Abstract
Background
In the US, cigarette flavorings are banned, with the exception of menthol. The cooling effects of menthol could facilitate the absorption of tobacco toxicants. We examined levels of biomarkers of tobacco exposure among US smokers of menthol and non-menthol cigarettes.
Methods
We studied 4,603 White, African-American, and Mexican-American current smokers ≥ 20 years of age who participated in the National Health and Nutrition Examination Survey from 1999 through 2010 and had data on cigarette type and serum cotinine, blood cadmium, and blood lead concentrations. Urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol) (NNAL) was studied in 1,607 participants with available measures.
Results
A total of 3,210 (74.3%) participants smoked non-menthol cigarettes compared to 1,393 (25.7%) participants who smoked menthol cigarettes. The geometric mean concentrations comparing smokers of non-menthol to menthol cigarettes were 163.1 vs. 175.9 ng/mL for serum cotinine; 0.95 vs. 1.02 μg/L for blood cadmium; 1.87 vs. 1.75 μg/dL for blood lead; and 0.27 vs. 0.23 ng/mL for urine NNAL. After multivariable adjustment, the ratios (95% confidence interval [CI]) comparing smokers of menthol to non-menthol cigarettes were 1.03 (0.95, 1.11) for cotinine, 1.10 (1.04, 1.16) for cadmium, 0.95 (0.90, 1.01) for lead, and 0.81 (0.65, 1.01) for NNAL.
Conclusions
In a representative sample of US adult smokers, current menthol cigarette use was associated with increased concentration of blood cadmium, an established carcinogen and highly toxic metal, but not with other biomarkers.
Impact
These findings provide information regarding possible differences in exposure to toxic constituents among menthol cigarette smokers compared to non-menthol cigarette smokers.
Keywords: cigarette smoking, menthol, biomarkers
INTRODUCTION
Tobacco use is the leading preventable cause of premature death in the United States (1). The burden of tobacco-related disease, however, is not uniformly distributed across the population. It has been hypothesized that menthol cigarette use, particularly among African-American smokers, may contribute to these disparities. Menthol is an alcohol derived from peppermint oil or produced synthetically that is used as a flavoring agent in cigarettes and other consumer products (2). The cooling and anti-irritant effects of menthol could result in greater depth of inhalation and facilitate the absorption of tobacco toxicants (3-5). Various studies have investigated possible effects of menthol cigarette use on smoking behavior and topography (5-13), with one study showing increased puff volumes associated with menthol cigarette smoking compared to non-menthol cigarette smoking (7).
Mainstream cigarette smoke contains over 7,000 chemicals including many known carcinogens (14, 15). Some studies have assessed differences in biomarkers of intake of tobacco smoke constituents between menthol and nonmenthol cigarette smokers. Biomarkers evaluated include nicotine metabolites, carbon monoxide and tobacco-specific nitrosamines (6, 16-22). Some but not all studies found increased levels of cotinine (measured in serum and plasma) and carbon monoxide (measured by exhaled air carbon monoxide and blood carboxyhemoglobin) among menthol cigarette smokers compared to non-menthol cigarette smokers (5, 7, 16, 21, 23). Previous epidemiological studies examining differences in biomarker concentrations comparing menthol and non-menthol cigarette use have been limited to cotinine and carbon monoxide and restricted to White and African-American smokers only (5-7, 16-20, 23) including a study of 1,943 NHANES 2001- 2006 participants which found no differences in serum cotinine between menthol and non-menthol cigarette smokers (24). Little is known, however, about other tobacco biomarkers as well as differences for Mexican-Americans compared to Whites or African-Americans.
The objective of this study was to examine levels of tobacco related biomarkers comparing White, African-American and Mexican-American smokers of menthol and non-menthol cigarettes who participated in NHANES from 1999 through 2010. We included the following tobacco related biomarkers available in NHANES: serum cotinine, a metabolite of nicotine specific to tobacco smoke (25-27); blood cadmium and lead, toxic metals found in mainstream and sidestream tobacco smoke that are also found in other environmental sources (15, 28-30); and urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol) (NNAL), a metabolite of exposure to the specific tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (31-33). Polycyclic aromatic hydrocarbons (PAHs), including urine 1-pyrene, a toxic and carcinogenic tobacco combustion product, were measured only in a small subsample (NHANES 2001- 2004) and could not be incorporated in this study. Under the US Family Smoking Prevention and Tobacco Control Act, signed into law in 2009, cigarette flavorings are banned, with the exception of menthol. Evidence on the impact of menthol cigarettes on exposure to tobacco related toxicants as compared to non-menthol cigarettes could inform the Food and Drug Administration (FDA) about the regulation of menthol as a tobacco additive.
METHODS
Study Population
NHANES is conducted by the US National Center for Health Statistics (NCHS; Centers for Disease Control and Prevention [CDC], Atlanta, GA), using a complex multistage sampling design to obtain a representative sample of the civilian non-institutionalized US population. NHANES study protocols for the 1999-2010 survey years were approved by the National Center for Health Statistics Institutional Review Board, and oral and written informed consent was obtained from all participants. The overall participation rate for NHANES examinations was 77% for survey years 1999-2010. A total of 32,464 adults 20 years of age or older participated in NHANES between 1999 and 2010. We excluded 25,409 never or former smokers, 125 pregnant women, 727 participants missing serum cotinine, blood cadmium or blood lead measures, 253 participants missing cigarette type (non-menthol or menthol), 68 participants reporting smokeless tobacco (snuff or chewing tobacco) use, 581 participants of non-White, African-American or Mexican-American race as the number of menthol cigarette smokers was very small, and 698 participants were missing other relevant covariates, leaving 4,603 participants for this study. Current smokers included in this analysis were similar to the corresponding NHANES smoking population with respect to sociodemographic variables (data not shown). Urinary NNAL measures were only available in NHANES 2007- 2010 (N=1,607).
Participant Smoking Characteristics
Information on smoking was obtained with a self-reported questionnaire. Smoking characteristics included self-reported number of cigarettes smoked per day (during the past 5 days), cigarette type, time to first cigarette, age at initiation and smoking behavior in the home. Cigarette type was determined by the brand smoked at the time of the interview and was categorized by NCHS as menthol or non-menthol.
Tobacco Related Biomarkers
Serum cotinine, blood cadmium, blood lead and urine NNAL are biomarkers related to tobacco exposure available in the National Health and Nutrition Examination Survey (NHANES) (34-37). Serum cotinine was measured by an isotope-dilution high-performance liquid chromatography/ atmospheric pressure chemical ionization tandem mass spectrometric method. The limit of detection for serum cotinine was 0.05 ng/mL for NHANES 1999- 2000 and the first phase of NHANES 2001- 2002, and 0.015 ng/mL for the second phase of NHANES 2001- 2002 and for NHANES 2003- 2010. No observations were below the limit of detection. The half-life of serum cotinine is 16 hours (26).
Cadmium and lead were measured simultaneously in whole blood on a PerkinElmer Model SIMAA 6000 multielement atomic absorption spectrometer, with Zeeman background correction in 1999–2002 and on an inductively coupled plasma-mass spectrometer in 2003–2010 after confirmation of no metal contamination in the collection and storage materials. The limit of detection for blood cadmium was 0.3 μg/L for NHANES 1999–2002 and 0.2 μg/L for NHANES 2003–2010, resulting in 0.3% of observations below the limit of detection. The half life of cadmium in blood is 75- 128 days for a component of recent exposure and 7.4- 16 years for a component of chronic exposure (38). The limit of detection for blood lead was 0.3 μg/dL for NHANES 1999-2010, resulting in 1 observation below the limit of detection. The half-life of lead in blood is 30 days (39).
NNAL was measured using liquid chromatography linked to tandem mass spectrometry. The limit of detection for urine total NNAL was 0.6 pg/mL for NHANES 2007–2010, resulting in 0.6% of observations below the limit of detection. The half-life of NNAL in urine is 10- 16 days (40).
For biomarkers with concentrations below the limits of detection, a level equal to the limit of detection divided by the square root of two was imputed.
Other Variables
Self-reported information on sex, age, race/ethnicity and education was collected by interview. Race/ethnicity was subsequently categorized by NCHS as non-Hispanic White, non-Hispanic African-American, Mexican-American, other Hispanic, and other. Body mass index (BMI) was calculated by dividing measured weight in kilograms by measured height in meters squared. Urine creatinine, used to adjust for urine dilution in spot urine samples in statistical models for urinary NNAL, was determined using a Jaffé rate reaction measured with a CX3 analyzer.
Statistical Analysis
Descriptive statistics were stratified by race/ethnicity (White, African-American and Mexican-American) and/or cigarette type (menthol and non-menthol). Biomarker levels were right-skewed and log-transformed for the analyses. We estimated crude and multivariable adjusted ratios of geometric means of biomarkers of tobacco exposure (serum cotinine, blood cadmium and lead, and urine NNAL) comparing smokers of menthol cigarettes to non-menthol cigarette smokers. The ratios of the geometric means and their 95% confidence intervals were obtained by exponentiating the coefficients and standard errors from the linear regression models on log-transformed biomarker levels. We also estimated crude and multivariable adjusted ratios of geometric means of biomarkers of tobacco exposure (serum cotinine, blood cadmium and lead, and urine NNAL) in African-American and Mexican-American smokers compared to White smokers. Lastly, we estimated crude and multivariable adjusted ratios of geometric means of biomarkers of tobacco exposure (serum cotinine, blood cadmium and lead, and urine NNAL) comparing smokers of menthol cigarettes to non-menthol cigarette smokers stratified by race/ethnicity. Effect modification was examined using a product term of the indicator variables for the participants’ race/ethnicity and cigarette type smoked (menthol or non-menthol). The p-values for interaction were combined into a single p-value for interaction using the Wald test.
Multivariable models were adjusted first for sex, age (continuous), education (<high school/high school/>high school) and BMI (continuous) (Model 1). Second, we also adjusted for cigarettes smoked per day (continuous) (Model 2). For cadmium, lead and NNAL, we further adjusted for serum cotinine (log-transformed) (Model 3) to investigate whether the association between menthol and the biomarkers was independent of the total smoke exposure. In addition, models by cigarette type were adjusted for race/ethnicity (White/African-American/Mexican-American) and models by race/ethnicity were further adjusted for cigarette type (menthol/non-menthol) (Model 4).
Several sensitivity analyses were considered. For lead, we further adjusted for age of the house (according to the year the family home was built) as individuals living in older homes have been shown to have higher lead exposure (39). Age of the house was categorized as before 1950, 1950- 1978, after 1978 (year in which lead paint was banned in the US) and unknown (41) for 3,788 participants in NHANES 1999- 2008 with available data (data on housing characteristics were unavailable for NHANES 2009- 2010). Results were similar (data not shown). We also considered the impact of frequency of smoking by restricting analyses to participants reporting smoking everyday (N=3,993). Results were similar (data not shown).
All statistical analyses were performed using the survey package (42, 43) (version 3.24) in R software (44) (version 2.12.1) to account for the complex sampling design and weights in NHANES 1999- 2010 and to obtain appropriate estimates and standard errors. All statistical tests were 2-sided and confidence intervals were set at 95%.
RESULTS
A total of 3,210 (74.3%) participants smoked non-menthol cigarettes compared to 1,393 (25.7%) participants who smoked menthol cigarettes (Table 1). Menthol cigarettes were smoked by 19.4%, 72.3% and 11.0% of White, African-American and Mexican-American smokers, respectively (Supplementary Table S1). Smokers of menthol cigarettes were more likely to be female, younger, African-American, have less education, have a higher body mass index, to smoke fewer cigarettes per day and to allow smoking inside the home (Table 1). In bivariate analyses, blood cadmium concentrations were higher and blood lead concentrations were lower in smokers of menthol cigarettes compared to smokers of non-menthol cigarettes (Table 1). The geometric mean concentrations comparing smokers of non-menthol to menthol cigarettes were 163.1 vs. 175.9 ng/mL for serum cotinine; 0.95 vs. 1.02 μg/L for blood cadmium; 1.87 vs. 1.75 μg/dL for blood lead; and 0.27 vs. 0.23 ng/mL for urine NNAL.
Table 1.
Participant Characteristics by Cigarette Type
| Cigarette Type |
|||
|---|---|---|---|
| Characteristics | Non-menthol | Menthol | P-value |
| N | 3210 (74.3) | 1393 (25.7) | |
| Sex | |||
| Men | 1916 (57.0) | 711 (45.8) | <0.001 |
| Women | 1294 (43.0) | 682 (54.2) | |
| Age, y | 42.2 (0.3) | 40.7 (0.5) | 0.01 |
| Race/ethnicity | |||
| White | 2148 (87.0) | 508 (60.7) | <0.001 |
| African-American | 355 (4.8) | 789 (36.4) | |
| Mexican-American | 707 (8.2) | 96 (2.9) | |
| Education | |||
| <High school | 1241 (27.9) | 426 (24.2) | 0.02 |
| High school | 895 (31.7) | 473 (36.7) | |
| >High school | 1074 (40.4) | 494 (39.1) | |
| Body mass index, kg/m2 | 27.1 (0.1) | 27.9 (0.2) | <0.001 |
| Age at initiation, y | |||
| <15 | 804 (23.8) | 276 (19.6) | 0.09 |
| 15- 16 | 766 (26.3) | 308 (24.7) | |
| 17-19 | 883 (28.5) | 412 (30.7) | |
| ≥20 | 756 (21.5) | 397 (25.1) | |
| Frequency of Smoking | |||
| Everyday | 2764 (88.0) | 1229 (89.4) | 0.27 |
| Some days | 446 (12.0) | 164 (10.6) | |
| Cigarettes smoked per day | |||
| <5 | 546 (12.4) | 239 (13.9) | <0.001 |
| 5- 10 | 1007 (28.3) | 588 (38.6) | |
| 11-20 | 1187 (41.6) | 458 (37.0) | |
| ≥21 | 470 (17.7) | 108 (10.6) | |
| Time to first smoke, mina | |||
| ≤ 5 | 470 (31.9) | 227 (33.7) | 0.53 |
| 6-30 | 473 (33.8) | 185 (30.2) | |
| 31-60 | 253 (18.5) | 122 (18.9) | |
| > 60 | 253 (15.9) | 113 (17.1) | |
| Smoking allowed in home | |||
| Yes | 1988 (63.1) | 982 (67.8) | 0.03 |
| No | 1196 (36.2) | 402 (31.5) | |
| Serum cotinine, ng/mL | 163.1 (154.4, 172.2) | 175.9 (165.8, 186.6) | 0.08 |
| Blood cadmium, μg/L | 0.95 (0.92, 0.98) | 1.02 (0.98, 1.06) | 0.007 |
| Blood lead, μg/dL | 1.87 (1.81, 1.93) | 1.75 (1.67, 1.84) | 0.02 |
| Urine NNAL, ng/mLb | 0.27 (0.24, 0.31) | 0.23 (0.20, 0.26) | 0.07 |
Values represent No (weighted %) for categorical variables continuous variables, except for serum cotinine, blood cadm which geometric means (95% CI) are reported
Only available in NHANES 2001-2010 (N=1449 and 647 respectively)
Only available in NHANES 2007-2010 (N=1118 and 489 respectively)
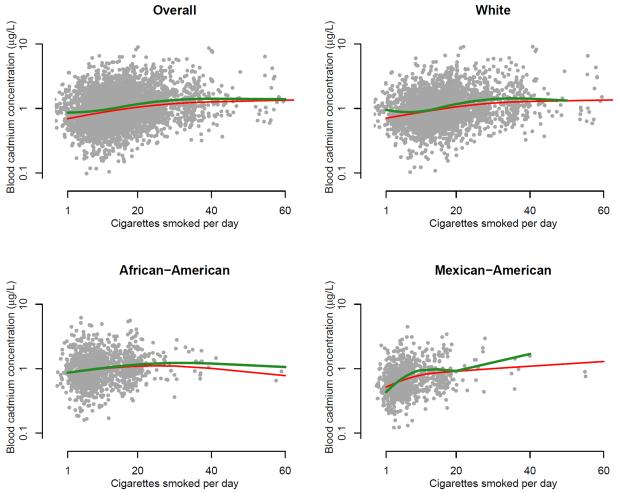
After adjustment for age, sex, education, BMI, cigarettes smoked per day and serum cotinine (for cadmium, lead and NNAL), the ratios (95% confidence interval) of the geometric means comparing menthol vs. non-menthol cigarette smokers were 1.24 (1.14, 1.34) for cotinine, 1.10 (1.05, 1.15) for cadmium, 1.02 (0.97, 1.07) for lead, and 0.76 (0.62, 0.92) for NNAL (Table 2, model 2 for serum cotinine and model 3 for cadmium, lead and NNAL). After further adjustment for race/ethnicity, the corresponding ratios were markedly decreased and no longer statistically significant for serum cotinine (1.03, 95% confidence interval 0.95, 1.11) but remained statistically increased for blood cadmium (1.10, 95% confidence interval 1.04, 1.17) (Table 2, model 4). Increased levels of blood cadmium concentrations in smokers of menthol cigarettes compared to non-menthol cigarette smokers were observed across the range of the number of cigarettes smoked per day overall and in analyses stratified by race/ethnicity (Figure 1). In stratified analyses by race/ethnicity, blood cadmium concentrations remained increased in all race/ethnic groups and there was no statistical effect modification (p for interaction 0.65) although the association was only statistically significant for White participants (Table 3). Serum cotinine concentrations were non-significantly increased in African-American and Mexican-American participants, but not in White participants, although there was no evidence for effect modification (p for interaction 0.36). Comparing menthol to non-menthol cigarette smokers by race/ethnicity stratum, blood lead concentrations were increased only in African-Americans (p for interaction 0.04), and urine NNAL concentrations were increased only in Mexican-Americans (p for interaction 0.001). After adjustment for age of the home for blood lead concentration, effect modification between type of cigarette and race/ethnicity was no longer significant (p for interaction 0.13).
Table 2.
Ratio (95% CI) of Geometric Mean of Biomarkers by Cigarette Type
| N | Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|
| Serum cotinine | |||||
| Non-menthol | 3210 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |
| Menthol | 1393 | 1.12 (1.03, 1.22) | 1.24 (1.14, 1.34) | — | 1.03 (0.95, 1.11) |
| Blood cadmium | |||||
| Non-menthol | 3210 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Menthol | 1393 | 1.09 (1.03, 1.15) | 1.15 (1.09, 1.20) | 1.10 (1.05, 1.15) | 1.10 (1.04, 1.16) |
| Blood lead | |||||
| Non-menthol | 3210 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Menthol | 1393 | 1.02 (0.98, 1.07) | 1.03 (0.98, 1.08) | 1.02 (0.97, 1.07) | 0.95 (0.90, 1.01) |
| Urine NNALa | |||||
| Non-menthol | 1118 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Menthol | 489 | 0.85 (0.67, 1.08) | 0.92 (0.72, 1.17) | 0.76 (0.62, 0.92) | 0.81 (0.65, 1.01) |
Model 1 adjusted for age, sex, education and body mass index
Model 2 further adjusted for cigarettes smoked per day
Model 3 further adjusted for serum cotinine (for blood cadmium, blood lead and urine NNAL)
Model 4 further adjusted for race/ethnicity
Only available in NHANES 2007-2010 (N= 1607) and further adjusted for urine creatinine
Table 4.
Ratio (95% CI) of Geometric Mean of Biomarkers by Race/Ethnicity
| N | Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|
| Serum cotinine | |||||
| White | 2656 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |
| African-American | 1144 | 1.16 (1.08, 1.24) | 1.55 (1.44, 1.68) | — | 1.53 (1.41, 1.66) |
| Mexican-American | 803 | 0.28 (0.24, 0.33) | 0.42 (0.36, 0.50) | 0.43 (0.36, 0.50) | |
| Blood cadmium | |||||
| White | 2656 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| African-American | 1144 | 0.99 (0.94, 1.04) | 1.16 (1.11, 1.23) | 1.07 (1.02, 1.11) | 1.01 (0.96, 1.07) |
| Mexican-American | 803 | 0.69 (0.64, 0.74) | 0.87 (0.81, 0.93) | 1.04 (0.96, 1.12) | 1.04 (0.97, 1.12) |
| Blood lead | |||||
| White | 2656 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| African-American | 1144 | 1.25 (1.19, 1.30) | 1.30 (1.24, 1.36) | 1.28 (1.22, 1.34) | 1.31 (1.24, 1.39) |
| Mexican-American | 803 | 1.12 (1.05, 1.20) | 1.19 (1.11, 1.27) | 1.22 (1.14, 1.31) | 1.22 (1.14, 1.31) |
| Urine NNALa | |||||
| White | 942 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| African-American | 414 | 0.69 (0.57, 0.83) | 1.04 (0.90, 1.20) | 0.72 (0.64, 0.80) | 0.78 (0.67, 0.90) |
| Mexican-American | 251 | 0.53 (0.36, 0.79) | 0.83 (0.64, 1.08) | 1.29 (1.13, 1.47) | 1.28 (1.12, 1.46) |
Model 1 adjusted for age, sex, education and body mass index
Model 2 further adjusted for cigarettes smoked per day
Model 3 further adjusted for serum cotinine (for blood cadmium, blood lead and urine NNAL)
Model 4 further adjusted for cigarette type (non-menthol/menthol)
Only available in NHANES 2007-2010 (N= 1607) and further adjusted for urine creatinine
Figure 1.
Blood cadmium concentrations by cigarettes smoked per day stratified by type of cigarette (menthol vs. non-menthol) overall and by race/ethnicity. Green and red lines represent geometric mean blood cadmium concentrations by cigarettes smoked per day modeled based on restricted quadratic splines with knots at 5th, 50th and 95th percentiles for menthol and non-menthol cigarette smokers, respectively. Results were adjusted for age, sex, education, body mass index, cigarettes smoked per day, serum cotinine, and race/ethnicity (for the overall). Scatterplots represent fully adjusted levels of blood cadmium concentrations and cigarettes smoked per day.
Table 3.
Ratio (95% CI) of Geometric Mean of Biomarkers by Cigarette Type and by Race/Ethnicity a
| N | Serum cotinine | Blood cadmium | Blood lead | Urine NNALb | |
|---|---|---|---|---|---|
| White | |||||
| Non-menthol | 2148 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Menthol | 508 | 1.00 (0.91, 1.10) | 1.11 (1.04, 1.19) | 0.94 (0.88, 1.00) | 0.79 (0.56, 1.10) |
| African-American | |||||
| Non-menthol | 355 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Menthol | 789 | 1.11 (0.98, 1.27) | 1.04 (0.95, 1.14) | 1.08 (1.00, 1.17) | 0.96 (0.80, 1.14) |
| Mexican-American | |||||
| Non-menthol | 707 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Menthol | 96 | 1.14 (0.81, 1.60) | 1.05 (0.92, 1.18) | 0.92 (0.79, 1.06) | 2.33 (1.40, 3.87) |
| P for interactionc | 0.36 | 0.65 | 0.04 | 0.001 |
Adjusted for age, sex, education, body mass index and cigarettes smoked per day
Only available in NHANES 2007-2010 (N= 942 White, 414 African-Americans, 251 Mexican-Americans) and further adjusted for urine creatinine
P for interaction by cigarette type and race/ethnicity was estimated using indicator variables that combined the participants’ race/ethnicity with their cigarette type (menthol or non-menthol) and combined using the Wald test.
After adjustment for demographics, cigarettes smoked per day and serum cotinine (for cadmium, lead and NNAL), the ratios (95% confidence interval) comparing African-American and Mexican-American smokers to White smokers were, respectively, 1.55 (1.44, 1.68) and 0.42 (0.36, 0.50) for serum cotinine; 1.07 (1.02, 1.11) and 1.04 (0.96, 1.12) for blood cadmium; 1.28 (1.22, 1.34) and 1.22 (1.14, 1.31) for blood lead; and 0.72 (0.64, 0.80) and 1.29 (1.13, 1.47) for NNAL (Table 4, model 2 for serum cotinine and model 3 for cadmium, lead and NNAL). Further adjustment for cigarette type resulted in similar associations by race/ethnicity for serum cotinine, blood lead and urine NNAL (Table 4, model 4). For blood cadmium, blood cadmium levels were no longer increased in African Americans compared to Whites (ratio 1.01, 95% CI 0.96, 1.07), suggesting that differences in blood cadmium levels between African Americans and Whites could be related to menthol cigarette smoking.
DISCUSSION
In a representative sample of US smokers who participated in NHANES 1999-2010, current menthol cigarette use was associated with increased concentration of blood cadmium, but not of other biomarkers. The association between menthol cigarette use and blood cadmium concentration persisted after adjustment for demographic factors, cigarettes smoked per day, serum cotinine and race/ethnicity and it was observed in all race/ethnic groups, although not always achieving statistical significance. For serum cotinine, however, the association with menthol cigarette use was no longer observed after adjustment for race/ethnicity. In subgroup analyses, menthol cigarette use was also associated with increased blood lead concentrations among African-American smokers and with increased urine NNAL among Mexican-American smokers. We had no a priori hypothesis regarding differences in the association of menthol cigarettes with lead and NNAL biomarkers by race/ethnic groups and these post-hoc findings need to be interpreted with caution.
Cadmium is a highly toxic and carcinogenic tobacco constituent. It is well established that tobacco smoke is a major source of cadmium exposure for the general population (37). The tobacco plant accumulates cadmium from contaminated soils and cadmium is further concentrated in the cigarette throughout the tobacco production process (45, 46). Little is known about differences in cadmium content of tobacco by cigarette type and any resulting differences in exposure for smokers of menthol vs. non-menthol cigarettes. In a 2011 reanalysis of tobacco industry documents, cadmium concentrations were found to be increased by more than 20% in smoke of test cigarettes with mostly menthol additives, compared to smoke of control test cigarettes containing tobacco only (47). These data were derived from test cigarettes, and although they were designed to match an industry standard and to be representative of the type of cigarettes that are sold around the world (48), little is known regarding cadmium content of commercial menthol cigarettes. Cadmium is a carcinogen that causes cancers of the lung and prostate (49). Cadmium, at relatively low levels of exposure including levels found in this study population, has been associated with increased risk of cardiovascular diseases, kidney diseases and bone diseases (50-54, 54-56).
In our study, after adjustment for race/ethnicity we found no differences in serum cotinine concentrations between menthol and non-menthol cigarettes smokers. Two studies, one conducted in 161 African-American and White adult smokers and the other conducted in 37 African-American and White female smokers, found higher cotinine concentrations among menthol cigarette smokers (7, 16). The majority of studies, however, found no difference in serum cotinine concentrations comparing smokers of menthol vs. non-menthol cigarettes (6, 17-20). The study by Caraballo et. al. used data from 1,943 African-American and White smokers who participated in NHANES 2001- 2006 and also found no differences in serum cotinine between menthol and non-menthol cigarette smokers after adjustment for cigarettes smoked per day (24).
Relatively little is known about differences in NNAL concentrations, a metabolite of NNK, in menthol versus non-menthol cigarettes. We found more than a 2-fold increase in urinary total NNAL comparing menthol to non-menthol cigarette smokers among Mexican-Americans. In analyses by race/ethnicity, NNAL concentrations were lower in African-Americans and higher in Mexican Americans compared to Whites after adjustment for sociodemographic factors, cigarettes per day and serum cotinine, and cigarette type. Little is known about racial/ethnic differences in NNAL metabolism. Previous studies comparing urinary NNAL, including one study in participants from NHANES 2007- 2008, found no association with menthol cigarette use (17, 18, 57). However, these studies were limited to African-American and White smokers. It will be important to evaluate the consistency of the findings for cigarette type and NNAL among other populations including Hispanic/Latino populations.
In NHANES 1999-2010, 72% of African- American smokers smoked menthol cigarettes. In NHANES 2001- 2008 menthol cigarette use was associated with increased odds of self-reported history of stroke (OR: 2.25, 95% confidence interval: 1.33- 3.78) (58). Other studies of health risks have shown no differences in risks for cardiovascular (59, 60), cancer (59, 61-65) and respiratory outcomes (59, 60) among smokers of menthol and non-menthol cigarettes. Two studies (66, 67), moreover, have found lower lung cancer risk among menthol smokers. However, there are limitations in the design of these studies (e.g., challenges in comparing health risks of two toxic products in which patterns of use could have changed over time). The Tobacco Products Scientific Advisory Committee (TPSAC) review found evidence for a role of menthol in initiation and cessation (13) with menthol cigarette use being associated with less successful smoking cessation and lower quit rates (60, 68-71), which could further contribute to racial disparities.
This study, characterized by the rigorous quality control measures of NHANES, was conducted in a large representative sample of US adult smokers. We were able to examine the relationship of menthol cigarette use with several tobacco related biomarkers, including established tobacco carcinogens, under actual smoking conditions rather than laboratory conditions. Previous studies have been limited to African-American and White smokers; therefore this study is strengthened by the inclusion of Mexican-American smokers. This study has some limitations. The relatively small number of White and Mexican American menthol smokers and of African-American non-menthol cigarette smokers reduces the precision of race/ethnicity specific results. Also, although smoking is a significant source of human exposure to cadmium and lead (28, 29, 37, 72, 73), these compounds are not specific to tobacco smoke and other sources, such as diet and ambient air, are important sources for the general population. However, among smokers, tobacco smoke is the dominant source of cadmium exposure. No information was available regarding whether cigarette type had changed over time. The use of menthol cigarettes, however, has been shown to be relatively constant over time and individuals are unlikely to switch between cigarette types (59, 60, 74). Further research needs to identify whether differences in biomarker concentrations between menthol and non-menthol cigarette use arise from differences in tobacco concentrations or could be related to topography. TPSAC, however, found no compelling evidence on topography and if topography was the main reason we would expect to see elevated levels across all biomarkers (13).
Conclusions
In a representative sample of the US population, higher concentrations of blood cadmium, an established carcinogen and highly toxic metal, were found in smokers of menthol cigarettes compared to smokers of non-menthol cigarettes, although differences were not found for other biomarkers. We also observed increased concentrations of blood lead and urine NNAL among African-American and Mexican-American menthol cigarette smokers, respectively, compared to their non-menthol cigarette smoking counterparts. These results provide additional information regarding possible harms of menthol cigarette use.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by the US National Cancer Institute (R03CA153959). Miranda R Jones was also supported by the Cardiovascular Epidemiology Institutional Training from the National Heart, Lung and Blood Institute (T32HL007024).
Footnotes
Disclosure of Potential Conflicts of Interests: No potential conflicts of interest were disclosed.
REFERENCES
- (1).U.S. Department of Health and Human Services . The health consequences of smoking: a report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2004. [Google Scholar]
- (2).Giovino GA, Sidney S, Gfroerer JC, O’Malley PM, Allen JA, Richter PA, et al. Epidemiology of menthol cigarette use. Nicotine Tob Res. 2004;6(Suppl 1):S67–S81. doi: 10.1080/14622203710001649696. [DOI] [PubMed] [Google Scholar]
- (3).Benowitz NL, Herrera B, Jacob P., III Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004;310(3):1208–15. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- (4).Perez-Stable EJ, Herrera B, Jacob P, III, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152–6. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- (5).Jarvik ME, Tashkin DP, Caskey NH, McCarthy WJ, Rosenblatt MR. Mentholated cigarettes decrease puff volume of smoke and increase carbon monoxide absorption. Physiol Behav. 1994;56(3):563–70. doi: 10.1016/0031-9384(94)90302-6. [DOI] [PubMed] [Google Scholar]
- (6).Ahijevych K, Gillespie J, Demirci M, Jagadeesh J. Menthol and nonmenthol cigarettes and smoke exposure in black and white women. Pharmacol Biochem Behav. 1996;53(2):355–60. doi: 10.1016/0091-3057(95)02034-9. [DOI] [PubMed] [Google Scholar]
- (7).Ahijevych K, Parsley LA. Smoke constituent exposure and stage of change in black and white women cigarette smokers. Addict Behav. 1999;24(1):115–20. doi: 10.1016/s0306-4603(98)00031-8. [DOI] [PubMed] [Google Scholar]
- (8).Caskey NH, Jarvik ME, McCarthy WJ, Rosenblatt MR, Gross TM, Carpenter CL. Rapid smoking of menthol and nonmenthol cigarettes by black and white smokers. Pharmacol Biochem Behav. 1993;46(2):259–63. doi: 10.1016/0091-3057(93)90350-3. [DOI] [PubMed] [Google Scholar]
- (9).McCarthy WJ, Caskey NH, Jarvik ME, Gross TM, Rosenblatt MR, Carpenter C. Menthol vs nonmenthol cigarettes: effects on smoking behavior. Am J Public Health. 1995;85(1):67–72. doi: 10.2105/ajph.85.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Nil R, Battig K. Separate effects of cigarette smoke yield and smoke taste on smoking behavior. Psychopharmacology (Berl) 1989;99(1):54–9. doi: 10.1007/BF00634452. [DOI] [PubMed] [Google Scholar]
- (11).Pickworth WB, Moolchan ET, Berlin I, Murty R. Sensory and physiologic effects of menthol and non-menthol cigarettes with differing nicotine delivery. Pharmacol Biochem Behav. 2002;71(1-2):55–61. doi: 10.1016/s0091-3057(01)00623-2. [DOI] [PubMed] [Google Scholar]
- (12).Lawrence D, Cadman B, Hoffman AC. Sensory properties of menthol and smoking topography. Tob Induc Dis. 2011;9(Suppl 1):S3. doi: 10.1186/1617-9625-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Tobacco Products Scientific Advisory Committee (TPSAC) Menthol cigarettes and public health: review of the scientific evidence and recommendations. Food and Drug Administration; Rockville, MD: 2011. [Google Scholar]
- (14).U.S. Department of Health and Human Services . How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2010. [Google Scholar]
- (15).Smith CJ, Perfetti TA, Garg R, Hansch C. IARC carcinogens reported in cigarette mainstream smoke and their calculated log P values. Food Chem Toxicol. 2003;41(6):807–17. doi: 10.1016/s0278-6915(03)00021-8. [DOI] [PubMed] [Google Scholar]
- (16).Clark PI, Gautam S, Gerson LW. Effect of menthol cigarettes on biochemical markers of smoke exposure among black and white smokers. Chest. 1996;110(5):1194–8. doi: 10.1378/chest.110.5.1194. [DOI] [PubMed] [Google Scholar]
- (17).Heck JD. Smokers of menthol and nonmenthol cigarettes exhibit similar levels of biomarkers of smoke exposure. Cancer Epidemiol Biomarkers Prev. 2009;18(2):622–9. doi: 10.1158/1055-9965.EPI-08-0550. [DOI] [PubMed] [Google Scholar]
- (18).Muscat JE, Chen G, Knipe A, Stellman SD, Lazarus P, Richie JP., Jr. Effects of menthol on tobacco smoke exposure, nicotine dependence, and NNAL glucuronidation. Cancer Epidemiol Biomarkers Prev. 2009;18(1):35–41. doi: 10.1158/1055-9965.EPI-08-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Mustonen TK, Spencer SM, Hoskinson RA, Sachs DP, Garvey AJ. The influence of gender, race, and menthol content on tobacco exposure measures. Nicotine Tob Res. 2005;7(4):581–90. doi: 10.1080/14622200500185199. [DOI] [PubMed] [Google Scholar]
- (20).Signorello LB, Cai Q, Tarone RE, McLaughlin JK, Blot WJ. Racial differences in serum cotinine levels of smokers. Dis Markers. 2009;27(5):187–92. doi: 10.3233/DMA-2009-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Williams JM, Gandhi KK, Steinberg ML, Foulds J, Ziedonis DM, Benowitz NL. Higher nicotine and carbon monoxide levels in menthol cigarette smokers with and without schizophrenia. Nicotine Tob Res. 2007;9(8):873–81. doi: 10.1080/14622200701484995. [DOI] [PubMed] [Google Scholar]
- (22).Hoffman AC. The health effects of menthol cigarettes as compared to non-menthol cigarettes. Tob Induc Dis. 2011;9(Suppl 1):S7. doi: 10.1186/1617-9625-9-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Miller GE, Jarvik ME, Caskey NH, Segerstrom SC, Rosenblatt MR, McCarthy WJ. Cigarette mentholation increases smokers’ exhaled carbon monoxide levels. Experimental and Clinical Psychopharmacology. 1994;2(2):154–60. [Google Scholar]
- (24).Caraballo RS, Holiday DB, Stellman SD, Mowery PD, Giovino GA, Muscat JE, et al. Comparison of serum cotinine concentration within and across smokers of menthol and nonmenthol cigarette brands among non-Hispanic black and non-Hispanic white U.S. adult smokers, 2001-2006. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1329–40. doi: 10.1158/1055-9965.EPI-10-1330. [DOI] [PubMed] [Google Scholar]
- (25).Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- (26).Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;(192):29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Perez-Stable EJ, Benowitz NL, Marin G. Is serum cotinine a better measure of cigarette smoking than self-report? Prev Med. 1995;24(2):171–9. doi: 10.1006/pmed.1995.1031. [DOI] [PubMed] [Google Scholar]
- (28).Galazyn-Sidorczuk M, Brzoska MM, Moniuszko-Jakoniuk J. Estimation of Polish cigarettes contamination with cadmium and lead, and exposure to these metals via smoking. Environ Monit Assess. 2008;137(1-3):481–93. doi: 10.1007/s10661-007-9783-2. [DOI] [PubMed] [Google Scholar]
- (29).Kalcher K, Kern W, Pietsch R. Cadmium and lead in the smoke of a filter cigarette. Sci Total Environ. 1993;128(1):21–35. doi: 10.1016/0048-9697(93)90177-8. [DOI] [PubMed] [Google Scholar]
- (30).Pappas RS, Polzin GM, Zhang L, Watson CH, Paschal DC, Ashley DL. Cadmium, lead, and thallium in mainstream tobacco smoke particulate. Food Chem Toxicol. 2006;44(5):714–23. doi: 10.1016/j.fct.2005.10.004. [DOI] [PubMed] [Google Scholar]
- (31).Church TR, Anderson KE, Caporaso NE, Geisser MS, Le CT, Zhang Y, et al. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol Biomarkers Prev. 2009;18(1):260–6. doi: 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11(6):559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- (33).Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23(6):907–22. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- (34).Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–48. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- (35).Bernert JT, Pirkle JL, Xia Y, Jain RB, Ashley DL, Sampson EJ. Urine concentrations of a tobacco-specific nitrosamine carcinogen in the U.S. population from secondhand smoke exposure. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2969–77. doi: 10.1158/1055-9965.EPI-10-0711. [DOI] [PubMed] [Google Scholar]
- (36).Lee MG, Chun OK, Song WO. Determinants of the blood lead level of US women of reproductive age. J Am Coll Nutr. 2005;24(1):1–9. doi: 10.1080/07315724.2005.10719436. [DOI] [PubMed] [Google Scholar]
- (37).Tellez-Plaza M, Navas-Acien A, Caldwell KL, Menke A, Muntner P, Guallar E. Reduction in cadmium exposure in the United States population, 1988-2008: the contribution of declining smoking rates. Environ Health Perspect. 2012;120(2):204–9. doi: 10.1289/ehp.1104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Jarup L, Rogenfelt A, Elinder CG, Nogawa K, Kjellstrom T. Biological half-time of cadmium in the blood of workers after cessation of exposure. Scand J Work Environ Health. 1983;9(4):327–31. doi: 10.5271/sjweh.2404. [DOI] [PubMed] [Google Scholar]
- (39).Agency for Toxic Substances and Disease Registry . Toxicological profile for lead. Aug, 2007. [PubMed] [Google Scholar]
- (40).Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, Sobczak A, et al. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob Res. 2011;13(3):202–8. doi: 10.1093/ntr/ntq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Apostolou A, Garcia-Esquinas E, Fadrowski JJ, McLain P, Weaver VM, Navas-Acien A. Secondhand tobacco smoke: a source of lead exposure in US children and adolescents. Am J Public Health. 2012;102(4):714–22. doi: 10.2105/AJPH.2011.300161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Lumley T. Analysis of complex survey samples. Journal of Statistical Software. 2004;9(1):1–19. [Google Scholar]
- (43).Lumley T. Survey: analysis of complex survey samples. R package version 3.24. 2011 Nov 10; updated 2011; cited. Available from: URL: http://cran.r-project.org/web/packages/survey/index/html.
- (44).R Development Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2011 Nov 10; updated 2010; cited. Available from: URL: http://www.R-project.org/
- (45).Gutenmann WH, Bache CA, Lisk DJ, Hoffmann D, Adams JD, Elfving DC. Cadmium and nickel in smoke of cigarettes prepared from tobacco cultured on municipal sludge-amended soil. J Toxicol Environ Health. 1982;10(3):423–31. doi: 10.1080/15287398209530264. [DOI] [PubMed] [Google Scholar]
- (46).Smith CJ, Livingston SD, Doolittle DJ. An international literature survey of “IARC Group I carcinogens” reported in mainstream cigarette smoke. Food Chem Toxicol. 1997;35(10-11):1107–30. doi: 10.1016/s0278-6915(97)00063-x. [DOI] [PubMed] [Google Scholar]
- (47).Wertz MS, Kyriss T, Paranjape S, Glantz SA. The toxic effects of cigarette additives. Philip Morris’ project mix reconsidered: an analysis of documents released through litigation. PLoS Med. 2011;8(12):e1001145. doi: 10.1371/journal.pmed.1001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Carmines EL. Evaluation of the potential effects of ingredients added to cigarettes. Part 1: cigarette design, testing approach, and review of results. Food Chem Toxicol. 2002;40(1):77–91. doi: 10.1016/s0278-6915(01)00084-9. [DOI] [PubMed] [Google Scholar]
- (49).International Agency for Research on Cancer (IARC) IARC Monographs on the evaluation of carcinogenic risk to humans. Vol. 58: Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. Cadmium; Lyon: Feb, 1993. [PMC free article] [PubMed] [Google Scholar]
- (50).Gallagher CM, Kovach JS, Meliker JR. Urinary cadmium and osteoporosis in U.S. Women >or= 50 years of age: NHANES 1988-1994 and 1999-2004. Environ Health Perspect. 2008;116(10):1338–43. doi: 10.1289/ehp.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Gallagher CM, Chen JJ, Kovach JS. Environmental cadmium and breast cancer risk. Aging (Albany NY) 2010;2(11):804–14. doi: 10.18632/aging.100226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Gallagher CM, Meliker JR. Blood and urine cadmium, blood pressure, and hypertension: a systematic review and meta-analysis. Environ Health Perspect. 2010;118(12):1676–84. doi: 10.1289/ehp.1002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Peters JL, Perlstein TS, Perry MJ, McNeely E, Weuve J. Cadmium exposure in association with history of stroke and heart failure. Environ Res. 2010;110(2):199–206. doi: 10.1016/j.envres.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, Guallar E. Cadmium Exposure and All Cause and Cardiovascular Mortality in the US General Population. Environ Health Perspect. 2012 doi: 10.1289/ehp.1104352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Hwangbo Y, Weaver VM, Tellez-Plaza M, Guallar E, Lee BK, Navas-Acien A. Blood cadmium and estimated glomerular filtration rate in Korean adults. Environ Health Perspect. 2011;119(12):1800–5. doi: 10.1289/ehp.1003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, et al. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol. 2009;170(9):1156–64. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Xia Y, Bernert JT, Jain RB, Ashley DL, Pirkle JL. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in smokers in the United States: NHANES 2007-2008. Biomarkers. 2011;16(2):112–9. doi: 10.3109/1354750X.2010.533288. [DOI] [PubMed] [Google Scholar]
- (58).Vozoris NT. Mentholated cigarettes and cardiovascular and pulmonary diseases: a population-based study. Arch Intern Med. 2012;172(7):590–1. doi: 10.1001/archinternmed.2012.320. [DOI] [PubMed] [Google Scholar]
- (59).Murray RP, Connett JE, Skeans MA, Tashkin DP. Menthol cigarettes and health risks in Lung Health Study data. Nicotine Tob Res. 2007;9(1):101–7. doi: 10.1080/14622200601078418. [DOI] [PubMed] [Google Scholar]
- (60).Pletcher MJ, Hulley BJ, Houston T, Kiefe CI, Benowitz N, Sidney S. Menthol cigarettes, smoking cessation, atherosclerosis, and pulmonary function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arch Intern Med. 2006;166(17):1915–22. doi: 10.1001/archinte.166.17.1915. [DOI] [PubMed] [Google Scholar]
- (61).Brooks DR, Palmer JR, Strom BL, Rosenberg L. Menthol cigarettes and risk of lung cancer. Am J Epidemiol. 2003;158(7):609–16. doi: 10.1093/aje/kwg182. [DOI] [PubMed] [Google Scholar]
- (62).Carpenter CL, Jarvik ME, Morgenstern H, McCarthy WJ, London SJ. Mentholated cigarette smoking and lung-cancer risk. Ann Epidemiol. 1999;9(2):114–20. doi: 10.1016/s1047-2797(98)00042-8. [DOI] [PubMed] [Google Scholar]
- (63).Hebert JR, Kabat GC. Menthol cigarette smoking and oesophageal cancer. Int J Epidemiol. 1989;18(1):37–44. doi: 10.1093/ije/18.1.37. [DOI] [PubMed] [Google Scholar]
- (64).Kabat GC, Hebert JR. Use of mentholated cigarettes and lung cancer risk. Cancer Res. 1991;51(24):6510–3. [PubMed] [Google Scholar]
- (65).Kabat GC, Hebert JR. Use of mentholated cigarettes and oropharyngeal cancer. Epidemiology. 1994;5(2):183–8. doi: 10.1097/00001648-199403000-00008. [DOI] [PubMed] [Google Scholar]
- (66).Blot WJ, Cohen SS, Aldrich M, McLaughlin JK, Hargreaves MK, Signorello LB. Lung cancer risk among smokers of menthol cigarettes. J Natl Cancer Inst. 2011;103(10):810–6. doi: 10.1093/jnci/djr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Rostron B. Lung Cancer Mortality Risk for U.S. Menthol Cigarette Smokers. Nicotine Tob Res. 2012 doi: 10.1093/ntr/nts014. [DOI] [PubMed] [Google Scholar]
- (68).Gandhi KK, Foulds J, Steinberg MB, Lu SE, Williams JM. Lower quit rates among African American and Latino menthol cigarette smokers at a tobacco treatment clinic. Int J Clin Pract. 2009;63(3):360–7. doi: 10.1111/j.1742-1241.2008.01969.x. [DOI] [PubMed] [Google Scholar]
- (69).Hyland A, Garten S, Giovino GA, Cummings KM. Mentholated cigarettes and smoking cessation: findings from COMMIT. Community Intervention Trial for Smoking Cessation. Tob Control. 2002;11(2):135–9. doi: 10.1136/tc.11.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Okuyemi KS, Ahluwalia JS, Ebersole-Robinson M, Catley D, Mayo MS, Resnicow K. Does menthol attenuate the effect of bupropion among African American smokers? Addiction. 2003;98(10):1387–93. doi: 10.1046/j.1360-0443.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- (71).Okuyemi KS, Ebersole-Robinson M, Nazir N, Ahluwalia JS. African-American menthol and nonmenthol smokers: differences in smoking and cessation experiences. J Natl Med Assoc. 2004;96(9):1208–11. [PMC free article] [PubMed] [Google Scholar]
- (72).Nordberg GF, Noqawa K, Nordberg M, Friberg L. Cadmium. In: Elsevier, editor. Handbook on the toxicology of metals. Amsterdam: 2007. pp. 445–86. [Google Scholar]
- (73).Richter PA, Bishop EE, Wang J, Swahn MH. Tobacco smoke exposure and levels of urinary metals in the U.S. youth and adult population: the National Health and Nutrition Examination Survey (NHANES) 1999-2004. Int J Environ Res Public Health. 2009;6(7):1930–46. doi: 10.3390/ijerph6071930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Roper Organization A study of smokers’ habits and attitudes with special emphasis on low tar and menthol cigarettes volume I. Research, March 1979. 2011 Nov 11; updated 2009; cited. Available from: URL: http://legacy.library.ucsf.edu/tid/zvt46b00.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.