Abstract
On the basis of the recently published results of a clinical trial comparing 12 and 36 months of imatinib in adjuvant therapy for gastrointestinal stromal tumors (GISTs), which demonstrated clinical benefit of longer imatinib treatment in terms of delaying recurrences and improving overall survival, both the US Food and Drug Administration and the European Medicines Agency have updated their recommendations and approved 36 months of imatinib treatment in patients with v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT)-positive GISTs (also known as CD117-positive GISTs) at high risk of recurrence after surgical resection of a primary tumor. This article discusses patient selection criteria for extended adjuvant therapy with imatinib, different classifications of risk of recurrence, and assessment of the response to therapy.
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract, with a mean annual incidence of 10–15 cases per million people, affecting mainly older individuals at a median age of 55–65 years [1–4]. Radical surgery is the treatment of choice in primary resectable GISTs, but almost all GISTs are associated with a risk of recurrence, and approximately 40–50 % of patients with potentially curative resections develop recurrent or metastatic disease [5, 6]. Classic cytotoxic chemotherapy is ineffective in advanced cases. Radiotherapy has restricted efficacy in the management of GISTs, principally because the tumor location is surrounded by dose-limiting vital organs. The prognosis of patients with inoperable or metastatic GISTs was poor until the beginning of the 21st century, when significant progress in understanding the molecular pathogenesis of GISTs resulted in development of a treatment that has become a model of targeted therapy in oncology. The introduction of imatinib mesylate (Gleevec™ or Glivec®; Novartis), a small-molecule selective inhibitor of receptor tyrosine kinases, has revolutionized the treatment of GISTs, both in the adjuvant setting and in advanced (i.e., inoperable and/or metastatic) cases. On the basis of recently published results of a clinical trial comparing 12 and 36 months of adjuvant imatinib therapy [7], demonstrating clinical benefit of longer imatinib treatment in terms of delaying recurrences and improving overall survival (OS), both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have updated their recommendations and approved 36 months of imatinib treatment in patients with v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT)-positive GISTs (also known as CD117-positive GISTs) at high risk of recurrence after surgical resection of the primary tumor.
Clinical and Molecular Features of GISTs
GISTs may originate anywhere in the gastrointestinal tract—most frequently in the stomach, followed by the small intestine. They comprise a heterogeneous group of tumors ranging from small lesions with clinically benign behavior to highly aggressive malignant tumors [8–10]. Metastases develop mainly in the liver or intraperitoneally and may even occur more than 10 years after surgery on the primary lesion, necessitating long-term follow-up of GIST patients [9, 11]. GISTs are believed to arise from progenitors related to the interstitial cells of Cajal, which are the pacemakers for peristalsis [12–14]. Approximately 85–95 % of GISTs express KIT, which is currently used for routine immunohistochemical diagnosis [15]. Other well-established immunohistochemical markers used for differential diagnosis include DOG1 [Discovered on GIST-1; encoded by the ANO1 (anoctamin 1, calcium activated chloride channel) gene], CD34 (a hematopoietic progenitor stem-cell antigen), smooth muscle actin, S100 protein, and desmin (a muscle cell marker) [16–21]. Characteristic genomic alterations in both benign and malignant GISTs mainly involve chromosomal losses of 1p, 14q, and 22q. Additional cytogenetic abnormalities present in metastatic GISTs involve losses of chromosomes 13q, 15q, and 18, and partial deletions of 11p and 9p [including tumor suppressor genes CDKN2A (cyclin-dependent kinase inhibitor 2A) and CDKN2B], as well as gains of 5p, 8q, and 17q [22–28].
Approximately 75–80 % of sporadic GISTs harbor KIT-activating mutations, and another 5–13 % of sporadic GISTs carry platelet-derived growth factor receptor, alpha polypeptide (PDGFRA)-activating mutations [29, 30]. About two thirds of all mutations in GISTs occur at the 5′ end of KIT exon 11. Less common primary mutation sites in KIT include the 3′ end of exons 11 and 9. The most frequently mutated region in PDGFRA is exon 18, typically exhibiting the p.D842V substitution.
Approximately 10–15 % of GISTs do not present detectable mutations in KIT or PDGFRA [29–40]. KIT/PDGFRA wild-type GISTs arise mainly from the stomach and are characterized by distinct clinical and pathological features, including predominant incidence in young female patients, epithelioid morphology, frequent lymphovascular invasion and lymph node metastases, and unpredictable clinical behavior. Wild-type GISTs carry inactivating mutations in genes coding for mitochondrial succinate dehydrogenase (SDH) complex II subunits A, B, C, and D, which are components of the Krebs cycle and the respiratory chain. Additionally, this subgroup of GISTs express insulin-like growth factor 1 receptor (IGF1R). Wild-type GISTs are commonly associated with Carney’s triad, Carney-Stratakis syndrome, or neurofibromatosis type 1 [41–51].
Table 1 summarizes the most important molecular features of GISTs in terms of KIT and PDGFRA mutational status.
Table 1.
Molecular classification of gastrointestinal stromal tumors (GISTs) according to v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT) and platelet-derived growth factor receptor, alpha polypeptide (PDGFRA) mutational status
| Genotype | Features |
|---|---|
| KIT mutations (75–80 % of sporadic GISTs) | |
| Exon 11 | Most common mutation in sporadic GISTs (65–70 %); present in tumors localized at all gastrointestinal sites; best response to imatinib; also reported in familial GISTs |
| Exon 9 | More common in GISTs originating from the small bowel/colon; intermediate/dose-dependent response to imatinib in advanced GISTs |
| Exon 13 | Present in tumors localized at all gastrointestinal sites; observed clinical responses to imatinib; reported in familial GISTs; more often as secondary mutations in imatinib-resistant tumors |
| Exon 17 | Present in tumors localized at all gastrointestinal sites; observed clinical responses to imatinib (except for p.D816V); reported in familial GISTs; more often as secondary mutations in imatinib-resistant tumors |
| PDGFRA mutations (5–13 % of sporadic GISTs) | |
| Exon 12 | Present in tumors localized at all gastrointestinal sites; observed clinical responses to imatinib |
| Exon 14 | Only a few cases described in the literature; more common in GISTs originating from the stomach |
| Exon 18 | More common in GISTs originating from the stomach, usually with epithelioid morphology; often related to indolent clinical behavior; p.D842V is the most common and is resistant to imatinib; other exon 18 mutations are sensitive to imatinib |
| KIT/PDGFRA wild type | Frequent in pediatric GISTs; poor response to imatinib; typical for GISTs related to neurofibromatosis type 1, Carney’s triad (gastric GIST + pulmonary chondroma ± paraganglioma), or Carney-Stratakis syndrome (GIST + paraganglioma, characterized by mutations in genes encoding SDH subunits SDHA, SDHB, SDHC, SDHD), and/or IGF1R expression |
IGF1R insulin-like growth factor 1 receptor, SDH succinate dehydrogenase
Imatinib Mesylate Therapy for Advanced GISTs
Imatinib mesylate was initially developed for the treatment of chronic myelogenous leukemia, to specifically inhibit the tyrosine kinase activity of breakpoint cluster region–c-abl oncogene 1, non-receptor tyrosine kinase (BCR–ABL) fusion oncoprotein [52]. However, in preclinical studies, it was demonstrated that imatinib also inhibited the activity of KIT, PDGFRA/B, ABL1, and ABL2 (also known as ARG) tyrosine kinases [53, 54], which encouraged examination of imatinib therapy for other neoplasms driven by constitutive receptor tyrosine kinase activation. The first report describing imatinib treatment in a GIST patient with multiple metastatic lesions demonstrated a dramatic response to this therapy [55]. As early as 2002, imatinib was registered for treatment of advanced GISTs (i.e. in metastatic and/or recurrent and/or inoperable disease). The results of several clinical trials confirmed the high efficacy of imatinib in the treatment of GISTs in the majority of patients with inoperable/metastatic disease [56–60], prolonging median survival from 10–19 months (historical data) to approximately 5 years. Two large, parallel, very similar international studies comparing a standard imatinib dose of 400 mg daily with a high dose of 800 mg daily demonstrated a similar response rate and OS with the two imatinib doses but better progression-free survival (PFS) in the high-dose treatment arm [60–62]. Moreover, data from these trials have shown that the response of GISTs with KIT exon 9 mutations depends on the dose of the drug, and that these patients benefit from a higher dose (800 mg daily) of imatinib, demonstrating significantly longer PFS (18 months) than patients receiving a standard dose of 400 mg daily (6 months) [39]. Unfortunately the spectacular activity of imatinib is time limited, and secondary resistance develops in the majority of patients [11, 61].
Adjuvant Imatinib Mesylate Therapy for GISTs
Although the treatment of choice in primary resectable localized GISTs is radical resection with negative margins, almost half of the patients ultimately develop recurrent or metastatic disease after potentially curative surgery [63]. Therefore, the idea of adjuvant therapy with imatinib after primary resection has been evoked to delay or prevent relapse and to prolong patients’ survival. The role of imatinib therapy in the adjuvant setting has been evaluated in several phase II and III clinical trials, namely ACOSOG Z9000 [98] and Z9001 [76] (conducted by the American College of Surgeons Oncology Group), SSGXVIII/AIO [7, 65] (conducted by the Scandinavian Sarcoma Group and the Sarcoma Group of the Arbeitsgemeinschaft Internistische Onkologie XVIII), RTOG S0132 [95] (conducted by the Radiation Therapy Oncology Group), and EORTC 62024 (conducted by the European Organization for Research and Treatment of Cancer) [99]. Table 2 presents the most important clinical trials of adjuvant imatinib in GISTs. Data from the phase III ACOSOG Z9001 trial [76] evaluating 1 year of adjuvant therapy with imatinib 400 mg daily versus placebo in patients after microscopically radical (R0) resection of GISTs at least 3 cm in diameter showed a significant reduction in the risk of recurrence from 17 to 2 % at 1 year (during 20 months of follow-up) [p = 0.0001], with a hazard ratio (HR) of 0.35. Although the treatment was well tolerated, no significant impact on OS was demonstrated, thus implying that adjuvant imatinib delays rather than prevents relapse. The eligibility criteria for this trial were clearly inadequate because more than 40 % of patients had tumors between 3 and 6 cm in size, which in the majority were at low risk of relapse and did not require adjuvant therapy after surgery. Nevertheless, in 2008, imatinib was approved for use in adjuvant therapy after resection of primary GISTs in patients at significant risk of relapse. Importantly, the initial approval lacked definite guidance concerning the optimal duration of treatment and risk assessment criteria.
Table 2.
The most important clinical trials of adjuvant therapy with imatinib in gastrointestinal stromal tumors (GISTs)
| Study | Study design | No. of patients | Major eligibility criteria | Results | |
|---|---|---|---|---|---|
| Primary endpoint | Secondary endpoints | ||||
| ACOSOG Z9000, DeMatteo et al. 2009 [98] | One arm, open, multicenter; imatinib 400 mg daily for 1 year | 107 | Primary GIST KIT-positive after radical resection; high risk of relapse: tumor size ≥10 cm OR tumor rupture OR <5 intraperitoneal metastases | OS at median follow-up of 4 years; 1-year OS: 99 %; 2-year OS: 97 %; 3-year OS: 97 % | RFS at median follow-up of 4 years; 1-year RFS: 94 %; 2-year RFS: 73 %; 3-year RFS: 61 % |
| Kang et al. 2009 [97] | One arm, open, multicenter, prospective; imatinib 400 mg daily for 2 years | 47 | Primary GIST with exon 11 KIT mutation after radical resection; high risk of relapse: tumor size ≥10 cm OR mitotic index ≥10/50 HPFs OR tumor size ≥5 cm and mitotic index ≥5/50 HPFs | RFS at median follow-up of 26.9 months; 1-year RFS: 97.7 %; 2-year RFS: 92.7 % | |
| Li et al. 2011 [93]a | Open, non-randomized, prospective, one center; imatinib 400 mg daily for 3 years versus observation | 56 (imatinib), 49 (observation) | Primary GIST KIT-positive after resection; intermediate or high risk of recurrence (NIH classification): tumor size >5 cm AND/OR mitotic index >5/50 HPFs | Significantly better RFS in the imatinib arm as compared with observation at median follow-up of 45 months (HR 0.188, 95 % CI 0.085–0.417; p < 0.001); 1-year RFS: 100 versus 90 %; 2-year RFS: 96 versus 57 %; 3-year RFS: 89 versus 48 % | Significantly decreased risk of death due to GIST with adjuvant imatinib therapy in comparison with observation at median follow-up of 45 months (HR 0.254, 95 % CI 0.070–0.931; p = 0.025) |
| Jiang et al. 2011 [92]a | Non-randomized, one center, prospective; imatinib 400 mg daily for 5 years versus observation | 35 (imatinib), 55 (observation) | Primary GIST KIT-positive after R0 resection; high risk of relapse (modified NIH classification) | Significantly better RFS with imatinib as compared with observation at median follow-up of 44.0 months (HR 0.122, 95 % CI 0.041–0.363; p < 0.001); 1-year RFS: 100 versus 70.9 %; 2-year RFS: 88.0 versus 37.8 %; 3-year RFS: 88.0 versus 27.5 % | |
| ACOSOG Z9001, DeMatteo et al. 2009 [76, 86] | Double-blind, placebo-controlled, randomized, multicenter; imatinib 400 mg daily versus placebo for 1 year | 359 (imatinib), 354 (placebo) | Primary GIST KIT-positive after radical resection; tumor size ≥3 cm; low, intermediate, or high risk of relapse | Significant improvement in 1-year RFS in the imatinib arm (98 %) as compared with placebo (83 %); median follow-up time 19.7 months; HR 0.35; p < 0.0001 | Lack of statistically significant difference in 1-year OS between study arms (HR 0.66; p = 0.47) |
| SSGXVIII/AIO, Joensuu et al. 2012 [7, 65]a | Two arms, open, randomized, multicenter, prospective; imatinib 400 mg daily for 1 versus 3 years | 200 (1 year), 200 (3 years) | Primary GIST KIT-positive after radical resection; high risk of relapse (modified NIH classification): tumor size >10 cm OR mitotic index >10/50 HPFs OR mitotic index >5/50 and tumor size >5 cm OR tumor rupture | Significant improvement in RFS with 3-year imatinib therapy as compared with 1-year therapy at median follow-up of 54 months (HR 0.46, 95 % CI 0.32–0.65; p < 0.0001); 5-year RFS: 65.6 versus 47.9 % | Significant improvement in OS with 3-year imatinib therapy as compared with 1-year therapy at median follow-up of 54 months (HR 0.45, 95 % CI 0.22–0.89; p = 0.019); 5-year OS: 92.0 versus 81.7 % |
| EORTC 62024, Hohenberger at al. 2012 [99] | Two arms, open, randomized, multicenter, prospective; imatinib 400 mg daily for 2 years versus observation | 906 | Primary GIST KIT-positive after radical resection; intermediate or high risk of relapse (NIH classification): tumor size >5 cm AND/OR mitotic index >5/50 HPFs | Time to imatinib failure at relapse (changed from OS) | RFS, OS, safety: results are expected in 2013 |
ACOSOG American College of Surgeons Oncology Group, AIO Arbeitsgemeinschaft Internistische Onkologie, CI confidence interval, EORTC European Organization for Research and Treatment of Cancer, HPFs high-powered fields, HR hazard ratio, KIT v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog, NIH National Institutes of Health, OS overall survival, R0 microscopically radical resection of the tumor, RFS recurrence-free survival, SSG Scandinavian Sarcoma Group
aStudies evaluating adjuvant therapy with imatinib for at least 3 years
Only recent updates of the European Society for Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) guidelines have included the recommendation for 36 months of adjuvant imatinib therapy in adult patients with KIT-positive GISTs at high risk of relapse. However, the optimal duration of imatinib therapy is still unknown.
The latest FDA and EMA approvals for imatinib were based on the results of the SSGXVIII/AIO trial, which demonstrated that prolonged treatment extends both recurrence-free survival (RFS) and OS [64]. Data from the SSGXVIII/AIO trial, comparing 12 and 36 months of adjuvant imatinib treatment after resection of GISTs in patients with a high risk of recurrence, were first presented in 2011 at the 47th Annual Meeting of the American Society of Clinical Oncology. In the 36-month treatment arm, a significant improvement was observed in terms of both RFS (5-year RFS: 65.6 vs. 47.9 %; p < 0.0001) and OS (5-year OS: 92.0 vs. 81.7 %; p = 0.01; HR 0.45). The treatment was most effective in patients carrying KIT exon 11 mutations. The study demonstrated that prolonged imatinib treatment was generally well tolerated, and the most common adverse events included anemia, leukopenia, periorbital edema, fatigue, nausea, diarrhea, muscle cramps, and elevated blood lactate dehydrogenase levels. More patients discontinued imatinib therapy in the 36-month treatment arm than in the 12-month arm, for reasons other than GIST recurrence (25.8 vs. 12.6 %; p < 0.001) [7, 65].
Assessment of the Risk of Recurrence after Primary Surgery, and Patient Selection for Extended Adjuvant Imatinib Therapy
Evaluation of the risk factors for recurrence after primary surgery is essential for reliable prognosis, scheduling of follow-up, and identification of patients who may potentially benefit from adjuvant therapy. The main criteria taken into account in a few existing risk stratification systems include the tumor site, size, mitotic index, and tumor rupture; however, the uniform risk criteria remain difficult to determine.
The National Institutes of Health (NIH) consensus criteria formulated in 2001 provided the first evidence-based categorization and a practical scheme for risk assessment in the clinical course of this disease. This risk classification was based on the tumor size and mitotic rate [evaluated per 50 high-powered fields (HPFs)] as the most reliable prognostic factors [66]. This scheme was complemented in 2006 by Miettinen and Lasota from the Armed Forces Institute of Pathology (AFIP), who recognized the significance of the tumor location as an independent prognostic factor in GISTs. They created a new risk assessment scheme (recommended by the NCCN and commonly used) which reflected better prognosis of gastric GISTs compared with intestinal GISTs of the same mitotic index and size [21, 67–70] (Table 3 and Fig. 1). The same prognostic factors were taken into account in the nomogram created by Gold et al. [71], which seems to vaguely outperform the NIH and NCCN–AFIP criteria. Moreover, it has been demonstrated that tumor rupture (either spontaneous or iatrogenic) is an important risk factor, which strongly correlates with the risk of recurrence in GISTs [72, 73]. This observation has led to the development of modified NIH criteria and novel non-linear risk stratification systems, including prognostic contour maps and heat maps, constructed on the basis of the tumor size, site, mitotic index, and incidence of tumor rupture [73–75]. These features may provide even more accurate estimation of the risk of recurrence and are appropriate for individualizing risk stratification for adjuvant therapy in GISTs. Subgroup analysis of the ACOSOG Z9001 trial confirmed that the major clinical benefit of adjuvant therapy was limited to the group of patients at high risk of relapse according to the NCCN–AFIP criteria (an improvement in 2-year RFS from 41 to 77 %; p < 0.0001) [76].
Table 3.
National Comprehensive Cancer Network (NCCN)–Armed Forces Institute of Pathology (AFIP) risk criteria after resection of primary gastrointestinal stromal tumors (GISTs), according to Miettinen and Lasota [9]
| Tumor parameters | Primary tumor location and risk of recurrence | ||||
|---|---|---|---|---|---|
| Size | Mitotic index | Stomach | Duodenum | Small intestine | Rectum |
| ≤2 cm | ≤5/50 HPFs | 0 % | 0 % | 0 % | 0 % |
| >2 cm, ≤5 cm | Very low (1.9 %) | Low (8.3 %) | Low (4.3 %) | Low (8.5 %) | |
| >5 cm, ≤10 cm | Low (3.6 %) | High (34 %) | Intermediate (24 %) | High (57 %) | |
| >10 cm | Intermediate (12 %) | High (52 %) | |||
| ≤2 cm | >5/50 HPFs | Insufficient data | Insufficient data | High (50 %) | High (52–71 %) |
| >2 cm, ≤5 cm | Intermediate (16 %) | High (50–86 %) | High (73–90 %) | ||
| >5 cm, ≤10 cm | High (55–86 %) | ||||
| >10 cm | |||||
HPFs high-powered fields
Fig. 1.
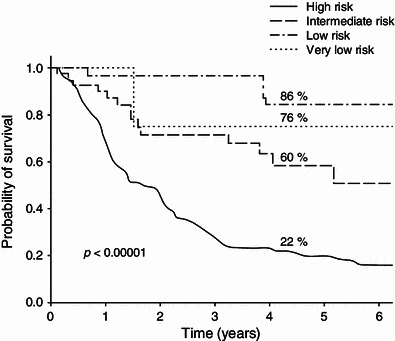
Recurrence-free survival in small-bowel gastrointestinal stromal tumors (GISTs), according to National Comprehensive Cancer Network (NCCN)–Armed Forces Institute of Pathology (AFIP) risk categories (based on the authors’ own data from 659 primary GISTs after radical resection, presented during the European Society of Surgical Oncology conference [100])
In addition to clinicopathological factors, molecular features may also present added value to risk stratification of GISTs. However, they have not been included in the present risk assessment guidelines. Several studies have demonstrated better prognosis for patients harboring KIT exon 11 point mutations or insertions, as well as PDGFRA exon 18 mutations. On the other hand, tumors carrying KIT exon 11 deletions (especially involving codons 557 or 558) and KIT exon 9 duplications are associated with an aggressive disease course [30, 77–83]. It has also been proposed that genomic complexity, defined by a genomic index determined by array comparative genomic hybridization, may serve as a useful adjunct to the current risk stratification systems, which are often uninformative in the case of intermediate-risk patients [84, 85].
It is worth noting that the updated FDA and EMA approvals for 36 months of imatinib treatment apply to patients who specifically meet the inclusion criteria determined in the SSGXVIII/AIO trial [7, 65]. In that trial, imatinib treatment was initiated within the first 12 weeks after primary surgery. Patients were eligible for the trial if they had KIT-positive GISTs and demonstrated at least one of the following features: longest tumor diameter >10 cm, mitotic index >10/50 HPFs, longest tumor diameter >5 cm and mitotic index >5/50 HPFs, or tumor rupture prior to or at the time of surgery. This classification represents a modified NIH risk-stratification system, complemented with tumor rupture as an independent prognostic factor [75]. Tumor location was excluded from the risk assessment criteria in this study. Gastric GISTs constituted approximately half of the cases in both the 12- and 36-month arms, followed by small-intestine GISTs (37 and 31 % of cases, respectively), and GISTs located in the colon or rectum constituted 8 and 10 % of cases, respectively. In 7 % of patients in each arm, the tumor was in another location or the location was unspecified.
Benefit of and Resistance to Adjuvant Imatinib Therapy
The results of the SSGXVIII/AIO trial [65] demonstrated that mutational analysis of GISTs may have predictive value for the clinical response to adjuvant imatinib therapy, similar to data observed in the metastatic setting. From the molecular point of view, resistance to imatinib has its origins in KIT/PDGFRA mutational status. Data reported by Joensuu and colleagues [65] showed that patients with KIT exon 11 mutations benefit the most from prolonged adjuvant treatment. Similar data were shown for patients treated in the ACOSOG Z9001 trial [86]; the 2-year RFS rate was 91 % for patients treated with adjuvant imatinib harboring KIT exon 11 mutations, as compared with 65 % in a group of patients with the same genotype receiving placebo (p < 0.0001).
On the other hand, primary imatinib resistance in the adjuvant setting has been demonstrated especially in cases carrying a PDGFRA exon 18 p.D842V mutation, presumably because of the structural alterations at the imatinib binding site. This mutation is detected in approximately 10 % of operable GISTs [75, 87], especially in tumors originating from the stomach (exceeding 20 % of cases in this location) [30]. Adjuvant imatinib should not be recommended in cases of GISTs harboring a PDGFRA exon 18 p.D842V mutation. In the ACOSOG Z9001 trial [86], adjuvant imatinib therapy had no positive impact on RFS in this subgroup of patients.
Interestingly, it has been demonstrated that patients with advanced GISTs harboring mutations in KIT exon 9 may benefit from an imatinib dose increase to 800 mg daily [62]. This indicates that patients with this mutation may be underdosed when receiving 400 mg of imatinib daily, but it has never been examined in any clinical trial in the adjuvant setting. In wild-type GISTs, the tumor size and mitotic index poorly predict clinical outcome; therefore, current risk stratification systems seem to be inapplicable in this subgroup of patients [50, 51]. Moreover, wild-type GISTs present a limited response to imatinib treatment, in comparison with GISTs carrying imatinib-sensitive mutations. Adjuvant imatinib efficacy in KIT exon 9 mutants and wild-type GISTs warrants further study; however, the numbers of patients in these subgroups are usually small, and so statistical significance is difficult to reach when these categories are analyzed [86]. Nevertheless, KIT and PDGFRA genotyping in GISTs should be performed routinely in the adjuvant setting, since it may help to tailor the treatment to patients who are more likely to respond to imatinib therapy, or to exclude patients with imatinib-resistance mutations [86, 88].
In the SSGXVIII/AIO trial, patients were monitored for their response to imatinib with contrast-enhanced computed tomography or magnetic resonance imaging at 6-month intervals for the first 7 years and annually thereafter. An initial staging examination was performed within 28 days before the introduction of imatinib treatment. Blood biochemistry and cell counts were performed at 1- to 3-month intervals in the course of the treatment [65]. GIST relapse is usually observed at the highest frequency within the first 2 years after completion of adjuvant treatment; therefore, regular imaging in this period is especially important for early detection of recurrence [64, 76]. The majority of patients who develop GIST recurrence after completion of adjuvant imatinib respond to an imatinib rechallenge regardless of the prior treatment duration [64]. On the basis of the clinical behavior of advanced GISTs, it may be anticipated that in patients who relapse during adjuvant treatment or within the first few weeks after completion of adjuvant treatment, an increased dose of imatinib or introduction of another tyrosine kinase inhibitor, such as sunitinib, may be beneficial because these cases are probably primarily imatinib resistant. However, no clinical trial has addressed this hypothesis as yet [64]. Generally, only a few patients in the SSGXVIII/AIO trial developed GIST recurrence during imatinib treatment (2 % of patients in the 12-month arm and 6 % of patients in the 36-month arm). This suggests that acquired resistance to adjuvant imatinib (related mainly to occurrence of secondary KIT/PDGFRA mutations) is infrequent in this patient population [7, 65].
The optimal duration of imatinib therapy is not yet known. We still do not know if adjuvant imatinib therapy can cure a patient by preventing relapse or can only delay it. In the metastatic setting, interruption of imatinib therapy has been associated with disease relapse at a median of 6 months after stopping imatinib after 1, 3, or 5 years of treatment [89, 90]. The significant improvement in OS associated with 3 years versus 1 year of adjuvant imatinib in the SSGXVIII/AIO trial [7, 65] was based on the limited number of deaths that occurred at median follow-up of 54 months, and so longer follow-up is needed to confirm the OS advantage related to 3-year adjuvant imatinib therapy.
Future of Adjuvant Imatinib Therapy
There are still several unresolved issues concerning future use of adjuvant imatinib in GISTs. In the coming years, adjuvant imatinib treatment for at least 3 years will be standard therapy in high-risk GIST patients harboring sensitive mutations. In intermediate-risk patients, adjuvant imatinib should be considered, provided there is better characterization of individual prognostic features. The role of adjuvant imatinib therapy in patients with wild-type GISTs or KIT exon 9 mutations should be better defined, and the appropriate initial dose of imatinib—400 or 800 mg daily in patients with KIT exon 9 mutants—must be established. The optimal duration of adjuvant imatinib therapy beyond 3 years requires further investigation and should preferably be determined on the basis of randomized controlled trials. Furthermore, the optimal follow-up schedule after discontinuation of the therapy is not well established. The only issue that seems to be incontestable in the immediate future is the necessity for genotyping of every primary GIST considered for adjuvant therapy [91].
Conclusions
Despite the striking efficacy of imatinib, recurrent or metastatic GIST is still not a curable disease. This implies that prevention of disease recurrence following surgical resection of the primary tumor is the key to further improvement of the clinical outcomes of patients affected by GISTs. Three years of adjuvant imatinib treatment, as opposed to 1 year of treatment, significantly reduced the risk of recurrence and improved OS in patients with KIT-positive GISTs at high risk of recurrence after surgery [7]. Currently, 3 years of adjuvant treatment for patients at high risk of recurrence may be considered as a standard of care. However, it is not clear whether patients who are classified as intermediate risk should be treated with adjuvant imatinib. Results from several phase II studies support the idea that at least 2 years of adjuvant imatinib treatment is beneficial for intermediate-risk GISTs (especially those harboring KIT exon 11 mutations) and may be considered in this subgroup of patients [92–97]. On the other hand, patients with very low-risk or low-risk tumors are likely to be cured by surgery alone and should not receive adjuvant imatinib.
Beyond risk assessment for proper selection of patients for adjuvant imatinib therapy, mutational status also has a predictive value for clinical response to the therapy. It may help to tailor the treatment to patients carrying more sensitive mutations, such as KIT exon 11 mutations, or to exclude patients with imatinib-resistance mutations, such as a PDGFRA p.D842V mutation. Thus, KIT and PDGFRA genotyping of patients with GISTs is obligatory in the adjuvant setting [86, 88].
Acknowledgments
Joanna Przybyl conducts a project within the International PhD Projects Program (MPD) of the Foundation for Polish Science, and it is co-financed by the European Union Regional Development Fund.
Conflict of Interest Disclosures
Piotr Rutkowski has received honoraria and travel grants from Novartis, has received honoraria from Pfizer, and has been a member of an advisory board for Novartis. The other authors have no conflicts of interest that are directly relevant to the content of this article.
References
- 1.Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era: a population-based study in western Sweden. Cancer. 2005;103(4):821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 2.Monges G, Bisot-Locard S, Blay JY, et al. The estimated incidence of gastrointestinal stromal tumors in France: results of PROGIST study conducted among pathologists. Bull Cancer. 2010;97(3):E16–E22. doi: 10.1684/bdc.2010.1041. [DOI] [PubMed] [Google Scholar]
- 3.Cassier PA, Ducimetière F, Lurkin A, et al. A prospective epidemiological study of new incident GISTs during two consecutive years in Rhone Alpes region: incidence and molecular distribution of GIST in a European region. Br J Cancer. 2010;103(2):165–170. doi: 10.1038/sj.bjc.6605743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mastrangelo G, Coindre JM, Ducimetière F, et al. Incidence of soft tissue sarcoma and beyond: a population-based prospective study in 3 European regions. Cancer. 2012;118(21):5339–5348. doi: 10.1002/cncr.27555. [DOI] [PubMed] [Google Scholar]
- 5.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutkowski P, Debiec-Rychter M, Ruka W. Gastrointestinal stromal tumors: key to diagnosis and choice of therapy. Mol Diagn Ther. 2008;12(3):131–143. doi: 10.1007/BF03256278. [DOI] [PubMed] [Google Scholar]
- 7.Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265–1272. doi: 10.1001/jama.2012.347. [DOI] [PubMed] [Google Scholar]
- 8.Miettinen M, Lasota J. Gastrointestinal stromal tumors: definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438(1):1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 9.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23(2):70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Sandberg AA, Bridge JA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: desmoplastic small round-cell tumors. Cancer Genet Cytogenet. 2002;138(1):1–10. doi: 10.1016/S0165-4608(02)00680-5. [DOI] [PubMed] [Google Scholar]
- 11.Rutkowski P, Debiec-Rychter M, Nowecki ZI, et al. Different factors are responsible for predicting relapses after primary tumors resection and for imatinib treatment outcomes in gastrointestinal stromal tumors. Med Sci Monit. 2007;13(11):CR515–CR522. [PubMed] [Google Scholar]
- 12.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 13.Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152(5):1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 14.Min KW, Leabu M. Interstitial cells of Cajal (ICC) and gastrointestinal stromal tumor (GIST): facts, speculations, and myths. J Cell Mol Med. 2006;10(4):995–1013. doi: 10.1111/j.1582-4934.2006.tb00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, et al. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11(8):728–734. [PubMed] [Google Scholar]
- 16.Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol. 2011;104(8):865–873. doi: 10.1002/jso.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang GH, Srivastava A, Kim YE, et al. DOG1 and PKC-theta are useful in the diagnosis of KIT-negative gastrointestinal stromal tumors. Mod Pathol. 2011;24(6):866–875. doi: 10.1038/modpathol.2011.11. [DOI] [PubMed] [Google Scholar]
- 18.Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol. 2009;33(9):1401–1408. doi: 10.1097/PAS.0b013e3181a90e1a. [DOI] [PubMed] [Google Scholar]
- 19.Liegl B, Hornick JL, Corless CL, et al. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009;33(3):437–446. doi: 10.1097/PAS.0b013e318186b158. [DOI] [PubMed] [Google Scholar]
- 20.Sciot R, Debiec-Rychter M, Daugaard S, et al. Distribution and prognostic value of histopathologic data and immunohistochemical markers in gastrointestinal stromal tumours (GISTs): an analysis of the EORTC phase III trial of treatment of metastatic GISTs with imatinib mesylate. Eur J Cancer. 2008;44(13):1855–1860. doi: 10.1016/j.ejca.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen M, Makhlouf H, Sobin LH, et al. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30(4):477–489. doi: 10.1097/00000478-200604000-00008. [DOI] [PubMed] [Google Scholar]
- 22.el-Rifai W, Sarlomo-Rikala M, Miettinen M, et al. DNA copy number losses in chromosome 14: an early change in gastrointestinal stromal tumors. Cancer Res. 1996;56(14):3230–3233. [PubMed] [Google Scholar]
- 23.El-Rifai W, Sarlomo-Rikala M, Andersson LC, et al. DNA sequence copy number changes in gastrointestinal stromal tumors: tumor progression and prognostic significance. Cancer Res. 2000;60(14):3899–3903. [PubMed] [Google Scholar]
- 24.Kim NG, Kim JJ, Ahn JY, et al. Putative chromosomal deletions on 9P, 9Q and 22Q occur preferentially in malignant gastrointestinal stromal tumors. Int J Cancer. 2000;85(5):633–638. doi: 10.1002/(SICI)1097-0215(20000301)85:5<633::AID-IJC6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Gunawan B, Schulten HJ, von Heydebreck A, et al. Site-independent prognostic value of chromosome 9q loss in primary gastrointestinal stromal tumours. J Pathol. 2004;202(4):421–429. doi: 10.1002/path.1537. [DOI] [PubMed] [Google Scholar]
- 26.Meza-Zepeda LA, Kresse SH, Barragan-Polania AH, et al. Array comparative genomic hybridization reveals distinct DNA copy number differences between gastrointestinal stromal tumors and leiomyosarcomas. Cancer Res. 2006;66(18):8984–8993. doi: 10.1158/0008-5472.CAN-06-1972. [DOI] [PubMed] [Google Scholar]
- 27.Assamaki R, Sarlomo-Rikala M, Lopez-Guerrero JA, et al. Array comparative genomic hybridization analysis of chromosomal imbalances and their target genes in gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2007;46(6):564–576. doi: 10.1002/gcc.20439. [DOI] [PubMed] [Google Scholar]
- 28.Wozniak A, Sciot R, Guillou L, et al. Array CGH analysis in primary gastrointestinal stromal tumors: cytogenetic profile correlates with anatomic site and tumor aggressiveness, irrespective of mutational status. Genes Chromosomes Cancer. 2007;46(3):261–276. doi: 10.1002/gcc.20408. [DOI] [PubMed] [Google Scholar]
- 29.Lasota J, Miettinen M. KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs) Semin Diagn Pathol. 2006;23(2):91–102. doi: 10.1053/j.semdp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Wozniak A, Rutkowski P, Piskorz A, et al. Prognostic value of KIT/PDGFRA mutations in gastrointestinal stromal tumours (GIST): Polish Clinical GIST Registry experience. Ann Oncol. 2012;23(2):353–360. doi: 10.1093/annonc/mdr127. [DOI] [PubMed] [Google Scholar]
- 31.Ernst SI, Hubbs AE, Przygodzki RM, et al. KIT mutation portends poor prognosis in gastrointestinal stromal/smooth muscle tumors. Lab Invest. 1998;78(12):1633–1636. [PubMed] [Google Scholar]
- 32.Taniguchi M, Nishida T, Hirota S, et al. Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res. 1999;59(17):4297–4300. [PubMed] [Google Scholar]
- 33.Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61(22):8118–8121. [PubMed] [Google Scholar]
- 34.Singer S, Rubin BP, Lux ML, et al. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol. 2002;20(18):3898–3905. doi: 10.1200/JCO.2002.03.095. [DOI] [PubMed] [Google Scholar]
- 35.Antonescu CR, Sommer G, Sarran L, et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res. 2003;9(9):3329–3337. [PubMed] [Google Scholar]
- 36.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 37.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22(18):3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 38.Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23(23):5357–5364. doi: 10.1200/JCO.2005.14.068. [DOI] [PubMed] [Google Scholar]
- 39.Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42(8):1093–1103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 40.Cho S, Kitadai Y, Yoshida S, et al. Deletion of the KIT gene is associated with liver metastasis and poor prognosis in patients with gastrointestinal stromal tumor in the stomach. Int J Oncol. 2006;28(6):1361–1367. [PubMed] [Google Scholar]
- 41.Pantaleo MA, Astolfi A, Indio V, et al. SDHA loss-of-function mutations in KIT-PDGFRA wild-type gastrointestinal stromal tumors identified by massively parallel sequencing. J Natl Cancer Inst. 2011;103(12):983–987. doi: 10.1093/jnci/djr130. [DOI] [PubMed] [Google Scholar]
- 42.Gaal J, Stratakis CA, Carney JA, et al. SDHB immunohistochemistry: a useful tool in the diagnosis of Carney-Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol. 2011;24(1):147–151. doi: 10.1038/modpathol.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gill AJ, Chou A, Vilain R, et al. Immunohistochemistry for SDHB divides gastrointestinal stromal tumors (GISTs) into 2 distinct types. Am J Surg Pathol. 2010;34(5):636–644. doi: 10.1097/PAS.0b013e3181d6150d. [DOI] [PubMed] [Google Scholar]
- 44.Italiano A, Chen CL, Sung YS, et al. SDHA loss of function mutations in a subset of young adult wild-type gastrointestinal stromal tumors. BMC Cancer. 2012;12(1):408. doi: 10.1186/1471-2407-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pantaleo MA, Nannini M, Astolfi A, et al. A distinct pediatric-type gastrointestinal stromal tumor in adults: potential role of succinate dehydrogenase subunit A mutations. Am J Surg Pathol. 2011;35(11):1750–1752. doi: 10.1097/PAS.0b013e318230a523. [DOI] [PubMed] [Google Scholar]
- 46.Lasota J, Wang Z, Kim SY, et al. Expression of the receptor for type I insulin-like growth factor (IGF1R) in gastrointestinal stromal tumors: an immunohistochemical study of 1078 cases with diagnostic and therapeutic implications. Am J Surg Pathol. 2013;37(1):114–119. doi: 10.1097/PAS.0b013e3182613c86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner AJ, Remillard SP, Zhang YX, et al. Loss of expression of SDHA predicts SDHA mutations in gastrointestinal stromal tumors. Mod Pathol. (Epub 2012 Sep 7). [DOI] [PubMed]
- 48.Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci USA. 2011;108(1):314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stratakis CA, Carney JA. The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): molecular genetics and clinical implications. J Intern Med. 2009;266(1):43–52. doi: 10.1111/j.1365-2796.2009.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miettinen M, Wang ZF, Sarlomo-Rikala M, et al. Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol. 2011;35(11):1712–1721. doi: 10.1097/PAS.0b013e3182260752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, Smyrk TC, Young WF, Jr, et al. Gastric stromal tumors in Carney triad are different clinically, pathologically, and behaviorally from sporadic gastric gastrointestinal stromal tumors: findings in 104 cases. Am J Surg Pathol. 2010;34(1):53–64. doi: 10.1097/PAS.0b013e3181c20f4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Druker BJ. Inhibition of the Bcr-Abl tyrosine kinase as a therapeutic strategy for CML. Oncogene. 2002;21(56):8541–8546. doi: 10.1038/sj.onc.1206081. [DOI] [PubMed] [Google Scholar]
- 53.Okuda K, Weisberg E, Gilliland D, et al. ARG tyrosine kinase activity is inhibited by STI571. Blood. 2001;97(8):2440–2448. doi: 10.1182/blood.V97.8.2440. [DOI] [PubMed] [Google Scholar]
- 54.Savage DG, Antman KH. Imatinib mesylate: a new oral targeted therapy. N Engl J Med. 2002;346(9):683–693. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- 55.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344(14):1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 56.van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet. 2001;358(9291):1421–1423. doi: 10.1016/S0140-6736(01)06535-7. [DOI] [PubMed] [Google Scholar]
- 57.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 58.Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target: results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39(14):2006–2011. doi: 10.1016/S0959-8049(02)00836-5. [DOI] [PubMed] [Google Scholar]
- 59.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26(4):620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 60.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 61.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 62.Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28(7):1247–53. [DOI] [PMC free article] [PubMed]
- 63.Rutkowski P, Nowecki ZI, Michej W, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol. 2007;14(7):2018–2027. doi: 10.1245/s10434-007-9377-9. [DOI] [PubMed] [Google Scholar]
- 64.Joensuu H. Adjuvant treatment of GIST: patient selection and treatment strategies. Nat Rev Clin Oncol. 2012;9(6):351–358. doi: 10.1038/nrclinonc.2012.74. [DOI] [PubMed] [Google Scholar]
- 65.Joensuu H, Eriksson M, Hatrmann J, et al. Twelve versus 36 months of adjuvant imatinib (IM) as treatment of operable GIST with a high risk of recurrence: final results of a randomized trial (SSGXVIII/AIO) J Clin Oncol. 2011;29(18 Suppl):LBA1. [Google Scholar]
- 66.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33(5):459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 67.Miettinen M, Furlong M, Sarlomo-Rikala M, et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001;25(9):1121–1133. doi: 10.1097/00000478-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Miettinen M, Kopczynski J, Makhlouf HR, et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27(5):625–641. doi: 10.1097/00000478-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 70.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 71.Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10(11):1045–1052. doi: 10.1016/S1470-2045(09)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rutkowski P, Nowecki Z, Michej W, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumors (GISTs) Ann Surg Oncol. 2007;14:2018–2027. doi: 10.1245/s10434-007-9377-9. [DOI] [PubMed] [Google Scholar]
- 73.Rutkowski P, Bylina E, Wozniak A, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour: the impact of tumour rupture on patient outcomes. Eur J Surg Oncol. 2011;37(10):890–896. doi: 10.1016/j.ejso.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 74.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39(10):1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 75.Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265–274. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 76.DeMatteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andersson J, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors with KIT exon 11 deletions are associated with poor prognosis. Gastroenterology. 2006;130(6):1573–1581. doi: 10.1053/j.gastro.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 78.Lasota J, Jasinski M, Sarlomo-Rikala M, et al. Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol. 1999;154(1):53–60. doi: 10.1016/S0002-9440(10)65250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lasota J, Dansonka-Mieszkowska A, Sobin LH, et al. A great majority of GISTs with PDGFRA mutations represent gastric tumors of low or no malignant potential. Lab Invest. 2004;84(7):874–883. doi: 10.1038/labinvest.3700122. [DOI] [PubMed] [Google Scholar]
- 80.Lasota J, vel Dobosz AJ, Wasag B, et al. Presence of homozygous KIT exon 11 mutations is strongly associated with malignant clinical behavior in gastrointestinal stromal tumors. Lab Invest. 2007;87(10):1029–1041. doi: 10.1038/labinvest.3700628. [DOI] [PubMed] [Google Scholar]
- 81.Lasota J, Dansonka-Mieszkowska A, Stachura T, et al. Gastrointestinal stromal tumors with internal tandem duplications in 3′ end of KIT juxtamembrane domain occur predominantly in stomach and generally seem to have a favorable course. Mod Pathol. 2003;16(12):1257–1264. doi: 10.1097/01.MP.0000097365.72526.3E. [DOI] [PubMed] [Google Scholar]
- 82.Wardelmann E, Losen I, Hans V, et al. Deletion of Trp-557 and Lys-558 in the juxtamembrane domain of the c-kit protooncogene is associated with metastatic behavior of gastrointestinal stromal tumors. Int J Cancer. 2003;106(6):887–895. doi: 10.1002/ijc.11323. [DOI] [PubMed] [Google Scholar]
- 83.Martin J, Poveda A, Llombart-Bosch A, et al. Deletions affecting codons 557–558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS) J Clin Oncol. 2005;23(25):6190–6198. doi: 10.1200/JCO.2005.19.554. [DOI] [PubMed] [Google Scholar]
- 84.Chibon F, Lagarde P, Salas S, et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat Med. 2010;16(7):781–787. doi: 10.1038/nm.2174. [DOI] [PubMed] [Google Scholar]
- 85.Lagarde P, Perot G, Kauffmann A, et al. Mitotic checkpoints and chromosome instability are strong predictors of clinical outcome in gastrointestinal stromal tumors. Clin Cancer Res. 2012;18(3):826–838. doi: 10.1158/1078-0432.CCR-11-1610. [DOI] [PubMed] [Google Scholar]
- 86.Corless CL, Ballman KV, Antonescu C, et al. Relation of tumor pathologic and molecular features to outcome after surgical resection of localized primary gastrointestinal stromal tumor (GIST): results of the intergroup phase III trial ACOSOG Z9001 [abstract] J Clin Oncol. 2010;28(15 Suppl):10006. [Google Scholar]
- 87.Emile JF, Brahimi S, Coindre JM, et al. Frequencies of KIT and PDGFRA mutations in the MolecGIST prospective population-based study differ from those of advanced GISTs. Med Oncol. 2012;29(3):1765–1772. doi: 10.1007/s12032-011-0074-y. [DOI] [PubMed] [Google Scholar]
- 88.Casali PG, Blay JY. ESMO/CONTICANET/EUROBONET Consensus Panel of Experts. Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up [abstract] Ann Oncol. 2010;21(Suppl 5):v98–v102. doi: 10.1093/annonc/mdq208. [DOI] [PubMed] [Google Scholar]
- 89.Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol. 2007;25(9):1107–1113. doi: 10.1200/JCO.2006.09.0183. [DOI] [PubMed] [Google Scholar]
- 90.Le Cesne A, Ray-Coquard I, Bui BN, et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol. 2010;11(10):942–949. doi: 10.1016/S1470-2045(10)70222-9. [DOI] [PubMed] [Google Scholar]
- 91.Reichardt P, Blay J-Y, Boukovinas I, et al. Adjuvant therapy in primary GIST: state-of-the-art. Ann Oncol. 2012;23(11):2776–2781. doi: 10.1093/annonc/mds198. [DOI] [PubMed] [Google Scholar]
- 92.Jiang WZ, Guan GX, Lu HS, et al. Adjuvant imatinib treatment after R0 resection for patients with high-risk gastrointestinal stromal tumors: a median follow-up of 44 months. J Surg Oncol. 2011;104(7):760–764. doi: 10.1002/jso.22010. [DOI] [PubMed] [Google Scholar]
- 93.Li J, Gong JF, Wu AW, et al. Post-operative imatinib in patients with intermediate or high risk gastrointestinal stromal tumor. Eur J Surg Oncol. 2011;37(4):319–324. doi: 10.1016/j.ejso.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 94.Huang H, Liang H, Zhan ZL, et al. Surgical outcomes of gastrointestinal stromal tumors of the stomach: a single unit experience in the era of targeted drug therapy. Med Oncol. 2012;29(2):941–947. doi: 10.1007/s12032-011-9888-x. [DOI] [PubMed] [Google Scholar]
- 95.Wang D, Zhang Q, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol. 2012;19(4):1074–1080. doi: 10.1245/s10434-011-2190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McAuliffe JC, Hunt KK, Lazar AJ, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol. 2009;16(4):910–919. doi: 10.1245/s10434-008-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kang B, Lee J, Ryu M, et al. A phase II study of imatinib mesylate as adjuvant treatment for curatively resected high-risk localized gastrointestinal stromal tumors [abstract] J Clin Oncol. 2009;27(15 Suppl):E21515. [Google Scholar]
- 98.DeMatteo RP, Owzar K, Antonescu CR, et al. Efficacy of adjuvant imatinib mesylate following complete resection of localized, primary gastrointestinal stromal tumor (GIST) at high risk of recurrence: the US Intergroup phase II trial ACOSOG Z9000 [abstract no. 8]. 2008 Gastrointestinal Cancers Symposium, Orlando.
- 99.Hohenberger P, Rutkowski P, Bonvalot S, et al. Analysis of the quality of reporting surgical procedures in patients undergoing resection for primary gastrointestinal stromal tumors: a reporting tool derived from the EORTC–STBSG 62024 trial [abstract no. 10096] J Clin Oncol. 2012;30(15 Suppl):10096. [Google Scholar]
- 100.Rutkowski P, Wozniak A, Osuch C, et al. Utility of NCCN-AFIP (Miettinen-Lasota) risk criteria for assessment of recurrence risk of gastrointestinal stroma tumours (GIST) originating from stomach and small bowel—analysis of Polish Clinical GIST Registry [abstract no. 19] Eur J Surg Oncol. 2010;36:799. [Google Scholar]


