Abstract
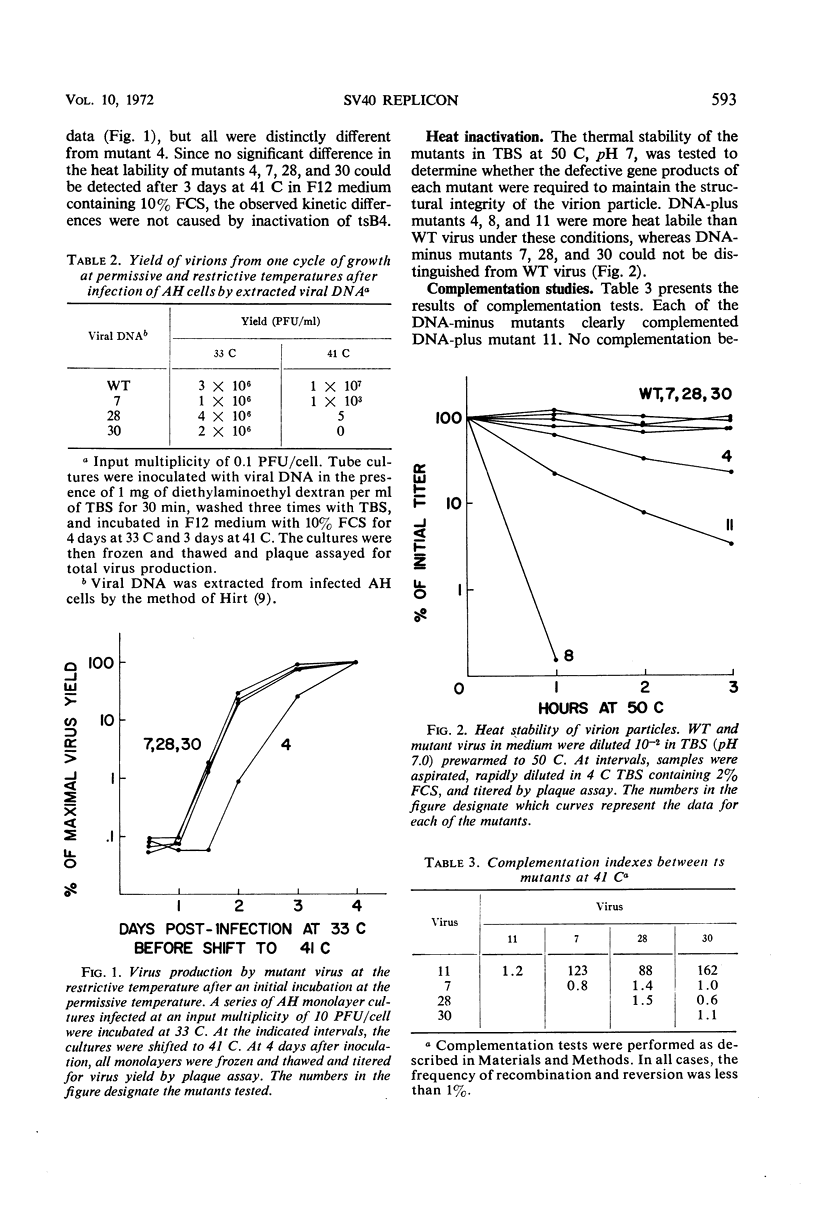
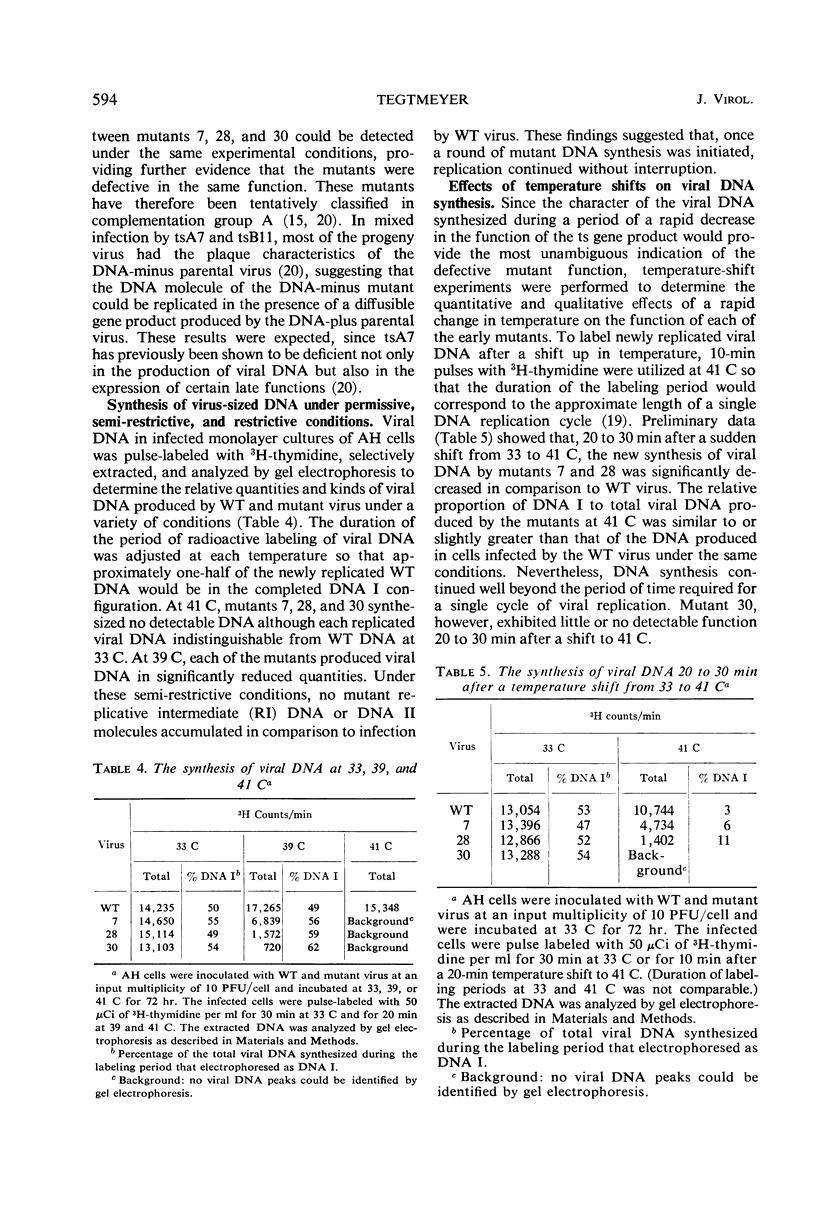
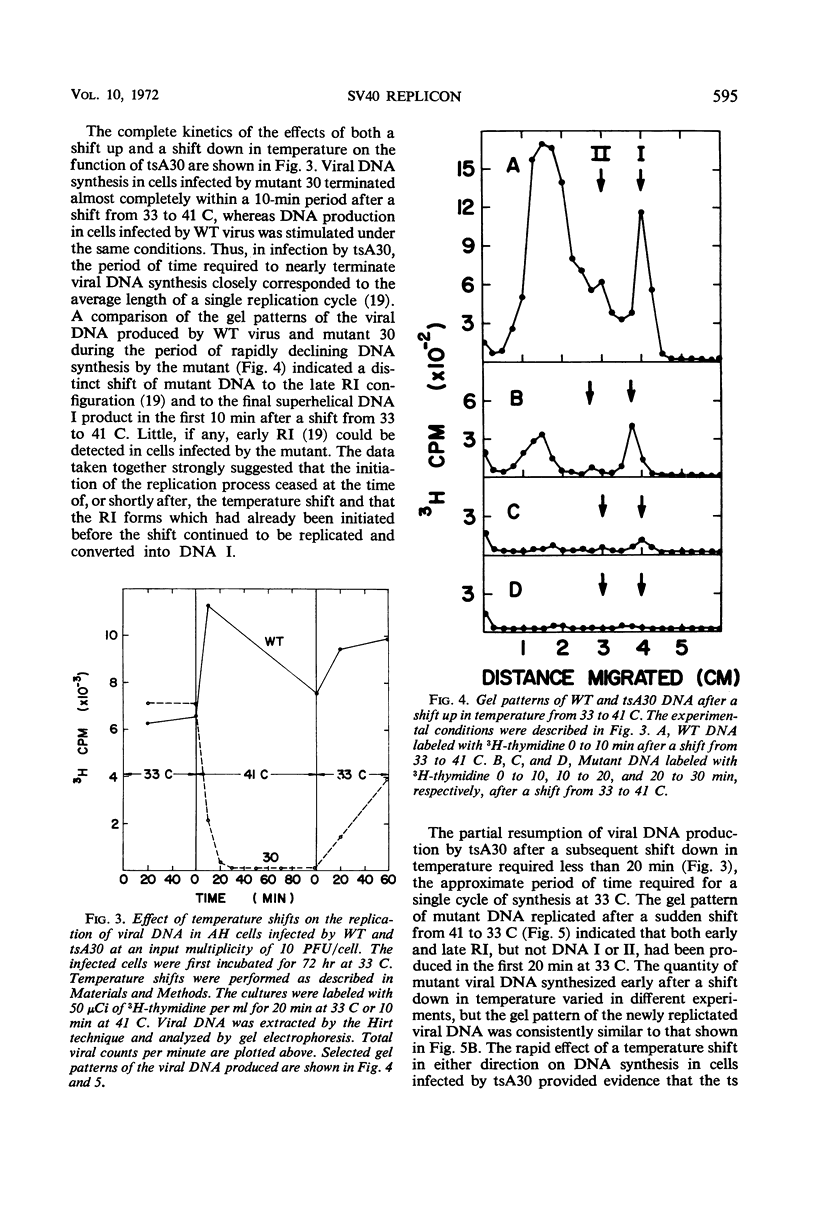
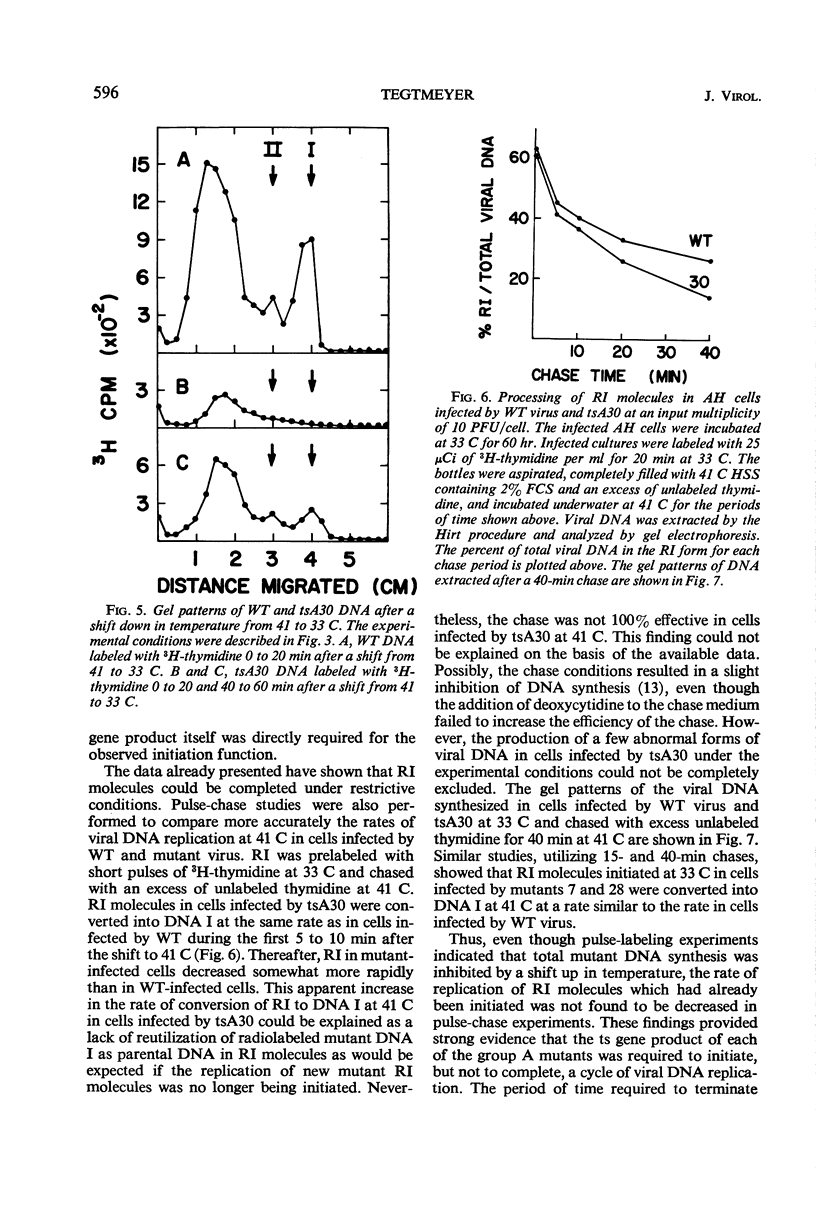
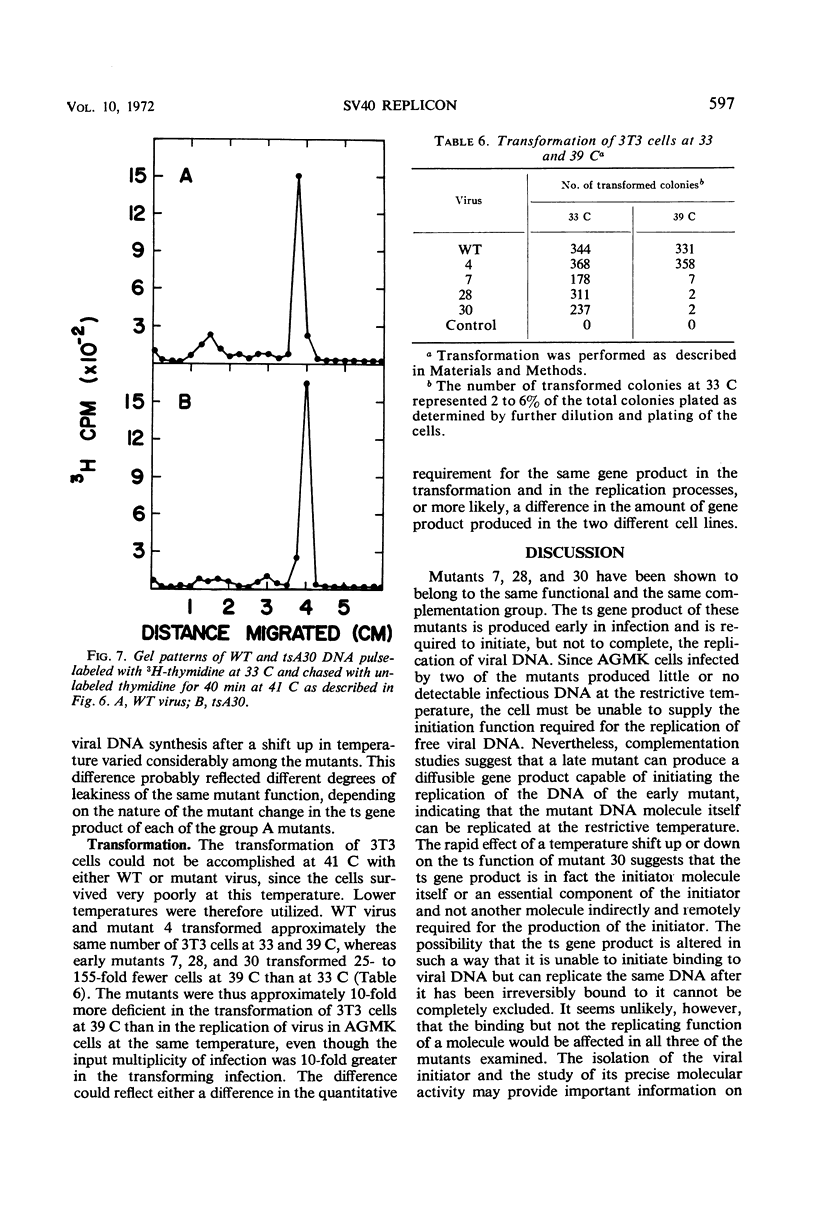
Three temperature-sensitive (ts) mutants of simian virus 40 (SV40) in complementation group A (tsA7, tsA28, tsA30) have been isolated and characterized in permissive and restrictive host cells. At 41 C in the AH line of African green monkey kidney cells, the mutants are deficient in an early function required to produce infectious viral deoxyribonucleic acid (DNA). Temperature-shift experiments and analysis of SV40 viral DNA replication by gel electrophoresis have provided strong evidence that the ts gene product of the three mutants is directly required to initiate each new round of viral DNA replication but is not required to complete a cycle which has already begun. The synthesis of mutant DNA molecules themselves can be initiated by a nonmutant gene product in viral complementation studies at 41 C. The cell, however, cannot substitute a host function to provide the initiator required for the replication of free viral DNA. The viral initiator is also required to establish the stable transformation of 3T3 cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK P. H., ROWE W. P. SV-40 INDUCED PROLIFERATION OF TISSUE CULTURE CELLS OF RABBIT, MOUSE, AND PORCINE ORIGIN. Proc Soc Exp Biol Med. 1963 Dec;114:721–727. doi: 10.3181/00379727-114-28780. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- GUENALP A. GROWTH AND CYTOPATHIC EFFECT OF RUBELLA VIRUS IN A LINE OF GREEN MONKEY KIDNEY CELLS. Proc Soc Exp Biol Med. 1965 Jan;118:85–90. [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Gershon D., Sachs L., Winocour E. The induction of cellular DNA synthesis by simian virus 40 in contact-inhibited and in x-irradiated cells. Proc Natl Acad Sci U S A. 1966 Sep;56(3):918–925. doi: 10.1073/pnas.56.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Dulbecco R. Induction of DNA synthesis by SV40. Proc Natl Acad Sci U S A. 1966 Aug;56(2):736–740. doi: 10.1073/pnas.56.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry P., Black P. H., Oxman M. N., Weissman S. M. Stimulation of DNA synthesis in mouse cell line 3T3 by Simian virus 40. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1170–1176. doi: 10.1073/pnas.56.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Defendi V. Integration of simian virus 40 deoxyribonucleic acid into the deoxyribonucleic acid of permissive monkey kidney cells. J Virol. 1972 Apr;9(4):705–707. doi: 10.1128/jvi.9.4.705-707.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Nathans D., Danna K. J. Specific origin in SV40 DNA replication. Nat New Biol. 1972 Apr 19;236(68):200–202. doi: 10.1038/newbio236200a0. [DOI] [PubMed] [Google Scholar]
- REICHARD P., CANELLAKIS Z. N., CANELLAKIS E. S. Studies on a possible regulatory mechanism for the biosynthesis of deoxyribonucleic acid. J Biol Chem. 1961 Sep;236:2514–2519. [PubMed] [Google Scholar]
- Robb J. A., Martin R. G. Genetic analysis of simian virus 40. I. Description of microtitration and replica-plating techniques for virus. Virology. 1970 Aug;41(4):751–760. doi: 10.1016/0042-6822(70)90439-3. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Tegtmeyer P., Martin R. G., Kit S. Proposal for a uniform nomenclature for simian virus 40 mutants. J Virol. 1972 Mar;9(3):562–563. doi: 10.1128/jvi.9.3.562-563.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWEET B. H., HILLEMAN M. R. The vacuolating virus, S.V. 40. Proc Soc Exp Biol Med. 1960 Nov;105:420–427. doi: 10.3181/00379727-105-26128. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Dohan C., Jr, Reznikoff C. Inactivating and mutagenic effects of nitrosoguanidine on simian virus 40. Proc Natl Acad Sci U S A. 1970 Jul;66(3):745–752. doi: 10.1073/pnas.66.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Macasaet F. Simian virus 40 deoxyribonucleic acid synthesis: analysis by gel electrophoresis. J Virol. 1972 Oct;10(4):599–604. doi: 10.1128/jvi.10.4.599-604.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Green H. High frequency of SV40 transformation of mouse cell line 3T3. Virology. 1966 Apr;28(4):756–759. doi: 10.1016/0042-6822(66)90261-3. [DOI] [PubMed] [Google Scholar]



