Abstract
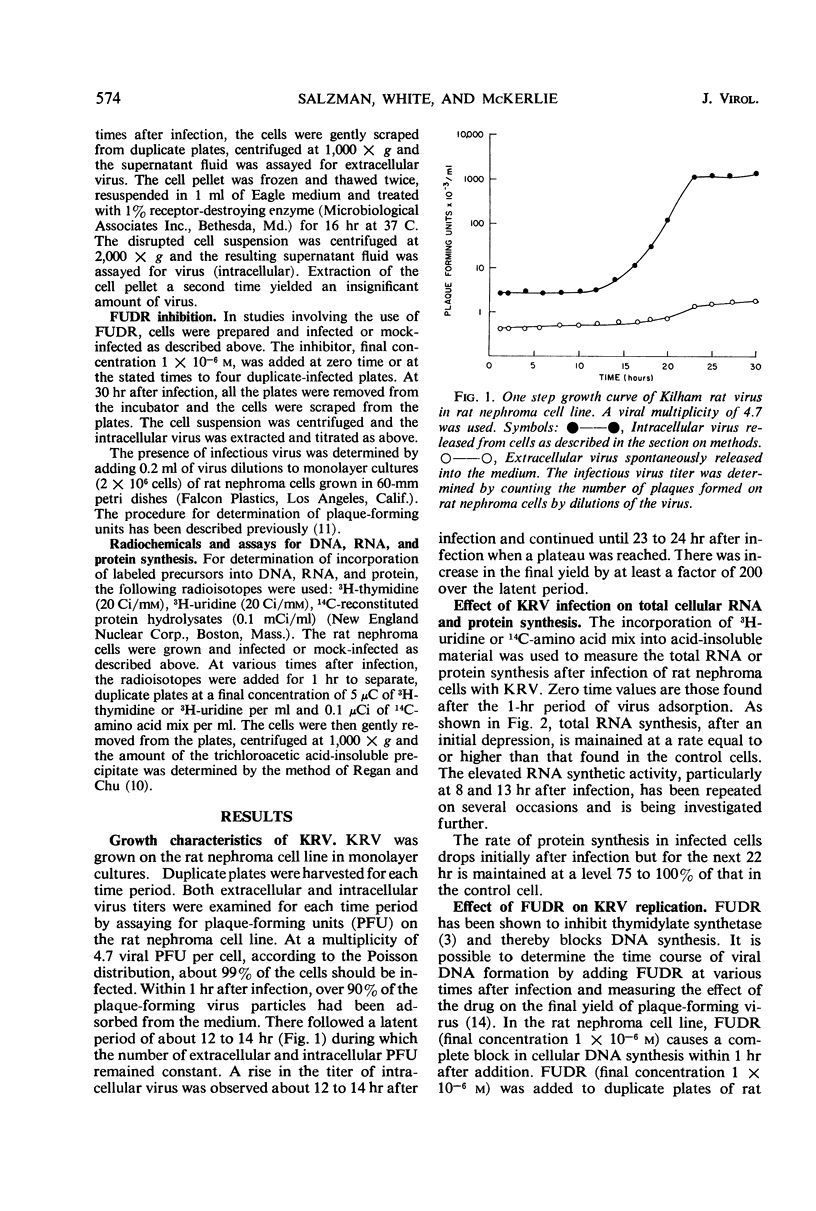
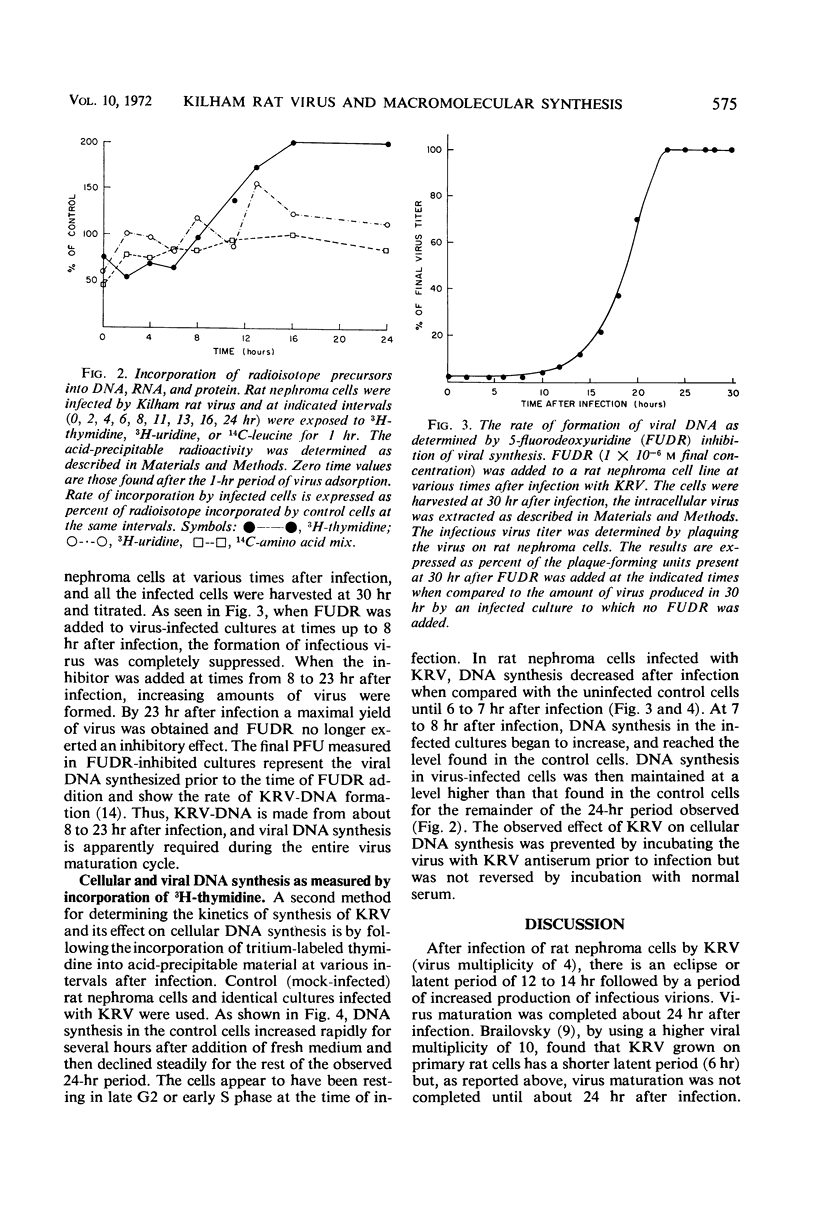
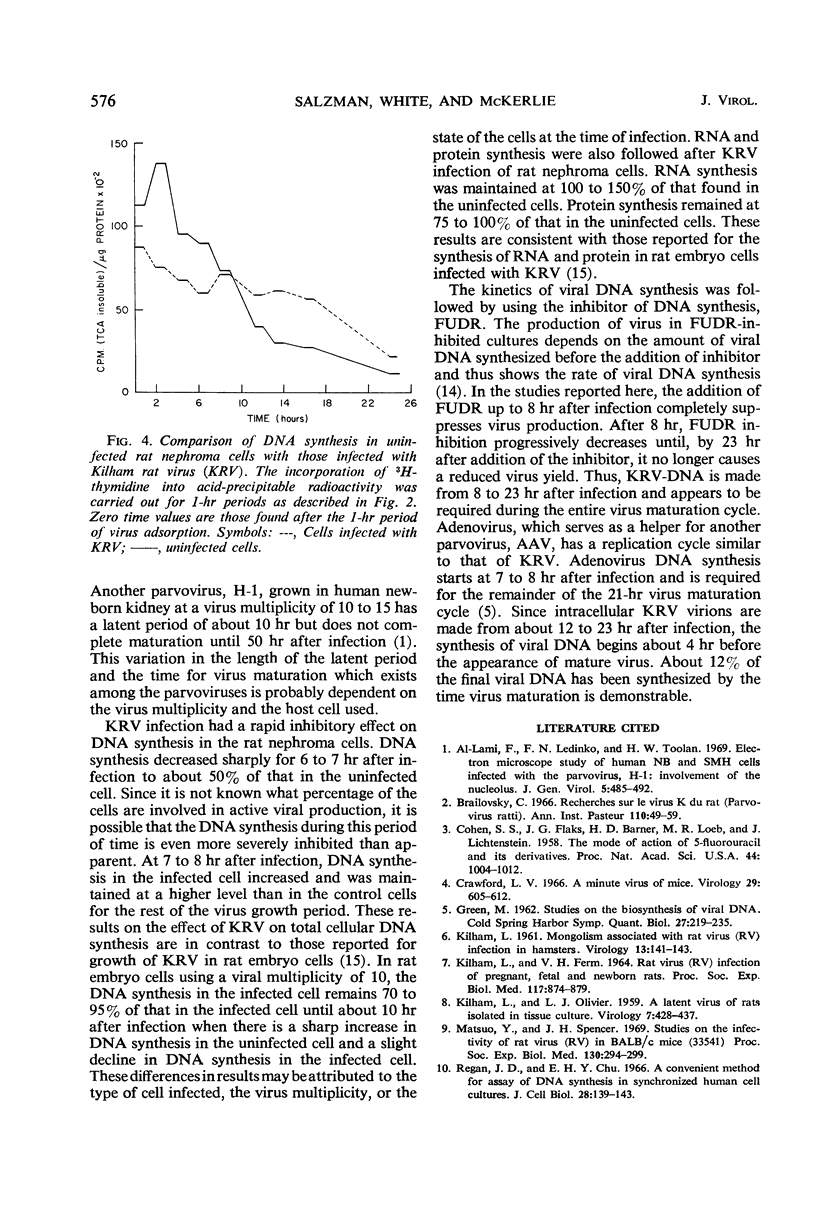
Kilham rat virus (KRV) is adsorbed into the rat nephroma cell within 1 hr after infection. There follows a latent period of about 12 hr during which less than 1% of the input infectious virus can be accounted for. New infectious virions can be detected at about 12 hr and the maximal yield of virus is attained by 23 hr after infection. The increase in final virus yield is about 200-fold over that found in the latent period. During this 23-hr period of virus growth, the rate of protein synthesis remains 75 to 100% of that in the uninfected cell. Ribonucleic acid (RNA) synthesis during this period is maintained at 100 to 150% of that found in the control cells. The addition of the inhibitor of deoxyribonucleic acid (DNA) synthesis, 5-fluoro-deoxyuridine (FUDR), up to 8 hr after infection completely suppresses virus production. After 8 hr, viral DNA production has started and FUDR inhibition progressively decreases until by 23 hr the addition of the inhibitor no longer causes a reduced virus yield. Viral DNA synthesis once initiated is required for the remainder of the 23-hr virus cycle. Viral DNA synthesis probably begins about 4 hr before the production of infectious virions. In the KRV-infected cells, DNA synthesis decreased sharply for 6 to 7 hr after infection in comparison to the uninfected cell. At 7 to 8 hr after infection, DNA synthesis in the infected cell increased and was maintained at a higher level than in the control cells for the rest of the virus growth period.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brailovsky C. Recherches sur le virus K du rat (Parvovirus ratti). I. Une méthode de titrage par plages et son application à l'étude du cycle de multiplication du virus. Ann Inst Pasteur (Paris) 1966 Jan;110(1):49–59. [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V. A minute virus of mice. Virology. 1966 Aug;29(4):605–612. doi: 10.1016/0042-6822(66)90284-4. [DOI] [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- KILHAM L., FERM V. H. RAT VIRUS (RV) INFECTIONS OF PREGNANT, FETAL AND NEWBORN RATS. Proc Soc Exp Biol Med. 1964 Dec;117:874–879. doi: 10.3181/00379727-117-29723. [DOI] [PubMed] [Google Scholar]
- KILHAM L. Mongolism associated with rat virus (RV) infection in hamsters. Virology. 1961 Jan;13:141–143. doi: 10.1016/0042-6822(61)90043-5. [DOI] [PubMed] [Google Scholar]
- KILHAM L., OLIVIER L. J. A latent virus of rats isolated in tissue culture. Virology. 1959 Apr;7(4):428–437. doi: 10.1016/0042-6822(59)90071-6. [DOI] [PubMed] [Google Scholar]
- Matsuo Y., Spencer H. J. Studies on the infectivity of rat virus (RV) in BALB-c mice. Proc Soc Exp Biol Med. 1969 Jan;130(1):294–299. doi: 10.3181/00379727-130-33541. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Chu E. H. A convenient method for assay of DNA synthesis in synchronized human cell cultures. J Cell Biol. 1966 Jan;28(1):139–143. doi: 10.1083/jcb.28.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN N. P. The rate of formation of vaccinia deoxyribonucleic acid and vaccinia virus. Virology. 1960 Jan;10:150–152. doi: 10.1016/0042-6822(60)90015-5. [DOI] [PubMed] [Google Scholar]
- Salzman L. A., Jori L. A. Characterization of the Kilham rat virus. J Virol. 1970 Feb;5(2):114–122. doi: 10.1128/jvi.5.2.114-122.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. L., Kakefuda T. Linear, single-stranded deoxyribonucleic acid isolated from Kilham rat virus. J Virol. 1971 Jun;7(6):830–835. doi: 10.1128/jvi.7.6.830-835.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. L. Structural proteins of Kilham rat virus. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1551–1556. doi: 10.1016/0006-291x(70)90564-4. [DOI] [PubMed] [Google Scholar]
- TOOLAN H. W. Experimental production of mongoloid hamsters. Science. 1960 May 13;131(3411):1446–1448. doi: 10.1126/science.131.3411.1446. [DOI] [PubMed] [Google Scholar]
- Tennant R. W. Inhibition of mitosis and macromolecular synthesis in rat embryo cells by Kilham rat virus. J Virol. 1971 Oct;8(4):402–408. doi: 10.1128/jvi.8.4.402-408.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



