Abstract
The efficacy of influenza vaccination in patients treated with rituximab is a clinically important question. Rheumatology clinics are populated with patients receiving rituximab for a broad array of disorders. Although several studies have explored the efficacy of other vaccines in rituximab-treated populations, results have been conflicting. We wished to define influenza vaccine efficacy in a rituximab-treated cohort. We examined 17 evaluable subjects treated with rituximab for rheumatologic conditions. T cell subsets, B cells subsets, T cell function, and B cell function were evaluated at specific time points along with hemagglutinination inhibition titers after receiving the standard inactivated influenza vaccine. T cell subset counts were significantly different than controls but did not change with rituximab. B cells depleted in all patients but were in various stages of recovery at the time of vaccination. Influenza vaccine responsiveness was poor overall, with only 16% of subjects having a four-fold increase in titer. Pre-existing titers were retained throughout the study, however. The ability to respond to the influenza vaccine appeared to be related to the degree of B cell recovery at the time of vaccination. This study emphasizes that antibody responses to vaccine are impaired in subjects treated with rituximab and supports the concept that B cell recovery influences influenza vaccine responsiveness.
Keywords: Rituxmab, influenza, antibody, HAI titer, B cell
Introduction
In the United States, influenza infections cause an excess of 225,000 hospitalizations a year and 36,000 deaths per year [1,2]. Mortality and hospitalization are not evenly distributed across all ages. Infants and the elderly are two recognized high risk populations while chronic illness and pregnancy represent additional high risk populations [3-12]. As a public health measure, influenza vaccination is widely recommended for patients with chronic illness and for those who are immunologically vulnerable [13]. The vaccine changes yearly to match the prevailing circulating strains and induces a protective antibody response. The critical antibodies are thought to be directed to the hemagglutinin molecule and to interfere with viral entry [14-16]. Seroprotection is usually defined as a hemagglutination inhibition (HAI) titer of ≥1:40. This is based on data of young healthy vaccine recipients and it is clear that a titer of 1:40 is not similarly protective in the elderly and other immune compromised populations [17-24]. Without larger studies to define HAI titers providing clinical protection in specific populations, most studies continue to use 1:40 as the threshold for seroprotection. The goal of vaccination is to provide clinical protection and most studies utilize antibody responses as the relevant surrogate marker. Nevertheless, T cell responses occur and have been demonstrated to offer modest protection in murine models.
In patients with chronic autoimmune diseases, influenza and other infections represent a major source of morbidity and mortality [25-30]. Both the disease itself as well as immunosuppressive therapies can contribute to increased morbidity associated with infection. One of the cornerstones of prevention is influenza vaccination in immune compromised individuals. In general, studies of influenza vaccination in cohorts of patients with autoimmune diseases have suggested reduced but detectable vaccine responses [31-35]. Both medications and the autoimmune disease itself can contribute to globally poor vaccine responses [36,33,31,34,35]. As the use of rituximab has increased in most rheumatology clinics, the question of vaccine efficacy in rituximab-treated populations has become more important. There are limited data on the influenza vaccine specifically. Several studies have attempted to define effects on vaccine responses with conflicting results [33,37-40,36,41]. The variable response rates in these studies may have been due to different schedules of vaccination after rituximab. Repopulation of the B cell compartment is a dynamic and variable process [42]. Vaccination of patients with lymphoma treated with rituximab has likewise had limited efficacy [43,44]. We undertook a prospective study to investigate whether laboratory predictors of influenza responsiveness could be identified in a cohort of rheumatology patients treated with rituximab. We found that those patients who had undetectable circulating B cells were much less likely to respond, while there were modest responses to the inactivated influenza vaccine among patients who had any level of detectable B cells. We also found that pre-existing HAI titers were stable in individual patients over time.
Methods
Subjects
Twenty-five subjects who were on active rituximab therapy for autoimmune disease were enrolled in this prospective study of influenza vaccine effectiveness. We assessed baseline immunologic parameters, within four weeks of rituximab administration, on the day of vaccination (7-9 months after rituximab) and at 2 months and six months after vaccination. Subject received the inactivated influenza vaccine as part of their routine care. This study was approved by the Institutional Review Board. Demographic characteristics are listed in Table 1. An adult control cohort from the same city, which has in part been previously reported, had parallel studies performed on the day of vaccination and 4-8 weeks afterward [45]. We age-matched the controls to the study population. Demographic characteristics are listed in Table 1.
Table 1.
Demographic characteristics of the study population
| Patients | Number (n=17) | Percent |
|---|---|---|
| Female | 16 | 94% |
| Caucasian | 11 | 65% |
| African American | 4 | 24% |
| Asian | 2 | 12% |
| Rheumatoid arthritis* | 8 | 47% |
| Sjögren's syndrome* | 6 | 35% |
| SLE* | 2 | 12% |
| Polymyositis | 2 | 12% |
| Wegener's vasculitis | 1 | 6% |
| Controls | 15 | |
| Female | 8 | 53% |
| Caucasian | 11 | 73% |
Two subjects had two concurrent diagnoses (rheumatoid arthritis and SLE, rheumatoid arthritis and Sjögren's syndrome)
Vaccines
Four seasonal vaccines were used: For 2006-2007, A/New Caledonia/20/99 (H1N1)-like virus; A/Wisconsin/67/2005 (H3N2)-like virus; B/Malaysia/2506/2004-like virus. For 2007-2008, A/Solomon Islands/3/2006 (H1N1)-like virus; A/Wisconsin/67/2005 (H3N2)-like virus; B/Malaysia/2506/2004-like virus. For 2008-2009, A/Brisbane/59/2007 (H1N1)-like virus; A/Brisbane/10/2007, (H3N2)-like virus; B/Florida/4/2006-like virus. For 2009-2010, A/Brisbane/59/2007 (H1N1)-like virus; A/Brisbane/10/2007 (H3N2)-like virus; B/Brisbane/60/2008-like virus.
Immunologic Assessments
A standard hemagglutination inhibition (HAI) assay optimized for the vaccine administered to each patient was used to define antibody responses to the vaccine [46]. Flow cytometry for T cell and B cell subsets was performed. Fixed stained T cells were run on an LSR II (BD Biosciences) and analyzed using FlowJo software (TreeStar). Approximately 200,000 to 500,000 total events were collected per assay. CD4 Naïve cells were defined as CD45RA+CD31+. CD4 Central Memory T cells were defined as CD27+CD45RO+CCR7+. CD4 Effector Memory T cells were defined as CD45RO+/CD27+/CCR7-. CD4 Reverted Memory T cells were defined as CD45RA+/CD31-/CCR7+. CD8 Naïve cells were defined as CD45RA+CD31+. CD8 Central Memory T cells were defined as CD27+CD45RO+CCR7+. CD8 Effector Memory T cells were defined as CD45RO+/CD27+/CCR7-. CD8 Reverted Memory T cells were defined as CD45RA+/CD31-/CCR7+.
Flow cytometry of B cell subsets was performed using fresh venous whole blood anti-coagulated with EDTA as described previously [42]. B cells were defined as CD19+ lymphocytes. Analyses were performed on a FACSCalibur with CellQuest software (Version 5.2.1, Becton Dickenson, San Jose, CA). CD19+ lymphocytes were analyzed for CD27 and IgM. The absolute B cell count was obtained by multiplying the absolute lymphocyte count by the CD19+ fraction. A minimum of 150μL of blood was analyzed. The limit of detection was <0.67 B cells/μL. Our criterion for complete B cell depletion at week 4 after rituximab was an absolute B cell count of ≤5 B cells/μL [47].
To examine functional responses to the vaccine, T cell ELISPOTs and B cell ELISPOTs were performed. A cocktail of influenza proteins (Protein Sciences, Meriden, CT) matched to the vaccine year was used as specific antigen (at 5 μg/mL) in a standard γ-interferon T cell ELISPOT assay 58. PMA and ionomycin (combined at 5 μg/mL each) were used as a positive control. This assay examined a range of epitopes, and was HLA-dependent. Foreign antigen sources were avoided to minimize background. The B cell ELISPOT defined the frequency of memory B cells activated by influenza to produce antibody 59,60. PBMC were stimulated for 6 days with pokeweed mitogen at 1:100,000, SAC at 1:10,000 and CpG-2006 at 6mg/ml (Sigma Aldrich St. Louis, MO). Following the stimulation period, cells were treated for six hours with the influenza protein cocktail described above (at 0.5 μg/mL). Finally, IgG production assessed by quantification of effector cells using ImmunoSpot (CTL, version 4) software. Proliferation assays utilized carboxyfluorescein succinimidyl ester (CFSE)-loaded cells stimulated for five days with season-specific influenza virus. Flow cytometry was used to define the divided cells.
Statistical analyses
The χ2 test was used for the comparisons of responder frequency and seroconversion. Descriptive statistics were performed using the Mann-Whitney method. Two-tailed p values are reported. Significance was set at p≤0.05.
Results
Patient Characteristics
Twenty-five subjects were originally enrolled in this study to define predictors of influenza vaccine serologic responses. The demographic characteristics of the study cohort are given in Table 1. Patient diagnoses and B cell counts on the day of vaccination are provided in Table 2. As anticipated, the majority of the subjects were female (96%) and Caucasian (60%). The mean age was 49 years and the underlying diseases were diverse, with rheumatoid arthritis being the most common diagnosis (40%). Five subjects withdrew from the study: three were unwilling to receive the vaccine and two had increasing illness. Three subjects were withdrawn by the overseer due to failure to complete the essential time points, leaving 17 subjects for analysis. A subset of 12 patients with synchronized studies were used to define vaccine responder status. Enrollment into this study required only that the subject was expected to start rituximab specifically for their autoimmune disease. All subjects received the inactivated year-specific influenza vaccine as part of their routine care. Adult age-matched controls had the same laboratory studies performed at baseline and at 4-8 weeks after vaccination. Immunologic evaluations were obtained at the time of enrollment, at four weeks after a course of rituximab, at the time of vaccination (7-9 months after rituximab), two months and four months after vaccination. The immunologic assessments included year-specific HAI titers to the influenza vaccine, T cell subsets, B cell subsets, T cell proliferation, T cell cytokine production, B cell IgG production, and T cell repertoire analysis by spectratyping.
Table 2.
Clinical characteristics
| Disease group | Additional medications on the day of vaccination | Mean B cell count (cells/μl of whole blood ± SD) on the day of vaccination |
|---|---|---|
| Rheumatoid arthritis | Leflunomide, etanercept, prednisone | 1 ± 1 |
| Sjögren's | Hydroxychloroquine, ibuprofen, leflunomide, prednisone, methotrexate | 37.6 ± 63.5 |
| SLE | Hydroxychloroquine, prednisone, methotrexate | 1 ± 0 |
| Polymyositis | Methotrexate, hydroxychloroquine prednisone | 7 ± 8.5 |
| Wegener's | None | 1 |
B cell reconstitution
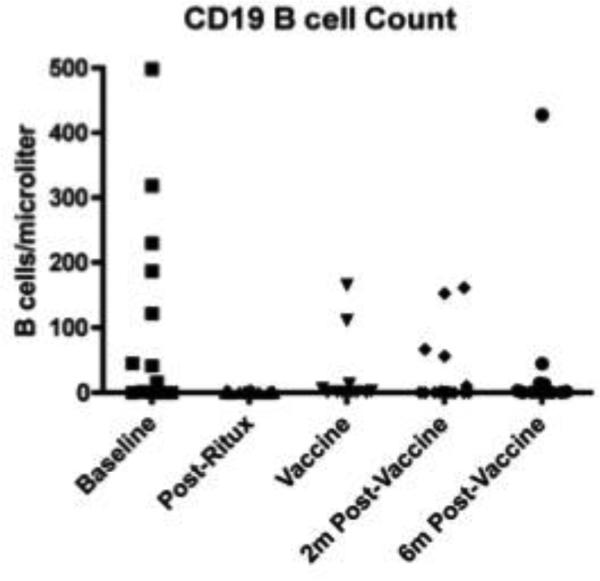
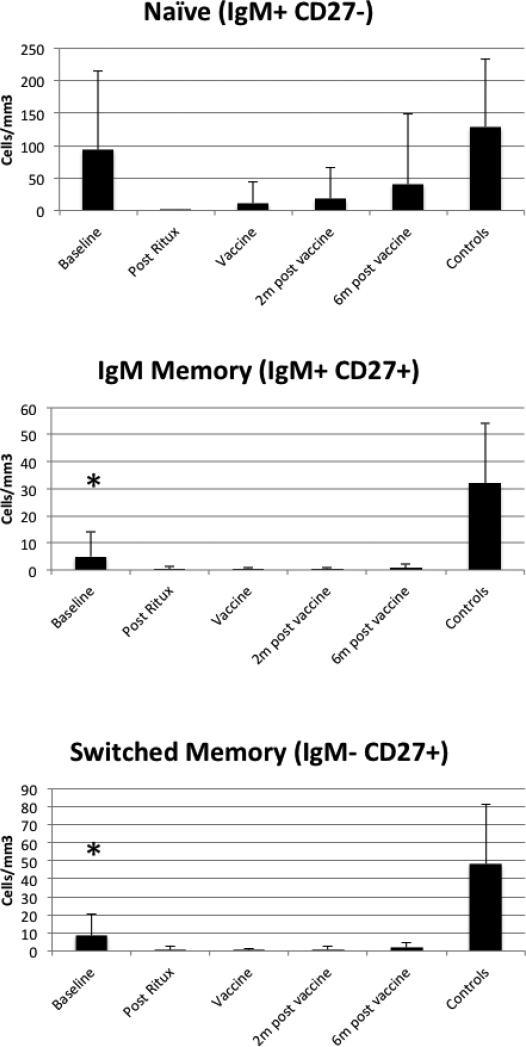
Three patients had received rituximab prior to study entry. The remainder were naïve to rituximab. We first analyzed B cell subsets by flow cytometry to characterize the pattern of B cell reconstitution. Depletion after rituximab was complete in these subjects (Figure 1) and no patient failed to deplete. At 7-9 months after rituximab, some subjects had reconstitution of their naïve B cells but switched memory (CD19+CD27+, IgM-) and non-switched memory (CD19+CD27+IgM+) B cells remained less than 10% of their starting values throughout the entire time frame (Figure 2).
Figure 1. B cell depletion kinetics.
Total B cells (CD19+) were measured at baseline, 4 weeks after rituximab, the day of vaccine administration (7-9 months after rituximab administration), 2 months after vaccination and 6 months after vaccination. B cells depleted well in all patients but recovery was variable.
Figure 2. B cell subsets.
B cell subsets were defined using flow cytometry. The mean and standard deviation are shown and the baseline evaluations for the controls are shown at the right of each graph. Baseline levels were compared between patients and controls. The IgM memory and switched memory subsets were different from controls at baseline (p<0.001 for both). On the day of vaccination, memory subset counts were significantly lower in patients than controls with p<0.001.
T cells in Subjects
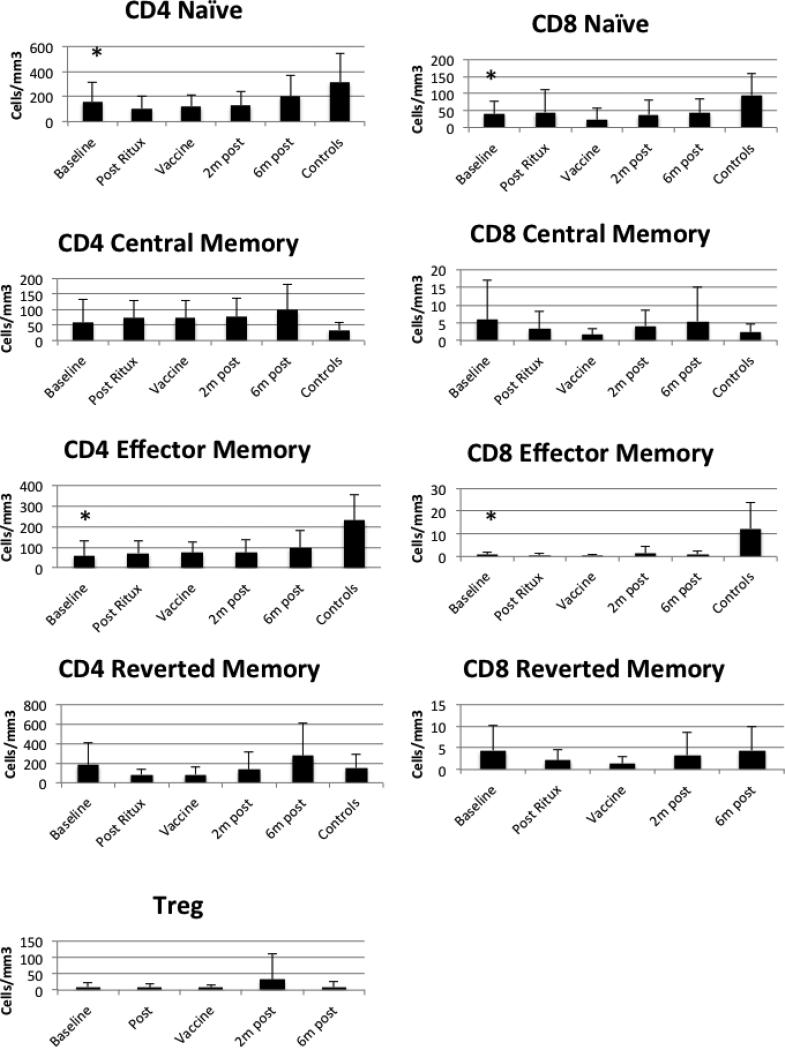
All patients were on additional immunologically-modifying interventions in addition to rituximab (Table 2). On the day of vaccination, four patients were on low dose prednisone, four were on hydroxychloroquine, two were on leflunomide, one was on azathioprine and one was on methotrexate. To examine this cohort for evidence of T cell compromise, we initially examined T cell subsets over the same time points. We compared the T cells to the control group (Figure 3). Naïve and effector CD4 and CD8 T cells were lower in patients than controls at baseline. We could not discern a direct effect of the Rituximab. To assess T cell function, we analyzed CD4 and CD8 responses to influenza using purified cells and a γ-interferon ELISPOT. Responses were comparable to controls at baseline but there were no significant increases after vaccination (data not shown). Similarly, we measured proliferation after PHA and influenza stimuli and we did not detect a response after vaccination in the rituximab-treated cohort (data not shown). There were no changes in the T cell repertoire as detected by spectratyping over the time points (data not shown). Globally, the T cell compartment was less altered in the patients compared to controls than the B cell compartment, as expected.
Figure 3. T cell subsets.
T cell subsets were defined using flow cytometry. The mean and standard deviation are shown at each time point and the baseline evaluations for the controls are shown on the far right of each graph. Baseline levels were compared between patients and controls and the asterisks indicate results statistically different from controls. The p values for CD4 naïve and effector subsets were p=0.05 and p<0.01 respectively. For CD8 naïve and effector subsets, the p values were p=0.01 and p<0.01 respectively.
Influenza Vaccine Responses
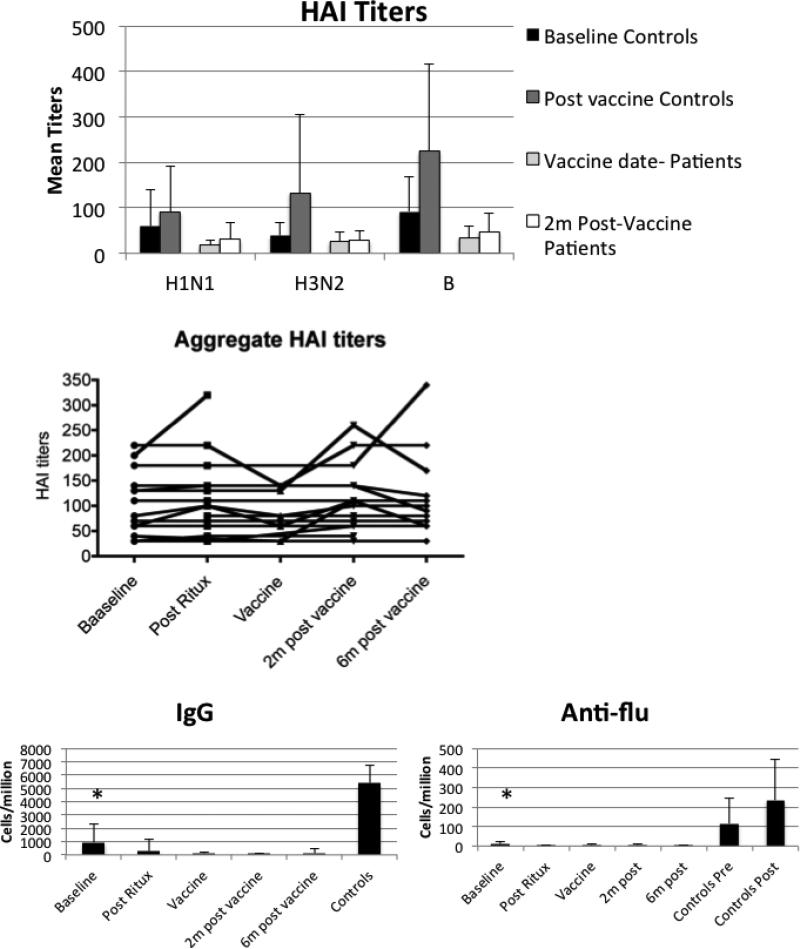
To define the rate of vaccine response, the rate of seroconversion was used. This represents a four-fold increase in titer after vaccination. Only two of 12 patients had a four-fold response and it was to a single serotype in both cases. These two patients had rheumatoid arthritis and polymyositis. In contrast, 10/15 of the adult controls had seroconversion after vaccination (p=0.009) and in most cases it was to multiple serotypes. In spite of the failure to respond to the vaccine, pre-existing aggregate titers (defined as the sum of titers to the three serotypes) were retained over a long time frame (Figure 4)
Figure 4. Antibody production.
Antibody responses to the vaccine were measured using the hemagglutination inhibition assay matched for each year of the vaccine (top panel). Mean titers and standard deviation are shown in the graph. Post vaccine responses in the patients were significantly lower than controls with p<0.001 for all three serotypes. In addition, the difference between the pre- and post-vaccine titers in the patients were non-significant. The existing HAI titers in the patients varied little over the course of the study. The second panel uses a sum of the individual HAI serotypes in the graph. Each line represents an individual study patient. We also assessed global IgG production and anti-influenza responses using a B cell ELISPOT assay. Although baseline levels are lower in patients than controls, there is detectable antibody production in this assay. Nevertheless, all time points exhibited significantly less total IgG and influenza-specific responses than controls.
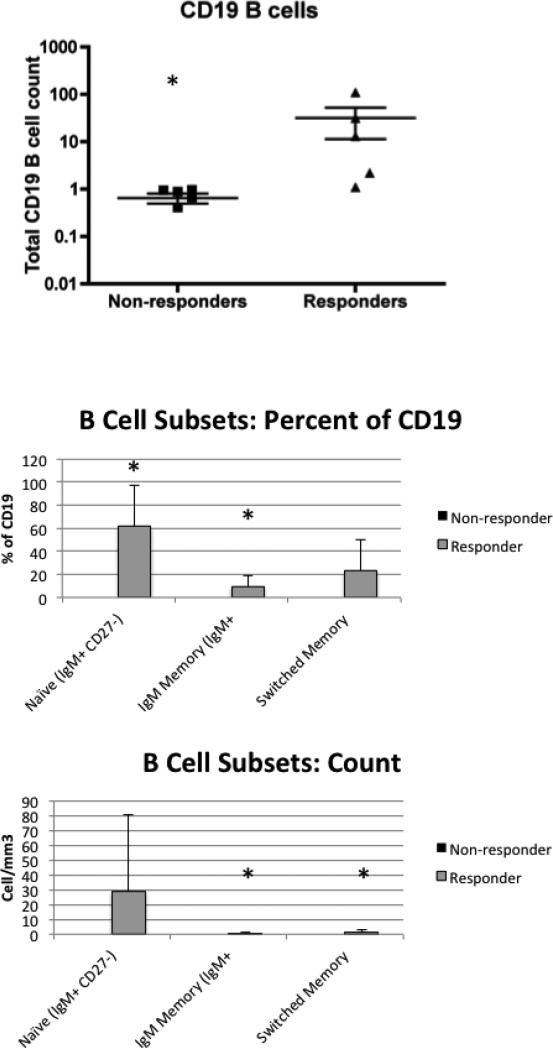
To understand the determinants of the response to the inactivated influenza vaccination, we first defined B cell function. Overall, there were low numbers of antibody-secreting cells detected in a B cell ELISPOT assay (Figure 4). To examine correlates of the vaccine response, we identified five patients who had any detectable response to the vaccine. We defined responders as those who had a two-fold increase to at least one serotype at the two-month time point after vaccination. These five patients had rheumatoid arthritis (X2), polymyositis and Sjögren's syndrome (X2). We compared the T cell subsets defined in Figure 3 between responders and non-responders. There were no significant differences in T cell counts between the two groups (data not shown). However, the absolute B cell numbers were higher in responders compared to non-responders (p=0.004) (Figure 5). This was not restricted to a single B cell subset (Figure 5). Responders had higher numbers of IgM and switched memory B cells compared to non-responders.
Figure 5. Characteristics of the responder group.
Within the cohort, we compared B cell subsets on the day of vaccination between responders and nonresponders. The mean and standard error are shown. The non-responders are shown in dark grey but the values are so low, they appear superimposed on the X axis. The naïve B cell and IgM memory percentages were lower in nonresponders than responders with p<0.01 and p<0.05 respectively. The absolute counts were lower in non-responders than responders for IgM memory and switched memory B cell subsets with p<0.05 for IgM memory and P<0.01 for switched memory B cells. Although the responders had variable B cell counts, the non-responders uniformly had <1 B cell/mm3. Asterisks indicate significant differences.
Discussion
The protection from influenza infection relies on innate defenses, humoral immunity and cytotoxic T cell responses. In murine models, T cell responses are more likely to be cross-protective, but they offer modest protection from severe disease [48-53]. In contrast, humoral immunity offers limited cross-protection against other serotypes but is strongly protective against infection [54-57]. Therefore, we focused on antibody responses.
This study was designed to determine whether there was a threshold B cell count that could predict serologic influenza vaccine responsiveness. In fact, patients with B cell counts of >1 cell/mm3 did have some response to the vaccine (two-fold rise in titer to one serotype), while those subjects who had B cell counts <1 cell/mm3 did not respond. This was a small study, however, and many of the B-cell counts clustered right around the threshold of one. Additional studies to test the predictive value of this threshold will be required. Recent progress has been made in identifying predictors of vaccine responses in healthy donors [58]. Specifically for influenza, the ability to stratify immunologically vulnerable subjects to maximize the effective use of the vaccine in resource-limited situations would be desirable. Repletion of the B cell compartment after rituximab is highly variable and influenza vaccine is available seasonally, making it difficult to develop standard protocols useful for all patients. Therefore identification of a laboratory marker would be extremely valuable.
Previous studies have identified diminished responses to various vaccines after rituximab treatment. Some studies have found an association of vaccine responses with the degree of B cell depletion [47,43,37] while others have not found such an association [36,38,39]. Our study is consistent with an association of B cell depletion and compromised vaccine responses. Differences between study results may be related to the specific vaccine utilized, the degree of priming, and the extent to which T cells provide help.
Based on this study, the uniform use of the inactivated influenza vaccine in patients treated with rituximab must be considered of limited serologic efficacy. Clinical efficacy due to T cell responses is possible although in this cohort, vaccine-specific T cell responses were minimal [59,60]. In subjects with detectable peripheral blood B cells, the vaccine may induce a serologic response, however, patients should be cautioned that protection may not be complete. Since pre-existing titers to influenza were not affected, consideration should be given to vaccinating patients before treatment with rituximab.
Acknowledgements
The authors would like to thank the patients and the physicians who contributed to this study. The skills of Dr. Yang-Zhu Du, Lytia Fisher and Kristal Dow are gratefully acknowledged. Supported in part by NIH grant NO1-AI-50024, the Arthritis Foundation, the American Autoimmunity Related Disease Association, and R01 AR34156 and R01 AI063626.
Footnotes
Conflict of Interest
The authors declare that have no conflict of interest.
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. doi:joc21709 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 3.Neuzil KM, Wright PF, Mitchel EF, Jr., Griffin MR. The burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr. 2000;137(6):856–864. doi: 10.1067/mpd.2000.110445. doi:S0022-3476(00)25105-6 [pii] 10.1067/mpd.2000.110445. [DOI] [PubMed] [Google Scholar]
- 4.Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr., Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342(4):225–231. doi: 10.1056/NEJM200001273420401. doi:MJBA-420401 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Neuzil KM, Reed GW, Mitchel EF, Jr., Griffin MR. Influenza-associated morbidity and mortality in young and middle-aged women. JAMA. 1999;281(10):901–907. doi: 10.1001/jama.281.10.901. doi:joc81147 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Neuzil KM, Coffey CS, Mitchel EF, Jr., Griffin MR. Cardiopulmonary hospitalizations during influenza season in adults and adolescents with advanced HIV infection. J Acquir Immune Defic Syndr. 2003;34(3):304–307. doi: 10.1097/00126334-200311010-00008. [DOI] [PubMed] [Google Scholar]
- 7.Dushoff J, Plotkin JB, Viboud C, Earn DJ, Simonsen L. Mortality due to influenza in the United States--an annualized regression approach using multiple-cause mortality data. Am J Epidemiol. 2006;163(2):181–187. doi: 10.1093/aje/kwj024. doi:kwj024 [pii] 10.1093/aje/kwj024. [DOI] [PubMed] [Google Scholar]
- 8.Groenwold RH, Hoes AW, Hak E. Impact of influenza vaccination on mortality risk among the elderly. Eur Respir J. 2009;34(1):56–62. doi: 10.1183/09031936.00190008. doi:09031936.00190008 [pii] 10.1183/09031936.00190008. [DOI] [PubMed] [Google Scholar]
- 9.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935–1944. doi: 10.1056/NEJMoa0906695. doi:NEJMoa0906695 [pii] 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 10.Kim YJ, Boeckh M, Englund JA. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med. 2007;28(2):222–242. doi: 10.1055/s-2007-976494. doi:10.1055/s-2007-976494. [DOI] [PubMed] [Google Scholar]
- 11.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9(8):493–504. doi: 10.1016/S1473-3099(09)70175-6. doi:S1473-3099(09)70175-6 [pii] 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malhotra A, Krilov LR. Influenza and respiratory syncytial virus. Update on infection, management, and prevention. Pediatric Clinics of North America. 2000;47(2):353–372. doi: 10.1016/s0031-3955(05)70211-x. [DOI] [PubMed] [Google Scholar]
- 13.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, Cox NJ. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. doi:rr5908a1 [pii] [PubMed] [Google Scholar]
- 14.Keitel WA, Couch RB, Cate TR, Hess KR, Baxter B, Quarles JM, Atmar RL, Six HR. High doses of purified influenza A virus hemagglutinin significantly augment serum and nasal secretion antibody responses in healthy young adults. J Clin Microbiol. 1994;32(10):2468–2473. doi: 10.1128/jcm.32.10.2468-2473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel) 2003;115:63–73. [PubMed] [Google Scholar]
- 16.Bachi T, Gerhard W, Yewdell JW. Monoclonal antibodies detect different forms of influenza virus hemagglutinin during viral penetration and biosynthesis. Journal of Virology. 1985;55(2):307–313. doi: 10.1128/jvi.55.2.307-313.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beyer WE, Palache AM, Luchters G, Nauta J, Osterhaus AD. Seroprotection rate, mean fold increase, seroconversion rate: which parameter adequately expresses seroresponse to influenza vaccination? Virus Research. 2004;103(1-2):125–132. doi: 10.1016/j.virusres.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Reilly A, Kersun LS, McDonald K, Weinberg A, Jawad AF, Sullivan KE. The efficacy of influenza vaccination in a pediatric oncology population. J Pediatr Hematol Oncol. 2010;32(5):e177–181. doi: 10.1097/MPH.0b013e3181d869f3. doi:10.1097/MPH.0b013e3181d869f3. [DOI] [PubMed] [Google Scholar]
- 19.Couch RB. Seasonal inactivated influenza virus vaccines. Vaccine. 2008;26(Suppl 4):D5–9. doi: 10.1016/j.vaccine.2008.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123(7):518–527. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- 21.Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272(21):1661–1665. [PubMed] [Google Scholar]
- 22.Nichol KL. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine. 2003;21(16):1769–1775. doi: 10.1016/s0264-410x(03)00070-7. doi:S0264410X03000707 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Ruben FL. Inactivated influenza virus vaccines in children. Clin Infect Dis. 2004;38(5):678–688. doi: 10.1086/382883. doi:10.1086/382883 CID32328 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Zangwill KM, Belshe RB. Safety and efficacy of trivalent inactivated influenza vaccine in young children: a summary for the new era of routine vaccination. Pediatr Infect Dis J. 2004;23(3):189–197. doi: 10.1097/01.inf.0000116292.46143.d6. doi:00006454-200403000-00002 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Fessler BJ. Infectious diseases in systemic lupus erythematosus: risk factors, management and prophylaxis. Best Pract Res Clin Rheumatol. 2002;16(2):281–291. doi: 10.1053/berh.2001.0226. doi:10.1053/berh.2001.0226 S1521694201902268 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Aydintug AO, Jedryka-Goral A, de Ramon E, Fernandez-Nebro A, Galeazzi M, Haga HJ, Mathieu A, Houssiau F, Ruiz-Irastorza G, Ingelmo M, Hughes GR. Morbidity and mortality in systemic lupus erythematosus during a 5-year period. A multicenter prospective study of 1,000 patients. European Working Party on Systemic Lupus Erythematosus. Medicine (Baltimore) 1999;78(3):167–175. doi: 10.1097/00005792-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Symmons DP. Mortality in rheumatoid arthritis. Br J Rheumatol 27 Suppl. 1988;1:44–54. [PubMed] [Google Scholar]
- 28.Phillip R, Luqmani R. Mortality in systemic vasculitis: a systematic review. Clin Exp Rheumatol. 2008;26(5 Suppl 51):S94–104. doi:2458 [pii] [PubMed] [Google Scholar]
- 29.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–2285. doi: 10.1001/jama.295.19.2275. doi:295/19/2275 [pii] 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 30.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287–2293. doi: 10.1002/art.10524. doi:10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 31.Crowe SR, Merrill JT, Vista ES, Dedeke AB, Thompson DM, Stewart S, Guthridge JM, Niewold TB, Franek BS, Air GM, Thompson LF, James JA. Influenza vaccination responses in human systemic lupus erythematosus: Impact of clinical and demographic features. Arthritis Rheum. 2011;63(8):2396–2406. doi: 10.1002/art.30388. doi:10.1002/art.30388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Porto F, Lagana B, Biselli R, Donatelli I, Campitelli L, Nisini R, Cardelli P, Rossi F, D'Amelio R. Influenza vaccine administration in patients with systemic lupus erythematosus and rheumatoid arthritis. Safety and immunogenicity. Vaccine. 2006;24(16):3217–3223. doi: 10.1016/j.vaccine.2006.01.028. doi:S0264-410X(06)00042-9 [pii] 10.1016/j.vaccine.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Gabay C, Bel M, Combescure C, Ribi C, Meier S, Posfay-Barbe K, Grillet S, Seebach JD, Kaiser L, Wunderli W, Guerne PA, Siegrist CA. Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03-adjuvanted pandemic influenza vaccine: a prospective, open-label, parallel-cohort, single-center study. Arthritis Rheum. 2011;63(6):1486–1496. doi: 10.1002/art.30325. doi:10.1002/art.30325. [DOI] [PubMed] [Google Scholar]
- 34.Elkayam O, Amir S, Mendelson E, Schwaber M, Grotto I, Wollman J, Arad U, Brill A, Paran D, Levartovsky D, Wigler I, Caspi D, Mandelboim M. Efficacy and safety of vaccination against pandemic 2009 influenza A (H1N1) virus among patients with rheumatic diseases. Arthritis Care Res (Hoboken) 2011;63(7):1062–1067. doi: 10.1002/acr.20465. doi:10.1002/acr.20465. [DOI] [PubMed] [Google Scholar]
- 35.Fomin I, Caspi D, Levy V, Varsano N, Shalev Y, Paran D, Levartovsky D, Litinsky I, Kaufman I, Wigler I, Mendelson E, Elkayam O. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis. 2006;65(2):191–194. doi: 10.1136/ard.2005.036434. doi:ard.2005.036434 [pii] 10.1136/ard.2005.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oren S, Mandelboim M, Braun-Moscovici Y, Paran D, Ablin J, Litinsky I, Comaneshter D, Levartovsky D, Mendelson E, Azar R, Wigler I, Balbir-Gurman A, Caspi D, Elkayam O. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis. 2008;67(7):937–941. doi: 10.1136/ard.2007.077461. doi:ard.2007.077461 [pii] 10.1136/ard.2007.077461. [DOI] [PubMed] [Google Scholar]
- 37.Arad U, Tzadok S, Amir S, Mandelboim M, Mendelson E, Wigler I, Sarbagil-Maman H, Paran D, Caspi D, Elkayam O. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine. 2011;29(8):1643–1648. doi: 10.1016/j.vaccine.2010.12.072. doi:S0264-410X(10)01845-1 [pii] 10.1016/j.vaccine.2010.12.072. [DOI] [PubMed] [Google Scholar]
- 38.van Assen S, Holvast A, Benne CA, Posthumus MD, van Leeuwen MA, Voskuyl AE, Blom M, Risselada AP, de Haan A, Westra J, Kallenberg CG, Bijl M. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010;62(1):75–81. doi: 10.1002/art.25033. doi:10.1002/art.25033. [DOI] [PubMed] [Google Scholar]
- 39.Bingham CO, 3rd, Looney RJ, Deodhar A, Halsey N, Greenwald M, Codding C, Trzaskoma B, Martin F, Agarwal S, Kelman A. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62(1):64–74. doi: 10.1002/art.25034. doi:10.1002/art.25034. [DOI] [PubMed] [Google Scholar]
- 40.Pescovitz MD, Torgerson TR, Ochs HD, Ocheltree E, McGee P, Krause-Steinrauf H, Lachin JM, Canniff J, Greenbaum C, Herold KC, Skyler JS, Weinberg A. Effect of rituximab on human in vivo antibody immune responses. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.08.008. doi:S0091-6749(11)01246-2 [pii] 10.1016/j.jaci.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yri OE, Torfoss D, Hungnes O, Tierens A, Waalen K, Nordoy T, Dudman S, Kilander A, Wader KF, Ostenstad B, Ekanger R, Meyer P, Kolstad A. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118(26):6769–6771. doi: 10.1182/blood-2011-08-372649. doi:10.1182/blood-2011-08-372649. [DOI] [PubMed] [Google Scholar]
- 42.Sutter JA, Kwan-Morley J, Dunham J, Du YZ, Kamoun M, Albert D, Eisenberg RA, Luning Prak ET. A longitudinal analysis of SLE patients treated with rituximab (anti-CD20): factors associated with B lymphocyte recovery. Clin Immunol. 2008;126(3):282–290. doi: 10.1016/j.clim.2007.11.012. doi:S1521-6616(07)01408-8 [pii] 10.1016/j.clim.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Bedognetti D, Zoppoli G, Massucco C, Zanardi E, Zupo S, Bruzzone A, Sertoli MR, Balleari E, Racchi O, Messina M, Caltabiano G, Icardi G, Durando P, Marincola FM, Boccardo F, Ferrarini M, Ansaldi F, De Maria A. Impaired response to influenza vaccine associated with persistent memory B cell depletion in non-Hodgkin's lymphoma patients treated with rituximab-containing regimens. J Immunol. 2011;186(10):6044–6055. doi: 10.4049/jimmunol.1004095. doi:jimmunol.1004095 [pii] 10.4049/jimmunol.1004095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horwitz SM, Negrin RS, Blume KG, Breslin S, Stuart MJ, Stockerl-Goldstein KE, Johnston LJ, Wong RM, Shizuru JA, Horning SJ. Rituximab as adjuvant to high-dose therapy and autologous hematopoietic cell transplantation for aggressive non-Hodgkin lymphoma. Blood. 2004;103(3):777–783. doi: 10.1182/blood-2003-04-1257. doi:10.1182/blood-2003-04-1257 2003-04-1257 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Jawad AF, Prak EL, Boyer J, McDonald-McGinn DM, Zackai E, McDonald K, Sullivan KE. A Prospective Study of Influenza Vaccination and a Comparison of Immunologic Parameters in Children and Adults with Chromosome 22q11.2 Deletion Syndrome (DiGeorge Syndrome/Velocardiofacial Syndrome). Journal of clinical immunology. 2011;31(6):927–935. doi: 10.1007/s10875-011-9569-8. doi:10.1007/s10875-011-9569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levin MJ, Song LY, Fenton T, Nachman S, Patterson J, Walker R, Kemble G, Allende M, Hultquist M, Yi T, Nowak B, Weinberg A. Shedding of live vaccine virus, comparative safety, and influenza-specific antibody responses after administration of live attenuated and inactivated trivalent influenza vaccines to HIV-infected children. Vaccine. 2008;26(33):4210–4217. doi: 10.1016/j.vaccine.2008.05.054. doi:S0264-410X(08)00645-2 [pii] 10.1016/j.vaccine.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albert D, Dunham J, Khan S, Stansberry J, Kolasinski S, Tsai D, Pullman-Mooar S, Barnack F, Striebich C, Looney RJ, Prak ET, Kimberly R, Zhang Y, Eisenberg R. Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Annals of the rheumatic diseases. 2008;67(12):1724–1731. doi: 10.1136/ard.2007.083162. doi:10.1136/ard.2007.083162. [DOI] [PubMed] [Google Scholar]
- 48.Stevenson PG, Hawke S, Bangham CR. Protection against lethal influenza virus encephalitis by intranasally primed CD8+ memory T cells. J Immunol. 1996;157(7):3065–3073. [PubMed] [Google Scholar]
- 49.Kreijtz JH, Bodewes R, van Amerongen G, Kuiken T, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine. 2007;25(4):612–620. doi: 10.1016/j.vaccine.2006.08.036. doi:S0264-410X(06)00986-8 [pii] 10.1016/j.vaccine.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 50.Benton KA, Misplon JA, Lo CY, Brutkiewicz RR, Prasad SA, Epstein SL. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or gamma delta T cells. J Immunol. 2001;166(12):7437–7445. doi: 10.4049/jimmunol.166.12.7437. [DOI] [PubMed] [Google Scholar]
- 51.Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 52.Powell TJ, Strutt T, Reome J, Hollenbaugh JA, Roberts AD, Woodland DL, Swain SL, Dutton RW. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol. 2007;178(2):1030–1038. doi: 10.4049/jimmunol.178.2.1030. doi:178/2/1030 [pii] [DOI] [PubMed] [Google Scholar]
- 53.Stevenson PG, Hawke S, Bangham CR. Protection against influenza virus encephalitis by adoptive lymphocyte transfer. Virology. 1997;232(1):158–166. doi: 10.1006/viro.1997.8535. doi:S0042-6822(97)98535-4 [pii] 10.1006/viro.1997.8535. [DOI] [PubMed] [Google Scholar]
- 54.Rangel-Moreno J, Carragher DM, Misra RS, Kusser K, Hartson L, Moquin A, Lund FE, Randall TD. B cells promote resistance to heterosubtypic strains of influenza via multiple mechanisms. J Immunol. 2008;180(1):454–463. doi: 10.4049/jimmunol.180.1.454. doi:180/1/454 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Virelizier JL. Host defenses against influenza virus: the role of anti-hemagglutinin antibody. J Immunol. 1975;115(2):434–439. [PubMed] [Google Scholar]
- 56.Doherty PC, Gerhard W. Breakdown of the blood--cerebrospinal fluid barrier to immunoglobulin in mice injected intracerebrally with a neurotropic influenza A virus. Post-exposure treatment with monoclonal antibody promotes recovery. J Neuroimmunol. 1981;1(3):227–237. doi: 10.1016/0165-5728(81)90027-8. [DOI] [PubMed] [Google Scholar]
- 57.Kris RM, Yetter RA, Cogliano R, Ramphal R, Small PA. Passive serum antibody causes temporary recovery from influenza virus infection of the nose, trachea and lung of nude mice. Immunology. 1988;63(3):349–353. [PMC free article] [PubMed] [Google Scholar]
- 58.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, Kennedy K, Wu H, Bennouna S, Oluoch H, Miller J, Vencio RZ, Mulligan M, Aderem A, Ahmed R, Pulendran B. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–125. doi: 10.1038/ni.1688. doi:ni.1688 [pii] 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forrest BD, Pride MW, Dunning AJ, Capeding MR, Chotpitayasunondh T, Tam JS, Rappaport R, Eldridge JH, Gruber WC. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clinical and vaccine immunology : CVI. 2008;15(7):1042–1053. doi: 10.1128/CVI.00397-07. doi:10.1128/CVI.00397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176(10):6333–6339. doi: 10.4049/jimmunol.176.10.6333. doi:176/10/6333 [pii] [DOI] [PubMed] [Google Scholar]