Abstract
The superior efficacy of bariatric surgery compared with intensive medical treatment in reversing metabolic disease is now well accepted, but the critical mechanisms remain unknown. Unlike dieting, which triggers strong counter-regulatory responses such as hunger and craving, certain obesity surgeries appear to permanently reset the level of defended body weight. Understanding the molecular mechanisms behind successful surgery would thus go a long way in developing future “knifeless” treatment options. Major candidates include changes in gut-brain signaling by hormones, bile acids, and other still unidentified factors. By re-sensitizing homeostatic regulatory circuits in the hypothalamus and hedonic-motivational processing in cortico-limbic systems to internal signals, bariatric surgery could thus lead to a state of being content with less.
Keywords: Obesity surgery, brain, Roux-en-Y gastric bypass surgery, hypothalamus, food reward
Does bariatric surgery “reset” homeostatically regulated optimal body weight?
Although bariatric surgery has a long history, it has only recently reached prominent status among available treatments of an ever increasing prevalence of obesity. Aided by the failure to develop highly efficient and safe new drugs, bariatric surgery, particularly Roux-en-Y gastric bypass surgery (RYGB, see Glossary), is now considered the most effective treatment for morbid obesity and obesity-associated type-2 diabetes 1, 2. It is also clear that surgery cannot be the ultimate answer to the global obesity epidemic, but understanding the molecular and behavioral mechanisms of surgery-induced beneficial effects may lead to future “knifeless” treatments. Despite numerous hypothesized candidate mechanisms, no one mechanism has been unambiguously demonstrated to underlie the beneficial effects of gastric bypass surgery on weight control and diabetes resolution. This is not surprising in light of the complex changes induced by the various surgeries such as progressive adaptive changes in structure and function of the rearranged gastrointestinal tractas well as in gut-brain communication (Fig. 1). Here, we highlight some of these candidate mechanisms by reviewing the relevant literature in view of the two competing concepts in the control of energy balance, homeostatic and cognitive-emotional (non-homeostatic) regulation.
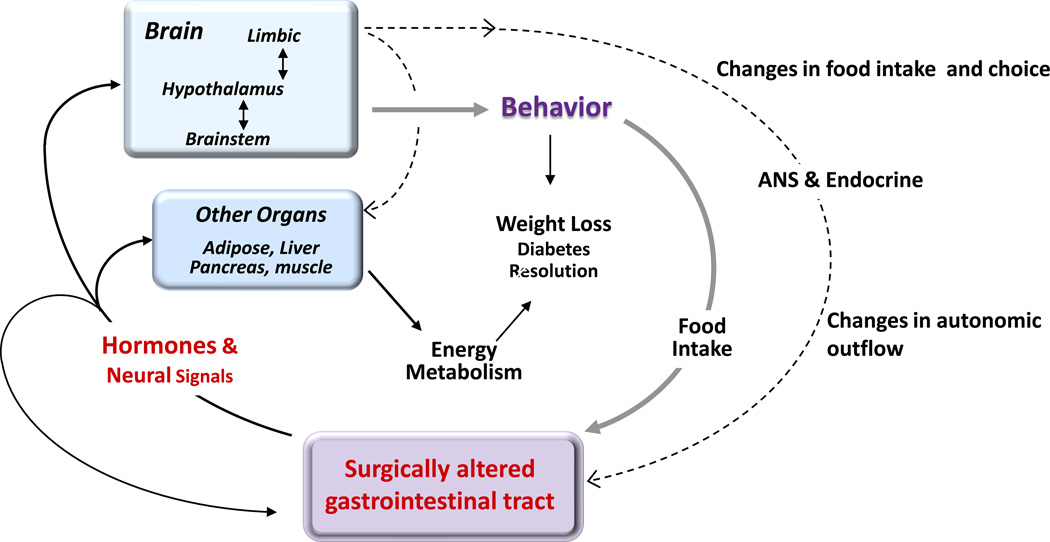
Fig. 1. Flow of information potentially involved in the physiological and behavioral consequences of gastric bypass surgery.
The primary surgical insult in the gut leads to progressive adaptive changes in structure (e.g. mucosal hypertrophy) and function (e.g. shift in microbiota composition, hormone release patterns, and bile acid metabolism). These combined changes signal to other organs, such as the liver, adipose tissue, pancreas, muscle, and brain, through either the circulatory or nervous system and ultimately lead to changes in energy intake, food choice, and energy expenditure. Changes in signaling to the brain not only affect food intake, but also autonomic and endocrine outflow back to the gut as well as to the other organs. ANS: Autonomic nervous system.
The hypothalamus has long been implicated in the regulation of homeostatic functions, particularly the defense of optimal body weight and adiposity 3. The discovery of leptin has spurred a flurry of research providing a basic neural and molecular blueprint of the hypothalamic “homeostatic regulator” 4, 5 (Fig. 2). A population of leptin-sensitive neurons in the arcuate nucleus of the hypothalamus expressing the potent orexigenic peptides Neuropeptide Y (NPY) and Agouti-related protein (AgRP) is the primary driver of food intake 6, 7. Another population of ARC neurons expressing proopiomelanocortin (POMC) and cocaine and amphetamine regulated transcript (CART) exert a tonic inhibitory influence on food intake that is necessary for appropriate regulation of energy homeostasis 8. These two neuron populations are not just sensitive to leptin, but also to a number of signals conveying the overall availability of fuels that either (1) circulate in the plasma, (2) are ready to be absorbed from the gastrointestinal tract, and (3) are stored as glycogen or fat. Availability of fuels in the near future is signaled from the gut by gastrointestinal hormones such as Ghrelin, GLP-1, and PYY 9, 10. In addition, gut signals can influence activity of neurons in the arcuate nucleus via ascending projections from the dorsal vagal complex.
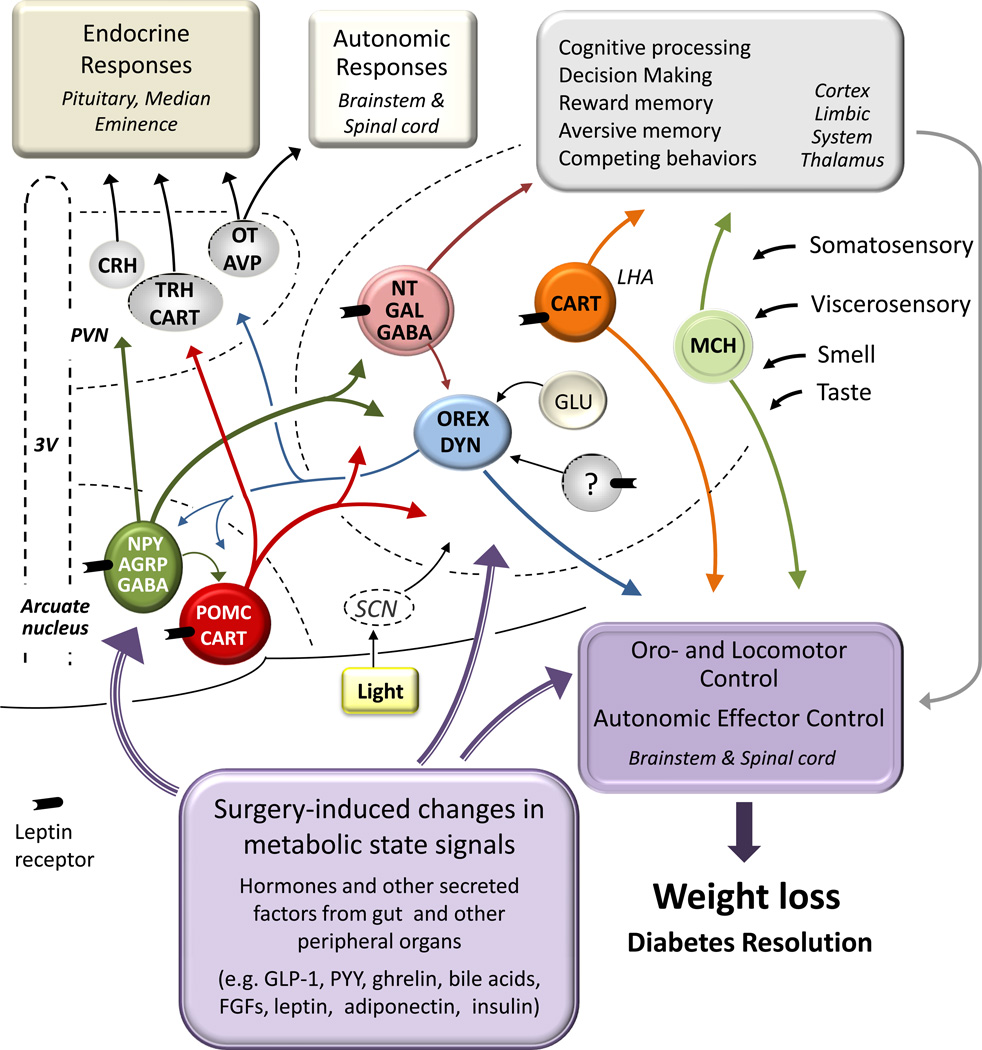
Fig. 2. Potential mediation of beneficial effects of bariatric surgery by the classical hypothalamic homeostatic regulator of energy balance.
Primary neurons in the arcuate nucleus sensitive to leptin and other metabolic state signals connect with secondary neurons in other parts of the hypothalamus to orchestrate activation of appropriate behavioral, autonomic, and endocrine anabolic and catabolic effector pathways. Major secondary neurons include Thyrotropin-releasing (TRH) and corticotrophin-releasing hormone (CRH) expressing neurons in the paraventricular nucleus (PVN) engaging the neuroendocrine axis, oxytocin (OT) expressing neurons in the PVN as well as orexin (Orex), melanin-concentrating hormone (MCH) and cocaine and amphetamine-regulated transcript (CART) expressing neurons in the lateral hypothalamic area (LHA) projecting to autonomic outflow pathways and oromotor and locomotor control areas in the caudal brain stem and spinal cord. Most of the lateral hypothalamic secondary neurons also project to corticolimbic structures involved in reward as well as cognitive and emotional processes. By acting on components of this regulatory system, bariatric surgery may be able to reset the defended level of body weight/adiposity to a less obese state. NPY, Neuropeptide Y; AGRP, agoutirelated protein; GABA, gamma-aminobutyric acid; POMC, proopiomelanocortin; NT, neurotensin; Gal, galanin; Dyn, dynorphin; SCN, suprachiasmatic nucleus; 3V, third Ventricle; GLP-1, glucagon-like peptide-1; PYY, peptide tyrosine tyrosine; FGFs, fibroblast growth factors (Modified after 92)
Although basomedial hypothalamic neurons respond to a variety of circulating nutrient signals, their ability to regulate food intake is dependent on downstream neuronal targets that reside in other hypothalamic areas, in particular the paraventricular nucleus (PVN) and lateral/perifornical hypothalamic areas (LHA) (see 11 for a review) (Fig. 2). These two brain regions are classically associated with the regulation of food intake and autonomic output, and each brain area contains a variety of neuropeptides associated with energy balance control. The prevailing model suggests that input from NPY/AgRP neurons is opposed by input from POMC neurons; this “metabolic” information is integrated with input from additional brain areas, and these downstream neurons in turn project widely to third and higher order neurons located in many areas of the brain and spinal cord 12. Clearly, the caudal brainstem is also participating in the regulation of body weight mediated by hormonal and neural feedback signals from the gut and leptin from adipose tissue 13, 14.
Numerous recent studies have shown that obesity is associated with impaired function of this homeostatic regulator. It is presently unclear whether this impairment is a preexisting condition to the development of obesity, or whether it is a secondary effect of the obese state. One of the leading hypotheses suggests that sustained energy-surplus, particularly in the form of saturated fats, corrupts mitochondrial function through maladaptive pro-inflammatory and redox-signaling and leads to deficient nutrient signaling, necessary for the down-regulation of energy intake and up-regulation of energy expenditure 15–17. It implies that, at a critical point, the impairment becomes permanent and obesity is no longer reversible but is actively defended by an elevated “set point” of the regulator. Major support for this view comes from the fact that in obese subjects, simple calorie-restriction to levels in lean individuals (also known as dieting) triggers strong counterregulatory mechanisms characterized by increased hunger and reduced energy expenditure 18. Obviously, any treatment that prevents this defense mechanism in spite of reduced energy intake would be ideal to treat obesity. Because many bariatric surgery patients appear to not be hungry anymore, or lose the desire to eat 19, 20, in spite of their reduced calorie intake, certain types of bariatric surgery could represent such ideal treatment.
Effects of bariatric surgery on hypothalamic set point mechanisms
Although the theoretical concept of defending a certain level or set-point of body weight/adiposity is widely used, there is no convenient measure to demonstrate its presence. Because of the strong anabolic and catabolic effects of manipulating the basomedial hypothalamic AGRP/NPY and POMC/CART neurons, the expression level of these peptides has often been used to show the “state” of the regulator in action, with increased AGRP/NPY-expression and/or decreased POMC-expression indicating an energy depleted or “hungry” state as seen after prolonged food deprivation 21, and decreased AGRP/NPY-expression and /or increased POMC-expression indicating an energy replete or “nonhungry” state.
Lessons from rodent models of bariatric surgeries
Few studies have examined hypothalamic peptide expression in rodent models of bariatric surgeries. In two studies, rats were subjected to duodenal-jejunal bypass 22, 23. NPY-expression in both studies and AGRP-expression in one study was increased 10–50 days after surgery compared with sham-surgery. Thus, their basomedial hypothalamus had the signature of a “hungry” brain, similar to what is typically observed after severe food deprivation 21, and indicating that no set point shift had occurred. In a model of RYGB in Sprague-Dawley rats, immunohistochemically detectable NPY in the ARC and PVH was reduced 10 days after surgery and in pair-fed controls, compared to sham-surgery 24. Assuming that depletion of NPY terminals in the PVN indicates increased activity of NPY-signaling and expression, these findings also support the conclusion that the rats were “hungry” and no shift in set point had occurred during this acute weight loss phase.
In contrast, Long Evans rats subjected to sleeve gastrectomy showed unchanged AGRP, NPY, POMC, MC4-, and leptin-receptor expression in the mediobasal hypothalamus, both at 10 days after surgery, when food intake was significantly reduced, and at 35 days after surgery, when food intake had returned to normal levels 25. In rats pair-fed to the sleeve gastrectomized group, AGRP-expression was significantly increased at 35 days, but not 10 days after surgery compared with sham surgery. Although there was a non-significant trend (− 40%) for decreased expression of POMC after 10 days 25, these findings suggest that the rats were not “hungry” in spite of the greatly reduced food intake and body weight/adiposity at 10 days after surgery, supporting the idea of a changed set point. Clearly, a comprehensive study assessing the temporal profile of changes in NPY, AGRP, and POMC expression comparing the different types of surgery is necessary to obtain a clearer picture. However, changes in expression levels of hypothalamic peptides do not by themselves demonstrate a crucial involvement in the effects of bariatric surgeries on energy balance – this can only come from interventional studies.
Bariatric surgery outcome in carriers of obesity-susceptibility loci
Downstream melanocortin-signaling via melanocortin-4 receptors (MC4R) is a crucial effector arm of the homeostatic regulator with strong effects on food intake and energy expenditure 26. Pharmacological agonism at the MC4R powerfully suppresses, while antagonism stimulates food intake, and MC4R null mice are hyperphagic and develop obesity 26, 27. The potential role of this signaling system was recently tested in mouse and rat models of MC4-receptor deficiency and in humans with mutations in the MC4-receptor gene. In the mouse, it was concluded that the MC4-receptor is required for the weight-reducing effects of RYGB surgery 28, while in the rat, it was concluded that the MC4-receptor is not required for vertical sleeve gastrectomy to have its full effect on body weight 29. In the mouse study, however, the initial 20% weight loss after surgery was identical in MC4R knockout and wildtype animals, suggesting that surgery-induced weight loss had nothing to do with MC4R-signaling, but that later weight-regain was attenuated with intact MC4R-siganling. Linking MC4R function to success with bariatric surgeries in humans also led to mixed results. Complete MC4R deficiency in one subject 30, and carrying MC4R variants in eight patients 31 impaired the weight-loss response to gastric banding. In contrast, no effects on the effectiveness of RYGB were found in patients with heterozygous MC4R mutations resulting in only partial loss of function 28, 32. Finally, patients carrying a rare variant of MC4R (I251L) that is negatively associated with obesity lost more weight after RYGB 33.
Some studies expanded their bariatric surgery patient characterization to include, in addition to MC4R, allelic variants of other obesity-susceptibility loci such as FTO (fat mass and obesity-associated gene), INSIG2 (insulin induced gene 2), and leptin receptor 34–36. Again, the outcomes of these studies were contradictory. While one study in 200 patients concluded that there is no influence of genetic susceptibility on excess weight loss after RYGB 34, another study in 1000 morbidly obese patients concluded that high allelic burden of four obesity SNPs is associated with poorer weight loss following RYGB 35. In other reports carrying out gastric bypass surgery in obese subjects with known hypothalamic injury induced by epithelial neoplasms called craniopharyngiomas, it was concluded that the effects on food intake and weight loss may not essentially rely on hypothalamic mechanisms 37, 38.
Because one of the key molecular changes in obesity is decreased sensitivity of hypothalamic neurons to leptin and insulin, reversing this resistance would be the most direct way to reset the defended level of adiposity. In fatty Zucker rats which are completely leptin resistant due to a leptin receptor mutation, RYGB surgery is still able to reduce body weight 39. Clearly, calorie restriction-induced weight loss alone can partially reverse leptin and insulin resistance 40, but it does not seem to fully reset the defended level of body weight, as indicated by the typical weight regain when forced calorie-restriction is lifted 18. Although sleeve gastrectomy induced significant weight loss, it did not improve leptin sensitivity or leptin receptor expression beyond the pair-fed group 25.
Taken together, from analyses of hypothalamic peptide expression and functionality of their immediate downstream signaling pathways, there is limited and inconsistent support for the notion that bariatric surgery changes the set point of the homeostatic regulator. Failure to support this idea may be due to the design of the experiment, particularly the timing of measurements in rodent models, and insufficient numbers of subjects in human studies.
Arguably the best test of set-point theory is to observe the behavioral response to body weight/adiposity perturbations. If after a period of imposed weight loss or gain, body weight returns promptly to pre-perturbation levels by increasing or decreasing energy intake, respectively, it can be said that this preferred body weight level is defended. In support of such a set point, sleeve-gastrectomized rats that had lost additional body weight by restricting their calorie intake promptly returned to their prerestriction body weight by increasing energy intake 25. This suggests that sleeve gastrectomized rats are able to substantially increase their food intake when necessary, but they chose not to do so to defend their lower body weight set point. However, similar experiments have not been done on other rodent bariatric surgery models, or in human subjects. Furthermore, the critical reciprocal test of forced over-feeding has not been carried out. Because these tests require either under-nutrition in already undernourished animals, often with signs of micronutrient malabsorption, or over-nutrition which can only be done by forced feeding (gavage) into a severely altered gastrointestinal tract, they are not without problems.
Potential signals mediating the effects of bariatric surgery on brain homeostatic functions
The surgically altered gut can signal to the brain directly or indirectly (via other organs such as the liver and pancreas) by two basic routes, the circulation and neural pathways 41 (Fig. 1). Because postprandial levels of the gut hormones Glucagon-like peptide-1 (GLP-1), and Peptide YY (PYY) have consistently been found to be dramatically increased after both RYGB 42, 43 and vertical sleeve gastrectomy 44, they have received by far the most attention as potential mediators of the beneficial effects of bariatric surgeries on glucose and body weight homeostasis 45. GLP-1 in particular could play a significant role in both the rapid improvement of glucose homeostasis and suppression of food intake through its multiple actions on the pancreas, caudal brainstem 14, and basomedial hypothalamus 46. To more directly implicate a critical role for exaggerated GLP-1 signaling, it will be important to demonstrate reduced beneficial effects of RYGB and VSG on body weight and glucose homeostasis in GLP-1 receptor null mice and during chronic pharmacological blockade of GLP-1 receptor signaling.
Ghrelin, another gut hormone that can directly affect activity of mediobasal hypothalamic neurons 47 may also not be critical, as vertical sleeve gastrectomy was just as efficient to suppress body weight in ghrelin-deficient mice 48.
Both RYGB and sleeve gastrectomy also result in increased levels of circulating bile acids 49–52 that signal through the nuclear receptor FXR and the membrane receptor TGR5 to a number of organs 53–56. The conjugated bile acid tauroursodeoxycholic acid (TUDCA), which decreases endoplasmatic reticulum (ER) stress, is a potent leptin-sensitizer in the hypothalamus of obese mice 57, likely resulting in a change of body weight set-point. The feedback control loop that regulates the total pool of bile acids also involves FXR-mediated stimulation of fibroblast growth factors-19 (FGF19) in humans and FGF15 in mice 53, and levels of FGF19 as well as FGF21 are significantly increased after RYGB surgery 49. FGF21 is known to improve glucose and body weight homeostasis through multiple pathways 58–60. The powerful anorexic and body weight lowering effects of monoclonal antibodies to the FGF1c-receptor that have partial agonist activity 61 suggest the intriguing possibility that the bile acid – FGF signaling pathway may be crucial for the success of RYGB and sleeve gastrectomy. In addition, bile acid signaling through the membrane receptor TGR5 has been found to increase brown fat thermogenesis 62 and GLP-1 secretion 63. Thus, bile acid-signaling is involved in fine-tuning energy homeostasis and its role in mediating the beneficial effects of bariatric surgeries may be very fruitful.
Effects of bariatric surgery on food reward mechanisms
A fundamental unanswered question is whether neural systems of reward, cognition, and emotion (Fig. 3) that are also involved in the control of food intake and energy expenditure, are part of an extended homeostatic regulator or whether they operate independently or non-homeostatically 64. If the latter were true, one could expect that the homeostatic regulator would compensate for any energy surfeit caused by increased food consumption and/or lower physical activity associated with the modern lifestyle. Considering the unabated obesity epidemic and Western diet-fed rodent models, this is clearly not the case. The concept of an extended homeostatic system is more plausible. It further suggests that there is no fixed set-point for body weight or adiposity, but that its defense is flexible and depends at least to some extent on the environment 65. It would follow that not just inputs and disturbances to the classical homeostatic neural circuitry in hypothalamus and brainstem, but also to the extended circuitry in reward, cognitive, and emotional brain areas, can result in body weight changes. This interpretation receives increasing support from studies demonstrating that classical “homeostatic” hormones such as leptin, insulin, and gut hormones can change food intake by their specific action in reward and associated brain areas 66–73, and see 74 for a review. Although reward and cognitive brain functions are directly driven by conditioned and unconditioned stimuli from the environment, such as ease of availability, palatability, social context etc., it is clear that metabolic state can powerfully modulate the incentive salience of such stimuli 75. Adopting this view of an expanded homeostatic regulation system, not only alterations in “classical” homeostatic hypothalamic and brainstem circuitry, but also in sensory and corticolimbic structures representing reward and cognitive functions, can cause overeating and obesity, and bariatric surgeries could act via these systems to reverse obesity (Fig. 3).
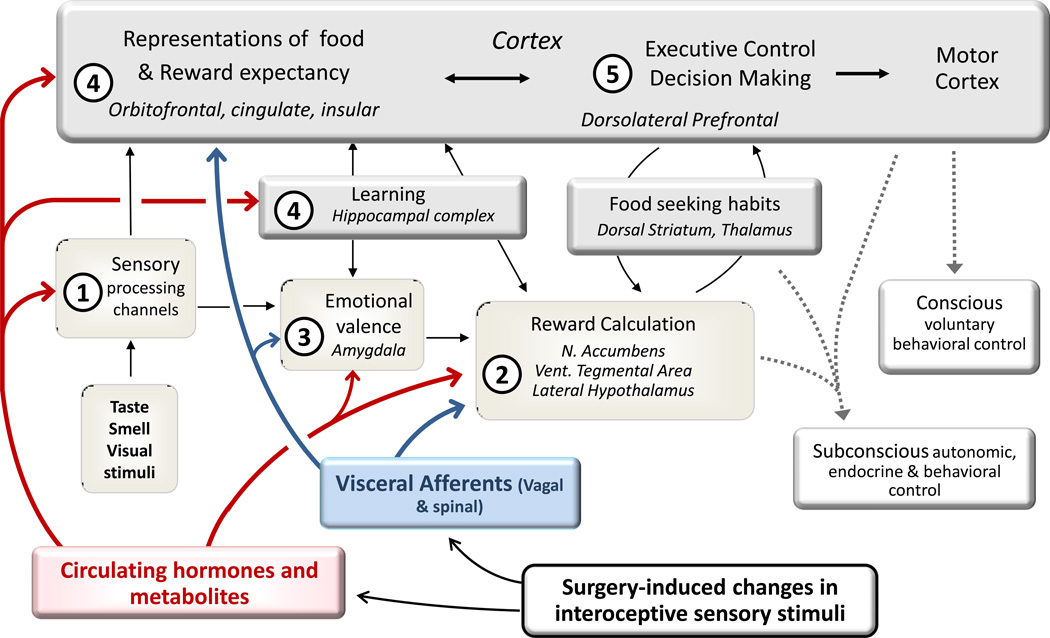
Fig. 3. Potential effects of bariatric surgery on brain areas involved in reward.
Brain areas involved inreward, cognitive, and emotional functions contributing to the control of food intake and representing the expanded homeostatic system regulating energy balance. Changes in circulating hormones and metabolites as well as changes in neuronal inputs from visceral afferents may affect (1) processing of sensory information all along the specific input pathways, (2) reward computation in the mesocorticolimbic dopamine system including the nucleus accumbens, (3) emotional valence computation in the amygdala, (4) formation and modification of “food memories” in insular and prefrontal cortex mediated by the hippocampal complex, and (5) decision making and executive control.
Studies in post-bariatric surgery patients, although still relatively anecdotal and uncontrolled, start to provide clear support for a role of reward and cognition. Several studies noted an RYGB surgery-induced shift from high-calorie to lower calorie foods 76–78, and a lowering of sweet taste detection threshold 79. That these surgery-induced changes in food choice may depend on alterations in brain reward functions was suggested by using the “Power of Food Scale”, a measure of hedonic appetite. It was shown that when tested with food present in the environment (but not available) and food readily available but not tasted, obese subjects scored significantly higher (more hedonic hungry) than lean subjects and that the exaggerated response was normalized 6 months after RYGB surgery 19. Using functional magnetic resonance imaging (fMRI) and PET-imaging with dopamine receptor ligands, it was possible to associate such behavioral changes with specific brain structures involved in reward processing and decision making 80–82.
Studies in rodent models have begun to identify changes in reward behaviors and their underlying neural circuits. In several obese rat models of RYGB surgery, it was shown that taste preference for sweet and oily stimuli, which is shifted to the right (higher concentrations) in obese rats, is reversed after RYGB surgery 83–85. Similar effects were found with oily stimuli (intralipid) in a non-obese rat model after RYGB 86. Because calorie restriction-induced weight loss in high-fat-fed obese rats without any surgery resulted in a similar left shift of the sucrose and corn oil concentration response curves 87, the effect of RYGB is likely secondary to weight loss and not some specific effect of RYGB. It will be interesting to identify the mechanisms by which obesity and RYGB change hedonic evaluation. Given the new concept of “taste in the gut” 88 it may involve obesity-associated blunting of vagal sensory functions 89 or, alternatively, processing of taste information along the gustatory pathways as well as hedonic processing in cortico-limbic brain areas. It Is likely that these changes in acute taste responsiveness to sweet and oily stimuli are at least partly responsible for the gradual shift in food choice from high-calorie to low-calorie foods observed in several rodent models of RYGB 90 and VSG 91 as well as in humans 77, 78.
Concluding remarks and outstanding questions
Finding the mechanisms by which some bariatric surgeries so rapidly resolve diabetes and effectively reduce body weight has turned out to be more difficult than anticipated. This is likely due to the complexity of the signaling changes that take place after such surgeries. Not only is there a large number of changes in humoral and neural signaling to many other organs, but dynamical changes occur over time and cannot all be captured in purely cross-sectional studies. Future research in both clinical and animal models needs to take such dynamic changes into account. There is much hope that the specific molecular mechanisms responsible for the beneficial effects of bariatric surgeries will be revealed by applying the emerging murine models to genetically altered animals. However, pitfalls associated with compensatory re-wiring in knockout models, particularly of hypothalamic regulatory circuits, need to be considered. Thus, targeted pharmacologic or genetic interventions at various times after surgery, will remain an important component of the tool kit. Furthermore, because in rodent models weight loss is rather rapid, new methodologies will be required to clearly distinguish weight loss-dependent from weight loss-independent mechanisms. Also, it is difficult to distinguish compensatory feeding induced by the rapid weight loss form primary surgery-induced effects on feeding during the immediate postoperative period.
Novel non-invasive imaging methods to assess brain functions in a longitudinal fashion would be most desirable. Because in many rodent models energy intake returns to near normal levels, the second phase may be mainly characterized by increased levels of energy expenditure, but interpretational problems associated with appropriate scaling to total body mass or fat-free mass have prevented clear consensus.
Thus, although bariatric surgery has significantly improved the quality od life in many obese patients, it is still not clear whether these subjects are truly happy with eating less, or whether they secretly long for food but can’t eat. A lot more definitive studies with improved methodologies will be necessary to fully understand the mechanisms involved in either scenario.
Acknowledgements
Supported by the National Institutes of Health grants DK047348 and DK 071082
Glossary
- Duodenal-jejunal bypass
is a surgical procedure that bypasses the duodenum and upper jejunum, but not the stomach. The cut distal end of the jejunum is anastomosed to the pyloric sphincter of the stomach and the proximal end is anastomosed to the ileum. The stomach retains its reservoir function, but nutrients are emptied directly into more distal portions of the jejunum.
- Gastric banding
restricts nutrient flow into the stomach by placing an adjustable band around the cardia of the stomach, but does not change the flow of nutrients emptied from the stomach.
- Roux-en-Y gastric bypass surgery (RYGB)
both restricts gastric volume and re-routes nutrient flow in the small intestine. The cut distal end of the mid-jejunum is anastomosed to a small gastric pouch that is surgically separated from the main body of the stomach. The proximal end of the cut jejunum is anastomosed with the ileum for drainage of bile and pancreatic juices (biliopancreatic limb). Thus, nutrients flow from the gastric pouch directly into more distal parts of the jejunum (the Roux-limb)
- Sleeve gastrectomy
also called gastric sleeve, is a surgical procedure in which a large portion of the stomach is surgically removed resulting to about 25% reduction of its original size. The remaining open edges are attached together to form a sleeve. The procedure, that is performed laparoscopically, permanently reduces the size of the stomach and is used as a means for weight loss therapy in extremely obese patients.
- Set-point theory
states that body weight and fat mass are regulated by a mechanism similar to a thermostat regulating room temperature. It assumes that adiposity signals such as leptin feedback to an adipostat in the hypothalamus to defend a certain level of fat mass.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mingrone G, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N. Engl. J. Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 2.Schauer PR, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N. Engl. J. Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brobeck JR. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol. Rev. 1946;26:541–559. doi: 10.1152/physrev.1946.26.4.541. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MW, et al. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 5.Cowley MA, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 6.Luquet S, et al. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 7.Krashes MJ, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 2011 doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morton GJ, et al. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 9.Kohno D, et al. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52:948–956. doi: 10.2337/diabetes.52.4.948. [DOI] [PubMed] [Google Scholar]
- 10.Riediger T, et al. Peptide YY directly inhibits ghrelin-activated neurons of the arcuate nucleus and reverses fasting-induced c-Fos expression. Neuroendocrinology. 2004;79:317–326. doi: 10.1159/000079842. [DOI] [PubMed] [Google Scholar]
- 11.Berthoud H-R. Multiple neural systems controlling food intake and body weight. Neurosci. Biobehav. Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 12.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu. Rev. Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 13.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes MR, et al. Caudal brainstem processing is sufficient for behavioral, sympathetic and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008 doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Souza CT, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 16.Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams LM. Hypothalamic dysfunction in obesity. Proc. Nutr. Soc. 2012;71:521–533. doi: 10.1017/S002966511200078X. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum M, et al. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J. Clin. Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultes B, et al. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am. J. Clin. Nutr. 2010;92:277–283. doi: 10.3945/ajcn.2009.29007. [DOI] [PubMed] [Google Scholar]
- 20.Miras AD, et al. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am. J. Clin. Nutr. 2012;96:467–473. doi: 10.3945/ajcn.112.036921. [DOI] [PubMed] [Google Scholar]
- 21.Paulsen SJ, et al. Gene expression profiling of individual hypothalamic nuclei from single animals using laser capture microdissection and microarrays. J. Neurosci. Methods. 2009;177:87–93. doi: 10.1016/j.jneumeth.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Nadreau E, et al. Effects of the biliopancreatic diversion on energy balance in the rat. Int J Obes (Lond) 2006;30:419–429. doi: 10.1038/sj.ijo.0803166. [DOI] [PubMed] [Google Scholar]
- 23.Warne JP, et al. Metabolic and neuroendocrine consequences of a duodenal-jejunal bypass in rats on a choice diet. Ann. Surg. 2009;249:269–276. doi: 10.1097/SLA.0b013e3181961d5d. [DOI] [PubMed] [Google Scholar]
- 24.Romanova IV, et al. Neurobiologic changes in the hypothalamus associated with weight loss after gastric bypass. J. Am. Coll. Surg. 2004;199:887–895. doi: 10.1016/j.jamcollsurg.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Stefater MA, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138:2426–2436. doi: 10.1053/j.gastro.2010.02.059. 2436 e2421-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balthasar N, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Butler AA, Cone RD. Knockout models resulting in the development of obesity. Trends Genet. 2001;17:S50–S54. doi: 10.1016/s0168-9525(01)02481-7. [DOI] [PubMed] [Google Scholar]
- 28.Hatoum IJ, et al. Melanocortin-4 Receptor Signaling Is Required for Weight Loss after Gastric Bypass Surgery. J. Clin. Endocrinol. Metab. 2012 doi: 10.1210/jc.2011-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mul JD, et al. Effect of vertical sleeve gastrectomy in melanocortin receptor 4-deficient rats. Am J Physiol Endocrinol Metab. 2012;303:E103–E110. doi: 10.1152/ajpendo.00159.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aslan IR, et al. Bariatric surgery in a patient with complete MC4R deficiency. Int J Obes (Lond) 2011;35:457–461. doi: 10.1038/ijo.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potoczna N, et al. Gene variants and binge eating as predictors of comorbidity and outcome of treatment in severe obesity. J. Gastrointest. Surg. 2004;8:971–981. doi: 10.1016/j.gassur.2004.09.032. discussion 981-972. [DOI] [PubMed] [Google Scholar]
- 32.Aslan IR, et al. Weight loss after Roux-en-Y gastric bypass in obese patients heterozygous for MC4R mutations. Obes. Surg. 2011;21:930–934. doi: 10.1007/s11695-010-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirshahi UL, et al. The MC4RI251L) allele is associated with better metabolic status and more weight loss after gastric bypass surgery. J. Clin. Endocrinol. Metab. 2011;96:E2088–E2096. doi: 10.1210/jc.2011-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goergen M, et al. Influence of obesity-susceptibility loci (MC4R and INSIG2) on the outcome of weight loss and amelioration of co-morbidity in obese patients treated by a gastric-bypass. Bull. Soc. Sci. Med. Grand. Duche Luxemb. 2011:7–24. [PubMed] [Google Scholar]
- 35.Still CD, et al. High allelic burden of four obesity SNPs is associated with poorer weight loss outcomes following gastric bypass surgery. Obesity (Silver Spring) 2011;19:1676–1683. doi: 10.1038/oby.2011.3. [DOI] [PubMed] [Google Scholar]
- 36.Parikh M, et al. Frequencies of obesity susceptibility alleles among ethnically and racially diverse bariatric patient populations. Surg Obes Relat Dis. 2012 doi: 10.1016/j.soard.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultes B, et al. Distal gastric bypass surgery for the treatment of hypothalamic obesity after childhood craniopharyngioma. Eur. J. Endocrinol. 2009;161:201–206. doi: 10.1530/EJE-09-0079. [DOI] [PubMed] [Google Scholar]
- 38.Bingham NC, et al. Bariatric surgery in hypothalamic obesity. Front Endocrinol (Lausanne) 2012;3:23. doi: 10.3389/fendo.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meirelles K, et al. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann. Surg. 2009;249:277–285. doi: 10.1097/SLA.0b013e3181904af0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelley DE, et al. Effects of moderate weight loss and orlistat on insulin resistance, regional adiposity, and fatty acids in type 2 diabetes. Diabetes Care. 2004;27:33–40. doi: 10.2337/diacare.27.1.33. [DOI] [PubMed] [Google Scholar]
- 41.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol. Motil. 2008;20(Suppl 1):64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin AC, et al. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151:1588–1597. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korner J, et al. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chambers AP, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.le Roux CW, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 46.Sandoval DA, et al. Arcuate GLP-1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008 doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briggs DI, et al. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151:4745–4755. doi: 10.1210/en.2010-0556. [DOI] [PubMed] [Google Scholar]
- 48.Chambers AP, et al. The Effects of Vertical Sleeve Gastrectomy in Rodents are Ghrelin Independent. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jansen PL, et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig. Dis. 2011;29:48–51. doi: 10.1159/000324128. [DOI] [PubMed] [Google Scholar]
- 50.Pournaras DJ, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simonen M, et al. Conjugated Bile Acids Associate with Altered Rates of Glucose and Lipid Oxidation after Roux-en-Y Gastric Bypass. Obes. Surg. 2012;22:1473–1480. doi: 10.1007/s11695-012-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cummings BP, et al. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 2012;153:3620–3632. doi: 10.1210/en.2012-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porez G, et al. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J. Lipid Res. 2012;53:1723–1737. doi: 10.1194/jlr.R024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keitel V, et al. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia. 2010;58:1794–1805. doi: 10.1002/glia.21049. [DOI] [PubMed] [Google Scholar]
- 55.Castro-Caldas M, et al. Tauroursodeoxycholic Acid Prevents MPTP-Induced Dopaminergic Cell Death in a Mouse Model of Parkinson's Disease. Mol. Neurobiol. 2012 doi: 10.1007/s12035-012-8295-4. [DOI] [PubMed] [Google Scholar]
- 56.Schubring SR, et al. The bile steroid chenodeoxycholate is a potent antagonist at NMDA and GABA(A) receptors. Neurosci. Lett. 2012;506:322–326. doi: 10.1016/j.neulet.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 57.Ozcan L, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Sarruf DA, et al. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59:1817–1824. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu AL, et al. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci Transl Med. 2011;3:113ra126. doi: 10.1126/scitranslmed.3002669. [DOI] [PubMed] [Google Scholar]
- 60.Kralisch S, Fasshauer M. Fibroblast growth factor 21: effects on carbohydrate and lipid metabolism in health and disease. Curr Opin Clin Nutr Metab Care. 2011;14:354–359. doi: 10.1097/MCO.0b013e328346a326. [DOI] [PubMed] [Google Scholar]
- 61.Sun HD, et al. Monoclonal antibody antagonists of hypothalamic FGFR1 cause potent but reversible hypophagia and weight loss in rodents and monkeys. Am J Physiol Endocrinol Metab. 2007;292:E964–E976. doi: 10.1152/ajpendo.00089.2006. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe M, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 63.Thomas C, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shin AC, et al. An expanded view of energy homeostasis: neural integration of metabolic, cognitive, and emotional drives to eat. Physiol. Behav. 2009;97:572–580. doi: 10.1016/j.physbeh.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Speakman JR, et al. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Dis Model Mech. 2011;4:733–745. doi: 10.1242/dmm.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leinninger GM, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Opland DM, et al. Modulation of the mesolimbic dopamine system by leptin. Brain Res. 2010;1350:65–70. doi: 10.1016/j.brainres.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leinninger GM, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dossat AM, et al. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J. Neurosci. 2011;31:14453–14457. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skibicka KP, et al. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–137. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 71.Konner AC, et al. Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell Metab. 2011;13:720–728. doi: 10.1016/j.cmet.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 72.Speed N, et al. Impaired striatal Akt signaling disrupts dopamine homeostasis and increases feeding. PLoS ONE. 2011;6:e25169. doi: 10.1371/journal.pone.0025169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunn JP, et al. Relationship of dopamine type 2 receptor binding potential with fasting neuroendocrine hormones and insulin sensitivity in human obesity. Diabetes Care. 2012;35:1105–1111. doi: 10.2337/dc11-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berthoud HR. Interactions between the "cognitive" and "metabolic" brain in the control of food intake. Physiol. Behav. 2007;91:486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 75.Berridge KC, et al. Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kenler HA, et al. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am. J. Clin. Nutr. 1990;52:87–92. doi: 10.1093/ajcn/52.1.87. [DOI] [PubMed] [Google Scholar]
- 77.Olbers T, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann. Surg. 2006;244:715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thirlby RC, et al. Effect of Roux-en-Y gastric bypass on satiety and food likes: the role of genetics. J. Gastrointest. Surg. 2006;10:270–277. doi: 10.1016/j.gassur.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 79.Burge JC, et al. Changes in patients' taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J. Am. Diet. Assoc. 1995;95:666–670. doi: 10.1016/S0002-8223(95)00182-4. [DOI] [PubMed] [Google Scholar]
- 80.Ochner CN, et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann. Surg. 2011;253:502–507. doi: 10.1097/SLA.0b013e318203a289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steele KE, et al. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes. Surg. 2010;20:369–374. doi: 10.1007/s11695-009-0015-4. [DOI] [PubMed] [Google Scholar]
- 82.Dunn JP, et al. Decreased dopamine type 2 receptor availability after bariatric surgery: preliminary findings. Brain Res. 2010;1350:123–130. doi: 10.1016/j.brainres.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shin AC, et al. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 2011;35:642–651. doi: 10.1038/ijo.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hajnal A, et al. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G967–G979. doi: 10.1152/ajpgi.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tichansky DS, et al. Decrease in sweet taste in rats after gastric bypass surgery. Surg. Endosc. 2011;25:1176–1181. doi: 10.1007/s00464-010-1335-0. [DOI] [PubMed] [Google Scholar]
- 86.le Roux CW, et al. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1057–R1066. doi: 10.1152/ajpregu.00139.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shin AC, et al. "Liking" and "wanting" of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1267–R1280. doi: 10.1152/ajpregu.00314.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reimann F, et al. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15:421–431. doi: 10.1016/j.cmet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 89.de Lartigue G, et al. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinol Metab. 2011;301:E187–E195. doi: 10.1152/ajpendo.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng H, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1273–R1282. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilson-Perez HE, et al. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berthoud HR, Munzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: From electrical self-stimulation to opto-genetics. Physiol. Behav. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]