Abstract
Design and synthesis of efficient drug delivery systems are of vital importance for medicine and healthcare. Materials innovation and nanotechnology have synergistically fueled the advancement of drug delivery. Innovation in material chemistry allows the generation of biodegradable, biocompatible, environment-responsive, and targeted delivery systems. Nanotechnology enables control over size, shape and multi-functionality of particulate drug delivery systems. In this review, we focus on the materials innovation and processing of drug delivery systems and how these advances have shaped the past and may influence the future of drug delivery.
Keywords: Commercialized drug delivery system, nanoparticle, polyplex, natural polymers, polymer-drug conjugate, combinatorial chemistry, microfluidics, particle replication in non-wetting template, step-flash imprint lithography
Introduction
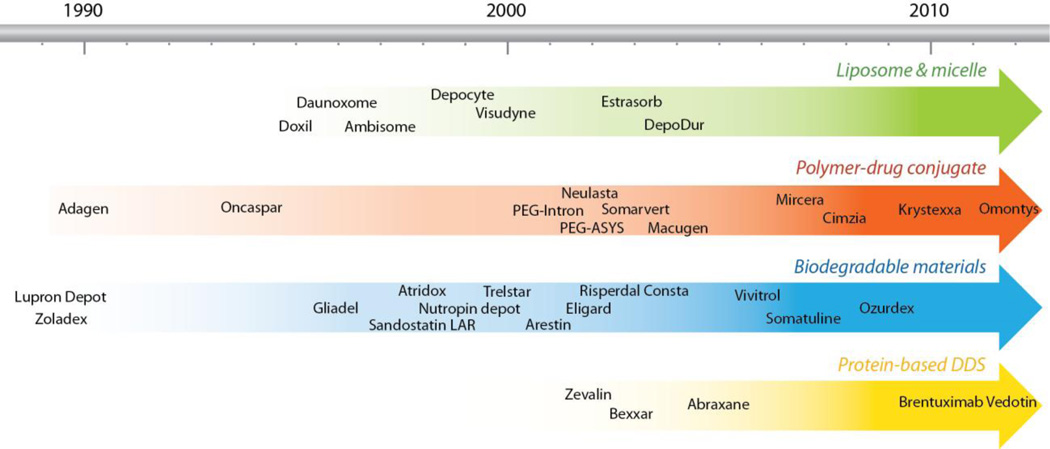
Drug delivery is a field of vital importance to medicine and healthcare. Controlled drug delivery improves bioavailability by preventing premature degradation and enhancing uptake, maintains drug concentration within the therapeutic window by controlling the drug release rate, and reduces side effects by targeting to disease site and target cells. Since the first FDA approval of drug delivery system (DDS), Liposomal amphotericin B, in 1990, more than 10 DDS are now commercially available to treat diverse diseases ranging from cancer to fungal infection and to muscular degeneration (Figure 1, Table 1) [1]. In improving therapeutic efficacy, DDS has benefited tens of millions of patients by relieving suffering and prolonging life. They have also changed the economics of drug development. Packaging an existing drug into controlled release formulations may not only improve its performance but also extend its patent life as a new product. The average cost and time required to develop a new DDS (approximately $20–50 million and 3–4 years) is significantly lower than that for a new drug (approximately $500 million and over 10 years) [2]. Not surprisingly the US market for advanced DDS has grown from $75 million in 2001 to $121 billion in 2010 [3]; the annual worldwide market for polymer-based controlled release system alone is estimated to be $60 billion in 2010 [4].
Figure 1.
Timeline showing FDA approved DDS in the market.
Table 1.
Examples of FDA approved DDS in the market.
| Product name and category |
Year of approval |
Technology | Indication |
|---|---|---|---|
| Liposome & micelle | |||
| ➢ Doxil | 1995 | PEGylated liposomal doxorubicin | Various types of cancer |
| ➢ Daunoxome | 1996 | Liposomal daunorubicin | Advanced HIV-associated Kaposi's sarcoma |
| ➢ Ambisome | 1997 | Liposomal amphotericin B | Fungal infections |
| ➢ Depocyt | 1999 | Liposomal cytarabine | Lymphomatous meningitis |
| ➢ Visudyne | 2000 | Liposomal verteporfin | Age-related macular degeneration |
| ➢ Estrasorb | 2003 | Estradiol micellar nanoparticles | Moderate to severe vasomotor symptoms of menopause |
| ➢ DepoDur | 2004 | Liposomal morphine sulfate | Postoperative pain |
| Polymer-drug conjugate | |||
| ➢ Adagen | 1990 | PEGylated adenosine deaminase | Adenosine deaminase deficiency causing severe combined immunodeficiency disease |
| ➢ Oncaspar | 1994 | PEGylated L-asparaginase | Acute lymphoblastic leukemia |
| ➢ PEG-intron | 2001 | PEGylated interferon alfa-2b | Chronic hepatitis C |
| ➢ PEG-ASYS | 2002 | PEGylated interferon alfa-2a | Chronic hepatitis C & B |
| ➢ Neulasta | 2002 | PEGylated granulocyte colony-stimulating factor analog | Neutropenia |
| ➢ Somavert | 2003 | PEGylated recombinant analogue of the human growth hormone | Acromegaly |
| ➢ Macugen | 2004 | Pegylated anti-VEGF aptamer | Age-related macular degeneration |
| ➢ Mircera | 2007 | PEGylated erythropoetin receptor activators | Anaemia associated with chronic kidney disease |
| ➢ Cimzia | 2008 | PEGylated tumor necrosis factor alpha inhibitor | Crohn's disease |
| ➢ Krystexxa | 2010 | PEGylated urate oxidase | Gout |
| ➢ Omontys | 2012 | PEGylated peginesatide | Anemia caused by chronic kidney disease |
| Biodegradable materials | |||
| ➢ Zoladex | 1989 | PLGA/goserelin acetate | Prostate and breast cancer |
| ➢ Lupron Depot | 1989 | PLGA/leuprolide acetate | Prostate cancer and endometriosis |
| ➢ Gliadel | 1996 | Polifeprosan 20/carmustine | High-grade & recurrent glioblastoma multiforme |
| ➢ Sandostatin LAR | 1998 | PLGA-glucose/octreotide acetate | Acromegaly |
| ➢ Atridox | 1998 | PLA/doxycycline hyclate | Periodontal disease |
| ➢ Nutropin depot | 1999 | PLGA/recombinant human growth hormone | Growth hormone deficiency |
| ➢ Trelstar | 2000 | PLGA/triptorelin pamoatea | Advanced prostate cancer |
| ➢ Arestin | 2001 | PLGA/minocycline | Adult periodontitis |
| ➢ Eligard | 2002 | PLGA/leuprolide acetate | Advanced prostate cancer |
| ➢ Risperdal Consta | 2003 | PLGA/risperidone | Schizophrenia & bipolar I Disorder |
| ➢ Vivitrol | 2006 | PLGA/naltrexone | Alcohol dependence & opioid dependence |
| ➢ Somatuline | 2007 | PLGA/lanreotide | Acromegaly |
| ➢ Ozurdex | 2009 | PLGA/dexamethasone | Macular edema |
| Protein-based DDS | |||
| ➢ Zevalin | 2002 | Anti-CD20 monoclonal antibody/yttrium-90 | Non-Hodgkin's lymphoma |
| ➢ Bexxar | 2003 | Anti-CD20 monoclonal antibody/iodine-131. | non-Hodgkin's lymphoma |
| ➢ Abraxane | 2005 | Albumin/paclitaxel | Breast cancer |
| ➢ Brentuximab Vedotin | 2011 | Anti-CD30 monoclonal antibody/monomethyl auristatin E | Hodgkin lymphoma & systemic anaplastic large cell lymphoma |
Source: U S Food and Drug Administration website (http://www.accessdata.fda.gov/scripts/cder/drugsatfda/). Websites of various pharmaceutical companies supplying the drugs.
Exciting advances in genomics and systems biology have continued to identify new molecular targets. Future therapeutics will be increasingly nucleic acid (plasmid DNA, siRNA, mRNA, and aptamer) and peptidic (small peptide and protein) in nature. They have to act intracellularly, although the polar nature of nucleic acids and proteins hinders their cellular entry. They are also typically more fragile than small molecules. Drug delivery will hence play an increasingly important role in realizing the full potential of the next generation of therapeutics.
Innovations in materials chemistry have initially fueled the development of DDS, creating carriers that are biodegradable, biocompatible, targeting, and stimulus-responsive. Nanotechnology has joined forces in the past decade. The realization that size and shape of nanoparticles (NPs) can help navigate biological carriers has stimulated the application of nanofabrication technologies, both top-down and bottom-up, to develop more effective particulate DDS. For instance, the size of NPs determines their biodistribution. Whereas particles smaller than 20nm will be cleared from circulation via reticuloendothelial system (RES) within a few hours when injected intravenously, larger ones will be trapped in the liver and the spleen within minutes [5, 6]. In a study of accumulation of long-circulating polymeric micelles at tumor site, Kataoka and colleagues show only those smaller than 30 nm could effectively penetrate poorly permeable pancreatic tumors [7]. Fabricating techniques such as nanoprecipitation, emulsion-based phase inversion, microfluidics-based self-assembly, layer-by-layer synthesis, and nanoimprinting have been used to generate particulate DDS to deliver a wide range of drugs. To fully realize the potential of a particulate DDS with controlled size and shape, nanofabrication and nanomanufacturing will play a more prominent role in the future. In this article, we will first briefly review DDS development from a materials perspective. This will be followed by a more detailed discussion on recent innovative fabrication techniques for DDS. It is our belief that when innovations in fabrication can catch up with those in materials design the development of DDS will enter a new era of improving healthcare.
1. Advances in materials design
1.1. Early FDA approved drug carriers
Design and synthesis of various biocompatible materials has driven the progress of DDS in the past few decades. Liposome has been the most successful candidate in clinical applications. Most of the FDA approved DDS are liposome or lipid-based (Figure 1, table 1) [1]. Moreover, polymer-drug conjugates, especially those conjugated with polyethylene glycol (PEG) to reduce protein adsorption and aggregation, is now a standard method to increase circulation and bioavailability of biomacromolecules such as antibodies. FDA approval of paclitaxel and albumin conjugates in replacement of the traditional Cremophor™ represents the first successful clinical example of using biomacromolecules to facilitate drug delivery (Table 1). In a randomized, open-labeled trial of 460 patients, the new formulation performed better than Taxol alone and showed significantly reduced side effect even at a 50% higher dose [8].
1.1.1. Biodegradable drug carrier: PLGA
Biodegradable polymers are often considered as alternatives to lipids for their improved in vivo stability. The degradability could be tuned to control the rate of drug release. Among all, poly (lactic acid) (PLA), poly(glycol acid) (PGA) and their copolymers, poly (lactide-co-glycolide) (PLGA) are the most widely used for DDS development because of their biodegradability, biocompatibility and ease of processing [9, 10]. PLGA NPs are used to deliver various bioactive agents, such as small drugs [11–14], peptides and proteins [15–17], and recently plasmid DNA [18, 19]. The size of PLGA particles can be adjusted [20, 21] by changing the chemical composition as well as the fabrication method. Drug release rate from PLGA NPs could be controlled by varying the molecular weight of PLA [22–24], which determines the degradation rate of the vesicle. PLGA NPs can be easily modified to accommodate targeting motif, PEGylation and environment-responsive elements. Incorporation of D enantiomer in PLA segments leads to the formation of stereocomplexes with improved mechanical property and slower degradation [25, 26]. In one study, stererocomplexes are used to form nanofibers with paclitaxel and provide a controlled release of the drug for more than 30 days [27]. All these support the notion that PLGA is a versatile system for drug delivery. It has been a popular platform for new DDS development in the past and will continue to be so in the future because many PLGA-based DDS are already on the market (Figure 1, Table 1).
1.1.2. Other FDA approved biodegradable DDS
A vast array of biodegradable polymers, ranging from synthetic to natural and to hybrid, have been studied and developed for drug delivery, many of which are highlighted in this theme issue and in recent reviews. Polyanhydride is another class of biodegradable polymers whose application as DDS has enjoyed clinical success. To overcome the undesirable bulk-degrading behavior of polyesters, polyanhydrides are designed to undergo surface-degradation. This is made possible by the hydrolytically labile anhydride linkage, which combines with a hydrophobic backbone, restricts biodegradation at the surface since the rate of hydrolysis at the surface is much faster than the rate of water penetration into the core of the sample. This in principle can lead to a steadier release kinetics if the release mechanism is controlled by the carrier degradation. First approved in 1996, Gliadel®, a polyanhydride wafer placed in the resection cavity after brain tumor surgery for the delivery of carmustine to patients with recurrent malignant glioma showed modest improvement in survival [28]. In 2003, Gliadel® received additional FDA approval to treat patients with newly diagnosed malignant glioma as adjunct to surgery and radiation therapy (Table 1). Recent phase III trials further investigated its use as first-line treatment when followed by concomitant radiochemotherapy with temozolomide [29]. Although the treatment is still associated with significant toxicity due to its aggressive nature, the benefit of controlled drug delivery is implicated in these trials. As diverse properties of drugs used for various therapies demand carriers of different properties to achieve optimal delivery, many biodegradable polymers have been proposed to satisfy the need [30–34]. Challenges to gain regulatory approval have slowed the progress but the tremendous benefits of controlled drug delivery should see the emergence of other new biodegradable DDS eventually.
1.2. Block copolymers
Block copolymers have captivated the imagination of researchers since the early days of drug delivery because of their remarkable chemical flexibility [35]. Depending on the choice of building blocks, they could assemble to nanostructures in the form of micelles, electrostatic complexes, or polymersomes. The versatility in forming colloidally stable nanoparticles is appealing for passive tumor targeting utilizing the EPR effect. In late 1980s and early 1990s, two groups independently reported the successful development of micellar DDS [36]. One is from Kataoka et al., which is an A-B block copolymer comprised of PEG and poly(aspartic acid) modified by 4-phenyl-1-butanol to enhance the hydrophobicity. This system is under phase II clinical investigation to deliver paclitaxel (NK105) and doxorubicin (NK911). The other one, which is a poly(propylene oxide)- poly(ethylene oxide)- poly(propylene oxide) (PEO-PPO-PEO) (Pluronic) triblock copolymer developed by Kabanov et al. to deliver doxorubicin, is under phase III clinical evaluation in Canada. Other than amphiphilic block copolymers, PEG-polycation block copolymers are also of great interest due to their ability to condense nucleic acids into nanosized polyplex with protective and biocompatible PEG shell. Block copolymers of PEG-PEI, PEG-polylysine have been investigated by Kataoka et. al. in the 1990s [37].
A major drawback of polymeric micelles is their relative instability in blood upon systematic administration, which leads to rapid dissociation and burst release. To tackle this problem, various innovative approaches have been used to engineering the micelle core with different chemistry: a) Increase the hydrophobicity of the core by attaching pendant groups to the backbone, such as fatty acid, benzyl groups, cholesterol [38–40]; b) Introduce hydrogen-bond interaction in the core. For example, Hedrick and Yang et al. have created an amphiphilic PEO-PCL based polymer containing urea functional group in the side chain that shows improved kinetic stability of micelles in the presence of a destabilizing agent [41]; c) Promote electrostatic interactions by introducing oppositely charged groups in the micelle core, such as PEO with either poly (L-lysine) or poly(aspartic acid) [37]; d) Crosslink the micelle core by thermal or photo-induced polymerization. For instance, Kataoka et al. synthesized PEO-PLA block copolymers having polymerizable methacryloyl pendant groups at the PLA block [42, 43]. Upon micelle formation, the methacryloyal groups are crosslinked thermally or photochemically. The final micelle structure is stable at high temperature and in organic solvent. By incorporating reversible crosslinkers such as di-sulfide bonds, polymeric micelles can be made stable in the blood stream but dissemble in reductive environment inside the cell [44]. Furthermore, with a chemical structure that affords better miscibility with the encapsulated cargo, higher loading level can be achieved [45, 46].
The shell of polymeric micelles could also be modified according to the application. For example, the micellar shell can be crosslinked to alter the permeability of the micelle and control drug release rate. A poly(acrylic acid) shell could be crosslinked with diamino compounds to form amide bonds in a condensation reaction. The hydrophilic shell could also be functionalized with various ligands (antibodies, small organic molecules, carbohydrates, peptides or polymers) to promote specific binding to cancer cells. The creation of polymeric micelles with cationic surface allows co-delivery of nucleic acids and small organic drugs. Yang et al. has synthesized a cationic amphiphilic block copolymer, which comprises poly(N-methyldietheneamine sebacate) (PMDS) as the cationic block for siRNA and plasmid binding, and a cholesterol pendant group to increase the hydrophobicity of the core for efficient drug loading [47]. The cationic core-shell micelle structures could efficiently deliver both cargos in vivo and exhibit synergistic effects to suppress tumor growth upon intra-tumor injection in a breast cancer model. A triblock copolymer, poly (ethylene glycol) –β-poly (ε-caprolactone)-β-poly(2-aminoethyl ethylene phosphate) (mPEG-β-PCL-β-PPEEA) is made by Wang et. al. to co-deliver Plk1 siRNA and paclitaxel [48]. Synergistic tumor suppression is observed in a dose-dependent manner following systematic administration. More such co-delivery systems have been investigated by various groups [49–53]. Though in vivo stability and the risk of exposing nucleic acids on micellelar surface to enzymatic degradations needs to be further addressed, these systems provide promising alternatives to liposomes for the co-delivery of hydrophilic macromolecule and small lipophilic drugs.
1.3. Polymer drug conjugates
In addition to encapsulation and non-covalent complexation, conjugation to a polymeric carrier via a liable linker presents another attractive approach to alter and optimize the pharmacokinetics of therapeutic agents. Many early polymer-drug conjugates have exclusively focused on the delivery of commercialized anti-cancer drugs, such as paclitaxel, doxorubicin and camptothecin [54]. Since the size of polymer-drug conjugates could be controlled by adjusting the molecular weight of the polymer, it could be optimized to maximize the benefits of enhanced permeability effect (EPR) at leaky tumor vasculature. Moreover, conjugating drugs to a polymeric carrier can enhance solubility of hydrophobic drugs, extend drug circulation in vivo and enhance uptake by addition of targeting motifs to the polymer. With 16 such systems (excluding PEG conjugates) entering clinical trials [55], the last decade has witnessed the rapid development and clinical validation of polymer-drug conjugates. Most of these clinical studies have adopted N-(2-hydroxypropyl) methacrylamide (HPMA) or poly (L-glutamic acid) (PGA) as the carrier. Originally developed by Kopecek and colleagues [56, 57], the HPMA-doxorubicin conjugate comprises doxorubicin linked to a 30 kD backbone via an enzyme degradable peptide in the sidechain, with about 8 wt % drug content [57]. After structural validation and promising preclinical results, HPMA-doxorubicin entered into a Phase I clinical trial in 1990 and that marked the first milestone of development of polymer-drug conjugates. In contrast to HPMA, PGA contains a biodegradable backbone and allows for higher drug content (37% for PGA-paclitaxel conjugate) [58, 59]. Conjugates of PGA with paclitaxel and camptothecin are also under clinical evaluation [60, 61]. Regulatory approval of these systems is however slow. Many polymer-drug conjugates show unexpected release behavior and side effects in clinical testing except PGA-paclitaxel conjugates (Xyotax). Early interpretation of phase III clinical trial of Xyotax concluded with no therapeutic activity [62, 63]. However, subsequent analysis did show that Xyotax could improve survival rate in women although not in men [54, 64]. An estrogen-dependent cathepsin B activity, the enzyme responsible for degrading PGA backbone, is the key player for these gender-dependent responses. The finding indicates the importance of using suitable biomarkers, which may be different from those for the naked drug, in the development of DDS.
In parallel with clinical validation of the first-generation polymer-drug conjugates, several innovative strategies of creating polymer-drug conjugates have been proposed recently. Cheng et. al. used a drug-initiated, controlled polymerization protocol to form polylactide (PLA)-drug conjugates. Drugs including paclitaxel [65], doxorubicin [66], cyclosporine A [67] and camptothecin [68] were conjugated to PLA via a hydrolysable ester linker. High drug content (10–30 wt %) was obtained and controlled release was observed. Reversible addition–fragmentation chain transfer (RAFT) polymerization, a technique that can achieve excellent control over polymer molecular weight and composition, has also been applied to produce HPMA-SN-38 (a camptothecin analog) conjugate [69]. The polymer-conjugate displays good aqueous solubility and similar anticancer effect of free drug. These one-step synthetic approaches simplify the manufacturing process as compared to conventional post-synthesis conjugation methods. However, they also limit the linker choice between polymer and drugs, which potentially diminishes the control over drug release rate. More innovative chemistries to expand the linker library will further increase the appeal of polymer-drug conjugates.
1.4. Novel use of nature polymers
Natural polymers derived from biological systems including protein, DNA, and polysaccharides are biocompatible and biodegradable polymers. They possess low toxicity and potentially favorable pharmacokinetics in the circulation. Use of natural polymers as drug carriers has a long history. The use of polysaccharides [70] such as heparin, chondroitin sulfate, and chitosan [70, 71] as carriers, and coupled with the use of antibody [72, 73] and transferrin [73, 74] as targeting motif has all brought significant clinical benefits. Nonetheless, the tremendous potential of natural polymers as drug carriers is still under-represented and deserves more attention. An interesting direction has been the use of nucleic acid in drug delivery. For instance, there is a growing interest in the use of DNA or RNA aptamer as new targeting motif in replacement of antibody [75, 76]. Aptamers, the conjugation chemistry of which is easier to control and confine, are smaller in size, easier and cheaper to synthesize and purify, and less immunogenic. Furthermore, nucleic acid itself as a biomaterial and building block may be an interesting platform for delivery applications [77]. There are no nanoscale interactions more elegant than those governing nucleic acids by the Watson-Crick pairing rules. The infinite combinations of DNA/RNA base pairs and their remarkable molecular recognition capability can give rise to interesting nanostructures that are only limited by our imagination. Creative assembly of nucleic acids has fashioned a plethora of 2D and 3D nanostructures with precisely controlled size, shape, and spatial functionalization. Various DNA-based nanostructures suitable for drug encapsulation have been invented, such as DNA nanotubes [78, 79] and DNA nanoboxes [80]. The nanobox which is about 35 nm in size has been designed to have a lid that can be locked or opened with a molecular “key”. This interesting design allows for controlled release of small chemicals. Other DNA nano-architectures have been used to deliver various bioagents such as doxorubicin [81], CpG [82, 83], and siRNA [81, 83]. The susceptibility of these DNA nanostructures to premature nuclease degradation may limit their utility, but modified nucleic acids have proved much more stable [84, 85]. In another case, modification of protein has led to a novel carrier design. Liu et. al. from Harvard mutated the green fluorescence protein (GFP) to change from a wild-type with a net of seven negatively charged groups to a polypeptide with 36 positively charged groups. The mutations do not alter the GFP structure and fluorescence intensity, and yet offer great chemical resistance to enzyme degradation and denaturation. These supercharged GFP are used to deliver various proteins [86, 87] and DNA/siRNA [87] into different cells. Not only serving a delivery function, the intrinsic fluorescence from GFP also allows for tracking of the delivered cargo. This example highlights the potential of polypeptides as drug carriers, which is being increasingly pursued by drug delivery researchers using protein engineering.
1.5. Recombinant protein-based drug carriers
Recombinant proteins bear the intrinsic advantages of natural biopolymers for medical application as they are biodegradable and potentially biocompatible if the artificial sequence is not antigenic. Genetic engineering allows precise control over structural and functional properties of recombinant proteins, such as their molecular weight, hydrophobicity, targeting motif, secondary structures, and drug conjugation sites. Two broadly studied recombinant protein systems are the elastomer-like proteins (ELPs), and silk-like proteins (SLP).
ELP is a family of recombinant proteins derived from the elastomeric domain of extracellular matrix protein elastin. This short hydrophobic domain, comprised of five amino acids (GVGVP), is the basic building block of ELPs [88]. Moreover, by substituting the second amino acid in the pentapeptide, ELPs can undergo reversible and rapid phase transition in response to temperature. Chilkoti and colleagues have designed a series of ELP with distinct transition temperatures as drug carriers [89–92]. In one system, ELP-peptide fusion protein was conjugated to doxorubicin [91], which formed micelles and aggregated in the tumor microenvironment under hyperthermic, leading to increased accumulation at tumor site. In another study, the effect of hyperthermia-induced micelle formation was exploited to present multivalent targeting motifs to enhance cellular uptake [92]. Chaikof and colleagues have also developed multiblock ELP for drug delivery in the configuration of NP, hydrogel or film depending on the multiblock composition and processing method [93–96].
Silk protein is a native block copolymer with alternating large hydrophobic and hydrophilic blocks. The hydrophobic block is generally a repetitive sequence conserved with short-chain amino acids, such as glycine and alanine. The hydrophilic block is less conserved and usually contains non-repetitive sequences rich in charged amino acids. The hydrophilic domain is often substituted with other peptide sequences to achieve specific function for drug delivery. The length of the hydrophobic domain could also be tuned to yield protein NPs with reproducible sizes for drug and gene delivery [97, 98]. A recent study demonstrated that a SLP recombinant protein endowed with a cell penetrating peptide could achieve transfection efficiency 45 times higher than that of poly(ethyleneimine) (PEI) [99].
1.6. Smart DDS
Much of innovations in materials design for drug delivery manifest in producing smart DDS that are able to release the therapeutic payload on-demand. Stimuli-responsive polymers mimic the behavior of biological molecules where external stimuli or changes in local environment can trigger a change in property: conformation, solubility, shape, charge, and size. Drug release can be regulated not only in a spatial manner via targeting, but also in a temporal manner when the external stimuli are applied. Various chemical (pH, ionic strength), physical (temperature, light, electricity, magnetic field, ultrasound, mechanical stress etc.), and biological (enzymes, proteins) signals have been used as the triggering stimuli [100]. Numerous monomers have been discovered to possess sensitivity to a specific stimulus. They can be assembled to a homo-polymer with tunable sensitivity to one signal or to a co-polymer responding to multiple stimuli. Smart polymer coupled with targeting ligand can probably best minimize off-target effects and maximize programmability. Smart DDS has been covered in excellent reviews elsewhere [101–104].
1.7. Combinatorial chemistry and polymer library
Structure-function relationship for material design in drug delivery has generally been derived from empirical deduction of available systems, and only with limited structural variations coming out from a single laboratory. A systematic approach is required. Systematic study is difficult for polymeric systems, not only because the synthesis is tedious, but also due to the intrinsic polydispersity of most polymers. Rapid progress in combinatorial chemistry enables the fine-tuning of drug carrier characteristics at the molecular level. In early days, combinatorial chemistry is used to synthesize and screen biocompatible polymers for coating of medical implants and devices. One approach was described by Kohn and coworkers, who built a library of 112 polyacrylates from 14 tyrosine-derived diphenols and eight diacids with slight differences in polymer bulkiness, flexibility, hydrophobicity, and cellular response that affected their potential utility in medical implant applications [105]. Recently, combinatorial chemistry was attempted to generate a library of potential gene carriers. Akinc et. al. used a simple synthetic scheme to produce a library of over 1200 lipid-like polymers by combinatorial chemistry to delivery siRNA in vivo [106]. In this study, a collection of alkyl acrylates or acrylamides was reacted with different diamines via Michael’s addition to produce thousands of siRNA delivery vectors with diverse physiochemical properties. A follow-up study used improved chemistry to synthesize another library of lipid-like polymers to reduce dosage of siRNA required for in vivo study [107]. Though there is a conflict in the optimal alkyl length for siRNA delivery between these two studies, they share some common conclusions such as retention of a secondary amine and possession of either two long amide tails or several smaller amide tails for high siRNA transfection efficiency in Hela cells. In a separate study, Weissleder et. al. generated a small library of 146 NPs each multivalently presenting unique small molecules on surface [108]. These magnetofluorescent NPs were coated with dextran and functionalized with primary amine for conjugating small molecules for targeting. With this library, the group was able to identify small molecules that produced differential affinity for different cell lines in vitro and in vivo, without knowing a priori the molecular receptors involved.
Chemical composition greatly influences physicochemical properties of NPs, which in turn governs the pharmacokinetics and bio-distribution of particulate DDS. Nonetheless, fabrication or formulation also plays an equally essential part for optimal delivery. As discussed earlier, structure-function relationship is essential for design, characterization and validation of DDS development. However, if the formulation is flawed in producing inferior products, the structure-function relationship would be inaccurate and even misleading. For example, the optimal N/P ratio of polyplexes for nonviral gene delivery determined by bulk mixing might be inaccurate if such formulation produces highly heterogeneous and unstable polyplexes within the same mixture. With the same materials chemistry, fabrication may also enable generation of NPs with different characteristics such as sizes, shapes, and drug loading efficiencies and release profiles, which impacts the therapeutic outcome. Moreover, fabrication methods directly influence the cost, ease of purification, reproducibility and scalability of a drug, which are also important considerations in pharmaceutical development. As it is envisioned that NPs will become the dominant DDS for intracellular delivery, and nanomedicine has captured the imagination of scientists and layman alike, herein we will discuss the fabrication of NPs, the development of which is fueled by nanotechnology innovations. Since conventional fabrication methods for particulate DDS are less reviewed elsewhere, we will also spend some time describing them before introducing the recent innovations in fabrication such as microfluidics and imprinting technologies.
2. Conventional NP fabrication
2.1. One-step NP formation: Nanoprecipitation
Nanoprecipitation (Figure 2a) is one of the most commonly used NP fabrication methods, which accounts for more than 50% of the NPs reported for drug delivery [106]. It is performed by adding an organic solution which contains the polymer and lipophilic drugs into an aqueous solution in a drop-wise manner under constant stirring. NPs containing drugs form instantaneously as the polymer diffuses into the aqueous phase. The miscibility of the solvent with water is the most critical parameter governing the outcome [109]. The rate of polymer addition and stirring speed also influence the size and drug loading level. Particle size formed by this method is usually around 200 nm, typically smaller than those produced by other processes. The method can be applied to a wide range of polymers [110], peptides [111] and amphiphilic cyclodextrins [112], although scale up would be inefficient due to the nature of drop-wise addition.
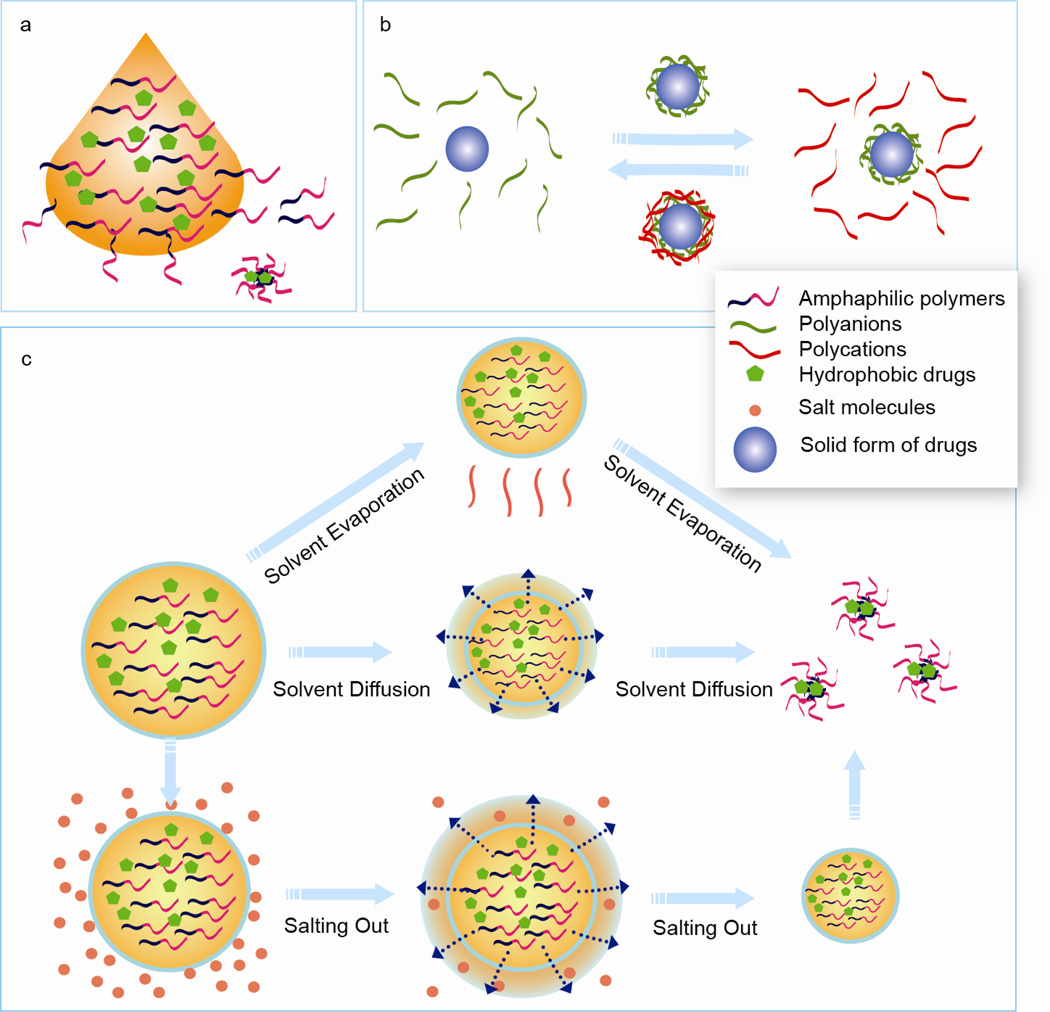
Figure 2.
Conventional nanoparticle fabrication methods. a) Nanoprecipitation. Polymer dissolved in organic solvent is added to an aqueous solution in a dropwise manner under constant agitation. Nanoparticles containing drugs form instantaneously as the polymer diffuses to the aqueous phase. b) Layer-by-Layer assembly. Solid form of drugs are used as the core. A polymer layer is first adsorbed onto the drug colloidal template by incubating in polymer solution and transferred to the oppsitely charged polymer solution for additional layering. This process is repeated until nanoparticles of desired sizes are formed. c) Emulsion-based two step methods. Emulsified oil-in-water droplets containing polymer and drugs are formed in the first step. In the second step, different methods are applied to remove the solvent and precipitate nanoparticles. Top pannel: Solvent evapoaration method. Solvents are gradually evaporated under vacuum and high pressure. Middle panel: Solvent diffusion method. The solvent used to prepare emulsion drops is partially miscible with water. When the emulsion droplets are diluted with water containing stablizer, organic solvent rapidly diffuses out from the droplets, leading to condensation of the materials within and formation of polymer nanoparticles. Bottom pannel: Salting out. The solvent used to prepare polymer and drug solution is totally miscible with water. Emulsification is conducted with aqueous phase containing high concentration of salt. The saturated aqueous phase prevents solvent from mixing with water. The emulsified droplets are then diluted in water. A sudden drop of salt concentration in continous phase causes extraction of organic solvent and precipiation of polymer drug nanoparticles.
2.2. Two-step NP formation: Emulsification-based methods
Many fabrication methods for NP preparation adopt a two-step process. In the first step, the organic phase containing the polymers and drugs are vigorously agitated or sonicated in the aqueous phase to form emulsified droplets. Depending on the emulsified system (from nanoemulsion to macroemulsions) used, the eventual particle size and drug loading varies. Double emulsions could also be used to prepare core-shell vesicular structures. Following emulsification, various methods are used to precipitate the polymer to form dense drug-loaded particles (Figure 2b).
2.2.1. Emulsification-solvent evaporation
Emulsification-solvent evaporation is the most common method reported to prepare NPs [109]. In this method, polymer is dissolved in a volatile solvent (such as dichloromethane, chloroform) and emulsified in an aqueous phase. Formation of NPs is achieved by the evaporation of the solvent under reduced pressure [113]. Nevertheless, this is a slow process compared to nanoprecipitation which happens in milliseconds. For instance, it requires 80 min to evaporate 10 mL of ethyl acetate from a 50 mL aqueous emulsion. During the first 40 min 90% of the solvent is removed but it takes another 40 min to completely get rid of the rest [114]. The size of NPs drops to minimal during the first 40 min, and increases in the second 40 min due to coalescence of emulsion droplets. Thus, the coalescence determines the final particle size which is largely dependent on the evaporation condition. This highlights the importance of optimizing processing conditions in determining quality of particulate DDS. Adjusting solvent evaporation condition such as temperature and pressure would improve the quality. The use of a surfactant such as sodium dodecyl sulphate (SDS) or poly(vinyl alcohol) (PVA) would also minimize the coalescence effect and produce smaller NPs [115].
Emulsion-solvent evaporation is widely used to encapsulate lipophilic drugs. However, the loading level for hydrophilic drugs, such as proteins and peptides is generally poor due to diffusion of the hydrophilic drug into the aqueous phase before the polymer can solidify to entrap the drug [116]. To overcome this problem, water-in-oil-in-water (W/O/W) double emulsion can be used to reduce the loss and also to preserve the bioactivity of delicate drugs such as proteins in the aqueous phase. Typically, the primary emulsion is formed by ultrasound treatment of a mixture of aqueous phase containing therapeutics and organic phase containing the polymer and an organic surfactant serving as the stabilizer for water-in-oil (W/O) emulsion. The second emulsion is formed by sonicating a mixture of the organic phase containing dispersed W/O emulsions and aqueous phase containing a hydrophilic stabilizer. Sonication duration for the second step is more critical in determining the final particle size as compared to that of the first step and surfactant concentration [117]. Longer sonication time significantly reduces the size of the particles and produces smaller polydispersity, but this has to be balanced against the potential risk of damaging the drug.
2.2.2. Emulsification-solvent diffusion
Emulsification-solvent diffusion is another widely used method to prepare drug-loaded NPs. In this method, the polymer is dissolved in a partially water-miscible solvent (such as benzyl alcohol and propylene carbonate) which is pre-saturated with water [118]. A typical emulsification method is then used to produce oil-in-water (O/W) emulsion droplets from the water-polymer saturated solvent. The dispersed droplets are then diluted by a large amount of water containing a stabilizer. The diffusion of organic solvent out from the droplets leads to the condensation of the materials within the droplet and formation of NPs. The solvent extraction process takes places within a few milliseconds, causing a drop in particle size. In general, the diameter of particles prepared by this method is around 150 nm. Due to the fast solvent extraction kinetics and well-defined solvent-water interaction, physical properties of NPs prepared with this method is highly reproducible and the polydispersity is significantly lower than NPs prepared by other conventional methods [119].
2.2.3. Emulsification-salting Out
Emulsification-salting out is a derivative of the emulsification-solvent diffusion method. The organic solvent used is totally miscible with water, such as acetone. The polymer-containing solvent is emulsified in an aqueous phase containing high concentration of salt (magnesium chloride, calcium chloride) or sucrose [120]. The saturated aqueous solution prevents acetone from mixing with water. Dilution of the emulsion droplets in a large amount of water results in an abrupt drop of salt concentration of the continuous phase, leading to the extraction of organic solvent and precipitation of NPs. This method works exclusively for lipophilic drugs. The choice of salting-out agent greatly impacts particle size and drug encapsulation efficiency, whereas mechanical mixing, stabilizer concentration have less profound effect. Zhang et. al. compared the solvent-diffusion and salting-out methods to prepare poly(trimethylene carbonate) (PTMC) NPs containing a lipophilic drug. Particle size from salting-out is smaller but drug loading efficiency is also lower [121].
2.3. Layer-by-layer synthesis
Layer-by-layer (LBL) NP preparation (Figure 2c) makes use of the electrostatic interaction between oppositely charged polyelectrolytes, such as polylysine, chitosan, gelatin B, or poly(ethyleneimine) (PEI) complexing with sodium alginate, poly(acrylic acid) (PAA), dextran sulfate, hyaluronic acid, chondroitin sulfate or heparin. Solid form of bioactive agents is often used as the core to grow the vesicular structure. A polymer layer is first adsorbed onto the colloidal template by incubation in the polymer solution, washed, and transferred to the oppositely charged polymer solution. Repeat of the cycle leads to a multi-layered coating that can control the release kinetics. Calcium phosphate nano-crystal, which could be dissolved away by chloric acid, is frequently used as the core. This method can be used to encapsulate various bioactive agents, such as paclitaxel [122], tamoxifen [123], vitamins [124], insulin [124], antigenic peptides [125], and nucleic acids [126]. In the latter case, oral delivery of siRNA against TNF-α and suppression of inflammation was achieved by LBL assembly of PEI and siRNA onto a β1,3-d-glucan core extracted from yeast. In this method, particle size and drug release rate could be controlled by controlling the thickness of the shell.
3. Microfluidic platforms for DDS fabrication
Conventional NP fabrication techniques are prone to polydispersity and batch-to-batch variations. For instance, the final size of NPs generated by the emulsion-based techniques is directly determined by the size of the emulsion droplets, which itself could be very heterogeneous in bulk mixing. While heterogeneity remains an insurmountable obstacle in bulk preparation of DDS, microfluidics, the manipulation of fluid in nano/picoliter scale channels, presents exciting opportunities to improve the fabrication and manufacturing of particulate DDS. The general benefits of conducting reaction in microfluidics include but not limited to rapid mixing of reagents, homogeneous reaction environment, flexibility for multi-step reaction design, enhanced processing accuracy and efficiency, better heat transfer due to high surface-to-volume ratio, miniaturization, and cost savings from reduced consumption of reagents [127]. In particular, the rapid mixing feature, flexibility for multi-step reaction and efficient processing, as we shall see later, contribute to the improved fabrication process of particulate DDS in three aspects. First, microfluidics is a versatile platform that can be used to synthesize various types of drug carriers. Second, microfluidics-aided synthesis exhibits better controllability over the physical properties of drug carriers. Third, microfluidic possesses are amenable to scale-up and on-line quality control, factors important for translation and commercialization.
Most of the microfluidics devices nowadays are fabricated in either glass [128–143] or polydimethylsiloxane (PDMS) [144–167]. Silicon [168–170], perfluoropolyether [171], polymethyl methacrylate (PMMA) [172, 173], polyurethane [174], polycarbonate [175] and stainless steel [176] microfluidics devices are also reported. In the commonest approach, two streams containing continuous and disperse phases are infused into two separate inlets, and the disperse phase is confined into isolated droplets or narrow stream at T-junction, in flow-focusing and concentric capillaries. The three most common types of microfluidics design is shown in Figure 3. The droplet configuration would produce spherical particulate DDS in a discrete manner whereas the focused-stream configuration would promote self-assembly at the interface of the two adjacent streams in a continuous manner.
Figure 3.

Common types of microfluidics design. (a) Flow focusing (b) T-junction (c) Concentric capillaries.
3.1. Microfluidics as versatile platform for DDS synthesis
3.1.1. Nano DDS
Due to the intrinsic limitation of bulk synthesis, the adoption of microfluidics for synthesis of nano-sized DDS has become an attractive alternative [169]. Self-assembly of liposome in microfluidics system is demonstrated [168–170]. Lipids dissolved in isopropyl alcohol (IPA) in the disperse phase is channeled through two streams of buffer solutions of continuous phase in a flow-focusing device where dilution of IPA occurs through mixing of fluids at the interface. Liposome particles (50–200 nm in diameter) highly homogenous in size are precipitated at downstream of the channel (Figure 4a). Using the same approach, monodisperse PLGA-PEG NPs ranging from 20 to 100 nm have also been synthesized [146] [147]. Drugs such as docetaxel and platinum (IV) prodrug have been encapsulated as an example. By selecting two immiscible phases containing different precursor molecules, lipid-polymer hybrid DDS are prepared in flow focusing device by dissolving hydrophilic PEG or PNIPAM in aqueous continuous phase and hydrophobic lipids in the organic phase. The formation of PLGA/lecithin/PEG NPs (35–180 nm) and lipid-PNIPAM (150–300 nm) are reported [148, 177]. Apart from precipitation reaction, recently the synthesis of polymer-drug conjugates using microfluidics system has also been demonstrated. Heparin–folic acid–retinoic acid (HFR) bioconjugates (100 nm) with high drug coupling ratio and uniform size distribution could be produced efficiently after thorough mixing of two reactants introduced into a microchannel [171]. Reduced reaction time and high drug conjugation efficiency is attained in a single-step assembly reaction.
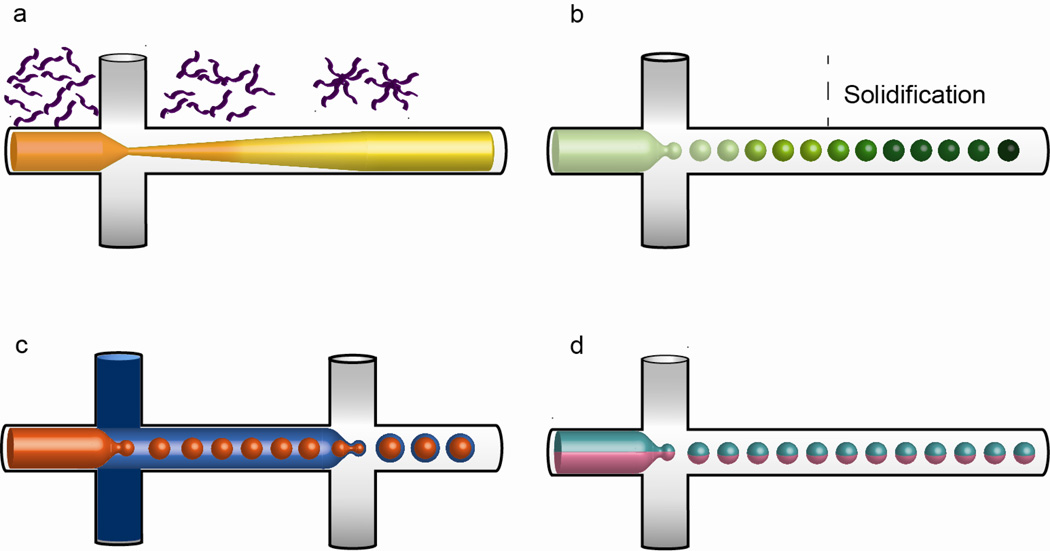
Figure 4.
Schematic illustration of the microfluidics preparation process of various drug carriers. (a) Nanocomplex. During hydrodynamic flow focusing, precursors self-assemble into nanoparticles when precursor-solvent solution is mixed with buffer, in which the precursor is poorly soluble. The process occurs in three stages involving nucleation of nanoparticles, growth through aggregation and stabilization. (b) Microparticle/microsphere. Disperse phase is broken up by the continuous phase to form droplets which in turn give rise to microparticles upon solidification. (c) Core-shell structure. Sequential encapsulation generates double emulsion after which the shell is solidified to produce a shell layer with a liquid core. (d) Janus particle. The disperse phase comprises two distinct inputs is broken up by the continuous phase to produce particle with two distinct phases.
While microfluidics facilitates the preparation of nano-sized DDS by rapidly mixing the precursor solution with buffer, the fabrication of DNA nanocomplexes such as lipoplex or polyplex for nonviral gene delivery of DNA, siRNA could also benefit from the same platform [132, 144, 145]. Polyplexes (250 nm) and lipoplexes (200 nm) formed in picoliter droplets by using a flow-focusing microfluidics device show smaller size, narrower size distribution, lower cytotoxicity, and higher transfection efficiency compared to those formed by bulk mixing, demonstrating the appeal of self-assembly in a small volume [144, 145].
3.1.2. Microparticles / microspheres
The capability of microfluidics to generate discrete droplets is leveraged to fabricate microparticulate DDS. A range of organic (PLA [151, 161] and PLGA [156, 157]) and inorganic (chitosan [172, 173], poly(N-isopropylacrylamide) pNIPAAM [133, 134, 152, 155], hydrogelator [153], silk protein [135], pectin [175], hydrazide and aldehyde-functionalized carbohydrates [158], dextranhydroxyethyl methacrylate (dex-HEMA) [166] and silica [154]) materials have been investigated for microparticle formation through generation of liquid precursor droplets in microfluidics prior to solidification by solvent extraction or induced polymerization (Figure 4b). Furthermore, porous microparticles are also fabricated with the help of microfluidics to facilitate drug release. The use of poly(ethylene glycol)-b-polylactide (PEG–PLA), which is oil-soluble, as gel matrix in O/W emulsion leads to the formation of a reverse micelle structure with a small amount of water encapsulated within, creating pores upon freeze drying [151]. PEG has also been used as porogens during the formation of porous pNIPAAM microparticles [155].
3.1.3. Core-shell structures
W/O/W is useful to prepare core-shell structures. However, conventional emulsification techniques used to prepare core-shell structure generate droplets with great variation in sizes. The possibility of adopting microfluidics for production of core-shell structure has been explored. Utada et. al. first reported the formation of W/O/W double emulsion droplets from a concentric microcapillary device [131]. Such W/O/W droplets/capsules could be used to trap and release insulin [178] and antibiotics [130] by either diffusion of substances through the oily membrane or disruption of the droplet structure under shear stress [179]. Using this method, DDS such as microscale polymersomes could be fabricated with precise control over their size [128]. In other studies, microcapsules with alginate, PLA or a polyelectrolyte shell comprising anionic platinum NPs and cationic diazoresin, are prepared by forming single emulsion followed by ionic crosslinking of the surface of the capsule, solvent evaporation or coating with polyelectrolytes [129, 140, 180].
3.1.4. Smart drug delivery core-shell structures
Microfluidics has also been used to produce smart capsules for drug delivery. Using pNIPAAM along with crosslinker (BIS) as the disperse phase and hexadecane containing photoinitiator as the continuous phase, thermosensitive pNIPAAM capsule could be generated in a single emulsion system by applying UV light to photopolymerize pNIPAAM precursor droplets from outside after the photoinitiators have diffused into contact with the monomer [167]. Change of capsule volume is induced by subjecting the capsule to different temperatures, a mechanism that could potentially be used to trigger the release of drugs. Using the double emulsion approach, thermosensitive microcapsules could be made by coating polyacrylamide microgel as the inner core with (pNIPAAM) precursor followed by crosslinking to form microgel capsule [181]. A stimulus-responsive colloidosome which exhibits 80% decrease in volume when actuated is fabricated by assembling pNIPAAM microgels at the interface of W/O emulsion [141]. Moreover, a pH-responsive capsule can be generated from an O/W/O emulsion template comprising chitosan in the middle water phase and oil-soluble crosslinker in the inner oil phase [142]. Chitosan is crosslinked at the inner O/W interface as the inner oil contacts with the middle chitosan layer during transition in the microfluidics device. The chitosan capsules displays an acid-sensitive burst release of cargo from the inner core by decomposition of the shell layer. Monodisperse polymersomes are fabricated using the similar approach in W/O/W emulsion when PEG-b-PLA adsorbs at the oil–water interfaces. The encapsulation and triggered release upon osmotic shock of 4000 Da dextran molecules is described. Finally, a “smart” magnetic field-sensitive drug delivery device is constructed with high molecular weight chitosan embedded with magnetite particles as the capsule layer [149]. The release of drug (aspirin in this case) from the inner core is sparked by an AC magnetic field that causes the compression and extension oscillation of the capsule, resembling the pumping action of drug. The drug release behavior could be tuned by simply varying the applied magnetic field.
3.1.5. Janus particles
In addition to conventional drug-encapsulated microparticles, Janus particles, which have an interesting anisotropic structure and are difficult to prepare by conventional methods, could be readily fabricated in microfluidics. Janus particles are fabricated by co-injecting two immiscible streams in the disperse phase and solidifying droplets generated at T-junction (Figure 4d) [174]. These particles are attractive since they can be used to co-deliver two drugs with distinct properties or a drug and an imaging marker. As a demonstration, water soluble (doxorubicin hydrochloride) and water insoluble (paclitaxel) drugs are encapsulated in a single Janus particle. Each drug is separately released from the particles [176].
3.1.6. Multi-shells, chamber particles and microfibers
Multi-shell and multi-chamber particles, which have been shown to be efficiently produced on microfluidics platform, represent novel drug delivery vehicles whose potential has yet to be fully explored [138]. A multi-shell particle allows multiple drugs or a diagnostic agent along with the drug to be encapsulated at different layers for combination drug therapy or theranostics. As an example, a multilayer gas liposphere is fabricated in a microfluidics system [160]. The liposphere contains an oil layer incorporated with hydrophobic drugs wrapping around a gas core intended as a contrast agent for ultrasound imaging. The lipid-PEG layer in exterior can be functionalized for targeted delivery. Multi-shell particles could prove useful in cancer therapy where combination drug therapy is more effective to eradicate tumor cells and as theranostic agents where the site of drug delivery can be pinpointed by imaging [147]. A multi-chamber particle can serve similar functions by encapsulating more than one inner sphere within a particle. A thermosensitive hydrogel encapsulating multiple inner oil droplets is fabricated by microfluidics, and the release of both hydrophobic (carried by the oil phase) and hydrophilic molecules (associated with the aqueous hydrogel phase) is demonstrated [161]. Furthermore, microfluidics system allows precise control of the composition of inner spheres [137].
Another potential DDS that can be fabricated on a microfluidics platform in a simple and cost-effective manner is drug-loaded microfibers. Such a fibrous structure can be useful in surgical reconstruction where diffusion of drug can improve wound healing and minimize scarring [182]. The production of alginate [162, 163], PLA [182], an amphiphilic copolymer [159] and poly(ethylene glycol) diacrylate (PEG-DA) [164] fibers have been reported by flowing a precursor solution into microchannels under laminar flow conditions to induce fiber formation by crosslinking, precipitation or polymerization, respectively. Among them, alginate fibres encapsulating silver NPs are successfully fabricated for application as wound dressing to stimulate healing while inhibiting microorganism growth [163]. The tensile strength of fibers, drug loading efficiency and degradation properties are parameters that need to be optimized to present its appeal as a novel DDS.
3.2. Microfluidics offers precise control over drug carrier synthesis
In addition to its versatile nature, microfluidics platform offers another advantage over existing processing methods by exhibiting high degree of control of DDS preparation in five aspects: particle size, shape, shell thickness of capsule, drug loading efficiency and release rate. The following sections illustrate how the precise control is achieved in each aspect.
3.2.1. Control of particle size
The most important benefit of using microfluidics to fabricate particulate DDS is the ability to precisely control particle size. The NPs or microparticles generated from microfluidics platform typically have a narrower size distribution than those acquired by conventional methods [146, 157]. By two ways can microfluidics platform facilitate the fabrication of monodisperse particles. a) Using a T-junction or flow-focusing device, the breakup of the disperse phase by the continuous phase is periodic and predictable, leading to discrete and consistently sized droplets formed at the junction. The size of droplets can be conveniently controlled via altering the flow rates of continuous and disperse phases. These droplets subsequently are solidified to give microspheres or undergo further encapsulation to generate uniform core-shell structure. In the case of nanocomplex synthesis, it is hypothesized that the charge neutralization between the cationic gene carrier and the negatively charged nucleic acid would be more complete by confining the two components in picoliter droplet, yielding nanocomplexes more uniform and compact while exhausting any unreacted cationic gene carrier that is often cytotoxic. b) In hydrodynamic focusing, the fluid stream to be mixed flowing along the central microfluidics channel is confined into a narrow stream after encountering two adjacent streams at higher flow rates. The diblock copolymers or lipids dissolved in solvent self assembles as solubility decreases via solvent dilution through mixing with buffer in adjacent streams, the process of which takes place in micron scale. In theory, the efficiency of mixing by diffusion is length dependent. In reactors with a characteristic length scale larger than 1mm, mixing by diffusion is inefficient whereas mixing time drops significantly as diffusion distance falls below 100 um [127]. Microfluidics channels, usually designed with width of 100 um or less, represent an efficient and readily accessible reactor for rapid mixing of fluids. The three stages of carrier fabrication by self-assembly (nucleation, growth through aggregation and stabilization after a characteristic aggregation time scale) are well controlled in the microfluidics channel, resulting in carriers with highly reduced polydispersity.
3.2.2. Control of particle shape
In addition to particle size, the effect of particle shape on drug carrier performance has recently gained more attention because of its influence on tissue biodistribution and endocytic uptake [183, 184]. Conventional soft lithography can be used to fabricate non-spherical particles but the throughput is limited by the mold size. Using a combined microscope projection lithography and microfluidics approach, however, the fabrication process for non-spherical objects could be rendered continuous which obviously appeals to clinical translation and product manufacturing [165]. In this approach, a mask with desired features is inserted in the microscope to generate a mask-defined UV light beam projection on the monomer stream flowing in a PDMS microfluidics device above. Particles with desired shape are polymerized and advect along the unpolymerized monomer stream to make way for subsequent polymerization to occur. Particles with a variety of shapes can be efficiently generated. These examples showcase another attractive feature of microfluidics-aided drug carrier synthesis, one that can be leveraged to mass produce drug carriers with various shapes to optimize their biological performance.
3.2.3. Control of particle shell thickness
In microcapsule, the shell represents a diffusion barrier to control the sustained release from the capsule. The drug release profile associated with microcapsules of varying shell thickness would be inhomogeneous, which would be undesirable if uncontrolled. In the double emulsion approach, the thickness of the capsule shell can be easily controlled via optimizing the design of microfluidics device or simply altering the flow rate adopted in the encapsulation process. PLA microcapsules with a shell thickness as thin as 80 nm could be reproducibly and effectively produced on microfluidics platform, which is otherwise not achievable in bulk preparation process [139]. The precise control of the capsule shell thickness afforded by microfluidics enables tuning of the drug release profile of microcapsules for different controlled drug release applications.
3.2.4. Control of drug loading efficiency
Compared with conventional fabrication methods for microspheres which resulted in a drug encapsulation efficiency (EE) of 50-90% [150], several studies that looked into drug loading efficiency associated with a microfluidics approach consistently reported an EE of 95% or greater [150, 173]. This is a direct result of generating isolated emulsion droplets during microparticle or microcapsule synthesis where no drug is lost from the droplets. In the case of nano DDS synthesis, one study reported an EE of 21–45% for PLGA-PEG NPs fabricated in bulk (emulsification-solvent diffusion) while a microfluidics approach achieved an EE of 28–51% [146]. This could be explained by the effect of different mixing rates. When the mixing rate is slow, the time scale of NP assembly is smaller than the time scale of solvent diffusion. Thus in bulk mixing NP starts assembling when the solvent concentration is still high and results in drugs (e.g. docetaxel) escaping the encapsulation process. In the microfluidics approach the time scales of the two processes are comparable and hence more drugs can be encapsulated within the carrier. This advantage would be of particular importance when one deals with expensive drugs.
3.2.5. Control of drug release rate
While the successful demonstration of microfluidics-aided fabrication of different types of delivery vehicles is prevalent, there has been little effort devoted to systematically characterizing the drug release kinetics profile of the particles generated from a microfluidics versus the conventional approach. The study carried out by Q. Xu et. al. compared the kinetics of drug release of PLGA microparticles generated from the two approaches [157]. The monodisperse particles prepared using microfluidics release drug more slowly and have a smaller initial burst effect than those observed in conventional polydisperse particles. The authors attributed the effect to more uniform drug distribution in the microfluidics-produced particles; hence drug release by degradation of the former would be more gradual. Similarly, Karnik et. al. reported the microfluidic-aided self-assembled PLGA-PEG NPs had slower and smaller initial burst of drug release than those fabricated by conventional approaches [146]. Again rapid mixing pertaining to microfluidics approach may lead to a more uniform drug distribution inside the particles so that drug release is steadier by uniform degradation of the particles. These studies serve to justify the use of microfluidics over conventional approaches in controlling the drug release rate.
3.3. Future perspective: scale-up production and versatility
As we have seen, microfluidics platform shows promise to revolutionize particulate DDS synthesis. However, research development is merely the first milestone before the process can be translated into industry-scale manufacturing. In order for the technique to be widely adopted, two requirements must be satisfied: scalability and versatility. Industrial application of microfluidics platform for drug carrier synthesis requires a high throughput production system. Effort has been made to construct devices that support generation of particles/capsules at high rates [143, 185, 186]. Incorporation of parallel generators in a single chip is one way to achieve mass production. Up to 128 droplet generators can be fabricated in a 4 cm X 4 cm chip to produce a throughput of 0.3 kg/h of monodisperse acrylic microspheres [185]. As miniaturization is a key feature of microfluidics device, tens or hundreds of these chips, occupying far less space than conventional synthesis equipment, can be made to operate simultaneously to manufacture DDS.
The drug fabrication process has also to be versatile in such a way that DDS with diverse properties can be efficiently fabricated and sorted. Current microfluidics-based synthesis is largely passive, where the particle properties are tuned by changing the flow rates of two immiscible phases. One of the limitations is that any change at input has a long response time; for instance, transition from dripping to jetting in droplet production sets a limit on the frequency of generation [187]. Recently an automated active droplet production system was proposed to address the issue by using valves to externally control the infusion of two phases so that the droplet volume and volume of continuous phase separating each droplet can be precisely and sharply tuned [187]. This can potentially increase versatility of the microfluidics drug carrier synthesis platform as carrier properties can be properly adjusted. Throughput will suffer but likely be addressable in the future.
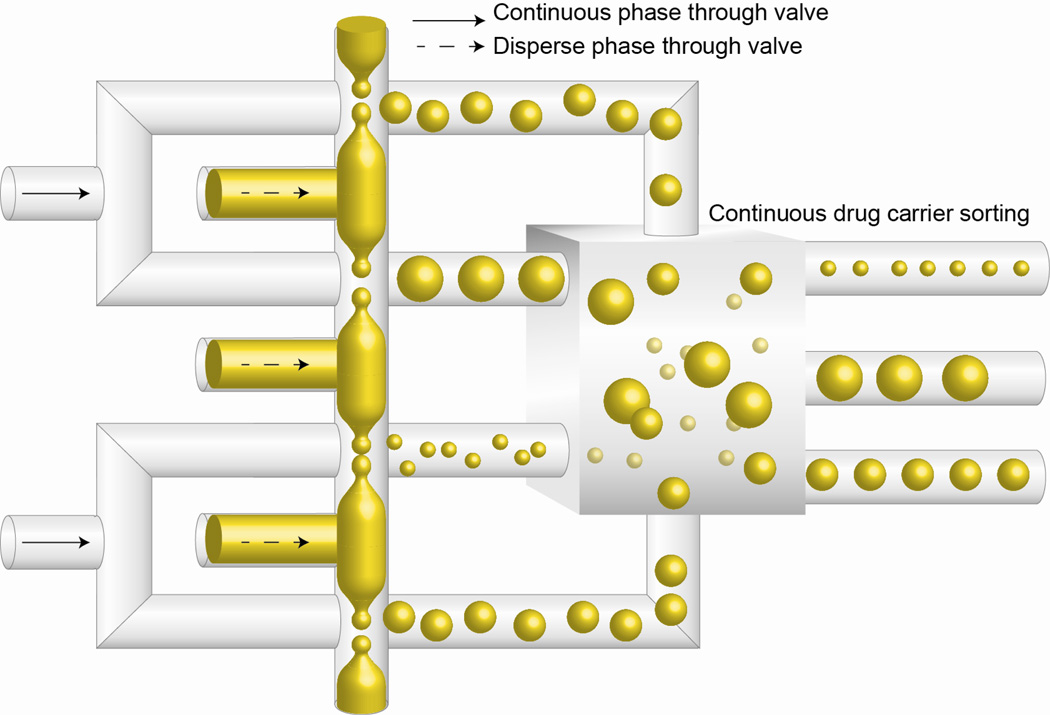
Following the synthesis of drug carriers varying in size or shell thickness, steps need to be taken to separate them according to their properties. One of the attractive features of microfluidics is the flexibility for multi-step processing; hence functions such as particle sorting can be incorporated into the system conveniently. In fact, continuous particle sorting based on size has been carried out in microfluidics using optical lattice [188], sedimentation [189], hydrodynamic filtration [190], and lateral displacement [191]. In these examples, mixture of particles of different sizes is channeled through the device, and subjected to physical force the magnitude of which depends on their size. Subsequently, the separate streams containing particles identical in size are collected in different reservoirs. Furthermore, the particle-sorting step can also be used as quality control, which ensures the process of synthesizing drugs on microfluidics platform is robust and reproducible by generating drug carriers with properties that are within an acceptable range. Even though properties including particle size, shell thickness, drug loading level are already tightly controlled in the microfluidics synthesis process, additional monitoring and screening steps would increase the value of the entire drug processing system. The integration of high throughput particle synthesis and sorting “on chip” can be seen as one of the greatest potential of microfluidics-aided drug carrier synthesis platform, and ultimately be adopted to advance DDS development (Figure 5).
Figure 5.
Potential platform for customized drug carrier synthesis and sorting. Mass production of microdroplets is achieved by incorporation of multiple droplet generators, with valves installed at the inlets of continuous and disperse phases to precisely control the size of particles. A continuous sorting component is included to sort particles according to their properties.
4. Top-down NP fabrication
The physical properties of DDS would affect drug delivery efficiency. While the influence of particle size on drug delivery efficiency has been well characterized, it is not until recently that the role of particle shape on drug delivery has been revealed. Rod-like structures demonstrate the highest cellular uptake efficiency, followed by spheres, cylinders and cubes [183, 184]. Shape-specific influence on particle circulation has also been discussed. Filamentous micelles have been shown to circulate 10 times longer than their spherical counterpart [192]. Nonetheless, the precise role of particle shape in drug delivery has not been elucidated due to the limited studies available. This is partly attributed to the lack of easy and robust methods in producing NPs with various shapes [193].
Early attempts to study the effect of particle shape on bioavailability have stretched spherical NPs into ellipsoidal particles [194]. Embedded in a PVA film, the spherical particles deform when the film is heated, stretched, and quenched. The heating temperature required to deform the particles is determined by the glass transition temperature of the polymeric carrier, which is around 90°C for PLGA and 140°C for polystyrene (PS) [195]. The high temperature greatly limits the type of bioactive agents that could be encapsulated in these deformed particles. One advantage of this method is that it produces non-spherical particles with exactly the same volume. Nonetheless, the process is highly material-specific and has only been demonstrated for PLGA and PS particles with very limited scalability [184].
Conventional NP synthesis typically relies on bottom-up approaches. As discussed earlier, the capacity to achieve large size differences and shape variation is greatly limited by the nature of the self-assembly process. In consequence, top-down methods that can produce particulate DDS with well-controlled size and shape are attractive.
4.1. Particle replication in non-wetting template (PRINT)
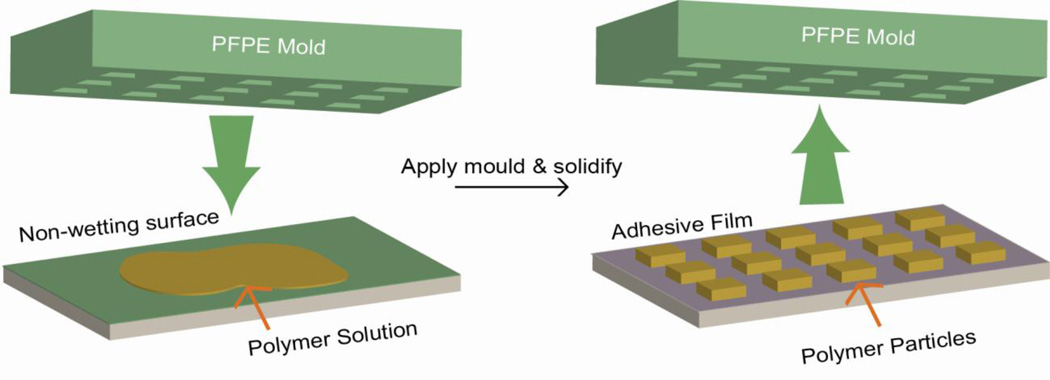
PRINT (Figure 6), first introduced in 2005, is a top-down technique to fabricate monodisperse particles with precise particle structures [196]. A non-wetting perfluoropolyether (PFPE) elastomeric mold containing wells or cavities of predefined shape and size is used to fabricate the particles. Polymer liquid solution containing the cargo is confined in the cavities by pressure applied between the mold and the PFPE surface, followed by crosslinking or solvent evaporation. The low surface energy of PFPE prevents the overflow of polymer solution to non-cavities region, leading to well-isolated NP formation. With this method, particles from 80 nm to 20 um have been fabricated with PLA, PEG hydrogels, proteins and stimuli-responsive polymers with disulfide [197] or silyl ether linkers [198]. Particles with various structures, such as circular disc, cube, rod, cone, and others have been made [196, 197, 199], with cargos ranging from chemotherapeutics [200] to imaging fluorophores [201]. With the PRINT technology, Gratton et. al. generated a library of PEG-based particles with a wide array of size and shape to study cellular uptake [183]. They also demonstrated that rod-like particles were more readily to be engulfed by Hela cells compared to particles of other structures.
Figure 6.
Particle Replication In Non-wetting Template (PRINT). A non-wetting PFPE mold with cavities of predesigned patterns is pressed against a polymer solution deposited on another non-wetting surface. The liquid polymer solution is then solidified by applying pressure or temperature. The solidified particles could be recovered from the mold by using an adhesive film.
4.2. Step-flash imprint lithography (S-FIL)
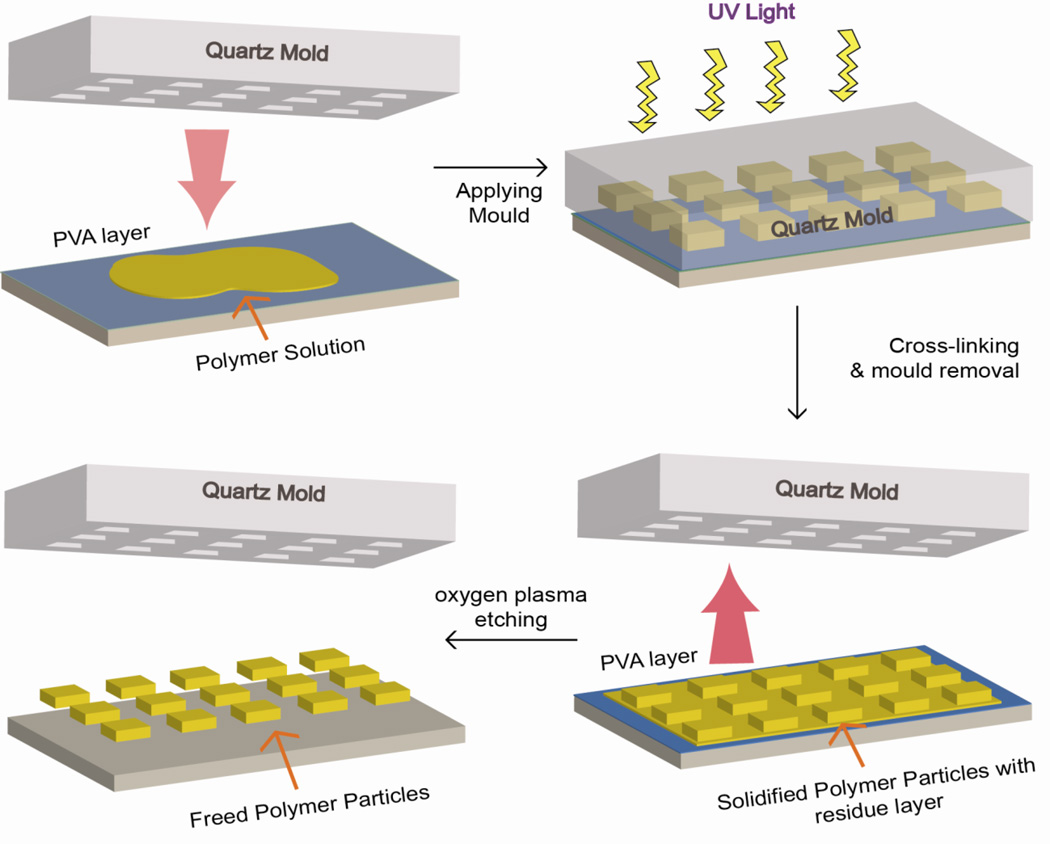
S-FIL (Figure 7) is a commercially available nanoimprint technique that uses a quartz template with predesigned patterns for particle synthesis. Polymer solution containing cross-linkable PEG-DA is added to the cavities of template and polymerized via UV light. A PVA layer is deposited beneath the polymer layer and on top of a silica wafer for the release of imprinted particles. Glangchai et. al. used this method to fabricate stimuli-responsive NPs of 50–400 nm and various shapes [202]. By incorporating an enzymatic degradable peptide GFLGK between PEG and diacrylate, the particles release plasmid DNA upon enzyme treatment in vitro. S-FIL relied on oxygen plasma treatment to release the NPs from PVA layer [202]. On one hand, it does not involve any mechanical stretch and maximally preserve the structure of NPs. On the other, oxygen plasma generates large quantity of reactive oxygen species and free radicals, which could damage biological materials such as DNA and protein and induces polymer degradation [203]. Furthermore, this method is restricted to photo-crosslinkable polymers and two dimensional shapes.
Figure 7.
Step-Flash Imprint Lithography (S-FIL). A quartz mold with cavities of predesigned shapes is pressed against a photo-crosslinkable monomer solution on top of a silica wafer. A PVA layer is put beneath the polymer solution for the release of imprinted particles. The monomers are cross linked by applying UV light. Residual layer from crosslinking reaction is removed by oxygen plasma etching and particles are freed by dissolving away the PVA layer.
4.3. Microfluidics-based top-down approaches
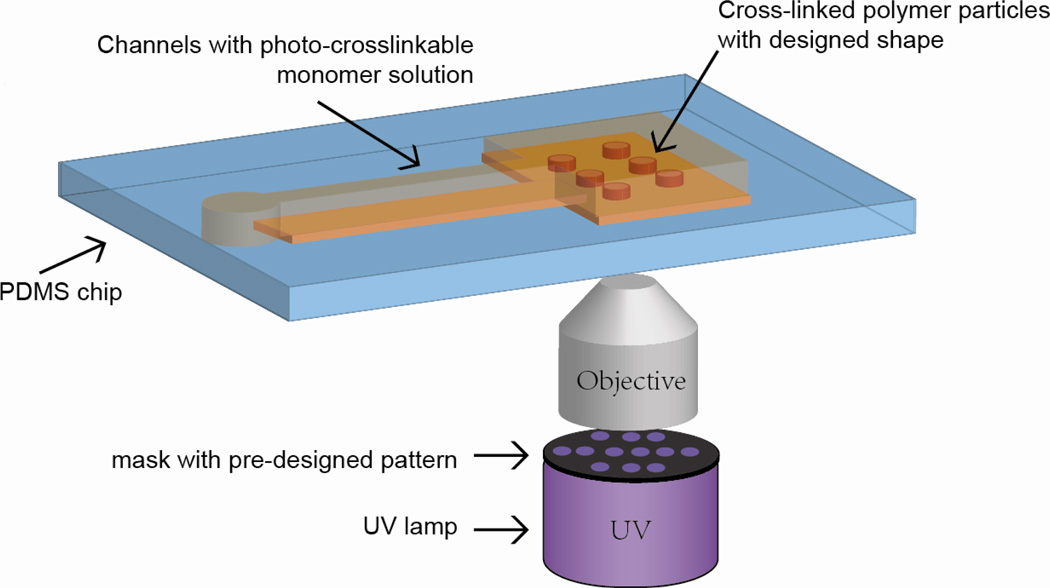
Dendukuri et. al. used continuous flow photolithography (CFL) (Figure 8) to fabricate particles of complex shapes [165]. A stream of PEGDA containing a photoinitiator is continuously channeled through a microfluidic device. A photomask with defined shapes is placed underneath the channel while pulses of UV light are applied. Particles of defined shapes are formed via cross-linking and flushed to the outlet for collection because of the continuous flow. This method eliminates the requirement of further harvesting steps and the particles could be purified by centrifugation and re-suspension in water. However, due to the continuous flow nature, the final shape is usually deformed from the designed structure, reducing geometrical resolution of the particles. To increase the resolution, the same group has developed stop flow lithography technique (SFL) [204, 205]. In SFL, a three-way solenoid valve is applied to control the flow inside the channels to stop briefly during each exposure for the polymerization to complete. Complex shapes with high resolution are formed, including toroid, hexongonal and worm-like structures. The high resolution and ease of harvesting and purification render this method a promising tool for top-down particle synthesis. However, it is also limited to photo-crosslinkable systems. In addition, solvent vulnerability of PDMS channels poses severe barrier for extension of the method to fabricate other particles. The dimensions of particles synthesized from this method are in the micrometer scale, which is primarily restricted by the resolution of current photolithography techniques.
Figure 8.
Continuous Flow Photolithography. A stream of photo-crosslinkable monomer solution continuously flows through the rectangular channel of microfluidic device. A photomask with defined patterns is placed underneath through which pulses of UV light are applied. Particles of defined shapes are formed via cross-linking reaction and are flushed out for collection.
5. Challenges and future perspectives
In summary, novel materials synthesis, introduction of environmental-sensitive polymers, successful adoption of natural polymers as carrier and improved understanding of the structure-function relationship have together transformed DDS development. The parallel advancement in bottom-up and top-down nanofabrication enables precise control over size and shape of particulate DDS. Moreover, the use of microfluidics in formulating DDS opens up the possibility for automation and scaling up, as well as on-chip particle screening and analysis.
However, many challenges remain. From the materials perspective, the majority of smart delivery systems works well in vitro but fails in more complicated in vivo environment. Furthermore, the challenge to integrate programmability, targetability, and environmental responsiveness remains formidable. From the fabrication perspective, conventional techniques have the advantage of easy scale-up, but lose accuracy in control over particle characteristics. Top-down approaches, in contrast, offer precise control over particle size and shape. However, up till now they are applicable to only a few systems, and with the exception of PRINT are difficult to scale up. Although microfluidics holds promise in addressing these issues, current lithographic techniques limit the manipulation of fluids in the micro-scale. In the context of nanomedicine “nanofluidics” would be desirable for DDS fabrication. When innovations in fabrication catch up with those of materials design, the goal for constructing and translating multifunctional, precisely controlled, and biocompatible DDS will be one step closer to reality.
Acknowledgements
This work is funded by NSF EEC-0425626, NIH HL089764 and HL109442. YZ and HFC are grateful for fellowship support from the Agency for Science, Technology and Research (Singapore) and the Sir Edward Youde Memorial Fund Council (Hong Kong), respectively.
Abbreviations
- CFL
continuous flow photolithography
- DDS
drug delivery system
- dex-HEMA
dextranhydroxyethyl methacrylate
- EE
encapsulation efficiency
- ELPs
elastomer-like proteins
- EPR
enhanced permeability effect
- GFP
green fluorescence protein
- HFR
heparin–folic acid–retinoic acid
- HPMA
N-(2-hydroxypropyl) methacrylamide
- IPA
isopropyl alcohol
- LBL
layer-bylayer
- NPs
nanoparticles
- O/W
oil-in-water
- PAA
poly(acrylic acid)
- PDMS
polydimethylsiloxane
- PEG
polyethylene glycol
- PEG-DA
poly(ethylene glycol) diacrylate
- PEG–PLA
poly(ethylene glycol)-b-polylactide
- PEI
poly(ethyleneimine)
- PEO-PPO-PEO
poly(propylene oxide)- poly(ethylene oxide)- poly(propylene oxide)
- PFPE
perfluoropolyether
- PGA
poly(glycol acid)/ poly (L-glutamic acid)
- PLA
poly(lactic acid)/polylactide
- PLGA
poly (lactide-co-glycolide)
- PMMA
polymethyl methacrylate
- pNIPAAM
poly(N-isopropylacrylamide)
particle replication in non-wetting template
- PS
polystyrene
- PTMC
poly(trimethylene carbonate)
- PVA
poly(vinyl alcohol)
- RAFT
reversible addition–fragmentation chain transfer
- RES
reticuloendothelial system
- S-FIL
step-flash imprint lithography
- SDS
sodium dodecyl sulphate
- SFL
stop flow lithography technique
- SLP
silk-like proteins
- W/O
water-in-oil
- W/O/W
water-in-oil-in-water
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 2.Verma RKG. Sanjay Current status of drug delivery technologies and future directions. Pharmaceutical technology on-line. 2001;25:4. [Google Scholar]
- 3.Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59:478–490. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Pornpattananangku D, Hu CM, Huang CM. Development of nanoparticles for antimicrobial drug delivery. Curr Med Chem. 2010;17:585–594. doi: 10.2174/092986710790416290. [DOI] [PubMed] [Google Scholar]
- 5.Schipper ML, Iyer G, Koh AL, Cheng Z, Ebenstein Y, Aharoni A, Keren S, Bentolila LA, Li J, Rao J, Chen X, Banin U, Wu AM, Sinclair R, Weiss S, Gambhir SS. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small. 2009;5:126–134. doi: 10.1002/smll.200800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrstensen H, Muller RH, Muller BW. Particle size, surface hydrophobicity and interaction with serum of parenteral fat emulsions and model drug carriers as parameters related to RES uptake. Clin Nutr. 1992;11:289–297. doi: 10.1016/0261-5614(92)90006-c. [DOI] [PubMed] [Google Scholar]
- 7.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 8.Sajja HK, East MP, Mao H, Wang YA, Nie S, Yang L. Development of multifunctional nanoparticles for targeted drug delivery and noninvasive imaging of therapeutic effect. Curr Drug Discov Technol. 2009;6:43–51. doi: 10.2174/157016309787581066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain R, Shah NH, Malick AW, Rhodes CT. Controlled drug delivery by biodegradable poly(ester) devices: different preparative approaches. Drug Dev Ind Pharm. 1998;24:703–727. doi: 10.3109/03639049809082719. [DOI] [PubMed] [Google Scholar]
- 10.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-coglycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 11.Yoo HS, Lee KH, Oh JE, Park TG. In vitro and in vivo anti-tumor activities of nanoparticles based on doxorubicin-PLGA conjugates. J Control Release. 2000;68:419–431. doi: 10.1016/s0168-3659(00)00280-7. [DOI] [PubMed] [Google Scholar]
- 12.Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Karydas AG, Ithakissios DS. PLGA-mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J Control Release. 2002;79:123–135. doi: 10.1016/s0168-3659(01)00530-2. [DOI] [PubMed] [Google Scholar]
- 13.Fontana G, Licciardi M, Mansueto S, Schillaci D, Giammona G. Amoxicillin-loaded polyethylcyanoacrylate nanoparticles: influence of PEG coating on the particle size, drug release rate and phagocytic uptake. Biomaterials. 2001;22:2857–2865. doi: 10.1016/s0142-9612(01)00030-8. [DOI] [PubMed] [Google Scholar]
- 14.Suh H, Jeong B, Rathi R, Kim SW. Regulation of smooth muscle cell proliferation using paclitaxelloaded poly(ethylene oxide)-poly(lactide/glycolide) nanospheres. J Biomed Mater Res. 1998;42:331–338. doi: 10.1002/(sici)1097-4636(199811)42:2<331::aid-jbm19>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Carino GP, Jacob JS, Mathiowitz E. Nanosphere based oral insulin delivery. J Control Release. 2000;65:261–269. doi: 10.1016/s0168-3659(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 16.Panyam J, Dali MM, Sahoo SK, Ma W, Chakravarthi SS, Amidon GL, Levy RJ, Labhasetwar V. Polymer degradation and in vitro release of a model protein from poly(D,L-lactide-co-glycolide) nanoand microparticles. Journal of Controlled Release. 2003;92:173–187. doi: 10.1016/s0168-3659(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 17.van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm Res. 2000;17:1159–1167. doi: 10.1023/a:1026498209874. [DOI] [PubMed] [Google Scholar]
- 18.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. Faseb J. 2002;16:1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 19.Prabha S, Labhasetwar V. Critical determinants in PLGA/PLA nanoparticle-mediated gene expression. Pharm Res. 2004;21:354–364. doi: 10.1023/b:pham.0000016250.56402.99. [DOI] [PubMed] [Google Scholar]
- 20.Elamanchili P, Lutsiak CM, Hamdy S, Diwan M. Samuel, "Pathogen-mimicking" nanoparticles for vaccine delivery to dendritic cells. J Immunother. 2007;30:378–395. doi: 10.1097/CJI.0b013e31802cf3e3. [DOI] [PubMed] [Google Scholar]
- 21.Lu JM, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, Chen C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;9:325–341. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faisant N, Siepmann J, Benoit JP. PLGA-based microparticles: elucidation of mechanisms and a new, simple mathematical model quantifying drug release. Eur J Pharm Sci. 2002;15:355–366. doi: 10.1016/s0928-0987(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 23.Crotts G, Park TG. Protein delivery from poly(lactic-co-glycolic acid) biodegradable microspheres: release kinetics and stability issues. J Microencapsul. 1998;15:699–713. doi: 10.3109/02652049809008253. [DOI] [PubMed] [Google Scholar]
- 24.Kranz H, Ubrich N, Maincent P, Bodmeier R. Physicomechanical properties of biodegradable poly(D,L-lactide) and poly(D,L-lactide-co-glycolide) films in the dry and wet states. J Pharm Sci. 2000;89:1558–1566. doi: 10.1002/1520-6017(200012)89:12<1558::aid-jps6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Shirahama H, Ichimaru A, Tsutsumi C, Nakayama Y, Yasuda H. Characteristics of the biodegradability and physical properties of stereocomplexes between poly(L-lactide) and poly(D-lactide) copolymers. Journal of Polymer Science Part A: Polymer Chemistry. 2005;43:438–454. [Google Scholar]
- 26.Tsuji H. Poly(lactide) stereocomplexes: formation, structure, properties, degradation, and applications. Macromol Biosci. 2005;5:569–597. doi: 10.1002/mabi.200500062. [DOI] [PubMed] [Google Scholar]
- 27.Tan JP, Kim SH, Nederberg F, Appel EA, Waymouth RM, Zhang Y, Hedrick JL, Yang YY. Hierarchical supermolecular structures for sustained drug release. Small. 2009;5:1504–1507. doi: 10.1002/smll.200801756. [DOI] [PubMed] [Google Scholar]
- 28.Lawson HC, Sampath P, Bohan E, Park MC, Hussain N, Olivi A, Weingart J, Kleinberg L, Brem H. Interstitial chemotherapy for malignant gliomas: the Johns Hopkins experience. Journal of neurooncology. 2007;83:61–70. doi: 10.1007/s11060-006-9303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bock HC, Puchner MJ, Lohmann F, Schutze M, Koll S, Ketter R, Buchalla R, Rainov N, Kantelhardt SR, Rohde V, Giese A. First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurgical review. 2010;33:441–449. doi: 10.1007/s10143-010-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Z, Wang J, Mao HQ, Leong KW. Polyphosphoesters in drug and gene delivery. Adv Drug Deliv Rev. 2003;55:483–499. doi: 10.1016/s0169-409x(03)00040-1. [DOI] [PubMed] [Google Scholar]
- 31.Wang YC, Yuan YY, Du JZ, Yang XZ, Wang J. Recent progress in polyphosphoesters: from controlled synthesis to biomedical applications. Macromol Biosci. 2009;9:1154–1164. doi: 10.1002/mabi.200900253. [DOI] [PubMed] [Google Scholar]
- 32.Bourke SL, Kohn J. Polymers derived from the amino acid L-tyrosine: polycarbonates, polyarylates and copolymers with poly(ethylene glycol) Adv Drug Deliv Rev. 2003;55:447–466. doi: 10.1016/s0169-409x(03)00038-3. [DOI] [PubMed] [Google Scholar]
- 33.Karp JM, Langer R. Development and therapeutic applications of advanced biomaterials. Current opinion in biotechnology. 2007;18:454–459. doi: 10.1016/j.copbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman AS. The origins and evolution of "controlled" drug delivery systems. Journal of controlled release : official journal of the Controlled Release Society. 2008;132:153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Gong J, Chen M, Zheng Y, Wang S, Wang Y. Polymeric micelles drug delivery system in oncology. Journal of controlled release : official journal of the Controlled Release Society. 2012;159:312–323. doi: 10.1016/j.jconrel.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Harada A, Kataoka K. Formation of Polyion Complex Micelles in an Aqueous Milieu from a Pair of Oppositely-Charged Block-Copolymers with Poly(Ethylene Glycol) Segments. Macromolecules. 1995;28:5294–5299. [Google Scholar]
- 38.Lavasanifar A, Samuel J, Sattari S, Kwon GS. Block copolymer micelles for the encapsulation and delivery of amphotericin B. Pharm Res. 2002;19:418–422. doi: 10.1023/a:1015127225021. [DOI] [PubMed] [Google Scholar]
- 39.Falamarzian A, Lavasanifar A. Chemical modification of hydrophobic block in poly(ethylene oxide) poly(caprolactone) based nanocarriers: effect on the solubilization and hemolytic activity of amphotericin B. Macromol Biosci. 2010;10:648–656. doi: 10.1002/mabi.200900387. [DOI] [PubMed] [Google Scholar]
- 40.Falamarzian A, Lavasanifar A. Optimization of the hydrophobic domain in poly(ethylene oxide)- poly(varepsilon-caprolactone) based nano-carriers for the solubilization and delivery of Amphotericin B. Colloids Surf B Biointerfaces. 2010;81:313–320. doi: 10.1016/j.colsurfb.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 41.Kim SH, Tan JP, Nederberg F, Fukushima K, Colson J, Yang C, Nelson A, Yang YY, Hedrick JL. Hydrogen bonding-enhanced micelle assemblies for drug delivery. Biomaterials. 2010;31:8063–8071. doi: 10.1016/j.biomaterials.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 42.Iijima M, Nagasaki Y, Okada T, Kato M, Kataoka K. Core-polymerized reactive micelles from heterotelechelic amphiphilic block copolymers. Macromolecules. 1999;32:1140–1146. [Google Scholar]
- 43.Kim JH, Emoto K, Iijima M, Nagasaki Y, Aoyagi T, Okano T, Sakurai Y, Kataoka K. Core-stabilized polymeric micelle as potential drug carrier: Increased solubilization of taxol. Polym Advan Technol. 1999;10:647–654. [Google Scholar]
- 44.Kakizawa Y, Harada A, Kataoka K. Environment-sensitive stabilization of core-shell structured polyion complex micelle by reversible cross-linking of the core through disulfide bond. J Am Chem Soc. 1999;121:11247–11248. [Google Scholar]
- 45.Nagarajan R, Barry M. Unusual Selectivity in Solubilization by Block Copolymer Micelles. Abstr Pap Am Chem S. 1986;191 287-COLL. [Google Scholar]
- 46.Shuai X, Ai H, Nasongkla N, Kim S, Gao J. Micellar carriers based on block copolymers of poly(epsilon-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J Control Release. 2004;98:415–426. doi: 10.1016/j.jconrel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Gao S, Ye WH, Yoon HS, Yang YY. Co-delivery of drugs and DNA from cationic coreshell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater. 2006;5:791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 48.Sun TM, Du JZ, Yao YD, Mao CQ, Dou S, Huang SY, Zhang PZ, Leong KW, Song EW, Wang J. Simultaneous delivery of siRNA and paclitaxel via a "two-in-one" micelleplex promotes synergistic tumor suppression. Acs Nano. 2011;5:1483–1494. doi: 10.1021/nn103349h. [DOI] [PubMed] [Google Scholar]
- 49.Kaneshiro TL, Lu ZR. Targeted intracellular codelivery of chemotherapeutics and nucleic acid with a well-defined dendrimer-based nanoglobular carrier. Biomaterials. 2009;30:5660–5666. doi: 10.1016/j.biomaterials.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 50.Zhu C, Jung S, Luo S, Meng F, Zhu X, Park TG, Zhong Z. Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA-PCL-PDMAEMA triblock copolymers. Biomaterials. 2010;31:2408–2416. doi: 10.1016/j.biomaterials.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 51.Xiong XB, Lavasanifar A. Traceable multifunctional micellar nanocarriers for cancer-targeted codelivery of MDR-1 siRNA and doxorubicin. Acs Nano. 2011;5:5202–5213. doi: 10.1021/nn2013707. [DOI] [PubMed] [Google Scholar]
- 52.Fan L, Li F, Zhang H, Wang Y, Cheng C, Li X, Gu CH, Yang Q, Wu H, Zhang S. Co-delivery of PDTC and doxorubicin by multifunctional micellar nanoparticles to achieve active targeted drug delivery and overcome multidrug resistance. Biomaterials. 2010;31:5634–5642. doi: 10.1016/j.biomaterials.2010.03.066. [DOI] [PubMed] [Google Scholar]
- 53.Wiradharma N, Zhang Y, Venkataraman S, Hedrick JL, Yang YY. Self-assembled polymer nanostructures for delivery of anticancer therapeutics. Nano Today. 2009;4:302–317. [Google Scholar]
- 54.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 55.Vicent MJ, Ringsdorf H, Duncan R. Polymer therapeutics: Clinical applications and challenges for development Preface. Adv Drug Deliver Rev. 2009;61:1117–1120. doi: 10.1016/j.addr.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Kopecek J, Bazilova H. Poly[N-(2-Hydroxypropyl)Methacrylamide].1. Radical Polymerization and Copolymerization. Eur Polym J. 1973;9:7–14. [Google Scholar]
- 57.Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, Thomson AH, Murray LS, Hilditch TE, Murray T, Burtles S, Fraier D, Frigerio E, Cassidy J. C.R.C.P.I.I. Comm, Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: First member of a new class of chemotherapeutic agents - Drug-polymer conjugates. Clin Cancer Res. 1999;5:83–94. [PubMed] [Google Scholar]
- 58.Li C, Yu DF, Newman RA, Cabral F, Stephens LC, Hunter N, Milas L, Wallace S. Complete regression of well-established tumors using a novel water-soluble poly(L-glutamic acid) paclitaxel conjugate. Cancer Res. 1998;58:2404–2409. [PubMed] [Google Scholar]
- 59.Singer JW, Baker B, De Vries P, Kumar A, Shaffer S, Vawter E, Bolton M, Garzone P. Poly-(L)- glutamic acid-paclitaxel (CT-2103) [XYOTAX (TM)], a biodegradable polymeric drug conjugate - Characterization, preclinical pharmacology, and preliminary clinical data. Polymer Drugs in the Clinical Stage: Advantages and Prospects. 2003;519:81–99. doi: 10.1007/0-306-47932-X_6. [DOI] [PubMed] [Google Scholar]
- 60.Bhatt R, de Vries P, Tulinsky J, Bellamy G, Baker B, Singer JW, Klein P. Synthesis and in vivo antitumor activity of poly(l-glutamic acid) conjugates of 20S-camptothecin. J Med Chem. 2003;46:190–193. doi: 10.1021/jm020022r. [DOI] [PubMed] [Google Scholar]
- 61.Springett GM, Takimoto C, McNamara M, Doroshow JH, Syed S, Eastham E, Spriggs D, Pezzulli S, Michelson G, Dupont J. Phase I study of CT-2106 (polyglutamate camptothecin) in patients with advanced malignancies. Journal of Clinical Oncology. 2004;22:226s–226s. [Google Scholar]
- 62.Langer CJ, O'Byrne KJ, Socinski MA, Mikhailov SM, Lesniewski-Kmak K, Smakal M, Ciuleanu TE, Orlov SV, Dediu M, Heigener D, Eisenfeld AJ, Sandalic L, Oldham FB, Singer JW, Ross HJ. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. J Thorac Oncol. 2008;3:623–630. doi: 10.1097/JTO.0b013e3181753b4b. [DOI] [PubMed] [Google Scholar]
- 63.Paz-Ares L, Ross H, O'Brien M, Riviere A, Gatzemeier U, Von Pawel J, Kaukel E, Freitag L, Digel W, Bischoff H, Garcia-Campelo R, Iannotti N, Reiterer P, Bover I, Prendiville J, Eisenfeld AJ, Oldham FB, Bandstra B, Singer JW, Bonomi P. Phase III trial comparing paclitaxel poliglumex vs docetaxel in the second-line treatment of non-small-cell lung cancer. Brit J Cancer. 2008;98:1608–1613. doi: 10.1038/sj.bjc.6604372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Canal F, Sanchis J, Vicent MJ. Polymer-drug conjugates as nano-sized medicines. Current opinion in biotechnology. 2011;22:894–900. doi: 10.1016/j.copbio.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Tong R, Cheng JJ. Paclitaxel-initiated, controlled polymerization of lactide for the formulation of polymeric nanoparticulate delivery vehicles. Angew Chem Int Edit. 2008;47:4830–4834. doi: 10.1002/anie.200800491. [DOI] [PubMed] [Google Scholar]
- 66.Tong R, Cheng JJ. Ring-Opening Polymerization-Mediated Controlled Formulation of Polylactide- Drug Nanoparticles. J Am Chem Soc. 2009;131:4744–4754. doi: 10.1021/ja8084675. [DOI] [PubMed] [Google Scholar]
- 67.Azzi J, Tang L, Moore R, Tong R, El Haddad N, Akiyoshi T, Mfarrej B, Yang SM, Jurewicz M, Ichimura T, Lindeman N, Cheng JJ, Abdi R. Polylactide-cyclosporin A nanoparticles for targeted immunosuppression. Faseb J. 2010;24:3927–3938. doi: 10.1096/fj.10-154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen KJ, Tang L, Garcia MA, Wang H, Lu H, Lin WY, Hou S, Yin Q, Shen CKF, Cheng JJ, Tseng HR. The therapeutic efficacy of camptothecin-encapsulated supramolecular nanoparticles. Biomaterials. 2012;33:1162–1169. doi: 10.1016/j.biomaterials.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams CC, Thang SH, Hantke T, Vogel U, Seeberger PH, Tsanaktsidis J, Lepenies B. RAFT-Derived Polymer-Drug Conjugates: Poly(hydroxypropyl methacrylamide) (HPMA)-7-Ethyl-10- hydroxycamptothecin (SN-38) Conjugates. ChemMedChem. 2012;7:281–291. doi: 10.1002/cmdc.201100456. [DOI] [PubMed] [Google Scholar]
- 70.Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliver Rev. 2008;60:1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Felt O, Buri P, Gurny R. Chitosan: a unique polysaccharide for drug delivery. Drug Dev Ind Pharm. 1998;24:979–993. doi: 10.3109/03639049809089942. [DOI] [PubMed] [Google Scholar]
- 72.Friden PM, Walus LR, Musso GF, Taylor MA, Malfroy B, Starzyk RM. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc Natl Acad Sci U S A. 1991;88:4771–4775. doi: 10.1073/pnas.88.11.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li H, Qian ZM. Transferrin/transferrin receptor-mediated drug delivery. Med Res Rev. 2002;22:225–250. doi: 10.1002/med.10008. [DOI] [PubMed] [Google Scholar]
- 74.Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 75.Huang YF, Shangguan D, Liu H, Phillips JA, Zhang X, Chen Y, Tan W. Molecular assembly of an aptamer-drug conjugate for targeted drug delivery to tumor cells. Chembiochem. 2009;10:862–868. doi: 10.1002/cbic.200800805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bagalkot V, Farokhzad OC, Langer R, Jon S. An Aptamer-Doxorubicin Physical Conjugate as a Novel Targeted Drug-Delivery Platform. Angewandte Chemie International Edition. 2006;45:8149–8152. doi: 10.1002/anie.200602251. [DOI] [PubMed] [Google Scholar]
- 77.Li THLH, Leong KW. Nucleic acid-based nanoengineering: novel structures for biomedical applications. Interface Focus. 2011;1:702–724. doi: 10.1098/rsfs.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ko S, Liu H, Chen Y, Mao C. DNA nanotubes as combinatorial vehicles for cellular delivery. Biomacromolecules. 2008;9:3039–3043. doi: 10.1021/bm800479e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilner OI, Orbach R, Henning A, Teller C, Yehezkeli O, Mertig M, Harries D, Willner I. Selfassembly of DNA nanotubes with controllable diameters. Nat Commun. 2011;2:540. doi: 10.1038/ncomms1535. [DOI] [PubMed] [Google Scholar]
- 80.Andersen ES, Dong M, Nielsen MM, Jahn K, Subramani R, Mamdouh W, Golas MM, Sander B, Stark H, Oliveira CL, Pedersen JS, Birkedal V, Besenbacher F, Gothelf KV, Kjems J. Self-assembly of a nanoscale DNA box with a controllable lid. Nature. 2009;459:73–76. doi: 10.1038/nature07971. [DOI] [PubMed] [Google Scholar]
- 81.Roh YH, Lee JB, Kiatwuthinon P, Hartman MR, Cha JJ, Um SH, Muller DA, Luo D. DNAsomes: Multifunctional DNA-based nanocarriers. Small. 2011;7:74–78. doi: 10.1002/smll.201000752. [DOI] [PubMed] [Google Scholar]
- 82.Li J, Pei H, Zhu B, Liang L, Wei M, He Y, Chen N, Li D, Huang Q, Fan C. Self-assembled multivalent DNA nanostructures for noninvasive intracellular delivery of immunostimulatory CpG oligonucleotides. Acs Nano. 2011;5:8783–8789. doi: 10.1021/nn202774x. [DOI] [PubMed] [Google Scholar]
- 83.Afonin KA, Grabow WW, Walker FM, Bindewald E, Dobrovolskaia MA, Shapiro BA, Jaeger L. Design and self-assembly of siRNA-functionalized RNA nanoparticles for use in automated nanomedicine. Nat Protoc. 2011;6:2022–2034. doi: 10.1038/nprot.2011.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alahari SK, Dean NM, Fisher MH, Delong R, Manoharan M, Tivel KL, Juliano RL. Inhibition of expression of the multidrug resistance-associated P-glycoprotein of by phosphorothioate and 5' cholesterol-conjugated phosphorothioate antisense oligonucleotides. Mol Pharmacol. 1996;50:808–819. [PubMed] [Google Scholar]
- 85.Jeong JH, Mok H, Oh YK, Park TG. siRNA conjugate delivery systems. Bioconjug Chem. 2009;20:5–14. doi: 10.1021/bc800278e. [DOI] [PubMed] [Google Scholar]
- 86.Cronican JJ, Thompson DB, Beier KT, McNaughton BR, Cepko CL, Liu DR. Potent Delivery of Functional Proteins into Mammalian Cells in Vitro and in Vivo Using a Supercharged Protein. ACS Chemical Biology. 2010;5:747–752. doi: 10.1021/cb1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McNaughton BR, Cronican JJ, Thompson DB, Liu DR. Mammalian cell penetration, siRNA transfection, and DNA transfection by supercharged proteins. Proc Natl Acad Sci U S A. 2009;106:6111–6116. doi: 10.1073/pnas.0807883106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frandsen JL, Ghandehari H. Recombinant protein-based polymers for advanced drug delivery. Chemical Society Reviews. 2012;41:2696–2706. doi: 10.1039/c2cs15303c. [DOI] [PubMed] [Google Scholar]
- 89.MacKay JA, Chen M, McDaniel JR, Liu W, Simnick AJ, Chilkoti A. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat Mater. 2009;8:993–999. doi: 10.1038/nmat2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meyer DE, Shin BC, Kong GA, Dewhirst MW, Chilkoti A. Drug targeting using thermally responsive polymers and local hyperthermia. J Control Release. 2001;74:213–224. doi: 10.1016/s0168-3659(01)00319-4. [DOI] [PubMed] [Google Scholar]
- 91.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Chilkoti A. Thermal cycling enhances the accumulation of a temperature-sensitive biopolymer in solid tumors. Cancer Res. 2007;67:4418–4424. doi: 10.1158/0008-5472.CAN-06-4444. [DOI] [PubMed] [Google Scholar]
- 92.Dreher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, Chilkoti A. Temperature triggered self-assembly of polypeptides into multivalent spherical micelles. J Am Chem Soc. 2008;130:687–694. doi: 10.1021/ja0764862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sallach RE, Cui W, Wen J, Martinez A, Conticello VP, Chaikof EL. Elastin-mimetic protein polymers capable of physical and chemical crosslinking. Biomaterials. 2009;30:409–422. doi: 10.1016/j.biomaterials.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim W, Chaikof EL. Recombinant elastin-mimetic biomaterials: Emerging applications in medicine. Adv Drug Deliv Rev. 2010;62:1468–1478. doi: 10.1016/j.addr.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jordan SW, Haller CA, Sallach RE, Apkarian RP, Hanson SR, Chaikof EL. The effect of a recombinant elastin-mimetic coating of an ePTFE prosthesis on acute thrombogenicity in a baboon arteriovenous shunt. Biomaterials. 2007;28:1191–1197. doi: 10.1016/j.biomaterials.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 96.Wu X, Sallach RE, Caves JM, Conticello VP, Chaikof EL. Deformation responses of a physically cross-linked high molecular weight elastin-like protein polymer. Biomacromolecules. 2008;9:1787–1794. doi: 10.1021/bm800012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Numata K, Subramanian B, Currie HA, Kaplan DL. Bioengineered silk protein-based gene delivery systems. Biomaterials. 2009;30:5775–5784. doi: 10.1016/j.biomaterials.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Numata K, Kaplan DL. Silk-based delivery systems of bioactive molecules. Adv Drug Deliv Rev. 2010;62:1497–1508. doi: 10.1016/j.addr.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Numata K, Kaplan DL. Silk-Based Gene Carriers with Cell Membrane Destabilizing Peptides. Biomacromolecules. 2010 doi: 10.1021/bm101055m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim S, Kim JH, Jeon O, Kwon IC, Park K. Engineered polymers for advanced drug delivery. Eur J Pharm Biopharm. 2009;71:420–430. doi: 10.1016/j.ejpb.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He J, Qi X, Miao Y, Wu HL, He N, Zhu JJ. Application of smart nanostructures in medicine. Nanomedicine (Lond) 2010;5:1129–1138. doi: 10.2217/nnm.10.81. [DOI] [PubMed] [Google Scholar]
- 102.Tarahovsky YS. "Smart" liposomal nanocontainers in biology and medicine. Biochemistry (Mosc) 2010;75:811–824. doi: 10.1134/s0006297910070023. [DOI] [PubMed] [Google Scholar]
- 103.MacEwan SR, Callahan DJ, Chilkoti A. Stimulus-responsive macromolecules and nanoparticles for cancer drug delivery. Nanomedicine (Lond) 2010;5:793–806. doi: 10.2217/nnm.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 105.Brocchini S, James K, Tangpasuthadol V, Kohn J. A combinatorial approach for polymer design. J Am Chem Soc. 1997;119:4553–4554. [Google Scholar]
- 106.Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm. 2010;385:113–142. doi: 10.1016/j.ijpharm.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 107.Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DW, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A. 2010;107:1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 109.Vauthier C, Bouchemal K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm Res. 2009;26:1025–1058. doi: 10.1007/s11095-008-9800-3. [DOI] [PubMed] [Google Scholar]
- 110.Ganachaud F, Katz JL. Nanoparticles and nanocapsules created using the Ouzo effect: Spontaneous emulsification as an alternative to ultrasonic and high-shear devices. Chemphyschem. 2005;6:209–216. doi: 10.1002/cphc.200400527. [DOI] [PubMed] [Google Scholar]
- 111.Duclairoir C, Nakache E, Marchais H, Orecchioni AM. Formation of gliadin nanoparticles: Influence of the solubility parameter of the protein solvent. Colloid Polym Sci. 1998;276:321–327. [Google Scholar]
- 112.Skiba M, Wouessidjewe D, Puisieux F, Duchene D, Gulik A. Characterization of amphiphilic betacyclodextrin nanospheres. Int J Pharmaceut. 1996;142:121–124. [Google Scholar]
- 113.McGinity JW, O'Donnell PB. Preparation of microspheres by the solvent evaporation technique. Adv Drug Deliv Rev. 1997;28:25–42. doi: 10.1016/s0169-409x(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 114.Desgouilles S, Vauthier C, Bazile D, Vacus J, Grossiord J-L, Veillard M, Couvreur P. The Design of Nanoparticles Obtained by Solvent Evaporation: A Comprehensive Study. Langmuir. 2003;19:9504–9510. [Google Scholar]
- 115.Lemoine D, Preat V. Polymeric nanoparticles as delivery system for influenza virus glycoproteins. J Control Release. 1998;54:15–27. doi: 10.1016/s0168-3659(97)00241-1. [DOI] [PubMed] [Google Scholar]
- 116.Yang YY, Chung TS, Ng NP. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials. 2001;22:231–241. doi: 10.1016/s0142-9612(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 117.Bilati U, Allemann E, Doelker E. Sonication parameters for the preparation of biodegradable nanocapsules of controlled size by the double emulsion method. Pharm Dev Technol. 2003;8:1–9. doi: 10.1081/pdt-120017517. [DOI] [PubMed] [Google Scholar]
- 118.Leroux JC, Allemann E, Doelker E, Gurny R. New Approach for the Preparation of Nanoparticles by an Emulsification-Diffusion Method. European Journal of Pharmaceutics and Biopharmaceutics. 1995;41:14–18. [Google Scholar]
- 119.Moinard-Checot D, Chevalier Y, Briancon S, Fessi H, Guinebretiere S. Nanoparticles for drug delivery: Review of the formulation and process difficulties illustrated by the emulsion-diffusion process. J Nanosci Nanotechno. 2006;6:2664–2681. doi: 10.1166/jnn.2006.479. [DOI] [PubMed] [Google Scholar]
- 120.Allémann E, Gurny R, Doelker E. Preparation of aqueous polymeric nanodispersions by a reversible salting-out process: influence of process parameters on particle size. Int J Pharmaceut. 1992;87:247–253. [Google Scholar]
- 121.Zhang Z, Grijpma DW, Feijen J. Poly(trimethylene carbonate) and monomethoxy poly(ethylene glycol)-block-poly(trimethylene carbonate) nanoparticles for the controlled release of dexamethasone. J Control Release. 2006;111:263–270. doi: 10.1016/j.jconrel.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 122.Krol S, Diaspro A, Cavalleri O, Cavanna D, Ballario P, Grimaldi B, Filetici P, Ornaghi P, Gliozzi A. Nanocapsule - A novel tool for medicine and science. In: Buzaneva E, Scharff P, editors. Frontiers of multifunctional integrated nanosytems. the Netherlands: Academic Publishers; 2004. pp. 439–446. [Google Scholar]
- 123.Agarwal A, Lvov Y, Sawant R, Torchilin V. Stable nanocolloids of poorly soluble drugs with high drug content prepared using the combination of sonication and layer-by-layer technology. J Control Release. 2008;128:255–260. doi: 10.1016/j.jconrel.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 124.Dai Z, Heilig A, Zastrow H, Donath E, Mohwald H. Novel formulations of vitamins and insulin by nanoengineering of polyelectrolyte multilayers around microcrystals. Chemistry. 2004;10:6369–6374. doi: 10.1002/chem.200400579. [DOI] [PubMed] [Google Scholar]
- 125.Palankar R, Skirtach AG, Kreft O, Bedard M, Garstka M, Gould K, Mohwald H, Sukhorukov GB, Winterhalter M, Springer S. Controlled intracellular release of peptides from microcapsules enhances antigen presentation on MHC class I molecules. Small. 2009;5:2168–2176. doi: 10.1002/smll.200900809. [DOI] [PubMed] [Google Scholar]
- 126.Aouadi M, Tesz GJ, Nicoloro SM, Wang M, Chouinard M, Soto E, Ostroff GR, Czech MP. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.DeMello AJ. Control and detection of chemical reactions in microfluidic systems. Nature. 2006;442:394–402. doi: 10.1038/nature05062. [DOI] [PubMed] [Google Scholar]
- 128.Duncanson WJ, Lin T, Abate AR, Seiffert S, Shah RK, Weitz DA. Microfluidic synthesis of advanced microparticles for encapsulation and controlled release. Lab on a chip. 2012 doi: 10.1039/c2lc21164e. [DOI] [PubMed] [Google Scholar]
- 129.Lensen D, van Breukelen K, Vriezema DM, van Hest JC. Preparation of biodegradable liquid core PLLA microcapsules and hollow PLLA microcapsules using microfluidics. Macromol Biosci. 2010;10:475–480. doi: 10.1002/mabi.200900404. [DOI] [PubMed] [Google Scholar]
- 130.Okochi H, Nakano M. Preparation and evaluation of w/o/w type emulsions containing vancomycin. Adv Drug Deliv Rev. 2000;45:5–26. doi: 10.1016/s0169-409x(00)00097-1. [DOI] [PubMed] [Google Scholar]
- 131.Utada AS, Lorenceau E, Link DR, Kaplan PD, Stone HA, Weitz DA. Monodisperse double emulsions generated from a microcapillary device. Science. 2005;308:537–541. doi: 10.1126/science.1109164. [DOI] [PubMed] [Google Scholar]
- 132.Endres T, Zheng M, Beck-Broichsitter M, Samsonova O, Debus H, Kissel T. Optimising the selfassembly of siRNA loaded PEG-PCL-lPEI nano-carriers employing different preparation techniques. Journal of controlled release : official journal of the Controlled Release Society. 2012 doi: 10.1016/j.jconrel.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 133.Kim JW, Utada AS, Fernandez-Nieves A, Hu Z, Weitz DA. Fabrication of monodisperse gel shells and functional microgels in microfluidic devices. Angew Chem Int Ed Engl. 2007;46:1819–1822. doi: 10.1002/anie.200604206. [DOI] [PubMed] [Google Scholar]
- 134.Shah RKK, Agresti JW, Weitz JJ, Chu DA, Y L. Fabrication of monodisperse thermosensitive microgels and gel capsules in microfluidic devices. Soft Matter. 2008;4:2303–2309. [Google Scholar]
- 135.Breslauer DN, Muller SJ, Lee LP. Generation of monodisperse silk microspheres prepared with microfluidics. Biomacromolecules. 2010;11:643–647. doi: 10.1021/bm901209u. [DOI] [PubMed] [Google Scholar]
- 136.Chu LYK, Shah JY, Weitz RK, A D. Monodisperse Thermoresponsive Microgels with Tunable Volume-Phase Transition Kinetics. Adv. Funct. Mater. 2007;17:3499–3504. [Google Scholar]
- 137.Wang W, Xie R, Ju XJ, Luo T, Liu L, Weitz DA, Chu LY. Controllable microfluidic production of multicomponent multiple emulsions. Lab on a chip. 2011;11:1587–1592. doi: 10.1039/c1lc20065h. [DOI] [PubMed] [Google Scholar]
- 138.Chu LY, Utada AS, Shah RK, Kim JW, Weitz DA. Controllable monodisperse multiple emulsions. Angew Chem Int Ed Engl. 2007;46:8970–8974. doi: 10.1002/anie.200701358. [DOI] [PubMed] [Google Scholar]
- 139.Kim SH, Kim JW, Cho JC, Weitz DA. Double-emulsion drops with ultra-thin shells for capsule templates. Lab on a chip. 2011;11:3162–3166. doi: 10.1039/c1lc20434c. [DOI] [PubMed] [Google Scholar]
- 140.Ren PW, Ju XJ, Xie R, Chu LY. Monodisperse alginate microcapsules with oil core generated from a microfluidic device. Journal of colloid and interface science. 2010;343:392–395. doi: 10.1016/j.jcis.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 141.Shah RK, Kim JW, Weitz DA. Monodisperse stimuli-responsive colloidosomes by self-assembly of microgels in droplets. Langmuir. 2010;26:1561–1565. doi: 10.1021/la9041327. [DOI] [PubMed] [Google Scholar]
- 142.Liu L, Yang JP, Ju XJ, Xie R, Liu YM, Wang W, Zhang JJ, Niu CH, Chu LY. Monodisperse coreshell chitosan microcapsules for pH-responsive burst release of hydrophobic drugs. Soft Matter. 2011;7:4821–4827. [Google Scholar]
- 143.Romanowsky MB, Abate AR, Rotem A, Holtze C, Weitz DA. High throughput production of single core double emulsions in a parallelized microfluidic device. Lab on a chip. 2012;12:802–807. doi: 10.1039/c2lc21033a. [DOI] [PubMed] [Google Scholar]
- 144.Hsieh AT, Hori N, Massoudi R, Pan PJ, Sasaki H, Lin YA, Lee AP. Nonviral gene vector formation in monodispersed picolitre incubator for consistent gene delivery. Lab on a chip. 2009;9:2638–2643. doi: 10.1039/b823191e. [DOI] [PubMed] [Google Scholar]
- 145.Ho YP, Grigsby CL, Zhao F, Leong KW. Tuning physical properties of nanocomplexes through microfluidics-assisted confinement. Nano letters. 2011;11:2178–2182. doi: 10.1021/nl200862n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Karnik R, Gu F, Basto P, Cannizzaro C, Dean L, Kyei-Manu W, Langer R, Farokhzad OC. Microfluidic Platform for Controlled Synthesis of Polymeric Nanoparticles. Nano letters. 2008;8:2906–2912. doi: 10.1021/nl801736q. [DOI] [PubMed] [Google Scholar]
- 147.Kolishetti N, Dhar S, Valencia PM, Lin LQ, Karnik R, Lippard SJ, Langer R, Farokhzad OC. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc Natl Acad Sci U S A. 2010;107:17939–17944. doi: 10.1073/pnas.1011368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Valencia PM, Basto PA, Zhang L, Rhee M, Langer R, Farokhzad OC, Karnik R. Single-step assembly of homogenous lipid-polymeric and lipid-quantum dot nanoparticles enabled by microfluidic rapid mixing. Acs Nano. 2010;4:1671–1679. doi: 10.1021/nn901433u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gong X, Peng S, Wen W, Sheng P, Li W. Design and Fabrication of Magnetically Functionalized Core/Shell Microspheres for Smart Drug Delivery Advanced Functional Materials. 2008;18:1–6. [Google Scholar]
- 150.He TL, Zhang Q, Mu K, Luo X, Wang T, Luo Y, G A modified microfluidic chip for fabrication of paclitaxel-loaded poly(L-lactic acid) microspheres. Microfluid Nanofluid. 2011;10:1289–1298. [Google Scholar]
- 151.Watanabe TO, Kimura T, Y Continuous fabrication of monodisperse polylactide microspheres by droplet- particle technology using microfluidic emulsification and emulsion-solvent diffusion. Soft Matter. 2011;7:9894–9897. [Google Scholar]
- 152.Seiffert SW, A D. Controlled fabrication of polymer microgels by polymer-analogous gelation in droplet microfluidics. Soft Matter. 2010;6:3184–3190. [Google Scholar]
- 153.Chen W, Yang Y, Rinadi C, Zhou D, Shen AQ. Formation of supramolecular hydrogel microspheres via microfluidics. Lab on a chip. 2009;9:2947–2951. doi: 10.1039/b906254h. [DOI] [PubMed] [Google Scholar]
- 154.Lee IY, Cheng Y, Jeong Z, K H. Generation of Monodisperse Mesoporous Silica Microspheres with Controllable Size and Surface Morphology in a Microfluidic Device. Adv. Funct. Mater. 2008;18:4014–4021. [Google Scholar]
- 155.Huang S, Lin B, Qin J. Microfluidic synthesis of tunable poly-(N-isopropylacrylamide) microparticles via PEG adjustment. Electrophoresis. 2011;32:3364–3370. doi: 10.1002/elps.201100340. [DOI] [PubMed] [Google Scholar]
- 156.Hung LH, Teh SY, Jester J, Lee AP. PLGA micro/nanosphere synthesis by droplet microfluidic solvent evaporation and extraction approaches. Lab on a chip. 2010;10:1820–1825. doi: 10.1039/c002866e. [DOI] [PubMed] [Google Scholar]
- 157.Xu Q, Hashimoto M, Dang TT, Hoare T, Kohane DS, Whitesides GM, Langer R, Anderson DG. Preparation of monodisperse biodegradable polymer microparticles using a microfluidic flow-focusing device for controlled drug delivery. Small. 2009;5:1575–1581. doi: 10.1002/smll.200801855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kesselman LR, Shinwary S, Selvaganapathy PR, Hoare T. Synthesis of monodisperse, covalently cross-linked, degradable "smart" microgels using microfluidics. Small. 2012;8:1092–1098. doi: 10.1002/smll.201102113. [DOI] [PubMed] [Google Scholar]
- 159.Marimuthu MK, An S, J Amphiphilic triblock copolymer and a microfluidic device for porous microfiber fabrication. Soft Matter. 2010;6:2200–2207. [Google Scholar]
- 160.Hettiarachchi K, Zhang S, Feingold S, Lee AP, Dayton PA. Controllable microfluidic synthesis of multiphase drug-carrying lipospheres for site-targeted therapy. Biotechnology progress. 2009;25:938–945. doi: 10.1002/btpr.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jagadeesan D, Nasimova I, Gourevich I, Starodubtsev S, Kumacheva E. Microgels for the encapsulation and stimulus-responsive release of molecules with distinct polarities. Macromol Biosci. 2011;11:889–896. doi: 10.1002/mabi.201100045. [DOI] [PubMed] [Google Scholar]
- 162.Shin SJ, Park JY, Lee JY, Park H, Park YD, Lee KB, Whang CM, Lee SH. "On the fly" continuous generation of alginate fibers using a microfluidic device. Langmuir. 2007;23:9104–9108. doi: 10.1021/la700818q. [DOI] [PubMed] [Google Scholar]
- 163.Su J, Zheng Y, Wu H. Generation of alginate microfibers with a roller-assisted microfluidic system. Lab on a chip. 2009;9:996–1001. doi: 10.1039/b813518e. [DOI] [PubMed] [Google Scholar]
- 164.Choi CH, Yi H, Hwang S, Weitz DA, Lee CS. Microfluidic fabrication of complex-shaped microfibers by liquid template-aided multiphase microflow. Lab on a chip. 2011;11:1477–1483. doi: 10.1039/c0lc00711k. [DOI] [PubMed] [Google Scholar]
- 165.Dendukuri D, Pregibon DC, Collins J, Hatton TA, Doyle PS. Continuous-flow lithography for high-throughput microparticle synthesis. Nat Mater. 2006;5:365–369. doi: 10.1038/nmat1617. [DOI] [PubMed] [Google Scholar]
- 166.De Geest BG, Urbanski JP, Thorsen T, Demeester J, De Smedt SC. Synthesis of monodisperse biodegradable microgels in microfluidic devices. Langmuir. 2005;21:10275–10279. doi: 10.1021/la051527y. [DOI] [PubMed] [Google Scholar]
- 167.Choi CH, Jung JH, Kim DW, Chung YM, Lee CS. Novel one-pot route to monodisperse thermosensitive hollow microcapsules in a microfluidic system. Lab on a chip. 2008;8:1544–1551. doi: 10.1039/b804839h. [DOI] [PubMed] [Google Scholar]
- 168.Jahn A, Vreeland WN, Gaitan M, Locascio LE. Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing. J Am Chem Soc. 2004;126:2674–2675. doi: 10.1021/ja0318030. [DOI] [PubMed] [Google Scholar]
- 169.Jahn A, Vreeland WN, DeVoe DL, Locascio LE, Gaitan M. Microfluidic directed formation of liposomes of controlled size. Langmuir. 2007;23:6289–6293. doi: 10.1021/la070051a. [DOI] [PubMed] [Google Scholar]
- 170.Jahn A, Stavis SM, Hong JS, Vreeland WN, DeVoe DL, Gaitan M. Microfluidic mixing and the formation of nanoscale lipid vesicles. Acs Nano. 2010;4:2077–2087. doi: 10.1021/nn901676x. [DOI] [PubMed] [Google Scholar]
- 171.Tran TH, Nguyen CT, Kim DP, Lee YK, Huh KM. Microfluidic approach for highly efficient synthesis of heparin-based bioconjugates for drug delivery. Lab on a chip. 2012;12:589–594. doi: 10.1039/c1lc20769e. [DOI] [PubMed] [Google Scholar]
- 172.Xu JHZ, Lan H, Luo WJ, S G. A Novel Microfluidic Approach for Monodispersed Chitosan Microspheres with Controllable Structures. Adv. Healthcare Mater. 2012;1:106–111. doi: 10.1002/adhm.201100014. [DOI] [PubMed] [Google Scholar]
- 173.Xu JH, Li SW, Tostado C, Lan WJ, Luo GS. Preparation of monodispersed chitosan microspheres and in situ encapsulation of BSA in a co-axial microfluidic device. Biomedical microdevices. 2009;11:243–249. doi: 10.1007/s10544-008-9230-3. [DOI] [PubMed] [Google Scholar]
- 174.Nie Z, Li W, Seo M, Xu S, Kumacheva E. Janus and ternary particles generated by microfluidic synthesis: design, synthesis, and self-assembly. J Am Chem Soc. 2006;128:9408–9412. doi: 10.1021/ja060882n. [DOI] [PubMed] [Google Scholar]
- 175.Ogonczyk D, Siek M, Garstecki P. Microfluidic formulation of pectin microbeads for encapsulation and controlled release of nanoparticles. Biomicrofluidics. 2011;5:13405. doi: 10.1063/1.3569944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xie H, She ZG, Wang S, Sharma G, Smith JW. One-step fabrication of polymeric Janus nanoparticles for drug delivery. Langmuir. 2012;28:4459–4463. doi: 10.1021/la2042185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hong JS, Stavis SM, DePaoli Lacerda SH, Locascio LE, Raghavan SR, Gaitan M. Microfluidic directed self-assembly of liposome-hydrogel hybrid nanoparticles. Langmuir. 2010;26:11581–11588. doi: 10.1021/la100879p. [DOI] [PubMed] [Google Scholar]
- 178.Engel RH, Riggi SJ, Fahrenbach MJ. Insulin: intestinal absorption as water-in-oil-in-water emulsions. Nature. 1968;219:856–857. doi: 10.1038/219856a0. [DOI] [PubMed] [Google Scholar]
- 179.Muguet V, Seiller M, Barratt G, Ozer O, Marty JP, Grossiord JL. Formulation of shear rate sensitive multiple emulsions. Journal of controlled release : official journal of the Controlled Release Society. 2001;70:37–49. doi: 10.1016/s0168-3659(00)00314-x. [DOI] [PubMed] [Google Scholar]
- 180.Gokmen MT, De Geest BG, Hennink WE, Du Prez FE. "Giant" hollow multilayer capsules by microfluidic templating. ACS applied materials & interfaces. 2009;1:1196–1202. doi: 10.1021/am900055b. [DOI] [PubMed] [Google Scholar]
- 181.Seiffert S, Thiele J, Abate AR, Weitz DA. Smart microgel capsules from macromolecular precursors. J Am Chem Soc. 2010;132:6606–6609. doi: 10.1021/ja102156h. [DOI] [PubMed] [Google Scholar]
- 182.Lavin DM, Stefani RM, Zhang L, Furtado S, Hopkins RA, Mathiowitz E. Multifunctional polymeric microfibers with prolonged drug delivery and structural support capabilities. Acta biomaterialia. 2012;8:1891–1900. doi: 10.1016/j.actbio.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 183.Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. Journal of Controlled Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nisisako T, Torii T. Microfluidic large-scale integration on a chip for mass production of monodisperse droplets and particles. Lab on a chip. 2008;8:287–293. doi: 10.1039/b713141k. [DOI] [PubMed] [Google Scholar]
- 186.Kobayashi IW, Uemura Y, Nakajima K, M Microchannel emulsification for mass production of uniform fine droplets: integration of microchannel arrays on a chip. Microfluid Nanofluid. 2010;8:255–262. [Google Scholar]
- 187.Guzowski J, Korczyk PM, Jakiela S, Garstecki P. Automated high-throughput generation of droplets. Lab on a chip. 2011;11:3593–3595. doi: 10.1039/c1lc20595a. [DOI] [PubMed] [Google Scholar]
- 188.MacDonald MP, Spalding GC, Dholakia K. Microfluidic sorting in an optical lattice. Nature. 2003;426:421–424. doi: 10.1038/nature02144. [DOI] [PubMed] [Google Scholar]
- 189.Huh D, Bahng JH, Ling Y, Wei HH, Kripfgans OD, Fowlkes JB, Grotberg JB, Takayama S. Gravity-driven microfluidic particle sorting device with hydrodynamic separation amplification. Analytical chemistry. 2007;79:1369–1376. doi: 10.1021/ac061542n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yamada M, Seki M. Hydrodynamic filtration for on-chip particle concentration and classification utilizing microfluidics. Lab on a chip. 2005;5:1233–1239. doi: 10.1039/b509386d. [DOI] [PubMed] [Google Scholar]
- 191.Huang LR, Cox EC, Austin RH, Sturm JC. Continuous particle separation through deterministic lateral displacement. Science. 2004;304:987–990. doi: 10.1126/science.1094567. [DOI] [PubMed] [Google Scholar]
- 192.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Merkel TJ, Herlihy KP, Nunes J, Orgel RM, Rolland JP, DeSimone JM. Scalable, shape-specific, top-down fabrication methods for the synthesis of engineered colloidal particles. Langmuir. 2010;26:13086–13096. doi: 10.1021/la903890h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ho CC, Keller A, Odell JA, Ottewill RH. Preparation of monodisperse ellipsoidal polystyrene particles. Colloid & Polymer Science. 1993;271:469–479. [Google Scholar]
- 195.Champion JA, Katare YK, Mitragotri S. Making polymeric micro- and nanoparticles of complex shapes. Proc Natl Acad Sci U S A. 2007;104:11901–11904. doi: 10.1073/pnas.0705326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc. 2005;127:10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 197.Petros RA, Ropp PA, DeSimone JM. Reductively labile PRINT particles for the delivery of doxorubicin to HeLa cells. J Am Chem Soc. 2008;130:5008–5009. doi: 10.1021/ja801436j. [DOI] [PubMed] [Google Scholar]
- 198.Parrott MC, Luft JC, Byrne JD, Fain JH, Napier ME, Desimone JM. Tunable bifunctional silyl ether cross-linkers for the design of acid-sensitive biomaterials. J Am Chem Soc. 2010;132:17928–17932. doi: 10.1021/ja108568g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Euliss LE, DuPont JA, Gratton S, DeSimone J. Imparting size, shape, and composition control of materials for nanomedicine. Chem Soc Rev. 2006;35:1095–1104. doi: 10.1039/b600913c. [DOI] [PubMed] [Google Scholar]
- 200.Nunes J, Herlihy KP, Mair L, Superfine R, DeSimone JM. Multifunctional shape and size specific magneto-polymer composite particles. Nano letters. 2010;10:1113–1119. doi: 10.1021/nl904152e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Canelas DA, Herlihy KP, DeSimone JM. Top-down particle fabrication: control of size and shape for diagnostic imaging and drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:391–404. doi: 10.1002/wnan.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Glangchai LC, Caldorera-Moore M, Shi L, Roy K. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles. J Control Release. 2008;125:263–272. doi: 10.1016/j.jconrel.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 203.Mogul R, Bolapos, shakov AA, Chan SL, Stevens RM, Khare BN, Meyyappan M, Trent JD. Impact of Low-Temperature Plasmas on Deinococcusradiodurans and Biomolecules. Biotechnology progress. 2003;19:776–783. doi: 10.1021/bp025665e. [DOI] [PubMed] [Google Scholar]
- 204.Panda P, Ali S, Lo E, Chung BG, Hatton TA, Khademhosseini A, Doyle PS. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip. 2008;8:1056–1061. doi: 10.1039/b804234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dendukuri D, Gu SS, Pregibon DC, Hatton TA, Doyle PS. Stop-flow lithography in a microfluidic device. Lab Chip. 2007;7:818–828. doi: 10.1039/b703457a. [DOI] [PubMed] [Google Scholar]