Abstract
Context:
The cholesterol side-chain cleavage enzyme P450scc, encoded by CYP11A1, converts cholesterol to pregnenolone to initiate steroidogenesis. P450scc deficiency can disrupt adrenal and gonadal steroidogenesis, resembling congenital lipoid adrenal hyperplasia clinically and hormonally; only 12 such patients have been reported previously.
Objective:
We sought to expand clinical and genetic experience with P450scc deficiency.
Patients and Methods:
We sequenced candidate genes in 7 children with adrenal insufficiency who lacked disordered sexual development. P450scc missense mutations were recreated in the F2 vector, which expresses the fusion protein P450scc–Ferredoxin Reductase–Ferredoxin. COS-1 cells were transfected, production of pregnenolone was assayed, and apparent kinetic parameters were calculated. Previously described P450scc mutants were assayed in parallel.
Results:
Four of five Bedouin children in one kindred were compound heterozygotes for mutations c.694C>T (Arg232Stop) and c.644T>C (Phe215Ser). Single-nucleotide polymorphism analysis confirmed segregation of these mutations. The fifth kindred member and another Bedouin patient presented in infancy and were homozygous for Arg232Stop. A patient from Fiji presenting in infancy was homozygous for c.358T>C (Arg120Stop). All mutations are novel. As assayed in the F2 fusion protein, P450scc Phe215Ser retained 2.5% of wild-type activity; previously described mutants Leu141Trp and Ala269Val had 2.6% and 12% of wild-type activity, respectively, and Val415Glu and c.835delA lacked detectable activity.
Conclusions:
Although P450scc is required to produce placental progesterone required to maintain pregnancy, severe mutations in P450scc are compatible with term gestation; milder P450scc mutations may present later without disordered sexual development. Enlarged adrenals usually distinguish steroidogenic acute regulatory protein deficiency from P450scc deficiency, but only DNA sequencing is definitive.
Steroid hormone biosynthesis is initiated by the cholesterol side-chain cleavage enzyme (P450scc, encoded by the CYP11A1 gene), which resides on the inner mitochondrial membrane where it converts cholesterol to pregnenolone, the precursor of all steroid hormones (1). Adrenal and gonadal cells that express P450scc also express the steroidogenic acute regulatory protein (StAR), which facilitates the movement of cholesterol into the mitochondria (2). However, the placenta does not express StAR and produces progesterone by StAR-independent steroidogenesis (3). Defects in StAR cause lipoid congenital adrenal hyperplasia (CAH) (4), and milder nonclassic lipoid CAH has been described (5, 6). However, defects in P450scc would be expected to disrupt placental synthesis of progesterone, which is needed to suppress uterine contractility and thus prevent spontaneous abortion or premature labor. Nevertheless, 12 patients with CYP11A1 mutations have been described, including 6 with severe mutations devoid of detectable activity (7–11) and 6 with late-onset nonclassical forms secondary to mutations that retain partial activity (12–15). The clinical presentation and hormonal findings in classic and nonclassic P450scc-deficient patients are indistinguishable from those with classic and nonclassic lipoid CAH, but patients with lipoid CAH typically have massively enlarged adrenals, whereas those described with P450scc deficiency have had small adrenals. The reason why some P450scc-deficient fetuses reach term is not known. We now describe 7 additional patients with P450scc deficiency whose presentations ranged from severe neonatal adrenal crisis with wholly inactivating loss-of-function mutations in CYP11A1 to children who presented with normal male genitalia at up to 4 years of age.
Patients and Methods
Six Bedouin patients (5 from a single kindred) were studied at Soroka Medical Center, Beer Sheva, Israel, and a 10½-year-old girl from a Fijian island was studied at Mater Children's Hospital, Brisbane, Australia. Studies at both centers were approved by local ethics committees, and informed consent was obtained from the parents; the clinical and hormonal data are summarized in Table 1. All patients were born at term; the 6 Bedouin patients had normal birth weights, and the Fijian patient was small for gestational age. Patients 1, 6, and 7 presented with adrenal crises in the neonatal period, whereas the other 4 presented between 1 and 4 years of age. All the parents were healthy; the mothers of patients 1 and 7 had histories of previous spontaneous early miscarriages.
Table 1.
Clinical and Hormonal Dataa
| Patient |
|||||||
|---|---|---|---|---|---|---|---|
| 1 (IV.1) | 2 (IV.6) | 3 (IV.7) | 4 (IV.12) | 5 (IV.13) | 6 | 7 | |
| Origin | Bedouin | Bedouin | Bedouin | Bedouin | Bedouin | Bedouin | Fijian |
| Karyotype | 46,XX | 46,XY | ND | 46,XY | 46,XX | 46,XX | 46,XX |
| Genotype | c.694C>T/c.694C>T | c.694C>T/c.644T>C | ND; inferred by linkage | c.694C>T/c.644T>C | c.694C>T/c.644T>C | c.694C>T/c.694C>T | c.358T>C/c.358T>C |
| Amino acid change | R232X/R232X | R232X/F215S | R232X/F215S | R232X/F215S | R232X/R232X | R120X/R120X | |
| Birth: gestation | Post term | Twin at term | Twin at term | Term | Term | Term | Term |
| Weight, g | 2814 | 2890 | 2820 | 3000 | 3450 | 2690 | 1940 |
| Mom's previous abortions | 2 | None | None | None | None | None | 1 |
| Age, y | 3.7 | 15.75 | Died, 6.3 | 7.5 | 8.5 | 1 | 10.5 |
| Age at diagnosis, y | Neonate | 1.2 | 1.2 | 4.75 | 1.5 | Neonate | Neonate |
| Presentation | Adrenal crisis | convulsion | Vomiting, diarrhea, electrolyte abnormalities | Adrenal crisis | Vomiting, diarrhea, electrolyte abnormalities | Adrenal crisis | Adrenal crisis |
| External genitalia | Nl female | Small penis | Nl female | Nl male | Nl female | Nl female | Nl female |
| Adrenals size (imaging mode) | Undetected (U/S) | Small (CT) | ND | ND | Small (CT) | ND | Nl (U/S) |
| Cortisol, μg/dl (basal/post ACTH) | <0.3/<0.3 | 0.8/4.2 | ND/2.5 | 2.0/1.8 | 5.1/7.6 | <0.3/<0.3 | <0.3/<0.3 |
| ACTH, pmol/L | 1200 | 578 (1–10) | ND | ND | 1114 (1–10) | >278 | 265 (1–10) |
| Progesterone, ng/mL (basal/post ACTH) | 0.3 | <0.3 (0.6–1.22) | ND | <0.3 | 0.3/0.3 | ND | ND |
| Aldosterone, ng/dL | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| 17OH-Progesterone, ng/mL | <0.1 | ND | <0.1 | <0.1 | <0.1 | <0.1 | Nil |
| DHEAS, μg/mL | <15 (35–430) | 32 (80–560) | <15 (35–430) | <15 (35–430) | <15 (35–430) | <15 (35–430) | Nil |
| Δ4-Androstenedione, nmol/L | <0.4 | ND | ND | <0.4 (0.34–10.3) | <0.4 (0.34–10.3) | <0.4 | Nil |
| 11-Deoxycortisol, nmol/L | <0.2 (0–2.5) | <0.3 (0–2.5) | <0.3 (0–2.5) | <0.1 (0–2.5) | ND | ||
| FSH/LH | 32/0.8 | 22/72 | ND | 0.6/0.1 | 1.8/0.1 | ND | 45.2/10.4 |
Abbreviations: CT, computed tomography; DHEAS, dehydroepiandrosterone sulfate; ND, not done; Nl, normal; Nil, not detectable; U/S, ultrasound.
Hormonal values in parentheses are age-appropriate normal values.
All patients presented with findings of adrenal insufficiency, including hyponatremia, hyperkalemia, hyperpigmentation, and hypoglycemia (Table 1). All patients had very low serum concentrations of all measured steroids and high concentrations of ACTH, with poor responses of cortisol and 17-hydroxyprogesterone to ACTH stimulation. Urinary steroid chromatography performed in patients 1, 4, and 7 identified no urinary steroids. Adrenal imaging (by ultrasonography or computed tomography) revealed small adrenals in patients 1, 2, and 5; imaging was not done in patients 3, 4, and 6, and patient 7 reportedly had normal-sized adrenals by ultrasonography. The patients who were 46,XX were phenotypically female, and the two patients who were 46,XY were phenotypically male. Patient 2, a 16-year-old 46,XY male, had a penile length of 5 cm (fifteenth centile for a prepubertal male), testicular volumes of 5 and 4 ml, and no pubic hair. At age 13 years, a GnRH test showed prepubertal responses of LH and FSH; at 16 years, a GnRH test showed abnormally high levels of gonadotropins, suggesting hypergonadotropic hypogonadism; at this time, his testosterone concentration was 41 ng/dl (1.42 nmol/L), a low early pubertal value. However, this patient also suffered from aggressive leukemia beginning at 10 years of age and had received chemotherapy with cyclophosphamide, which probably contributed to his testicular failure. His twin sister (patient 3) died at 6 years with a brain tumor. Patient 7 also had high FSH levels at 10.5 years of age. All patients were treated with hydrocortisone (10–15 mg/m2/d) and fludrocortisone (100 μg/d) (monitored by the plasma renin activity).
Genetics
DNA from the Bedouin patients was extracted for sequencing CYP11A1. The PCR primers to amplify the exons and their flanking regions (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org) were designed by the use of the Primer3 website (http://frodo.wi.mit.edu/primer3/). Both mutations were found on exon 4, and the following primers were used to PCR amplify this exon: forward, 5′-AGCCAATTACAGATGAGGAATCTC, and reverse, 5′-GCTCCTTTGAAGAAGATCACAGAT. DNA from patient 7 was sent to the Karolinska Institute, Stockholm, Sweden, for sequencing the StAR and CYP11A1 genes. Mutations in CYP11A1 were compared with the reference cDNA sequence (16).
For the extended family that includes patients 1 to 5, whole genome genotyping was done with Affymetrix (Santa Clara, California) GeneChip Human Mapping SNP5 arrays, and genotype calls were made with Affymetrix Genotyping Console Software. Searching these microarray data with the Superlink version 1.7 Pedtool server (http://bioinfo.cs.technion.ac.il/superlink/) confirmed a subset of single-nucleotide polymorphisms (SNPs) with minor allele frequency >0.1 and an average distance of 30 kilobases, suggesting chromosome sharing among the patients, in the region of chromosome 15 containing the CYP11A1 gene (15: 66 260 894–88 632 886) (NCBI36/hg18).
Construction of P450scc expression plasmid
The Phe215Ser mutant of P450scc was recreated in the P450scc moiety of the F2 plasmid, which expresses the fusion protein NH2-P450scc–Ferredoxin–Reductase–Ferredoxin-COOH (17). PCR-based site-directed mutagenesis was performed using primers 5′-TCACTAACGTCATTTCTGGGGAGCGCCAGGG and 5′-CCCTGGCGCTCCCCAGAAATGACGTTAGTGA, digested with DpnI at 37°C overnight, and transformed into competent Escherichia coli DH5α. The accuracy of the mutagenesis was confirmed by DNA sequencing.
Functional assay of P450scc activity
Assays of P450scc activity were done as described (13). Using Effectene (QIAGEN, Valencia, California), COS-1 cells were transfected with the F2 construct carrying the wild-type (WT) P450scc moiety or the P450scc mutant Phe215Ser, and were cotransfected with a vector expressing Renilla luciferase to control for transfection efficiency. Previously constructed F2 vectors carrying the P450scc mutants Leu141Trp, Val415Glu, c.835delA, and Ala269Val (11, 13) were transfected into parallel cultures of COS-1 cells to permit a direct comparison of mutant P450scc activities. After 24 hours of transfection, the cells were incubated with 0.3, 1, 3, and 5 μM 22R-hydroxycholesterol for 24 hours, the media were collected, and the pregnenolone content of the culture medium was assayed by ELISA (ALPCO Diagnostics, Salem, New Hampshire); the sensitivity of the assay was 54 pg/ml. The relatively long 24-hour incubation time was used because the mutants had low activities. Experiments were done in triplicate, and data were normalized to Renilla luciferase activities. Michaelis-Menten analyses were done using GraphPad Prism version 3 (GraphPad Software, San Diego, California). Because the assays were done in whole cells and not with pure, quantitated enzymes, the calculated kinetic values are only apparent values; nevertheless, this approach permits reliable comparisons of activities as a percentage of WT. Statistical analyses were done using two-tailed, unpaired t tests.
Western blotting
COS-1 cells were harvested 48 hours after transfection with F2 vectors, washed with PBS, resuspended in 0.32 M sucrose, 0.1 mM EDTA, 10 mM Tris-HCl (pH 7.8) (SET buffer), and homogenized by passage through a 28-gauge needle. Unbroken cells and nuclei were removed by centrifugation at 735 g for 5 minutes at 4°C in an Eppendorf 5415C bench-top centrifuge, and the supernatant was recentrifuged at 735 g for 5 minutes. The supernatant was then centrifuged at 15 000 g for 10 minutes, the pellet was washed by resuspending in SET buffer and centrifuged at 15 000 g for 10 minutes, and the pellet containing crude mitochondria extract was resuspended in 20 mM potassium phosphate, 10% glycerol, 0.1 mM phenylmethylsulfonyl fluoride and dissolved in sodium dodecyl sulfate (SDS) gel sample buffer (10 mM Tris HCl [pH 6.8], 0.1% SDS, 10% glycerol). The amounts of mitochondrial proteins were normalized to the transfection efficiency, loaded onto 7% SDS-PAGE, displayed by electrophoresis, transferred onto polyvinylidene difluoride membrane, probed with anti-P450scc antisera (18) at 1:2000 dilution, and visualized by chemiluminescence.
Results
Genetics
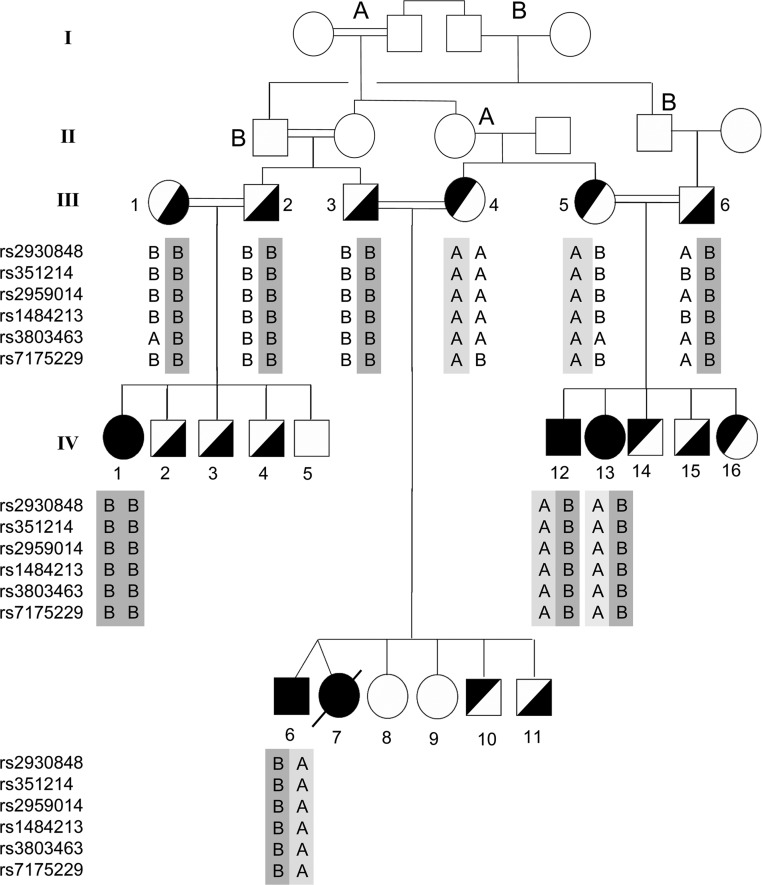
Because both males and females were affected in the extended Bedouin family, X-linked adrenal hypoplasia congenita (DAX1 mutations) was unlikely; hence, attention was focused on steroidogenic factors. The StAR genes of all patients examined were normal. Patients 1 to 6 were Bedouin, with patients 1 to 5 belonging to a single family (Figure 1); patient 7 was from Fiji. Bedouin patients 1 and 6 presented in infancy with adrenal crisis and were homozygous for a CYP11A1 mutation changing cytosine to thymidine at cDNA position 694, changing codon 232 from encoding arginine (CGA) to a stop codon (TGA) (Arg232Stop). Bedouin patients 2, 3, 4, and 5 had later clinical presentations (age 1.2–4.74 years) (Table 1), and were compound heterozygous for two CYP11A1 mutations. One allele carried the same Arg232Stop mutation seen in patients 1 and 6; the other allele carried a change from thymidine to cytosine at cDNA position 644, changing codon 215 from encoding phenylalanine (TTT) to serine (TCT) (Phe215Ser). The presence of two different mutations in this consanguineous family was confirmed by SNP arrays (Figure 1). The presence of the same Arg232Stop mutation in two apparently unrelated Bedouin families suggested this might be a common variant in this population. However, the c.694C>T (Arg232Stop) mutation was not found in 100 Bedouin chromosomes tested by TspR1 restriction endonuclease analysis. The other mutation (c.644T>C; Phe215Ser) found in the kindred in Figure 1 does not create or destroy a restriction endonuclease site. Patient 7 was homozygous for a novel change from cytosine to thymidine at cDNA position 358, changing codon 120 from arginine (CGA) to a stop codon (TGA). The parents of all the patients are apparently healthy and fertile.
Figure 1.
Family pedigree and haplotype analysis for patients 1 to 5. Data are shown for 6 SNPs: two within CYP11A1 (rs2959014 and rs1484213) and two on each side of the gene. These data show the chromosomal regions inherited from each parent. The mutation Arg232Stop is on the B chromosome indicated by dark gray (the B alleles), and the mutation Phe215Ser is on the A chromosome indicated by light gray (the A alleles). DNA was not available from individuals in generations I and II, but the A and B annotations indicate the probable segregation of the chromosomes bearing the mutations. Individuals III.1 and III.2 are shown as consanguineous because III.1 is reportedly related to individual I.4, but the relationship is not certain. All available individuals in generations III and IV were screened for the mutations; heterozygosity for Arg232Stop is designated by symbols filled on the right, and Phe215Ser is designated by symbols filled on the left. The NCBI genomic and protein reference sequences are NG_007973 and NP_000772 respectively.
Activities of the P450scc mutants
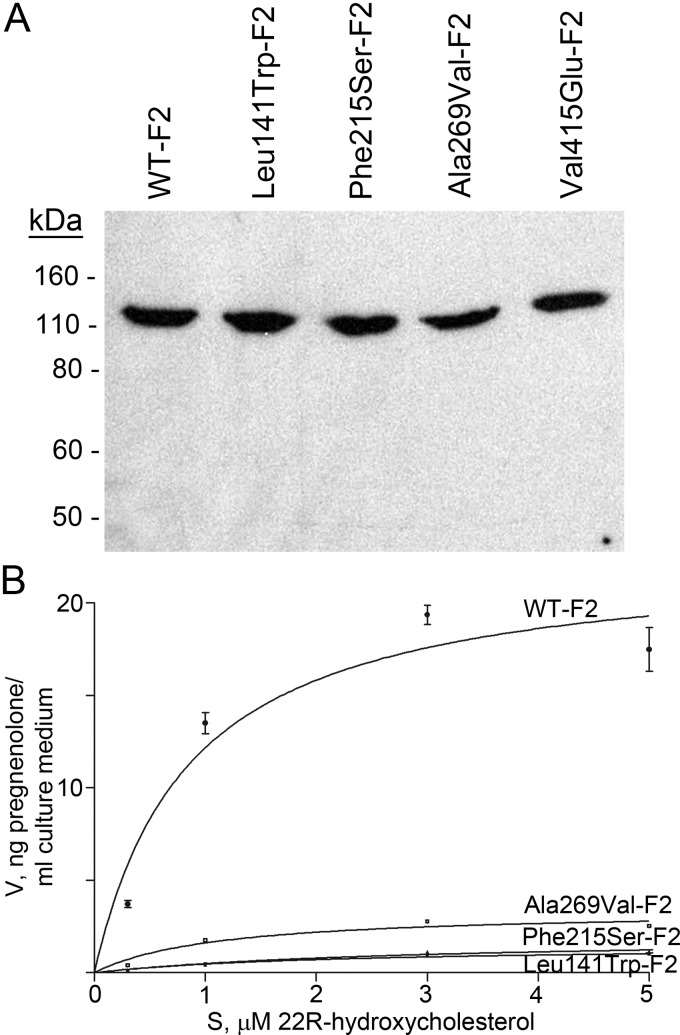
To evaluate the enzymologic consequences of the Phe215Ser missense mutation found in the mildly affected Bedouin patients, this mutation was recreated in the P450scc moiety of the vector expressing the F2 fusion protein (NH2-P450scc–Ferredoxin–Reductase–Ferredoxin-COOH), which effectively catalyzes P450scc activity (17). Western blotting showed that the transfected COS-1 cells expressed comparable levels of the WT and mutant F2 constructs (Figure 2A). The activity of the novel Phe215Ser mutant was compared with that of the WT and the previously described mutants c.835delA (9, 11, 13), Leu141Trp (11), Ala269Val (13), and Val415Glu (11). The constructs were transfected into COS-1 cells, incubated with 0.3μM to 5μM 22R-hydroxycholesterol for 24 hours, and assayed for the production of pregnenolone. Michaelis-Menten plots of these data (Figure 2B) were used to calculate the apparent Michaelis constant (Km) and the apparent maximum velocity (Vmax) and relative activity (Vmax/Km) compared with WT (Table 2). The Leu141Trp, Phe215Ser, and Ala269Val mutants had 2.6%, 2.5%, and 12% of WT activity, respectively. The Val415Glu and c.835delA mutants lacked detectable activity.
Figure 2.
Activities of the mutants. A, Western blot of mitochondrial proteins extracted from transfected COS-1 cells, probed with anti-P450scc antiserum. B, Michaelis-Menten analysis. P450scc fusion constructs for WT-F2, Leu141Trp-F2, Phe215Ser-F2, Ala269Val-F2, Val415Glu, and c.835delA were transfected into COS-1 cells for 24 hours and incubated with 0.3μM, 1μM, 3μM, and 5μM 22R-hydroxycholesterol for 24 hours. Pregnenolone production in the culture media was measured by ELISA. The data are shown as mean ± SD. Analyses were not done for Val415Glu and 835delA, because their activities were below the sensitivity of the assay.
Table 2.
Catalytic Activities Supported by P450scc F2 Fusion Constructs for WT and Mutants Leu141Trp, Phe215Ser, Ala269Val, Val415Glu, and c.835delA in the Conversion of 22R-Hydroxycholesterol to Pregnenolone in Transfected COS-1 Cellsa
| Km ± SD, μM | Vmax ± SD, ng/ml | Vmax/Km ± SD | % WT-F2 | |
|---|---|---|---|---|
| WT-F2 | 0.857 ± 0.064 | 22.6 ± 1.39 | 26.4 ± 0.465 | 100 |
| Leu141Trp-F2 | 2.13 ± 0.267 | 1.46 ± 0.112 | 0.692 ± 0.036 | 2.6 |
| P = .013 | P < .0001 | P < .0001 | ||
| Phe215Ser-F2 | 3.05 ± 0.919 | 2.00 ± 0.409 | 0.669 ± 0.058 | 2.5 |
| P = .0147 | P < .0001 | P < .0001 | ||
| Ala269Val-F2 | 1.11 ± 0.037 | 3.41 ± 0.025 | 3.06 ± 0.095 | 12 |
| P = .0039 | P < .0001 | P < .0001 | ||
| Val415Glu-F2b | ||||
| c.835delA-F2b |
The P values indicated the Km, Vmax, and Vmax/Km for the mutant F2 constructs were significantly different from WT-F2.
Activity below sensitivity of assay.
Structural modeling of the P450scc mutants
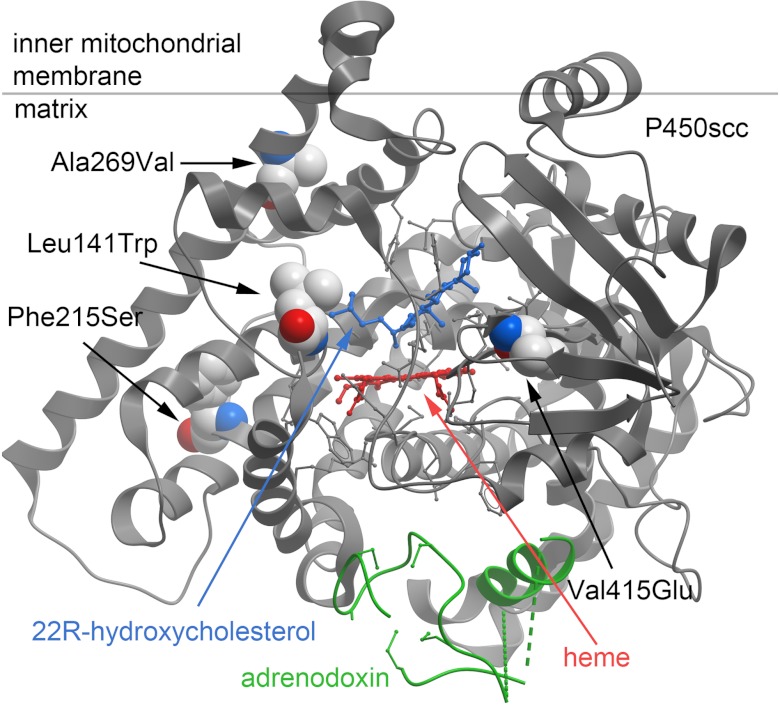
The crystal structures for bovine P450scc in complex with 22R-hydroxycholesterol (19) and for human P450scc in complex with its electron-donating partner, ferredoxin (20), now permit highly accurate modeling of P450scc missense mutations (Figure 3). Residue Leu141 is located in the loop between α-helices B′ and C and follows the active-site residue Leu140; Leu141 is conserved in many vertebrates but not in bovine and rat P450scc (20). The modeling suggests that the Leu141Trp substitution may hinder substrate binding, which would be consistent with the higher Km of this mutant for binding 22R-hydroxycholesterol. Residue Phe215 in α-helix E is conserved among many vertebrates but not in chicken, bullfrog, and zebrafish (20). Phe215 forms a hydrophobic interaction with residues in α-helix G. Substitution of a large hydrophobic amino acid for a smaller polar residue in the Phe215Ser mutant may destabilize the hydrophobic core, thus affecting enzymatic activity. Residue Ala269 in α-helix G makes a tight hydrophobic interaction with residues Ala236 and Met240 in α-helix F. The conservative Ala269Val substitution with an additional methyl group may destabilize the orientation of α-helices F and G, resulting in a loss of activity. The Val415Glu mutation occurs in β-strand 1–3; this substitution changes the residue's electrostatic charge and abolishes P450scc activity. The 3β-hydroxyl group of cholesterol does not interact directly with P450scc; instead, it binds to 2 water molecules, which participate in a hydrogen-bonded network with additional water molecules and polar residues His78, Tyr100, Asn249, and Gln416 (20). Thus, the mutation Val415Glu, which precedes Gln416, may alter the electrostatic charge of this network, thus affecting P450scc activity. None of the above residues participate directly in the interaction of P450scc with adrenodoxin (20).
Figure 3.
Model of human P450scc structure. The model is based on the crystal structure of human P450scc complexed with 22R-hydroxycholesterol (blue) and adrenodoxin (green); the heme is shown in red, and the boundary for the putative mitochondrial membrane is shown by the gray horizontal line. Residues Leu141, Phe215, Ala269, and Val415 are depicted as packed sphere images: white, carbon; red, carboxyl groups; blue, amine groups. The figure was created using Molsoft ICM MolBrowser (Molsoft LLC, La Jolla, California).
Discussion
Various congenital disorders of steroidogenesis and steroid receptors show that fetally produced steroids are not required for organogenesis, fetal development, or fetal welfare (1). However, progesterone suppresses uterine contractility and prevents spontaneous abortion and hence is essential for term pregnancy. In rodents and some other animals, the mother's corpus luteum of pregnancy produces progesterone throughout gestation; hence, rabbits with a spontaneous CYP11A1 deletion (21) and Cyp11a1 knockout mice (22) reach term without difficulty. However, human pregnancy is characterized by a second-trimester luteo-placental shift wherein the mother's corpus luteum involutes and the placenta takes over progesterone biosynthesis (23, 24). Thus, the endocrinology of human pregnancy would predict that mutations in P450scc, or any other factor required for placental progesterone biosynthesis, would be incompatible with term gestation. Nevertheless, beginning in 2001, 12 patients have been reported with CYP11A1 mutations causing P450scc deficiency. Most have had severe salt-wasting adrenal insufficiency (7–11) and P450scc mutations that lacked detectable activity, but some have had milder mutations resulting in later presentations with normal or nearly normal male sexual development (12–15). This report now brings the number of reported patients with P450scc deficiency to 19; these cases are summarized in Table 3. Review of these cases shows no clear relationship between the P450scc mutation and the duration of pregnancy; some infants (including our patients 1, 6, and 7) reached term despite having P450scc mutations devoid of activity. By contrast, a patient with severe mutation (premature stop codon) was born at 31 weeks of gestation (9). In several cases, including our cases 1 and 7, the mothers had histories of early miscarriages, suggesting that these pregnancies may have carried affected fetuses and were lost because of insufficient feto-placental progesterone. We have speculated that longer survival of maternal corpus luteum may provide the needed progesterone, thus explaining term deliveries in some affected pregnancies (11), but this hypothesis remains untested.
Table 3.
Reported Patients With P450scc Deficiency
| Patient | Mutations | Activity | Onset of Adrenal Failure | Adrenal Imaging | Karyotype | Genitalia | Maternal History; Miscarriage | Gestation | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 271insGD; not found | Nil | 2–4 y | Normal size on CT | 46,XY | Female with clitoromegaly | None | Term | 7 |
| 2 | R353W; A189V/splice | 7–9 mo | 46,XX | Nl female | None | Term | 8 | ||
| 3 | c.835delA; c.835delA | Nil | 9 d | Not seen on MRI or U/S | 46,XY | Nl female | Low estriol; 2 miscarriages | 31 wk | 9 |
| 4 | A359V; A359V | 11.7% | 21 mo | Not seen on MRI or U/S | 46,XY | Nl female | None | Term | 10 |
| 5 | L141W; V415E | 38.5%; Nil | 1–9 d | 46,XY | Nl female | None | Term | 11 | |
| 6 | c.835delA; IVS3+(2–3)insT | Nil; Nil | 8 d | Not seen on MRI or U/S | 46,XY | Nl female | Low estriol; 1 miscarriage | Term | 11 |
| 7 | L222P; L222P | 7% | 9 y | Nrml size on MRI and U/S | 46,XY | Cryptorchidism, midshaft hypospadias | Consanguineous twin died at 2.5 y | Term | 12 |
| 8a | c.835delA; A296V | Nil; 11% | 9.75 y | Small; stippled calcifications | 46,XY | Penis 2.3 cm; hypospadias, bifid scrotum | None? | Term | 13 |
| 9a | c.835delA; A296V | Nil; 11% | 12 mo | Small; punctuate calcifications | 46,XX | Nl female | None? | Term | 13 |
| 10a | R451W; R451W | 30% | 4.1 y | Normal on U/S | 46,XY | Nl male | NA? | NA? | 14 |
| 11a | R451W; R451W | 30% | 2.5 y | Normal on U/S | 46,XY | Nl male | NA? | Term | 14 |
| 12 | R360W; R405X | 30–38%; Nil | Neonatal period | Normal on U/S | 46,XY | Micropenis, hypospadias, chordee, cryptorchidism | Threatened miscarriage, treated with progesterone | Term | 15 |
| 13 (patient 1) | R232X; R232X | Nil | Neonatal period | Not seen on U/S | 46,XX | Nl female | Consanguineous, 2 miscariages | Post term | This report |
| 14a (patient 2) | R232X; F215S | Nil; 3.5% | 1.2 y | Small by CT | 46,XY | Small penis | Consanguineous | Twin at term | This report |
| 15a (patient 3) | R232X; F215S (inferred) | Nil; 3.5% (inferred) | 14 mo, died at 6.3 y | ND | ND | Nl female | Consanguineous | Twin at term | This report |
| 16a (patient 4) | R232X; F215S | Nil; 3.5% | 4.75 y | ND | 46,XY | Nl male | Consanguineous | Term | This report |
| 17a (patient 5) | R232X; F215S | Nil; 3.5% | 1.5 y | Small by CT | 46,XX | Nl female | Consanguineous | Term | This report |
| 18 (patient 6) | R232X; R232X | Nil | Neonatal period | ND | 46,XX | Nl female | Consanguineous, 2 miscarriages | Term | This report |
| 19 (patient 7) | R120X; R120X | Nil | Neonatal period | Normal on U/S | 46,XX | Nl female | Hyperemesis | 37 wk | This report |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; U/S, ultrasound; ND, not done.
Patients 8 and 9, 10 and 11, 13 and 14, and 15 and 16 are pairs of siblings.
The apparently normal development of male genitalia in our two 46,XY patients is puzzling in view of the very low residual activity (2.5%) of the mutant enzyme. Attempts to correlate the genotype reducing the mutant enzyme's activity with the patient's phenotype are problematic, in part because additional factors influence androgen synthesis and action and in part because there are substantial variations among different assays of P450scc activity. To reduce some of the variation in the published literature, we assayed three previously described P450scc mutants in parallel with our assays of the newly described Phe215Ser mutation. Using the same assay based on the F2 fusion protein of the P450scc system, we previously reported that the Leu141Trp mutant had 39% activity of WT activity (11), whereas we found only 2.6% in the present study. The basis of this difference is unclear. The previously reported patient was a compound heterozygote with Val415Glu on the other allele, which was wholly nonfunctional in both our previous study and in our present study and presented with adrenal crisis in the first week of life (11); hence, the present analysis showing low activity of Phe215Ser provides a better correlation with that patient's early adrenal failure. Because different assays at different times may yield different results, we interpret the present results as showing that the Leu141Trp is more severe than Phe215Ser, and that Ala269Val is substantially less severe; obviously, the clinical severity will depend on the mutation on the other allele.
When considering the patient with combined mineralocorticoid and glucocorticoid deficiency, an initial task is to determine whether or not the patient also has gonadal failure, which then suggests an early defect in steroidogenesis rather than an adrenal hypoplasia syndrome. The clinical diagnosis of P450scc deficiency resides on finding complete adrenal and gonadal failure with undetectable steroids in infancy or finding later adrenal failure, possibly with disordered sexual development in 46,XY patients. These clinical and hormonal findings are indistinguishable from those in patients with lipoid CAH due to StAR mutations, who have a similar spectrum of clinical severity from neonatal adrenal crisis to adult-onset glucocorticoid deficiency. Lipoid CAH is so named because of the classical finding of grossly enlarged, lipid-laden adrenals, whereas none of the P450scc-deficient patients reported to date has had adrenal enlargement. Nevertheless, a few patients with severe adrenal failure from StAR mutations have been described without adrenal enlargement (25). Thus, the only definitive way to distinguish deficiencies of P450scc and StAR reliably is by DNA sequencing.
The development of phenotypically normal male genitalia does not assure subsequent normal male reproductive capacity; hence, gonadal function should be monitored closely in patients of both sexes. Our patients 1, 2, and 7 had evidence of hypergonadotropic hypogonadism, and others have reported increased gonadotropins and subnormal responses of testosterone to human chorionic gonadotropin stimulation in patients with P450scc deficiency (11, 12). Unlike lipoid CAH, where the presence of StAR-independent steroidogenesis implies the two-hit model that explains the clinical findings (3, 26, 27), the mechanism by which patients with P450scc deficiency can develop normal male external genitalia and then subsequently have gonadal failure remains unclear.
Acknowledgments
We thank Ms. Izabella Damm for excellent technical assistance and Dr. Zoran Gucev for initially drafting Table 3.
This work was supported in part by National Institutes of Health Grant DK37922 (to W.L.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CAH
- Congenital adrenal hyperplasia
- SDS
- sodium dodecyl sulfate
- SNP
- single-nucleotide polymorphism
- StAR
- steroidogenic acute regulatory protein
- WT
- wild-type.
References
- 1. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller WL. StAR search: what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol Endocrinol. 2007;21:589–601 [DOI] [PubMed] [Google Scholar]
- 3. Bose HS, Sugawara T, Strauss JF, 3rd, Miller WL. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med. 1996;335:1870–1878 [DOI] [PubMed] [Google Scholar]
- 4. Lin D, Sugawara T, Strauss JF, 3rd, et al. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267:1828–1831 [DOI] [PubMed] [Google Scholar]
- 5. Baker BY, Lin L, Kim CJ, et al. Nonclassic congenital lipoid adrenal hyperplasia: a new disorder of the steroidogenic acute regulatory protein with very late presentation and normal male genitalia. J Clin Endocrinol Metab. 2006;91:4781–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Metherell LA, Naville D, Halaby G, et al. Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. J Clin Endocrinol Metab. 2009;94:3865–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tajima T, Fujieda K, Kouda N, Nakae J, Miller WL. Heterozygous mutation in the cholesterol side chain cleavage enzyme (P450scc) gene in a patient with 46,XY sex reversal and adrenal insufficiency. J Clin Endocrinol Metab. 2001;86:3820–3825 [DOI] [PubMed] [Google Scholar]
- 8. Katsumata N, Ohtake M, Hojo T, et al. Compound heterozygous mutations in the cholesterol side-chain cleavage enzyme gene (CYP11A) cause congenital adrenal insufficiency in humans. J Clin Endocrinol Metab. 2002;87:3808–3813 [DOI] [PubMed] [Google Scholar]
- 9. Hiort O, Holterhus PM, Werner R, et al. Homozygous disruption of P450 side-chain cleavage (CYP11A1) is associated with prematurity, complete 46,XY sex reversal, and severe adrenal failure. J Clin Endocrinol Metab. 2005;90:538–541 [DOI] [PubMed] [Google Scholar]
- 10. al Kandari H, Katsumata N, Alexander S, Rasoul MA. Homozygous mutation of P450 side-chain cleavage enzyme gene (CYP11A1) in 46, XY patient with adrenal insufficiency, complete sex reversal, and agenesis of corpus callosum. J Clin Endocrinol Metab. 2006;91:2821–2826 [DOI] [PubMed] [Google Scholar]
- 11. Kim CJ, Lin L, Huang N, et al. Severe combined adrenal and gonadal deficiency caused by novel mutations in the cholesterol side chain cleavage enzyme, P450scc. J Clin Endocrinol Metab. 2008;93:696–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rubtsov P, Karmanov M, Sverdlova P, Spirin P, Tiulpakov A. A novel homozygous mutation in CYP11A1 gene is associated with late-onset adrenal insufficiency and hypospadias in a 46,XY patient. J Clin Endocrinol Metab. 2009;94:936–939 [DOI] [PubMed] [Google Scholar]
- 13. Sahakitrungruang T, Tee MK, Blackett PR, Miller WL. Partial defect in the cholesterol side-chain cleavage enzyme P450scc (CYP11A1) resembling nonclassic congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab. 2011;96:792–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parajes S, Kamrath C, Rose IT, et al. A novel entity of clinically isolated adrenal insufficiency caused by a partially inactivating mutation of the gene encoding for P450 side chain cleavage enzyme (CYP11A1). J Clin Endocrinol Metab. 2011;96:E1798–E1806 [DOI] [PubMed] [Google Scholar]
- 15. Parajes S, Chan AO, But WM, et al. 2012 Delayed diagnosis of adrenal insufficiency in a patient with severe penoscrotal hypospadias due to two novel P450 side-change cleavage enzyme (CYP11A1) mutations (p.R360W; p.R405X). Eur J Endocrinol. 2012;167:881–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chung BC, Matteson KJ, Voutilainen R, Mohandas TK, Miller WL. Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta. Proc Natl Acad Sci U S A. 1986;83:8962–8966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harikrishna JA, Black SM, Szklarz GD, Miller WL. Construction and function of fusion enzymes of the human cytochrome P450scc system. DNA Cell Biol. 1993;12:371–379 [DOI] [PubMed] [Google Scholar]
- 18. Black SM, Szklarz GD, Harikrishna JA, Lin D, Wolf CR, Miller WL. Regulation of proteins in the cholesterol side-chain cleavage system in JEG-3 and Y-1 cells. Endocrinology. 1993;132:539–545 [DOI] [PubMed] [Google Scholar]
- 19. Mast N, Annalora AJ, Lodowski DT, Palczewski K, Stout CD, Pikuleva IA. Structural basis for three-step sequential catalysis by the cholesterol side chain cleavage enzyme CYP11A1. J Biol Chem. 2011;286:5607–5613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strushkevich N, MacKenzie F, Cherkesova T, Grabovec I, Usanov S, Park HW. Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc Natl Acad Sci U S A. 2011;108:10139–10143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang X, Iwamoto K, Wang M, Artwohl J, Mason JI, Pang S. Inherited congenital adrenal hyperplasia in the rabbit is caused by a deletion in the gene encoding cytochrome P450 cholesterol side-chain cleavage enzyme. Endocrinology. 1993;132:1977–1982 [DOI] [PubMed] [Google Scholar]
- 22. Hu MC, Hsu NC, El Hadj NB, et al. Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol Endocrinol. 2002;16:1943–1950 [DOI] [PubMed] [Google Scholar]
- 23. Csapo AI, Pulkkinen MO, Wiest WG. Effects of luteectomy and progesterone replacement therapy in early pregnant patients. Am J Obstet Gynecol. 1973;115:759–765 [DOI] [PubMed] [Google Scholar]
- 24. Csapo AI, Pulkkinen M. Indispensability of the human corpus luteum in the maintenance of early pregnancy. Luteectomy evidence. Obstet Gynecol Surv. 1978;33:69–81 [DOI] [PubMed] [Google Scholar]
- 25. Bose HS, Sato S, Aisenberg J, Shalev SA, Matsuo N, Miller WL. Mutations in the steroidogenic acute regulatory protein (StAR) in six patients with congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab. 2000;85:3636–3639 [DOI] [PubMed] [Google Scholar]
- 26. Fujieda K, Tajima T, Nakae J, et al. Spontaneous puberty in 46,XX subjects with congenital lipoid adrenal hyperplasia. Ovarian steroidogenesis is spared to some extent despite inactivating mutations in the steroidogenic acute regulatory protein (StAR) gene. J Clin Invest. 1997;99:1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bose HS, Pescovitz OH, Miller WL. Spontaneous feminization in a 46,XX female patient with congenital lipoid adrenal hyperplasia due to a homozygous frameshift mutation in the steroidogenic acute regulatory protein. J Clin Endocrinol Metab. 1997;82:1511–1515 [DOI] [PubMed] [Google Scholar]