Abstract
Vancomycin has been shown to affect tumor necrosis factor-alpha (TNF-α) pathways as an immunomodulator; this is thought to be separate from its function as an antibiotic [1]. Previous studies have shown that oral vancomycin (OV) is an effective treatment for concomitant primary sclerosing cholangitis (PSC) and inflammatory bowel disease (IBD) in children [2, 3]. Since both diseases are associated with immune dysfunction, we hypothesized that vancomycin’s therapeutic effect in IBD and PSC occurs through immunomodulation. Therefore, we examined the in vivo immunological changes that occur during OV treatment of 14 children with PSC and IBD. Within 3 months of OV administration, peripheral gamma-glutamyl transpeptidase (GGT) and alanine aminotransferase (ALT) concentrations, white blood cell (WBC) counts, and neutrophil counts normalized from elevated levels before treatment. Patients also demonstrated improved biliary imaging studies, liver biopsies and IBD symptoms and biopsies. Additionally, plasma transforming growth factor beta (TGF-β) levels were increased without concurrent shifts in Th1- or Th2-associated cytokine production. Peripheral levels of CD4+CD25hiCD127lo and CD4+FoxP3+ regulatory T (Treg) cells also increased in OV-treated PSC+IBD patients compared to pretreatment levels. A unique case study shows that the therapeutic effects of OV in the treatment of PSC+IBD do not always endure after OV discontinuation, with relapse of PSC associated with a decrease in blood Treg levels; subsequent OV retreatment was then associated with a rise in blood Treg levels and normalization of liver function tests (LFTs). Taken together, these studies support immune-related pathophysiology of PSC with IBD, which is responsive to OV.
Keywords: vancomycin, inflammatory bowel disease, primary sclerosing cholangitis, regulatory T cell
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a cholestatic disease characterized by chronic inflammation of the intrahepatic and/or extrahepatic bile ducts, often progressing to cirrhosis of the biliary tree, and eventually to liver failure [2–4]. Recent research indicates that onset and progression of PSC may result from immunologic dysfunction, complications associated with atypical environmental exposure, genetic predisposition, or a combination of these factors [5–8]. This multi-factorial disease etiology as well as the various manifestations of PSC (e.g. small/large duct PSC, IgG4-related PSC, IBD-associated PSC, and autoimmune hepatitis overlap PSC) has led to the implementation of many different treatment modalities to treat PSC (e.g. ursodeoxycholic acid and corticosteroid use), but a consensus therapy exhibiting clinical efficacy in trials has yet to be revealed.
We focused our studies on how OV might reduce inflammation in children with PSC and IBD through an immunomodulating effect on the lymphocytes. Related studies in murine models have demonstrated how specific directional selection of commensal microflora by OV administration may result in the induction of regulatory T (Treg) cells, an immunoregulatory cell type known for its anti-inflammatory properties [9–11]. Gut mucosal induction of Tregs has been implicated in the prevention and even reversal of intestinal and extraintestinal inflammatory processes [9, 12–14]. These findings and other similar reports [15–17] provide insight as to how OV administration may exhibit downstream anti-inflammatory properties in a disease setting such as PSC+IBD.
To better understand the therapeutic characteristics of OV treatment of pediatric PSC+IBD, this study assesses the clinical and immunological changes that occurred during their treatment with OV. We confirm, as previously reported [2, 3], that OV results in normalization of liver function test scores in these patients, and further describe normalizations in their total WBC, neutrophil, and serum auto-antibody titers. Our immunology assays reveal longitudinal elevations in their peripheral TGF-β levels and, further, demonstrate elevations in peripheral CD4+FoxP3+ and CD4+CD25(IL-2Rα)hiCD127(IL-7Rα)lo Treg levels that endure throughout the duration of treatment. This elevation is observed as early as 2 weeks post OV administration, reaching maximum levels by 3 months of daily treatment; however, it is not always sustained after discontinuation from OV. Significantly, in one case study, relapse of PSC+IBD following removal from OV coincides with a dramatic reduction in peripheral Treg levels. Our results suggest that the therapeutic properties of OV in the treatment of PSC+IBD may be directly linked to heterologous Treg induction.
METHODS
Human Subjects: Inclusion Criteria
14 subjects (12 male, 2 female; mean age =16.1 ± 4.7 yrs; range = 2–18 yrs) diagnosed with concomitant PSC and IBD (13 UC, 1 CD) were selected for inclusion in this study. All subjects were diagnosed with PSC by liver biopsy and imaging studies (endoscopic retrograde cholangiopancreatography (ERCP) and/or magnetic resonance cholangiopancreatography (MRCP)) and IBD by tissue biopsy and IBD serology. We define PSC patients with IBD on OV for ≥ 1 month as “Treated PSC.” The dosing of vancomycin was 50 mg/kg/day divided 3 times per day with a maximal dose of 1500 mg/day for those weighing ≥ 30 kg. Any cases of non-responsiveness resulted from non-compliance with medication; to date, all compliant patients have responded to OV treatment. Those subjects diagnosed with PSC but who were not on OV at the time of blood draw are defined as “Untreated PSC” patients.
PSC patients undergoing parallel treatments with other medications (e.g. steroid medications, Remicade) that could possibly affect their immune cell populations and phenotype were excluded from this study. Some patients were on steady treatment for their IBD with 5-aminosalicylic acid (5-ASA) and/or 6-mercaptopurine (6-MP) during the study but no changes were made in these medications before and while on OV. Upon enrollment, the patients on 6-MP still had clinical and histological signs of IBD in spite of the treatment, had resolution of their PSC and IBD inflammation on OV, and had similar changes in their immunologic studies as the others while on OV; these patients were therefore included in this study. As this study focuses on the therapeutic benefits of OV in the treatment of pediatric PSC+IBD, we choose to focus on subjects who have responded well to OV as reported by GGT and ALT normalization and symptomatic resolution by the time of these analyses.
Nine subjects were included as healthy controls in order to identify the method-specific baseline levels of peripheral regulatory T cells as determined by surface and intracellular staining. Healthy controls are children under the age of 18 who have no acute/chronic infection, no chronic disease, no medications, and no allergies.
Sample Collection
4–20mL of blood was collected into BD Vacutainer EDTA (BD Biosciences, Sparks, MD/USA) tubes at Sutter Medical Group in Sacramento, CA and the Lucile Packard Children’s Hospital at Stanford University. In some cases, blood was collected at other sites and shipped overnight at room temperature for next day processing. Previous work done in the lab has shown this process does not significantly affect Treg levels or fidelity (unpublished observations). Plasma used in the cytokine studies was isolated immediately following the blood draw and then shipped on dry ice. The plasma was from 6 patients in the original Cox et al study [2] and had been stored at −80°C in 1mL aliquots. For cell studies, peripheral blood mononuclear cells (PBMCs) were separated from blood collected into Vacutainer tubes containing EDTA by Ficoll (GE Healthcare Bio-Sciences AB, Uppsala/Sweden) gradient (density 1.077g/mL). PBMCs were isolated and washed twice at 1800RPM for 10 minutes and slow cooled from 4°C to −80°C over 24 hours in FBS (Life Sciences, Carlsbad, CA/USA) + 10% DMSO (Thermo Scientific, Rockford, IL/USA) at a concentration of 1–5×10^6 cells per milliliter. Cell samples were transferred from −80°C after step down cooling was complete to liquid nitrogen for long-term storage.
Plasma cytokine concentration measurement
Stored aliquots of subject plasma from the Cox et al study [2] were thawed, diluted to a concentration of 1:3 with PBS and centrifuged to remove any remaining particulate matter in preparation for measurements of cytokine concentrations. Diluted plasma samples were taken to the Human Immune Monitoring Core (HIMC) at Stanford University and cytokine concentrations were measured in duplicate on the Luminex 37-plex machine located at the facility. Plasma cytokine concentrations were measured at two timepoints: pre-initiation of OV, and 2–3 months post-OV initiation depending on sample availability (in most cases, OV dose was variably tapered after 3 months of treatment).
ALT, GGT and Complete Blood Count (CBC) analyses
Data from biochemical tests of liver function (n=6) and complete blood counts with differential (n=8) were tabulated and analyzed longitudinally. Data from such tests already published in the original Cox et al study [2] were not included.
Flow Cytometry and Cell Identification
PBMCs stored in liquid nitrogen were thawed for use at 37°C and immediately resuspended in RPMI-1640 + 10% FBS (Life Sciences) at 4°C. Prior to surface staining, cells were resuspended in 90uL cold PBS+1% FBS and incubated with 10uL FcR blocking agent (BD Biosciences) to prevent non-specific antibody binding. Cells were then stained with a panel of monoclonal antibodies against CD4[RPAT4] (BD Biosciences), CD25[BC96] (Biolegend, San Diego, CA/USA), CD127[HIL-7R-M21] (BD Biosciences), FoxP3[PCH101] (eBioscience, San Diego, CA/USA), and IL-10[JES3-19F1] (BD Biosciences). Stained samples were analyzed on a BD FACSCalibur flow cytometer. For intracellular stains, PBMCs were transferred to a 96 well plate and fixed using the BD Phosflow Fix and Perm/Wash Buffers (BD Biosciences) prior to staining. For extracellular experiments only, cell viability was assessed using a propidium iodide (PI) stain (BD Biosciences). Data from dead cells were not included in extracellular stain analyses. Compensation beads (BD Biosciences) were used during every experiment to accurately compensate samples during analysis. Tregs were identified as PI- CD4+CD25hi CD127lo cells as described previously [18] or CD4+FoxP3+.
Statistics
GGT, ALT
Changes in serum GGT and ALT were evaluated for significance using a Friedman test with Dunn’s multiple comparison.
Cytokine measurements
A Wilcoxon matched-pairs signed rank test for paired non-normal data was used to assess any significance in cytokine concentration fluctuation between the two time points considered.
Flow cytometry
Treg frequencies of healthy controls compared to untreated PSC+IBD or PSC+IBD+OV were evaluated for significance using a Mann-Whitney t test. Changes in Treg frequencies in pre- versus post-OV patients were analyzed using a Wilcoxon matched-pairs signed rank test.
Complete Blood Count
A Wilcoxon matched-pairs signed rank test was used to assess changes in peripheral count of white blood cells, neutrophils, and lymphocytes for significance.
RESULTS
Reduction of peripheral gamma-glutamyl transpeptidase, alanine aminotransferase concentrations and complete blood count (CBC) values in PSC+IBD patients on OV
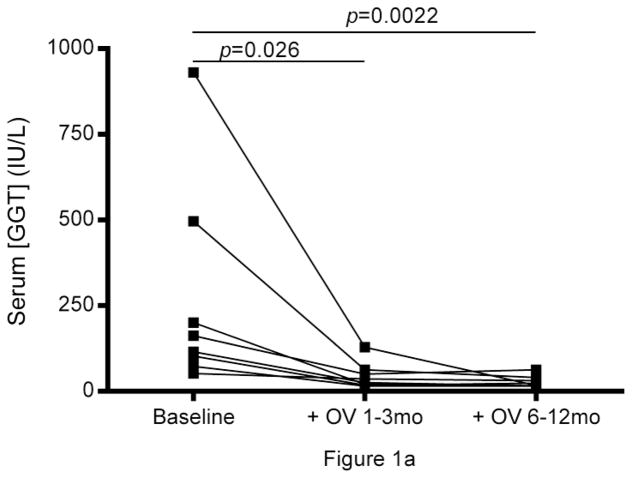
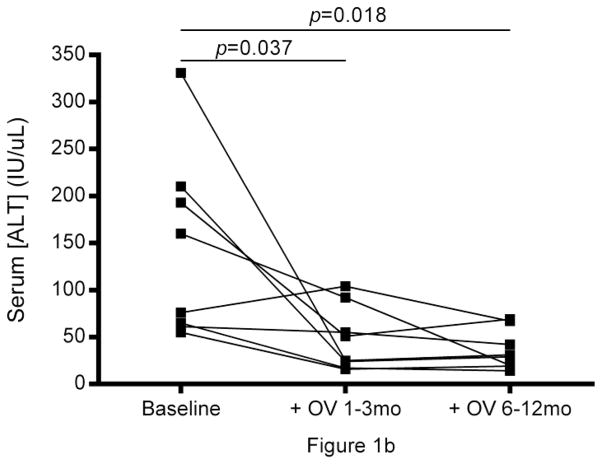
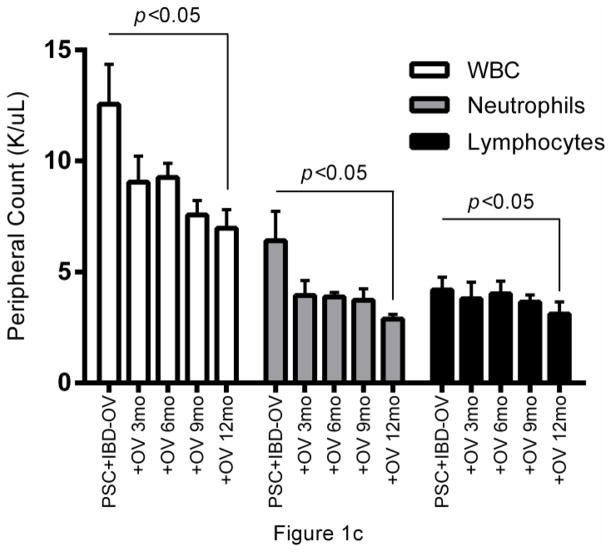
Abnormally high gamma-glutamyl transpeptidase (GGT) and alanine aminotransferase (ALT) were seen in all subjects prior to treatment with OV and normalized within 3 months of therapy and remained normal after 12 months of therapy. (Fig.1a, b). Improvement in symptoms associated with PSC and IBD, biliary imaging (MRCP), and biopsies of liver and intestine were observed, which coincided with a reduction in white blood cell counts (p<0.05, median Δ= −5.05) and a decrease in neutrophil population size (p<0.05, median Δ= −3.22) (Table 1, pFig.1c). Lymphocyte population sizes also decreased following OV administration (<0.05, median Δ= −0.75), though with a markedly smaller median difference between pre- and post-OV measurements.
Fig. 1.
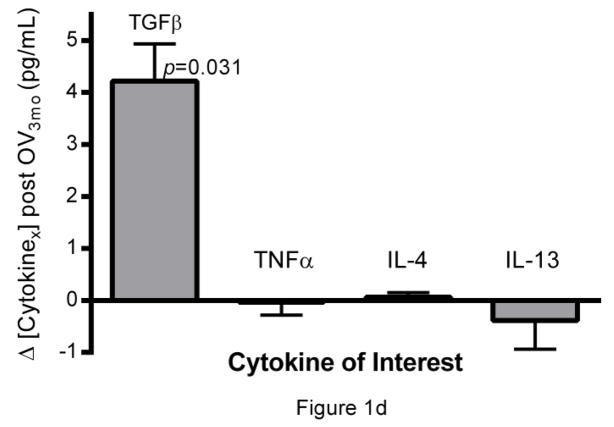
Longitudinal analysis of liver and immune function in PSC + IBD patients during OV treatment. Serum (a) gamma-glutamyl transpeptidase (GGT) and (b) alanine aminotransferase levels (IU/L) over 12 months of OV (n=8). (c) Peripheral count (K/uL) of WBC, neutrophils, and lymphocytes over 12 months of OV (n=6). (d) Longitudinal changes in cytokine production patterns from Th1 (TNFα), Th2 (IL-4, IL-13), and Treg (TGF-β) subtype cells before and 3 months post OV administration in a separate cohort of children with PSC+IBD (n=6) initially reported on in the original Cox et al study [2]
Table 1.
IBD/PSC diagnosis criteria and evidence of resolution during OV for 9 patients
| IBD/PSC Diagnosis Criteria | Evidence of Resolution post OV | |||||
|---|---|---|---|---|---|---|
| Subject | Sex | Age | Intestinal | Liver | Intestinal | Liver |
| 01 | M | 10y | Acute chronic colitis of colon | Chronic hepatitis (grade 2–3) with stage 2 fibrosis | No significant abnormality in intestinal biopsies; resolution of symptoms associated with IBD | Normal bile ducts; no evidence of inflammation; normalization of LFTs |
| 02 | M | 16y | Acute chronic colitis and cryptitis of the cecum, transverse and descending colon with quiescent colitis in sigmoid colon and rectum | Patchy portal tract fibrosis; cholangiolar proliferation; stage III fibrosis | Normal sigmoid, decscending, and transverse colon with good vascularity; minimal inflammation in cecum | Much less inflammation and fibrosis; preservation of portal tracts and overall architecture – stage II fibrosis |
| 03 | M | 3y | Colonic colitis on biopsy | Biliary tract outflow obstruction; chronic active inflammation of portal biliary tree | Asymptomatic for IBD and elimination of positive IBD serology. | No significant abnormality on biopsy – normal biliary tree and liver parenchyma and no evidence of PSC |
| 04 | M | 7y | Moderate acute and chronic inflammation with cryptitis throughout colon and rectum | Bile ductular proliferation; focal bridging fibrosis | Colonic tissues show no significant abnormality; asymptomatic for IBD | Minimal portal inflammation without any other visible abnormalities |
| 05 | F | 2y | Focal acute cryptitis throughout colon | Biliary cirrhosis with prominent cholangiolar and bile ductular proliferation; bridging fibrosis | Improvement in histologic features compared to previous biopsies; asymptomatic for IBD | Improved fibrosis but not cirrhosis. Reduced FIBROSpect score from 98 to 61. |
| 06 | M | 13y | Diffuse chronic and focal acute inflammation of colonic tissue; erythemea and pus in rectal and colonic tissues | Multifocal narrowing and beading of right and left hepatic ducts and branches with biliary strictures; hepatic parenchymal inflammation | No blood/mucus in stools and no abnormality in frequency of stools; no abdominal pain; asymptomatic for IBD | Resolution of biliary strictures; normal liver parenchyma |
| 07 | F | 6y | Evidence of chronic active colitis. Lamina propria expanded and occupied by lymphoplasmacytic infiltrate. Well developed crypt abscesses | Portal and periportal fibrosis. Portal areas expanded by significant inflammatory infiltrates | No diagnostic abnormalities in histological findings | Virtually no inflammation, fibrosis involving portal regions much reduced |
| 08 | M | 15y | Evidence of active colitis in all large bowel biopsies, including rectum. Also evidence of active inflammation with neutrophil infiltrate and small areas of cryptitis | Mild fibrosis and lymphoplasmacytic infiltrate in portal tracts | No evidence of significant abnormality in any biopsies | Liver appears essentially unremarkable. No longer portal fibrosis or inflammation |
| 09 | F | 9y | Crypt architectural distortion and increased lymphoplasmacytic inflammation; consistent with chronic IBD | Portal tracts with mixed inflammatory infiltrate composed predominantly of lymphocytes, also with neutrophils around ductules and in ducts. Evidence of periportal expansion/fibrosis | Marked improvement compared to prior biopsies. Only focal, minimal acute inflammatory changes noted | Portal tracts appear overall normal. No significant inflammation |
Fluctuations in Peripheral Cytokine Concentrations During OV Treatment
Of the cytokines assayed, TGF-β, an immunomodulatory cytokine known to be associated with Treg induction and function, was found to be elevated (p=0.031) after the first three months of OV (Fig.1d). Significant directional alterations in cytokines associated with Th1 (TNFα) and Th2 function (IL-4 and IL-13) were not observed (Fig.1d).
Increased Regulatory T (Treg) Cell Levels in PSC+IBD+OV
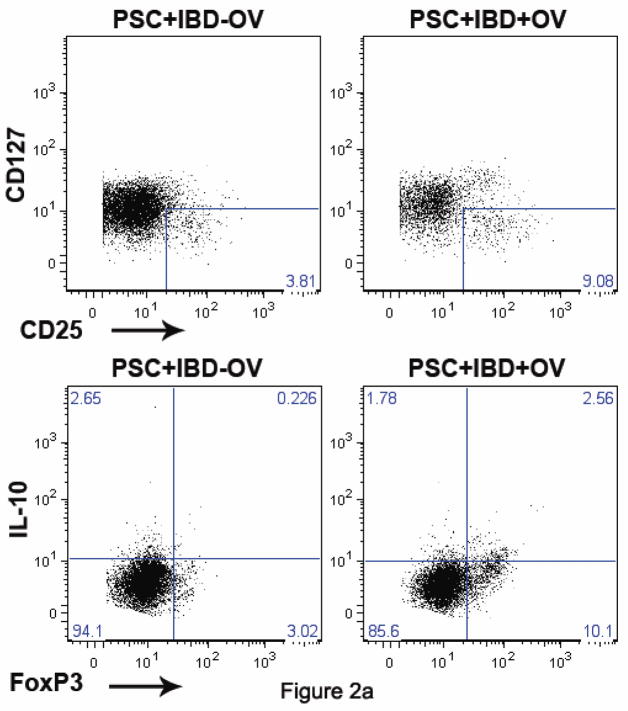
The elevation in peripheral TGF-β levels suggested a possible link between Tregs and OV treatment of pediatric PSC+IBD. Unfortunately, initial protocol for plasma studies did not involve storage of cell samples for further assay. Thus, cell samples were available from only 8 of the PSC+IBD±OV patients and assayed for Treg levels by flow cytometry. As Tregs are not a homogeneous cell class, we defined Tregs in two different ways: (1) CD4+ T cells that express high levels of the IL-2Rα (CD25) and low levels of the IL-7Rα (CD127) as described previously [18, 20] and (2) CD4+ T cells that express the canonical Treg-specific forkhead boxP3 (FoxP3) transcription factor (Fig.2a). Correlative analysis demonstrates that these two populations may have partially overlapping responses to OV, but are likely not identical (Fig.3). As a result, fluctuations in each are reported.
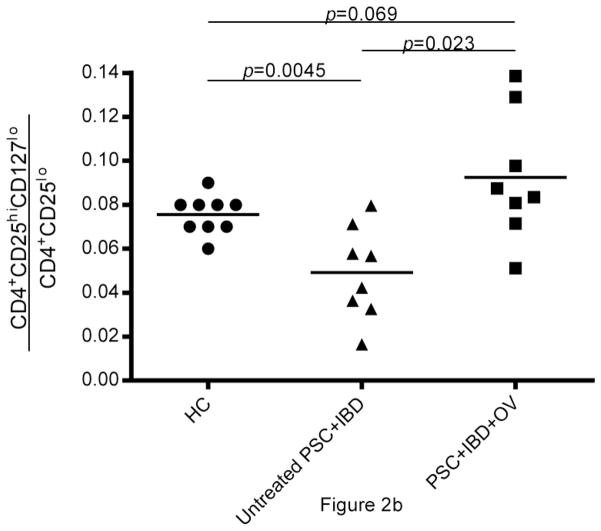
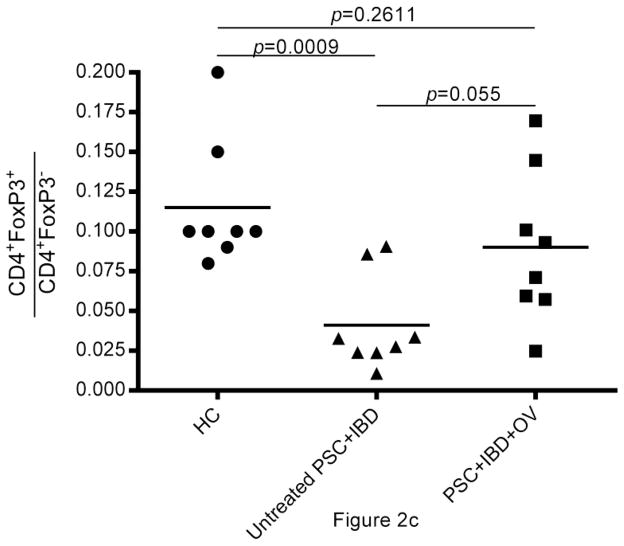
Fig. 2.
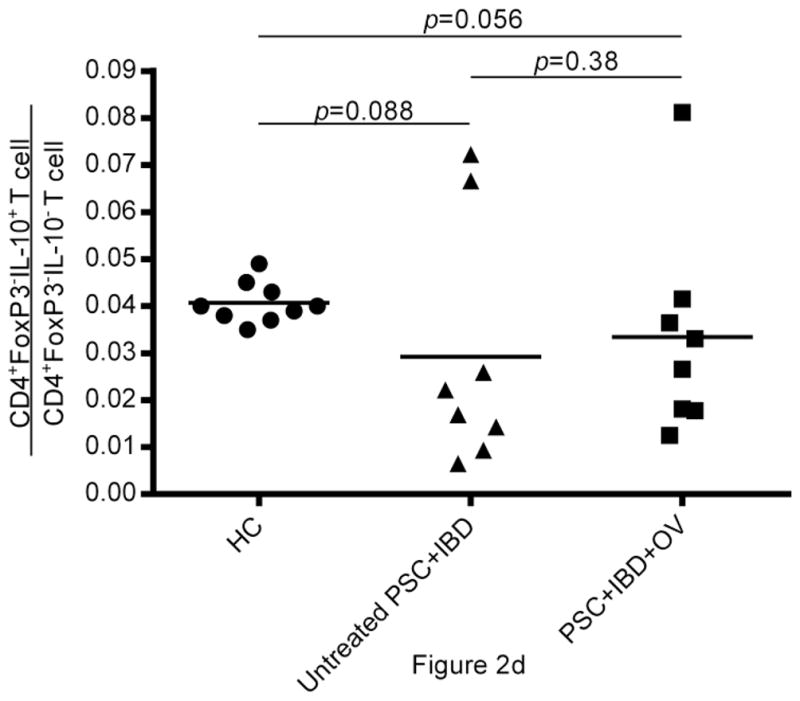
(a) Flow cytometric data from human PBMCs isolated from untreated PSC+IBD subjects and PSC+IBD+OV treated subjects assessing CD4+CD25hiCD127lo and CD4+FoxP3+IL-10+ Treg levels. Data shown for untreated and treated subjects is longitudinal and representative. Comparison of (b) CD4+CD25hiCD127lo and (c) CD4+FoxP3+ Treg levels in peripheral blood of healthy age and gender matched controls with PSC+IBD patients ± OV treatment. (d) IL-10 reservoirs in peripheral CD4+FoxP3- T cells
Fig. 3.
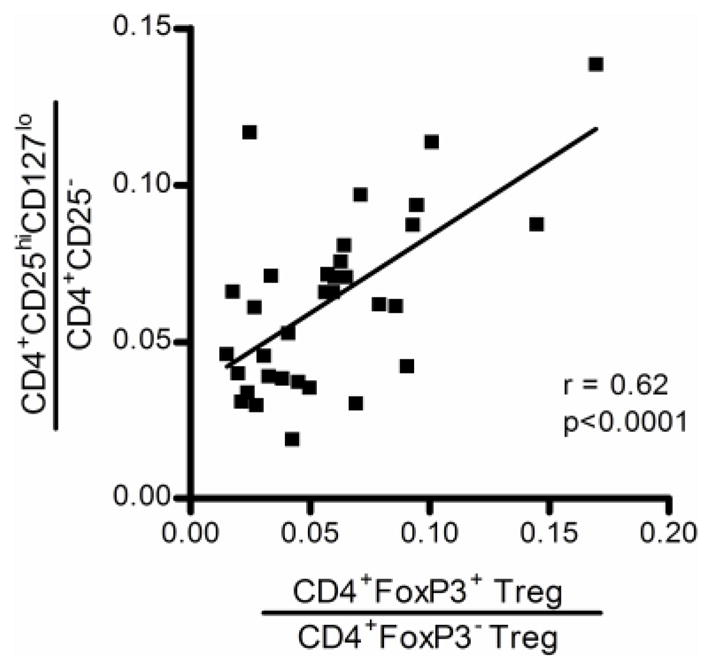
Correlative analysis of CD4+CD25hiCD127lo and CD4+FoxP3+ Treg responses throughout OV in PSC+IBD patients to assess their similarity
Analysis of composite data on the 8 PSC+IBD patient samples available for assay reveals that the proportion of CD4+ T cells made up of Tregs (hereon referred to as “Treg frequency”) is higher in PSC+IBD patients treated with OV than in untreated subjects (Fig.2b, c). CD4+CD25hiCD127lo Treg frequency) in PSC+IBD+OV subjects was higher (p=0.023) than in untreated PSC+IBD patients (mean = 0.049 ± 0.021; range = [0.0165, 0.0796) (Fig.2b). CD4+FoxP3+ Treg frequency in PSC+IBD+OV patients (mean = 0.090 ± 0.048; range = [0.025,0.170]) was also elevated compared to untreated PSC+IBD subjects (mean = 0.041 ± 0.030; range = [0.011,0.091]) (p=0.055) (Fig.2c). Both CD4+CD25hiCD127lo and CD4+FoxP3+ Treg frequencies in untreated PSC+IBD subjects were shown to be lower than in healthy controls (p=0.0045 and 0.0009, respectively) (Fig.2b, c) OV was not observed to affect total T cell population size (data not shown).
Case study of Treg induction in response to long term OV treatment of PSC+IBD
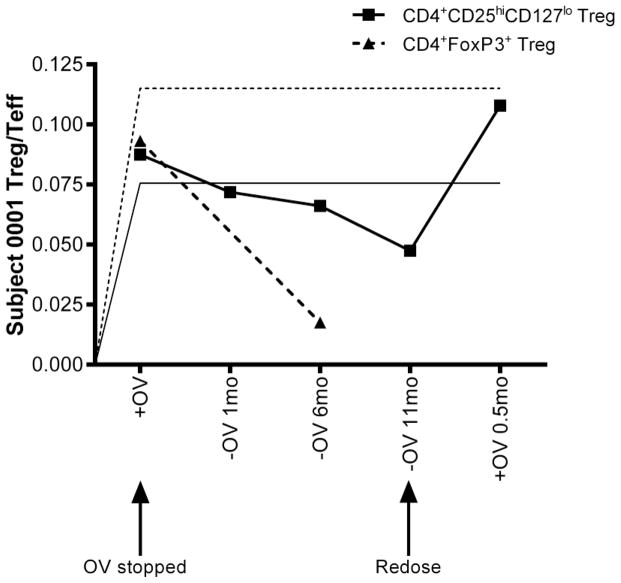
Subject 01 presented with hepatobiliary inflammation with stage II liver fibrosis characteristic of PSC, and colonic-restricted chronic inflammation and focal cryptitis consistent with UC. This subject had elevated GGT (601U/L) and ALT (515U/L) levels and complained of recurring abdominal pain, diarrhea, and vomiting. After two years of daily OV ingestion, repeat colonoscopy revealed complete normalization of colonic tissues and imaging studies demonstrated only minimal intrahepatic and extrahepatic ductal dilatation. Serology and blood chemistry reports showed a nearly 4-fold reduction in perinuclear anti-neutrophil cytoplasmic antibody (p-ANCA) levels from baseline, a trend observed in all subjects reported on in this study, and normalized GGT (24U/L) and ALT levels (19U/L). Immunology assays revealed a two-fold elevation in Treg levels compared to age-matched healthy and diseased controls. Subject 01 reported being asymptomatic. OV was then discontinued, resulting in recurrence and rediagnosis of PSC+IBD over the course of 9 months. Readministration of OV resolved both PSC and IBD within 2 weeks. Importantly, at all time points, patient condition was accurately reflected by elevations or reductions in peripheral Treg frequency (Fig.4)
Fig. 4.
Treg levels in a PSC+IBD patient with a unique OV experience (patient 01). Control CD4+CD25hiCD127lo Treg levels are indicated by a solid horizontal line while CD4+FoxP3+ control levels are indicated by a dashed horizontal line. This case study presents variation in Treg populations during recurrence of PSC+IBD after removal from OV that is successfully resolved with OV-readministration
Treg Specific IL-10 Production in the Periphery
Frequent reports of IL-10 as an effector of immune homeostasis at the level of the gut make it a cytokine of high interest in this study. Of particular interest are T cells that express IL-10 in the absence of FoxP3 (Tr1) as well as FoxP3+IL-10+ Tregs. Intracellular cytokine staining and analysis by flow cytometry was used to better understand the role of T cell specific IL-10 in the therapeutic outcome of OV treatment of PSC+IBD. OV was not observed to affect IL-10 levels in peripheral CD4+FoxP3- T cells. Compared to healthy controls, however, slightly lower IL-10 reservoirs were detected in both untreated (p=0.088) and treated (p=0.056) PSC+IBD patients (Fig.2d). IL-10 reservoirs were detectable in only a very small proportion of CD4+FoxP3+ T cells in the periphery (data not shown).
DISCUSSION
Primary sclerosing cholangitis (PSC) is a rare hepatobiliary disease characterized by chronic inflammation of the biliary tree that often progresses to cirrhosis and liver failure [2–4]. While the direct causes of PSC are unknown, recent studies suggest that the disease may primarily be of immunologic origins [5, 9, 21, 22]. Here, we report on a population of pediatric inflammatory bowel disease patients diagnosed with PSC (PSC+IBD), and observed resolution of PSC while administered daily doses of oral vancomycin (OV). Our studies support immunologic changes that occurred in these PSC+IBD patients during their disease resolution on daily dose OV.
All subjects reported on in this study experienced a reduction in peripheral gamma-glutamyl transpeptidase (GGT) and alanine aminotransferase (ALT) levels (Fig.1a, b) and improvement of biliary imaging, biopsies of the liver and intestine, and IBD symptoms while on OV (Table 1). Reduction in peripheral levels of these enzymes has been previously reported to correlate with improved liver health [2, 3]. In all reported cases, patients were clinically asymptomatic for both PSC and IBD as early as 2 weeks into, and throughout the duration of, their OV treatment.
Longitudinal CBC reports documented during a 12-month OV course were available for a small number of patients (n=6). Despite the low sample size, the data suggest that, in this patient population, OV leads to a reduction in WBC counts (p<0.05), possibly driven primarily by decreases in neutrophil populations (p<0.05) (Fig.1c). Further multiplex plasma cytokine analyses revealed elevations in TGF-β levels (p=0.031) by 3 months post-OV administration (Fig.1d), without concurrent elevations in cytokines associated with Th1 (TNFα) and Th2 (IL-4, IL-13) mediated responses (Fig.1d). While the source of this increase in TGF-β remains unknown, the role TGF-β plays in the induction and function of regulatory T cells is well documented [23–26].
Our group has previously published extensively on the development and function of Tregs, and has demonstrated a specialized role for Tregs in controlling inflammation at mucosal surfaces in human pathology, particularly with relation to allergic disorders of the airway [18, 27–29]. Additional studies further suggest Tregs may be involved in therapeutic processes associated with antibiotic-mediated directional selection of commensal bacteria [9, 12–16]. We thus measured Treg levels in two populations of PSC+IBD patients: those treated with OV and those who did not receive OV. Due to the controversy surrounding identification of Tregs [30], we tracked both CD4+CD25hiCD127lo Tregs [18, 20] and the canonical CD4+FoxP3+ Treg (Fig.2a). Using either definition, we found two-fold elevations in Treg frequencies (with respect to total non-Treg CD4+ T cell number) in OV-treated patients (Fig.2a–c). Longitudinal study of individual patients shows that peak Treg induction occurs within three months of OV administration, and endures steadily past 12 months on OV (data not shown). While T cell-specific release of IL-10 has been implicated in the maintenance of intestinal homeostasis [31, 32], we did not see alterations in IL-10 reservoirs in peripheral T cells during OV treatment.
As previously reported, most patients have recurrence of PSC when the vancomycin is discontinued [2, 3]. Analysis of a unique case study (Fig.4) provides further insight on immunologic changes that are associated with recurrence of PSC with IBD after stopping the OV. Within 9 months of stopping the OV, subject 01 experienced gradual recurrence of both PSC and IBD (diagnosed by biopsy and imaging), as well as a distinct reduction in Treg levels to below control values. Readministration of OV resolved clinical symptoms within two weeks. Measurement of Treg levels at each of these time points (during initial resolution, relapse, and recovery), are highly suggestive that increased peripheral Treg levels are associated with OV treatment of PSC+IBD in children.
Vancomycin, a D-Ala-D-Ala inhibitor, exhibits pharmacological activity against gram-positive bacteria, and uniquely, its poor absorption following oral delivery allows it maximal efficacy in the intestinal tissues. In 2000, Hessle et al published findings that gram-positive bacteria potently induce inflammatory IL-12 production, while gram-negative bacteria induce IL-10 production during stimulation of primary human PBMCs [33]. Additional studies have suggested gram-negative commensals, such as Bacteroides fragilis, provide protective fortifications against perturbations of intestinal homeostasis, particularly through Treg induction [16]. An analysis of changes in the microbiome during OV treatment is currently underway. To date, no complications from long-term OV treatment (e.g. vancomycin-resistant Enterococcus or C. difficile) have been observed.
Because of the absence of identifiable pathogens in stool studies and because vancomycin is both an immunomodulator and an antibiotic [1], it is reasonable to propose that vancomycin is having a two-fold effect. First, it may have a direct immunomodulatory effect on the T cell inflammatory process via the TNF-alpha pathway and downstream Treg induction. Second, it may also be driving commensal microbiota that are both qualitatively and quantitatively normal to a predominantly gram-negative composition, thereby shifting the cytokine profile of the gut and reversing an aberrant T cell inflammatory response. In this way, the vancomycin could be addressing both sides of a dysfunctional interaction between bacterial microbiota and the immune system, though a precise mechanism remains to be elucidated.
In conclusion, we show that OV-mediated disease resolution in pediatric PSC+IBD patients is associated with elevated peripheral TGF-β levels without alterations in Th1 or Th2 cytokine production patterns, and an increase in peripheral CD4+CD25hiCD127lo and CD4+FoxP3+ Tregs measurable as early as two-weeks post administration that is maximal and enduring by three months post-OV. Additional experimentation is necessary to confirm the mechanism by which OV alleviates symptoms associated with PSC and IBD and the role that T cells might play in driving pathology and/or recovery.
Institutional Review Board, Patient Consent, Clinical Trials Registry
The study protocols were approved by the Stanford University or Sutter Health Institutional Review Boards, and were performed in accordance with the 1964 Declaration of Helsinki and its later amendments. Written consent was obtained from one or both parents of all children and from all subjects who were ≥ 18 years of age before participating in this study; children older than 7 years of age signed a statement of assent. Details of this clinical trial are open to public access under ClinicalTrials.gov identifier: NCT01322386.
Acknowledgments
The project described in this publication was supported by the Stanford NIH/NCRR CTSA award number UL1 RR025744 and by the Lucile Packard Foundation for Children’s Health. The authors would like to thank the families of the Lucile Packard Foundation for Child Health, the Wadsworth family, the Stanford Digestive Disease Center, NIH P30 DK56339, and the Human Immune Monitoring Core of Stanford University for their generous support of these studies. The authors would additionally like to acknowledge the following individuals for their assistance with this report: Katharine Eng, M.D., Cameron McDonald-Hyman, and Ruhi Nath.
Sources of support: Stanford NIH/NCRR CTSA Award Number UL1 RR025744 (Cox); Lucile Packard Foundation for Child Health (Cox); Stanford Digestive Disease Center, NIH P30DK56339 (Nadeau); Vice Provost for Undergraduate Education (VPUE) Major Grant Award at Stanford University (Seki).
Footnotes
DISCLOSURES
The authors declare that they have no conflict of interest.
Contributor Information
David N. Abarbanel, Email: dabarb01@stanford.edu.
Scott M. Seki, Email: sseki@stanford.edu.
Yinka Davies, Email: daviesy@sutterhealth.org.
Natalie Marlen, Email: marlenn@sutterhealth.org.
Joseph A. Benavides, Email: dnaistheway@gmail.com.
Kathleen Cox, Email: kacox@lpch.org.
Kari C. Nadeau, Email: knadeau@stanford.edu.
Kenneth L. Cox, Email: kcox@lpch.org.
References
- 1.Howden BP, Smith DJ, Mansell A, Johnson PD, Ward PB, Stinear TP, et al. Different bacterial gene expression patterns and attenuated host immune responses are associated with the evolution of low-level vancomycin resistance during persistent methicillin-resistant Staphylococcus aureus bacteraemia. BMC microbiology. 2008;8:39. doi: 10.1186/1471-2180-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox KL, Cox KM. Oral vancomycin: treatment of primary sclerosing cholangitis in children with inflammatory bowel disease. Journal of pediatric gastroenterology and nutrition. 1998;27(5):580–3. doi: 10.1097/00005176-199811000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Davies YK, Cox KM, Abdullah BA, Safta A, Terry AB, Cox KL. Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: an immunomodulating antibiotic. Journal of pediatric gastroenterology and nutrition. 2008;47(1):61–7. doi: 10.1097/MPG.0b013e31816fee95. [DOI] [PubMed] [Google Scholar]
- 4.Mieli-Vergani GaDV. Unique features of primary sclerosing cholangitis in children. Curr Opin Gastroenterol. 26(3):4. doi: 10.1097/MOG.0b013e3283388f5b. [DOI] [PubMed] [Google Scholar]
- 5.Bowlus CL. Cutting edge issues in primary sclerosing cholangitis. Clinical reviews in allergy & immunology. 2011;41(2):139–50. doi: 10.1007/s12016-010-8221-3. [DOI] [PubMed] [Google Scholar]
- 6.Hov JR, Kosmoliaptsis V, Traherne JA, Olsson M, Boberg KM, Bergquist A, et al. Electrostatic modifications of the human leukocyte antigen-DR P9 peptide-binding pocket and susceptibility to primary sclerosing cholangitis. Hepatology. 2011;53(6):1967–76. doi: 10.1002/hep.24299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsen TH, Franke A, Melum E, Kaser A, Hov JR, Balschun T, et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010;138(3):1102–11. doi: 10.1053/j.gastro.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 8.Melum E, Franke A, Schramm C, Weismuller TJ, Gotthardt DN, Offner FA, et al. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nature genetics. 2011;43(1):17–9. doi: 10.1038/ng.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183(10):6041–50. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell host & microbe. 2008;4(4):337–49. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–27. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–4. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482(7385):395–9. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strauch UG, Obermeier F, Grunwald N, Gurster S, Dunger N, Schultz M, et al. Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut. 2005;54(11):1546–52. doi: 10.1136/gut.2004.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(27):12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu B, Tonkonogy SL, Sartor RB. Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice. Gastroenterology. 2011;141(2):653–62. 62, e1–4. doi: 10.1053/j.gastro.2011.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen KD, Vanichsarn C, Fohner A, Nadeau KC. Selective deregulation in chemokine signaling pathways of CD4+CD25(hi)CD127(lo)/(-) regulatory T cells in human allergic asthma. The Journal of allergy and clinical immunology. 2009;123(4):933–9. e10. doi: 10.1016/j.jaci.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siedlar M, et al. Vancomycin down-regulates lipopolysaccharide-induced tumour necrosis factor alpha (TNF alpha) production and TNF alpha-mRNA accumulation in human blood monocytes. Immunopharmacology. 1997;35(3):7. doi: 10.1016/s0162-3109(96)00156-7. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. The Journal of experimental medicine. 2006;203(7):1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichtman SN, Okoruwa EE, Keku J, Schwab JH, Sartor RB. Degradation of endogenous bacterial cell wall polymers by the muralytic enzyme mutanolysin prevents hepatobiliary injury in genetically susceptible rats with experimental intestinal bacterial overgrowth. The Journal of clinical investigation. 1992;90(4):1313–22. doi: 10.1172/JCI115996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtman SN, Sartor RB, Keku J, Schwab JH. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98(2):414–23. doi: 10.1016/0016-5085(90)90833-m. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura A, Wakabayashi Y, Mori T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. Journal of biochemistry. 2010;147(6):781–92. doi: 10.1093/jb/mvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of experimental medicine. 2003;198(12):1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Chen D, et al. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 - precursors. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(10):1614–27. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Kim YM, Munoz A, Hwang PH, Nadeau KC. Migration of regulatory T cells toward airway epithelial cells is impaired in chronic rhinosinusitis with nasal polyposis. Clin Immunol. 2010;137(1):111–21. doi: 10.1016/j.clim.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen KD, Vanichsarn C, Nadeau KC. Impaired IL-10-dependent induction of tolerogenic dendritic cells by CD4+CD25hiCD127lo/- natural regulatory T cells in human allergic asthma. American journal of respiratory and critical care medicine. 2009;180(9):823–33. doi: 10.1164/rccm.200905-0761OC. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen KD, Vanichsarn C, Nadeau KC. TSLP directly impairs pulmonary Treg function: association with aberrant tolerogenic immunity in asthmatic airway. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology. 2010;6(1):4. doi: 10.1186/1710-1492-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmetterer KG, Neunkirchner A, Pickl WF. Naturally occurring regulatory T cells: markers, mechanisms, and manipulation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(6):2253–76. doi: 10.1096/fj.11-193672. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 32.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–58. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Hessle C, Andersson B, Wold AE. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infection and immunity. 2000;68(6):3581–6. doi: 10.1128/iai.68.6.3581-3586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]