvβ3 integrin in cell–matrix interactions also coordinates a strong response to growth factors, underscoring the importance of this integrin for tissue regeneration, angiogenesis and tumor metastasis [188]. An involvement of αvβ3 integrins in insulin signaling was discovered when IRS-1, a cytoplasmic signal transduction mediator of the insulin and insulin-like growth factor 1 (IGF-1) receptors, was found to co-immunoprecipitate with αvβ3 integrin [188].
Later, a 100 kDa protein, identified as the insulin receptor (IR) beta chain by immunoprecipitation of complexes of αvβ3 integrin with anti-IR antibodies, followed by blotting with anti-phosphotyrosine (anti-pY) antibodies [189]. The integrin association typically requires growth factor stimulation of the receptors.
IGF-1 plays a key role in macrophage migration during the onset of tissue inflammation. IGF-1 induced recruitment of αvβ3 integrins, as well as translocation of the integrin adaptor protein phospho-paxillin to focal adhesion sites is also documented [190]. Experiments with pharmacological inhibitors of Akt, PKC and p38 MAP kinase revealed that IGF-1 facilitated migration requires IGF-1/IGF-1R-mediated PI3-kinase/PKC/p38-dependent integrin inside-out signaling, but does not require MMP activation. Similarly, αvβ3 integrin localization in the core of focal adhesions and involvement of αvβ3 integrin signaling pathways was found in an IGF-1 mediated migration of extravillous trophoblast (EVT) cells [191].
Doxorubicin-loaded human serum albumin nanoparticles conjugated with an antibody targeting αvβ3-positive M21 melanoma cells exhibited enhanced cytotoxicity as compared to free doxorubicin [192]. However, there is a practical alternative other than using expensive antibodies: ligands containing an RGD tripeptide active site can be recognized by all five αV integrins, two β1 integrins (α5, α8) and αIIbβ3 [193], and they are therefore useful tools for targeting integrins. When PEGylated-copolymer-coated iron oxide nanoparticles conjugated with a cyclic peptide containing this RGD sequence was administrated in mouse model for targeting αvβ3, successful tumor homing in-vivo was perceived in a subcutaneous U87MG glioblastoma xenograft model by magnetic resonance imaging (MRI) imaging [194]. Similar integrin-specific binding was achieved on human umbilical vein endothelial cells by paramagnetic liposomes conjugated with the cyclic RGD peptide [195]. siRNA encapsulated into RGD-labeled chitosan nanoparticles led to targeted silencing of multiple growth-promoting genes in nude mice bearing SKOV3ip1, HeyA8, and A2780 cells [196]. RGD-modified adenovirus expressing a therapeutic thymidine kinase suicide gene and a somatostatin receptor complexed to In-111 pentetreotide has demonstrated safety, potential efficacy, and possible gene transfer imaging capability in a Phase I clinical trial [197]. These studies support the possible clinical application of RGD-integrin targeting for imaging tumors and treatment of angiogenesis.
3.6. Transferrin receptor (TfR) nanoparticle targeting
As mentioned previously, Tfs are glycoproteins that bind free iron in the serum, and facilitate iron uptake into proliferating cells by binding to TfR on the cell surface. TfR is overexpressed in malignant cells due to the increased requirement for iron [198]. Upon binding to Tf, TfR is endocytosed into acidic compartments where the iron dissociates. This characteristic makes TfR a valuable target for compartment-specific drug delivery, particularly in cancer treatment.
Active targeting with PEGylated gold nanoparticles conjugated with Tf led to internalization into tumor induced Neuro2A cancer cells that have abundant TfR expression [116], exhibiting satisfactory TfR-targeting specificity in vivo. Comparison of the efficacy of the anti-cancer drug, hydroxycamptothecin, loaded into different drug carriers against sarcoma-180 tumors in mice showed that PEGylated non-ionic surfactant-based liposomes conjugated with Tf had significantly stronger inhibition of tumor growth compared with non-targeted PEGylated liposomes, non-coated liposomes and free hydroxycamptothecin. Moreover, such efficacy was achieved without any side effect, such as decreased body weight [199]. A similar study compared the efficacy of doxorubicin loaded into stealth liposomes against Heps tumors in mice [200]. It also showed that PEGylated liposomes conjugated with Tf inhibited the tumor more potently than the non-targeted analogs. These studies all demonstrate increased drug delivery efficiency in vivo by Tf targeting. A strong advantage of Tf targeting, as opposed to use of signaling ligands, is due to its lack of complicating downstream signaling effects since it functions largely as a nutrient uptake mechanism.
Two TfR targeting liposomal systems have entered clinical trials [201]: MBP-426, a liposome conjugated with Tf for the delivery of oxaliplatin, has completed a Phase I clinical trial (2006, NCT00355888). It has also reached a Phase Ib/II clinical trial in second-line patients with gastric, gastroesophageal, or esophageal adenocarcinoma (2009, NCT00964080). SGT-53 is a liposome encapsulating plasmid DNA coding for wild type p53, with the conjugation of an anti-TfR single-chain variable fragment (TfRscFv) for targeting TfR. SGT-53 is currently in a Phase Ib clinical trial (2007, NCT00470613) in patients with advanced solid tumors.
A recent study showed that siRNA encapsulated in polymer nanoparticles conjugated with Tf caused a specific gene inhibition (reduction in mRNA and protein) by an RNAi mechanism of action when administered via systemic administration in Phase I clinical trial [202]. This study performed on patients with metastatic melanoma is the first example of a dose-dependent accumulation of targeted nanoparticles in human tumors [202], indicating the effectiveness of TfR targeting in a clinical application.
3.7. Low-density lipoprotein receptor (LDLR) nanoparticle targeting
Certain rapidly proliferating cancer cells have a high demand for cholesterol for cell membrane synthesis and therefore overexpress the LDL receptor [203, 204]. Thus the LDL receptor is regarded as a potential target that may facilitate the delivery and endocytosis of drug carriers into cancer cells.
LDL-mediated targeting of liposomes to leukemic lymphocytes in vitro was reported as early as 1985 [3]. Recently, siRNA delivery by LDL nanoparticles also showed enhanced gene silencing in HepG2 cells compared to their analog without LDL [205]. In vivo studies of LDL-receptor targeting also have given promising results: nanoparticles conjugated with LDL and near-infrared dyes showed specific tumor-targeting functions in mice, suggesting their value in enhanced optical cancer imaging [206]. Use of this receptor, like TfR, is theoretically less prone to complicating side effects since it too involves nutritional uptake.
4. Multivalent targeting
Multivalency is a term that describes the simultaneous interaction of multiple recognition elements on one molecular entity with multiple receptors on another entity. Multivalent ligands present multiple copies of the same recognition element (homomultimer) or a combination of different recognition elements (heteromultimer) that are connected via, or displayed upon, the central scaffold. The recognition element of a multivalent ligand can be a carbohydrate, peptide, protein, or small molecule – any moiety that binds to a receptor. The scaffold is the main determining factor of the structure of the multivalent ligand – it also dictates how easily the structural features can be varied. The most critical structural features are the shape, flexibility, size, valency and orientation of the individual recognition elements – they often dramatically influence activity and mechanism of action [207–210]. For example, in the processes that are activated by the receptor clustering that precedes endocytosis, the most potent multivalent ligands will be those that contain many closely spaced recognition elements, whereas in the systems that rely on transcytosis, the scaffold size and selectivity of the interaction of each individual receptor-recognition element pair may also dictate the efficiency of its transport.
An important concept underlies the design of drug carriers based on multivalent ligands: many cell surface receptors are modular, with binding of the recognition element and signaling performed by structurally distinct and spatially segregated domains (Figure 4). Hence, interaction of the multivalent carrier with the extracellular region of the receptor may inexplicably activate the cell signaling cascade and alter the effect of the cargo. Because substrate binding and signaling activity are linked for many cell surface receptors, careful consideration of the receptor structure, receptor-receptor communication and crosstalk of the intracellular signals is required in order to orchestrate the overall response. For example, a multivalent display of the recognition element that is acting alone as an antagonist often leads to receptor clustering, resulting in use of the entire multivalent structure as an agonist [211]. Conversely, it has been proposed that multiple agonist recognition elements displayed in an orientation that holds receptors in an unproductive state for signaling conformations can result in a multivalent antagonist [212]. Likewise, ligand-induced intracellular sequestering of the signaling proteins can also result in an antagonist or agonist-like effects [213, 214].
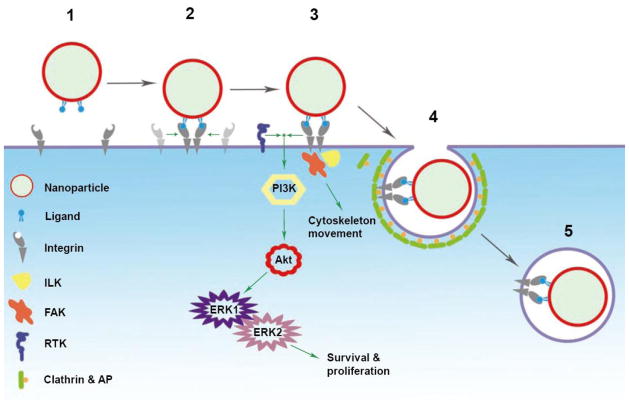
Figure 4. Multivalent Targeting of Integrin Receptors Activates Signaling.
Phase 1, the cargo bearing ligands approaches the plasma membrane bearing receptors; Phase 2, the ligands bind the recognition domain of the receptors and trigger polymerization of receptors; Phase 3, the spatially segregated intracellular domains of the receptors initiate downstream signaling; Phase 4, the cargo falls into the invagination formed by the plasma membrane; Phase 5, the cargo is internalized for subsequent intracellular trafficking. Binding of nanoparticle to integrins can activate signaling pathways and subsequently affect cell proliferation, differentiation or migration. Integrins synergize with other cell surface receptors, such as receptor protein tyrosine kinases, to activate signaling via ERK1/2 cascade. ILK – integrin-linked kinase, FAK – focal adhesion kinase, RTK – receptor tyrosine kinase.
Design of multivalent drug carriers can be accomplished through conjugation of multiple recognition elements onto an appropriate dendrimer, polymer, micelle or nanoparticle scaffold [215–217]. Overall, such constructs show enhanced binding affinity towards the target due to their distinct interaction mechanisms as compared to the monovalent ligand [209, 216, 218]. For example, ligands with multiple copies of a recognition element can bind to multiple binding sites on a single oligomeric receptor, such as an immunoglobulin or some lectins, by chelation or sub-site binding [219–221]. In both situations, the translational entropy cost is paid with the first receptor–ligand contact and subsequent binding interactions proceed without additional penalties in translational entropy [222]. For certain receptors that possess binding sub-sites in addition to their primary site of interaction, multivalent ligands may gain binding energy from contacting these secondary sites. In the situations where the multivalent ligand occupies more than one binding site—whether it is a subsite or a primary site within an oligomeric receptor—the ligand typically will bind with an increased affinity [223].
Another mechanism by which a multivalent ligand can act as an inhibitor is through steric stabilization; the steric bulk of the ligand may preclude the engagement of a bound receptor with an opposing viral particle or cell [224]. This aspect of multivalency is particularly attractive in the design of inhibitors of binding interactions between cellular and viral surfaces. Still another mode of interaction involves ligand-induced clustering of the multiple receptors on the cell surface, a process that is facilitated in the fluid lipid bilayer where two-dimensional diffusion of receptors is readily observed. Such clustering of the receptors often activates intracellular signaling and is extensively studied for the interaction of the integrin receptors with multivalent ligands with RGD-type peptide as recognition elements (vide supra) [225–227]. Lastly, statistical rebinding [228], where bulky multivalent ligands with low diffusion rate first dissociate but then bind again with the different recognition element to the same single-site receptor represents another interaction mechanism that can lead to an increase in binding affinity.
Homomultivalent ligands display multiple copies of the same recognition element and are often misclassified through the use of the more general term “multivalent”. The multiple recognition elements can be either directly conjugated to a drug delivery vehicle, or to a backbone that is, in turn, attached to a delivery vehicle. In both situations, the spacing of the ligand, its orientation, the chemical nature of the linker backbone, and the length of the linker all have been shown to influence binding kinetics and binding cooperativity. For example, early studies by Kessler and coworkers, homodimers of somatostatin analogs conjugated to aliphatic linkers and spaced 16–18 angstrom apart showed 150 times increased affinity over the monomer, but as the linker length varied, the apparent affinity of the homodimers decreased [229]. Using multiple copies of α-melanocortin stimulating hormone tethered via spacers of different length and rigidity, Hruby, Gillies et al. showed that the structure of the spacer, its length, conformational flexibility and the orientation of the recognition element have an impact on the binding affinity of the ligand [230]. In addition, authors noticed that the enhanced cooperativity of the multivalent interaction (larger Hill coefficient) is largely dependent on the number of recognition elements and less dependent on the properties of the spacer.
The reports of quantitative, accurate and systematic studies of the binding equilibrium between homomultivalent nanoparticles and their target receptors are rare, probably due to the inherent difficulty in performing such assays where high binding constants must be measured under equilibrium conditions in the presence of multiple secondary interactions. Nevertheless, recent report on binding of dendrimer-based folate nanoparticles to surface-immobilized folate binding protein (FBP) provided important insights into the nature of such interactions [231]. The authors have shown that dissociation constants (KD) between the homomultivalent nanoparticle and FBP, as measured by surface plasmon resonance (SPR) experiments, are dramatically enhanced (~2,500- to 170,000-fold over folic acid) and largely dependent upon the optimum number of folate ligands per particle. Examination of SPR data revealed a linear increase in the equilibrium association constant (Ka) as a function of the number of folates, indicating that the association to the surface does not exhibit any cooperativity. However, the decrease in equilibrium dissociation constant (KD) was exponential as a function of the number of folates, demonstrating the cooperativity upon dissociation from the surface. Furthermore, cell-based assays showed enhanced uptake of the higher-affinity nanoparticles in KB cells. Interestingly, the rate of endocytosis was similar for all nanoparticles regardless of the number of ligands, suggesting that the observed enhancement of KD, through multivalency, not an enhanced rate of endocytosis, is the key factor contributing to the improved rate of uptake of these drug delivery platforms. The effect of the linker was not reported in this work, however, the follow-up study with homomultivalent nanoparticles based on methotrexates conjugated to the same dendrimer suggests that the optimum length and conformational flexibility of the linker is important in functional assays such as inhibition of the catalytic activity of dihydrofolate reductase [232].
Multivalent ligands can also consist of dissimilar recognition elements. Such constructs are termed heteromultivalent and can recognize and simultaneously bind different types of cell surface receptors, potentially providing higher specificity toward a cellular phenotype that displays a particular receptor combination [233–236]. Numerous examples of multivalent targeting have been reported, such as heteromultimers of Tf-TfR plus RGD-integrin, and aptamer-nucleolin/tenascin-C plus RGD-integrin combinations. Both approaches demonstrated high specificity for in vitro delivery of nanoparticles [237, 238]. Furthermore, multivalent targeting also showed higher in vivo effectiveness as compared to monovalent ligands: nanoparticles using Tf-TfR + RGD-integrin increased the retinal delivery of nanoparticles that were administered intravenously [239].
One particularly cogent example of the increased efficacy of heteromultivalent targeting involves the use of cyclic RGD peptide and antagonist peptide anginex (Anx) conjugated to paramagnetic or fluorescent liposomes in targeting of αvβ3 integrins and galectin-1, respectively [240]. Galectin-1 has been identified as an important factor in the signaling of endothelial cell proliferation, migration, and apoptosis and it was proposed as a molecular marker for target-specific imaging of angiogenesis. Using optical techniques and MRI it was shown that the heteromultivalent approach produced synergistic targeting effects with dramatically elevated uptake of nanoparticles into endothelial cells as compared to single-ligand, homomultivalent targeting [240].
Multivalency is, without a doubt, an important concept guiding the design of nanoparticles for drug delivery. Due to the paucity of the reported examples and complex role of multivalent interactions in cell-nanoparticle recognition, there are no clear guidelines for the precise placement of the interacting ligands in order to achieve the desired rate of internalization and localization and to minimize the off-target effects that arise from nonspecific binding. Given the important role of the structure of the nanoparticle itself, ultimately the success of the multivalent targeting strategy may come from the integration of the nanoparticle design with parallel synthesis, resulting in a library of nanoparticles that can be subjected to a screen against different cell lines for the desired property, (specificity, rate of internalization, etc.). Such an approach could facilitate rapid development of functional nanomaterials that utilize multivalent interactions for applications such as targeting specific cell types, differentiating cell lines, and detecting distinct cellular states.
5. Conclusion
Considerable advances have been made from the time when potential of receptor-mediated endocytotic targeting inferred 25 years ago was realized with the first successful attempts to enhance drug internalization through ligand conjugation. However the complexities inherent in the trafficking pathways themselves, the possibility of alteration of trafficking processes depending on the ligand concentration and other factors such as multivalency, and the growing body of knowledge regarding the diversity of ways in which the scaffolds themselves, nanoparticles, interact with different cells and tissues will keep drug delivery scientists busy for some time to come.
Acknowledgments
SX was supported by NIH RO1 EY016985. CTO was supported by NIH grants RO1 EY016985 and R21 EB012281. SHA acknowledges support from NIH grants RO1 EY016985, RO1 EY011386, RO1 EY017293 and 5UL1RR031986. BO was supported by NSF grant 0748838 and NIH grants R21 CA129388 and R21 HL094969.
Abbreviations
- AML
acute myelogenous leukemia
- AP
adaptor protein
- APCs
antigen-presenting cells
- Arf
ADP-ribosylation factor
- CLIC
clathrin independent carriers
- CtxB
cholera toxin B
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EPR
enhanced permeability and retention
- ER
endoplasmic reticulum
- ESCRT
endosomal sorting complex required for transport
- FBP
folate binding protein
- FcRn
neonatal Fc receptor
- FR
folate receptor
- GEEC
GPI-anchored protein-enriched early endosomal compartment
- GPCR
G-protein coupled receptor
- GPI-AP
glycosylphosphatidyl inositol anchored protein
- HFE
familial hemochromatosis protein
- IGF
insulin-like growth factor
- IgG
immunoglobulin G
- LDL
low-density lipoprotein
- LDLR
low-density lipoprotein receptor
- MMP
matrix metalloproteinase
- PACS1
phosphofurin acidic cluster sorting protein
- pIgR
polymeric immunoglobulin receptor
- Rabs
ras-like proteins from brain
- SNARE
receptor for soluble N-ethylmaleimide sensitive factor attachment proteins
- SPR
surface plasmon resonance
- StxB
Shiga toxin B
- SV40
simian virus 40
- Tf
transferrin
- TfR
transferrin receptor
- TGF
transforming growth factor
- TGN
trans-Golgi network
- TTP
TfR trafficking protein
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sutherland R, Delia D, Schneider C, Newman R, Kemshead J, Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981;78:4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Widera A, Norouziyan F, Shen WC. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv Drug Deliv Rev. 2003;55:1439–1466. doi: 10.1016/j.addr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Vidal M, Sainte-Marie J, Philippot JR, Bienvenue A. LDL-mediated targeting of liposomes to leukemic lymphocytes in vitro. EMBO J. 1985;4:2461–2467. doi: 10.1002/j.1460-2075.1985.tb03957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajendran L, Knolker HJ, Simons K. Subcellular targeting strategies for drug design and delivery. Nat Rev Drug Discov. 2010;9:29–42. doi: 10.1038/nrd2897. [DOI] [PubMed] [Google Scholar]
- 5.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145:182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearse BM, Crowther RA. Structure and assembly of coated vesicles. Annu Rev Biophys Biophys Chem. 1987;16:49–68. doi: 10.1146/annurev.bb.16.060187.000405. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- 8.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 9.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 10.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 11.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reider A, Wendland B. Endocytic adaptors--social networking at the plasma membrane. J Cell Sci. 2011;124:1613–1622. doi: 10.1242/jcs.073395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brodsky FM. Diversity of Clathrin Function: New Tricks for an Old Protein. Annu Rev Cell Dev Biol. 2012 doi: 10.1146/annurev-cellbio-101011-155716. [DOI] [PubMed] [Google Scholar]
- 14.Bonazzi M, Vasudevan L, Mallet A, Sachse M, Sartori A, Prevost MC, Roberts A, Taner SB, Wilbur JD, Brodsky FM, Cossart P. Clathrin phosphorylation is required for actin recruitment at sites of bacterial adhesion and internalization. J Cell Biol. 2011;195:525–536. doi: 10.1083/jcb.201105152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo JW, Mitragotri S. Polymer particles that switch shape in response to a stimulus. Proc Natl Acad Sci U S A. 2010;107:11205–11210. doi: 10.1073/pnas.1000346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khormaee S, Chen R, Park JK, Slater NK. The influence of aromatic side-chains on the aqueous properties of pH-sensitive poly(L-lysine iso-phthalamide) derivatives. J Biomater Sci Polym Ed. 2010;21:1573–1588. doi: 10.1163/092050609X12519805626194. [DOI] [PubMed] [Google Scholar]
- 17.Kyriakides TR, Cheung CY, Murthy N, Bornstein P, Stayton PS, Hoffman AS. pH-sensitive polymers that enhance intracellular drug delivery in vivo. J Control Release. 2002;78:295–303. doi: 10.1016/s0168-3659(01)00504-1. [DOI] [PubMed] [Google Scholar]
- 18.Wu QJ, Zhu XC, Xiao X, Wang P, Xiong da K, Gong CY, Wang YS, Yang L, Wei YQ. A novel vaccine delivery system: biodegradable nanoparticles in thermosensitive hydrogel. Growth Factors. 2011;29:290–297. doi: 10.3109/08977194.2011.624517. [DOI] [PubMed] [Google Scholar]
- 19.Zhan F, Chen W, Wang Z, Lu W, Cheng R, Deng C, Meng F, Liu H, Zhong Z. Acid-activatable prodrug nanogels for efficient intracellular doxorubicin release. Biomacromolecules. 2011;12:3612–3620. doi: 10.1021/bm200876x. [DOI] [PubMed] [Google Scholar]
- 20.Cross KJ, Langley WA, Russell RJ, Skehel JJ, Steinhauer DA. Composition and functions of the influenza fusion peptide. Protein Pept Lett. 2009;16:766–778. doi: 10.2174/092986609788681715. [DOI] [PubMed] [Google Scholar]
- 21.Bavdek A, Kostanjsek R, Antonini V, Lakey JH, Dalla Serra M, Gilbert RJ, Anderluh G. pH dependence of listeriolysin O aggregation and pore-forming ability. FEBS J. 2012;279:126–141. doi: 10.1111/j.1742-4658.2011.08405.x. [DOI] [PubMed] [Google Scholar]
- 22.Terra RM, Guimaraes JA, Verli H. Structural and functional behavior of biologically active monomeric melittin. J Mol Graph Model. 2007;25:767–772. doi: 10.1016/j.jmgm.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12:362–375. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wernick NL, Chinnapen DJ, Cho JA, Lencer WI. Cholera toxin: an intracellular journey into the cytosol by way of the endoplasmic reticulum. Toxins (Basel) 2010;2:310–325. doi: 10.3390/toxins2030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandvig K, Bergan J, Dyve AB, Skotland T, Torgersen ML. Endocytosis and retrograde transport of Shiga toxin. Toxicon. 2010;56:1181–1185. doi: 10.1016/j.toxicon.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Bitler BG, Goverdhan A, Schroeder JA. MUC1 regulates nuclear localization and function of the epidermal growth factor receptor. J Cell Sci. 2010;123:1716–1723. doi: 10.1242/jcs.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polishchuk R, Di Pentima A, Lippincott-Schwartz J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat Cell Biol. 2004;6:297–307. doi: 10.1038/ncb1109. [DOI] [PubMed] [Google Scholar]
- 28.Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol. 2002;3:944–955. doi: 10.1038/nrm972. [DOI] [PubMed] [Google Scholar]
- 29.Tesar DB, Cheung EJ, Bjorkman PJ. The chicken yolk sac IgY receptor, a mammalian mannose receptor family member, transcytoses IgY across polarized epithelial cells. Mol Biol Cell. 2008;19:1587–1593. doi: 10.1091/mbc.E07-09-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He W, Ladinsky MS, Huey-Tubman KE, Jensen GJ, McIntosh JR, Bjorkman PJ. FcRn-mediated antibody transport across epithelial cells revealed by electron tomography. Nature. 2008;455:542–546. doi: 10.1038/nature07255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Predescu SA, Predescu DN, Malik AB. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2007;293:L823–842. doi: 10.1152/ajplung.00436.2006. [DOI] [PubMed] [Google Scholar]
- 32.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 33.Johansen FE, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor and IgA transport: new advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol. 2011;4:598–602. doi: 10.1038/mi.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herve F, Ghinea N, Scherrmann JM. CNS delivery via adsorptive transcytosis. AAPS J. 2008;10:455–472. doi: 10.1208/s12248-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchs R, Ellinger I. Endocytic and transcytotic processes in villous syncytiotrophoblast: role in nutrient transport to the human fetus. Traffic. 2004;5:725–738. doi: 10.1111/j.1600-0854.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 36.Altschuler Y, Hodson C, Milgram SL. The apical compartment: trafficking pathways, regulators and scaffolding proteins. Curr Opin Cell Biol. 2003;15:423–429. doi: 10.1016/s0955-0674(03)00084-x. [DOI] [PubMed] [Google Scholar]
- 37.Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 38.Mostov K, Su T, ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol. 2003;5:287–293. doi: 10.1038/ncb0403-287. [DOI] [PubMed] [Google Scholar]
- 39.Jones EA, Waldmann TA. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest. 1972;51:2916–2927. doi: 10.1172/JCI107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodewald R. pH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J Cell Biol. 1976;71:666–669. doi: 10.1083/jcb.71.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simister NE, Story CM, Chen HL, Hunt JS. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996;26:1527–1531. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- 42.Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol. 1996;157:3317–3322. [PubMed] [Google Scholar]
- 43.Jerdeva GV, Tesar DB, Huey-Tubman KE, Ladinsky MS, Fraser SE, Bjorkman PJ. Comparison of FcRn- and pIgR-mediated transport in MDCK cells by fluorescence confocal microscopy. Traffic. 2010;11:1205–1220. doi: 10.1111/j.1600-0854.2010.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzaban S, Massol RH, Yen E, Hamman W, Frank SR, Lapierre LA, Hansen SH, Goldenring JR, Blumberg RS, Lencer WI. The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J Cell Biol. 2009;185:673–684. doi: 10.1083/jcb.200809122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, Blumberg RS, Lencer WI. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104:903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci U S A. 2011;108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben Suleiman Y, Yoshida M, Nishiumi S, Tanaka H, Mimura T, Nobutani K, Yamamoto K, Takenaka M, Aoganghua A, Miki I, Ota H, Takahashi S, Matsui H, Nakamura M, Blumberg RS, Azuma T. Neonatal Fc receptor for IgG (FcRn) expressed in the gastric epithelium regulates bacterial infection in mice. Mucosal Immunol. 2012;5:87–98. doi: 10.1038/mi.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bitonti AJ, Dumont JA, Low SC, Peters RT, Kropp KE, Palombella VJ, Stattel JM, Lu Y, Tan CA, Song JJ, Garcia AM, Simister NE, Spiekermann GM, Lencer WI, Blumberg RS. Pulmonary delivery of an erythropoietin Fc fusion protein in non-human primates through an immunoglobulin transport pathway. Proc Natl Acad Sci U S A. 2004;101:9763–9768. doi: 10.1073/pnas.0403235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dumont JA, Bitonti AJ, Clark D, Evans S, Pickford M, Newman SP. Delivery of an erythropoietin-Fc fusion protein by inhalation in humans through an immunoglobulin transport pathway. J Aerosol Med. 2005;18:294–303. doi: 10.1089/jam.2005.18.294. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J Neuroimmunol. 2001;114:168–172. doi: 10.1016/s0165-5728(01)00242-9. [DOI] [PubMed] [Google Scholar]
- 52.Deane R, Sagare A, Hamm K, Parisi M, LaRue B, Guo H, Wu Z, Holtzman DM, Zlokovic BV. IgG-assisted age-dependent clearance of Alzheimer’s amyloid beta peptide by the blood-brain barrier neonatal Fc receptor. J Neurosci. 2005;25:11495–11503. doi: 10.1523/JNEUROSCI.3697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, Eden PA, Anderson CL. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 54.Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197:315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomilin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries JP, Saenger W, Krausslich HG, Shupliakov O, Robinson PJ, McCluskey A, Haucke V. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell. 2011;146:471–484. doi: 10.1016/j.cell.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 56.Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 57.Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gkouvatsos K, Papanikolaou G, Pantopoulos K. Regulation of iron transport and the role of transferrin. Biochim Biophys Acta. 2012;1820:188–202. doi: 10.1016/j.bbagen.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 59.Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9:217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt PJ, Toran PT, Giannetti AM, Bjorkman PJ, Andrews NC. The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell Metab. 2008;7:205–214. doi: 10.1016/j.cmet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tosoni D, Puri C, Confalonieri S, Salcini AE, De Camilli P, Tacchetti C, Di Fiore PP. TTP specifically regulates the internalization of the transferrin receptor. Cell. 2005;123:875–888. doi: 10.1016/j.cell.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 62.Chen J, Wang J, Meyers KR, Enns CA. Transferrin-directed internalization and cycling of transferrin receptor 2. Traffic. 2009;10:1488–1501. doi: 10.1111/j.1600-0854.2009.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norouziyan F, Shen WC, Hamm-Alvarez SF. Tyrphostin A8 stimulates a novel trafficking pathway of apically endocytosed transferrin through Rab11-enriched compartments in Caco-2 cells. Am J Physiol Cell Physiol. 2008;294:C7–21. doi: 10.1152/ajpcell.00372.2006. [DOI] [PubMed] [Google Scholar]
- 64.Descamps L, Dehouck MP, Torpier G, Cecchelli R. Receptor-mediated transcytosis of transferrin through blood-brain barrier endothelial cells. Am J Physiol. 1996;270:H1149–1158. doi: 10.1152/ajpheart.1996.270.4.H1149. [DOI] [PubMed] [Google Scholar]
- 65.Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002;3:256–266. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- 66.Maurer ME, Cooper JA. Endocytosis of megalin by visceral endoderm cells requires the Dab2 adaptor protein. J Cell Sci. 2005;118:5345–5355. doi: 10.1242/jcs.02650. [DOI] [PubMed] [Google Scholar]
- 67.Mishra SK, Jha A, Steinhauser AL, Kokoza VA, Washabaugh CH, Raikhel AS, Foster WA, Traub LM. Internalization of LDL-receptor superfamily yolk-protein receptors during mosquito oogenesis involves transcriptional regulation of PTB-domain adaptors. J Cell Sci. 2008;121:1264–1274. doi: 10.1242/jcs.025833. [DOI] [PubMed] [Google Scholar]
- 68.Morris SM, Cooper JA. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic. 2001;2:111–123. doi: 10.1034/j.1600-0854.2001.020206.x. [DOI] [PubMed] [Google Scholar]
- 69.Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002;21:4915–4926. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He G, Gupta S, Yi M, Michaely P, Hobbs HH, Cohen JC. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin, and AP-2. J Biol Chem. 2002;277:44044–44049. doi: 10.1074/jbc.M208539200. [DOI] [PubMed] [Google Scholar]
- 71.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kazazic M, Bertelsen V, Pedersen KW, Vuong TT, Grandal MV, Rodland MS, Traub LM, Stang E, Madshus IH. Epsin 1 is involved in recruitment of ubiquitinated EGF receptors into clathrin-coated pits. Traffic. 2009;10:235–245. doi: 10.1111/j.1600-0854.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 73.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 74.Madshus IH, Stang E. Internalization and intracellular sorting of the EGF receptor: a model for understanding the mechanisms of receptor trafficking. J Cell Sci. 2009;122:3433–3439. doi: 10.1242/jcs.050260. [DOI] [PubMed] [Google Scholar]
- 75.McNiven MA. Big gulps: specialized membrane domains for rapid receptor-mediated endocytosis. Trends Cell Biol. 2006;16:487–492. doi: 10.1016/j.tcb.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 76.Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9:639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 78.Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89:836–843. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 79.Jones AT. Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J Cell Mol Med. 2007;11:670–684. doi: 10.1111/j.1582-4934.2007.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 81.Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, Takehashi M, Tanaka S, Ueda K, Simpson JC, Jones AT, Sugiura Y, Futaki S. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol Ther. 2004;10:1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 82.Zaro JL, Rajapaksa TE, Okamoto CT, Shen WC. Membrane transduction of oligoarginine in HeLa cells is not mediated by macropinocytosis. Mol Pharm. 2006;3:181–186. doi: 10.1021/mp0500869. [DOI] [PubMed] [Google Scholar]
- 83.Nishimura S, Takahashi S, Kamikatahira H, Kuroki Y, Jaalouk DE, O’Brien S, Koivunen E, Arap W, Pasqualini R, Nakayama H, Kuniyasu A. Combinatorial targeting of the macropinocytotic pathway in leukemia and lymphoma cells. J Biol Chem. 2008;283:11752–11762. doi: 10.1074/jbc.M708849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McNiven MA, Thompson HM. Vesicle formation at the plasma membrane and trans-Golgi network: the same but different. Science. 2006;313:1591–1594. doi: 10.1126/science.1118133. [DOI] [PubMed] [Google Scholar]
- 85.McNiven MA, Ridley AJ. Focus on membrane dynamics. Trends Cell Biol. 2006;16:485–486. doi: 10.1016/j.tcb.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 86.Lipardi C, Mora R, Colomer V, Paladino S, Nitsch L, Rodriguez-Boulan E, Zurzolo C. Caveolin transfection results in caveolae formation but not apical sorting of glycosylphosphatidylinositol (GPI)-anchored proteins in epithelial cells. J Cell Biol. 1998;140:617–626. doi: 10.1083/jcb.140.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hansen CG, Nichols BJ. Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol. 2010;20:177–186. doi: 10.1016/j.tcb.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 88.Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, Hancock JF, Parton RG. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, Ravid K, Pilch PF. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 2008;8:310–317. doi: 10.1016/j.cmet.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bastiani M, Parton RG. Caveolae at a glance. J Cell Sci. 2010;123:3831–3836. doi: 10.1242/jcs.070102. [DOI] [PubMed] [Google Scholar]
- 91.Nichols BJ. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat Cell Biol. 2002;4:374–378. doi: 10.1038/ncb787. [DOI] [PubMed] [Google Scholar]
- 92.Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 93.Sprenger RR, Fontijn RD, van Marle J, Pannekoek H, Horrevoets AJ. Spatial segregation of transport and signalling functions between human endothelial caveolae and lipid raft proteomes. Biochem J. 2006;400:401–410. doi: 10.1042/BJ20060355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 95.Hayer A, Stoeber M, Ritz D, Engel S, Meyer HH, Helenius A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J Cell Biol. 2010;191:615–629. doi: 10.1083/jcb.201003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Predescu D, Predescu S, Malik AB. Transport of nitrated albumin across continuous vascular endothelium. Proc Natl Acad Sci U S A. 2002;99:13932–13937. doi: 10.1073/pnas.212253499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tiruppathi C, Naqvi T, Wu Y, Vogel SM, Minshall RD, Malik AB. Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. Proc Natl Acad Sci U S A. 2004;101:7699–7704. doi: 10.1073/pnas.0401712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mehta D, Bhattacharya J, Matthay MA, Malik AB. Integrated control of lung fluid balance. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1081–1090. doi: 10.1152/ajplung.00268.2004. [DOI] [PubMed] [Google Scholar]
- 99.Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotechnol. 2007;25:327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hansen CG, Nichols BJ. Molecular mechanisms of clathrin-independent endocytosis. J Cell Sci. 2009;122:1713–1721. doi: 10.1242/jcs.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Howes MT, Kirkham M, Riches J, Cortese K, Walser PJ, Simpson F, Hill MM, Jones A, Lundmark R, Lindsay MR, Hernandez-Deviez DJ, Hadzic G, McCluskey A, Bashir R, Liu L, Pilch P, McMahon H, Robinson PJ, Hancock JF, Mayor S, Parton RG. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J Cell Biol. 2010;190:675–691. doi: 10.1083/jcb.201002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Howes MT, Mayor S, Parton RG. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr Opin Cell Biol. 2010;22:519–527. doi: 10.1016/j.ceb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 103.Mayor S, Rothberg KG, Maxfield FR. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994;264:1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- 104.Sabharanjak S, Mayor S. Folate receptor endocytosis and trafficking. Adv Drug Deliv Rev. 2004;56:1099–1109. doi: 10.1016/j.addr.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 105.Kalia M, Kumari S, Chadda R, Hill MM, Parton RG, Mayor S. Arf6-independent GPI-anchored protein-enriched early endosomal compartments fuse with sorting endosomes via a Rab5/phosphatidylinositol-3′-kinase-dependent machinery. Mol Biol Cell. 2006;17:3689–3704. doi: 10.1091/mbc.E05-10-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–423. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 107.Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eyster CA, Higginson JD, Huebner R, Porat-Shliom N, Weigert R, Wu WW, Shen RF, Donaldson JG. Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic. 2009;10:590–599. doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Slimane TA, Trugnan G, Van ISC, Hoekstra D. Raft-mediated trafficking of apical resident proteins occurs in both direct and transcytotic pathways in polarized hepatic cells: role of distinct lipid microdomains. Mol Biol Cell. 2003;14:611–624. doi: 10.1091/mbc.E02-08-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hughes JB, Berger C, Rodland MS, Hasmann M, Stang E, Madshus IH. Pertuzumab increases epidermal growth factor receptor down-regulation by counteracting epidermal growth factor receptor-ErbB2 heterodimerization. Mol Cancer Ther. 2009;8:1885–1892. doi: 10.1158/1535-7163.MCT-09-0291. [DOI] [PubMed] [Google Scholar]
- 111.Kumari S, Borroni V, Chaudhry A, Chanda B, Massol R, Mayor S, Barrantes FJ. Nicotinic acetylcholine receptor is internalized via a Rac-dependent, dynamin-independent endocytic pathway. J Cell Biol. 2008;181:1179–1193. doi: 10.1083/jcb.200709086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tesar DB, Tiangco NE, Bjorkman PJ. Ligand valency affects transcytosis, recycling and intracellular trafficking mediated by the neonatal Fc receptor. Traffic. 2006;7:1127–1142. doi: 10.1111/j.1600-0854.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71:409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 114.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 115.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci U S A. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Choi CH, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc Natl Acad Sci U S A. 2010;107:1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorg Med Chem. 2009;17:2950–2962. doi: 10.1016/j.bmc.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 118.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51:691–743. [PubMed] [Google Scholar]
- 119.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Jong WH, Borm PJ. Drug delivery and nanoparticles:applications and hazards. Int J Nanomedicine. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yadav KS, Chuttani K, Mishra AK, Sawant KK. Effect of Size on the Biodistribution and Blood Clearance of Etoposide-Loaded PLGA Nanoparticles. PDA J Pharm Sci Technol. 2011;65:131–139. [PubMed] [Google Scholar]
- 122.Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 2008;69:1–9. doi: 10.1016/j.ejpb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 123.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 124.Leikina E, Ramos C, Markovic I, Zimmerberg J, Chernomordik LV. Reversible stages of the low-pH-triggered conformational change in influenza virus hemagglutinin. EMBO J. 2002;21:5701–5710. doi: 10.1093/emboj/cdf559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simoes S, Moreira JN, Fonseca C, Duzgunes N, de Lima MC. On the formulation of pH-sensitive liposomes with long circulation times. Adv Drug Deliv Rev. 2004;56:947–965. doi: 10.1016/j.addr.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 126.Chen X, Lee HF, Zaro JL, Shen WC. Effects of receptor binding on plasma half-life of bifunctional transferrin fusion proteins. Mol Pharm. 2011;8:457–465. doi: 10.1021/mp1003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vecchione L, Jacobs B, Normanno N, Ciardiello F, Tejpar S. EGFR-targeted therapy. Exp Cell Res. 2011;317:2765–2771. doi: 10.1016/j.yexcr.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 128.Tseng CL, Su WY, Yen KC, Yang KC, Lin FH. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials. 2009;30:3476–3485. doi: 10.1016/j.biomaterials.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 129.Hadjipanayis CG, Machaidze R, Kaluzova M, Wang L, Schuette AJ, Chen H, Wu X, Mao H. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70:6303–6312. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Austin CD, De Maziere AM, Pisacane PI, van Dijk SM, Eigenbrot C, Sliwkowski MX, Klumperman J, Scheller RH. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell. 2004;15:5268–5282. doi: 10.1091/mbc.E04-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leonard D, Hayakawa A, Lawe D, Lambright D, Bellve KD, Standley C, Lifshitz LM, Fogarty KE, Corvera S. Sorting of EGF and transferrin at the plasma membrane and by cargo-specific signaling to EEA1-enriched endosomes. J Cell Sci. 2008;121:3445–3458. doi: 10.1242/jcs.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leslie M. EGF is internalized and degraded. J Cell Biol. 2005;1:3. [Google Scholar]
- 133.Liu D, Liu F, Liu Z, Wang L, Zhang N. Tumor specific delivery and therapy by double-targeted nanostructured lipid carriers with anti-VEGFR-2 antibody. Mol Pharm. 2011;8:2291–2301. doi: 10.1021/mp200402e. [DOI] [PubMed] [Google Scholar]
- 134.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 135.Kroeze WK, Sheffler DJ, Roth BL. G-protein-coupled receptors at a glance. J Cell Sci. 2003;116:4867–4869. doi: 10.1242/jcs.00902. [DOI] [PubMed] [Google Scholar]
- 136.Hild W, Pollinger K, Caporale A, Cabrele C, Keller M, Pluym N, Buschauer A, Rachel R, Tessmar J, Breunig M, Goepferich A. G protein-coupled receptors function as logic gates for nanoparticle binding and cell uptake. Proc Natl Acad Sci U S A. 2010;107:10667–10672. doi: 10.1073/pnas.0912782107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 138.Insel PA, Tang CM, Hahntow I, Michel MC. Impact of GPCRs in clinical medicine: monogenic diseases, genetic variants and drug targets. Biochim Biophys Acta. 2007;1768:994–1005. doi: 10.1016/j.bbamem.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou M, Nakatani E, Gronenberg LS, Tokimoto T, Wirth MJ, Hruby VJ, Roberts A, Lynch RM, Ghosh I. Peptide-labeled quantum dots for imaging GPCRs in whole cells and as single molecules. Bioconjug Chem. 2007;18:323–332. doi: 10.1021/bc0601929. [DOI] [PubMed] [Google Scholar]
- 140.Hartmann LC, Keeney GL, Lingle WL, Christianson TJ, Varghese B, Hillman D, Oberg AL, Low PS. Folate receptor overexpression is associated with poor outcome in breast cancer. Int J Cancer. 2007;121:938–942. doi: 10.1002/ijc.22811. [DOI] [PubMed] [Google Scholar]
- 141.Iwakiri S, Sonobe M, Nagai S, Hirata T, Wada H, Miyahara R. Expression status of folate receptor alpha is significantly correlated with prognosis in non-small-cell lung cancers. Ann Surg Oncol. 2008;15:889–899. doi: 10.1245/s10434-007-9755-3. [DOI] [PubMed] [Google Scholar]
- 142.Chen H, Ahn R, Van den Bossche J, Thompson DH, O’Halloran TV. Folate-mediated intracellular drug delivery increases the anticancer efficacy of nanoparticulate formulation of arsenic trioxide. Mol Cancer Ther. 2009;8:1955–1963. doi: 10.1158/1535-7163.MCT-09-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao X, Li H, Lee RJ. Targeted drug delivery via folate receptors. Expert Opin Drug Deliv. 2008;5:309–319. doi: 10.1517/17425247.5.3.309. [DOI] [PubMed] [Google Scholar]
- 144.Gabizon A, Tzemach D, Gorin J, Mak L, Amitay Y, Shmeeda H, Zalipsky S. Improved therapeutic activity of folate-targeted liposomal doxorubicin in folate receptor-expressing tumor models. Cancer Chemother Pharmacol. 2010;66:43–52. doi: 10.1007/s00280-009-1132-4. [DOI] [PubMed] [Google Scholar]
- 145.Nichols B. Endocytosis of lipid-anchored proteins: excluding GEECs from the crowd. J Cell Biol. 2009;186:457–459. doi: 10.1083/jcb.200907119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lakhan SE, Sabharanjak S, De A. Endocytosis of glycosylphosphatidylinositol-anchored proteins. J Biomed Sci. 2009;16:93. doi: 10.1186/1423-0127-16-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Saeed AO, Magnusson JP, Moradi E, Soliman M, Wang W, Stolnik S, Thurecht KJ, Howdle SM, Alexander C. Modular construction of multifunctional bioresponsive cell-targeted nanoparticles for gene delivery. Bioconjug Chem. 2011;22:156–168. doi: 10.1021/bc100149g. [DOI] [PubMed] [Google Scholar]
- 148.Liu L, Zheng M, Renette T, Kissel T. Modular Synthesis of Folate Conjugated Ternary Copolymers: Polyethylenimine-graft-Polycaprolactone-block-Poly(ethylene glycol)-Folate for Targeted Gene Delivery. Bioconjug Chem. 2012 doi: 10.1021/bc300025d. [DOI] [PubMed] [Google Scholar]
- 149.Werner ME, Karve S, Sukumar R, Cummings ND, Copp JA, Chen RC, Zhang T, Wang AZ. Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials. 2011;32:8548–8554. doi: 10.1016/j.biomaterials.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lu PL, Chen YC, Ou TW, Chen HH, Tsai HC, Wen CJ, Lo CL, Wey SP, Lin KJ, Yen TC, Hsiue GH. Multifunctional hollow nanoparticles based on graft-diblock copolymers for doxorubicin delivery. Biomaterials. 2011;32:2213–2221. doi: 10.1016/j.biomaterials.2010.11.051. [DOI] [PubMed] [Google Scholar]
- 151.Dohmen C, Edinger D, Frohlich T, Schreiner L, Lachelt U, Troiber C, Radler J, Hadwiger P, Vornlocher HP, Wagner E. Nanosized Multifunctional Polyplexes for Receptor-Mediated SiRNA Delivery. ACS Nano. 2012;6:5198–5208. doi: 10.1021/nn300960m. [DOI] [PubMed] [Google Scholar]
- 152.Li H, Lu Y, Piao L, Wu J, Liu S, Marcucci G, Ratnam M, Lee RJ. Targeting human clonogenic acute myelogenous leukemia cells via folate conjugated liposomes combined with receptor modulation by all-trans retinoic acid. Int J Pharm. 2010;402:57–63. doi: 10.1016/j.ijpharm.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 154.Aplin AE, Howe A, Alahari SK, Juliani RL. Signal transduction and signal modulation by cell adhesion receptors: The role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacological Reviews. 1998;50:197–263. [PubMed] [Google Scholar]
- 155.Gottschalk KE, Kessler H. The structures of Integrins and integrin-ligand complexes: Implications for drug design and signal transduction. Angewandte Chemie-International Edition. 2002;41:3767–3774. doi: 10.1002/1521-3773(20021018)41:20<3767::AID-ANIE3767>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 156.Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 157.Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–747. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shi F, Sottile J. Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci. 2008;121:2360–2371. doi: 10.1242/jcs.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: Regulation through alpha(1)beta(1) and alpha(2)beta(1) integrins. Proceedings Of The National Academy Of Sciences Of The United States Of America. 1997;94:13612–13617. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Senger DR, Perruzzi CA, Streit M, Koteliansky VE, de Fougerolles AR, Detmar M. The alpha(1)beta(1) and alpha(2)beta(1) Integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. American Journal Of Pathology. 2002;160:195–204. doi: 10.1016/s0002-9440(10)64363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Whelan MC, Senger DR. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. Journal Of Biological Chemistry. 2003;278:327–334. doi: 10.1074/jbc.M207554200. [DOI] [PubMed] [Google Scholar]
- 163.Garmy-Susini B, Jin H, Zhu YH, Sung RJ, Hwang R, Varner J. Integrin alpha(4)beta(1)-VCAM-1-mediated adhesion between endothelial and mural cells is required for blood vessel maturation. Journal Of Clinical Investigation. 2005;115:1542–1551. doi: 10.1172/JCI23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang JT, Rayburn H, Hynes RO. Embryonic Mesodermal Defects In Alpha(5) Integrin-Deficient Mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 165.Goh KL, Yang JT, Hynes RO. Mesodermal defects and cranial neural crest apoptosis in alpha 5 integrin-null embryos. Development. 1997;124:4309–4319. doi: 10.1242/dev.124.21.4309. [DOI] [PubMed] [Google Scholar]
- 166.Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, Hynes RO. Central roles of alpha(5)beta(1) integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arteriosclerosis Thrombosis And Vascular Biology. 2002;22:927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- 167.Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin alpha 5 beta 1 with the central cell-binding domain of fibronectin. American Journal Of Pathology. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo CR, Pihlajaniemi T, Alitalo K, Vuori K. Interaction of endostatin with integrins implicated in angiogenesis. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2001;98:1024–1029. doi: 10.1073/pnas.031564998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, Plow EF. A mechanism for modulation of cellular responses to VEGF: Activation of the integrins. Molecular Cell. 2000;6:851–860. [PubMed] [Google Scholar]
- 170.Stupack DG, Cheresh DA. Integrins and angiogenesis. Current Topics In Developmental Biology. 2004;64:207–238. doi: 10.1016/S0070-2153(04)64009-9. [DOI] [PubMed] [Google Scholar]
- 171.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 172.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. beta 3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. Journal Of Clinical Investigation. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang XZ, Sheppard D, Hynes O, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta(3) integrin or beta(3) and beta(5) integrins. Nature Medicine. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 174.Robinson SD, Reynolds LE, Wyder L, Hicklin DJ, Hodivala-Dilke KM. beta 3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arteriosclerosis Thrombosis And Vascular Biology. 2004;24:2108–2114. doi: 10.1161/01.ATV.0000143857.27408.de. [DOI] [PubMed] [Google Scholar]
- 175.Reynolds AR, Reynolds LE, Nagel TE, Lively JC, Robinson SD, Hicklin DJ, Bodary SC, Hodivala-Dilke KM. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta(3)-integrin-deficient mice. Cancer Research. 2004;64:8643–8650. doi: 10.1158/0008-5472.CAN-04-2760. [DOI] [PubMed] [Google Scholar]
- 176.McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, Shani M, Dvorak HF, Wolburg H, Bader BL, Dvorak AM, Hynes RO. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alpha v integrins. Molecular And Cellular Biology. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhu JW, Motejlek K, Wang DN, Zang KL, Schmidt A, Reichardt LF. beta 8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of alpha v integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 179.Hiran TS, Mazurkiewicz JE, Kreienberg P, Rice FL, LaFlamme SE. Endothelial expression of the alpha 6 beta 4 integrin is negatively regulated during angiogenesis. Journal Of Cell Science. 2003;116:3771–3781. doi: 10.1242/jcs.00681. [DOI] [PubMed] [Google Scholar]
- 180.Nikolopoulos SN, Blaikie P, Yoshioka T, Guo WJ, Giancotti FG. Integrin beta 4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–483. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 181.Eliceiri BP, Cheresh DA. The role of alpha v integrins during angiogenesis: insights into potential mechanisms of action and clinical development. Journal Of Clinical Investigation. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Brooks PC, Stromblad S, Sanders LC, vonSchalscha TL, Aimes RT, StetlerStevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 183.Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes & Development. 1999;13:1055–1066. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- 184.Koivunen E, Wang BC, Ruoslahti E. Phage Libraries Displaying Cyclic-Peptides With Different Ring Sizes - Ligand Specificities Of The Rgd-Directed Integrins. Bio-Technology. 1995;13:265–270. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- 185.Pasqualini R, Koivunen E, Ruoslahti E. alpha v Integrins as receptors for tumor targeting by circulating ligands. Nature Biotechnology. 1997;15:542–546. doi: 10.1038/nbt0697-542. [DOI] [PubMed] [Google Scholar]
- 186.Boger DL, Goldberg J, Silletti S, Kessler T, Cheresh DA. Identification of a novel class of small-molecule antiangiogenic agents through the screening of combinatorial libraries which function by inhibiting the binding and localization of proteinase MMP2 to integrin alpha(v)beta(3) Journal Of The American Chemical Society. 2001;123:1280–1288. doi: 10.1021/ja003579+. [DOI] [PubMed] [Google Scholar]
- 187.Arap W, Pasqualini R, Ruoslahti E. Chemotherapy targeted to tumor vasculature. Curr Opin Oncol. 1998;10:560–565. doi: 10.1097/00001622-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 188.Vuori K, Ruoslahti E. ASSOCIATION OF INSULIN-RECEPTOR SUBSTRATE-1 WITH INTEGRINS. Science. 1994;266:1576–1578. doi: 10.1126/science.7527156. [DOI] [PubMed] [Google Scholar]
- 189.Schneller M, Vuori K, Ruoslahti E. alpha v beta 3 integrin associates with activated insulin and PDGF beta receptors and potentiates the biological activity of PDGF. Embo J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Furundzija V, Fritzsche J, Kaufmann J, Meyborg H, Fleck E, Kappert K, Stawowy P. IGF-1 increases macrophage motility via PKC/p38-dependent alpha v beta 3-integrin inside-out signaling. Biochem Biophys Res Commun. 2010;394:786–791. doi: 10.1016/j.bbrc.2010.03.072. [DOI] [PubMed] [Google Scholar]
- 191.Kabir-Salmani M, Shiokawa S, Akimoto Y, Sakai K, Nagamatsu S, Nakamura Y, Lotfi A, Kawakami H, Iwashita M. alpha(v)beta(3) integrin signaling pathway is involved in insulin-like growth factor I-stimulated human extravillous trophoblast cell migration. Endocrinology. 2003;144:1620–1630. doi: 10.1210/en.2002-220886. [DOI] [PubMed] [Google Scholar]
- 192.Wagner S, Rothweiler F, Anhorn MG, Sauer D, Riemann I, Weiss EC, Katsen-Globa A, Michaelis M, Cinatl J, Jr, Schwartz D, Kreuter J, von Briesen H, Langer K. Enhanced drug targeting by attachment of an anti alphav integrin antibody to doxorubicin loaded human serum albumin nanoparticles. Biomaterials. 2010;31:2388–2398. doi: 10.1016/j.biomaterials.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 193.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen K, Xie J, Xu H, Behera D, Michalski MH, Biswal S, Wang A, Chen X. Triblock copolymer coated iron oxide nanoparticle conjugate for tumor integrin targeting. Biomaterials. 2009;30:6912–6919. doi: 10.1016/j.biomaterials.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kluza E, van der Schaft DW, Hautvast PA, Mulder WJ, Mayo KH, Griffioen AW, Strijkers GJ, Nicolay K. Synergistic targeting of alphavbeta3 integrin and galectin-1 with heteromultivalent paramagnetic liposomes for combined MR imaging and treatment of angiogenesis. Nano Lett. 2010;10:52–58. doi: 10.1021/nl902659g. [DOI] [PubMed] [Google Scholar]
- 196.Han HD, Mangala LS, Lee JW, Shahzad MM, Kim HS, Shen D, Nam EJ, Mora EM, Stone RL, Lu C, Lee SJ, Roh JW, Nick AM, Lopez-Berestein G, Sood AK. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin Cancer Res. 2010;16:3910–3922. doi: 10.1158/1078-0432.CCR-10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kim KH, Dmitriev I, O’Malley JP, Wang M, Saddekni S, You Z, Preuss MA, Harris RD, Aurigemma R, Siegal GP, Zinn KR, Curiel DT, Alvarez RD. A phase I clinical trial of Ad5.SSTR/TK.RGD, a novel infectivity-enhanced bicistronic adenovirus, in patients with recurrent gynecologic cancer. Clin Cancer Res. 2012;18:3440–3451. doi: 10.1158/1078-0432.CCR-11-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 199.Hong M, Zhu S, Jiang Y, Tang G, Pei Y. Efficient tumor targeting of hydroxycamptothecin loaded PEGylated niosomes modified with transferrin. J Control Release. 2009;133:96–102. doi: 10.1016/j.jconrel.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 200.Li X, Ding L, Xu Y, Wang Y, Ping Q. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int J Pharm. 2009;373:116–123. doi: 10.1016/j.ijpharm.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 201.Daniels TR, Bernabeu E, Rodriguez JA, Patel S, Kozman M, Chiappetta DA, Holler E, Ljubimova JY, Helguera G, Penichet ML. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim Biophys Acta. 2012;1820:291–317. doi: 10.1016/j.bbagen.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shao T, Li X, Ge J. Target drug delivery system as a new scarring modulation after glaucoma filtration surgery. Diagn Pathol. 2011;6:64. doi: 10.1186/1746-1596-6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Corbin IR, Zheng G. Mimicking nature’s nanocarrier: synthetic low-density lipoprotein-like nanoparticles for cancer-drug delivery. Nanomedicine (Lond) 2007;2:375–380. doi: 10.2217/17435889.2.3.375. [DOI] [PubMed] [Google Scholar]
- 205.Jin H, Lovell JF, Chen J, Lin Q, Ding L, Ng KK, Pandey RK, Manoharan M, Zhang Z, Zheng G. Mechanistic Insights into LDL Nanoparticle-Mediated siRNA Delivery. Bioconjug Chem. 2012;23:33–41. doi: 10.1021/bc200233n. [DOI] [PubMed] [Google Scholar]
- 206.Corbin IR, Chen J, Zheng G. Functionalizing low-density lipoprotein nanoparticles for in vivo near-infrared optical imaging of cancer. Proc SPIE. 2007;6626:1–7. [Google Scholar]
- 207.Roy R, Page D, Perez SF, Bencomo VV. Effect of shape, size, and valency of multivalent mannosides on their binding properties to phytohemagglutinins. Glycoconj J. 1998;15:251–263. doi: 10.1023/a:1006945028547. [DOI] [PubMed] [Google Scholar]
- 208.Kiessling LL, Gestwicki JE, Strong LE. Synthetic multivalent ligands as probes of signal transduction. Angew Chem Int Ed Engl. 2006;45:2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kiessling LL, Strong LE, Gestwicki JE. Principles for multivalent ligand design. Annual Reports in Medicinal Chemistry. 2000;35:321–330. [Google Scholar]
- 210.Kitov PI, Paszkiewicz E, Sadowska JM, Deng Z, Ahmed M, Narain R, Griener TP, Mulvey GL, Armstrong GD, Bundle DR. Impact of the nature and size of the polymeric backbone on the ability of heterobifunctional ligands to mediate shiga toxin and serum amyloid p component ternary complex formation. Toxins (Basel) 2011;3:1065–1088. doi: 10.3390/toxins3091065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Carrithers MD, Lerner MR. Synthesis and characterization of bivalent peptide ligands targeted to G-protein-coupled receptors. Chem Biol. 1996;3:537–542. doi: 10.1016/s1074-5521(96)90144-1. [DOI] [PubMed] [Google Scholar]
- 212.Graef IA, Holsinger LJ, Diver S, Schreiber SL, Crabtree GR. Proximity and orientation underlie signaling by the non-receptor tyrosine kinase ZAP70. EMBO J. 1997;16:5618–5628. doi: 10.1093/emboj/16.18.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nishizumi H, Horikawa K, Mlinaric-Rascan I, Yamamoto T. A double-edged kinase Lyn: a positive and negative regulator for antigen receptor-mediated signals. J Exp Med. 1998;187:1343–1348. doi: 10.1084/jem.187.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Torigoe C, Inman JK, Metzger H. An unusual mechanism for ligand antagonism. Science. 1998;281:568–572. doi: 10.1126/science.281.5376.568. [DOI] [PubMed] [Google Scholar]
- 215.Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem Rev. 2002;102:555–578. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- 216.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angewandte Chemie-International Edition. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 217.Barkey NM, Tafreshi NK, Josan JS, De Silva CR, Sill KN, Hruby VJ, Gillies RJ, Morse DL, Vagner J. Development of Melanoma-Targeted Polymer Micelles by Conjugation of a Melanocortin 1 Receptor (MC1R) Specific Ligand. Journal of Medicinal Chemistry. 2011;54:8078–8084. doi: 10.1021/jm201226w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kiessling LL, Pohl NL. Strength in numbers: non-natural polyvalent carbohydrate derivatives. Chem Biol. 1996;3:71–77. doi: 10.1016/s1074-5521(96)90280-x. [DOI] [PubMed] [Google Scholar]
- 219.Kitov PI, Sadowska JM, Mulvey G, Armstrong GD, Ling H, Pannu NS, Read RJ, Bundle DR. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature. 2000;403:669–672. doi: 10.1038/35001095. [DOI] [PubMed] [Google Scholar]
- 220.Fan EK, Zhang ZS, Minke WE, Hou Z, Verlinde C, Hol WGJ. High-affinity pentavalent ligands of Escherichia coli heat-labile enterotoxin by modular structure-based design. Journal of the American Chemical Society. 2000;122:2663–2664. [Google Scholar]
- 221.Zhang ZS, Merritt EA, Ahn M, Roach C, Hou Z, Verlinde C, Hol WGJ, Fan E. Solution and crystallographic studies of branched multivalent ligands that inhibit the receptor-binding of cholera toxin. Journal of the American Chemical Society. 2002;124:12991–12998. doi: 10.1021/ja027584k. [DOI] [PubMed] [Google Scholar]
- 222.Jencks WP. On the attribution and additivity of binding energies. Proc Natl Acad Sci U S A. 1981;78:4046–4050. doi: 10.1073/pnas.78.7.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Howorka S, Nam J, Bayley H, Kahne D. Stochastic detection of monovalent and bivalent protein-ligand interactions. Angew Chem Int Ed Engl. 2004;43:842–846. doi: 10.1002/anie.200352614. [DOI] [PubMed] [Google Scholar]
- 224.Mammen M, Dahmann G, Whitesides GM. Effective inhibitors of hemagglutination by influenza virus synthesized from polymers having active ester groups. Insight into mechanism of inhibition. J Med Chem. 1995;38:4179–4190. doi: 10.1021/jm00021a007. [DOI] [PubMed] [Google Scholar]
- 225.Hood J, Bednarski M, Frausto R, Guccione S, Reisfeld R, Xiang R, Cheresh D. Tumor regression by targeted gene delivery. Cancer Gene Therapy. 2003;10:49. [Google Scholar]
- 226.Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, Cheresh DA. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- 227.Miyamoto S, Akiyama SK, Yamada KM. SYNERGISTIC ROLES FOR RECEPTOR OCCUPANCY AND AGGREGATION IN INTEGRIN TRANSMEMBRANE FUNCTION. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- 228.Hlavacek WS, Percus JK, Percus OE, Perelson AS, Wofsy C. Retention of antigen on follicular dendritic cells and B lymphocytes through complement-mediated multivalent ligand-receptor interactions: theory and application to HIV treatment. Mathematical Biosciences. 2002;176:185–202. doi: 10.1016/s0025-5564(02)00091-3. [DOI] [PubMed] [Google Scholar]
- 229.Kessler H, Schudok M, Haupt A. Peptides 1988: proceedings of the 20th European peptide symposium; September 4–9, 1988; Tübingen, W. de Gruyter. 1989. [Google Scholar]
- 230.Vagner J, Handl HL, Gillies RJ, Hruby VJ. Novel targeting strategy based on multimeric ligands for drug delivery and molecular imaging: homooligomers of alpha-MSH. Bioorganic & Medicinal Chemistry Letters. 2004;14:211–215. doi: 10.1016/j.bmcl.2003.09.079. [DOI] [PubMed] [Google Scholar]
- 231.Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR, Holl MMB. The binding avidity of a nanoparticle-based multivalent targeted drug delivery platform. Chemistry & Biology. 2007;14:107–115. doi: 10.1016/j.chembiol.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 232.Li MH, Choi SK, Thomas TP, Desai A, Lee KH, Kotlyar A, Holl MMB, Baker JR. Dendrimer-based multivalent methotrexates as dual acting nanoconjugates for cancer cell targeting. European Journal of Medicinal Chemistry. 2012;47:560–572. doi: 10.1016/j.ejmech.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Martinez-Veracoechea FJ, Frenkel D. Designing super selectivity in multivalent nano-particle binding. Proc Natl Acad Sci U S A. 2011;108:10963–10968. doi: 10.1073/pnas.1105351108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Josan JS, Handl HL, Sankaranarayanan R, Xu LP, Lynch RM, Vagner J, Mash EA, Hruby VJ, Gillies RJ. Cell-Specific Targeting by Heterobivalent Ligands. Bioconjugate Chemistry. 2011;22:1270–1278. doi: 10.1021/bc1004284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Xu LP, Vagner J, Josan J, Lynch RM, Morse DL, Baggett B, Han HY, Mash EA, Hruby VJ, Gillies RJ. Enhanced targeting with heterobivalent ligands. Molecular Cancer Therapeutics. 2009;8:2356–2365. doi: 10.1158/1535-7163.MCT-08-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lee YS, Fernandes S, Kulkarani V, Mayorov A, Davis P, Ma SW, Brown K, Gillies RJ, Lai J, Porreca F, Hruby VJ. Design and synthesis of trivalent ligands targeting opioid, cholecystokinin, and melanocortin receptors for the treatment of pain. Bioorganic & Medicinal Chemistry Letters. 2010;20:4080–4084. doi: 10.1016/j.bmcl.2010.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Quan CY, Chang C, Wei H, Chen CS, Xu XD, Cheng SX, Zhang XZ, Zhuo RX. Dual targeting of a thermosensitive nanogel conjugated with transferrin and RGD-containing peptide for effective cell uptake and drug release. Nanotechnology. 2009;20:335101. doi: 10.1088/0957-4484/20/33/335101. [DOI] [PubMed] [Google Scholar]
- 238.Ko HY, Choi KJ, Lee CH, Kim S. A multimodal nanoparticle-based cancer imaging probe simultaneously targeting nucleolin, integrin alphavbeta3 and tenascin-C proteins. Biomaterials. 2011;32:1130–1138. doi: 10.1016/j.biomaterials.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 239.Singh SR, Grossniklaus HE, Kang SJ, Edelhauser HF, Ambati BK, Kompella UB. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16:645–659. doi: 10.1038/gt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kluza E, van der Schaft DWJ, Hautvast PAI, Mulder WJM, Mayo KH, Griffioen AW, Strijkers GJ, Nicolay K. Synergistic Targeting of alpha(v)beta(3) Integrin and Galectin-1 with Heteromultivalent Paramagnetic Liposomes for Combined MR Imaging and Treatment of Angiogenesis. Nano Letters. 2010;10:52–58. doi: 10.1021/nl902659g. [DOI] [PubMed] [Google Scholar]