Abstract
Rationale:
Intermedin (IMD) is a novel peptide expressed in trophoblast cells in human placenta and enhances the invasion, migration, and human leukocyte antigen class I, G (HLA-G) expression in first-trimester HTR-8SV/neo cells. We recently reported that infusion of IMD antagonist in pregnant rats is detrimental to pregnancy outcome, resulting in impaired fetoplacental growth and deformed placental vasculature.
Objective:
This study was undertaken to assess expression of IMD and its involvement in human implantation and early placentation and assess whether its expression is altered in spontaneous abortion.
Findings and Conclusions:
We demonstrate for the first time that IMD is present in day 5 embryonic secretome; villous and decidual expression of IMD is higher at 6–8 weeks after a decline as gestation advances toward the second trimester; first-trimester spontaneous abortion is associated with a lower expression of IMD in serum, villi, and decidua; IMD stimulates the invasive capacity of first-trimester primary Extravillous cytotrophoblast cells; and IMD decreases elevated levels of tumor suppressor Kangia-1 in decidual explants from first-trimester spontaneous abortion. In conclusion, this study is the first to demonstrate a potential involvement of IMD in human embryo implantation and placental development via regulation of trophoblast invasion at the maternal-fetal interface and suggests a physiological role for this novel peptide in establishment of human pregnancy.
Intermedin (IMD), also known as adrenomedullin 2, is a novel peptide, recently discovered simultaneously by 2 groups in 2004 (1, 2). IMD is expressed in many organ systems, including but not limited to the gastrointestinal tract, lungs, cardiovascular and renal systems, hypothalamus, pituitary gland, adrenal gland, placenta, uterus, and ovaries (2–7). IMD functions systemically as a potent vasodilator, delays gastric emptying, and enhances angiogenesis (5–7).
We reported that the expression of IMD is higher in day 9 implantation sites and in day 15 placenta compared with days 18–22 of rat pregnancy (4). Plasma IMD levels are higher during rat pregnancy, and IMD treatment reduces the mean arterial pressure in pregnant rats (8). Furthermore, we showed that infusion of IMD antagonist (IMD17–47) in early rat gestation decreases weights of the implantation sites and results in fetoplacental growth restriction in later gestation (4, 9). In addition, IMD17–47 infusion down-regulates factors important for embryo implantation and early placental development, such as matrix metalloproteinases-2/9, vascular endothelial growth factor, and placental growth factor in day 9 rat gestation (4). Therefore, these studies provide evidence for a potential role of IMD in rat embryo implantation and fetoplacental growth. However, it is not known whether IMD has a physiological role in human pregnancy.
We reported earlier that IMD is expressed in first-, second-, and third-trimester human placentas and in syncytiotrophoblasts, cytotrophoblasts, and villous endothelial cells (3). In addition, we showed that IMD increases the invasive index and migration of trophoblast cells (HTR-8SV/neo) in the expression of human leukocyte antigen class I, G (HLA-G), a marker of the invasive phenotype of trophoblasts and that these effects of IMD are mediated through MAPK3/1 transduction pathway. This suggests a potential involvement of IMD in human placental development in human pregnancy (10).
Human embryo invasion and implantation is a very intricate and coordinated process, which is not completely understood. Optimal trophoblast cell invasion into the uterine deciduas forms the basis of successful pregnancy, causing the rearrangement of the uterine spiral arteries necessary for high conductance of spiral artery to accommodate the huge increase in blood flow to support the growing pregnancy (11). This process is mediated through an intricate balance of invasion promoting factors in villi and inhibitory factors in decidua (11–14). An aberrant expression of these factors results in abnormal trophoblast invasion causing defective spiral artery remodeling resulting in pathological pregnancy states, such as intrauterine growth restriction, preeclampsia, and spontaneous abortion (14–17). Kangia-1 (KAI-1), a tumor suppressor protein that inhibits trophoblast invasion, has recently been shown to be expressed in decidua, and its expression is up-regulated in spontaneous abortion (18, 19).
We hypothesize that IMD increases the invasive capacity of first-trimester cytotrophoblast cells, that IMD levels in serum and placenta are lower in pathological pregnancy, and that IMD treatment decreases the elevated decidual KAI-1 associated with spontaneous abortions. Therefore, the objectives of this study was to assess the expression profile of IMD in first-trimester human pregnancy, assess the pathology-related changes in its expression in spontaneous abortion, assess the effect of IMD on the invasive potential of primary extravillous cytotrophoblast cells (EVCTs) from elective abortion, and to identify whether IMD treatment inhibits the elevated levels of decidual KAI-1 in spontaneous abortions.
Materials and Methods
Human subjects
This study was approved by the Institutional Review Board at the University of Texas Medical Branch at Galveston. Abortion tissues (5–14 weeks) and blood were obtained at 5–14 weeks of gestation from consented elective (before administration of any drug) and spontaneous abortion patients at Planned Parenthood Houston and at onsite University of Texas Medical Branch at Galveston clinics in Galveston, respectively, within 30 minutes after the abortion. Spontaneous abortion tissues were collected from patients diagnosed for fetal death by ultrasound typically within a few hours or days prior to evacuation of uterine contents. Blood was collected before conducting the elective abortion and at the time when patient arrived at the hospital in cases of spontaneous abortion. Plasma was separated and stored at −80°C until used for immunoassay [enzyme immunoassay (EIA)] using an EIA kit as per the instructions of the manufacturer (Phoenix Pharmaceuticals, Belmont, California). Placental tissues were carefully dissected to exclude any membrane contamination, flash frozen for RNA isolation, embedded in optimum cutting temperature compound to make 5- to 7-μm-thick sections for immunohistochemical studies, and used for cells isolation and explant cultures. Gestational ages were calculated from the date of the last menstrual period with either ultrasound or clinical confirmation. Patients with maternal infection, chromosomal abnormalities, or any kind of physical insult were excluded.
Blastocyst culture medium
Blastocyst culture medium was obtained from the Center of Reproductive Medicine (Webster, Texas). Approximately 30–50 μL of discarded Quinn's Advantage blastocyst medium (Sage, Trumbull, Connecticut) was obtained after culturing of 2–5 fresh blastocysts in the medium for approximately 48 hours prior to embryo transfer or storage. Discarded blastocyst culture media were also collected from 2–5 frozen embryos, which were thawed for approximately 2–4 hours in 30–50 μL of Quinn's Advantage blastocyst medium. The discarded fresh and frozen blastocyst culture media were stored at −80°C until used.
Isolation and culture of primary EVCTs
The EVCTs were isolated from the villous tissue per published methods (20), with an additional purification step involving the use of magnetically labeled antibodies. Briefly, an EVCT-enriched fraction obtained after the Percoll gradient purification was purified further to eliminate any residual leukocyte and macrophage contamination by negative selection with magnetically labeled CD45 and CD14 antibody (Advance Magnetics Inc, Cambridge, Massachusetts). Cells were finally purified by positive selection of magnetically labeled CK7-positive cells, and purity of EVCTs was tested by immunocytochemical staining and flow cytometry with antibodies for CK7 and HLA-G.
Immunofluorescent staining
Optimum cutting temperature-embedded frozen tissue sections or primary EVCTs were fixed with methanol and acetone mixture (1:1) for 15 minutes and washed twice for 5 minutes in PBS containing Tween 20 washing solution and processed for immunofluorescent staining and analyzed as reported earlier (9). The antibodies used were for CK7, vimentin (Dako, Carpinteria, California), HLA-G (Santa Cruz Biotechnology, Santa Cruz, California), and IMD (Alpha Diagnostics, San Antonio, Texas) at a dilution of 1:150 as reported earlier or as per the manufacturer's instructions (3). Briefly, chicken antimouse IgG-Alexa Fluor 594 (Molecular Probes, Eugene, Oregon) was used for the detection of the first primary antibody. Absence of antibody, preimmune serum, or mouse IgG (Ready-to-Use; Dako) was used as a negative control. Mouse IgG1 (Dako), IMD antibody neutralized by IMD peptide, and secondary antibody alone were also used as a negative control (data not shown).
Isolation of total RNA and quantitative real-time PCR
Total RNA was isolated from tissues using TRIzol (Life Technologies, Grand Island, New York). Total RNAs were digested with deoxyribonuclease I (catalog number 79254; QIAGEN Inc, Valencia, California), followed by cleanup procedures using an RNeasy minikit (catalog number 74104; QIAGEN) as per the manufacturer's instructions, and cDNA was made as reported earlier (9).
Quantitative real-time quantitative RT-PCR using SYBR Green (Bio-Rad Laboratories, Hercules, California) was performed on a CFX96 real-time PCR detection system (catalog number 184-5096; Bio-Rad Laboratories) for IMD and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using primer gene-specific primer assays (SABiosciences, Frederick, Maryland). Amplification of GAPDH served as an endogenous control. Reactions were incubated at 95°C for 10 minutes and cycled according to the following parameters: 95°C for 30 seconds (melt) and 60°C for 1 minute (anneal/extend) for a total of 40 cycles. A negative control without cDNA was performed to test the primer specificity. The relative gene expression was calculated by use of the threshold cycle GAPDH/threshold cycle target gene. For no-RT control, nuclease-free water was used in place of the reverse transcriptase.
Invasion assay
Invasion of the primary EVCTs was assessed using the Culture 96-well BME cell invasion assay (Trevigen, Gaithersburg, Maryland) per the manufacturer's instruction. In brief, purified primary EVCTs were serum starved for 24 hours prior to the invasion assay. The EVCTs were then seeded onto the BME-coated invasion chambers at 0.5 × 105 cells/well and on inserts without BME and treated with IMD (10−9 to 10−7 M) in the presence or absence of IMD17–47(10−5M). Serum containing 5% and 10% fetal bovine serum was used as a positive control. The EVCTs were then incubated with treatments for 24 hours at 37°C. At the end of the incubation period, the EVCTs were washed, and calcein adrenomedullin was added to each well and incubated for 1 hour. After the final incubation, the receiver plate was read using a fluorescent top reader with filters set to 485 nm for excitation and 520 nm for emission (BMG Labtech, Ortenberg, Germany). The invasive index was calculated as per the manufacturer's instructions and as reported earlier (3).
Decidual explant culture
Decidual tissue from women undergoing spontaneous abortions in the first trimester of pregnancy (∼50 mg/well) were incubated in serum-free medium for 12 hours followed by treatment with IMD (10−7 to 10−9 M) in the presence and absence of IMD antagonist (IMD17–47) in serum-free media. The treatments were stopped after 24 hours, and tissues were collected for RNA extraction.
Enzyme immunoassay
Stored serum samples and blastocyst secretomes were analyzed for IMD concentration with the help of an EIA kit specific for human IMD as per the manufacturer's instruction (Phoenix Pharmaceuticals).
Statistical analysis
The data were statistically analyzed with Prism version 4.00 for Windows (GraphPad Software, San Diego, California; www.graphpad.com). The significance within the groups was analyzed by a Student's t test and a Bonferroni's multiple-comparison test. Analysis of trends via the smoothing splines model was used to analyze IMD levels in villous and decidual tissues. Values were considered significant at P < .05.
Results
Expression of IMD in the secretome of blastocyst culture media
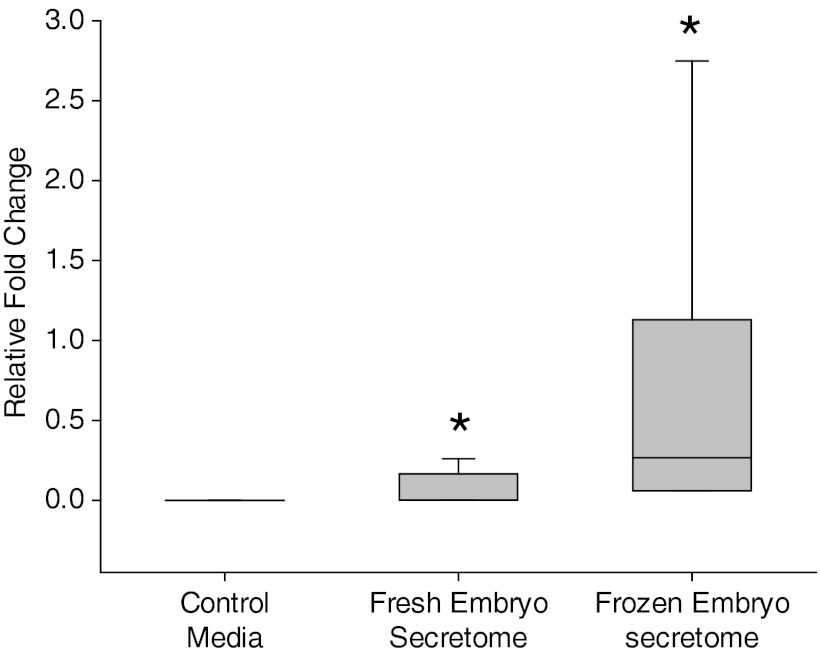
To establish a time line of IMD expression in early pregnancy, discarded blastocyst media secretome was collected and analyzed via an EIA for the presence of IMD. Figure 1 shows that IMD is present in the secretome of day 5 after fertilization fresh (n = 11) and frozen/thawed blastocysts (n = 16), demonstrating its presence as early as the blastocyst stage of pregnancy. IMD levels were found to be statistically higher (P < .05) in frozen/thawed embryos as compared with fresh embryos (Figure 1).
Figure 1.
Expression of immunoreactive IMD in blastocyst secretome. Mean fold change in the expression of IMD in the secretome of day 5 fresh (n = 11) and frozen blastocysts (n = 16) was assessed in the blastocyst culture media after 48 hours of culture. As shown, IMD production in the secretome of embryos is statistically higher than the control (P < .05). Asterisk (*) indicates significant difference between the fresh and the frozen blastocyst culture medium.
Pathology-related changes in serum IMD levels
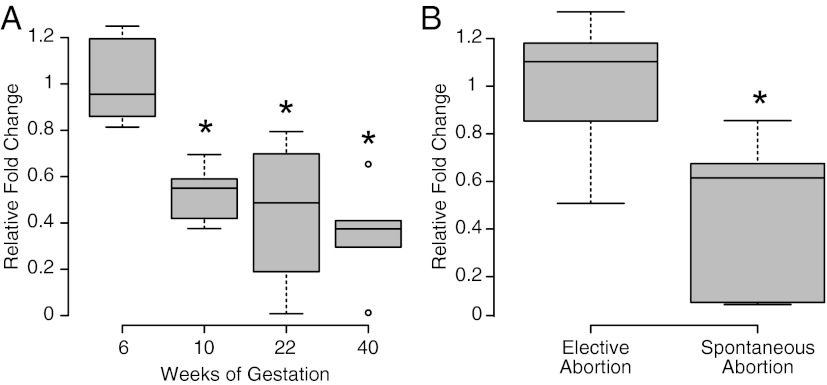
Serum IMD levels were found to be higher at 6 ± 1 weeks' gestation (n = 9), decreasing significantly as gestation advanced toward term, as shown in Figure 2A at 10 ± 1 weeks (n = 6), 22 ± 1 weeks (n = 5), and 40 weeks (n = 4) (P ≤ .05). When comparing total IMD serum levels in first-trimester spontaneous abortion (9 ± 1 weeks, n = 13) with age-matched elective abortions, IMD levels are significantly lower in patients with spontaneous abortions (P < .05).
Figure 2.
Mean fold change in Immunoreactive IMD levels in serum during pregnancy. A, IMD immunoreactivity in serum in different weeks of gestation is significantly higher at 6 ± 1 wk compared with 10 ± 1 weeks (n = 6), 22 ± 1 weeks (n = 5), and 40 ± 1 weeks (n = 4) (P < .05). B, Serum IMD levels in spontaneously aborted pregnancies at 9 ± 1 weeks are significantly lower compared with age-matched elective abortions (P < .05; n = 13). Asterisk (*) indicates a significant difference between the groups.
Expression of IMD immunoreactivity in first-trimester villous and decidual tissue:
As shown in Figure 3, IMD protein immunoreactivity is higher at 7 weeks of gestation compared with 10 weeks of gestation in the electively terminated villous tissue (Figure 3A) as well as decidual tissue (Figure 3B) (n = 3, ×400 magnification).
Figure 3.
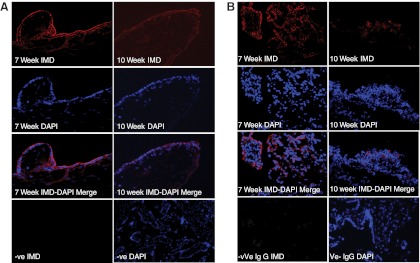
Expression of IMD immunoreactivity in first-trimester placental villi and decidua. A, IMD immunoreactivity in electively aborted villous tissues collected from 7 and 10 weeks of gestation (n = 3). B, IMD immunolocalization in decidua collected from 7 and 10 weeks of gestation (n = 3). IgG was used as negative control and DAPI was used for blue nuclear staining (×400 magnification). DAPI, 4′,6′-diamino-2-phenylindole.
Expression of IMD mRNA in first-trimester villous and decidual tissue
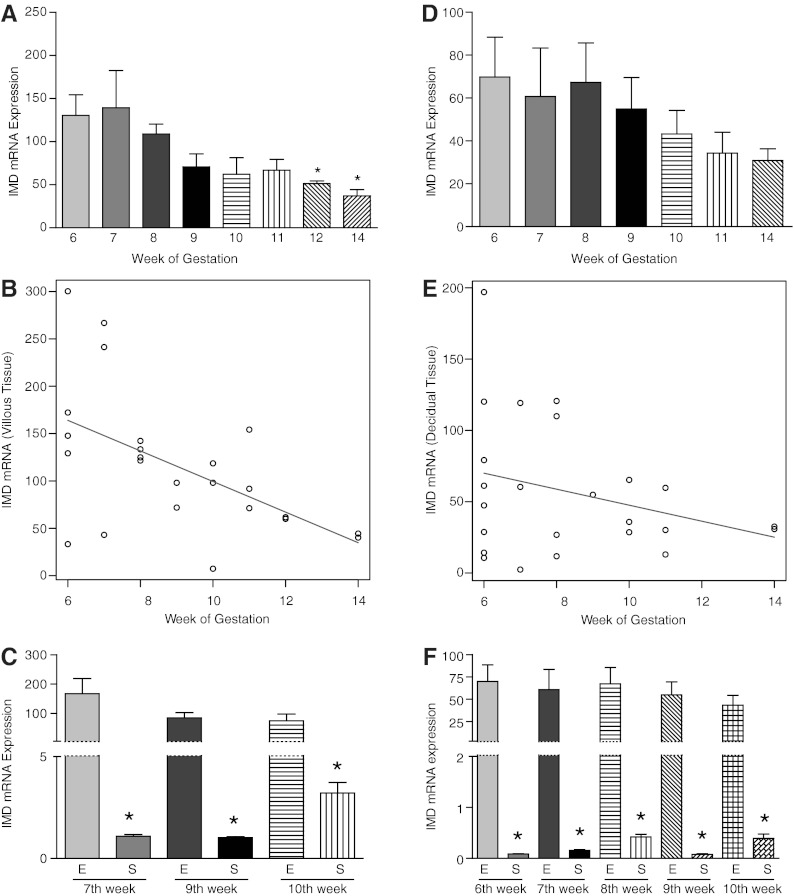
Intermedin mRNA levels were assessed by gestational age using quantitative RT-PCR in Figure 4. As shown, IMD is expressed at all weeks' gestation (6–14 wk, n = 2–5) in both villous and decidual tissue. IMD levels in electively terminated villous tissue are significantly higher at 6 (n = 5) and 7 weeks (n = 3) compared with week 12 (n = 3) and week 14 (n = 2), suggesting decreases with advancing gestation (P < .05) (Figure 4A). An analysis of the trend of IMD mRNA levels from 6 to 14 weeks in electively terminated villous tissue shows an exponential decrease in IMD levels (P = .04; Figure 4B). Although not as many time points were evaluated, IMD levels in spontaneously aborted villous tissue at 7, 9, and 10 weeks (P < .001, n = 3–4 at each week) were noted to have an inverse relationship to the IMD mRNA levels in comparison with age-matched electively aborted villous tissue. However, these levels were greater than 100-fold lower at all weeks of gestation in the spontaneously aborted compared with electively aborted villous tissue (P < .05; Figure 4C).
Figure 4.
Expression of IMD mRNA in first-trimester placental villi and decidua. A, IMD expression in elective first-trimester villous tissue showing a decline from 6 to 14 weeks of gestation (n = 5, 3, 4, 2, 3, 3, 2, and 2, respectively), with a statistical significance between 6 and 7 weeks of gestation compared with 12–14 weeks [P < .01]. B, Trend analysis showing an overall decay trend of IMD mRNA expression in first-trimester villi (P = .04). C, Comparison of IMD levels in villous tissue from spontaneously aborted (S) tissue at 7, 9, and 10 weeks (P < .001, n = 3–4 at each week) compared with age-matched electively (E) aborted villous tissue. IMD levels in age-matched electively terminated pregnancies showing an inverse relationship with decrease in IMD levels in elective abortions and increase in spontaneous abortions with increasing gestational age. D, IMD expression in elective first-trimester decidual tissue showing a decline from 6 to 14 weeks of gestation (n = 5, 3, 4, 2, 3, 3, 2, and 2, respectively). E, Trend analysis showing an overall decay trend of IMD mRNA expression in first-trimester decidua (P = .9). F, IMD levels in spontaneously (S) aborted decidual tissue from 6, 7, 8, 9, and 10 weeks of gestation (P < .001, n = 3–4 at each week) compared with age-matched electively (E) aborted decidual tissue. Asterisk (*) indicates a significant difference between the groups.
Similarly, expression of IMD mRNA in electively terminated decidual tissue showed higher IMD mRNA levels at 6 weeks of gestation (n = 5) (Figure 4D) compared with week 10 onward (n = 2–5, P = .054). An analysis of the trend in IMD mRNA levels from 7 to 14 weeks in electively terminated decidual tissue (Figure 4E) also shows a decreasing trend in the expression of IMD in decidua; however, the change does not reach the level of statistical significance (P = .9). Comparison of IMD mRNA levels in spontaneous abortion at 6, 7, 8, 9, and 10 weeks of gestation (n = 3–4 at each week) with age-matched elective abortion tissues (Figure 4F) shows that decidual IMD levels are lower in spontaneous abortion at all weeks tested (Figure 4F, P < .001). These data suggest IMD may have an important role for normal progression of pregnancy in the first trimester of human gestation.
Effect of IMD on invasion of primary EVCT
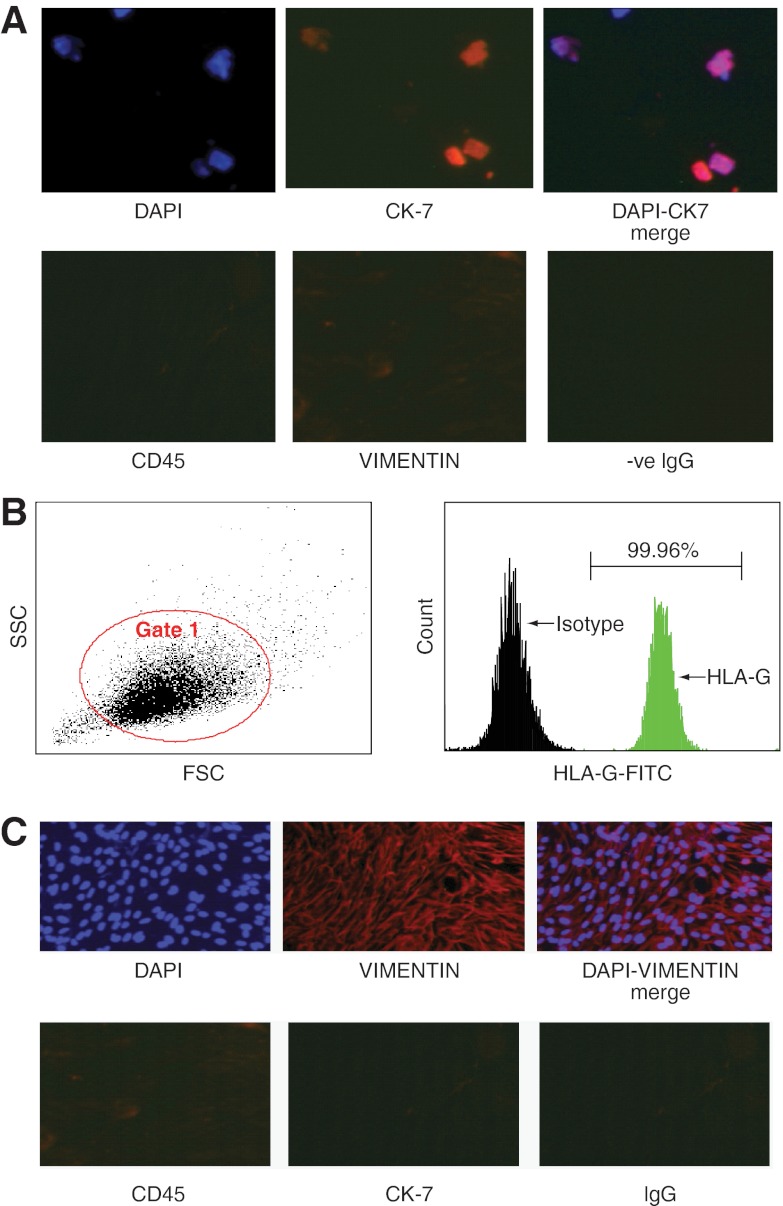
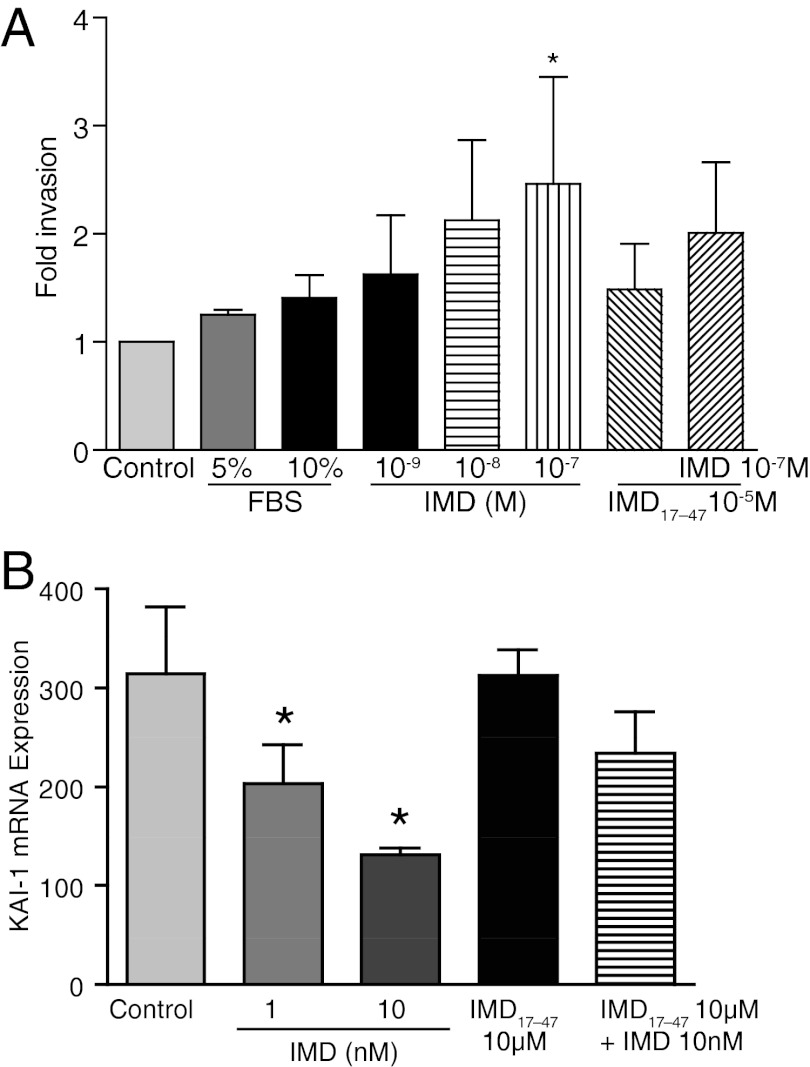
The invasive potential of IMD was assessed on first trimester EVCTs isolated from elective abortions. The purified EVCTs used were greater than 99% positive for HLA-G and CK7 (Figure 5). Similar to the effects of IMD observed in HTR S-SV/neo cells (3), Figure 6A demonstrates that IMD increases the invasive index of the primary EVCTs up to a maximum of 2.4-fold as compared with the untreated control EVCTs (P < .05, n = 3). The addition of IMD17–47 blunted this response.
Figure 5.
Immunofluorescent characterization of primary EVCTs and DSCs. A, Primary EVCTs isolated from first-trimester elective abortion tissues were stained with vimentin, CK7, or CD45 antibody for immune-fluorescent detection or IgG as negative control. DAPI was used for blue nuclear staining. Purified EVCTs show greater than 99% staining for CK7 and negative staining for vimentin or CD45 (×400 magnification). DAPI, 4′,6′-diamino-2-phenylindole. B, Flow cytometric analysis of EVCT cell purity showing side-scatter and forward-scatter characteristics of the isolated cells and percentage of isolated cells positive for the HLA-G marker after detection with specific antibody conjugated to fluorescein isothiocyanate. As shown, greater than 99% of the purified EVCTs were HLA-G positive trophoblast cells. C, Purified primary DSCs isolated from first trimester decidua were stained with Vimentin, CK-7, or CD45 antibody for immunofluorescent detection and DAPI being used for blue nuclear staining. IgG was used as negative control. Greater than 99% cells are stained positively for vimentin and negative for CK-7 or CD45 (×400 magnification).
Figure 6.
Effects of IMD treatment on the invasive potential of primary EVCT and decidual expression of KAI-1 mRNA. A, Effect of IMD on invasion of primary extravillous cytotrophoblast cells of placental villi from elective abortions. Primary EVCTs (15 000 cells/well in RPMI + 2% BSA) were seeded in 96-well Matrigel-coated plates and treated with either 5% fetal bovine serum, 10% BSA, or IMD in presence or absence of IMD17–47 or IMD17–47 alone. After 24 hours, cells were treated with calcein AM and read for fluorescence at 494/517 nm. The y-axis represents the fold change in number of labeled invaded cells compared with the control (normalized to 1). Asterisk (*) indicates significant difference compared with the control untreated cells (n = 3; P ≤ .05). B, Effect of IMD treatment on the expression of KAI-1 mRNA in decidual explants from first-trimester spontaneous abortion. Quantitative RT-PCR analysis shows that IMD decreases the expression of KAI-1 mRNA. As shown, IMD (10−7 to 10−9 M) dose dependently down-regulates KAI-1 mRNA expression in decidua, and its effects are inhibited in the presence of IMD17–47. Asterisk (*) indicates significant differences compared with untreated explants (P < .05, n = 3).
Effect of IMD on the expression of tumor suppressor KAI-1 in spontaneous abortion decidual tissue
KAI-1 inhibits trophoblast invasion and decidual expression of KAI1is elevated in spontaneous abortion (18). Figure 6B shows that IMD (10−7 to 10−9 M) dose dependently inhibits the expression of KAI-1 in first-trimester decidual explants in culture from spontaneous abortion, and this effect is antagonized by IMD17–47 (P < .05).
Discussion
The major findings of this study are as follows: 1) IMD is found in the secretome of day 5 postfertilization fresh and frozen blastocysts; 2) IMD levels are significantly lower in the serum of spontaneously aborted compared with electively aborted pregnancies; 3) IMD is present in both villous and decidual tissues in the first-trimester placenta at all weeks of gestation; 4) IMD levels are highest during early weeks of gestation in villi and decidua of elective abortions, trending toward a decline with advancing gestation in the first trimester, whereas IMD levels in villous and decidual tissue of spontaneous abortions are lower during the early weeks of gestation and increase as pregnancy progresses toward the second trimester; 5) expression of IMD in villous and decidual tissues is substantially greater in electively terminated normal pregnancies as compared with pathological spontaneous abortions; 6) IMD increases the invasive capacity of primary EVCTs by 2.4-fold, and IMD17–47 antagonist blunts this response; and 7) IMD lowers the expression of KAI-1 mRNA in first-trimester decidual tissues from spontaneous abortions.
In this study, we show for the first time that IMD is expressed in human pregnancy as early as day 5 after fertilization in the embryonic secretome from both freshly cultured embryo and cultured frozen embryos. However, as shown in Figure 1, IMD levels were higher in the secretome of frozen embryos. There are several reports showing that frozen embryos lead to healthier babies with better success rates than fresh embryo transfer (21, 22). We have recently shown that infusion of IMD antagonist from day 3 pregnant rats is detrimental to the growth of implantation sites and causes a decline in metalloproteinases and placental growth factors. Therefore, these data suggest that IMD may be involved in the developmental stages of blastocyst, and thus, IMD treatment may prove beneficial to the outcome of assisted fertilization and further improve the success rates of fresh embryo transfer (4).
Although the probability for a potential spontaneous abortion cannot be ruled out completely in women who opt for elective abortion, we used serum and tissues from women who had elective abortion in early gestation to identify IMD expression profile in early human pregnancy. Figure 2 shows serum levels of IMD are significantly higher at 6 ± 1 weeks of gestation after a declining trend as gestation advanced toward term (Figure 2A). The decrease in IMD levels toward the end of the first trimester corresponds with completion of implantation and placentation, suggesting a major role for IMD during early stages of pregnancy. IMD-related peptides, calcitonin gene-related peptide, and adrenomedullin are also shown to play a role in human pregnancy. Interestingly, calcitonin gene-related peptide as well as adrenomedullin increases throughout pregnancy with a peak in the third trimester, whereas IMD levels are higher in early stages of gestation suggesting IMD's role in early human pregnancy (23–25). We further assessed the serum levels of IMD in first-trimester spontaneous abortion to determine whether serum IMD levels are associated with pathological states of pregnancy. Figure 2B shows IMD serum levels are significantly lower in the first-trimester spontaneous abortions compared with age-matched elective abortions. This suggests that an abnormal trophoblast invasion decreases IMD levels or the effect of lower IMD levels in these spontaneous abortions could possibly be a cause. However, the limitations of the studies involving tissues from spontaneous abortions in human pregnancy cannot be ignored. Future studies, perhaps in a nonhuman primate model, are required to address the relationship of the lower IMD levels in spontaneous abortion with the occurrence of pathology.
Furthermore, this study shows that IMD is present not only in serum, but also in both the villous and decidual tissues in first-trimester human pregnancy. A similar trend of IMD levels was also observed in the villous and decidual tissues, with IMD immunoreactivity (Figure 3) and mRNA (Figure 4) levels being highest at early weeks of the first trimester after a steady decline in both the tissues (Figure 4, A and B) as pregnancy advanced towards second trimester. A relatively low oxygen environment characterizes blastocyst implantation, placentation, and early embryonic development (26) The IMD gene possess hypoxia response elements in its promoter region (27), and as shown in Figure 4, IMD expression is higher in the earlier weeks of the first trimester in human pregnancy. This reinforces our concept that IMD is important in early human gestation. Conversely, villous and decidual tissues of pathological spontaneous abortion were noted to have IMD levels that were low at 6 weeks of gestation, showing an increasing trend with advancing first trimester (Figure 4, C and F). This may suggest a compensatory increase in IMD levels in spontaneous abortions that begin with low IMD levels in the early weeks of gestation and have higher levels toward the end of the first trimester but are still unable to reach optimal conditions to sustain normal pregnancy, resulting in spontaneous abortion in the first trimester. When comparing IMD levels in electively terminated abortions vs spontaneous abortions, IMD levels are significantly higher at all weeks of gestation in both villous and decidual tissue in elective abortions (Figure 4, C and F).
Abnormal trophoblast invasion is 1 of the major causes of pathological pregnancies, such as recognized spontaneous abortions, preeclampsia, and intrauterine growth restriction (28). Because IMD is expressed in villous and decidual tissues and we reported earlier that IMD promotes the invasive potential of HTR cells, it was imperative to confirm the stimulatory effect of IMD in primary EVCTs isolated from first-trimester elective abortions. Figure 6A shows that IMD increases the invasion of primary trophoblast cells by 2.4-fold compared with the untreated EVCTs. Therefore, this study confirms our previous studies done in HTR-8sv/neo cells that IMD increases the invasive index of trophoblast cell to facilitate human placental development (3).
Greater than 100-fold lower expression of IMD in spontaneous abortion (Figure 4, C and F) compared with normal elective abortions suggests that suboptimal levels of IMD in first-trimester human pregnancy may be a potential cause for abnormal trophoblast invasion and placentation, leading to pregnancy complications that originate due to abnormal trophoblast invasion such as spontaneous abortion, preeclampsia, and intrauterine growth restriction. Optimal trophoblast invasion is mediated by an intricate balance between the trophoblast invasion promoting factors and decidual invasion inhibitory factors. KAI-1 is a tumor suppressor protein recently shown to be expressed in decidual tissue, which inhibits trophoblast invasion into the decidua. KAI-1 expression is elevated significantly in spontaneous abortions (18, 19). Figure 6 shows that IMD dose dependently suppresses the expression of KAI-1 in first-trimester decidual explants from spontaneous abortions and that these effects are inhibited by IMD17–47, suggesting a potential role for IMD in inhibiting the expression of decidual KAI-1 to facilitate trophoblast invasion. Expression of IMD in villous and decidual tissues suggests an autocrine/paracrine role for IMD in mediating trophoblast invasion via regulating embryonic and uterine factors to facilitate early placental development.
In conclusion, this study demonstrates that IMD is expressed in the day 5 blastocyst stage in human pregnancy. As implantation and placentation proceeds, IMD levels are higher in the earlier weeks of first-trimester placenta and decline as pregnancy advances toward the second trimester. In addition, this study suggests a physiological role for IMD in trophoblast invasion in early placental development and its association with first-trimester spontaneous abortion in human pregnancy. Future studies are needed to identify the mechanism of IMD-stimulated trophoblast invasion to facilitate placental development in human pregnancy.
Acknowledgments
We thank Elizabeth Powell for her incredible administrative assistance; Dr Vickie Schnell (Center of Reproductive Medicine, Webster, Texas) for the collection and use of discarded embryo secretomes.
This work was supported by the National Institutes of Health through Grant HD054867 (to M.C.) and Grants HL58144 and HL102866 (to C.Y.).
Disclosure Summary: The authors report no conflict of interest.
Footnotes
- EIA
- Enzyme immunoassay
- EVCT
- extravillous cytotrophoblast cell
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- HLA-G
- human leukocyte antigen class I, G
- IMD
- intermedin
- KAI-1
- Kangia-1.
References
- 1. Roh J, Chang CL, Bhalla A, Klein C, Hsu SY. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem. 2004;279(8):7264–7274 [DOI] [PubMed] [Google Scholar]
- 2. Takei Y, Inoue K, Ogoshi M, Kawahara T, Bannai H, Miyano S. Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett. 2004;556(1–3):53–58 [DOI] [PubMed] [Google Scholar]
- 3. Chauhan M, Yallampalli U, Dong YL, Hankins GD, Yallampalli C. Expression of adrenomedullin 2 (ADM2)/intermedin (IMD) in human placenta: role in trophoblast invasion and migration. Biol Reprod. 2009;81(4):777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chauhan M, Elkins R, Balakrishnan M, Yallampalli C. Potential role of intermedin/adrenomedullin 2 in early embryonic development in rats. Regul Pept. 2011;170(1–3):65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith RS, Jr, Gao L, Bledsoe G, Chao L, Chao J. Intermedin is a new angiogenic growth factor. Am J Physiol Heart Circ Physiol. 2009;297(3):H1040–H1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takahashi K, Kikuchi K, Maruyama Y, et al. Immunocytochemical localization of adrenomedullin 2/intermedin-like immunoreactivity in human hypothalamus, heart and kidney. Peptides. 2006;27(6):1383–1389 [DOI] [PubMed] [Google Scholar]
- 7. Takahashi K, Morimoto R, Hirose T, Satoh F, Totsune K. Adrenomedullin 2/intermedin in the hypothalamo-pituitary-adrenal axis. J Mol Neurosci. 2011;43(2):182–192 [DOI] [PubMed] [Google Scholar]
- 8. Chauhan M, Ross GR, Yallampalli U, Yallampalli C. Adrenomedullin-2, a novel calcitonin/calcitonin-gene-related peptide family peptide, relaxes rat mesenteric artery: influence of pregnancy. Endocrinology. 2007;148(4):1727–1735 [DOI] [PubMed] [Google Scholar]
- 9. Chauhan M, Yallampalli U, Reed L, Yallampalli C. Adrenomedullin 2 antagonist infusion to rats during midgestation causes fetoplacental growth restriction through apoptosis. Biol Reprod. 2006;75(6):940–947 [DOI] [PubMed] [Google Scholar]
- 10. Chauhan M, Balakrishnan M, Yallampalli U, et al. Adrenomedullin 2/intermedin regulates HLA-G in human trophoblasts. Biol Reprod. 2011;85(6):1232–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kam EP, Gardner L, Loke YW, King A. The role of trophoblast in the physiological change in decidual spiral arteries. Hum Reprod. 1999;14(8):2131–2138 [DOI] [PubMed] [Google Scholar]
- 12. Burrows TD, King A, Loke YW. Trophoblast migration during human placental implantation. Hum Reprod Update. 1996;2(4):307–321 [DOI] [PubMed] [Google Scholar]
- 13. Godbole G, Suman P, Gupta SK, Modi D. Decidualized endometrial stromal cell derived factors promote trophoblast invasion. Fertil Steril. 2011;95(4):1278–1283 [DOI] [PubMed] [Google Scholar]
- 14. Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69(1):1–7 [DOI] [PubMed] [Google Scholar]
- 15. Goldman-Wohl D, Yagel S. Regulation of trophoblast invasion: from normal implantation to pre-eclampsia. Mol Cell Endocrinol. 2002;187(1–2):233–238 [DOI] [PubMed] [Google Scholar]
- 16. Bose P, Kadyrov M, Goldin R, et al. Aberrations of early trophoblast differentiation predispose to pregnancy failure: lessons from the anti-phospholipid syndrome. Placenta. 2006;27(8):869–875 [DOI] [PubMed] [Google Scholar]
- 17. Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187(5):1416–1423 [DOI] [PubMed] [Google Scholar]
- 18. Li MQ, Hou XF, Shao J, Tang CL, Li DJ. The DSCs-expressed CD82 controls the invasiveness of trophoblast cells via integrinbeta1/MAPK/MAPK3/1 signaling pathway in human first-trimester pregnancy. Biol Reprod. 2010;82(5):968–979 [DOI] [PubMed] [Google Scholar]
- 19. Gellersen B, Briese J, Oberndorfer M, et al. Expression of the metastasis suppressor KAI1 in decidual cells at the human maternal-fetal interface: regulation and functional implications. Am J Pathol. 2007;170(1):126–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Handschuh K, Guibourdenche J, Tsatsaris V, et al. Human chorionic gonadotropin expression in human trophoblasts from early placenta: comparative study between villous and extravillous trophoblastic cells. Placenta. 2007;28(2–3):175–184 [DOI] [PubMed] [Google Scholar]
- 21. Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995–2006. Fertil Steril. 2010;94(4):1320–1327 [DOI] [PubMed] [Google Scholar]
- 22. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96(2):344–348 [DOI] [PubMed] [Google Scholar]
- 23. Yallampalli C, Chauhan M, Thota CS, Kondapaka S, Wimalawansa SJ. Calcitonin gene-related peptide in pregnancy and its emerging receptor heterogeneity. Trends Endocrinol Metab. 2002;13(6):263–269 [DOI] [PubMed] [Google Scholar]
- 24. Li L, Tang F, O WS. Coexpression of adrenomedullin and its receptor component proteins in the reproductive system of the rat during gestation. Reprod Biol Endocrinol. 2010;8:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang X, Green KE, Yallampalli C, Dong YL. Adrenomedullin enhances invasion by trophoblast cell lines. Biol Reprod. 2005;73(4):619–626 [DOI] [PubMed] [Google Scholar]
- 26. Lunghi L, Ferretti ME, Medici S, Biondi C, Vesce F. Control of human trophoblast function. Reprod Biol Endocrinol. 2007;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfeil U, Aslam M, Paddenberg R, et al. Intermedin/adrenomedullin-2 is a hypoxia-induced endothelial peptide that stabilizes pulmonary microvascular permeability. Am J Physiol Lung Cell Mol Physiol. 2009;297(5):L837–L845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cartwright JE, Fraser R, Leslie K, Wallace AE, James JL. Remodelling at the maternal-fetal interface: relevance to human pregnancy disorders. Reproduction. 2010;140(6):803–813 [DOI] [PubMed] [Google Scholar]