Abstract
Context:
Use of a single set of thyroid function tests to define subclinical hypothyroidism may lead to misclassification over time and could influence findings from longitudinal studies.
Objective:
We assessed the risks of coronary heart disease (CHD), heart failure (HF), and cardiovascular (CV) death in older adults with persistent subclinical hypothyroidism.
Design, Setting, and Participants:
The study included 679 subclinically hypothyroid and 4184 euthyroid U.S. individuals at least 65 yr old enrolled in the Cardiovascular Health Study and not taking thyroid preparations.
Main Outcome Measure:
We measured the 10-yr risk of incident CHD, HF, and CV death from persistent subclinical hypothyroidism, overall and stratified by degree of TSH elevation (4.5–6.9, 7.0–9.9, and 10.0–19.9 mU/liter).
Results:
There was no association between persistent subclinical hypothyroidism and incident CHD [hazard ratio (HR), 1.12; 95% confidence interval (CI), 0.93–1.36], HF (HR, 1.05; 95% CI, 0.97–1.27), or CV death (HR, 1.07; 95% CI, 0.87–1.31) in adjusted analyses in which subclinical hypothyroidism was modeled as a time-varying exposure using up to four serial thyroid function tests. When subclinical hypothyroidism was stratified by degree of TSH elevation, no significant associations were found in any stratum. Findings were similar in fixed exposure analyses in which only participants with testing 2 yr apart were considered, with no association between persistent or transient subclinical hypothyroidism and incident CHD, HF, or CV death.
Conclusions:
Our data do not support increased risk of CHD, HF, or CV death in older adults with persistent subclinical hypothyroidism.
The clinical significance of subclinical hypothyroidism in the elderly is undetermined, with various cohort studies reporting increased, decreased, or no health risk from untreated subclinical hypothyroidism (1). The relationship between subclinical hypothyroidism and coronary heart disease (CHD) and heart failure (HF) is particularly controversial. A recent meta-analysis of 11 major cohort studies reported increased risk of CHD events only in those with more severe subclinical hypothyroidism, represented by TSH levels of 10 mU/liter or higher and of CHD mortality in those with TSH levels of 7 mU/liter or higher (2). There was no evidence of a difference by age in risk of CHD associated with subclinical hypothyroidism. Similarly, three published studies of HF risk in elderly participants show an increased risk only at TSH levels above 7 mU/liter (3) or 10 mU/liter (4, 5), respectively.
Previous analyses from observational studies have used a single set of thyroid function tests to define subclinical hypothyroidism. However, thyroid function test results may change over time, with resolution to euthyroidism in a large proportion of those with mild subclinical hypothyroidism and progression to overt hypothyroidism in those with higher TSH levels that suggest severe subclinical hypothyroidism (6). Therefore, the use of a single set of thyroid function tests to define subclinical hypothyroidism may lead to significant misclassification over time and could influence longitudinal findings for risk of health outcomes. Using data from the Cardiovascular Health Study (CHS), a large cohort study of community-dwelling individuals aged 65 yr and older, we sought to assess the risks of CHD, HF, and cardiovascular (CV) death in community-dwelling older adults with persistent subclinical hypothyroidism.
Subjects and Methods
Study population
The CHS is a population-based, longitudinal study of risk factors for the development of CV disease in 5888 adults aged 65 yr or older (7). Enrollment of an original cohort of 5201 adults occurred between May 1989 and June 1990, and an additional cohort of 687 African-Americans was enrolled in 1992–1993. Eligible individuals were identified from an age- and sex-stratified random sample of the Medicare eligibility rosters in four U.S. communities: Washington County, Maryland; Allegheny County, Pennsylvania; Sacramento County, California; and Forsyth County, North Carolina. To be eligible, individuals had to be noninstitutionalized, expecting to remain in the area for the following 3 yr, not in active treatment for cancer, not wheelchair-bound at home, not requiring a proxy respondent at entry, and capable of providing medical consent. Household members of the sampled individual were recruited, if eligible. The institutional review boards of all four sites and the coordinating center at the University of Washington in Seattle approved the study. All participants gave informed consent.
Annual study visits through 1999 included a detailed medical history, physical examination, and assessment of health status. Blood was drawn after a 12-h fast, and serum was processed and stored in −70 C freezers for future testing.
Thyroid function testing
TSH testing was performed on 3678 baseline samples from the original cohort using a third-generation assay with a functional sensitivity of 0.008 mU/liter (LumaTag hTSH; Nichols Institute, San Juan Capistrano, CA) as previously described (8), and in participants from both cohorts with available serum at the 1992–1993 (n = 3996), 1994–1995 (n = 4005), and 1996–1997 (n = 3371) visits using a third-generation assay with a functional sensitivity of 0.005 mU/liter (Elecsys 2010 analyzer; Roche Diagnostics, Indianapolis, IN) as previously described (6). A validation study was performed across the range of TSH values using both assays in 48 CHS participants. We examined Bland-Altman plots, and in regression models, the intercept was −0.14 and the slope was 1.04. The correlation coefficient was 0.99. The mean (sd) TSH values were 7.56 mU/liter (10.5) in the original assay and 7.39 mU/liter (10.0) in the later assay (P = 0.45). Free T4 concentrations were assessed at all time-points in participants with abnormal TSH levels, using a direct monoclonal antibody assay (Amerlex-MAB; Amersham International, Amersham, UK) at the 1989–1990 visit and a chemiluminescent immunoassay (Elecsys 2010; Roche Diagnostics) at all other time-points.
Ascertainment of events
Participants have been contacted semiannually regarding hospitalizations or new occurrences of CV events since the study's inception. Provisional diagnoses of CHD and HF based on interview, review of medical records, and other support documents have been reviewed and adjudicated at periodic meetings of the study's morbidity and mortality subcommittee (9). This subcommittee had no knowledge of a participant's thyroid function. CHD was defined as the occurrence of angina, myocardial infarction, coronary angioplasty, bypass surgery, or CHD death. HF events were defined on the basis of diagnosis from a physician and consideration of symptoms, signs, chest radiographs, and treatment (current prescription for a diuretic agent and digitalis or a vasodilator). Information about deaths was obtained through medical records, death certificates, autopsy and coroner reports. CV deaths were defined as those due to atherosclerosis (including peripheral vascular disease), CHD, cerebrovascular events, and other CV causes.
Assessment of covariates
Thyroid medication use was assessed annually via examination of medication bottles. Hypertension was defined as systolic blood pressure greater than 140 mm Hg, or diastolic blood pressure greater than 90 mm Hg, or self-reported hypertension and use of medications. Diabetes was defined as fasting blood glucose greater than 126 mg/dl or use of oral hypoglycemic drugs or injectable insulin. Lipids, C-reactive protein, and creatinine were measured with standard assays (10, 11).
Statistical analysis
Study participants were classified into groups based on each of their thyroid function tests (TFTs) over time. Euthyroidism was defined as a TSH concentration of 0.45–4.50 mU/liter, and subclinical hypothyroidism was defined as a TSH concentration of more than 4.50 mU/liter and less than 20 mU/liter with a normal free T4 concentration (8). Participants who were in one of these two groups at the time of their first TFT and who were not taking thyroid hormone preparations at the time of the first TFT were included in analyses. Participants with prevalent CHD or HF at the time of their first TFT were excluded from analyses of time to CHD or HF, respectively.
Characteristics of study participants at the time of their first TFTs were compared between the two groups using a t test or χ2 as appropriate. Incidence rates were calculated by dividing the total number of each event by the person-years at risk for that event. Kaplan-Meier plots were used to display the cumulative incidence of CHD, HF, and CV death by thyroid status. Multivariable Cox proportional hazards regression models were used to estimate the hazard ratios (HRs) associated with subclinical hypothyroidism compared with euthyroidism for each of CHD, HF, and CV death, adjusting for age, sex, race, and initiation of thyroid medication use during follow-up. Additional CHD models with adjustment for smoking, body mass index (BMI), diabetes, hypertension, low-density lipoprotein (LDL) cholesterol, ln(C-reactive protein), and creatinine elevation greater than 1.4 mg/dl, and HF models with these covariates plus prevalent CHD were examined with no difference in results. Results were also similar among the group of CHS participants who never received any thyroid supplementation. Participants who died or were lost to follow-up before having an event were censored at the date of death or last contact. Initial models included follow-up through June of 2008, but violations in model assumptions that require proportionality of risk occurred after 10 yr. Therefore, in final models, follow-up was censored at 10 yr after the first TSH value. Results from models in which follow-up time was unlimited up to 19 yr vs. censored at 10 yr did not differ. Models with both incident and recurrent events did not differ from those with incident events; only incident events are considered. Statistical tests for interactions between thyroid status group and sex and thyroid status group and age above or below 85 yr were assessed, with no evidence for interaction.
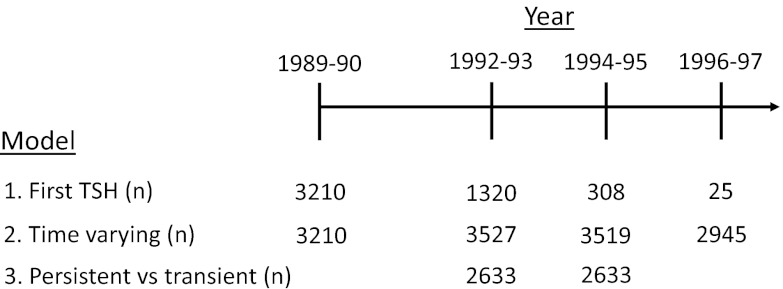
Subclinical hypothyroidism was modeled three different ways, and consistency across model types was examined. In the first model, only the first TSH was used to classify participants as subclinically hypothyroid or euthyroid, without noting subsequent changes in thyroid testing over time. Analyses were performed using the entire group with subclinical hypothyroidism and stratified by degree of TSH elevation (4.5–6.9, 7.0–9.9, and 10.0–19.9 mU/liter). There were 4863 participants available for these analyses. In the second model, thyroid status was updated over time to allow for changes in status during follow-up. Analyses stratified by degree of TSH elevation were also performed using this model. Of the 4863 participants included in these analyses, 926 had only one set of TFTs, 980 had exactly two measurements, 1513 had exactly three measurements, and 1444 had TFT measurements at all four time-points. When comparing participants who had only one or two sets of TFTs with those with three or four TFTs, those with fewer sets of TFTs were older (75.6 vs. 72.1 yr), were more likely to be men (48 vs. 41%), and had more prevalent comorbid disease (25 vs. 17% with CHD, 8 vs. 3% with HF, and 19 vs. 13% with diabetes) (all P < 0.001). In the final model, persistent subclinical hypothyroidism was examined as a fixed exposure in which persistently subclinically hypothyroid and transiently subclinically hypothyroid participants were compared with persistently euthyroid participants. Thyroid status was determined on the basis of thyroid function testing from the 1992–1993 and 1994–1995 visits. Participants were classified as persistently subclinically hypothyroid or persistently euthyroid if they met criteria at both tests and had no history of thyroid medication use. Those who were subclinically hypothyroid in 1992–1993 and euthyroid in 1994–1995 without thyroid medication use were classified as transiently subclinically hypothyroid. There were an insufficient number of participants to perform these analyses with stratification by degree of TSH elevation. Figure 1 presents a timeline reporting the number of participants included in each analysis by time-point of thyroid function test measurement.
Fig. 1.
Timeline of thyroid function test measurements for each analysis. n, The number of participants who were euthyroid or subclinically hypothyroid, not taking thyroid medications, and who were included at each of four possible time-points. For model 1, one set of thyroid function tests was required for inclusion in analyses, using the first thyroid function test measurement available, and the total number of participants was 4863. For model 2, the number of included participants was also 4863, although additional thyroid function tests at all study visits after the first thyroid function test measurement were included in the model. Model 3 required a set of thyroid function tests at both the 1992–1993 and 1994–1995 visits, which was available in 2633 participants.
Results
At the time of the first TSH measurement, there were 679 individuals with subclinical hypothyroidism and 4184 with euthyroidism (Table 1). Compared with the euthyroid group, those with subclinical hypothyroidism were slightly older, more likely to be female and Caucasian, and less likely to smoke. They were not more likely to have prevalent CHD or HF. During the 10-yr follow-up period, 225 (33.1%) of the participants in the subclinical hypothyroidism group and 174 (4.2%) in the euthyroid group initiated thyroid hormone medication.
Table 1.
Characteristics of individuals with subclinical hypothyroidism and euthyroidism at first TSH measurement
| Characteristic | Subclinical hypothyroidism | Euthyroidism | P value |
|---|---|---|---|
| n | 679 | 4184 | |
| Age (yr) | 74.1 (5.9) | 73.4 (5.7) | 0.004 |
| Male | 260 (38.3) | 1881 (45.0) | 0.001 |
| Caucasian | 619 (91.2) | 3420 (81.7) | <0.001 |
| Current smoker | 53 (7.8) | 492 (11.8) | 0.002 |
| BMI (kg/m2) | 26.7 (4.9) | 26.6 (4.6) | 0.62 |
| LDL cholesterol (mg/dl) | 128 (34) | 130 (35) | 0.62 |
| C-reactive protein (mg/liter)a | 2.62 (9.31) | 2.55 (8.30) | 0.53 |
| Hypertension | 381 (56.2) | 2446 (58.5) | 0.26 |
| Diabetes mellitus | 110 (16.2) | 636 (15.2) | 0.50 |
| High creatinine (>1.4 mg/dl) | 54 (7.9) | 366 (8.8) | 0.48 |
| CHD | 138 (20.5) | 821 (19.6) | 0.67 |
| HF | 37 (5.5) | 204 (4.9) | 0.52 |
| TSH (mU/liter) | 6.7 (2.6) | 2.1 (0.97) | N/Ab |
Data are expressed as number (percentage), except for age, BMI, LDL cholesterol, C-reactive protein, and TSH, which are expressed as mean (sd). N/A, Not available.
Geometric mean and t test of mean difference on the natural log scale.
Groups defined by this measure.
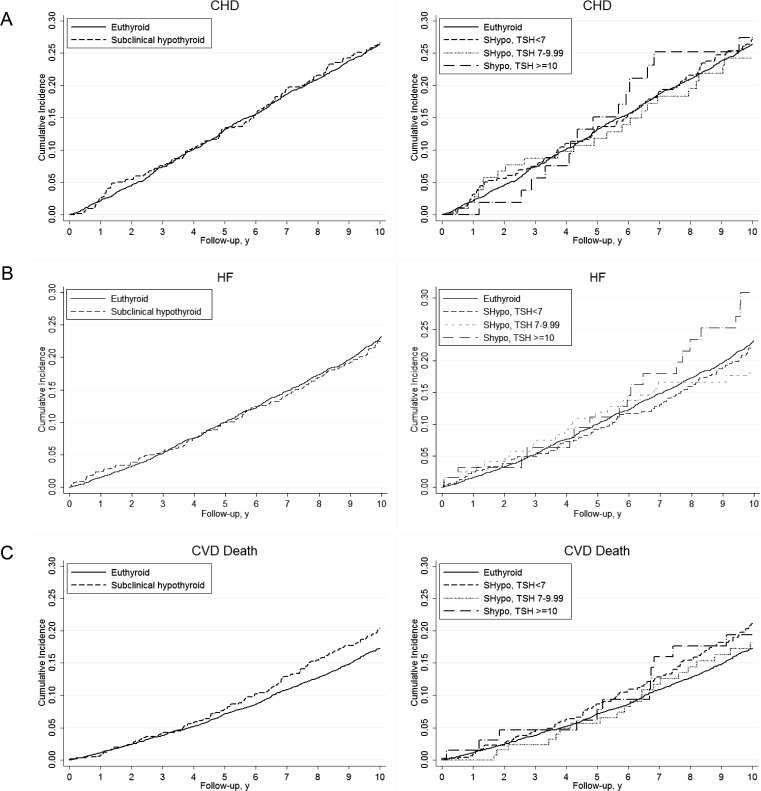
In the first model, in which only the first TSH was used to define thyroid status, there was no difference in incident CHD, HF, or CV death between participants with subclinical hypothyroidism and euthyroidism (Fig. 2; P value for all log-rank tests > 0.05). No association was seen between subclinical hypothyroidism and incident CHD [HR, 1.04; 95% confidence interval (CI), 0.86–1.26], HF (HR, 0.95; 95% CI, 0.78–1.15), or CV mortality (HR, 1.06; 95% CI, 0.86–1.30) in multivariable models with 10-yr follow-up (Table 2). Additional analyses stratified by degree of TSH elevation (4.5–6.9, 7.0–9.9, and 10.0–19.9 mU/liter) showed no increase in risk of any of these CV events by subgroup of subclinical hypothyroidism (Fig. 2 and Table 2). P values for interactions between thyroid hormone use and CV outcomes were not significant. The results for CV mortality were unchanged when further adjusted for CHD or HF at TSH baseline.
Fig. 2.
Cumulative incidence over 10 yr of follow-up of CHD (A), HF (B), and CV death (C), comparing subclinical hypothyroidism and euthyroidism at first TSH measurement, overall, and by TSH strata. All comparisons are not statistically significant.
Table 2.
Ten-year incidence of CHD, HF, and CV mortality by thyroid status defined by first TSH measurement
| Euthyroid | Subclinical hypothyroid |
||||
|---|---|---|---|---|---|
| Overall | TSH 4.5–6.9 | TSH 7.0–9.9 | TSH 10.0–19.9 | ||
| CHD | |||||
| No. of events/no. at risk | 795/3363 | 130/541 | 93/382 | 23/105 | 14/54 |
| Incidence (95% CI) per 1000 person-years | 30.0 (28.0–32.2) | 30.4 (25.6–36.1) | 31.0 (25.3–37.9) | 27.7 (18.4–41.6) | 31.9 (18.9–53.9) |
| HR (95% CI) | 1.0 | 1.04 (0.86–1.26) | 1.06 (0.85–1.32) | 0.96 (0.63–1.46) | 1.07 (0.62–1.84) |
| HF | |||||
| No. of events/no. at risk | 804/3980 | 126/642 | 87/455 | 21/123 | 18/64 |
| Incidence (95% CI) per 1000 person-years | 25.3 (23.6–27.1) | 24.5 (20.6–29.2) | 23.9 (19.4–29.5) | 21.5 (14.0–33.0) | 34.5 (21.7–54.7) |
| HR (95% CI) | 1.0 | 0.95 (0.78–1.15) | 0.95 (0.76–1.18) | 0.79 (0.51–1.23) | 1.25 (0.77–2.05) |
| CV death | |||||
| No. at risk | 645/4184 | 124/679 | 91/483 | 21/131 | 12/65 |
| Incidence (95% CI) per 1000 person-years | 18.3 (16.9–19.7) | 21.8 (18.3–26.0) | 22.6 (18.4–27.8) | 19.2 (12.5–29.4) | 21.2 (12.0–37.3) |
| HR (95% CI) | 1.0 | 1.06 (0.86–1.30) | 1.16 (0.92–1.45) | 0.83 (0.53–1.29) | 0.82 (0.45–1.48) |
Adjusted for age, sex, race, and initiation of thyroid medication during follow-up period.
Similarly, in the time-varying model, in which thyroid status was updated with subsequent TSH measurements, there was no association between subclinical hypothyroidism and incident CHD (HR, 1.12; 95% CI, 0.93–1.36), HF (HR, 1.05; 95% CI, 0.87–1.27), or CV mortality (HR, 1.07; 95% CI, 0.87–1.31) (Table 3). Analyses stratified by degree of TSH elevation (4.5–6.9, 7.0–9.9, and 10.0–19.9 mU/liter) again showed no increase in risk of any of these CV events by subgroup of subclinical hypothyroidism. Additionally, adjusting for CHD or HF at the time of the first TFT did not alter findings for CV death.
Table 3.
Ten-year incidence of CHD, HF, and CV mortality by time-varying thyroid status, using up to four thyroid function tests
| Euthyroid | Subclinical hypothyroid |
||||
|---|---|---|---|---|---|
| Overall | TSH 4.5–6.9 | TSH 7.0–9.9 | TSH 10.0–19.9 | ||
| CHD | |||||
| Person-years | 26,627 | 3,887 | 2,782 | 719 | 306 |
| No. of events | 788 | 130 | 94 | 26 | 10 |
| Incidence (95% CI) per 1000 person-years | 29.6 (27.6–31.7) | 33.4 (28.2–39.7) | 33.8 (27.6–41.4) | 36.2 (24.6–53.1) | 25.9 (13.9–48.1) |
| HR (95% CI) | 1.0 | 1.12 (0.93–1.36) | 1.12 (0.90–1.39) | 1.26 (0.85–1.87) | 0.85 (0.45–1.87) |
| HF | |||||
| Person-years | 31,887 | 4,731 | 3,377 | 883 | 470 |
| No. of events | 790 | 128 | 96 | 21 | 11 |
| Incidence (95% CI) per 1000 person-years | 24.8 (23.1–26.6) | 27.1 (22.8–32.2) | 28.4 (23.3–34.7) | 23.8 (15.5–36.5) | 23.4 (13.0–42.3) |
| HR (95% CI) | 1.0 | 1.05 (0.87–1.27) | 1.12 (0.90–1.38) | 0.89 (0.58–1.38) | 0.80 (0.44–1.47) |
| CV death | |||||
| Person-years | 35,348 | 5,250 | 3,766 | 980 | 503 |
| No. of events | 639 | 116 | 82 | 22 | 12 |
| Incidence (95% CI) per 1000 person-years | 18.1 (16.7–19.5) | 22.1 (18.4–26.5) | 21.8 (17.5–27.0) | 22.4 (14.8–34.1) | 23.9 (13.5–42.0) |
| HR (95% CI) | 1.0 | 1.07 (0.87–1.31) | 1.10 (0.87–1.38) | 1.04 (0.68–1.60) | 0.92 (0.51–1.65) |
Adjusted for age, sex, race, and initiation of thyroid medication during follow-up period.
In the fixed exposure model, thyroid status was defined on two consecutive measurements 2 yr apart (1992–1993 and 1994–1995), and those with persistent subclinical hypothyroidism (mean TSH, 7.2 ± 2.9 mU/liter in 1992–1993) and transient subclinical hypothyroidism (mean TSH, 5.6 ± 1.3 mU/liter in 1992–1993) were compared with individuals with persistent euthyroidism. There was no association between persistent or transient subclinical hypothyroidism and incident CHD (HR, 1.37; 95% CI, 1.00–1.87; and HR, 1.00; 95% CI, 0.65–1.53, respectively), HF (HR, 0.89; 95% CI, 0.63–1.25; and HR, 1.01; 95% CI, 0.68–1.49, respectively), and CV death (HR, 0.97; 95% CI, 0.64–1.48; and HR, 0.99; 95% CI, 0.70–1.39, respectively) (Table 4).
Table 4.
Ten-year incidence of CHD, HF, and CV mortality by thyroid status defined by TSH measurements at 1992–1993 and 1994–1995 visits
| Euthyroid | Persistent subclinical hypothyroid | Transient subclinical hypothyroid | |
|---|---|---|---|
| CHD | |||
| No. of events/no. at risk | 431/1749 | 51/156 | 22/91 |
| Incidence (95% CI) per 1000 person-years | 32.9 (30.0–36.2) | 46.1 (35.1–60.7) | 32.1 (21.2–48.8) |
| HR (95% CI) | 1.0 | 1.37 (1.00–1.87) | 1.00 (0.65–1.53) |
| HF | |||
| No. of events/no. at risk | 476/2155 | 42/193 | 27/117 |
| Incidence (95% CI) per 1000 person-years | 29.3 (26.8–32.1) | 29.4 (21.7–39.8) | 30.5 (20.9–44.4) |
| HR (95% CI) | 1.0 | 0.89 (0.63–1.25) | 1.01 (0.68–1.49) |
| CV death | |||
| No. of events/no. at risk | 394/2298 | 45/206 | 24/129 |
| Incidence (95% CI) per 1000 person-years | 21.4 (19.4–23.6) | 27.7 (20.7–37.1) | 23.2 (15.5–34.6) |
| HR (95% CI) | 1.0 | 0.97 (0.64–1.48) | 0.99 (0.70–1.39) |
Adjusted for age, sex, race, and initiation of thyroid medication during follow-up period.
Discussion
In this large population-based study of adults aged 65 yr or older, we report no association between persistent subclinical hypothyroidism and CHD, HF, or CV mortality. There was also no evidence of a gradient of risk or dose-response relationship by degree of TSH elevation, although the number of participants in the category of severe subclinical hypothyroidism (TSH, 10.0–19.9 mU/liter) was relatively small, with only 54–65 participants at risk for the events studied.
In a previous analysis using a single set of baseline TFTs obtained in 2639 euthyroid and 496 subclinically hypothyroid CHS participants, we found no association between subclinical hypothyroidism and CHD or CV death (8). Our findings are unchanged in this update of the single time-point analysis, which includes a larger number of CHS participants (4184 euthyroid and 679 with subclinical hypothyroidism). We have previously shown in the CHS cohort that only 56% of those with subclinical hypothyroidism have persistence 2 yr later, and a significant minority (34%) revert to euthyroidism during that time frame (6). This finding raised concern that our analyses using a single time-point to define subclinical hypothyroidism were diluted by individuals with transient subclinical hypothyroidism, potentially masking an effect of persistent subclinical hypothyroidism and biasing our results toward the null.
To address this issue of misclassification, we performed two additional sets of analyses. In the first, we included all of the available data, with up to four sets of TFTs spanning an 8-yr period. In this model, thyroid status was updated over time to allow for changes in status during follow-up. Strengths of this model included the use of multiple time-points and statistical power from inclusion of all data. However, not all participants had measurements at all time-points, which could lead to residual misclassification. In addition, this model assumes that the change in CV risk occurs immediately after the change in thyroid function test, which is biologically unlikely. In the second model, we selected participants from the two visits with the largest number of TFT measurements and categorized them as persistently subclinically hypothyroid, transiently subclinically hypothyroid, or persistently euthyroid. This is a simpler model conceptually and provides precise definitions of thyroid status, but it results in a smaller sample size and possible selection bias. The concordance of results between two models with different underlying assumptions supports the robustness of our findings of a null association.
Results from other studies vary from showing increased risk (12, 13), decreased risk (14), or no association (3, 8, 15) between subclinical hypothyroidism and CHD. An individual participant level meta-analysis of seven cohorts with CHD event data confirmed significant heterogeneity in CHD risk across studies (2). Previously, it had been suggested that participant age would explain this heterogeneity, with increased risk exclusively in younger individuals with subclinical hypothyroidism (1). However, in subgroup analysis of this meta-analysis, there was no evidence of a gradient of risk by age, and age stratification failed to resolve the heterogeneity, whereas TSH stratification successfully did so, with increased CHD risk for those with a TSH level of 10–19.9 mU/liter. Additional analyses examining CV mortality showed an increased risk in those with TSH levels of 7 mU/liter or higher (2). One of our analyses suggested a possible association between persistent subclinical hypothyroidism and CHD with a hazard ratio of 1.37 and a greater point estimate than that of the transient subclinical hypothyroidism group, suggesting greater risk from longer duration of subclinical hypothyroidism. However, the CI included one and should be considered in light of the multiple comparisons performed.
One other cohort study has reported increased risk of HF at a TSH threshold of 7 mU/liter (3), and a prior analysis in CHS reported increased HF risk at a threshold of 10 mU/liter (4). The CIs were wide for the estimate in the prior CHS analysis (HR, 1.88; 95% CI, 1.05–3.34), and with analysis of a larger sample of CHS participants, this association has decreased in magnitude and is no longer statistically significant. A recent secondary analysis of a clinical trial of pravastatin in older individuals with CV risk factors or CV disease found an increased risk of HF at a TSH of more than 10 mU/liter, but this finding should be interpreted with caution because there were only four HF events in this TSH stratum (5). An additional analysis of persistent subclinical hypothyroidism 6 months later suggested elevated HF risk for those with TSH greater than 10 mU/liter, although there were only three HF events in this group. Recently, a meta-analysis that includes these studies has been published, which shows increasing risk of HF with TSH levels of 7.0 mU/liter or higher (16). We found no increased HF risk in subclinical hypothyroidism in any model, overall or by TSH stratum. Subclinically hypothyroid individuals with higher TSH levels, particularly above 10 mU/liter, are at increased risk of overt hypothyroidism and thyroid hormone initiation (6). We excluded individuals taking thyroid hormone at baseline, who represent inadequately treated overt hypothyroidism, and accounted for thyroid hormone initiation in all of our analyses. Possible explanations for the discrepancy between our findings and prior studies is our exclusion of individuals with inadequately treated overt hypothyroidism, who may have a higher CV risk; our upper cutoff of 20 mU/liter to define subclinical hypothyroidism; and our community-dwelling population, rather than one enriched with individuals with CV disease. In addition, the stratified analyses from the meta-analysis suggest that the participants included in our study population may represent a lower risk group with subclinical hypothyroidism because they were of older age, included blacks, and were without baseline HF, all groups with no or attenuated associations with HF in this meta-analysis (16). One caveat to consider with all studies is the small number of participants with subclinical hypothyroidism at the highest TSH levels, from the perspectives of statistical power and clinical impact, because they represent only 10–15% of individuals with subclinical hypothyroidism.
Inherent in the determination of CV risk from untreated subclinical hypothyroidism is the expectation that levothyroxine treatment will attenuate any risk. Although several randomized clinical trials have shown improvement of CV risk factors with treatment (as reviewed in Ref. 1), there are no trials with sufficient power to examine CV endpoints. In the absence of these data, a recent analysis of the United Kingdom General Practitioner Research Database has shown that those aged 40–70 yr with subclinical hypothyroidism who initiated therapy had fewer fatal and nonfatal CV events than those who did not, although the levothyroxine effect was not present in those older than 70 yr (17). These results highlight the importance of considering age in the treatment of subclinical hypothyroidism. However, it is important to note that the decisions both to perform thyroid function testing and to treat subclinical hypothyroidism once detected are not made by physicians randomly, and residual confounding may account for some of all of the observed associations.
A major strength of our study is the use of a large population-based cohort that was specifically designed to examine CV risk factors, with a long duration of follow-up for events. All of the CV events were adjudicated based on objective information collected during examination and review of hospital and physician's records and CMS data. All thyroid function testing was performed using banked samples, and results were not provided to study participants or their physicians. This is the first study to incorporate multiple thyroid function tests in the same individual over time. More than one modeling strategy was employed to assess the robustness of findings to modeling assumptions, and thyroid hormone initiation was incorporated into all analyses. However, our findings may not be applicable to populations younger than age 65 yr, and we had limited power to evaluate CV risk in subgroups whose TSH levels were 7.0 mU/liter or greater. We limited the duration of follow-up to 10 yr because a biologically relevant effect should appear within that time frame, the likelihood of misclassification of thyroid status increases with greater duration from the last TSH measurement, and model assumptions were violated with longer follow-up. The CHS is an observational study, and thus we are unable to evaluate the risks and benefits of treating subclinical hypothyroidism in this study.
Clinical implications
CHS is the first cohort study to employ repeated measures of thyroid function in examining CV risk. Our data suggest that subclinical hypothyroidism, either transient or persistent, does not represent a risk factor for CHD, HF, or CV mortality in men and women aged 65 and older. We also did not find any subsets of older individuals with subclinical hypothyroidism to target for therapy based on degree of TSH elevation or duration of subclinical hypothyroidism. Our findings do not support the treatment of subclinical hypothyroidism to prevent CV disease or HF in older people.
Acknowledgments
The research reported in this article was supported by Grant R01AG-032317 from the National Institute on Aging (NIA); contracts HHSN268201200036C, N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute; with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through Grants AG-023629, AG-15928, AG-20098, and AG-027058 from the NIA. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 508
- BMI
- Body mass index
- CHD
- coronary heart disease
- CI
- confidence interval
- CV
- cardiovascular
- HF
- heart failure
- HR
- hazard ratio
- LDL
- low-density lipoprotein
- TFT
- thyroid function test.
References
- 1. Biondi B, Cooper DS. 2008. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 29:76–131 [DOI] [PubMed] [Google Scholar]
- 2. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J. 2010. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304:1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB, Bauer DC. 2005. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 165:2460–2466 [DOI] [PubMed] [Google Scholar]
- 4. Rodondi N, Bauer DC, Cappola AR, Cornuz J, Robbins J, Fried LP, Ladenson PW, Vittinghoff E, Gottdiener JS, Newman AB. 2008. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health Study. J Am Coll Cardiol 52:1152–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nanchen D, Gussekloo J, Westendorp RG, Stott DJ, Jukema JW, Trompet S, Ford I, Welsh P, Sattar N, Macfarlane PW, Mooijaart SP, Rodondi N, de Craen AJ. 2012. Subclinical thyroid dysfunction and the risk of heart failure in older persons at high cardiovascular risk. J Clin Endocrinol Metab 97:852–861 [DOI] [PubMed] [Google Scholar]
- 6. Somwaru LL, Rariy CM, Arnold AM, Cappola AR. 2012. The natural history of subclinical hypothyroidism in the elderly: the Cardiovascular Health Study. J Clin Endocrinol Metab 97:1962–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. 1991. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1:263–276 [DOI] [PubMed] [Google Scholar]
- 8. Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. 2006. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 295:1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. 1995. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 5:278–285 [DOI] [PubMed] [Google Scholar]
- 10. Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. 1995. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 41:264–270 [PubMed] [Google Scholar]
- 11. Cushman M, Psaty BM, Macy E, Bovill EG, Cornell ES, Kuller LH, Tracy RP. 1996. Correlates of thrombin markers in an elderly cohort free of clinical cardiovascular disease. Arterioscler Thromb Vasc Biol 16:1163–1169 [DOI] [PubMed] [Google Scholar]
- 12. Walsh JP, Bremner AP, Bulsara MK, O'Leary P, Leedman PJ, Feddema P, Michelangeli V. 2005. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med 165:2467–2472 [DOI] [PubMed] [Google Scholar]
- 13. Razvi S, Weaver JU, Vanderpump MP, Pearce SH. 2010. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham Survey cohort. J Clin Endocrinol Metab 95:1734–1740 [DOI] [PubMed] [Google Scholar]
- 14. Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. 2004. Thyroid status, disability and cognitive function, and survival in old age. JAMA 292:2591–2599 [DOI] [PubMed] [Google Scholar]
- 15. Boekholdt SM, Titan SM, Wiersinga WM, Chatterjee K, Basart DC, Luben R, Wareham NJ, Khaw KT. 2010. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 72:404–410 [DOI] [PubMed] [Google Scholar]
- 16. Gencer B, Collet TH, Virgini V, Bauer DC, Gussekloo J, Cappola AR, Nanchen D, den Elzen WP, Balmer P, Luben RN, Iacoviello M, Triggiani V, Cornuz J, Newman AB, Khaw KT, Jukema JW, Westendorp RG, Vittinghoff E, Aujesky D, Rodondi N. 2012. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation 126:1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Razvi S, Weaver JU, Butler TJ, Pearce SH. 2012. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 172:811–817 [DOI] [PubMed] [Google Scholar]