Abstract
The central melanocortin system plays an essential role in the regulation of energy metabolism. Key to this regulation is the sensing and responses of neurons expressing proopiomelanocortin (POMC) and agouti-related protein (AgRP) to blood-borne metabolic signals. Recent evidence has demonstrated that POMC- and AgRP-neurons are not simply mirror opposites of each other in function and responsiveness to metabolic signals, nor are they exclusively first-order neurons. These neurons act as central transceivers, receiving both hormonal and neural signals, integrating and transmitting this information to peripheral tissues via the autonomic nervous system to coordinate whole body energy metabolism. This review focuses on most recent developments obtained from rodent studies on the function, metabolic regulation and circuitry of the central melanocortin system.
Keywords: Agouti-related protein, proopiomelanocortin, melanocortin receptor, energy metabolism, feeding circuits, leptin, obesity
Basic Components of the Central Melanocortin System
The central melanocortin system is comprised of neurons that express two of the five identified melanocortin receptor (MCR) subtypes (see glossary), namely the MC3R and MC4R, and neurons that express their endogenous agonists (notably POMC derived α-melanocyte stimulating hormone, α-MSH) and antagonists (AgRP). AgRP is exclusively expressed in a group of neurons in the arcuate nucleus of the mediobasal hypothalamus, and these neurons also co-express Neuropeptide Y (NPY) and the classical neurotransmitter γ-aminobutyric acid (GABA) [1, 2]. Since AgRP is the unique signature molecule in these neurons when compared with the more widespread expression of NPY and GABA, these neurons are termed AgRP neurons hereafter.
POMC expression is similarly restricted in neurons within the arcuate nucleus, but can also be found in the nucleus of the solitary tract in the hindbrain. Some POMC neurons also co-express cocaine and amphetamine related transcript (CART) [3], however the importance of CART in feeding regulation is controversial [4]. Aside from the expression of the signature POMC peptide, POMC neurons in the arcuate nucleus consist of distinct subpopulations of GABAergic and glutamatergic neurons [5, 6], although the GABAergic nature of these neurons is debated [7]. POMC and AgRP neurons project in parallel to many of the same brain regions [1, 3]. As receptors for α-MSH and AgRP neuropeptides, the MC4R is widely expressed in the brain, while MC3R is mostly expressed within the hypothalamus, with limited expression in the thalamus and brain stem [8].
One special property of the arcuate nucleus could be its vicinity to the median eminence, a circumventricular organ. Fenestrated capillaries are present in the median eminence, and considerable vascular permeability is observed in the arcuate nucleus [9-12]. Furthermore, the ventricular wall of the 3rd ventricle next to the median eminence and the arcuate nucleus is lined with specialized ependymal cells called tanycytes [13]. In contrast to the tanycytes of the median eminence, tanycytes at the periphery of the arcuate nucleus are devoid of efficient tight junction complexes, which may facilitate access of molecules from cerebral spinal fluid to the arcuate parenchyma [10]. This unique anatomical feature of the arcuate nucleus raises the possibility that some of the resident cells, including AgRP and POMC neurons, may have more direct access to circulating metabolic signals from the periphery. The functional significance of this property awaits further study.
Melanocortinergic Neurons in Feeding Regulation
The importance of melanocortin in control of energy balance is indisputable. This is demonstrated by the essential roles of POMC and MC4R in regulation of feeding and body weight. Loss of function of POMC or MC4R results in severe hyperphagia and profound obesity in rodents and humans [3]. In fact, mutations in Mc4r gene represent the most common cause of monogenic obesity in humans [14, 15]. There is site-specific action of MC4R; MC4R in the paraventricular hypothalamus and the amygdala control food intake, whilst MC4R in cholinergic neurons regulates energy expenditure [16, 17]. In contrast to MC4R, the role of MC3R in the pathogenesis of human obesity remains unclear, although loss of function of MC3R in rodents results in increased adiposity and reduced lean mass [18, 19]. Mc3r null mice also exhibit defects in fasting-induced re-feeding and a Cushing-like syndrome with elevated basal corticosterone levels, alteration in adipose tissue distribution and bone remodeling [20]. The MC3R has also been suggested to govern feeding-associated behaviors, including food anticipatory activity and the behavioral adaptations to restricted feeding [21, 22].
In contrast to the paramount importance of POMC and MC4R, the role of AgRP neuropeptide in feeding regulation has become the focus of recent debate. It is well known that central administration of AgRP potently stimulates feeding, and transgenic overexpression of Agrp results in severe obesity [3]. However, mice lacking the Agrp gene, the Npy gene, or both do not exhibit alterations in feeding or body weight [23], while mice deficient in GABA release from AgRP neurons show mild reduction of body weight on a chow diet [24]. These results indicate that AgRP, NPY and GABA may play redundant roles in the regulation of food intake and body weight, notably in the face of life-long deficiency of one of the components. The recent development of inducible genetic and imaging tools has allowed cell type-specific, reversible and temporal manipulation of neuronal activity. An optogenetic approach using light-activated cation channel channelrhodopsin-2 shows that acute activation of AgRP neurons evokes, while selective stimulation of POMC neurons inhibits, feeding [25]. Similarly, using technology involving activation of designer receptors by designer drugs, acute activation of AgRP neurons rapidly induces feeding and ultimately increases fat stores [26]. Interestingly, continuation of evoked feeding requires ongoing stimulation of AgRP neurons, suggesting that activity in downstream circuits is tied to dynamic AgRP neuron activity [25]. Consistently, acute ablation of AgRP neurons results in severe anorexia and weight loss [27, 28]. Taken together, the above findings firmly establish a crucial role of AgRP neurons in feeding regulation.
Elucidation of downstream circuitry that mediates the orexigenic effects of AgRP neurons has been an important area of recent research. A new circuit has been recently proposed whereby a subpopulation of neurons in the hindbrain parabrachial nucleus integrates GABAergic input from arcuate AgRP neurons, and glutamatergic input from the nucleus of solitary tract of the hindbrain [29]. Thus, AgRP neurons, by projecting to the hindbrain from the arcuate nucleus, may link long-term adiposity signals acting in the arcuate nucleus, with short-term gastrointestinal satiety signals acting in the nucleus of solitary trait, a region that receives afferent vagal signals from the gastrointestinal tract. Intriguingly, a separate study suggests that acute evoked feeding induced by photo-stimulation of AgRP neurons does not require a circuit linking AgRP neurons to the hindbrain parabrachial nucleus, but instead requires inhibition of oxytocin neurons in the paraventricular hypothalamus [30]. Similarly, inhibition of POMC neurons is also dispensable for acute evoke feeding by activation of AgRP neurons, although this circuit likely regulates long-term feeding responses [25, 30]. Future studies are required to dissect how distinct circuits interact to regulate acute and long term food intake under different physiological conditions.
A significant recent development is the notion that AgRP neurons may regulate feeding via a melanocortin-independent mechanism. Inhibition of MCRs is not necessary for evoked feeding responses elicited by acute activation of AgRP neurons, suggesting that AgRP is not necessary for acute feeding behavior [25]. Instead, NPY and GABA, both components of AgRP neurons, are important modulators of evoked feeding [30]. In addition, starvation caused by acute ablation of AgRP neurons has been attributed to a melanocortin-independent, but GABA-dependent, mechanism [29, 31, 32]. Together, the above studies suggest that GABA and NPY play an important role in mediating the orexigenic function of AgRP neurons, and that the explicit necessity of the AgRP neuropeptide in feeding regulation is once again put into question. Whether AgRP neuropeptide, the namesake of the AgRP neurons, may have any non-redundant function in metabolic regulation requires further studies.
Feeding-Independent Metabolic Regulation via the Autonomic Nervous System
In addition to feeding control, melanocortinergic neurons also participate in the regulation of energy metabolism in peripheral tissues via feeding-independent mechanisms, most notably by regulation of the autonomic nervous system. The sympathetic nervous system (SNS) has significant influence on white adipose tissue (WAT) function. Central AgRP administration has been shown to reduce WAT sympathetic activity, whereas melanocortin agonists increase sympathetic activity and the phosphorylation of the essential lipolytic enzyme hormone sensitive lipase [33-35], although the exact WAT depot affected depends on the species studied. This is supported by studies in human subjects, where intranasal application of an MC4R agonist causes subcutaneous WAT lipolysis [36]. Evidence also supports the notion that the MC4R can control brown adipose tissue (BAT) thermogenesis via the SNS. Central injections of synthetic MCR agonist melanotan II can increase BAT uncoupling protein-1 mRNA expression and increase BAT temperature, an effect that can be blocked by BAT sympathectomy [37, 38]. Accordingly, central infusion of AgRP reduces sympathetic activity and BAT temperature [39]; blockade of MC4R in the dorsomedial hypothalamus also blunts BAT thermogenesis induced by melanotan II [40]. Collectively these data demonstrate that increasing MC4R signaling at multiple brain sites can increase BAT thermogenesis via SNS activation.
In contrast, dissecting the contribution of MC4R signaling to sympathetic or parasympathetic outflow regulation has been a challenge in understanding the autonomic control of hepatic glucose production (HPG). AgRP neurons have been shown to mediate insulin's suppressive effect on HPG, an effect that is independent of feeding and body weight [41, 42]. While previous studies implicate the parasympathetic nervous system as the mediator in this regulation [43], this conclusion has been disputed [44]. A recent study shows that genetic restoration of MC4R expression in cholinergic sympathetic preganglionic neurons of Mc4r null mice is sufficient to attenuate hyperglycemia and HPG, without drastically affecting food intake [17]. This finding indicates that MC4R signaling regulates heptic glucose homeostasis by activating the SNS. Further studies are required to determine whether suppression of HPG by insulin action on AgRP neurons is mediated by a melanocortin-dependent or independent mechanism.
Direct and Indirect Hormonal Regulation of Melanocortinergic Neurons
Situated in the arcuate nucleus next to a circumventricular organ, AgRP or POMC neurons are ideally positioned to sense blood-borne metabolic signals. Indeed, these neurons have been shown to be regulated by a number of hormonal signals, including leptin, insulin, estrogen, ghrelin, glucagon-like peptide 1, peptide YY, fatty acids, glucocorticoids and proinflammatory cytokines [4, 45-49]. Among these signals, leptin regulation of melanocortinergic neurons has been a focus of interest. POMC and AgRP neurons are direct targets of leptin regulation, and hence are called first-order targets by leptin. The long form of leptin receptor is expressed in subsets of POMC and AgRP neurons, and leptin administration rapidly stimulates phosphorylation of STAT3, a key leptin signaling pathway, in these neurons [3]. Additionally, increasing evidence suggests that POMC and AgRP neurons are regulated by leptin via indirect mechanisms. Leptin induces dynamic changes of excitatory and inhibitory synaptic densities onto POMC and AgRP neurons, suggesting that leptin regulates these neurons indirectly by acting on their presynaptic neurons [50]. This notion is supported by recent finding that leptin reduces inhibitory tone onto postsynaptic POMC neurons by working directly on presynaptic GABAergic neurons [7]. This mechanism may have functional consequences in the development of obesity, since obesity-prone rats have a greater number of inhibitory inputs onto POMC neurons than obesity-resistant rats [51]. Thus, the traditional first-order POMC and AgRP neurons are also second- or higher-order neurons regulated by leptin. Conversely, the traditional second-order neurons, including MC4R neurons, can also be under the direct “first-order” control of leptin [52]. The identities of the leptin-responsive neurons that project onto POMC and AgRP neurons are not completely understood, however a subset of inhibitory synaptic inputs onto POMC neurons originate from AgRP neurons and contain AgRP, NPY and/or GABA [2, 7].
Similar to leptin, other metabolic hormones such as insulin, estrogen and ghrelin also exert direct and indirect effects on arcuate melanocortinergic neurons. For example, insulin directly inhibits POMC firing rates via insulin receptor action on POMC neurons [53] but indirectly inhibits POMC activity by suppression of ventromedial hypothalamus neurons that project excitatory input onto POMC neurons [54]. Estrogen receptor alpha is expressed in POMC neurons, the removal of which results in hyperphagia and weight gain, demonstrating cell-autonomous action of estrogen on POMC neurons [55]. However, estrogen also induces synaptic remodeling onto POMC neurons, suggesting that estrogen may regulate POMC activity indirectly via other inter-neurons [56]. Ghrelin acts directly on the growth hormone secretagogue receptors (GHSR) expressed on AgRP neurons to stimulate firing frequency and feeding [57]. In addition, ghrelin also acts on other pre-synaptic neurons to cause the release of glutamate onto AgRP neurons, possibly generating a positive feedback loop to prolong ghrelin action [58].
Taken together, AgRP and POMC neurons sense and integrate diverse metabolic signals by acting as first-order neurons. Additionally these neurons are also higher-order neurons, whose activities are influenced by other neurons that are under the direct control of metabolic hormones. This mode of regulation may allow metabolic fine-turning or feedback regulation in response to changes of physiologic conditions. It is worth noting that POMC neurons exhibit considerable heterogeneity. POMC neurons consist of distinct subpopulations of GABAergic and glutamatergic neurons [5, 6], and the acute responses to leptin and insulin are largely segregated into distinct subpopulations of POMC neurons [53]. Future studies are needed to determine how diverse metabolic and neural signals, direct and indirect, are integrated by distinct subpopulations of melanocortinergic neurons, and how these signals further prorogate to their downstream effector neurons.
Requirement of Melanocortinergic Neurons for Leptin Function
One major function of leptin is to convey body's energy status to the brain. With starvation, plasma leptin concentrations decrease precipitously, which leads to suppression of Pomc expression and stimulation of Agrp expression, changes that can be prevented by leptin replacement [3]. Although starvation also stimulates the expression of other orexigenic neuropeptides, such as melanin-concentrating hormone and orexin, in the lateral hypothalamus, their corresponding neurons do not express functional leptin receptors and are not activated by leptin [59]. In light of the severe obesity phenotypes displayed by leptin, POMC or MC4R deficiency, a long-held notion has been that leptin exerts its anorexigenic effects by directly stimulating POMC neurons while inhibiting AgRP neurons [3]. However, this notion has been recently challenged using data generated from genetic models. First, by using a genetically targeted and easily detectable reporter system, the long form of the leptin receptor is found to be expressed in many hypothalamic and extra-hypothalamic regions [60, 61]. Second, while obese Mc4r null mice do not respond to the anorectic effects of leptin, non-obese Mc4r null mice do, suggesting that MC4R signaling is not an exclusive target of leptin action and that factors resulting from obesity contribute to leptin resistance in this mouse model [62]. Third, genetic removal of leptin receptors from POMC or AgRP neurons only produces mild obesity [63, 64]. In stark contrast, removal of leptin receptors from a wide-range of neurons expressing neuronal nitric oxide synthase-1 (NOS1) produces hyperphagic obesity, decreased energy expenditure and hyperglycemia, approaching the extent seen in whole-body leptin receptor null mice [65]. Together, these studies indicate that melanocortinergic neurons may play a much smaller role than previously anticipated in mediating leptin's effects on appetite and body weight. However, one must be cautious in interpreting data exclusively from genetic models since these mutant mice may have developed compensatory mechanisms that may not faithfully describe the normal contribution of these neurons to physiology. The generation of inducible Cre-lox systems may help to address this issue. It should also be acknowledged that upon removal of direct leptin action on POMC or AgRP neurons, these neurons could still receive indirect regulation from leptin via other leptin-responsive presynaptic neurons, making POMC and AgRP neurons indirect mediators for leptin's anorexigenic effects.
In addition to the regulation of appetite and energy expenditure, leptin also independently governs many other metabolic processes. For example, leptin has been recently shown to act in the brain to suppress hepatic triglyceride levels via activation of the SNS [66]. Two recent studies demonstrate that restoration of leptin receptor expression in POMC neurons of leptin receptor deficient mice completely normalizes blood glucose levels [67, 68]. It remains to be determined whether distinct subpopulations of POMC neurons mediate different aspects of POMC functions or distinct metabolic effects of leptin. Nevertheless, these studies raise an intriguing possibility that melanocortinergic neurons may play a predominant role in mediating leptin's effects on the autonomic control of lipid and glucose metabolism in peripheral tissues.
Distinct Responses of AgRP and POMC Neurons to Metabolic Signals
AgRP and POMC neurons are situated in close proximity to each other in the arcuate nucleus and project in parallel to a number of brain regions. At the receptor level, AgRP and POMC-derived peptides have opposing actions. AgRP and POMC neurons also have broadly opposing functions on feeding and body weight and they are regulated in opposite directions upon changes of energy status, such as in starvation. While overwhelming evidence indicates that AgRP and POMC neurons act to counter-balance each other to adjust appetite, they are not simply mirror opposites of each other in their responses to metabolic stimuli. One notable example is the response of these two neuronal populations to ghrelin. Ghrelin levels rise before meals and after fasting [69-71]. The GHSR is expressed on the vast majority of AgRP neurons, but on very few POMC neurons [57, 72]. Ghrelin stimulates AgRP neuronal activity but has no direct effect on POMC activity, although ghrelin could still exert inhibitory effects on POMC neurons indirectly via AgRP neurons [24, 57, 69, 73, 74]. Consistently, the orexigenic effects of ghrelin are lost in mice that lack Agrp and Npy, indicating that ghrelin exerts its orexigenic effects by activating AgRP neurons [24, 75]. Furthermore, AgRP but not POMC neurons have dendritic spines, and fasting-induced increase of glutamatergic input onto AgRP neurons is paralleled by an increase in AgRP spine density [76]. AgRP and POMC neurons may also differ in nutrient sensing. Palmitic acid, a saturated fatty acid, mediates insulin resistance in hypothalamic neurons by activating protein kinase C (PKC)-θ in diet-induced obesity [77]. Interestingly, PKC-θ is expressed in discrete neuronal populations of the arcuate nucleus, specifically in AgRP but not in POMC neurons [77].
Taken together, the above studies suggest that AgRP and POMC neurons may differ in their abilities to sense and respond to certain hormonal and metabolic signals, and they may play distinct roles in mediating the actions of these signals. The basis for this differential regulation is current not known. One possibility is the closer anatomical proximity of AgRP neurons to the median eminence compared with POMC neurons, which could expose AgRP neurons to greater concentrations of blood-borne substances. AgRP neurons project onto POMC neurons and suppress POMC neuronal activity and α-MSH release [2], although the reverse has not been demonstrated. While the change of AgRP neuronal activity could impact AgRP and α-MSH release thereby altering MC4R activity, AgRP neurons also regulate feeding via a melanocortin-independent, GABA-dependent mechanism [25, 29, 32]. The release of NPY from AgRP neurons can also act on non-MC4R neurons via interaction with the Y receptors. Thus upon stimulation, AgRP neurons can transmit signals to a number of different targets via both melanocortin-dependent and independent mechanisms.
Dysregulation of Melanocortinergic Neurons in Obesity
Considerable effort is focused on understanding how the function of melanocortinergic neurons is influenced by environmental changes, particularly diet-induced obesity. Although obesity is the result of complex interactions among multiple hormonal and neuronal pathways, a number of studies have indicated that melanocortinergic neurons contribute to dysregulation of leptin action and the etiology of obesity. Here we briefly describe some recent progress in this area.
The effect of cellular stress on melanocortinergic function
Recent studies suggest that high fat feeding increases cellular stress, which alters melanocortinergic function. Previous work shows that increased endoplasmic reticulum (ER) stress and activation of the unfolded protein response in the hypothalamus of obese mice inhibits leptin signaling [78, 79]. Interestingly, proliferation of peroxisomes, which are derived from the ER, is increased in the hypothalamus of diet-induced obese animals [80]. Scavenging of reactive oxygen species (ROS) by peroxisomes suppresses POMC neuronal firing while stimulates AgRP neuronal activity [80]. High fat feeding also induces reactive gliosis in the mediobasal hypothalamus and elevates the expression of inflammatory cytokines [51, 81, 82], creating a local inflammatory environment that could dysregulate melanocortinergic function. Noticeably, autophagy, an indicator of cellular stress and neuronal injury [83, 84], is markedly elevated in the POMC neurons of diet-induced obese animals and neuronal injury is evident in POMC neurons upon short-term consumption of a high fat diet [82]. However, autophagy in POMC neurons is required for appropriate axonal outgrowth, neuropeptide expression and leptin signaling, and selective deletion of autophagy gene Atg7 in POMC neurons leads to obesity [85-87]. Similarly, autophagy in AgRP neurons is required for appropriate AgRP neuronal function during starvation [88]. Thus, it remains to be determined whether high fat diet-induced autophagy in melanocortinergic neurons represents a protective mechanism to maintain neuronal homeostasis or if it is a pathological cause of neuronal injury and dysregulation. Collectively the above studies suggest that increased neuronal stress and injury are closely associated with impairment of hypothalamic neuronal function in obesity.
New players in leptin signaling and leptin resistance
Several novel signaling pathways have been recently implicated in leptin signaling and may be involved in the development of cellular leptin resistance, a hallmark feature of obesity [89]. Elevation of adenosine 3', 5'-monophosphate (cAMP) levels impairs multiple leptin signaling cascades within the hypothalamus [90]. Epac, a cAMP-regulated guanine nucleotide exchange factor for the small G protein Rap1, is activated in diet-induced obesity, and it blunts leptin-induced depolarization of POMC neurons [90]. Furthermore, protein tyrosine phosphatase 1B (PTP1B) acts as a negative regulator of leptin and insulin signaling, and its deletion from POMC neurons increases energy expenditure and improves glucose homeostasis [91]. Interestingly, removal of another phosphatase, SH2 domain-containing protein tyrosine phosphatase-2 (SHP2), from POMC neurons results in the opposite phenotype [91]. Recently, the G-protein-coupled receptor Gpr17 is shown to be a transcriptional target of FOXO1 in AgRP neurons, and a novel modulator of food intake [42]. Future studies are needed to dissect functional interactions among these novel signaling components and to determine their roles in modulation of melanocortinergic function under physiological and pathological conditions.
While impairment of leptin action is likely the result of multiple mechanisms induced by nutrient excess, one player that contributes to hypothalamic leptin resistance could be leptin itself. Under conditions of negative energy balance, the decline in plasma leptin levels is detected by the brain and triggers adaptive responses to increase appetite and lower energy expenditure. Such responses are intact even in obese individuals, where the adaptive responses triggered by weight loss can be prevented by replacement doses of leptin administration [92]. In contrast, under conditions of chronic positive energy balance (e.g. prolonged consumption of fat rich diets) the resultant elevation in circulating leptin concentration is inevitably met with the development of leptin resistance, attenuating the anorexic effects of the elevated leptin. Evidence suggests that chronic hyperleptinemia arising from obesity, but not the obesity alone, is required for the development of leptin resistance [93]. Accordingly, over-expression of leptin receptors in POMC neurons [94] and transgenic overexpression of leptin [95-98] predisposes mice to leptin resistance and diet-induced obesity. Elevated expression of suppressor of cytokine signaling 3 (SOCS3), a transcriptional target of STAT3, may serve as a negative feedback inhibitor of leptin signaling. SOCS3 expression is increased by leptin and in diet-induced obesity, but is reduced in leptin-deficient mice [65]. Transgenic upregulation of SOCS3 in POMC neurons not only inhibits STAT3 signaling but also suppresses other signaling pathways, including mTOR signaling [99]. Indeed, constitutive activation of STAT3 in POMC neurons causes obesity, which is attributed to increased feedback inhibition by SOCS3 and impairment of other signaling pathways [100]. It should be noted that SOCS3 can also be induced by STAT3-independent signaling mechanisms [90, 101].
The contribution of increased leptin action to leptin resistance may appear counter-intuitive at first glance. However, consistent with the essential role of leptin in adaptive responses to starvation, leptin-brain communication may have evolved to defend body adiposity which could be advantageous in times of famine [102]. Thus, the development of leptin resistance may allow body adiposity to be maintained at elevated levels in the face of hyperleptinemia, consistent with the physiological role of leptin to preserve body adiposity. Interestingly, obesity and starvation, two seemingly opposite physiological conditions, share some neuroendocrine similarities. This includes reduced leptin action via lower plasma leptin concentrations or acquired leptin resistance, increased firing frequency of AgRP neurons and decreased activity of POMC neurons [80]. Thus, modulation of AgRP and POMC neuronal function in obesity may represent one adaptive mechanism to dampen central leptin action in the face of chronic hyperleptinemia.
Concluding Remarks
Over the past decade, our understanding of the central melanocortin system has advanced considerably. Gone is the notion that POMC and AgRP neurons are simply first-order neurons responding in broadly opposing manners to the same metabolic and hormonal stimuli. It is becoming clear that POMC and AgRP neurons respond to diverse metabolic signals that elicit both unique and opposing physiological effects. These neurons are first-order targets by peripheral metabolic signals, but concurrently they are second- or greater-order neurons. Furthermore, these neurons regulate diverse metabolic processes via melanocortin-dependent and melanocortin-independent mechanisms. In this regard, central melanocortinergic neurons are transceivers of hormonal and neuronal information, receiving, integrating and transmitting this information to other brain regions and peripheral tissues to maintain whole-body energy homeostasis.
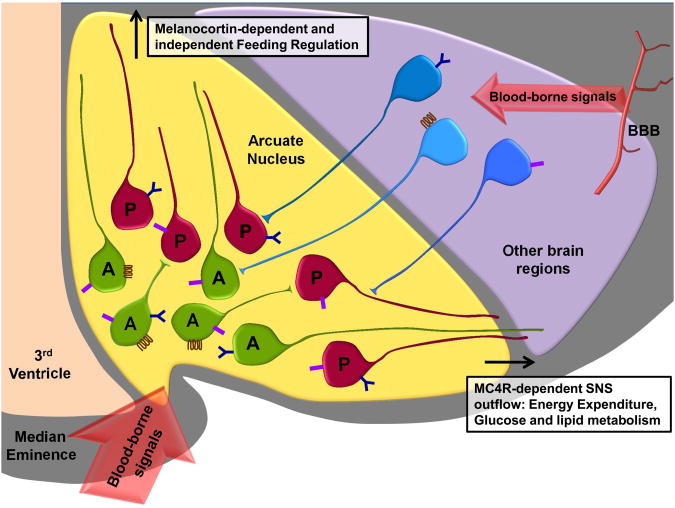
Figure 1. Arcuate melanocortinergic neurons serve as metabolic transceivers to regulate energy balance and peripheral energy metabolism.
The POMC neurons (depicted as red cells) and AgRP neurons (depicted as green cells) are in the vicinity of median eminence, a circumventricular organ that exhibits higher permeability to blood-borne metabolic signals. Blood borne signals also gain access to neurons in other regions of the brain by crossing the blood-brain barrier (BBB). The POMC neurons and AgRP neurons express a variety of different receptors (depicted by different shapes and colors on the cell surface), some of which are also expressed by neurons in other regions of the brain (depicted as blue cells). For example, AgRP neurons express the growth hormone secretagogue receptor (GHSR, the ghrelin receptor), but POMC neurons do not. In contrast, both POMC and AgRP neurons express the leptin receptor and insulin receptor, which are also expressed by some extra-arcuate neuronal subtypes. Hence, in addition to under direct hormonal regulation, the AgRP and POMC neurons are influenced by other neurons that express hormone receptors. AgRP neurons express AgRP, NPY and GABA, while POMC neurons are comprised of distinct subpopulations of GABAergic and glutamatergic neurons and they show heterogeneous responses to leptin and insulin. POMC and AgRP neurons project to the paraventricular nucleus, the parabrachial nucleus and possibly other regions of the brain to regulate feeding via MC4R dependent and independent mechanisms. POMC and AgRP neurons also act on MC4R to regulate sympathetic outflow motor neurons to affect locomotor activity and glucose homeostasis.
Box: Outstanding Questions
What are the identities of the pre-synaptic neurons that can modulate AgRP and POMC neuronal function, and how are these pre-synaptic neurons regulated?
Are all POMC and AgRP neurons created equal? Are there anatomical and functional distinctions among subpopulations of neurons that express the same neuropeptides?
Are diverse peripheral metabolic signals integrated by the same or distinct subpopulations of melanocortinergic neurons?
What are the overlapping and unique functions of neuropeptides and classical small neurotransmitters that are co-expressed from the same melanocortinergic neurons?
Are there distinct downstream target neurons that mediate effects of melanocortinergic neurons on feeding, energy expenditure, hepatic glucose production and lipid metabolism?
What are the specific roles of the parasympathetic and sympathetic nervous system in mediating melanocortin's metabolic functions in different tissues?
Are there site-specific or process-specific alterations of melanocortinergic functions in obesity? What is the temporal regulation of these alterations?
Acknowledgments
J.P.W and A.W.X are supported by research grants from the NIH NIDDK (R01DK080427 and R01DK089094).
Glossary
- Agouti-related protein (AgRP)
AgRP is a neuropeptide that binds to the melanocortin receptor MC4R and MC3R. In the brain, AgRP expression is restricted in a subset of neurons within the arcuate nucleus.
- alpha-Melanocyte stimulating hormone (α-MSH)
An anorexigenic neuropeptide that is synthesized within the arcuate nucleus and brain stem and derived from POMC.
- Anorexigenic
or appetite suppressant, a substance that reduces appetite and food consumption, resulting in weight loss.
- Circumventricular organ
Any of the structures in or near the base of the brain that differ from normal brain tissue in having capillaries that lack the usual blood-brain barrier and thus are not isolated from certain compounds in the blood.
- First-order neurons
In the melanocortin system, “first-order” neurons are those neurons that directly detect and respond to a hormone or metabolite.
- Ghrelin
An orexigenic stomach-derived hormone that binds to the growth hormone secretagogue receptor and stimulates appetite.
- Insulin
A hormone released from the β-cells of the pancreas that regulates blood glucose levels, but has complex acute and chronic actions within the brain.
- Leptin
A hormone released from adipocytes that circulates in proportion to body fat mass and has broadly anorexigenic roles on energy balance, as well as roles in glucose and lipid metabolism that are independent from its actions on food intake.
- Melanocortin 4 receptor (MC4R)
A receptor with widespread distribution within the brain and spinal cord. This receptor is best known to mediate anorexigenic effects exerted by agonist α-MSH. Mutation of MC4R is the most common form of inherited human obesity with a 2.5% prevalence in obese adults.
- Melanocortin 3 receptor (MC3R)
A receptor that has broad distribution in the brain, but is distinct in its distribution and ontogeny compared to the MC4R.
- Neuropeptide Y (NPY)
A neuropeptide that is widely expressed with the brain and acts on the Y receptors (Y1, Y2, Y4, Y5). Within the arcuate nucleus, NPY is co-expressed with AgRP in the same neurons.
- Orexigenic
or appetite stimulant, a naturally derived (such as a hormone) or synthetic compound that increases appetite and food consumption. Certain neuropeptides, such as ghrelin, orexin and neuropeptide Y, are considered orexigenic.
- Parasympathetic nervous system
Part of the autonomic nervous system, the main nerves of which are the vagus nerves that originate in the medulla oblongata. Preganglionic parasympathetic neurons synapse with a few postganglionic neurons close to, or in, the target organ. Pre- and post-ganglionic neurons use mainly acetylcholine as the neurotransmitter.
- Proopiomelanocortin (POMC)
A polypeptide post-translationally processed by prohormone convertases to generate small peptides, including adrenocorticotrophin (ACTH) and the α-, β- and γ-melanocyte stimulating hormones (MSHs).
- Second (or higher) order neurons
neurons that, while not directly detecting a hormone or metabolite, receive inputs from “first order” neurons either directly (“second-order”) or indirectly (“higher order”) that have directly responded to a hormone or metabolite, thus propagating the signal.
- Sympathetic nervous system
Part of the autonomic nervous system, the preganglionic motor neurons of which arise in the spinal cord. Preganglionic sympathetic neurons use acetylcholine, whereas postganglionic neurons use catecholamines as neurotransmitters.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Broberger C, et al. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowley MA, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 3.Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet. 2002;3:589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- 4.Simpson KA, et al. Hypothalamic regulation of food intake and clinical therapeutic applications. Arq Bras Endocrinol Metabol. 2009;53:120–128. doi: 10.1590/s0004-27302009000200002. [DOI] [PubMed] [Google Scholar]
- 5.Hentges ST, et al. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci. 2009;29:13684–13690. doi: 10.1523/JNEUROSCI.3770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarvie BC, Hentges ST. Expression of GABAergic and glutamatergic phenotypic markers in hypothalamic proopiomelanocortin neurons. J Comp Neurol. 2012;520:3863–3876. doi: 10.1002/cne.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vong L, et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morita S, Miyata S. Different vascular permeability between the sensory and secretory circumventricular organs of adult mouse brain. Cell Tissue Res. 2012 doi: 10.1007/s00441-012-1421-9. [DOI] [PubMed] [Google Scholar]
- 10.Mullier A, et al. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol. 2010;518:943–962. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciofi P, et al. Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus. Endocrinology. 2009;150:5509–5519. doi: 10.1210/en.2009-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faouzi M, et al. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology. 2007;148:5414–5423. doi: 10.1210/en.2007-0655. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez EM, et al. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- 14.Farooqi IS, O'Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab. 2008;4:569–577. doi: 10.1038/ncpendmet0966. [DOI] [PubMed] [Google Scholar]
- 15.Ranadive SA, Vaisse C. Lessons from extreme human obesity: monogenic disorders. Endocrinol Metab Clin North Am. 2008;37:733–751. x. doi: 10.1016/j.ecl.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balthasar N, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Rossi J, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao YX. Mutations in the melanocortin-3 receptor (MC3R) gene: Impact on human obesity or adiposity. Curr Opin Investig Drugs. 2010;11:1092–1096. [PubMed] [Google Scholar]
- 19.Begriche K, et al. The role of melanocortin neuronal pathways in circadian biology: a new homeostatic output involving melanocortin-3 receptors? Obes Rev. 2009;10(2):14–24. doi: 10.1111/j.1467-789X.2009.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renquist BJ, et al. Melanocortin-3 receptor regulates the normal fasting response. Proc Natl Acad Sci U S A. 2012;109:E1489–1498. doi: 10.1073/pnas.1201994109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton GM, et al. The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci. 2008;28:12946–12955. doi: 10.1523/JNEUROSCI.3615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begriche K, et al. Homeostastic and non-homeostatic functions of melanocortin-3 receptors in the control of energy balance and metabolism. Physiol Behav. 2011;104:546–554. doi: 10.1016/j.physbeh.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian S, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong Q, et al. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aponte Y, et al. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krashes MJ, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luquet S, et al. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 28.Gropp E, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 29.Wu Q, et al. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atasoy D, et al. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Q, et al. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc Natl Acad Sci U S A. 2008;105:2687–2692. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Q, et al. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brito MN, et al. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148:5339–5347. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- 34.Shrestha YB, et al. Central melanocortin stimulation increases phosphorylated perilipin A and hormone-sensitive lipase in adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2010;299:R140–149. doi: 10.1152/ajpregu.00535.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nogueiras R, et al. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117:3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wellhoner P, et al. Intranasal application of the melanocortin 4 receptor agonist MSH/ACTH(4-10) in humans causes lipolysis in white adipose tissue. Int J Obes (Lond) 2012;36:703–708. doi: 10.1038/ijo.2011.105. [DOI] [PubMed] [Google Scholar]
- 37.Williams DL, et al. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology. 2003;144:4692–4697. doi: 10.1210/en.2003-0440. [DOI] [PubMed] [Google Scholar]
- 38.Vaughan CH, et al. Characterization of a novel melanocortin receptor-containing node in the SNS outflow circuitry to brown adipose tissue involved in thermogenesis. Brain Res. 2011;1411:17–27. doi: 10.1016/j.brainres.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yasuda T, et al. Hypothalamic melanocortin system regulates sympathetic nerve activity in brown adipose tissue. Exp Biol Med (Maywood) 2004;229:235–239. doi: 10.1177/153537020422900303. [DOI] [PubMed] [Google Scholar]
- 40.Enriori PJ, et al. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konner AC, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Ren H, et al. FoxO1 Target Gpr17 Activates AgRP Neurons to Regulate Food Intake. Cell. 2012;149:1314–1326. doi: 10.1016/j.cell.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pocai A, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 44.van den Hoek AM, et al. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes. 2008;57:2304–2310. doi: 10.2337/db07-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Jonghe BC, et al. Melanocortin control of energy balance: evidence from rodent models. Cell Mol Life Sci. 2011;68:2569–2588. doi: 10.1007/s00018-011-0707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Y, et al. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann N Y Acad Sci. 2011;1243:1–14. doi: 10.1111/j.1749-6632.2011.06248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krasnow SM, Marks DL. Neuropeptides in the pathophysiology and treatment of cachexia. Curr Opin Support Palliat Care. 2010;4:266–271. doi: 10.1097/SPC.0b013e32833e48e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banks WA. The blood-brain barrier: connecting the gut and the brain. Regul Pept. 2008;149:11–14. doi: 10.1016/j.regpep.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belgardt BF, et al. Hormone and glucose signalling in POMC and AgRP neurons. J Physiol. 2009;587:5305–5314. doi: 10.1113/jphysiol.2009.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinto S, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 51.Horvath TL, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A. 2010;107:14875–14880. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghamari-Langroudi M, et al. Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin. Proc Natl Acad Sci U S A. 2011;108:355–360. doi: 10.1073/pnas.1016785108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams KW, et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klockener T, et al. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat Neurosci. 2011;14:911–918. doi: 10.1038/nn.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Y, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao Q, et al. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 57.Andrews ZB, et al. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, et al. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leinninger GM, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leshan RL, et al. Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring) 2006;14(5):208S–212S. doi: 10.1038/oby.2006.310. [DOI] [PubMed] [Google Scholar]
- 61.Scott MM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marsh DJ, et al. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 63.Balthasar N, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 64.van de Wall E, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leshan RL, et al. Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat Med. 2012 doi: 10.1038/nm.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warne JP, et al. Impairment of Central Leptin-Mediated PI3K Signaling Manifested as Hepatic Steatosis Independent of Hyperphagia and Obesity. Cell Metab. 2011;14:791–803. doi: 10.1016/j.cmet.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Huo L, et al. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9:537–547. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berglund ED, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakazato M, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 70.Cummings DE, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 71.Zigman JM, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Willesen MG, et al. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- 73.Villanueva EC, et al. Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status. Endocrinology. 2009;150:4541–4551. doi: 10.1210/en.2009-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cowley MA, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 75.Chen HY, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 76.Liu T, et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benoit SC, et al. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. J Clin Invest. 2009;119:2577–2589. doi: 10.1172/JCI36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hosoi T, et al. Endoplasmic reticulum stress induces leptin resistance. Mol Pharmacol. 2008;74:1610–1619. doi: 10.1124/mol.108.050070. [DOI] [PubMed] [Google Scholar]
- 79.Ozcan L, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 80.Diano S, et al. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med. 2011;17:1121–1127. doi: 10.1038/nm.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Souza CT, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 82.Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chu CT. Autophagic stress in neuronal injury and disease. J Neuropathol Exp Neurol. 2006;65:423–432. doi: 10.1097/01.jnen.0000229233.75253.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coupe B, et al. Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metab. 2012;15:247–255. doi: 10.1016/j.cmet.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaushik S, et al. Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO Rep. 2012;13:258–265. doi: 10.1038/embor.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quan W, et al. Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Endocrinology. 2012;153:1817–1826. doi: 10.1210/en.2011-1882. [DOI] [PubMed] [Google Scholar]
- 88.Kaushik S, et al. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab. 2011;14:173–183. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Myers MG, Jr, et al. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21:643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fukuda M, et al. Induction of leptin resistance by activation of cAMP-Epac signaling. Cell Metab. 2011;13:331–339. doi: 10.1016/j.cmet.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Banno R, et al. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest. 2010;120:720–734. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosenbaum M, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knight ZA, et al. Hyperleptinemia is required for the development of leptin resistance. PLoS ONE. 2010;5:e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gamber KM, et al. Over-expression of leptin receptors in hypothalamic POMC neurons increases susceptibility to diet-induced obesity. PLoS ONE. 2012;7:e30485. doi: 10.1371/journal.pone.0030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rico L, et al. Targeted overexpression of leptin to keratinocytes in transgenic mice results in lack of skin phenotype but induction of early leptin resistance. Endocrinology. 2005;146:4167–4176. doi: 10.1210/en.2005-0156. [DOI] [PubMed] [Google Scholar]
- 96.Tanaka T, et al. Skeletal muscle AMP-activated protein kinase phosphorylation parallels metabolic phenotype in leptin transgenic mice under dietary modification. Diabetes. 2005;54:2365–2374. doi: 10.2337/diabetes.54.8.2365. [DOI] [PubMed] [Google Scholar]
- 97.Ogus S, et al. Hyperleptinemia precipitates diet-induced obesity in transgenic mice overexpressing leptin. Endocrinology. 2003;144:2865–2869. doi: 10.1210/en.2002-0178. [DOI] [PubMed] [Google Scholar]
- 98.Qiu J, et al. Transgenic mice overexpressing leptin accumulate adipose mass at an older, but not younger, age. Endocrinology. 2001;142:348–358. doi: 10.1210/endo.142.1.7909. [DOI] [PubMed] [Google Scholar]
- 99.Reed AS, et al. Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes. 2010;59:894–906. doi: 10.2337/db09-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ernst MB, et al. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J Neurosci. 2009;29:11582–11593. doi: 10.1523/JNEUROSCI.5712-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prentice AM, et al. Evolutionary origins of the obesity epidemic: natural selection of thrifty genes or genetic drift following predation release? Int J Obes (Lond) 2008;32:1607–1610. doi: 10.1038/ijo.2008.147. [DOI] [PubMed] [Google Scholar]