Abstract
The lateral habenula (LHb) is part of the habenular complex in the dorsal diencephalon. The LHb is an important regulator of several neurotransmitter systems in the midbrain; disturbances in this regulation may contribute to mood disorders, abnormalities in cognition, drive, and addiction. Owing to the critical role this nucleus plays in modulating activity of midbrain nuclei, there has been a rapid increase in studies targeting the LHb in the recent years. In this review, we describe studies using traditional approaches to elucidate the function of this brain region, such as lesion, electrical and chemical stimulation, electrophysiology and in vivo microdialysis. We have selected a variety of illustrative studies to discuss each of these methods. Next, we describe studies using methods that are based upon recent advances in molecular biology techniques including recent results from our laboratory using the Designer Receptor Exclusively Activated by Designer Drug (DREADD) technology. Using a Gi/o-coupled DREADD, we found that inhibition of the LHb reduces depression-like behavior in the forced swim test in a manner that suggests enhanced serotonergic activity. The emerging picture reveals that the LHb is likely to be a critical node in the network of subcortical nuclei that regulate aversive learning, motivation, stress responses, etc. We describe how recently developed methods have advanced the study of the LHb and are leading research of this brain region in promising new directions.
Keywords: Designer receptors exclusively activated by designer drugs (DREADDs), Viral-mediated gene transfer, FLEX switch strategy, Optogenetic, Pharmacogenetic
1. Introduction
The habenular complex in the dorsal diencephalon is located on either side of the vertebrate third ventricle bilaterally. The habenular complex consists of medial and lateral sub-divisions that are morphologically and functionally distinct and have divergent connections with other brain regions (Klemm, 2004; Sutherland, 1982). Here, we focus on the lateral habenula (LHb), a brain region that is currently under intense scrutiny by neuroscientists. The LHb receives afferent projections via the stria medullaris from the limbic forebrain, which is innervated by the cerebral cortex, basal ganglia, lateral hypothalamus, and parts of the extended amygdala, among other brain regions (Geisler and Trimble, 2008). LHb efferents (via the fasciculus retroflexus) target brainstem nuclei, including the dopaminergic ventral tegmental area (VTA) and substantia nigra pars compacta, the serotonergic dorsal and median raphe nuclei, the cholinergic laterodorsal tegmentum and the GABAergic rostromedial tegmental nucleus (RMTg) (Araki et al., 1988; Geisler and Trimble, 2008; Herkenham and Nauta, 1979; Jhou et al., 2009b). Functionally, LHb lesions increase dopamine turnover in striatal and cortical terminal regions (Lecourtier et al., 2008; Lisoprawski et al., 1980; Nishikawa et al., 1986) while local electrical stimulation of the LHb inhibits spontaneous firing of VTA dopamine neurons (Christoph et al., 1986; Ji and Shepard, 2007). Serotonin neurons in the dorsal raphe nucleus are also inhibited by electrical stimulation of the LHb (Park, 1987; Wang and Aghajanian, 1977). Thus, the LHb forms a connective locus between the cortex and the midbrain that restrains the activity of several midbrain nuclei (Balcita-Pedicino et al., 2011; Brinschwitz et al., 2010; Hikosaka et al., 2008). As a result, the LHb is known to be involved in a variety of physiological and pathological responses, like reward (Gomita and Gallistel, 1982), reward error processing (Matsumoto and Hikosaka, 2007), stress (Del Bel et al., 1998; Kazi et al., 2004; Wirtshafter et al., 1994), and clinical disorders like major depression (Sartorius et al., 2010), schizophrenia (Sandyk, 1992; Shepard et al., 2006), and addiction (Beretta et al., 2012; Brown et al., 2010; James et al., 2011; Zhang et al., 2005).
The inhibitory impact of this nucleus was perplexing since most LHb projection neurons appear to be excitatory, glutamatergic neurons. However, this contradiction may be resolved by the recognition that LHb neurons frequently synapse onto GABAergic neurons, in the VTA (Omelchenko et al., 2009) or in the recently identified RMTg (Jhou et al., 2009a; Jhou et al., 2009b), which would in turn inhibit dopaminergic neuronal activity. This is intriguing since deficits in an extended network from cortical to subcortical regions, specifically in the downward inhibitory control is thought to be an important feature of several neuropsychiatric conditions including mood disorders and affective disorders (Price and Drevets, 2012; Volkow and Baler, 2012).
Our goal here is to review the various methodological approaches that have been used to study the function of the LHb. The reviewed studies include those that explored the LHb using lesion, electrical and chemical stimulation, in vivo microdialysis and electrophysiological approaches. We also discuss newer optogenetic and pharmacogenetic techniques and briefly highlight their advantages and disadvantages. Finally, we propose the use of viral-mediated gene transfer of DREADDs (Designer Receptors Exclusively Activated by Designer Drugs) to further explore the function of the LHb in complex emotional behaviors and describe recent data from our laboratory demonstrating that DREADDs can serve as useful tools to manipulate LHb neuronal activity.
2. Methodologies used to study functions of the LHb
2.1 Electrophysiology
In a seminal study using an electrophysiological approach, Matsumoto and Hikosaka (2007) demonstrated that negative prediction errors result in activation of the LHb. In this study, rhesus monkeys were implanted with electrodes in the LHb (as well as in dopaminergic areas) and trained to perform right and left eye saccades that were recorded by search coils surgically implanted under the conjunctiva. A saccade to one side predicted a reward, while a saccade to the other side did not. The authors found that saccades to the reward-paired side were faster than the unpaired side, and increased dopamine neuronal activity was recorded at reward delivery and a decrease in dopamine neuronal activity was recorded when reward was not delivered. Interestingly, neuronal activity recorded from neurons in the LHb decreased at reward delivery and increased when the reward was not delivered, suggesting that LHb and dopamine neuronal activity are inversely related. Matsumoto and Hikosaka also demonstrated that in addition to negative error prediction signals, other negative emotional states such as air puffs to the face of rhesus monkeys, result in similar electrophysiological responses in LHb neurons to those described above (Matsumoto and Hikosaka, 2009). We suspect that LHb hyperactivity in response to negative prediction errors and the subsequent disruption of dopaminergic signaling plays an important role in addictive disorders, especially during withdrawal and relapse phases.
A combination of electrophysiological and anatomical tools was used by Hikosaka and colleagues to identify an inhibitory nucleus, similar to the rat RMTg in monkeys (Hong et al., 2011). The monkey RMTg receives excitatory (glutamatergic) input from the LHb and stimulation of this nucleus inhibits midbrain dopamine neurons (Hong et al., 2011). A combination of these tools was also used by Malinow and colleagues to examine the cellular basis of depression (Li et al., 2011). The authors examined the synaptic circuitry of the LHb in an acute learned helplessness model of depression (induced by exposure to inescapable footshocks), and congenital learned helplessness model (produced by selective breeding of rats with high acute learned helplessness). They not only found that enhanced excitatory synaptic transmission onto VTA-projecting LHb neurons positively correlates with the degree of helpless behavior, but also that deep brain stimulation of the LHb, used clinically for treatment-resistant depression (Sartorius and Henn, 2007), acutely reverses learned helplessness. These results suggest that increased presynaptic transmission onto LHb neurons is likely involved in the pathogenesis of depression, as assessed in the learned helplessness model.
2.2 Electrical stimulation and lesion studies
Most earlier descriptions of habenular modulation of midbrain neurons are from studies using lesion or stimulation approaches to manipulate LHb activity. For instance, Christoph et al. electrically stimulated the LHb and observed inhibition of dopaminergic neurons in the VTA and substantia nigra compacta in single-unit extracellular recordings in these brain regions in anesthetized rats via the fasciculus retroflexus (Christoph et al., 1986). Ji and Shepard (2007) further explored the mechanism of inhibition of dopamine neurons by LHb stimulation. They found that the GABAA receptor antagonist bicuculline, but not the SK channel blocker apamin, attenuated the effect of LHb stimulation on dopamine neurons, suggesting that LHb induced suppression of dopamine is mediated, at least in part, by midbrain GABAergic neurons.
Analogous to the effect of LHb stimulation on midbrain dopamine, electrical stimulation of the LHb has also been reported to induce a net inhibitory effect on firing in serotonergic raphe neurons (Park, 1987; Wang and Aghajanian, 1977). Wang and Aghajanian further reported that this effect is blocked by the GABAA receptor antagonist picrotoxin, suggesting that this is a GABAA receptor-dependent effect. This observation was confirmed in a report by Ferraro et al. (1996). Thus, the LHb appears to function as a relay node with the ability to fine-tune the activity of midbrain monoaminergic neurons.
In addition to studying the anatomical connections of the LHb, scientists have performed lesion studies to determine the effect of loss of LHb function on various behaviors. For instance, Morissette and Boye (2008) examined the effect of electrolytic LHb lesions on brain stimulation reward. It is well known that stimulatory electrodes when placed in the network of the brain reward circuitry will induce very strong reinforcing effects (Olds and Milner, 1954; Routtenberg and Lindy, 1965). In this study, rats were implanted with monopolar stimulating electrodes in four brain regions that are known to support operant responding for brain stimulation reward; the lateral hypothalamus, VTA, dorsal raphe, or median raphe nucleus and were subsequently trained to nose poke for intracranial self-stimulation at one of the four sites. A lesioning electrode was also implanted in the ipsilateral habenula. The authors demonstrated that unilateral electrolytic lesions of the LHb induced long-lasting attenuations in the rewarding properties of intracranial self-stimulation. In contrast to this observation, it has also been previously demonstrated that stimulation of the LHb has rewarding properties in itself (Sutherland and Nakajima, 1981; Vachon and Miliaressis, 1992). The discrepancy in these observations is possibly due to multiple factors. The size of the lesion in most lesion studies is not exactly identical; with respect to the LHb, this may result in the lesions influencing different output neurons, such as neurons directed to the VTA versus the RMTg that are known to be distinct (Jhou et al., 2009b; Lavezzi and Zahm, 2011). In addition, the differences in the duration between the time of lesion and testing likely results in differences in the degree of neuronal degeneration and discrepancies in behavioral effects.
Yang et al. (2008) examined the behavioral effects of LHb lesions in rat models of depression. The authors used two rat models: (i) exposure to chronic mild stress and, (ii) chronic administration of the tricyclic antidepressant clomipramine in neonates that produced depressive phenotype in adulthood as assessed in the forced swim test. The authors demonstrated that rats with electrolytic lesions of the LHb demonstrated a decrease in immobility time and an increase in climbing time in the forced swim test, while rats with sham lesions did not. In vivo microdialysis studies indicated that serotonin levels in the dorsal raphe nucleus in animals with a ‘depressive’ phenotype were significantly lower than control animals. These results imply that dysfunction of LHb regulation of the dorsal raphe nucleus likely plays an important role in the pathogenesis of depressive disorders. Additional studies are warranted to determine the role of LHb on other midbrain structures, importantly the noradrenergic system in depression. Indeed, deep brain stimulation of the LHb, consisting of continuously delivered high frequency stimulation, has recently been used for the treatment of major depression in a therapy-refractory patient (Sartorius et al., 2010). To examine the cellular effects of deep brain stimulation on the LHb, Li et al. (2011) placed a stimulating electrode in the LHb delivering 7 stimulus 130 Hz trains separated by 40 ms millisecond intervals (similar to the deep brain stimulation protocol used clinically) and recorded synaptic transmission onto VTA-projecting LHb neurons in a slice preparation. Deep brain stimulation of the LHb significantly decreased excitatory synaptic transmission for the duration of stimulation that was reversed after cessation of the stimulus train. Using the same deep brain stimulation protocol described above, Li et al. further demonstrated reversal of the depressive phenotype in a rat model of learned helplessness. These findings point to a critical role of the LHb in the pathogenesis of major depression.
2.3 In vivo microdialysis
Despite the direct influence of the LHb on midbrain monoamine neurotransmitter systems that traditionally have been extensively studied using in vivo microdialysis, relatively few studies have used this technique to examine the effect of modulation of LHb neuronal activity on the extracellular release of these neurotransmitters.
LeCourtier et al. (2008) tested the hypothesis that the LHb maintains tonic inhibitory control of dopamine neurons in a microdialysis study in the rat brain. Activity of the LHb was transiently inhibited by local application of AMPA receptor antagonist LY293558 and extracellular dopamine levels were measured in the nucleus accumbens, lateral striatum, and prefrontal cortex. Reverse dialysis of LY293558 into the LHb produced an approximately 1.5-fold increase in the extracellular concentration of dopamine in the nucleus accumbens and striatum, while the increase in cortical extracellular dopamine was minimal. In contrast, electrical stimulation of the LHb had negligible effect on extracellular dopamine in these brain regions. Taken together, these results indicate that the increase in dopamine levels upon LHb inhibition are greater than the decrease in dopamine levels upon LHb stimulation, suggesting that tonic activity of the LHb may exert a near-maximal effect on neurotransmitter release in these brain regions.
Using a double-loop microdialysis technique (with 60% higher recovery rate for indoles compared to a single-loop dialysis probe), Kalen et al. (1989b) examined the effect of electrical stimulation of the LHb-dorsal raphe pathway on extra-synaptic levels of striatal serotonin in rats under halothane anesthesia. They determined, interestingly, that 15 Hz, 0.5 mA LHb stimulation produced an approximately 1.5-fold increase in extracellular striatal serotonin levels, which was completely blocked by ibotenic acid lesions of the ipsilateral fasciculus retroflexus, but not by silver knife transection of the interpeduncular nucleus (that carries major output projections from the medial habenula). Kalen et al further determined that the striatal increase in extracellular serotonin was completely abolished by injections of the glutamate antagonist kynurenic acid into the dorsal raphe. Further, raphe injections of the GABAA antagonist bicuculline enhanced the facilitatory effect of LHb stimulation on striatal serotonin. These results appear to be in direct conflict with results from lesion studies demonstrating an inhibitory effect of LHb stimulation on serotonin (Wang and Aghajanian, 1977). We speculate that technical differences in methodologies used, particularly the use of halothane anesthesia for the microdialysis experiments, may account for these differences. The push-pull technique was also used by Reisine et al. (1982) to demonstrate that the transmitters associated with the LHb projections to the dorsal raphe nucleus may be GABA and/or substance P. In a distinct set of experiments, Kalen et al (1989a) also determined that electrical stimulation of the LHb enhances hippocampal norepinephrine release via the locus coeruleus. Further, it appears that the effect of LHb stimulation on norepinephrine release is not specific to any terminal field since increases in extracellular norepinephrine are also seen in the caudate nucleus, nucleus accumbens, and prefrontal cortex (Cenci et al., 1992). Taken together, it appears that LHb stimulation activates the locus coeruleus, which subsequently enhances norepinephrine release in terminal fields, an effect that possibly plays a role in the sustained behavioral activation seen upon LHb stimulation (Nilsson et al., 1990).
2.4 Optogenetics
Recently, experimental strategies using engineered receptors activated by light or ‘opsins’ to modulate neuronal activity in vivo in discrete brain regions have gained popularity (Deisseroth et al., 2006; Zhang et al., 2006). Light activated cation channels such as channelrhodopsin-2 (ChR2) or chloride pumps such as halorhodopsin (NpHR) can be used to activate or inhibit cells of interest with remarkable spatial, temporal, and phenotypic precision (reviewed elsewhere in this issue). Transgenic strategies are providing increasingly more precise and controllable expression of these transgenes in mice and rats, whereas viral vectors can be used to introduce genes for engineered proteins into target brain regions in several species. Stimulation of ChR2 with pulsed blue light (450–490 nm, usually laser light delivered via implanted optical fibers) induces neuronal depolarization, while yellow light (580 nm) activates NpHR’s to induce neuronal hyperpolarization. A third class of optogenetic tools, called OptoXRs, are rhodopsin-GPCR chimeras that can induce metabotropic signaling upon light activation of the receptor (Airan et al., 2009). The use of light-sensitive engineered proteins are elegant and powerful tools for neuroscientists because of the temporal and spatial precision possible in activating or inhibiting neuronal firing; these characteristics make them especially suitable for studying the contribution of small, deep structures, such as the LHb, to complex behaviors. In particular, the problems associated with damaging or inadvertently modulating nearby structures are markedly reduced. Below, we describe recent studies using optogenetic techniques in combination with electrophysiology to study the LHb.
In an elegant set of studies, Stamatakis and Stuber (2012) examined the behavioral effects of selective activation of the LHb-RMTg projecting neurons. The authors introduced ChR2 fused to enhanced yellow fluorescent protein (EYFP) into the LHb using adeno-associated virus-mediated gene transfer and observed ChR2-EYFP expression in LHb terminals in the VTA and RMTg. Subsequent whole cell recordings from RMTg neurons in brain slices revealed that light pulses that selectively stimulated ChR2-EYFP expressing terminals resulted in inward currents that were blocked by the glutamate antagonist 6,7-dinitroquinoxaline-2,3-dione. Given that LHb neurons encode aversive-stimuli related information, the authors further examined whether exposure to an aversive stimulus modulates excitatory neurotransmission at LHb-RMTg synapses. Mice expressing ChR2-EYFP in LHb-RMTg fibers were exposed to intermittent, unpredictable footshocks (0.75 mA, 500 ms) for 20 minutes, and performed whole-cell patch-clamp electrophysiology 1h post-shock exposure. They found an increase in the frequency (but not amplitude) of miniature excitatory post-synaptic potentials, a decrease in paired-pulse ratio, and no difference in AMPA/NMDA ratio in response to optical stimulation. Taken together, these data suggest that exposure to footshock stress enhances activity of LHb neurons projecting to the RMTg. Stamatakis and Stuber further examined the behavioral effects of optically stimulating LHb-RMTg fibers and found, not surprisingly, that activating these projections not only induces passive avoidance and conditioned place aversion, but also disrupts positive reinforcement.
While Stamatakis and Stuber examined the functional relevance of LHb output neurons to the RMTg, Shabel et al. (2012) employed optogenetic strategies to determine the properties of neuronal inputs to the LHb from the basal ganglia. The authors used adeno-associated virus-mediated gene transfer to target ChR2 expressing yellow fluorescent protein to the entopeduncular nucleus and found that stimulation of entopeduncular nucleus axons in the LHb excites LHb neurons via glutamate receptors, thereby confirming that these inputs are glutamatergic, which is unusual for basal ganglia outputs. The authors optically stimulated these projections and found that activating this pathway produced an aversive effect in a place preference test. Owing to the role of the LHb in the pathophysiology of depressive behaviors, the authors further examined the ability of serotonin to modulate excitability of LHb neurons and found that serotonin has an inhibitory effect on the excitatory input from the entopeducular nucleus to the LHb. This suggests that negative prediction errors can be modulated by serotonin, a neurotransmitter implicated in mood disorders and depression, thus opening up promising new avenues for therapeutic interventions. In summary, results from optogenetic studies point to complex, bidirectional interactions between LHb and other subcortical nodes that modulate affective information.
3. Designer receptors exclusively activated by designer drugs (DREADDs)
Strategies using engineered receptors that are activated by light to manipulate LHb neuronal activity in vivo have been described in the previous section. Here, we will describe novel strategies using engineered receptors that are activated by synthetic ligands to modulate in vivo neuronal activity in the LHb in a defined spatial and temporal manner.
An approach steadily gaining in popularity is to use ligand-dependent engineered receptors called designer receptors exclusively activated by designer drugs (DREADDs) to selectively modulate neuronal activity. These receptors consist of a family of evolved muscarinic receptors that were engineered by molecular evolution and site-directed mutagenesis in the laboratory of Dr. Bryan Roth at the University of North Carolina. The DREADDs were designed to display high affinity for clozapine-N-oxide (CNO), an otherwise inert ligand while losing affinity for their original cognate endogenous ligand, acetylcholine (Armbruster et al., 2007; Rogan and Roth, 2011). Three DREADD receptors have been described to date, coupling to Gs, Gi, and Gq pathways, which are reviewed elsewhere in this issue. Following transduction of DREADDs into LHb neurons, systemic administration of CNO will stimulate these receptors and activate downstream signaling pathways that can activate (via Gs or Gq) or inhibit (via Gi) cells that express the receptors. However, these signaling events are more complex than simply inducing or inhibiting neuronal firing; rather, activating each of these pathways may also have significant impacts on gene expression, plasticity, and modulate other signaling pathways. While optogenetic receptors can be controlled instantaneously with the application of laser light, CNO can be administered systemically which will activate DREADDs on a time scale that is more typical for drug-receptor interactions (i.e. minutes to a few hours). Combined with viral-mediated gene transfer or transgenic expression, this is a powerful strategy to examine the role of neurons in a specific brain region on behavior because (i) the same methodology can be applied to stimulate as well as inhibit neuronal activity (using different DREADDs, even simultaneously), (ii) its precise anatomical accuracy since CNO will activate only expressed DREADD receptors without off-target effects, (iii) DREADDs can be activated “remotely” by intravenously or orally administered CNO, or by local CNO infusion if preferred, and (iv) activation of DREADDs is ligand-driven, and hence activation or inhibition of neurons is temporary, unlike a neurotoxic lesion, and can be timed to coincide with different aspects of behavior. While optogenetics allows subsecond precision in activating neurons or even axon terminals (Adamantidis et al., 2011; Stuber et al., 2011), the DREADD approach allows for brief, repeated activation of the engineered receptors at key points during a complex behavioral task without any implantation of fiberoptics, making it less invasive. In our laboratory, we have previously used hM4Di (Gi/o coupled DREADD) expressed with viral vectors to selectively target the direct or indirect striatal pathways to show that these pathways play opposing roles in psychostimulant-induced plasticity (Ferguson et al., 2011). It is intriguing that this DREADD affected behavioral plasticity associated with repeated amphetamine exposure without disrupting acute behavioral responses mediated by the striatal circuitry. We have also used both hM4Di and hM3D (Gq coupled DREADD) with viral vectors to demonstrate that inhibition and activation of the direct pathway has opposing effects on the plasticity involved in a decision-making task (Ferguson, 2012). Below, we propose strategies for using DREADDs to modulate LHb neuronal activity.
3.1 Single-virus approach
For this approach we used herpes simplex virus (HSV)-mediated gene transfer that we have used in our laboratory for about a decade; it is neuron-specific, allows a large transgene insert including phenotype-specific promoters, and is reliable (Barot et al., 2007; Ferguson et al., 2011; McDevitt et al., 2011). We coupled HSV-mediated gene transfer with the DREADD technology to study the effect of transient LHb inhibition on behavioral responses in the modified forced swim test (Detke et al., 1995; Porsolt et al., 1977). hM4Di viral vector (Figure 1) was injected into the LHb in male Sprague-Dawley rats (250–300g) using stereotaxic techniques as previously described (Clark et al., 2002). After a postoperative recovery period of two days, a modified forced swim test was performed. The FST container was a 40 cm tall plexiglass cylinder with a 20 cm diameter base. The cylinder was filled with water (25 degrees C) to 30 cm. A 15 minute pre-test was performed on Day 1 which involved placing the rat in the chamber for 15 min, followed by towel drying the rat and returning it to the home cage. Following the pre-test, rats were treated with vehicle or CNO, 1mg/kg, i.p. three times over the ensuing 24 hours in a between-subjects manner as previously described (Detke et al., 1995); briefly, each rat received either vehicle (2% DMSO in sterile water; n=7) or CNO (1 mg/kg, i.p.; n=5) thirty minutes and fifteen hours following the pre-test, and finally thirty minutes prior to the test (Day 2). The test was conducted four days following viral vector infusion based on prior data from our laboratory that examined onset and duration of HSV viral-vector induced gene expression (Barot et al., 2007). Thirty minutes following the last CNO injection, test sessions were conducted, that were procedurally identical to the pre-test session with the exception that the duration was 5 minutes. Behavior was videotaped and scored in a blinded fashion. A time-sampling method was used in which behavior was assessed every 5 seconds and scored as climbing, swimming or immobility. Each rat was tested once; immediately following the test, rats were euthanized and brains were dissected and subsequently analyzed for transgene expression. CNO significantly increased swim time and decreased immobility time, while behavioral responses in rats with missed LHb injections (n=3) were similar to controls (Figure 2). Increased immobility time in the forced swim test is widely accepted as an indicator of behavioral depression (Porsolt et al., 1977), and increased swimming as compared to climbing behavior is associated with enhanced serotonergic activity (Detke et al., 1995). These data suggest antidepressant-like effects of transient LHb silencing and provide the first demonstration that LHb neuronal activity can be successfully modulated using the DREADD technology.
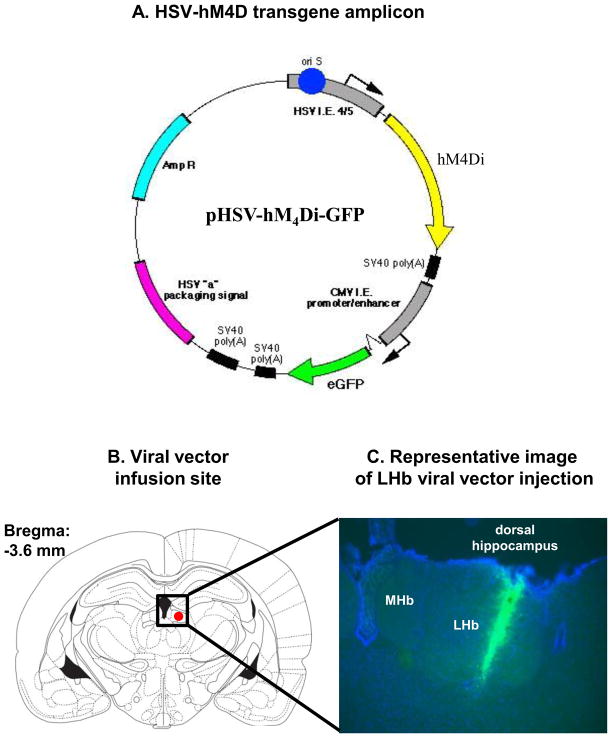
Figure 1. Virus-mediated gene transfer.
(A) Illustration of the herpes simplex virus-hM4Di green fluorescent protein transgene amplicon (B) Illustration of rat brain coordinates (Paxinos plate 32, −3.6 mm) used for viral vector infusion. The red oval depicts the target zone for the viral vector unilaterally. MHb: Medial habenula, LHb: Lateral habenula (C) Representative histological plate of demonstrating viral vector injection from a coronal section of the LHb four days after viral vector infusion.
Figure 2. Effects of HSV-hM4D mediated LHb inhibition on behavioral responses in the modified forced swim test.
Viral vector was injected into the LHb and a modified forced swim test was performed. Rats treated with CNO (1 mg/kg, i.p., n=5) demonstrated an increase in swim time and a decrease in immobility time compared to vehicle treated rats (n=7). Rats treated with CNO, but with missed LHb injections (lateral to the lateral boundary of the LHb; n=3) did not demonstrate reversal of behavioral effects in the forced swim test. p< 0.001, significantly differs from vehicle-treated group.
3.2 Dual-virus FLEX strategy
As stated previously, the LHb projects to the dopaminergic VTA, the serotonergic dorsal raphe nucleus, the GABAergic RMTg, and cholinergic laterodorsal tegmentum, and activation of each of these LHb projections likely results in distinct behavioral effects. We describe and demonstrate here the use of a recently developed dual virus FLEX (FLip and EXcise) switch strategy (Atasoy et al., 2008), a variant of the Cre-flox system that can potentially be used in rats to study the functional significance of each of these LHb projections. The viruses used for this technique encode a transgene that is doubly floxed by loxP sites, so that Cre inverts, but does not excise the transgene. The transgene is cloned into the construct in a 3′ to 5′ orientation relative to the promoter, and Cre recombines the DNA such that the transgene is now “flipped” into a 5′ to 3′ orientation to allow transcription. We injected a FLEX-green fluorescent protein (GFP) transgene with adeno-associated virus (Figure 3A) in the LHb of male Long-Evans rat (400–425g) and then injected canine adenovirus-2 (CAV-2) expressing Cre in neurons that project from LHb to the VTA. CAV-2 efficiently infects axon terminals and is retrogradely transported to the neuronal cell bodies in the LHb where it expresses Cre. Thus, the FLEX-GFP vector can only lead to GFP expression in neurons that are retrogradely infected with Cre, where GFP is flipped into the proper orientation to allow transcription (Figure 3B). We observe no leakage expression with this system whatsoever if Cre is not introduced. This general approach using floxed, inverted hM4Di, where the DREADD is fused to mCherry to monitor receptor expression, has been recently applied in mice with genetic/viral Cre expression (Krashes et al., 2011). We propose to use the above technique in rats, which are more amenable to complex behavioral tasks, to delineate the functional significance of LHb projections to each of its midbrain target nuclei.
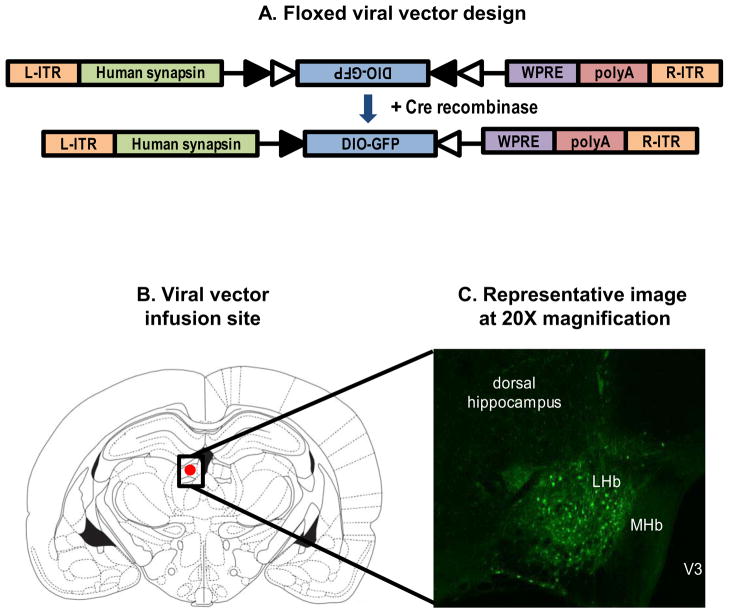
Figure 3. FLEX switch strategy.
(A) Illustration of the FLEX Viral vector design. L-ITR: Left inverted terminal repeat, R-ITR: Right inverted terminal repeat, WPRE: Woodchuck hepatitis post-transcriptional regulatory element (B) Illustration of LHb rat brain coordinates (Paxinos plate 32, −3.6 mm) used for viral vector infusion. The red oval depicts the target zone for the viral vector infusion. MHb: Medial habenula, LHb: Lateral habenula (C) Representative histological plate demonstrating viral vector injection from a coronal section of the LHb twenty four days after viral vector infusion (GFP signal is enhanced using an anti-GFP antibody).
4. Conclusions, Perspectives and Future Directions
The LHb is a small but intriguing brain region that appears to play an important role in integrating responses to aversive stimuli. The particular connections of the LHb have been elucidated but very little is now known about the relative contribution of each of these outputs to controlling motivated behavior and other responses to emotionally significant stimuli. In-depth microdialysis/voltametry studies to determine the effect of LHb manipulation on neurotransmitter release are warranted. Further, little is currently known about aberrant cellular processes in the LHb, knowledge of which can be crucial to determine the etiology of addictive disorders, mood disorders, depression, etc. and can guide towards development of therapeutics. Due to the small size of this brain region and the complexity of its inputs and outputs to controlling motivated behavior, we believe that engineered receptors will be especially useful for performing discrete, temporally specific manipulations to tease apart the functional neuroanatomy of this region. These new methods are particularly well-suited for studies of the LHb because it is possible to control the activity of these cells and observe the resulting changes in behavior while minimizing confounds associated with impacts on nearby brain regions or fiber tracts.
Acknowledgments
This work was supported by the National Institutes of Health DA106432 grant to J.F.N and University of Washington Alcohol and Drug Abuse Institute Research grant 65-6076 to S.G.N. The authors thank Denis Smirnov for proof-reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamantidis AR, et al. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan RD, et al. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- Araki M, et al. The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res. 1988;441:319–330. doi: 10.1016/0006-8993(88)91410-2. [DOI] [PubMed] [Google Scholar]
- Armbruster BN, et al. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, et al. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, et al. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barot SK, et al. 5-HT(1B) receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur J Neurosci. 2007;25:3125–3131. doi: 10.1111/j.1460-9568.2007.05568.x. [DOI] [PubMed] [Google Scholar]
- Beretta CA, et al. Habenula circuit development: past, present, and future. Front Neurosci. 2012;6:51. doi: 10.3389/fnins.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinschwitz K, et al. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience. 2010;168:463–476. doi: 10.1016/j.neuroscience.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Brown RM, et al. Identification of brain nuclei implicated in cocaine-primed reinstatement of conditioned place preference: a behaviour dissociable from sensitization. PLoS One. 2010;5:e15889. doi: 10.1371/journal.pone.0015889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA, et al. Regional differences in the regulation of dopamine and noradrenaline release in medial frontal cortex, nucleus accumbens and caudate-putamen: a microdialysis study in the rat. Brain Res. 1992;581:217–228. doi: 10.1016/0006-8993(92)90711-h. [DOI] [PubMed] [Google Scholar]
- Christoph GR, et al. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, et al. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22:4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, et al. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bel EA, et al. Differential expression of c-fos mRNA and Fos protein in the rat brain after restraint stress or pentylenetetrazol-induced seizures. Cell Mol Neurobiol. 1998;18:339–346. doi: 10.1023/A:1022505232618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, et al. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SPP, Neumaier JF. Elucidating the role of striatal pathways in operant responding and decision-making tasks using targeted viral mediated gene transfer of DREADD receptors in rats. 2012 Submitted. [Google Scholar]
- Ferraro G, et al. Lateral habenular influence on dorsal raphe neurons. Brain Res Bull. 1996;41:47–52. doi: 10.1016/0361-9230(96)00170-0. [DOI] [PubMed] [Google Scholar]
- Geisler S, Trimble M. The lateral habenula: no longer neglected. CNS Spectr. 2008;13:484–489. doi: 10.1017/s1092852900016710. [DOI] [PubMed] [Google Scholar]
- Gomita Y, Gallistel CR. Effects of reinforcement-blocking doses of pimozide on neural systems driven by rewarding stimulation of the MFB: a 14C-2-deoxyglucose analysis. Pharmacol Biochem Behav. 1982;17:841–845. doi: 10.1016/0091-3057(82)90369-0. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, et al. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28:11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, et al. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31:11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, et al. Propensity to ‘relapse’ following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience. 2011;199:235–242. doi: 10.1016/j.neuroscience.2011.09.047. [DOI] [PubMed] [Google Scholar]
- Jhou TC, et al. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, et al. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009b;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalen P, et al. Electrical stimulation of the lateral habenula increases hippocampal noradrenaline release as monitored by in vivo microdialysis. Exp Brain Res. 1989a;76:239–245. doi: 10.1007/BF00253642. [DOI] [PubMed] [Google Scholar]
- Kalen P, et al. Regulation of striatal serotonin release by the lateral habenula-dorsal raphe pathway in the rat as demonstrated by in vivo microdialysis: role of excitatory amino acids and GABA. Brain Res. 1989b;492:187–202. doi: 10.1016/0006-8993(89)90901-3. [DOI] [PubMed] [Google Scholar]
- Kazi JA, et al. Prolonged expression of c-Fos protein in the lateral habenular nucleus of the Japanese monkey (Macaca fuscata) after eye enucleation. Neurosignals. 2004;13:130–133. doi: 10.1159/000076566. [DOI] [PubMed] [Google Scholar]
- Klemm WR. Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit. 2004;10:RA261–73. [PubMed] [Google Scholar]
- Krashes MJ, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi HN, Zahm DJ. The mesopontine rostromedial tegmental nucleus: An integrative modulator of the reward system. Basal Ganglia. 2011;1:191–200. doi: 10.1016/j.baga.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, et al. Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. Eur J Neurosci. 2008;27:1755–1762. doi: 10.1111/j.1460-9568.2008.06130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisoprawski A, et al. Selective activation of the mesocortico-frontal dopaminergic neurons induced by lesion of the habenula in the rat. Brain Res. 1980;183:229–234. doi: 10.1016/0006-8993(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt RA, et al. Serotonin 1B autoreceptors originating in the caudal dorsal raphe nucleus reduce expression of fear and depression-like behavior. Biol Psychiatry. 2011;69:780–787. doi: 10.1016/j.biopsych.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette MC, Boye SM. Electrolytic lesions of the habenula attenuate brain stimulation reward. Behav Brain Res. 2008;187:17–26. doi: 10.1016/j.bbr.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Nilsson OG, et al. Acetylcholine release in the rat hippocampus as studied by microdialysis is dependent on axonal impulse flow and increases during behavioural activation. Neuroscience. 1990;36:325–338. doi: 10.1016/0306-4522(90)90429-8. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, et al. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–336. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, et al. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MR. Monosynaptic inhibitory postsynaptic potentials from lateral habenula recorded in dorsal raphe neurons. Brain Res Bull. 1987;19:581–586. doi: 10.1016/0361-9230(87)90075-x. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, et al. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Reisine TD, et al. Involvement of lateral habenula-dorsal raphe neurons in the differential regulation of striatal and nigral serotonergic transmission in cats. J Neurosci. 1982;2:1062–1071. doi: 10.1523/JNEUROSCI.02-08-01062.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A, Lindy J. Effects of the availability of rewarding septal and hypothalamic stimulation on bar pressing for food under conditions of deprivation. J Comp Physiol Psychol. 1965;60:158–161. doi: 10.1037/h0022365. [DOI] [PubMed] [Google Scholar]
- Sandyk R. Pineal and habenula calcification in schizophrenia. Int J Neurosci. 1992;67:19–30. doi: 10.3109/00207459208994773. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Henn FA. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses. 2007;69:1305–1308. doi: 10.1016/j.mehy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Sartorius A, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Shabel SJ, et al. Input to the lateral habenula from the Basal Ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012;74:475–81. doi: 10.1016/j.neuron.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard PD, et al. Schizophrenia in translation: the presence of absence: habenular regulation of dopamine neurons and the encoding of negative outcomes. Schizophr Bull. 2006;32:417–421. doi: 10.1093/schbul/sbj083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1117. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Nakajima S. Self-stimulation of the habenular complex in the rat. J Comp Physiol Psychol. 1981;95:781–791. doi: 10.1037/h0077833. [DOI] [PubMed] [Google Scholar]
- Vachon MP, Miliaressis E. Dorsal diencephalic self-stimulation: a movable electrode mapping study. Behav Neurosci. 1992;106:981–991. doi: 10.1037//0735-7044.106.6.981. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD. Neuroscience. To stop or not to stop? Science. 2012;335:546–548. doi: 10.1126/science.1218170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK. Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science. 1977;197:89–91. doi: 10.1126/science.194312. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, et al. Dopamine agonists and stress produce different patterns of Fos-like immunoreactivity in the lateral habenula. Brain Res. 1994;633:21–26. doi: 10.1016/0006-8993(94)91517-2. [DOI] [PubMed] [Google Scholar]
- Yang LM, et al. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Zhang F, et al. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–92. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- Zhang F, et al. Increased c-Fos expression in the medial part of the lateral habenula during cue-evoked heroin-seeking in rats. Neurosci Lett. 2005;386:133–137. doi: 10.1016/j.neulet.2005.06.008. [DOI] [PubMed] [Google Scholar]