Abstract
Aim
Periodontal diseases are associated with a variety of systemic diseases, including cardiovascular disease and stroke, and patients with periodontitis demonstrate elevated levels of anticardiolipin antibodies. We sought to determine if anticardiolipin antibodies from periodontitis patients induced monocyte chemotactic protein-1 production by human vascular endothelial cells.
Materials and Methods
IgG was purified from sera from 53 subjects, including chronic and aggressive periodontitis patients and periodontally healthy controls, with elevated or normal IgG anticardiolipin levels. In addition, anticardiolipin antibodies were specifically removed from some sera by immunoabsorption.
Results
We found that, irrespective of diagnostic category, IgG from subjects with elevated anticardiolipin induced significantly greater monocyte chemotactic protein-1 production by human vascular endothelial cells than IgG from those subjects with normal anticardiolipin titers. Removal of anticardiolipin from IgG preparations from periodontitis patients significantly reduced their ability to induce monocyte chemotactic protein-1.
Conclusions
Since elevated titers of anticardiolipin are found in a significantly greater proportion of patients with periodontitis than in periodontally healthy individuals, and these antibodies activate endothelial cells to produce monocyte chemotactic protein-1, they may explain some of the associations noted between periodontal infections and systemic conditions.
Keywords: anticardiolipin, periodontal disease, endothelial cells, antibodies, monocyte chemotactic protein-1
Introduction
Anticardiolipin antibodies are most commonly found in patients with autoimmune diseases such as systemic lupus erythematosis (SLE) or the antiphospholipid syndrome (APS) (Tripodi et al., 2011). Such patients are known to have increased incidence of thrombotic events as well as adverse pregnancy outcome (fetal involution) and are also thought to demonstrate early atherosclerosis (Kobayashi et al., 2005, Matsuura and Lopez, 2008). It is hypothesized that some of these manifestations of disease are in part a consequence of the interaction of autoantibodies, including β2-glycoprotein 1-dependent anticardiolipin antibodies (aCl), with the endothelium, leading to vascular inflammation and thrombosis. For example, aCl can interact directly with endothelial cells to upregulate adhesion molecules and induce production of cytokines such as MCP-1 and molecules such as tissue factor (Cho et al., 2002), and can behave as opsonins by interacting with modified low-density lipoproteins to enhance uptake of cholesterol into macrophages (Matsuura and Lopez, 2008). Additionally, it has been demonstrated that a variety of microbial pathogens can induce the production of cross-reactive aCl-like antibodies that can induce antiphospholipid syndrome-like symptoms in animal models (Blank et al., 2002a), leading to the hypothesis that some infectious diseases can promote APS-like symptoms, including accelerated atherosclerotic cardiovascular disease, in the absence of autoimmune disease (Artenjak et al., 2012).
There is substantial evidence that periodontitis contributes to the magnitude of systemic inflammation, and it has been hypothesized that this may constitute a mechanism whereby periodontal diseases could contribute to the development or progression of a variety of systemic conditions (Teles and Wang, 2011). Many studies demonstrate associations of periodontitis with cardiovascular disease (CVD), though data unequivocally demonstrating a cause and effect relationship between these conditions are lacking (Lockhart et al., 2012). Upregulation of the endothelium promoting egress of inflammatory cells from the bloodstream and into atheromatous lesions is a fundamental pathologic characteristic of CVD (Libby et al., 2009). Thus, assessment of endothelial function has been one approach that investigators have used to study associations of periodontitis with CVD and to assess the impact of periodontal therapy on CVD measures (Amar et al., 2003, Higashi et al., 2008, Pischon et al., 2007, Tonetti et al., 2007). These indirect approaches to assessment of CVD risk indicate that there is endothelial dysfunction associated with periodontitis that may be improved by therapy aimed at decreasing periodontal inflammation.
Our previous studies have indicated that a significant proportion of individuals with periodontitis demonstrate elevated serum concentrations of aCl (Schenkein et al., 2003). It has recently been demonstrated that oral bacterial pathogens may be cross-reactive with β2GP1, the target antigen of aCl (Wang et al., 2008) and that periodontal therapy can decrease systemic antibody levels of aCl (Gunupati et al., 2011). Our observation that periodontitis patients’ sera can contain elevated concentrations of aCl (Schenkein et al., 2003), and studies indicating that aCl can be associated with cardiovascular diseases even in patients without autoimmune disease (Artenjak et al., 2012) raise the question of whether the aCl found in periodontitis has pathogenic properties. The intent of this study was to evaluate the potential pathogenicity of aCl found in periodontitis patients by determining their ability to induce production of the key cytokine MCP-1 by endothelial cells.
Methods
Clinical Methods and Serum Samples
This study was approved by the Internal Review Board of Virginia Commonwealth University. Serum samples used for this study were from subjects described in previous publications (Schenkein et al., 2003, Schenkein et al., 2007). The subjects were identified at Virginia Commonwealth University School of Dentistry and included patients with generalized aggressive and chronic periodontitis as well as subjects without periodontitis. All participants were determined to be systemically healthy by medical history. Each participant received a comprehensive periodontal evaluation that included determination of pocket depth (PD), attachment loss (AL), plaque index (PI), (Silness and Löe, 1964) and gingival index (GI)(Löe and Silness, 1963) at 6 sites per tooth. At the time of examination a blood sample was taken and processed for serum which was stored at −70°C. Serum samples chosen for inclusion in this study were selected from those previously assayed for aCl content so as to provide sufficient numbers of sera within each diagnostic category to test hypotheses regarding the impact of diagnosis on aCl function. Sera testing positive for aCl from periodontally healthy subjects was very limited due to the low prevalence of positive tests within this group (Schenkein et al., 2003).
The diagnostic criteria were as follows. Subjects with normal periodontium (NP) included those of any age with no evidence of AL or pockets greater than 3 mm, other than in buccal or lingual areas of gingival recession. Subjects with Chronic Periodontitis (CP) were of age >25 years with AL 2 mm or greater on more than one tooth in any extent or severity pattern that is consistent with plaque level and age. The definition of Generalized Aggressive Periodontitis (GAgP) for the subjects in this report conform to current diagnostic guidelines for these diseases (Armitage, 1999) with the additional requirement that such patients had a history of disease onset prior to age 35. They presented with at least 8 teeth with 5 mm or more attachment loss at interproximal sites, at least 3 of the affected teeth were not first molars and incisors. The total initial sample number included 23 from subjects with CP, 15 samples from subjects with GAgP, and 21 NP subjects.
Purification of IgG and Depletion of aCl from IgG preparations
Purification of IgG from patients samples was accomplished using two methods, which proved to be equivalent with respect to IgG yield and purity. First, after samples were adjusted to 1M NaCl and the pH was adjusted to 7.8 by addition of .5M Na2HPO4, one mL of each sample was applied to a one mL column of Protein G Sepharose that was equilibrated in 1M NaCl, .05M PO4, pH 7.8. Elution was accomplished by addition of .1M Glycine .2M NaCl, pH 2.00, and eluted samples were immediately neutralized by addition of 1M NaOH. Subsequently, samples were dialyzed against PBS. In the second method, samples were dialyzed against .005M PO4, pH 7.8. and one mL of each sample was applied to a one mL column of DEAE Sephacel, equilibrated in .005M PO4, pH 7.8. Pass through fractions were collected and the column was washed until A280 was less than .02.
Following both isolation procedures fractions were assayed for anti CL using the Varelisa Cardiolipin IgG kit. (Phadia, Thermo Fisher Scientific, US). Positive fractions were pooled and concentrated, where necessary, using Amicon Ultra centrifugal filters (Millipore, Billerica, MA), then reassayed for anti CL. Total IgG was determined by ELISA using Nunc-Immuno Plate MaxiSorp Surface (NUNC, Thermo Fisher Scientific, US) coated with affinity purified goat anti human IgG (Jackson Immuno Research, West Grove, PA) diluted to 2 µg /mL in .005M PO4 pH 7.8. Plates were washed, and samples and standards were added. Following incubation plates were again washed and F(ab)’2 HRPO-tagged affinity purified goat anti-human IgG (Jackson Immuno Research) was added. Following further incubation, plates were again washed and developed using chromagen TMB. All incubations were for 30 minutes at room temperature.
Generation of an aCl immunoabsorbant was accomplished using the method of Pengo and Biasiolo (Pengo and Biasiolo, 1993). The aCl immunoabsorbant was placed in wells of MultiScreen-HTS Filter Plates (Millipore, Billerica, MA). Patient IgG samples were added to the wells and then were incubated with shaking for 60 minutes at room temperature. Filter plates were then mounted onto sterile tissue culture plates and centrifuged (500 × g) for 10 minutes. The resulting supernatants were assayed for IgG aCl and total IgG.
Following purification of the IgG samples it was noted that 6 samples, 3 each from the NP and CP group, contained contaminating LPS determined as described by Cho et al (Cho et al., 2002). Thus, the final sample number included 53 subjects (18 NP, 20 CP, and 15 GAgP).
Culturing of HUVEC cells
HUVEC cells (American Type Culture Collection, ATCC, Manassas, VA) at 1E6 in Vascular Cell Basal Medium (ATCC) supplemented with Endothelial Cell Growth Kit – VEGF (ATCC), were incubated at 37°C in 5% CO2 in Biocoat Gelatin Cellware (Becton Dickinson, Franklin Lakes, NJ) in 75 cm2 vented flasks and allowed to grow until confluent. Media were changed twice a week. When cells were confluent, plates were washed with Basal Medium to remove protein and replaced with Trypsin-EDTA 1X (Mediatech, Inc., Manassas, VA). Plates were incubated at 37°C for 10 minutes, and eluted cells were then washed in Basal Medium – VEGF, resuspended to 40 ml, and 200 ul of the suspension was added to each well on Biocoat 96 well plates (Becton Dickinson, Franklin Lakes, NJ). Plates were incubated at 37°C, 5% CO2 until HUVECs were confluent.
Experiments were carried out using a single HUVEC cell line utilizing second or third passage cells. 20 ug of IgG were added from each sample per well and incubated at 37°C in 5% CO2 for 18 h (Cho et al., 2002)Supernatants were removed and assayed for MCP-1 using a Quantikine MCP-1 ELISA.(R & D Systems, Minneapolis, MN). Triplicate cultures were established for each condition and mean results from the 3 cultures were determined.
Statistical methods
Comparisons of demographic characteristics and periodontal measures between groups and comparison of MCP-1 levels between subjects with elevated and normal aCl concentrations were accomplished by analysis of variance (ANOVA). Pre- and post-absorption levels of MCP-1 stimulation was analyzed by repeated measures ANOVA. Associations of IgG aCl and MCP-1 production were analyzed by linear regression analysis, and the impact of demographic factors was determined by multiple regression analysis.
Results
Sera from 59 subjects were chosen for analysis based upon periodontal diagnosis and previously measured concentrations of IgG aCl. Six IgG samples were found to contain contaminating LPS following purification and were not further analyzed. Table 1 illustrates the demographic and periodontal characteristics of the 53 subjects. 18 periodontally healthy subjects (NP), 20 CP subjects, and 15 GAgP subjects were studied. CP subjects were older than the NP and GAgP subjects. The composition of the groups by both race and sex were not significantly different. Periodontal measures differed significantly between the groups for PD and ALOSS, and NP subjects had more teeth than the AP subjects.
Table 1.
Demographic and clinical characteristics of the subject population
| NP1 (n=18) | CP 2 (n=20) | GAgP 3 (n=15) | p-value | |
|---|---|---|---|---|
| Age (SEM) | 38.2 (2.4) | 47.4 (2.3) | 32.0 (2.7) | P=.0002 |
| Race: % A, B, W 4 | 0, 22.2, 77.8 | 0, 35.0, 65.0 | 6.7, 53.3, 62.3 | P=.15 |
| Sex: %Female | 72.2 | 55.0 | 46.7 | P=.30 |
| Mean PD: mm (SEM) 5 | 2.00 (0.17) | 2.62 (0.16) | 3.90 (0.19) | P=.0001 |
| Mean ALOSS (mm) 5 | 0.37 (0.41) | 2.29 (0.38) | 3.70 (0.44) | P=.0001 |
| N teeth | 27.4 (1.41) | 22.9 (1.34) | 24.0 (1.55) | P=.07 |
NP: Normal Periodontium
CP: Chronic Periodontitis
GAgP: Generalized Aggressive Periodontitis
A, B, W: Asian, Black, White
PD: pocket depth; ALOSS: attachment loss
IgG was purified from subjects’ sera and added to cultures of HUVEC for assessment of stimulation of MCP-1 production. Figure 1 demonstrates that there is a significant association between the IgG aCl serum titer and MCP-1 production by HUVEC in response to IgG purified from those samples (p<.0001, r2=.48). We further determined the association between clinically elevated levels of aCl and stimulation of MCP-1 by comparing preparations from samples with >15 Units/ml to those with < 15 Units/ml. As seen in Figure 2, MCP-1 produced by HUVEC was significantly greater for IgG from sera containing elevated aCl titers than for those with normal aCl titers (420.7 pg/ml versus 152.1 pg/ml, p<.0001). We further noted that there was no influence of the demographic characteristics of the serum donors (age, race, sex, or smoking history) on the relationships between IgG aCl titer and MCP-1 production.
Figure 1.
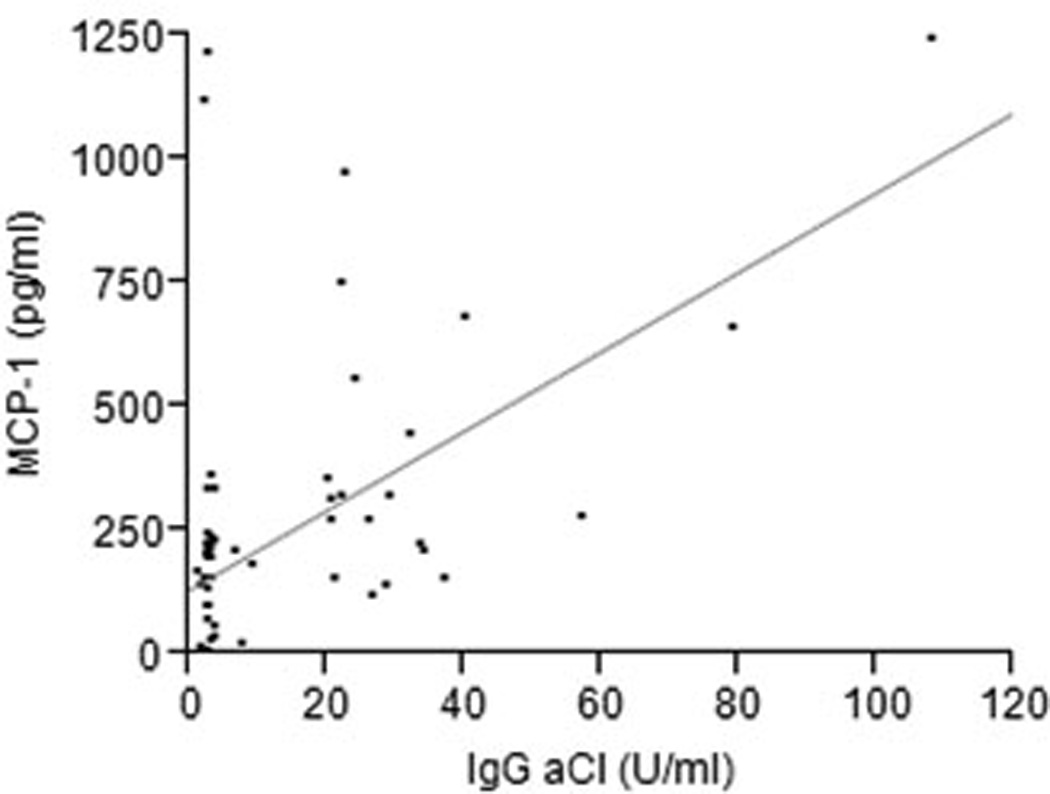
MCP-1 production by HUVEC following stimulation by IgG from systemically healthy subjects with or without periodontitis. 20 ug of IgG were added from each sample per well and incubated at 37°C in 5% CO2 for 24h. Experiments were carried out in triplicate and the mean concentration of MCP-1 in culture supernatants was determined. There was a significant association between the IgG aCl serum titer and MCP-1 production for all samples (p<.0001, r2=.48).
Figure 2.
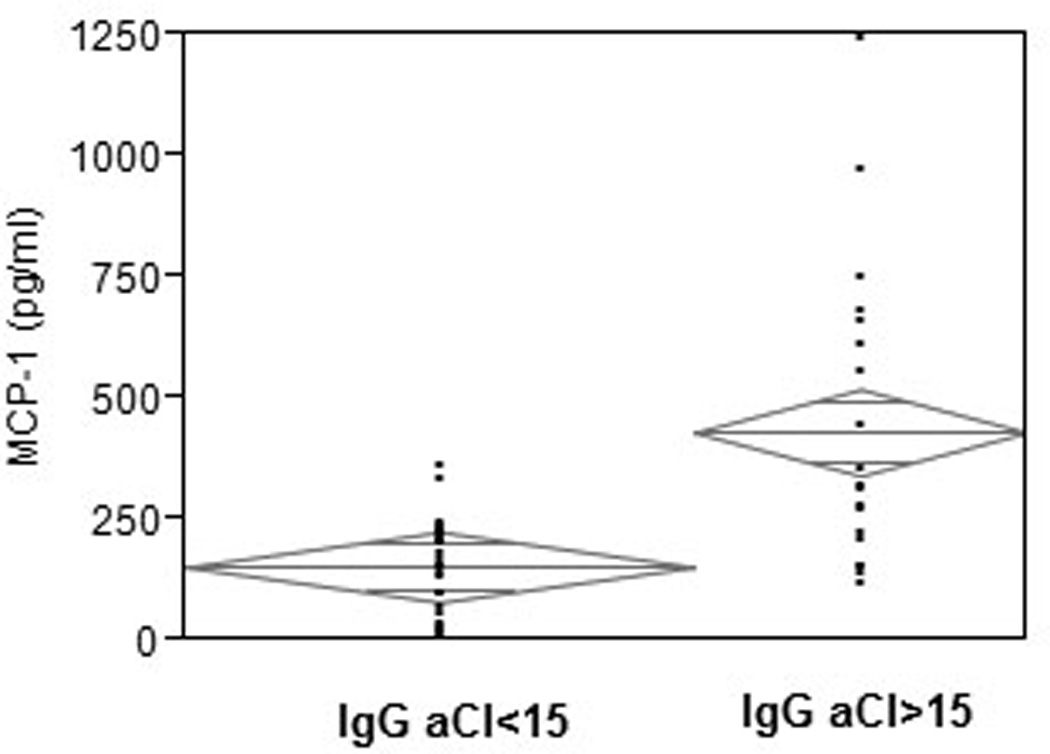
MCP-1 production by HUVEC following stimulation with IgG from subjects with elevated (IgG aCl >15 U/ml) or normal ((IgG aCl >15 U/ml) aCl titers. 20 ug of IgG were added from each sample per well and incubated at 37°C in 5% CO2 for 24h. Experiments were carried out in triplicate and the mean concentration of MCP-1 in culture supernatants was determined. MCP-1 produced by HUVEC was significantly greater for IgG from sera containing elevated aCl titers than for those with normal aCl titers (420.7 ug/ml versus 152.1 ug/ml, p<.0001). Diamonds illustrate mean values and confidence intervals.
We then assessed MCP-1 production by HUVEC within each diagnostic category for patients with normal and elevated IgG aCl. As shown in Table 2, for each diagnostic group, including the NP subjects, IgG from subjects with elevated aCl levels stimulated greater levels of MCP-1 than IgG from subjects with normal IgG aCl titers.
Table 2.
Stimulation of HUVEC production of MCP-1 by IgG from periodontal diagnostic groups according to IgG aCl serum levels
| Diagnosis1 | IgG aCl concentration |
N | MCP-1 (pg/ml) (SEM) |
p-value |
|---|---|---|---|---|
| NP | < 15 U/ml | 14 | 168.40 (39.6) | P=0.0167 |
| >15 U/ml | 4 | 393.26 (74.2) | ||
| CP | < 15 U/ml | 10 | 101.37 (89.2) | P=.0079 |
| >15 U/ml | 10 | 478.58 (89.2) | ||
| GAgP | < 15 U/ml | 8 | 187.16 (48.6) | P=.0175 |
| >15 U/ml | 7 | 393.26 (74.2) |
NP = Normal Periodontium; CP = Chronic Periodontitis; GAgP = Generalized Aggressive Periodontitis
Finally, to confirm that MCP-1 stimulation was due to IgG aCl content, IgG preparations from 4 CP subjects with varying aCl IgG titers were immunoabsorbed on a Sephadex column containing β2GP1 bound to cardiolipin. Table 3 illustrates the results of these studies. Removal of antibodies reactive with cardiolipin/β2GP1 resulted in significant depletion of IgG aCL content of the samples. Consequent stimulation of HUVEC cells with pre-absorption and post-absorption IgG preparations resulted in a significant decrease of MCP-1 production in the presence of the absorbed samples.
Table 3.
Effect of removal of aCl from IgG preparations on stimulation of MCP-1 production by HUVEC
| IgG aCl (U/mg IgG) | MCP-1 (pg/ml)±SEM | |||
|---|---|---|---|---|
| Pre-absorption | Post-absorption | Pre-absorption | Post-absorption | |
| 1.00 | 0.31 | 520.86 ± 54.55 | 84.76 ± 20.72 | P=.004 |
| 3.72 | 0.35 | 749.88 ± 94.76 | 93.07 ± 20.67 | P=.002 |
| 22.00 | 13.90 | 1384.39 ± 142.92 | 717.00 ± 167.89 | P=.03 |
| 3.12 | 0.41 | 766.50 ± 108.66 | 202.29 ± 5.49 | P=.007 |
Discussion
Our previous data indicated that aCl serum concentrations are elevated in a higher proportion of patients with either CP or GAgP than in NP subjects (Schenkein et al., 2003). In addition, our data have shown that elevation of aCl, especially in GAgP patients, is associated with elevated levels of soluble adhesion molecules indicative of endothelial cell activation and elevated C-reactive protein (Schenkein et al., 2007). Since these previous studies excluded, by history, patients with known autoimmune diseases or infections known to stimulate systemic production of aCl, these results raised the question of whether the antibodies detected in the immunoassay for aCl in periodontal disease patients had characteristic biological functions of genuine autoimmune antibodies as might be found in SLE or APS, or if they were simply non-functional cross-reactive antibodies. To begin to answer this question, we chose sera from subjects with normal and elevated IgG aCl and assessed the ability of IgG purified from these sera to stimulate endothelial cells to produce MCP-1. The results indicated a clear association of IgG from sera containing elevated titers of aCl with stimulation of endothelial cells to produce MCP-1. This association is found within the two periodontitis subject groups as well as within the periodontally healthy subjects. However, it should be noted that the prevalence of elevated aCl in the NP subject group is nearly identical to that found in the general population (approximately 5%), while the prevalence of elevated aCl in periodontitis subjects is significantly higher (15–20%) (Schenkein et al., 2003). Thus, periodontitis patients appear to be more prone to develop these antibodies and the antibodies likely have pathogenic properties.
Several studies have shown that patients with SLE, especially those demonstrating elevated antiphospholipid antibodies, are prone to accelerated atherosclerosis, and is has been hypothesized that the interaction of aCl and with the endothelium may play a critical mechanistic role explaining this association (Narshi et al., 2011). IgG from SLE and APS patients with elevated aCl titers have been shown to increased monocyte adhesion to endothelial cells (Simantov et al., 1995), to upregulate endothelial cell adhesion molecules (Gharavi et al., 2002, Pierangeli and Harris, 1994, Pierangeli et al., 1995), and to stimulate MCP-1 production (Cho et al., 2002). It is noteworthy that the association of antiphospholipids with accelerated atherosclerosis is a controversial concept and that several studies have failed to demonstrate this association (Artenjak et al., 2012).
There are significant differences between the clinical manifestations of APS and the systemic clinical conditions associated with periodontal infections. Patients with APS are prone to develop arterial and venous thrombi and women with APS frequently experience recurrent spontaneous abortion (Tripodi et al., 2011). In addition, there is some evidence for early atherosclerosis in these patients. Such patients usually have serum aCl levels in the very high range. On the other hand, periodontal infections are associated with preterm labor, low birth weight, atherosclerosis, endothelial dysfunction, and myocardial infarction, though cause and effect relationships are yet to be proven (Lockhart et al., 2012). The concentrations of aCl in sera from these patients, though clinically elevated (i.e. greater than levels found in 95 % of the general population), are largely in the moderate range. Thus, there are similarities between the sequellae of APS and conditions with increased risk in periodontitis patients but the severity of the associated clinical manifestations are quite different, as are the serum concentrations of these antibodies. Furthermore, APS appears to involve additional functional thrombotic defects and is frequently characterized by the appearance of additional autoantibodies that contribute to the onset of prothrombotic events. Nevertheless, it is difficult to ignore the similarities between these conditions, especially in view of data indicating that the antibodies found in periodontitis patients appear to have some proinflammatory effects on endothelial cells. The results are consistent with an interpretation that aCl in periodontitis patients could contribute to systemic disease in a manner consistent with the concentrations of antibodies found in these patients in comparison to those with true autoimmune disease.
We consider it very likely that aCl antibodies in periodontitis patients can be induced by oral microbial pathogens, and have preliminarily observed in our laboratory that P. gingivalis, a pathogen in periodontitis, can induce production of antibodies reactive with cardiolipin/β2GP1 complexes in both mice and rabbits (unpublished observations, 2012). It is known that aCl and APS-like symptoms can be induced by a variety of microbial pathogens (Avcin et al., 2008, Blank et al., 2002b, Garcia-Carrasco et al., 2009). This can occur by molecular mimicry due to the presence of microbial peptide sequences homologous to the target antigenic peptide for pathogenic aCl present on β2GP1 (Blank et al., 2002b). It has in fact been demonstrated that some periodontal microbial pathogens, including P. gingivalis, A. actinomycetemcomitans, and T. denticola have peptide sequences with sufficient homology to a key hexapeptide in β2GP1 to induce mutually cross-reactive antibodies with the potential to be pathogenic (Wang et al., 2008). There are additional studies showing that infections may trigger APS in other rheumatic diseases, suggesting a microbial origin for both pathogenic and non-pathogenic aCl (Amital et al., 2008). In periodontitis, this type of antibacterial immune response could be in part responsible for associations of periodontal infections with adverse cardiovascular and pregnancy outcomes in some patients, or they merely may be markers of events that co-occur with these conditions. We consider it unlikely that aCl in periodontitis patients are true autoantibodies in that the presence of autoimmune disease was an exclusion criterion for this study.
In summary, IgG from patients with periodontitis who also have elevated serum concentrations of aCl stimulates increased production of the key cytokine MCP-1 from HUVEC cultures. Since periodontitis patients with no evidence of systemic disease demonstrate a significantly higher prevalence of elevated serum levels of aCl compared to periodontally healthy subjects, and since these potentially pathogenic antibodies may be induced by a variety of periodontal pathogens, it is proposed that molecular mimicry with the production of aCl could be one pathogenic link between periodontal infection and systemic disease.
Clinical Relevance.
Scientific Rationale for the Study
Anticardiolipin antibodies, frequently found in periodontitis patients, are of unknown pathogenicity but can be associated with thrombosis, stroke, myocardial infarction, and adverse pregnancy outcomes.
Principal findings
We found that anticardiolipin from periodontitis patients stimulate endothelial cells to produce a cytokine, MCP-1, that is central to the development of atherosclerotic lesions.
Practical implications
The data indicate that it is possible that these antibodies are pathogenic and could explain some of the associations between periodontitis and systemic diseases. Tests for anticardiolipin are routine clinical assays, and could be incorporated into a risk profile for these patients.
Acknowledgements
The authors gratefully acknowledge Kimberly Hollaway for her expert clinical management of the subjects participating in this study.
Source of Funding Statement
This project was supported in part by grant RO1DE018125 from the National Institute of Dental and Craniofacial Research.
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest related to this paper.
References
- Amar S, Gokce N, Morgan S, Loukideli M, Van Dyke TE, Vita JA. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:1245–1249. doi: 10.1161/01.ATV.0000078603.90302.4A. [DOI] [PubMed] [Google Scholar]
- Amital H, Govoni M, Maya R, Meroni PL, Ori B, Shoenfeld Y, Tincani A, Trotta F, Sarzi-Puttini P, Atzeni F. Role of infectious agents in systemic rheumatic diseases. Clin Exp Rheumatol. 2008;26:S27–S32. [PubMed] [Google Scholar]
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- Artenjak A, Lakota K, Frank M, Cucnik S, Rozman B, Bozic B, Shoenfeld Y, Sodin-Semrl S. Antiphospholipid antibodies as non-traditional risk factors in atherosclerosis based cardiovascular diseases without overt autoimmunity. A critical updated review. Autoimmunity reviews. 2012 doi: 10.1016/j.autrev.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Avcin T, Canova M, Guilpain P, Guillevin L, Kallenberg CG, Tincani A, Tonon M, Zampieri S, Doria A. Infections, connective tissue diseases and vasculitis. Clin Exp Rheumatol. 2008;26:S18–S26. [PubMed] [Google Scholar]
- Blank M, Krause I, Fridkin M, Keller N, Kopolovic J, Goldberg I, Tobar A, Shoenfeld Y. Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. The Journal of clinical investigation. 2002a;109:797–804. doi: 10.1172/JCI12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M, Krause I, Fridkin M, Keller N, Kopolovic J, Goldberg I, Tobar A, Shoenfeld Y. Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. J Clin Invest. 2002b;109:797–804. doi: 10.1172/JCI12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CS, Cho ML, Chen PP, Min SY, Hwang SY, Park KS, Kim WU, Min DJ, Min JK, Park SH, Kim HY. Antiphospholipid antibodies induce monocyte chemoattractant protein-1 in endothelial cells. Journal of immunology. 2002;168:4209–4215. doi: 10.4049/jimmunol.168.8.4209. [DOI] [PubMed] [Google Scholar]
- Garcia-Carrasco M, Galarza-Maldonado C, Mendoza-Pinto C, Escarcega RO, Cervera R. Infections and the antiphospholipid syndrome. Clin Rev Allergy Immunol. 2009;36:104–108. doi: 10.1007/s12016-008-8103-0. [DOI] [PubMed] [Google Scholar]
- Gharavi AE, Pierangeli SS, Espinola RG, Liu X, Colden-Stanfield M, Harris EN. Antiphospholipid antibodies induced in mice by immunization with a cytomegalovirus-derived peptide cause thrombosis and activation of endothelial cells in vivo. Arthritis Rheum. 2002;46:545–552. doi: 10.1002/art.10130. [DOI] [PubMed] [Google Scholar]
- Gunupati S, Chava VK, Krishna BP. Effect of phase I periodontal therapy on anti-cardiolipin antibodies in patients with acute myocardial infarction associated with chronic periodontitis. Journal of periodontology. 2011;82:1657–1664. doi: 10.1902/jop.2011.110002. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Goto C, Jitsuiki D, Umemura T, Nishioka K, Hidaka T, Takemoto H, Nakamura S, Soga J, Chayama K, Yoshizumi M, Taguchi A. Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension. 2008;51:446–453. doi: 10.1161/HYPERTENSIONAHA.107.101535. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Lopez LR, Shoenfeld Y, Matsuura E. The role of innate and adaptive immunity to oxidized low-density lipoprotein in the development of atherosclerosis. Annals of the New York Academy of Sciences. 2005;1051:442–454. doi: 10.1196/annals.1361.086. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. Journal of the American College of Cardiology. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, Taubert KA, Newburger JW, Gornik HL, Gewitz MH, Wilson WR, Smith SC, Jr, Baddour LM. Periodontal Disease and Atherosclerotic Vascular Disease: Does the Evidence Support an Independent Association?: A Scientific Statement From the American Heart Association. Circulation. 2012 doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- Löe H, Silness J. Periodontal disease in preqnancy. I. Prevalence and severity. Acta Odont. Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Matsuura E, Lopez LR. Autoimmune-mediated atherothrombosis. Lupus. 2008;17:878–887. doi: 10.1177/0961203308093553. [DOI] [PubMed] [Google Scholar]
- Narshi CB, Giles IP, Rahman A. The endothelium: an interface between autoimmunity and atherosclerosis in systemic lupus erythematosus? Lupus. 2011;20:5–13. doi: 10.1177/0961203310382429. [DOI] [PubMed] [Google Scholar]
- Pengo V, Biasiolo A. Purification of anticardiolipin and lupus anticoagulant activities by using cardiolipin immobilized on agarose beads. Thrombosis research. 1993;72:423–430. doi: 10.1016/0049-3848(93)90242-g. [DOI] [PubMed] [Google Scholar]
- Pierangeli SS, Harris EN. Antiphospholipid antibodies in an in vivo thrombosis model in mice. Lupus. 1994;3:247–251. doi: 10.1177/096120339400300408. [DOI] [PubMed] [Google Scholar]
- Pierangeli SS, Liu XW, Barker JH, Anderson G, Harris EN. Induction of thrombosis in a mouse model by IgG, IgM and IgA immunoglobulins from patients with the antiphospholipid syndrome. Thrombosis and haemostasis. 1995;74:1361–1367. [PubMed] [Google Scholar]
- Pischon N, Hagewald S, Kunze M, Heng N, Christan C, Kleber BM, Muller C, Bernimoulin JP. Influence of periodontal therapy on the regulation of soluble cell adhesion molecule expression in aggressive periodontitis patients. Journal of periodontology. 2007;78:683–690. doi: 10.1902/jop.2007.060286. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Berry CR, Burmeister JA, Brooks CN, Barbour SE, Best AM, Tew JG. Anti-cardiolipin antibodies in sera from patients with periodontitis. Journal of dental research. 2003;82:919–922. doi: 10.1177/154405910308201114. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Best AM, Brooks CN, Burmeister JA, Arrowood JA, Kontos MC, Tew JG. Anti-cardiolipin and increased serum adhesion molecule levels in patients with aggressive periodontitis. Journal of periodontology. 2007;78:459–466. doi: 10.1902/jop.2007.060305. [DOI] [PubMed] [Google Scholar]
- Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odont. Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- Simantov R, LaSala JM, Lo SK, Gharavi AE, Sammaritano LR, Salmon JE, Silverstein RL. Activation of cultured vascular endothelial cells by antiphospholipid antibodies. The Journal of clinical investigation. 1995;96:2211–2219. doi: 10.1172/JCI118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles R, Wang CY. Mechanisms involved in the association between periodontal diseases and cardiovascular disease. Oral diseases. 2011;17:450–461. doi: 10.1111/j.1601-0825.2010.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. The New England journal of medicine. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- Tripodi A, de Groot PG, Pengo V. Antiphospholipid syndrome: laboratory detection, mechanisms of action and treatment. Journal of internal medicine. 2011;270:110–122. doi: 10.1111/j.1365-2796.2011.02362.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Nagasawa T, Chen Y, Ushida Y, Kobayashi H, Takeuchi Y, Umeda M, Izumi Y. Molecular mimicry of Aggregatibacter actinomycetemcomitans with beta2 glycoprotein I. Oral microbiology and immunology. 2008;23:401–405. doi: 10.1111/j.1399-302X.2008.00442.x. [DOI] [PubMed] [Google Scholar]


