Abstract
Context:
There is no effective treatment for systemic sclerosis and related fibrosing diseases. Recently the action of CYP11A1 on vitamin D3 was shown to produce biologically active 20S-hydroxyvitamin D [20(OH)D3] and 20,23(OH)2D3, 20,22(OH)2D3, and 17,20,23(OH)3D3.
Objectives:
Because 20(OH)D3 is noncalcemic (nontoxic) in vivo at very high doses, we evaluated its antifibrogenic activities both in vitro and in vivo. Because it is further metabolized by CYP11A1, we also tested preclinical utilities of its hydroxyderivatives, especially 20,23(OH)2D3.
Design:
Human dermal fibroblasts from scleroderma and normal donors were used to test the efficiency of hydroxyvitamin D derivatives in inhibiting TGF-β1-induced collagen and hyaluronan synthesis and inhibiting cell proliferation. The in vivo activity of 20(OH)D3 was tested using bleomycin-induced sclerosis in C57BL/6 mice.
Results:
20(OH)D3 and 20,23(OH)2D3 inhibited TGF-β1-induced collagen and hyaluronan synthesis similarly to 1,25(OH)2D3 in cultured human fibroblasts. Also, 20(OH)D3, 20,23(OH)2D3, and 1,25(OH)2D3 suppressed TGF-β1-induced expression of COL1A2, COL3A1, and hyaluronan synthase-2 mRNA, indicating that they regulate these matrix components at the transcriptional level. 20(OH)D3, 20,23(OH)2D3, 20,22(OH)2D3, and 17,20,23(OH)3D3 inhibited proliferation of dermal fibroblasts with comparable potency with 1,25(OH)2D3, with 20(OH)D2 being less active and 1α(OH)D3 being almost inactive. 20,23(OH)2D3 at 3 μg/kg had no effect on serum Ca++ or fibroblast growth factor-23 levels and did not cause any noticeable signs of morbidity. 20(OH)D3 markedly suppressed fibrogenesis in mice given sc bleomycin as demonstrated by total collagen content and hematoxylin and eosin staining of skin biopsies.
Conclusions:
20(OH)D3 is an excellent candidate for preclinical studies on scleroderma, with other CYP11A1-derived products of its metabolism deserving further testing for antibrogenic activity.
Calcitriol [1,25(OH)2D3], aside from regulating calcium metabolism, shows potent immunomodulatory, antiinflammatory, and antifibrogenic properties (1–3). Systemic sclerosis (SSc) and related fibrosing diseases are devastating and they shorten the life span of the patient. There are no known effective Food and Drug Administration-approved antifibrotic treatments for SSc and related fibrotic diseases (4, 5). Although 1,25(OH)2D3 is an excellent candidate for treatment of human autoimmune diseases, such as SSc (scleroderma), rheumatoid arthritis, multiple sclerosis, psoriasis, and systemic lupus erythematosus (1, 3–5), its application is severely limited by its toxic (calcemic) effects (2, 3). Therefore, there is a continuing effort to develop noncalcemic vitamin D analogs to treat autoimmune diseases (6, 7).
Recently a novel pathway of vitamin D metabolism, mediated by the action of cytochrome P450 side chain cleavage (P450scc, CYP11A1) was discovered (Supplemental Scheme 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org), which produces 20S-hydroxyvitamin D [20(OH)D3 and 20(OH)D2] as the first and main metabolites (8–10), with 20(OH)D3 being further hydroxylated by CYP11A1 to 20,23(OH)2D3 and 17,20,23(OH)3D3 (9, 11). This pathway can operate in vivo, with 20(OH)D3 serving as an endogenous product (12). These hydroxyderivatives of vitamin D3 induce the differentiation program in human keratinocytes and leukemia cells and inhibit nuclear factor-κβ activity (13–16), with 20(OH)D3 being noncalcemic in rats and mice at concentrations as high as 3.0 μg/kg (15) and 30 μg/kg (17), respectively.
This lack of in vivo toxicity at very high doses (17) and antiproliferative and potentially antiinflammatory activities (13, 14, 16), together with the enzymatic origin of these derivatives (11) defining them as natural products (12), prompted us to test their in vitro and in vivo antifibrogenic activities.
Materials and Methods
The experiments were approved by local Institutional Animal Care and Use Committee and institutional review board protocols committees. 20(OH)D3/D2 and 20,23(OH)2D3/17,20,23(OH)3D3 were produced enzymatically (9, 11). 1(OH)D3 and 1,25(OH)2D3 were from Sigma Chemical Co. (St Louis, Missouri).
In vitro experiments
Human dermal fibroblasts were isolated from biopsies of a scleroderma patient and from normal donors and were grown as described previously (18, 19). The confluent fibroblasts were preincubated in serum-free media for 24 hours, and vitamin D hydroxyderivatives (10−9 or 10−10 M) were added to 3 replicate wells 2 hours prior to addition of recombinant human TGF-β1 (R & D Systems, Minneapolis, Minnesota) to a final concentration of 5 ng/mL. To measure total collagen and hyaluronan production, after 48 hours of culture plate wells were pulsed for 24 hours with 1 μCi [3H]proline to assess collagen production (20) or 12.5 μCi [3H]acetate to assess hyaluronan production (19). To measure type I collagen protein, supernatants were collected after 48 hours of treatment and collagen I immunoreactivity was measured using an ELISA specific for type I collagen from Chondrex Corp (Redmond, Washington) (18).
Fibroblasts from 3 different donors were grown to confluency in a series of 100-mm-diameter tissue culture dishes in Eagle's MEM (EMEM) supplemented with 9% charcoal-stripped fetal calf serum and then cultured for 24 hours in serum-false EMEM to synchronize the cells. Medium was then changed to EMEM containing 1% charcoal-stripped fetal calf serum and ascorbic acid (50 μg/mL). To culture dishes were added 20(OH)D3, 20,23(OH)2D3, and 1,25(OH)2D3 at a final concentration of 10−8 or 10−10 M or ethanol (EtOH) vehicle at 1:10 000 or 1:1 million dilution to control for final EtOH content of the cultures. After 2 hours incubation, recombinant human TGF-β1 (R & D Systems) dissolved in PBS was added at a final concentration of 10 ng/mL, or PBS alone was added to appropriate cultures. After an additional 16 hours culture, total RNAs were isolated from fibroblast monolayers using Trizol reagent (Invitrogen, Carlsbad, California) and purified using an RNA easy cleanup kit (QIAGEN, Valencia, California). RNAs were quantified using NanoDrop-2000 (Thermo, Franklin, New Jersey), and the quality of RNA was checked using a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, California). Fifty nanograms of total RNA were used for Taq Man real-time (RT)-PCR using primers for human COL1A2, COL3A1, hyaluronan synthase (HAS)-1, HAS2, and glyceraldehyde-3-phosphate dehydrogenase (Applied Biosystems Life Technologies, Grand Island, New York). The Taqman RT-PCR reactions were carried out with an ABI 7900 real time PCR system [software: sequence detection system (Applied Biosystems Inc, Foster City, California)] using Applied Biosystems' standard protocol for RT-PCR and PCR. Samples were normalized to glyceraldehyde-3-phosphate dehydrogenase mRNA expression levels. Data for hydroxyvitamin D derivative treated fibroblast cultures are shown as ΔΔCT normalized to an endogenous reference (PBS+EtOH) vehicle culture.
Testing of antiproliferative activity on human dermal fibroblasts is described in the Supplemental Data.
Preclinical model of scleroderma
Female C57BL/6 mice 6 weeks old were purchased from Jackson Labs (Bar Harbor, Maine) and maintained on a regular laboratory chow diet. Groups of mice (5 each) received daily injections of either vehicle (50 μL sesame oil, ip, and 100 μL saline, sc); bleomycin (180 μg per 100 μL bleomycin, sc, and 50 μL sesame oil, ip); or bleomycin + 20(OH)D3 (180 μg per 100 μL bleomycin, sc, and 3 μg/kg of 20(OH)D3 per 50 μL sesame oil, ip) for 21 days. The skin was injected sc with bleomycin or vehicle daily within the same 1.5-cm2 area. On day 22 all mice were euthanized and skin biopsies of 1 cm circumference were taken and divided in two. One part was formalin fixed and processed for histopathological analysis, whereas the second part was frozen. The frozen skin samples were thawed and treated overnight with pepsin (0.1 mg/mL) and 0.5 M acetic acid at 4°C to remove terminal nonhelical telopeptides to release the collagen into solution. Total solubilized collagen was measured using a Sircol collagen assay kit (Biocolor Ltd, Carrickfergus, United Kingdom). The calcemic effects of 20(OH)D3 and 20,23(OH)2D3 were measured as described previously (18). Data are presented as means ± SEM and were analyzed with a Student's t test (for 2 groups) or ANOVA using Prism 4.00 (GraphPad, San Diego, California).
Testing calcemic effects
Female C57BL/6 mice, 6 weeks old, were purchased from Jackson Labs and maintained on a regular laboratory chow diet. Mice were divided into groups of 3 to receive either sterile sesame oil (50 μL; Sigma Chemical Co) or 3 μg/kg 20,23(OH)2D3 dissolved in 50 μL sterile sesame oil by daily ip injection for 14 days, as described previously (17, 18). On day 15 mice were euthanized and sera were taken for measurement of total Ca++ by atomic absorption spectrophotometry and fibroblast growth factor-23 by ELISA (Immuntopics, San Clemente, California), as described previously (18).
Docking analyses
Docking analyses were performed as described in the Supplemental Data using the crystal structure of the vitamin D receptor (VDR).
Statistical analyses
Differences in values obtained with the vitamin D hydroxyderivatives were compared with appropriate controls using an ANOVA, with P ≤ .05 considered statistically different.
Results and Discussion
The profibrotic activities of TGF-β1 have been implicated in the etiology of systemic sclerosis and related fibrosing diseases as well as the bleomycin skin fibrosis preclinical model of scleroderma (4, 5, 19, 21). Both 20(OH)D3 and 1,25(OH)2D3 at 10−10 M concentration significantly inhibited TGF-β1-induced total collagen protein and hyaluronan production by confluent cultures of normal human fibroblasts with comparable efficacy (Figure 1A). The fibroblasts remained confluent, maintained normal morphology, and their viability after treatments was greater than 90% by trypan blue exclusion. A similar inhibition was seen at 10−9 M (not shown). This effect of 20(OH)D3 on collagen production was further confirmed in dermal fibroblasts grown from involved skin from a patient with diffuse cutaneous SSc using an ELISA test, which showed that secreted type I collagen protein production was inhibited (Figure 1B). In additional experiments using another normal dermal fibroblast line, we observed inhibitory effects of 20(OH)D3, 20,23(OH)2D3, and 1,25(OH)2D3 on collagen synthesis (Figure 1C).
Figure 1.
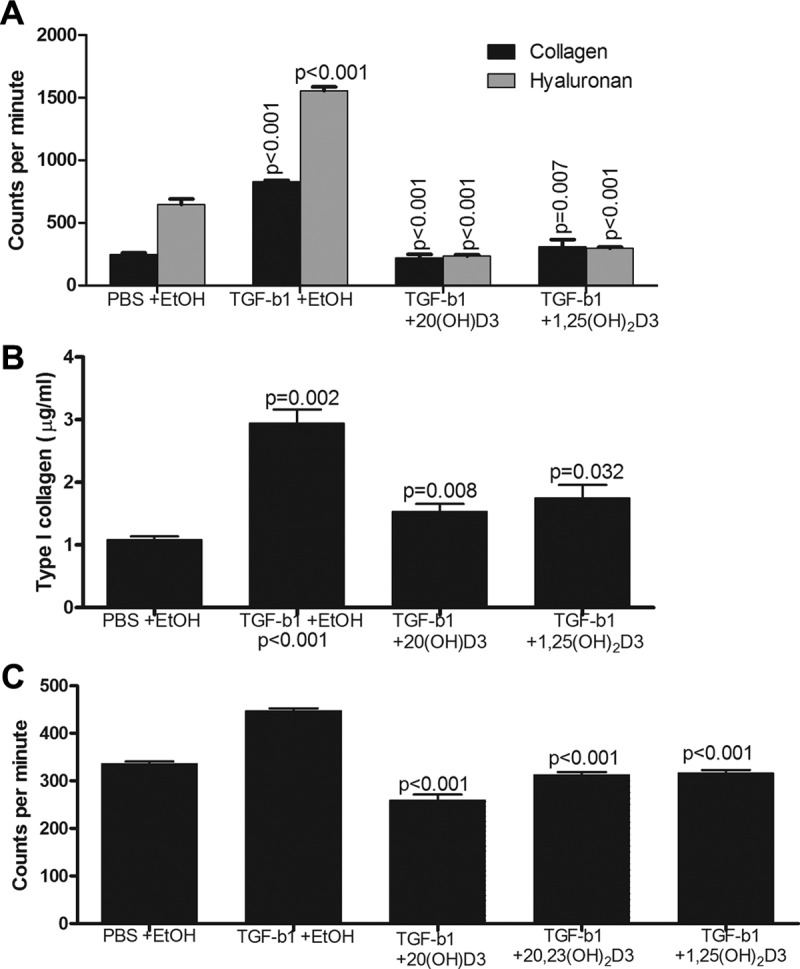
Vitamin D hydroxyderivatives inhibit total collagen and hyaluronan production stimulated by TGF-β1. Scleroderma human dermal fibroblast line 08 (A and B) or normal human dermal fibroblasts line 442 (C) were exposed to 20(OH)D3, 20,23(OH)2D3, or 1,25(OH)2D3 and then treated with TGF-β1 as described. Total collagen (A and C) and hyaluronan (A) and collagen type 1 (B) were measured versus PBS + EtOH and TGF-β1 + EtOH controls, respectively. Data are presented as mean ± SEM of 3 replicates, and P values for statistically significant differences between control and treatments are shown above the bars. Results were confirmed using 2 additional normal human dermal fibroblast lines (data not shown).
In studies using additional normal human fibroblasts, induced by TGF-β1, hydroxyderivatives of vitamin D caused the suppression of collagen and hyaluronan production at the transcriptional level (Figure 2). Specifically fibroblasts exhibited up-regulation of mRNAs for COL1A2 (P < .05), COL3A1 (P < .01), and HAS2 (P < .01) after 16 hours treatment with TGF-β1 that was significantly suppressed by either 10−8 or 10−10 M of 20(OH)D3, 20,23(OH)2D3, and 1,25(OH)2D3, which served as positive control for classical active form of vitamin D (Figure 2, A–C). The experiment was repeated with similar results (not shown). Under the conditions used, HAS1 mRNA expression was low and did not change after TGF-β1 treatment, preventing a reliable evaluation of vitamin D analogs modulatory effects (not shown).
Figure 2.
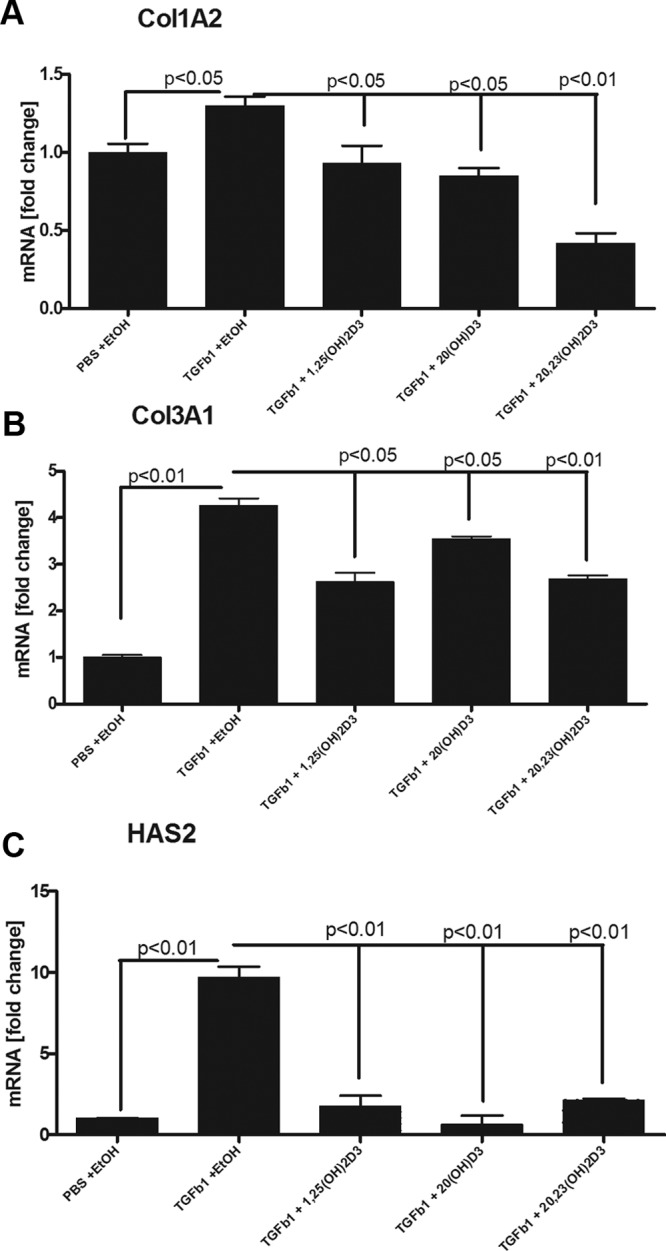
Vitamin D hydroxyderivatives down-regulate mRNAs of types I and III collagens and hyaluronan synthase 2 in TGFβ1-stimulated fibroblasts. Expression of COL1A2 mRNA in line Fib1 treated with 10−10 M vitamin D hydroxy-derivatives (A); COL3A1 mRNA in line Fib1 treated with 10−8M vitamin D hydroxy-derivatives (B); and HAS2 in line Z4 treated with 10−8 M vitamin D hydroxyderivatives (C) are shown. Results are expressed as mean ± SD, with P values of EtOH + TGF-β1 vs different treatments as indicated above connecting lines.
Although in this report, we have not explored mechanisms by which hydroxyvitamin D derivatives suppress TGF-β induced up-regulation of collagen gene expression, studies reported by others show that another hydroxyvitamin D derivative, 22-oxacalcitrol down-regulates levels of phosphorylated mothers against decapentaplegic homolog 2/3 (Smad2/3) and inhibits binding of phospho-Smad 3 to the proximal promoter region of the TGFβ-1 gene, thus attenuating TGF-β1 autoinduction (22, 23). This could reduce TGF-β1 influence on SP1 and AP-1 binding sequences regulating proα 1 (I) collagen promoter and proα2 (I) collagen gene expression (24).
It must be noted that 20(OH)D3 is noncalcemic in rats (15) and mice (17) at very high doses of at least 3 μg and 30 μ/kg, respectively. Since 20,23(OH)2D3 is a first and a major product of subsequent metabolism of 20(OH)D3 (Supplemental Scheme 1), we tested its calcemic activity in C57BL/6 mice. 20,23(OH)2D3 at 3 μg/kg had no effect on serum Ca++ or fibroblast growth factor-23 levels (Supplemental Table 1) and did not show any noticeable signs of morbidity. Similarly, histological analyses of heart and kidney showed no toxicity (not shown). In contrast, 1,25(OH)2D3 at an even lower dose of 2 μg/kg increased Ca++ up to 14.6 ± 0.5 mg/dL with secondary morbidity (17). Thus, 20(OH)D3 and 20,23(OH)2D3 are excellent candidates for further preclinical testing in models of human fibrosing diseases.
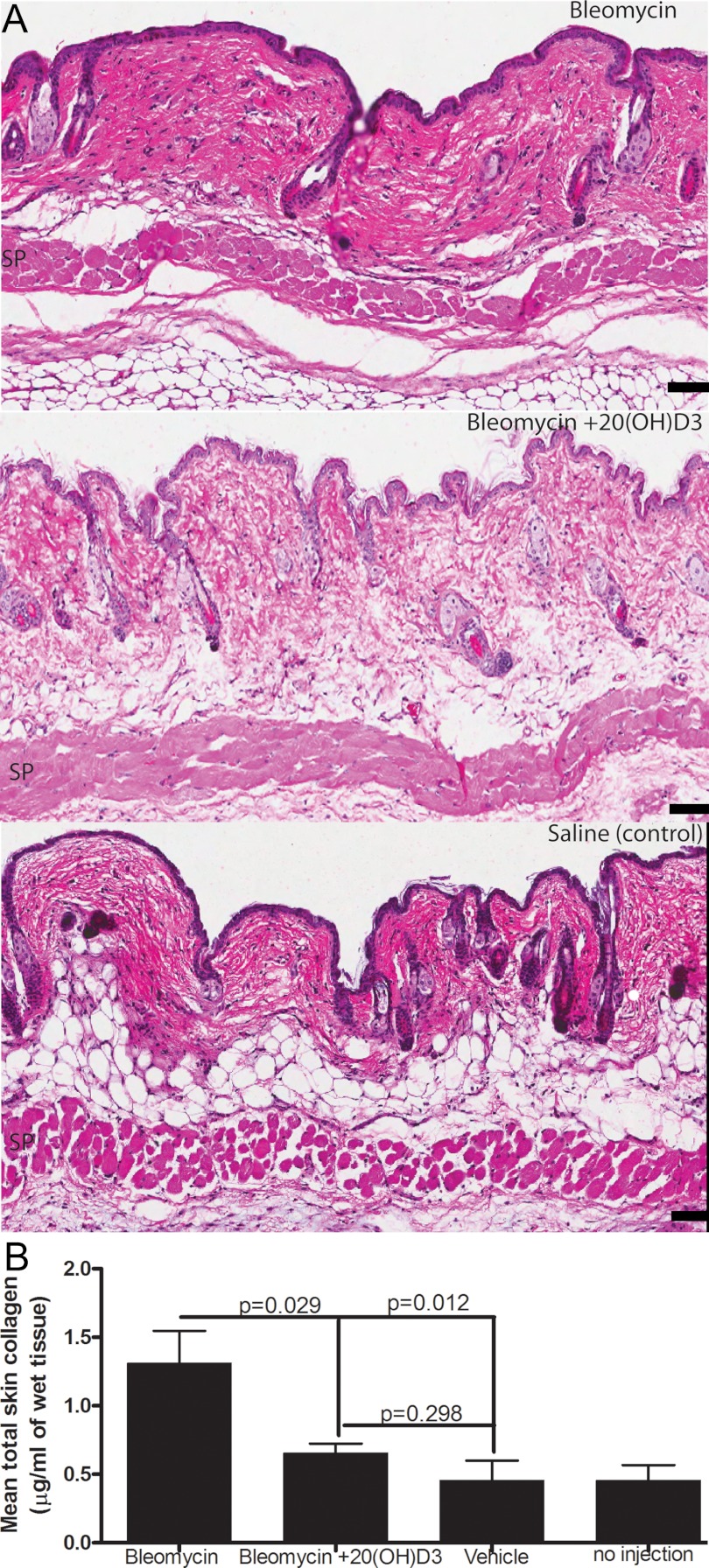
Because 20(OH)D3 is the main product of P450scc-mediated metabolism of vitamin D3 that dissociates from the catalytic site of the enzyme and is produced endogenously by skin epidermal keratinocytes (12), we focused our studies on testing its activity using a model of bleomycin-induced dermal sclerosis in mice. Although the mice injected with bleomycin showed skin hardening only on palpation, the skin of mice receiving concomitant 20(OH)D3 appeared to be normal by gross examination. Measurement of total collagen in skin biopsies showed that 20(OH)D3 inhibited normalized collagen content in mice given sc bleomycin (Figure 3B). Hematoxylin and eosin-stained slides of skin biopsies from mice with bleomycin-induced scleroderma also showed a marked reduction in collagen after treatment with 20(OH)D3 compared with those treated with sesame oil (Figure 3A). The bleomycin scleroderma model is characterized by an early infiltration of monocytes and neutrophils, which is replaced by intense fibrosis that increases from days 7–21 with minimal numbers of macrophages at day 21 (21). We were unable to discern a difference in the small number of mononuclear cells in skin sections of the different treatment groups. In view of the in vitro and in vivo results in this report, we conclude that 20(OH)D3 is an excellent candidate for further testing of its therapeutic utility in scleroderma or other skin-fibrosing diseases.
Figure 3.
20(OH)D3 attenuates the bleomycin-induced fibrosis in C57BL/6 mice. A, Hematoxylin and eosin-stained section (×80 magnification) show significant attenuation of bleomycin-induced fibrosis by 3 μg/kg of 20(OH)D3. SP, panniculus carnosus. B, 20(OH)D3 inhibits bleomycin-induced production of total solubilized collagen from skin fragments. Total collagen production is presented as mean ± SEM per treatment in micrograms per milligram of wet tissue, with indicated P values vs control. Scale bar, 75 μm.
Because 20(OH)D acts as a partial agonist on the VDR (15, 16), we performed docking experiments using the crystal structure of VDR, which showed that 20,23(OH)2D3 and 20(OH)D3 bind to the VDR in poses that overlap very well with native ligand, 1,25(OH)2D3, with relatively high docking scores (Supplemental Figure 1 and Supplemental Table 2). This supports that VDR is the main target for 20(OH)D3 and 20,23(OH)2D3 bioregulation. However, the final proof would require future careful testing using VDR−/− mice.
Because 20(OH)D3 and 20,23(OH)2D3 are further hydroxylated by P450scc in vitro and ex vivo to several minor products (Supplemental Scheme 1) (11, 12), we tested their activity in human dermal fibroblasts. Supplemental Figure 2 shows inhibition of proliferation of neonatal fibroblasts by 20(OH)D3, 20,23(OH)2D3, 20,22(OH)2D3, and 17,20,23(OH)3D3 with a potency similar to 1,25(OH)2D3, with 20(OH)D2 being less active and 1α(OH)D3 being almost inactive. This shows that subsequent metabolites of the P450scc-mediated pathway retain the biological activity of the parent 20(OH)D3 and that vitamin D3 hydroxyderivatives are more promising than vitamin D2 hydroxyderivatives in terms of therapeutic utility in fibrosing diseases.
Recently we detected production of 20(OH)D3, 20,23(OH)2D3, 20,22(OH)2D3, and 17,20,23(OH)3D3 in human epidermal keratinocytes, whether using endogenous or exogenous vitamin D3 as the substrate. The antifibrogenic activity of these novel vitamin D hydroxyderivatives documented in this paper opens up new exciting possibilities for the keratinocyte-based pathway as a regulator of dermal functions. In skin pathology one can envision topical application of noncalcemic 20(OH)D3 and 20,23(OH)2D3 to treat local fibrosing diseases such as morphea (localized scleroderma), keloid, or fibromatosis for which there is no effective treatment.
In conclusion, 20(OH)D3 is an excellent candidate for preclinical studies on scleroderma, with other CYP11A1-derived products of its metabolism deserving further testing for its ability to prevent and/or reverse fibrosis.
Acknowledgments
The excellent technical assistance of Patricia Wheller, MS, is acknowledged.
This work was supported by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants R01A052190 (to A.S.) and National Institutes of Health Grant 1R01AR056666-01A2 (to A.S.), a Merit Review Award from the US Department of Veterans Affairs (to A.E.P.). Additional partial support was also obtained from the Goodman Chair of Excellence in Medicine (to A.E.P.) of the University of Tennessee Health Science Center.
Disclosure Summary: The authors declare no conflict of interest.
Footnotes
- CYP11A1
- Cytochrome P450scc
- EMEM
- Eagle's MEM
- EtOH
- ethanol
- HAS
- hyaluronan synthase
- 20(OH)D2
- 20-hydroxyvitamin D2
- 1(OH)D3
- 1α-hydroxyvitamin D3
- 20(OH)D3
- 20S-hydroxyvitamin D3
- 1,25(OH)2D3
- 1α,25-dihydroxyvitamin D3
- 20,23(OH)2D3
- 20,23-dihydroxyvitamin D3
- 17,20,23(OH)3D3
- 17,20,23-trihydroxyvitamin D3
- P450scc
- P450 side chain cleavage
- RT
- real time
- SSc
- systemic sclerosis
- VDR
- vitamin D receptor.
References
- 1. Szodoray P, Nakken B, Gaal J, et al. The complex role of vitamin D in autoimmune diseases. Scand J Immunol. 2008;68:261–269 [DOI] [PubMed] [Google Scholar]
- 2. Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010;21:375–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 4. Varga J, Pasche B. Transforming growth factor β as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5:200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trojanowska M, Varga J. Molecular pathways as novel therapeutic targets in systemic sclerosis. Curr Opin Rheumatol. 2007;19:568–573 [DOI] [PubMed] [Google Scholar]
- 6. Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9:941–955 [DOI] [PubMed] [Google Scholar]
- 7. Carlberg C, Molnar F, Mourino A. Vitamin D receptor ligands: the impact of crystal structures. Exp Opin Ther Patents. 2012;22:417–435 [DOI] [PubMed] [Google Scholar]
- 8. Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1). Proc Natl Acad Sci USA. 2003;100:14754–14759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Slominski A, Semak I, Zjawiony J, et al. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slominski A, Semak I, Wortsman J, et al. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273:2891–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tuckey RC, Li W, Zjawiony JK, et al. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008;275:2585–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slominski AT, Kim TK, Shehabi HZ, et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 26(9):3901–3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM, Jr, Slominski AT. 20,23-Dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-κB activity in human keratinocytes. J Cell Physiol. 2010;223:36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zbytek B, Janjetovic Z, Tuckey RC, et al. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128:2271–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slominski AT, Janjetovic Z, Fuller BE, et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5:e9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slominski AT, Kim TK, Janjetovic Z, et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2011;300:C526–C541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang J, Slominski A, Tuckey RC, et al. 20-Hydroxyvitamin D3 inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–746 [PMC free article] [PubMed] [Google Scholar]
- 18. Slominski AT, Li W, Bhattacharya SK, et al. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J Invest Dermatol. 2011;131:1167–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Postlethwaite AE, Smith GN, Jr, Lachman LB, et al. Stimulation of glycosaminoglycan synthesis in cultured human dermal fibroblasts by interleukin 1. Induction of hyaluronic acid synthesis by natural and recombinant interleukin 1s and synthetic interleukin 1β peptide 163–171. J Clin Invest. 1989;83:629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raghow R, Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Transforming growth factor-β increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J Clin Invest. 1987;79:1285–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takagawa S, Lakos G, Mori Y, Yamamoto T, Nishioka K, Varga J. Sustained activation of fibroblast transforming growth factor-β/Smad signaling in a murine model of scleroderma. J Invest Dertmatol. 2003;121:41–50 [DOI] [PubMed] [Google Scholar]
- 22. Hirose M, Nishino T, Obata Y, et al. 22-Oxacalcitriol prevents progression of peritoneal fibrosis in a mouse model [published online ahead of print October 2, 2012]. Perit Dial Int. doi:10.3747/pdi.2011.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inoue K, Matsui I, Hamano T, et al. Maxacalcitol ameliorates tubulointerstitial fibrosis in obstructed kidneys by recruiting PPM1A/VDR complex to pSmad3. Lab Invest. 2012;92(12):1686–1697 [DOI] [PubMed] [Google Scholar]
- 24. Cutroneo KR. Human SP1 but not human AP1 binding to the TGF-β element in the 5′ flanking region of the rat PROα1(I)Collagen gene. Mol Bio Rep. 3:191–194 [DOI] [PubMed] [Google Scholar]