Abstract
Pancreatic cancer has a high mortality rate and alcoholism is a risk factor independent of smoking. We have shown that nicotinic acetylcholine receptors (nAChRs) regulate pancreatic ductal epithelia and pancreatic ductal adenocarcinoma (PDAC) cells in an autocrine fashion by stimulating their production of the stress neurotransmitters noradrenaline and adrenaline that signal through beta-adrenergic receptors (β-ARs). Our current study has investigated the modulation of this autocrine regulatory loop by chronic ethanol and explored the potential prevention of these effects by γ-amino butyric acid (GABA).
Using MTT assays, cell migration assays, western blotting, immunoassays, and gene knockdown of individual nAChRs in two PDAC cell lines and in immortalized human pancreatic duct epithelial cells, our data show that treatment for seven days with ethanol induced the protein expression and sensitivity of nAChRs α3, α5 and α7 resulting in increased production of noradrenaline and adrenaline which drive proliferation and migration via cAMP-dependent signaling downstream of β-ARs. Treatment with GABA prevented all of these responses to chronic ethanol, reducing cell proliferation and migration below base levels in untreated cells.
Our findings suggest that alcoholism induces multiple cAMP-dependent PDAC stimulating signaling pathways by up-regulating the protein expression and sensitivity of nAChRs that regulate stress neurotransmitter production. Moreover, our data identify GABA as a promising agent for the prevention of PDAC in individuals at risk due to chronic alcohol consumption.
Keywords: Chronic alcohol, nicotinic receptors, stress neurotransmitters, GABA, pancreatic cancer
Introduction
Pancreatic ductal adenocarcinoma (PDAC), which has phenotypic and functional features of pancreatic duct epithelial cells, is one of the most aggressive neoplastic diseases with a mortality rate near 100 % within one year of diagnosis (1). Smoking, chronic alcohol consumption and pancreatitis of any etiology, including alcoholism, are documented risk factors for PDAC (2, 3). Smoking and drinking are often correlated. However, a recent study has identified a significant association of chronic alcohol intake with pancreatic cancer mortality in never smokers, thus identifying chronic alcohol consumption as a PDAC risk factor independent of smoking (4).
Numerous publications have focused on the mechanisms of pancreatic cancer development and progression associated with exposure to nicotine, its nitrosated carcinogenic derivative NNK and other tobacco related carcinogens (5). Nicotine (6) and NNK (7, 8) are both high affinity agonists for nicotinic acetylcholine receptors (nAChRs) and this receptor family has been identified as important regulator of cell proliferation, apoptosis, migration and angiogenesis in the most common epithelial human cancers (9), including cancer of the lung (10–13), colon (14), breast (15), oral cavity (8), stomach (16) and pancreas (15, 17–19). It was iitially thought that cellular responses after treatment of cancer cells with nAChR agonists were caused by intracellular signaling pathways activated immediately downstream of nAChRs (9). However, emerging research suggests that most of these reported signaling events are in fact indirect responses caused by the nAChR-mediated synthesis and release of neurotransmitters by cancer cells and the epithelia from which they arise (9). In accord with these findings, we have recently shown that PDAC and pancreatic duct epithelial cells also synthesize and release the stress neurotransmitters adrenaline and noradrenaline in response to activation of nAChRs α3, α5 and α7 resulting in increased proliferation and migration via the activation of multiple cAMP-dependent signaling pathways downstream of β-ARs (18, 19).
It has been reported that exposure of immortalized pancreatic duct epithelial cells to ethanol in vitro enhanced NNK-induced activation of ERK1/2 and cell proliferation in a cAMP-dependent manner (20), while investigations in brain cells and recombinant nAChRs in oocytes have shown that chronic in vitro exposure to ethanol modulated the number and sensitivity of nAChRs (21, 22). These finding suggest that chronic exposure to ethanol may enhance pancreatic cancer -promoting beta-adrenergic pathways by modulating the expression and sensitivity of nAChRs. In the current experiments, we have therefore investigated the effects of chronic ethanol on the protein expression and sensitivity of nAChRs α3, α5 and α7 in immortalized pancreatic duct epithelial cells and two PDAC cell lines. Moreover, we have tested the potential inhibition of these responses by γ-amino butyric acid (GABA).
Materials and Methods
Chemicals, primers, antibodies and assay kits
The MTT (3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide) colorimetric assay kit was purchased from Sigma-Aldrich (St Louis, MO, USA). The CytoSelect Cell Migration Assay kit was purchased from Cell BioLabs, Inc. (San Diego, CA, USA). The 2-Cat ELISA Kit was purchased from Rocky Mountain Diagnostics Incorporation (Colorado Springs, CO, USA). The cAMP and PKA activity ELISA assays were purchased from Enzo Life Sciences (Farmingdale, NY, USA).
ELISA kits for Akt, ERK1/2, and CREB (Invitrogen Corporation, Carlsbad, CA, USA) are designed to detect pAKT at serine 473, pERK1/2 at threonine 202 and tyrosine 204 for ERK1 and threonine 185 and tyrosine 187 for ERK2, and pCREB at serine 133 respectively. The CycLex c-Src Kinase assay (MBL International, Woburn, MA, USA) is designed to detect p-Src at Tyrosine kinase-substrate-1. All four assays use the chromogenic tetra-methylbenzidine (TMB) substrate for signal detection.
Lipofectamine 2000 Reagent, stealth-1079 for the CHRNA3 gene, stealth-873 for the CHRNA5 gene, stealth-183 for the CHRNA7 gene, stealth RNAi Negative Control Low GC Duplex, and Opti-MEM I reduced serum medium 1X were purchased from Invitrogen Corporation (Carlsbad, CA, USA). The primer used to interfere with the α3 subunit mRNA was sense, GCU CUU CCA UGA ACC UCA AGG ACU A and antisense, UAG UCC UUG AGG UUC AUG GAA GAG C. The primer used to interfere with the α5 subunit mRNA was sense, GGG AGC AAA GGA AAC AGA ACC GAC A and antisense, UGU CGG UUC UGU UUC CUU UGC UCC C. The primer used to interfere with the α7 subunit mRNA was sense, GGA AGC UUU ACA AGG AGC UGG UCA A and antisense, UUG ACC AGC UCC UUG UAA AGC UUC C.
The antibodies anti-rabbit and anti-mouse were purchased from Cell Signaling (Danvers, MA, USA). The nicotinic acetylcholine receptor subunits α7 (56 kDa), α3 (57 kDa) and α5 (53 kDa) antibodies as well as β-actin (42 kDa) were all purchased from Abcam (Cambridge, MA, USA). Nicotine ((−)-Nicotine hydrogen tartrate salt, minimum 98 % TLC), γ-amino butyric acid (GABA) and isobutylmethylxanthine (IBMX) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ethanol was purchased from Fisher Scientific International (Fair Lawn, NJ, USA). The TE Buffer 1X was purchased from Promega Corporation (Madison, WI, USA). The lysis buffer used to extract proteins along with Pierce ECL western blotting substrate were purchased from Thermo Scientific (Rockford, IL, USA).
Maintenance and chronic treatments of cultured cells
The immortalized human pancreatic duct epithelial cell line, HPDE6-C7, was kindly provided to us in the year 2005 by Dr. Tsao (Division of Cellular and Molecular Biology, Department of Pathology, Ontario Cancer Institute/Princess Margaret Hospital, University of Toronto, Toronto, ON, Canada). This cell line was clonally established after transduction of the HPV16-E6E7 genes into primary cultures of pancreatic duct epithelial cells. The human PDAC cell lines Panc-1 (with activating point mutation in K-ras) and BxPC-3 (without ras mutation) were purchased in the year 2001 from the American Type Culture Collection (Manassas, VA, USA). We chose these particular PDAC cell lines for the current study to ensure inclusion of cells with and without ras mutations and because our laboratory has generated substantial information regarding nAChR-mediated regulation of these cell lines in vitro and in vivo (23–26). All three cell lines were last authenticated at the beginning of this project in the year 2010 by Research Animal Diagnostic Laboratory (RADIL, Columbia, MO, USA) by species-specific PCR evaluation.
Cell lines Panc-1 and BxPC-3 were maintained in DMEM and RPMI media respectively and were both supplemented with 10 % Fetal Bovine Serum (FBS). HPDE6-C7 cells were maintained in Keratinocyte Serum Free Medium (KSFM) supplemented with 25 mg/500 ml Bovine Pituitary Extract (BPE) and 2.5 μg/500 ml Epidermal Growth Factor (EGF) (GIBCO Invitrogen Corporation, Grand Island, NY, USA). All cell lines were grown without any antibiotics in an atmosphere of 5 % CO2, 99 % relative humidity and 37 °C.
Chronic treatment of cells in complete medium with ethanol (17 mM) and GABA (30 μM) was for 7 days. Fresh medium containing each drug was replaced every 24 hours. In the untreated control cells, the medium was replaced every 24 hours. Treatment groups were as follows:
Control: cells maintained for 7 days in complete medium without drugs;
Ethanol: cells treated with ethanol for 7 days;
Ethanol + GABA: cells treated simultaneously for 7 days with ethanol and GABA;
GABA: cells treated with GABA for 7 days.
The concentration (17 mM) of ethanol used is the equivalent of the legal blood alcohol limit of 0.08 % in the USA. The concentration of GABA (30 μM) is the equivalent of 21.7 mg/person/day and is within the recommended range of GABA as a nutritional supplement.
Protein analyses of nAChR subunits by western blotting
The protein expression of nAChR subunits α3, α5, and α7 was determined in cells chronically treated with ethanol, GABA or the combination of both. Protein samples were prepared using lysis buffer (RIPA Buffer 1X; Halt Protease Inhibitor Single-Use Cocktail, EDTA-Free (100X) (Thermo Scientific); 10 μl of 100 mM phenylmethysulfonylfluoride/ml RIPA; 10 μl of 100 mM Na3VO4/ml RIPA; 10 μl of 1 M NaF/ml RIPA). After heat denaturation, protein samples were electrophoresed using 12 % SDS gels (Invitrogen) and were then blotted onto membranes. The membranes were then blocked (5 % nonfat dry milk solution in 1X TBST) for one hour at room temperature. Membranes were then incubated overnight at 4 °C with the following primary antibodies: nAChR subunits α3, α5 and α7. The primary antibody β-actin was used as a loading control to ensure equal loading of proteins. All membranes were then washed (0.5 % Tween 20/TBS) and incubated with their respective fluorescent secondary antibodies for two hours. Protein bands were then visualized with enhanced chemiluminescence reagent (Pierce ECL Western Blotting Detection Substrate). Following background subtraction, mean densities of 2 rectangular areas of standard size per band from three independent westerns were determined and mean values and standard deviation (n = 6) of protein expression were calculated.
Assessment of responsiveness to ethanol by immunoassays for the detection of neurotransmitters
In order to test the hypothesis that chronic exposure to ethanol modulates the responsiveness of nAChR-mediated neurotransmitter production to ethanol itself, dose-response curves for ethanol were established in both unpretreated and chronically pretreated cells. Unpretreated cells from all three cell lines and cells pretreated for 7 days with 17 mM ethanol were exposed to a range of ethanol concentrations (1 μM, 50 μM, 500 μM, 1 mM, 17 mM and 35 mM) for 30 minutes prior to harvesting. EC50 values for ethanol were then compared in unpretreated versus ethanol pretreated cells. The culture media, containing the secreted stress neurotransmitters noradrenaline and adrenaline were then collected in 15 ml test tubes. The cells which contained synthesized intracellular catecholamines were lysed and harvested into 1.5 ml eppendorf tubes after a one time wash with warm 1X PBS. Total (secreted plus intracellular) stress neurotransmitters of five samples per treatment group was analyzed using 2-Cat ELISA kits following the vendors’ recommendations. Absorbance of samples was read using an uQuant Bio-Tek Instrument ELISA reader at 450 nm primary wavelength with a 630 nm reference wavelength.
Quantitative assessment of accumulated intracellular cAMP levels
Chronic treatment groups for this assay were identical to those of the above western blotting experiment. On the sixth day of treatment, cells were washed twice with warm 1X PBS then incubated for 30 minutes with 1 mM of the phosphodiesterase inhibitor isobutylmethylxanthine (IBMX) to prevent enzymatic breakdown of the cAMP formed. Once the 30 minutes incubation was over, cells were washed with 1X PBS twice then treated with 17 mM ethanol for 24 hours. The cells were then lysed and harvested into 1.5 ml eppendorf tubes following the cAMP ELISA kit’s instructions. Quantitative analyses of cAMP levels from five samples per treatment group were conducted using the cAMP ELISA kit following the vendor’s recommendations. Absorbance of samples was read using an uQuant Bio-Tek Instrument ELISA reader at 405 nm primary wavelength with a 550 nm reference wavelength.
Quantitative assessment of PKA activation and phosphorylation levels of signaling proteins by immunoassays
Following identical chronic treatments, the cells were lysed and harvested into 1.5 ml eppendorf tubes following ELISA kits’ instructions. Quantitative analyses of PKA activity, Akt, CREB, Src, and ERK1/2 phosphorylation from five samples per treatment group were conducted using PKA activity, Akt, CREB, c-Src, and ERK1/2 ELISA kits respectively following the vendors’ recommendations. Absorbance of samples was read using an uQuant Bio-Tek Instrument ELISA reader at 450 nm primary wavelength with a 630 nm reference wavelength.
Gene knockdown of the α3, α5 and α7-nAChRs
All three cell lines were grown for 24 hours in their respective complete media. After which they were switched to Opti-MEM I media and were divided into several groups. Groups 1 and 2 from each cell line were left untreated in Opti-MEM I media for 24 hours. Group 3 was transfected for 24 hours with stealth RNAi Negative Control Low GC Duplex. Groups 4 and 5 were transfected with either stealth-183, 1079 or 873 for the CHRNA7, 3, and 5 genes respectively for 24 hours in Opti-MEM I media. Once the 24 hours transfection was complete, all cells were switched into their respective complete media. Groups 1, 3 and 4 were left untreated for 7 days in complete media whereas groups 2 and 5 were treated with 17 mM ethanol for 7 days in complete media. All transfections were transient and were done using Lipofectamine 2000 reagent following the instructions of the manufacturer. Quantification of the expression of α3, α5 and α7-nAChRs for all groups were assessed via western blots at the end of the 7 days chronic exposure to ethanol for groups 2 and 5. Similar treatment groups to the one in this experiment were used to assess cell proliferation and migration of all three cell lines using MTT and cell migration assays following the procedure outlined below.
Assessment of cell proliferation by MTT assay
All three cell lines were seeded into 6-well plates at a density of 20,000 cells per well (n= 5) in their respective complete media. Cells were then left untreated or treated with ethanol, GABA or both for 7 days. The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide) colorimetric assay was then used for the assessment of cell proliferation following instructions provided by the vendor. Absorbance of samples was read using an uQuant Bio-Tek Instrument ELISA reader at a primary and reference wavelengths of 570 nm and 650 nm respectively.
Assessment of Cell migration by CytoSelect cell migration assay
The three cell lines were seeded onto the top chamber of polycarbonate membrane filter inserts (8 μM pore size) in 6-well plates at a density of 20,000 cells per well in their respective complete media. Using identical treatment groups as for the MTT assay (above), a cell migration assay kit was then used to assess the migratory ability of these cells following the vendor’s instructions. Optical density of cell samples was read at 560 nm using an uQuant Bio-Tek Instrument ELISA reader.
Statistical analysis of data
Using GraphPad Instat software (GraphPad, San Diego, CA, USA), all data presented in the figures as bar graphs were analyzed for statistically significant differences among treatment groups by One Way ANOVA and Tukey-Kramer multiple comparison test when the data followed a normal distribution. Data that did not pass normality tests were tested for significant differences by non-parametric Kruskal-Wallis ANOVA followed by Dunn’s multiple comparison test. GraphPad Prism 5 software (GraphPad, San Diego, CA, USA) was used to establish sigmoidal dose-response curves and to calculate EC50 values by nonlinear regression analysis. Data of the immunoassays are expressed as mean and +/− standard deviation of five samples per treatment group. Using NIH ImageJ, two densitometric determinations per band were assessed from three independent western blots (n=6) per protein and expressed as mean values and standard deviations.
Results
Effects of chronic ethanol and GABA on the protein expression of nAChRs
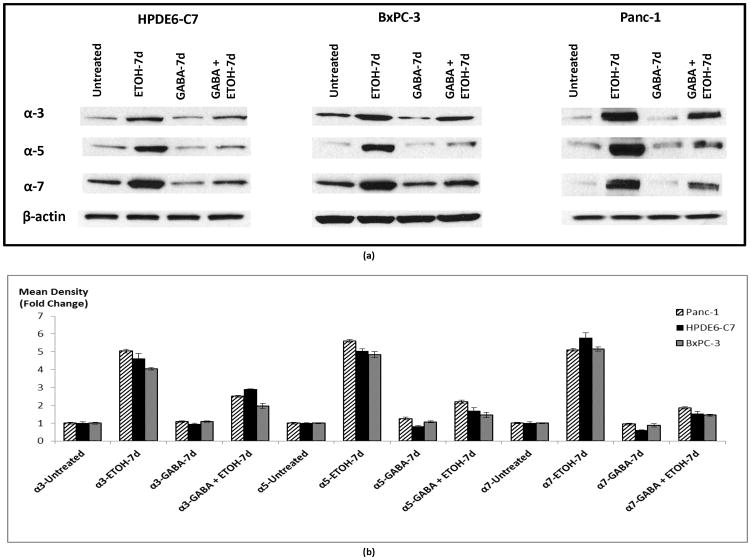
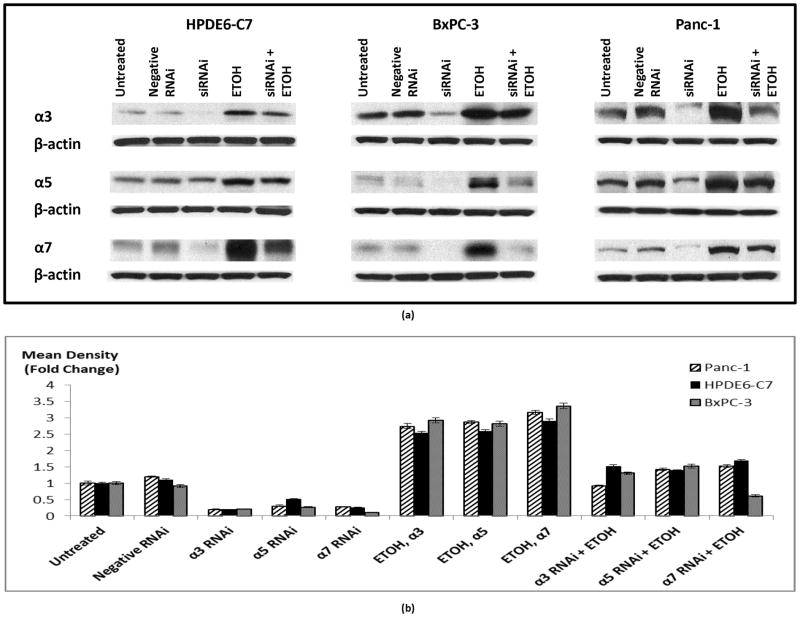
We have previously shown that nAChRs expressing the subunits α3, α5 and α7 regulate the proliferation of PDAC cell lines Panc-1 and BxPC-3 as well as immortalized pancreatic duct epithelial cells HPDE6-C7 by activating an autocrine stress neurotransmitter loop (18). In order to investigate a potential modulation of this regulatory loop by chronic ethanol, we tested the effects of chronic ethanol and GABA on the protein expression of these nAChRs. Our data show that chronic exposure to ethanol alone significantly induced (p < 0.0001) protein expression of nAChR subunits α3 (4.5 fold), α5 (5 fold) and α7 (5 fold). Simultaneous treatment of cells with GABA significantly (p < 0.0001) reduced these effects of ethanol (Figure 1).
Figure 1.
Western blots assessing protein expression of nAChR subunits in cells treated with 17 mM ethanol, 30 μM GABA or the combination of the two for 7 days. Protein β-actin was used as a loading control (a). The columns in figure (b) represent means and +/− SD of two mean density readings per band from three independent western blots (n = 6).
Concentration-dependent effects of chronic ethanol on total adrenaline and noradrenaline
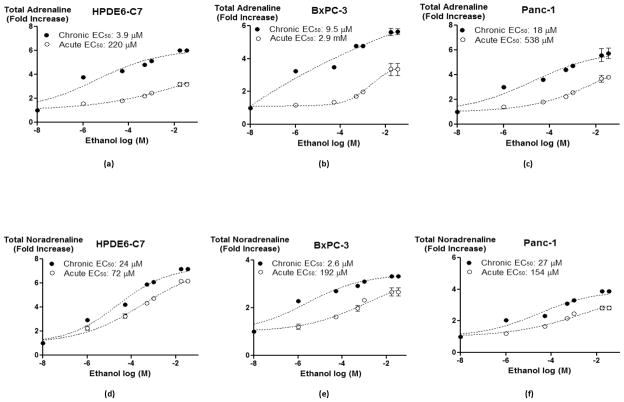
In order to assess the functional significance of the observed changes in chronic ethanol-induced protein expression of nAChRs on their effectors, we measured total (intracellular plus secreted) levels of the stress neurotransmitters noradrenaline and adrenaline in 7-day ethanol pretreated versus non-pretreated cells exposed to a range of ethanol concentrations (1 μM – 35 mM). As shown in figure 2, the concentration-dependent increases in total levels of the stress neurotransmitters noradrenaline and adrenaline were significantly (p < 0.0001) enhanced by 7-day pre-exposure to ethanol in all three cell lines and EC50 values of ethanol for these increases were significantly (p < 0.0001) reduced (Figure 2).
Figure 2.
Total (secreted and intracellular) adrenaline (a–c) and noradrenaline (d–f) levels in cells treated with single doses of ethanol from 1 μM to 35 mM for 30 minutes or pretreated with 17 mM ethanol for 7 days and then exposed to identical concentrations of ethanol. Data points are mean and +/− SD from five samples per treatment group.
Effects of chronic ethanol and GABA on intracellular accumulation of cAMP and PKA activation
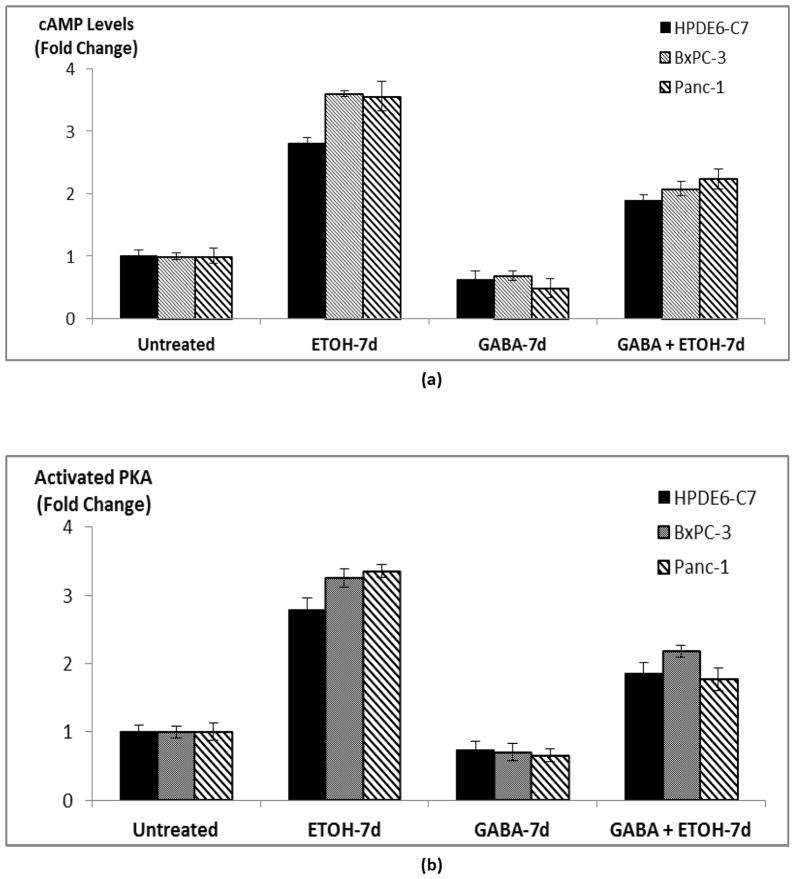
We have previously shown that nAChRs expressing subunits α3, α5 and α7 jointly regulate the synthesis and release of the stress neurotransmitters noradrenaline and adrenaline in PDAC cells and pancreatic duct epithelial cells (18). In turn, noradrenaline and adrenaline are β-AR agonists (27, 28) that increase intracellular cAMP levels via activation of the stimulatory G-protein Gαs of these receptors, resulting in the activation of PKA. (18). We therefore measured intracellular cAMP levels and activated PKA in the current experiments in order to assess the effects of chronic ethanol on these β-AR effectors. As figure 3 shows, chronic treatment of cells with ethanol significantly (p < 0.0001) increased intracellular cAMP levels (3–4 folds) in the three investigated cell lines while also significantly (p < 0.0001) increasing the levels of activated PKA (2.8–3.4 folds). Simultaneous exposure of these cells to GABA during the 7-day ethanol treatment period significantly (p < 0.0001) reduced both of these responses to ethanol (Figure 3). In addition, chronic exposure of cells to GABA alone significantly (p < 0.0001) suppressed base levels of cAMP and activated PKA in cells not pretreated with ethanol (Figure 3).
Figure 3.
ELISA assays showing cAMP levels (a) and PKA activity (b) in cells treated with 17 mM ethanol, 30 μM GABA or the combination of the two for 7 days. Data points are mean and +/− SD from five samples per treatment group.
Effects of chronic ethanol in the presence and absence of GABA on the phosphorylation of multiple signaling proteins
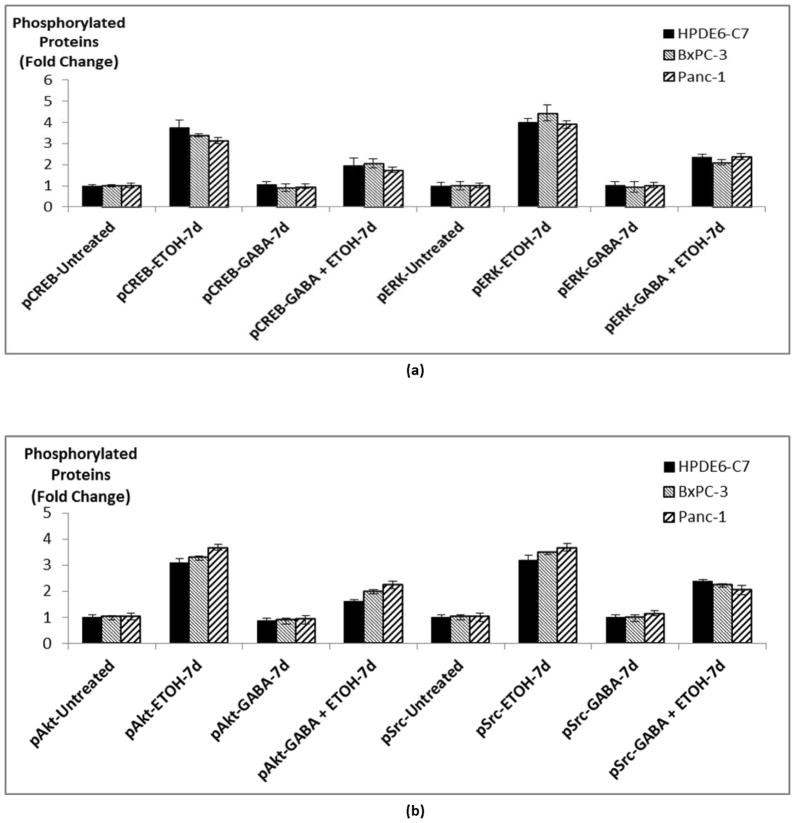
We have previously shown that activation of cAMP-dependent PKA results in the phosphorylation of multiple signaling proteins involved in the regulation of proliferation, angiogenesis, metastatic potential and apoptosis in PDAC cells (18, 24–26). We therefore assessed the potential modulation in phosphorylation levels of these signaling proteins by chronic ethanol in the presence and absence of simultaneous treatment with GABA. Our data show that chronic exposure of normal pancreatic duct epithelia and both PDAC cell lines to ethanol significantly induced (p < 0.0001) phosphorylation of ERK, CREB, Akt and Src (Figure 4). In analogy to our findings with cAMP and PKA, simultaneous exposure of the cells to GABA during the ethanol treatment period significantly (p < 0.0001) reduced each of these responses (Figure 4).
Figure 4.
ELISA assays showing phosphorylation of CREB and ERK (a) and of Src and Akt (b) in cells treated with 17 mM ethanol, 30 μM GABA or the combination of the two for 7 days. Data points are mean and +/− SD from five samples per treatment group.
Effects of nAChRs gene knockdown on ethanol-mediated regulation of cell proliferation and migration
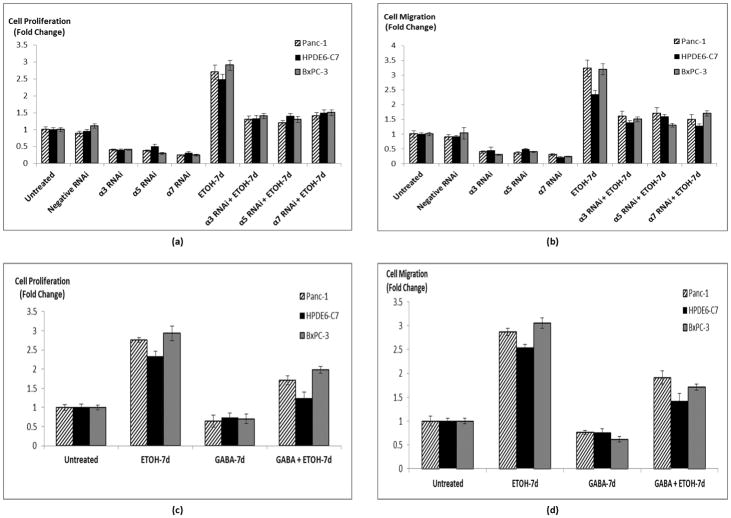
In order to determine if the protein induction of nAChRs α3, α5, and α7 by chronic ethanol shown in Figure 1 were associated with stimulation of proliferation and migration, we investigated the effects of gene knockdown of each of these nAChRs in the presence and absence of chronic ethanol. At the end of the 7 days ethanol pretreatment, the transient gene knockdown was still detectable by western blots (Figure 5). Gene knockdown of each of the three receptor subunits caused a significant reduction (p < 0.001) in base level cell proliferation (Figure 6a) and migration (Figure 6b) in all three cell lines. Chronic exposure to ethanol significantly (p < 0.001) increased cell proliferation (2.7 fold) and migration (3 fold) in each of the investigated cell lines (Figures 6a, b). Both of these responses to chronic ethanol were significantly (p < 0.001) reduced by gene knockdown of nAChRs α3, α5, or α7 in all three cell lines (Figures 6a, b).
Figure 5.
Western blots assessing the effects of gene knockdown on protein expression of nAChR subunits in the presence and absence of 7 days ethanol. Protein β-actin was used as a loading control (a). The columns in figure (b) represent means and +/− SD of two mean density readings per band from three independent western blots (n = 6).
Figure 6.
Effects of gene knockdown of nAChR subunits on cell proliferation (a) and migration (b) in the presence and absence of chronic ethanol. Cell proliferation (c) and migration (d) assays showing stimulation of both proliferation and migration upon ethanol exposure and a reversal of these effects upon exposure to GABA. The columns represent means and +/− SD of 5 samples per treatment group.
Effects of chronic ethanol in the presence and absence of GABA on cell proliferation and migration
Using an ethanol concentration (17 mM) equivalent to the legal blood alcohol limit of 0.08 % in the USA, we found that all three investigated cell lines responded with more than 2 fold increases (p < 0.0001) in the number of viable cells when exposed for 7 days to ethanol (Figure 6c). This response was significantly (p < 0.001) inhibited by simultaneous exposure of these cells to GABA (Figure 6c) at a concentration (30 μM) within the recommended daily dose of GABA as a nutritional supplement. In addition, 7-day exposure to GABA significantly (p < 0.0001) decreased the number of viable cells in the three cell lines not pre-treated with ethanol (Figure 6c). These findings were mirror-imaged by the results of cell migration assays that used identical treatment groups (Figure 6d), with more than 2.5 fold increase in migrated cells after 7-day treatment with ethanol (p < 0.0001) and significant (p < 0.0001) inhibition of this effect by GABA.
Discussion
Smoking and chronic alcohol consumption are often correlated and represent established risk factors for pancreatic cancer (1–3). Heavy alcohol consumption is thought to facilitate pancreatic cancer development in smokers by increasing the risk for diabetes and chronic pancreatitis which are independent risk factors for this malignancy (3). However, a recent study has identified chronic consumption of moderate amounts of alcohol in never smokers as an independent risk factor for pancreatic cancer (4). The mechanisms responsible for this effect of ethanol on pancreatic cancer are far from understood.
It has been shown that nicotine addiction and alcohol dependence are highly correlated (22, 29) and that chronic nicotine-induced upregulation of nAChR proteins in brain cells is significantly enhanced by simultaneous exposure to chronic ethanol (22). While nAChRs were initially thought to be uniquely expressed in the nervous system, more recent studies have identified these receptors in the majority of mammalian cells, including epithelia (30). In accord with these reports, we have recently shown that the stress neurotransmitters noradrenaline and adrenaline are synthesized and released by normal pancreatic duct epithelia and PDAC cells in vitro when nicotine binds to nAChRs expressing the subunits α3, α5, or α7 in these cells (18). In accord with the established function of noradrenaline and adrenaline as beta-adrenergic receptor agonists (27, 28), a single dose of nicotine thus increased cAMP and activated PKA, causing the phosphorylation of multiple signaling proteins (p-ERK, p-CREB, p-Akt, p-Src) frequently overexpressed in pancreatic cancer while also stimulating cell proliferation (18). We have also shown that exposure of immortalized pancreatic duct epithelial cells to a single dose of ethanol at a concentration (17 mM), equivalent to the legal blood alcohol limit (0.08 %) in the United States, stimulates cell proliferation by increasing intracellular cAMP levels, resulting in a significant induction of activated PKA and PKA-dependent phosphorylation of CREB and ERK1/2 (20). Our current study shows, for the first time, that chronic exposure to this moderate ethanol concentration significantly induces the protein expression of nAChRs α3, α5 and α7 in pancreatic duct epithelial and PDAC cells. The observed significant increases in total noradrenaline and adrenaline after chronic ethanol and associated significant decrease in EC50 values of ethanol required to stimulate the production of these neurotransmitters indicate functional sensitization of nAChRs α3, α5 and α7. The resulting significant inductions of activated PKA, CREB, ERK Akt and Src after chronic ethanol alone additionally identifies these signaling proteins as the driving forces of chronic ethanol-induced cell proliferation and migration in the three investigated cell lines.
The mechanisms of how chronic ethanol increased the protein expression and sensitivity of nAChRs in the current experiment remain to be elucidated. It is widely accepted that changes in nAChR number and sensitivity in response to chronic nAChR agonists are mediated by rapid posttranscriptional mechanisms without concomitant changes in mRNA levels (31). Ethanol is not a ligand for nAChRs but chronic exposure to ethanol modulates the responsiveness of these receptors to agonist by increasing the number of agonist binding sites (22). Epithelial cells produce the physiological nAChR agonist acetylcholine (30). Accordingly, chronic ethanol increased responsiveness of nAChRs α3, α5, and α7 to this physiological agonist in the current study. It has been suggested that ethanol-induced activation of PKC is involved in the increase in nAChR binding sites observed in PC12 cells after chronic exposure to ethanol (22). Alternatively, it has been shown that chronic ethanol increased the gene expression for enzymes that synthesize noradrenaline and adrenaline (32), a mechanism that may additionally have contributed to the ethanol-induced increase in stress neurotransmitter production in the current experiments. In turn, this may have triggered an increase in the posttranscriptional assembly and maturation of nAChRs, as these events are induced by increases in intracellular cAMP (31). Further studies are needed to obtain a more complete understanding of the complex mechanisms involved in ethanol-induced modulation of nAChRs in pancreatic cancer cells.
The importance of the observed in vitro effects of chronic ethanol on the beta-adrenergic signaling cascade for PDAC development is supported by findings that chronic treatment with ethanol and NNK induced the development of PDAC in hamsters (33). These tumors overexpressed nAChR proteins as well as p-CREB and p-ERK and were prevented by treatment with the beta-blocker propranolol (34). While our findings are in accord with reports from other laboratories that alcohol activates signaling proteins such as ERK, JNK, PKC and Stat3 in liver cells (35–39), they suggest that these proteins are downstream effectors of noradrenaline and adrenaline-induced beta-adrenergic signaling that is regulated by nAChRs.
Our findings suggest that changes in nAChR expression and function in the brain that are associated with alcohol dependence are also induced by chronic ethanol in PDAC cells and pancreatic duct epithelial cells where they cooperate to stimulate proliferation and migration, thus facilitating the development and progression of PDAC. Moreover, the strong inhibitory effects of GABA on the observed cellular responses to chronic ethanol identify this neurotransmitter, which is widely used as a nutritional supplement, as a promising agent for the prevention of PDAC in individuals at increased risk due to chronic consumption of alcohol. In light of the fact that the GABA-B receptor agonist baclofen has been identified as an effective therapeutic for alcohol dependence (40, 41), nutritional supplementation with GABA may not only have protective effects against PDAC development but simultaneously reduce the craving of alcohol withdrawal in dependent patients.
Beta-adrenergic signaling has emerged as an important regulatory cascade in some of the most common human malignancies, including cancer of the breast (42–44), colon (14, 45), prostate (46), stomach (16), ovary (47), pancreas (18, 24, 34, 48) and non-small cell lung cancer (49). In fact, several recent studies have shown improved clinical outcomes in breast cancer patients who received beta-blocker treatment for incidental cardiovascular disease while undergoing cancer therapy (42, 43). Moreover, we have reported that the beta-blocker propranolol prevents experimentally induced PDAC and non-small cell lung carcinoma in hamsters (34, 50). The significant activation of beta-adrenergic signaling by chronic ethanol via sensitization of nAChRs upstream of stress neurotransmitter production observed in the current experiments suggests that chronic consumption of alcohol may also promote the development and progression of non PDAC cancers that are under beta-adrenergic control and that GABA may have preventive effects on these cancers as well.
Acknowledgments
Grant Support
This project was supported by the National Cancer Institute grants R01CA042829 and RO1CA130888.
Footnotes
Conflict of Interest: None to declare
References
- 1.Duell EJ. Epidemiology and potential mechanisms of tobacco smoking and heavy alcohol consumption in pancreatic cancer. Mol Carcinog. 2012;51:40–52. doi: 10.1002/mc.20786. [DOI] [PubMed] [Google Scholar]
- 2.Genkinger JM, Spiegelman D, Anderson KE, Bergkvist L, Bernstein L, van den Brandt PA, et al. Alcohol intake and pancreatic cancer risk: a pooled analysis of fourteen cohort studies. Cancer Epidemiol Biomarkers Prev. 2009;18:765–76. doi: 10.1158/1055-9965.EPI-08-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowenfels AB, Maisonneuve P. Risk factors for pancreatic cancer. J Cell Biochem. 2005;95:649–56. doi: 10.1002/jcb.20461. [DOI] [PubMed] [Google Scholar]
- 4.Gapstur SM, Jacobs EJ, Deka A, McCullough ML, Patel AV, Thun MJ. Association of alcohol intake with pancreatic cancer mortality in never smokers. Arch Intern Med. 2011;171:444–51. doi: 10.1001/archinternmed.2010.536. [DOI] [PubMed] [Google Scholar]
- 5.Schuller HM, Al-Wadei HA. Neurotransmitter receptors as central regulators of pancreatic cancer. Future Oncol. 2010;6:221–8. doi: 10.2217/fon.09.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G. Structure and function of neuronal nicotinic acetylcholine receptors. Prog Brain Res. 1996;109:125–37. doi: 10.1016/s0079-6123(08)62094-4. [DOI] [PubMed] [Google Scholar]
- 7.Schuller HM, Orloff M. Tobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cells. Biochem Pharmacol. 1998;55:1377–84. doi: 10.1016/s0006-2952(97)00651-5. [DOI] [PubMed] [Google Scholar]
- 8.Arredondo J, Chernyavsky AI, Grando SA. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biol Ther. 2006;5:511–7. doi: 10.4161/cbt.5.5.2601. [DOI] [PubMed] [Google Scholar]
- 9.Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer. 2009;9:195–205. doi: 10.1038/nrc2590. [DOI] [PubMed] [Google Scholar]
- 10.Schuller HM. Cell type specific, receptor-mediated modulation of growth kinetics in human lung cancer cell lines by nicotine and tobacco-related nitrosamines. Biochem Pharmacol. 1989;38:3439–42. doi: 10.1016/0006-2952(89)90112-3. [DOI] [PubMed] [Google Scholar]
- 11.Maneckjee R, Minna JD. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines. Proc Natl Acad Sci U S A. 1990;87:3294–8. doi: 10.1073/pnas.87.9.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Wadei HA, Al-Wadei MH, Masi T, Schuller HM. Chronic exposure to estrogen and the tobacco carcinogen NNK cooperatively modulates nicotinic receptors in small airway epithelial cells. Lung Cancer. 2010;69:33–9. doi: 10.1016/j.lungcan.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH. Nicotine promotes cell proliferation via alpha7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol Appl Pharmacol. 2007;221:261–7. doi: 10.1016/j.taap.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Dasgupta P, Rizwani W, Pillai S, Kinkade R, Kovacs M, Rastogi S, et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124:36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin VY, Wu WK, Chu KM, Koo MW, Wong HP, Lam EK, et al. Functional role of beta-adrenergic receptors in the mitogenic action of nicotine on gastric cancer cells. Toxicol Sci. 2007;96:21–9. doi: 10.1093/toxsci/kfl118. [DOI] [PubMed] [Google Scholar]
- 17.Lazar M, Sullivan J, Chipitsyna G, Gong Q, Ng CY, Salem AF, et al. Involvement of osteopontin in the matrix-degrading and proangiogenic changes mediated by nicotine in pancreatic cancer cells. J Gastrointest Surg. 2010;14:1566–77. doi: 10.1007/s11605-010-1338-0. [DOI] [PubMed] [Google Scholar]
- 18.Al-Wadei MH, Al-Wadei HA, Schuller HM. Pancreatic cancer cells and normal pancreatic duct epithelial cells express an autocrine catecholamine loop that is activated by nicotinic acetylcholine receptors alpha3, alpha5, and alpha7. Mol Cancer Res. 2012;10:239–49. doi: 10.1158/1541-7786.MCR-11-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Wadei MH, Al-Wadei HA, Schuller HM. Effects of chronic nicotine on the autocrine regulation of pancreatic cancer cells and pancreatic duct epithelial cells by stimulatory and inhibitory neurotransmitters. Carcinogenesis. 2012;33:1745–53. doi: 10.1093/carcin/bgs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Askari MD, Tsao MS, Cekanova M, Schuller HM. Ethanol and the tobacco-specific carcinogen, NNK, contribute to signaling in immortalized human pancreatic duct epithelial cells. Pancreas. 2006;33:53–62. doi: 10.1097/01.mpa.0000226883.55828.e9. [DOI] [PubMed] [Google Scholar]
- 21.Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–80. [PubMed] [Google Scholar]
- 22.Dohrman DP, Reiter CK. Ethanol modulates nicotine-induced upregulation of nAChRs. Brain Res. 2003;975:90–8. doi: 10.1016/s0006-8993(03)02593-9. [DOI] [PubMed] [Google Scholar]
- 23.Al-Wadei HA, Al-Wadei MH, Ullah MF, Schuller HM. Celecoxib and GABA cooperatively prevent the progression of pancreatic cancer in vitro and in xenograft models of stress-free and stress-exposed mice. PLoS One. 2012;7:e43376. doi: 10.1371/journal.pone.0043376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weddle DL, Tithoff P, Williams M, Schuller HM. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22:473–9. doi: 10.1093/carcin/22.3.473. [DOI] [PubMed] [Google Scholar]
- 25.Askari MD, Tsao MS, Schuller HM. The tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone stimulates proliferation of immortalized human pancreatic duct epithelia through beta-adrenergic transactivation of EGF receptors. J Cancer Res Clin Oncol. 2005;131:639–48. doi: 10.1007/s00432-005-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuller HM, Al-Wadei HA, Majidi M. GABA B receptor is a novel drug target for pancreatic cancer. Cancer. 2008;112:767–78. doi: 10.1002/cncr.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallukat G. The beta-adrenergic receptors. Herz. 2002;27:683–90. doi: 10.1007/s00059-002-2434-z. [DOI] [PubMed] [Google Scholar]
- 28.Lefkowitz RJ. The superfamily of heptahelical receptors. Nat Cell Biol. 2000;2:E133–6. doi: 10.1038/35017152. [DOI] [PubMed] [Google Scholar]
- 29.Schlaepfer IR, Hoft NR, Ehringer MA. The genetic components of alcohol and nicotine co-addiction: from genes to behavior. Curr Drug Abuse Rev. 2008;1:124–34. doi: 10.2174/1874473710801020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–71. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol. 2009;78:756–65. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckendahl P, Kubovcakova L, Krizanova O, Pohorecky LA, Kvetnansky R. Ethanol consumption increases rat stress hormones and adrenomedullary gene expression. Alcohol. 2005;37:157–66. doi: 10.1016/j.alcohol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Schuller HM, Jorquera R, Reichert A, Castonguay A. Transplacental induction of pancreas tumors in hamsters by ethanol and the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1993;53:2498–501. [PubMed] [Google Scholar]
- 34.Al-Wadei HA, Al-Wadei MH, Schuller HM. Prevention of pancreatic cancer by the beta-blocker propranolol. Anticancer Drugs. 2009;20:477–82. doi: 10.1097/CAD.0b013e32832bd1e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Bao H, Sawyer S, Kunos G, Gao B. Effects of short and long term ethanol on the activation of signal transducer and activator transcription factor 3 in normal and regenerating liver. Biochem Biophys Res Commun. 1997;239:666–9. doi: 10.1006/bbrc.1997.7531. [DOI] [PubMed] [Google Scholar]
- 36.Hoek JB, Thomas AP, Rooney TA, Higashi K, Rubin E. Ethanol and signal transduction in the liver. FASEB J. 1992;6:2386–96. [PubMed] [Google Scholar]
- 37.Pandey SC. Protein kinase C: molecular and cellular targets for the action of ethanol. Alcohol Clin Exp Res. 1996;20:67A–71A. doi: 10.1111/j.1530-0277.1996.tb01749.x. [DOI] [PubMed] [Google Scholar]
- 38.Roivainen R, Hundle B, Messing RO. Ethanol enhances growth factor activation of mitogen-activated protein kinases by a protein kinase C-dependent mechanism. Proc Natl Acad Sci U S A. 1995;92:1891–5. doi: 10.1073/pnas.92.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeldin G, Yang SQ, Yin M, Lin HZ, Rai R, Diehl AM. Alcohol and cytokine-inducible transcription factors. Alcohol Clin Exp Res. 1996;20:1639–45. doi: 10.1111/j.1530-0277.1996.tb01710.x. [DOI] [PubMed] [Google Scholar]
- 40.Colombo G, Addolorato G, Agabio R, Carai MA, Pibiri F, Serra S, et al. Role of GABA(B) receptor in alcohol dependence: reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics. Neurotox Res. 2004;6:403–14. doi: 10.1007/BF03033315. [DOI] [PubMed] [Google Scholar]
- 41.Addolorato G, Leggio L, Ferrulli A, Cardone S, Bedogni G, Caputo F, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46:312–7. doi: 10.1093/alcalc/agr017. [DOI] [PubMed] [Google Scholar]
- 42.Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–38. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645–52. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drell TLt, Joseph J, Lang K, Niggemann B, Zaenker KS, Entschladen F. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res Treat. 2003;80:63–70. doi: 10.1023/A:1024491219366. [DOI] [PubMed] [Google Scholar]
- 45.Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61:2866–9. [PubMed] [Google Scholar]
- 46.Palm D, Lang K, Niggemann B, Drell TLt, Masur K, Zaenker KS, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer. 2006;118:2744–9. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- 47.Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–75. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuller HM, Al-Wadei HA, Ullah MF, Plummer HK., 3rd Regulation of pancreatic cancer by neuropsychological stress responses: a novel target for intervention. Carcinogenesis. 2012;33:191–6. doi: 10.1093/carcin/bgr251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Wadei HA, Plummer HK, 3rd, Ullah MF, Unger B, Brody JR, Schuller HM. Social stress promotes and gamma-aminobutyric acid inhibits tumor growth in mouse models of non-small cell lung cancer. Cancer Prev Res (Phila) 2012;5:189–96. doi: 10.1158/1940-6207.CAPR-11-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuller HM, Porter B, Riechert A. Beta-adrenergic modulation of NNK-induced lung carcinogenesis in hamsters. J Cancer Res Clin Oncol. 2000;126:624–30. doi: 10.1007/PL00008474. [DOI] [PMC free article] [PubMed] [Google Scholar]