Abstract
Context:
The gene for congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency, CYP21A2, is flanked by the gene encoding tenascin-X (TNXB), a connective tissue extracellular matrix protein that has been linked to both autosomal dominant and autosomal recessive Ehlers-Danlos syndrome (EDS). A contiguous deletion of CYP21A2 and TNXB has been described.
Objective:
The objective of the study was to determine the frequency and clinical significance of TNXB haploinsufficiency in CAH patients.
Design, Setting, and Participants:
A total of 192 consecutive unrelated CAH patients being seen as part of an observational study at the National Institutes of Health Clinical Center (Bethesda, MD) were prospectively studied during 2006–2010. Patients were evaluated for clinical evidence of EDS, including cardiac evaluation. DNA was analyzed by PCR, multiplex ligation-dependent probe amplification, Southern blot, and TNXB sequencing. Tenascin-X expression was evaluated by Western blot analysis of fibroblasts and immunostaining of the skin. CAH patients with TNXB haploinsufficiency were compared with age-matched CAH patients with normal TNXB (controls). Phenotyping of 7 parents with TNXB haploinsufficiency was performed.
Main Outcome Measures:
The frequency of TNXB haploinsufficiency among CAH patients and the frequency of EDS symptomatology among CAH patients with TNXB haploinsufficiency and controls.
Results:
TNXB haploinsufficiency, here termed CAH-X syndrome, was present in 7% of CAH patients. Twelve of 91 patients carrying a CYP21A2 deletion (13%) carried a contiguous deletion that extended into TNXB. One patient carried a TNXB premature stop codon. Twelve of 13 patients with CAH-X had EDS clinical features. Patients with CAH-X were more likely than age-matched controls to have joint hypermobility (P < .001), chronic joint pain (P = .003), multiple joint dislocations (P = .004), a structural cardiac valve abnormality by echocardiography (P = .02), and reduced tenascin-X expression by Western blot and immunostaining. A subset of parents had clinical findings.
Conclusions:
Clinical evaluation for connective tissue dysplasia should be routinely performed in CAH patients, especially those harboring a CYP21A2 deletion.
Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency is an autosomal recessive disease of adrenal steroidogenesis that is characterized by variable degrees of cortisol and aldosterone deficiency and androgen excess (1). The severity of clinical manifestations typically corresponds to the genotype and the degree of 21-hydroxylase enzyme impairment. The classic or severe form, with an incidence of approximately 1 case per 15 000 live births worldwide (1, 2), is characterized by prenatal virilization and genital ambiguity in newborn girls, postnatal virilization in both boys and girls, and adrenal insufficiency with or without salt wasting. A much more common mild or nonclassic form occurs in approximately one in 1000 in the general Caucasian population (3) and may be asymptomatic or associated with signs of androgen excess in childhood or early adulthood.
The gene encoding 21-hydroxylase, CYP21A2, and a highly homologous pseudogene, CYP21A1P, are mapped to the short arm of chromosome 6 within the human leukocyte antigen histocompatibility complex in a region of high gene density. The TNXB gene encoding tenascin-X, an extracellular matrix protein that is highly expressed in connective tissue, and a highly homologous pseudogene, TNXA, flank CYP21A2 and CYP21A1P, respectively. TNXB was originally identified because of its 3′ overlap with CYP21A2 (4). The first report of complete tenascin-X deficiency was a patient diagnosed with both CAH and Ehlers-Danlos syndrome (EDS), a hereditary disorder of connective tissue (5). Complete tenascin-X deficiency as a cause of classical EDS was subsequently demonstrated to follow an autosomal recessive pattern of inheritance (6). TNXB haploinsufficiency has been associated with hypermobility type EDS (7), characterized by hypermobility, joint subluxations, and chronic musculoskeletal pain.
Although the association of CAH and tenascin-X deficiency EDS was first reported more than a decade ago, there has not been a study to systematically evaluate a large cohort of CAH patients either for the presence of TNXB abnormalities at the molecular level or for the clinical features of EDS.
To investigate tenascin-X deficiency in CAH, we clinically evaluated 221 patients from 192 families with known CAH due to 21-hydroxylase deficiency for manifestations of EDS and completed molecular analysis of the CYP21A2 and TNXB genes. We performed extensive phenotyping of patients and parents identified as having TNXB mutations.
Materials and Methods
Participants
We studied 192 unrelated probands and their siblings for a total of 221 consecutive patients (97 males, 124 females, aged 1–65 years) with CAH due to 21-hydroxylase deficiency. All patients were enrolled in an ongoing prospective natural history study at the National Institutes of Health (NIH) Clinical Center (Bethesda, Maryland; Clinical trials no. NCT00250159). The diagnosis of 21-hydroxylase deficiency was based on hormonal evaluation (17-hydroxyprogesterone > 1200 ng/dL) and CYP21A2 genotyping (8), which was performed in all patients and 254 parents from 192 unrelated families. Approval was obtained from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. Written informed consent and assent were obtained for all individuals.
All patients were examined for joint and skin abnormalities prior to genotyping. Major and minor criteria used in the diagnosis of classical and hypermobile EDS were evaluated (9, 10). The Beighton 9-point scale was used to score joint hypermobility and was determined by one examiner (C.V.R.). A score of 1 was given for each fifth finger dorsiflexed more than 90°, each thumb apposed to the ipsilateral forearm, each elbow hyperextended more than 10°, each knee hyperextended more than 10°, and if the palms could be placed on the floor without bending the knees. Generalized hypermobility was defined as a Beighton score of 5 or greater for children and 4 or greater for postpubertal adolescents and adults (9). Skin was accessed for fragility (extensibility, thinness, scar formation, striae).
Transthoracic 2-dimensional echocardiography was performed in all subjects when possible (n = 161). Commonly encountered mild abnormalities, such as mild valvular regurgitation, were reported as no significant abnormality according to the American Society of Echocardiography (11). Magnetic resonance imaging of the heart, including the aortic valve, was subsequently performed on a 1.5T scanner in patients found to have TNXB haploinsufficiency. Parents with TNXB haploinsufficiency were invited to undergo a clinical and cardiac evaluation.
Mutation detection
PCR-based detection of chimeric TNXA/TNXB genes was performed as previously described (5). Multiplex ligation-dependent probe amplification (MLPA) was carried out for all 193 probands using SALSA MLPA KIT P155-B1 (MRC-Holland, Amsterdam, The Netherlands). The MLPA data were analyzed using Coffalyser 9.4 software (http://old.mlpa.com/coffalyser/). The principal promoter region covering the 5′ untranslated region and 200 bp upstream (12), 44 exons, and respective exon-flanking sequences of the TNXB gene were amplified and sequenced for patients with 1 or more joint or skin findings (n = 55) and all subjects who underwent a skin biopsy. Primers were designed by Primer3 (http://frodo.wi.mit.edu/primer3/) and are available upon request. The reference sequences of TNXB and TNXA are NG_008337.1 and NG_004658.2, respectively. Southern blots were performed as previously described (13).
Cell culture, Western blot analysis, and immunohistochemistry
Twelve CAH probands with TNXB haploinsufficiency and 19 age- and sex-matched CAH patients with a normal TNXB genotype consented for a skin biopsy. Skin fibroblasts from second through third passages were cultured to confluence in high glucose DMEM, 10% fetal bovine serum, penicillin, and streptomycin (Invitrogen, Carlsbad, California) at 37°C in 5% CO2 and lysed with radioimmunoprecipitation assay buffer (Pierce, Rockford, Illinois) containing protease and phosphatase inhibitors (Cocktail Sets I, II, III; Calbiochem, Gibbstown, New Jersey). Thirty micrograms of protein were loaded onto a 3%–8% NuPAGE Novex Tris-acetate precast gel (Invitrogen) and electrotransferred onto a polyvinyl difluoride membrane (Invitrogen). Proteins were blotted with a rabbit polyclonal antihuman tenascin-X (H-90) antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, California) or rabbit polyclonal antihuman β-tubulin antibody (1:2000; Cell Signaling Technology, Danvers, Massachusetts) overnight at 4°C, followed by 1 hour incubation with a secondary donkey antirabbit IgG enhanced chemiluminescence-horseradish peroxidase-linked antibody (1:5000; GE Healthcare, Piscataway, New Jersey) and visualized using an ECL Plus kit (GE Healthcare). NIH ImageJ software (Bethesda, Maryland) was used to quantify immunoblots.
Skin biopsies were fixed in 10% formalin before embedding in optimum cutting temperature compound (TissueTek, Torrance, California). Ten-micrometer cryosections were stained according to the Vectastain ABC kit (Vector Laboratories, Burlingame, California), quenched with 3% hydrogen peroxide in water at room temperature in the dark for 20 minutes and washed with 0.1% Triton X-100 in PBS for 10 minutes. A primary rabbit polyclonal antihuman tenascin-X (H-90) antibody (1:50; Santa Cruz Biotechnology) was added to the sections overnight at 4°C followed by a biotinylated antirabbit IgG secondary antibody for 1 hour at room temperature. Sections were processed according to the 3-3′-diaminobenzidine peroxidase substrate kit (Vector) and counterstained with Gill's hematoxylin no. 2 (Polysciences, Warrington, Pennsylvania).
Statistical analysis
Probands with CAH and TNXB haploinsufficiency were compared with age-matched CAH probands without TNXB mutations for the presence of clinical features suggestive of EDS. χ2 and independent-sample t tests were used (SPSS statistical software, version 12.0; SPSS Inc, Chicago, Illinois). Immunoblot group comparisons were made with the independent-sample t test. All P values are 2 tailed.
Results
TNXB mutations
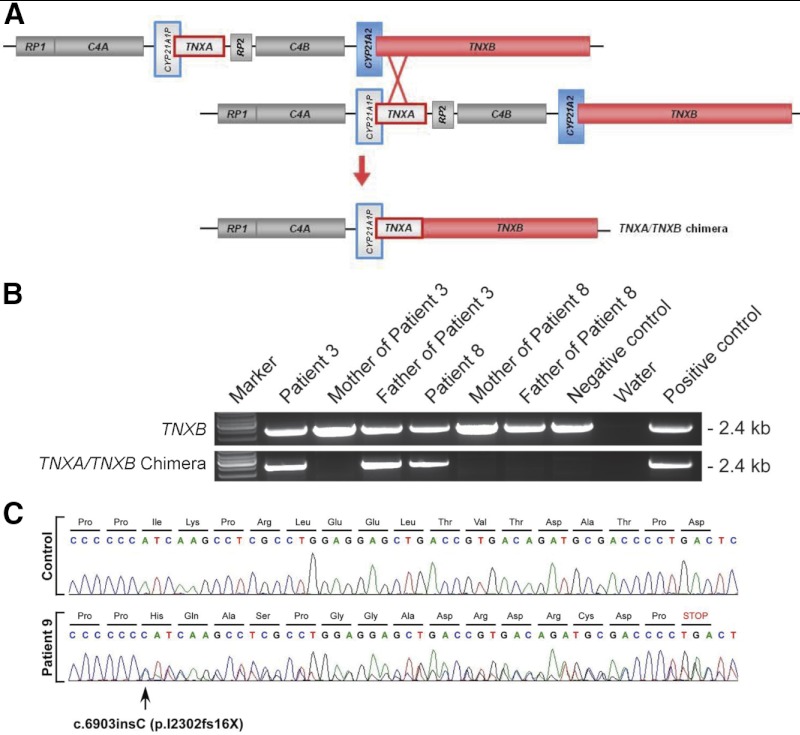
Of 192 unrelated CAH patients, we identified 13 patients with a deleterious TNXB mutation, termed here CAH-X syndrome. Overall, 91 CAH patients were either homozygous or heterozygous for a CYP21A2 deletion, and 12 of these deletions resulted in a chimeric TNXA/TNXB gene (Figure 1), representing a contiguous gene deletion syndrome. The remaining CYP21A2 deletions resulted in chimeric CYP21A1P/CYP21A2 genes, which do not affect TNXB. All chimeric TNXA/TNXB genes were identified by PCR (Figure 1B) and MLPA and confirmed by Southern blots. Sequencing the TNXB gene identified a premature stop codon mutation predicted to result in haploinsufficiency in 1 patient who was homozygous for the CYP21A2 mutation p.I172N (Figure 1C). Six novel missense mutations of unclear significance and 34 known polymorphisms (data not shown) were also identified. Eight TNXB mutations were confirmed to be inherited from a parent; 1 TNXA/TNXB chimera was de novo (Figure 1B).
Figure 1.
Mutation analysis of the TNXB gene in CAH-X patients. A, Schematic of the tenascin genes undergoing unequal crossover during meiosis resulting in a TNXA/TNXB chimera. CYP21A2 encodes the active 21-hydroxylase gene (blue); and TNXB encodes the active tenascin gene (red). Pseudogenes CYP21A1P and TNXA are in gray and are framed with the color of the corresponding functional gene. RP1 encodes a serine/threonine nuclear kinase gene (gray), and RP2 encodes the corresponding truncated pseudogene (gray). C4 encodes the fourth component of serum complement gene (gray). B, PCR-based identification of TNXA/TNXB chimera. Patient 8 is de novo. Negative control is a patient with intact CYP21A2 and TNXB genes. Positive control is a published carrier of TNXA/TNXB (13). C, TNXB sequencing chromatogram showing a heterozygous single-nucleotide insertion.
Expression of Tenascin-X in cultured fibroblasts and skin
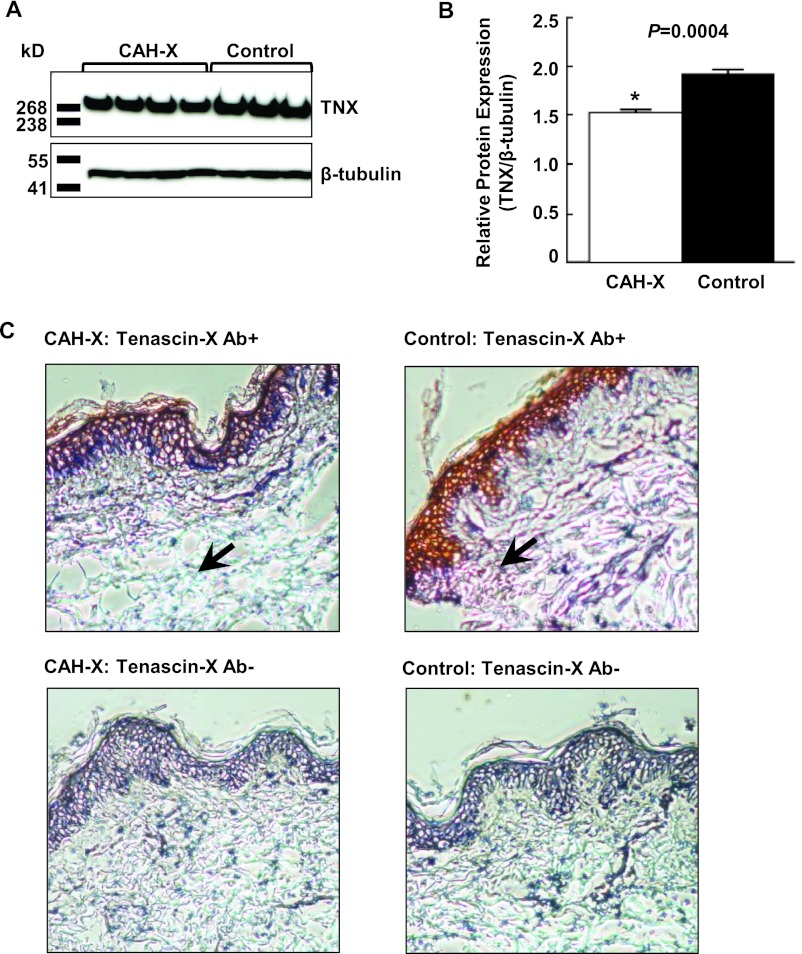
Western blot analysis of fibroblasts showed reduced tenascin-X expression in CAH-X syndrome patients relative to CAH controls (Figure 2, A and B). In addition, immunostaining of skin biopsies revealed reduced tenascin-X expression throughout the dermal layers in patients with CAH-X (Figure 2C).
Figure 2.
Analysis of tenascin-X protein expression. A, Representative Western blot analysis of skin fibroblasts. B, Reduced tenascin-X (TNX) expression in CAH-X patients relative to controls. TNX expression was quantified after normalizing to β-tubulin. C, Immunoperoxidase staining on 10-μm cryosections using a human TNX antibody. TNX staining throughout the dermal layer (arrows) is markedly reduced in CAH-X patients vs controls (upper panels). Representative sections in which primary antibody was omitted confirms antibody-specific staining (lower panels). Gill's hematoxylin no. 2 was used to counterstain (×10 magnification). Ab+, Human tenascin-X antibody; Ab−, primary antibody omitted.
Clinical findings in patients with TNXB haploinsufficiency
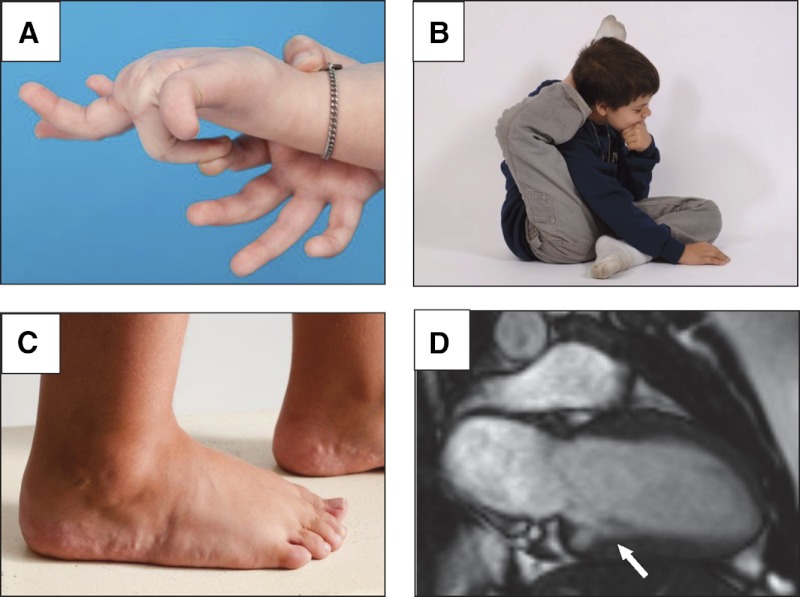
Twelve of 13 probands with CAH-X had clinical features of EDS (Table 1). Major diagnostic criteria for classical EDS, generalized joint hypermobility, or arthralgia for longer than 3 months in 4 or more joints, was present in 11 of 13 CAH-X probands (85%). Other clinical findings suggestive of EDS were commonly observed in CAH-X patients including hypermobility of small (Figure 3A) and large (Figure 3B) joints; piezogenic papules (Figure 3C); soft tissue rheumatism such as tenosynovitis, hernia, or prolapse; spondylosis; and functional bowel disorders (Table 1). The 2 oldest male patients (31 and 32 years old) had received bisphosphonate therapy for osteoporosis but did not have a history of glucocorticoid overtreatment. After eliminating common findings such as mild regurgitation or patent foramen ovale (n = 40, 45%), 6 probands had structural cardiac findings (Table 1). Echocardiography detected 2 structural valvular abnormalities in probands (P = .02 vs CAH controls). Cardiac magnetic resonance imaging detected the rare finding of a quadricuspid aortic valve (frequency of 3 in 10 923 [0.0003] in unpublished NIH experience), a congenital ventricular diverticulum (frequency of 38 in 10 923 [0.003] in unpublished NIH experience) (Figure 3D), and 4 other structural abnormalities in probands (Table 1).
Table 1.
Clinical Findings in 13 Probands and 2 Siblings With CAH-X Syndrome
| Patient No. | Sex and Age, y | CAH Phenotype | Hypermobility Scoreb | Other Joint Symptoms | Skin Findings | Additional Clinical Features |
|---|---|---|---|---|---|---|
| Patient 1 | F/8 | SW | 6 | Hypermobile fingers | Normal | LV diverticulum; chronic constipation; pes planus, piezogenic papules |
| Patient 2 | F/8 | SW | 8 | Hypermobile fingers; hip laxity | Normal | Quadricuspid aortic valve; bifid uvula; single kidney; pes planus; plantar fasciitis; scoliosis |
| Patient 3 | F/9 | NC | 6 | None | Easy bruising | Pes planus; piezogenic papules |
| Patient 4 | M/9 | SW | 8 | Hypermobile fingers; hip laxity | Easy bruising; loose skin | Gross motor delay; gastroesophageal reflux; rectal prolapse; elongated uvula with midline crease; piezogenic papules |
| Patient 5 | M/15 | SW | 0 | None | Striae | RV and LA enlargement |
| Brother of patient 5 | M/12 | SW | 3 | Hip laxity | Normal | |
| Patient 6 | M/17 | SW | 5 | Hypermobile fingers; multiple subluxations; chronic right knee pain | Extensive striae | |
| Patient 7 | F/18 | SV | 4 | Hypermobile fingers; multiple subluxations; chronic arthralgiaa; chronic tendonitis | Wide scars; androgenic alopecia | |
| Patient 8 | F/18 | SV | 6 | Hip laxity; dislocated knee; chronic arthralgiaa | Easy bruising; wide scars | |
| Patient 9 | M/18 | SW | 5 | None | Normal | LV enlargement; RV enlargement with decreased systolic function; RA enlargement |
| Sister of patient 9 | F/15 | SW | 1 | Hypermobile shoulders | Normal | |
| Patient 10 | F/22 | SW | 2 | Hypermobile fingers; chronic arthralgiaa | Easy bruising; thin skin; androgenic alopecia | LV enlargement |
| Patient 11 | F/31 | SW | 2 | Hip laxity; chronic arthralgiaa; shoulder subluxation | Easy bruising, wide scars | Diagnosed with fibromyalgia at age 24 |
| Patient 12 | M/31 | SW | 0 | Hypermobile fingers; multiple subluxations | Easy bruising | Osteoporosis |
| Patient 13 | M/32 | SV | 0 | Chronic arthralgiaa | Normal | Elongated anterior mitral valve leaflet; cervical spondylosis and osteoarthritis at age 27 y; osteoporosis |
Abbreviations: F, female; M, male; SW, salt wasting; SV, simple virilizing; NC, nonclassic; LV, left ventricle; RV, right ventricle; LA, left atrium; RA, right atrium.
Arthralgia is at least 3 months' duration.
Hypermobility score was assessed by the Beighton scale (9).
Figure 3.
Clinical findings in patients with CAH-X syndrome. A, Hypermobile small joints. B, Hypermobile large joints. C, Skin abnormalities such as piezogenic pedal papules, herniations of fat through the dermis that are commonly seen in patients with hereditary disorders of connective tissue. D, Cardiac abnormalities such as a left ventricular diverticulum.
When compared with age-matched CAH control patients, CAH-X patients were more likely to have generalized joint hypermobility as assessed by Beighton score (P < .001); hypermobility of large joints not assessed by Beighton, such as the hips and shoulders (P < .001); and hypermobility of the small joints of the hands (P = .007) (Table 2). Chronic joint pain and/or multiple joint subluxations were observed in 6 of 7 affected adults and in none of the patients aged 16 years or younger. Although easy bruising and striae were commonly reported by CAH-X patients, these were not different from unaffected CAH patients and could be confounded by glucocorticoid therapy. In general, skin was normal; however, widened scars were more commonly observed in CAH-X patients (P= .009), and 1 pediatric patient with CAH-X had loose, doughy skin.
Table 2.
Clinical Characteristics of CAH Probands Aged 7–35 Years Old According to TNXB Haploinsufficiency Carriership
| Variable | CAH-X (n = 13) | CAH Control (n = 103) | P Value |
|---|---|---|---|
| Age (mean ± sd) | 18.4 ± 8.9 | 17.0 ± 7.8 | .55 |
| Females [no. (%)] | 7 (53.8) | 59 (57.3) | 1.00 |
| Joints | |||
| Generalized joint hypermobility [no. (%)]a | 8 (61.5) | 14 (13.6) | <.001 |
| Hypermobility of large joints [no. (%)]b | 6 (46.2) | 5 (4.9) | <.001 |
| Hypermobility of small joints [no. (%)]c | 7 (53.8) | 18 (17.5) | .007 |
| More than 1 joint dislocation [no. (%)] | 3 (23.1) | 1 (0.97) | .004 |
| Arthralgia 4 or more joints [no. (%)]d | 4 (30.8) | 3 (2.9) | .003 |
| Arthralgia 1–3 joints or back pain [no. (%)]d | 4 (30.8) | 5 (4.9) | .009 |
| Skin | |||
| Easy bruising [no. (%)] | 6 (46.2) | 25 (24.3) | .11 |
| Loose skin [no. (%)] | 1 (7.7) | 0 | .11 |
| Striae [no. (%)] | 3 (23.1) | 18 (17.5) | .70 |
| Widened scar [no. (%)] | 4 (30.8) | 5 (4.9) | .009 |
| Androgenic alopecia (females) [no. (%)] | 2 (15.4) | 4 (6.8) | .14 |
Generalized hypermobility was defined as a Beighton score 5 of 9 or greater for children and 4 of 9 or greater for postpubertal adolescents and adults.
Hypermobility of large joints was defined as hypermobility of large joints not assessed in Beighton, shoulder, and hip.
Hypermobility of small joints defined as hypermobility of the hands or feet.
Arthralgia and back pain is of at least 3 months' duration.
Clinical findings of parents with TNXB haploinsufficiency
Seven parents with TNXB haploinsufficiency were available for clinical evaluation and displayed less symptomatology than CAH-X patients (Table 3). The mothers mostly had hypermobility, both affected fathers had chronic joint or back pain, but only 1 parent had chronic arthralgia in 4 or more joints. Two had cardiac abnormalities including 1 female (44 years old) with redundant anterior mitral valve leaflet and mitral prolapse and 1 male (54 years old) with a dilated aortic root of 43 mm (normal 20–36 mm) and atrial and ventricular enlargement.
Table 3.
Clinical Findings in 7 Parents With CAH Carriership and TNXB Haploinsufficiency
| Patient No. | Sex and Age, y | Hypermobility Scorea | Other Joint Symptoms | Skin Findings | Additional Clinical Features |
|---|---|---|---|---|---|
| Mother of patient 1 | F/30 | 6 | Hypermobile fingers; shoulder subluxation | Easy bruising | Pes planus |
| Mother of patient 2 | F/44 | 7 | Hypermobile fingers | Normal | Redundant anterior mitral leaflet and mitral prolapse; urethral prolapse at age 10 y; hiatus hernia; gastroesophageal reflux |
| Father of patient 3 | M/48 | 0 | Hypermobile in childhood; chronic arthralgia; chronic back pain | Normal | Varicose veins |
| Mother of patient 4 | F/41 | 5 | Hypermobile fingers; hip laxity; chronic back pain | Easy bruising | |
| Mother of patient 5 | F/54 | 0 | None | Normal | |
| Father of patient 9 | M/55 | 0 | Chronic elbow pain | Eczema | Dilated aortic root (43 mm); RV and RA enlargement; LA enlargement; pectus excavatum; lumbar spondylosis at age 39 y; gastroesophageal reflux; plantar fasciitis; wrist tendonitis |
| Mother of patient 10 | F/54 | 0 | None | Normal |
Abbreviations: M, male; F, female; RV, right ventricle; RA, right atrium; LA, left atrium.
Hypermobility score was assessed by the Beighton scale (9).
Discussion
We found that patients with CAH due to 21-hydroxylase deficiency commonly have TNXB haploinsufficiency with clinical evidence of a connective tissue dysplasia, thus defining a novel phenotype of 21-hydroxylase deficiency, termed here CAH-X syndrome. In our cohort, the prevalence of CAH-X syndrome was 7%, indicating that a significant subgroup of patients with CAH have a disorder that extends beyond the typical endocrine manifestations of the disease that are considered in patient management. Patients with CAH-X syndrome present with cardinal features of EDS such as joint hypermobility, especially during childhood, chronic joint pain and multiple joint dislocations in adulthood, soft tissue rheumatism, and other manifestations of tissue fragility. Midline defects such as cardiac structural abnormalities were observed in a subset of affected patients.
The CYP21A2 gene encoding 21-hydroxylase and the TNXB gene encoding tenascin-X are located within the human MHC class III region of chromosome 6p23.1 (14). Variation in the number of genes and the presence of highly homologous pseudogenes predispose this region to genetic recombination (15). Indeed, deleterious sequences in the pseudogene that are transferred to the functional gene by meiotic recombination account for approximately 95% of all CYP21A2 mutations (1), of which approximately 30% are large gene deletions (8, 16). In our cohort of CAH patients, we found that 13% of CYP21A2 deletions are due to a contiguous gene deletion that also impacts TNXB. However, we also described one CAH patient with a premature stop codon mutation in TNXB, suggesting that having a CYP21A2 mutation (ie, having had a recombination event in the RCCX module) may increase the risk of having a TNXB mutation.
TNXB was the first EDS gene described that does not encode a collagen or collagen-modifying enzyme but instead encodes a large extracellular matrix glycoprotein that is expressed in the dermis and in the connective tissue of skeletal muscle, the heart, and blood vessels (17). Tenascin-X has been implicated in the regulation of collagen deposition, and collagen cross-linking, and tenascin-X deficiency has been shown to reduce dermal elastic fiber length (18–20). Patients with autosomal recessive complete tenascin-X deficiency have features of classical EDS characterized by marked joint hypermobility, hyperelastic skin, and easy bruising, with normal wound healing (6, 21, 22). Additional features include muscle weakness and contractures with demonstration of abnormal muscle fibers by biopsy (23), bowel diverticulae, organ prolapse, obstetric complications (21, 24), and trachea rupture during anesthesia (25). TNXB haploinsufficiency, without concomitant CAH, results in hypermobility type EDS with variable expressivity (7). Neuromuscular abnormalities have been described in patients with TNXB haploinsufficiency, albeit less than in patients with complete tenascin-X deficiency (26).
In our patients with TNXB haploinsufficiency, joint hypermobility was a predominant clinical feature in addition to variable findings of chronic joint pain, soft tissue rheumatism, joint dislocations, piezogenic papules, and pes planus. Genitourinary or rectal prolapse was found in 2 of our subjects. Prior reports suggest that females with TNXB haploinsufficiency have more clinical features of EDS than males (7). Although we did observe some phenotypic variation, we did not observe a significant sex difference in EDS features in our CAH-X population or their affected parents. Females in general have more joint laxity than males; this was observed in our CAH cohort, despite their hormonal imbalances. None of our patients presented with muscle weakness. In addition to reflecting the severity of tenascin-X impairment, the absence of neuromuscular symptoms may be due to the relatively young age of our cohort. Such features may become apparent in longitudinal follow-up.
To our knowledge, 15 cases of complete tenascin-X deficient EDS cases have been reported (27); 2 patients had concomitant CAH (5, 6). TNXB haploinsufficiency has mostly been studied in the relatives of patients identified with autosomal recessive tenascin-X deficiency (7, 26). One case of CAH and TNXB haploinsufficiency, an 8-year-old female with marked joint hypermobility and a quadricuspid aortic valve, was reported by us (13); this patient is included here. Our cohort data provide further evidence that tenascin-X plays a direct role in the pathogenesis of EDS and that haploinsufficiency for a TNXB mutation, especially in the presence of CAH, results in a distinct phenotype similar to hypermobility-type EDS but also includes cardiac abnormalities. Our study also highlights the importance of a contiguous gene deletion in the phenotype of a monogenic disorder.
Cardiovascular abnormalities have been associated with various types of EDS, such as aortic rupture and dilation of the aorta in the vascular and classical types (9), whereas cardiac defects in hypermobility type EDS have been variable (28, 29). Severe mitral valve prolapse resulting in valve replacement was described in 2 patients with complete tenascin-X deficiency (21, 30). Our patients with TNXB haploinsufficiency were more likely to have a cardiac structural abnormality than CAH patients without TNXB haploinsufficiency. Moreover, 2 of our probands had rare or uncommon cardiac abnormalities, quadricuspid aortic valve, and a ventricular diverticulum, suggesting a developmental cardiac effect, whereas reduced collagen cross-linking may account for the observed chamber enlargement in some patients. Tenascin-X is expressed in the heart and large blood vessels (31, 32).
One strength of our study is our large population of well-characterized CAH controls without TNXB haploinsufficiency. In our study, parents with tenascin-X deficiency, who are carriers of CAH, displayed less joint symptomatology than adult patients with CAH-X. We did not evaluate a control population of parents. TNXB has been identified as an aldosterone-regulated gene (33) and patients with the severe salt-wasting form of CAH have aldosterone deficiency. The possibility that the endocrine milieu of CAH may influence the severity of EDS requires further study.
The availability of newborn testing has improved survival of CAH affected children; thus, we can estimate that approximately 20 000 persons in the United States are living with CAH. Once in the realm of pediatrics, CAH is now a multifaceted adult disease. There are many unresolved adverse clinical outcomes in CAH, many of which have been attributable to the multiple hormone imbalances characteristic of CAH, chronic glucocorticoid treatment, or a combination of hormonal factors. Our data suggest that some of these unresolved adverse outcomes may, in part, be related to tenascin-X deficiency. Poor quality of life was reported in a large cohort of CAH patients in the United Kingdom (34); however, chronic pain was not evaluated. Low bone mineral density (BMD) in adults with CAH has been attributed to increased cumulative glucocorticoid exposure (35). Low BMD is not a cardinal feature of EDS, but collagen is an important constituent of the bone matrix and altered collagen regulation could affect BMD (36). Patients with CAH may have increased risk of cardiovascular disease (35). Tenascin-X deficiency may exacerbate this risk. Further study regarding the possible role of tenascin-X in these unresolved clinical outcomes is warranted.
Patients with CAH due to 21-hydroxylase deficiency should be evaluated for connective tissue dysplasia, especially patients carrying a CYP21A2 deletion. Those with clinical symptomatology suggestive of hypermobility type EDS should be evaluated by a physiatrist. Treatment of musculoskeletal manifestations and physical therapy aimed at preventing joint instability may prevent the later development of joint dislocations and chronic musculoskeletal pain. Chronic joint pain, distinct from the pain associated with acute joint dislocations, is a serious long-term manifestation observed in our adult CAH-X patients and can be both physically and psychologically disabling. Patients with known or suspected CAH-X syndrome should also have a cardiac evaluation to determine whether there are any structural abnormalities. Because our study is cross-sectional and the affected persons in our study are relatively young, we do not yet know the frequency of serious adverse cardiac outcomes. The clinical significance of mild structural abnormalities in this study is unknown and needs to be assessed in a larger cohort.
Currently it is recommended that CYP21 genotyping be reserved for equivocal cases or for purposes of genetic counseling (35) and that clinical status should dictate the management of the patient. Similarly, we recommend that clinical evidence of hypermobility type EDS should dictate whether or not evaluation for tenascin-X deficiency should be pursued, with appropriate subspecialty referrals, including genetics with screening for tenascin-X haploinsufficiency. The field of genetics is rapidly changing and as genotyping becomes less expensive, more easily obtained, and more integrated into clinical practice, screening for tenascin-X deficiency may become a routine part of the CAH patient's evaluation. Our study demonstrates that more detailed genotyping is warranted to optimize the use of genetic testing in patient management. However, it is premature to recommend that all CAH patients be genetically screened for tenascin-X deficiency at this time.
The identification of CAH-X syndrome provides further insight into the complex clinical and genetic characteristics associated with CAH and promises to improve patient outcome through the development of focused medical management aimed at preventing long-term consequences.
Acknowledgments
We thank the patients and their parents for participating in this study. We also thank numerous fellows and the physical and occupational therapists of the Rehabilitation Medicine Department at the National Institutes of Health for their assistance in the clinical evaluation of these patients; Dr. Jiandong Yang for technical assistance of primer design for PCR and sequencing; Dr. Susan M. Krzysik-Walker for experimental support; Dr. Chack-Yung Yu (Department of Pediatrics, The Ohio State University, Columbus, OH) for providing probes for Southern blot; the National Institute on Aging Core Laboratory staff for DNA extraction and sample processing; and Dr. Josephine Egan for editorial review of the manuscript. D.P.M is a Commissioned Officer in the United States Public Health Service.
This work was supported by the Intramural Research Programs of the National Institutes of Health Clinical Center, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Heart, Lung, and Blood Institute, and The National Institute on Aging and, in part, by The Congenital Adrenal Hyperplasia Research, Education, and Support Foundation.
Disclosure Summary: W.C., R.M., Z.X., C.V.R., V.S., H.H., S.M.S., A.T.A., M.N., A.E.A., and N.B.M. have nothing to disclose. D.P.M. received research funding from Diurnal Limited in 2012.
Footnotes
- BMD
- Bone mineral density
- CAH
- congenital adrenal hyperplasia
- EDS
- Ehlers-Danlos syndrome
- MLPA
- multiplex ligation-dependent probe amplification.
References
- 1. Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–2136 [DOI] [PubMed] [Google Scholar]
- 2. Therrell BL, Jr, Berenbaum SA, Manter-Kapanke V, et al. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101:583–590 [DOI] [PubMed] [Google Scholar]
- 3. Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650–667 [PMC free article] [PubMed] [Google Scholar]
- 4. Morel Y, Bristow J, Gitelman SE, Miller WL. Transcript encoded on the opposite strand of the human steroid 21-hydroxylase/complement component C4 gene locus. Proc Natl Acad Sci USA. 1989;86:6582–6586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burch GH, Gong Y, Liu W, et al. Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nat Genet. 1997;17:104–108 [DOI] [PubMed] [Google Scholar]
- 6. Schalkwijk J, Zweers MC, Steijlen PM, et al. A recessive form of the Ehlers-Danlos syndrome caused by tenascin-X deficiency. N Engl J Med. 2001;345:1167–1175 [DOI] [PubMed] [Google Scholar]
- 7. Zweers MC, Bristow J, Steijlen PM, et al. Haploinsufficiency of TNXB is associated with hypermobility type of Ehlers-Danlos syndrome. Am J Hum Genet. 2003;73:214–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Finkielstain GP, Chen W, Mehta SP, et al. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2011;96:E161–E172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet. 1998;77:31–37 [DOI] [PubMed] [Google Scholar]
- 10. Grahame R, Bird HA, Child A. The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS). J Rheumatol. 2000;27:1777–1779 [PubMed] [Google Scholar]
- 11. Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802 [DOI] [PubMed] [Google Scholar]
- 12. Wijesuriya SD, Bristow J, Miller WL. Localization and analysis of the principal promoter for human tenascin-X. Genomics. 2002;80:443–452 [DOI] [PubMed] [Google Scholar]
- 13. Chen W, Kim MS, Shanbhag S, et al. The phenotypic spectrum of contiguous deletion of CYP21A2 and tenascin XB: quadricuspid aortic valve and other midline defects. Am J Med Genet A. 2009;149A:2803–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang Z, Mendoza AR, Welch TR, Zipf WB, Yu CY. Modular variations of the human major histocompatibility complex class III genes for serine/threonine kinase RP, complement component C4, steroid 21-hydroxylase CYP21, and tenascin TNX (the RCCX module). A mechanism for gene deletions and disease associations. J Biol Chem. 1999;274:12147–12156 [DOI] [PubMed] [Google Scholar]
- 15. Tusie-Luna MT, White PC. Gene conversions and unequal crossovers between CYP21 (steroid 21-hydroxylase gene) and CYP21P involve different mechanisms. Proc Natl Acad Sci USA. 1995;92:10796–10800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White PC, Vitek A, Dupont B, New MI. Characterization of frequent deletions causing steroid 21-hydroxylase deficiency. Proc Natl Acad Sci USA. 1988;85:4436–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mao JR, Bristow J. The Ehlers-Danlos syndrome: on beyond collagens. J Clin Invest. 2001;107:1063–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mao JR, Taylor G, Dean WB, et al. Tenascin-X deficiency mimics Ehlers-Danlos syndrome in mice through alteration of collagen deposition. Nat Genet. 2002;30:421–425 [DOI] [PubMed] [Google Scholar]
- 19. Veit G, Hansen U, Keene DR, et al. Collagen XII interacts with avian tenascin-X through its NC3 domain. J Biol Chem. 2006;281:27461–27470 [DOI] [PubMed] [Google Scholar]
- 20. Zweers MC, Dean WB, van Kuppevelt TH, Bristow J, Schalkwijk J. Elastic fiber abnormalities in hypermobility type Ehlers-Danlos syndrome patients with tenascin-X mutations. Clin Genet. 2005;67:330–334 [DOI] [PubMed] [Google Scholar]
- 21. Lindor NM, Bristow J. Tenascin-X deficiency in autosomal recessive Ehlers-Danlos syndrome. Am J Med Genet A. 2005;135:75–80 [DOI] [PubMed] [Google Scholar]
- 22. O'Connell M, Burrows NP, van Vlijmen-Willems MJ, Clark SM, Schalkwijk J. Tenascin-X deficiency and Ehlers-Danlos syndrome: a case report and review of the literature. Br J Dermatol. 2010;163:1340–1345 [DOI] [PubMed] [Google Scholar]
- 23. Voermans NC, Jenniskens GJ, Hamel BC, Schalkwijk J, Guicheney P, van Engelen BG. Ehlers-Danlos syndrome due to tenascin-X deficiency: muscle weakness and contractures support overlap with collagen VI myopathies. Am J Med Genet A. 2007;143A:2215–2219 [DOI] [PubMed] [Google Scholar]
- 24. Egging DF, van Vlijmen-Willems I, Choi J, et al. Analysis of obstetric complications and uterine connective tissue in tenascin-X-deficient humans and mice. Cell Tissue Res. 2008;332:523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Besselink-Lobanova A, Maandag NJ, Voermans NC, van der Heijden HF, van der Hoeven JG, Heunks LM. Trachea rupture in tenascin-X-deficient type Ehlers-Danlos syndrome. Anesthesiology. 2010;113:746–749 [DOI] [PubMed] [Google Scholar]
- 26. Voermans NC, van Alfen N, Pillen S, et al. Neuromuscular involvement in various types of Ehlers-Danlos syndrome. Ann Neurol. 2009;65:687–697 [DOI] [PubMed] [Google Scholar]
- 27. Hendriks AG, Voermans NC, Schalkwijk J, Hamel BC, van Rossum MM. 2011 Well-defined clinical presentation of Ehlers-Danlos syndrome in patients with tenascin-X deficiency: a report of four cases. Clin Dysmorphol. 2012;21(1):15–18 [DOI] [PubMed] [Google Scholar]
- 28. Dolan AL, Mishra MB, Chambers JB, Grahame R. Clinical and echocardiographic survey of the Ehlers-Danlos syndrome. Br J Rheumatol. 1997;36:459–462 [DOI] [PubMed] [Google Scholar]
- 29. McDonnell NB, Gorman BL, Mandel KW, et al. Echocardiographic findings in classical and hypermobile Ehlers-Danlos syndromes. Am J Med Genet A. 2006;140:129–136 [DOI] [PubMed] [Google Scholar]
- 30. Peeters AC, Kucharekova M, Timmermans J, et al. A clinical and cardiovascular survey of Ehlers-Danlos syndrome patients with complete deficiency of tenascin-X. Neth J Med. 2004;62:160–162 [PubMed] [Google Scholar]
- 31. Burch GH, Bedolli MA, McDonough S, Rosenthal SM, Bristow J. Embryonic expression of tenascin-X suggests a role in limb, muscle, and heart development. Dev Dyn. 1995;203:491–504 [DOI] [PubMed] [Google Scholar]
- 32. Bristow J, Tee MK, Gitelman SE, Mellon SH, Miller WL. Tenascin-X: a novel extracellular matrix protein encoded by the human XB gene overlapping P450c21B. J Cell Biol. 1993;122:265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fejes-Toth G, Naray-Fejes-Toth A. Early aldosterone-regulated genes in cardiomyocytes: clues to cardiac remodeling? Endocrinology. 2007;148:1502–1510 [DOI] [PubMed] [Google Scholar]
- 34. Arlt W, Willis DS, Wild SH, et al. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95:5110–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Speiser PW, Azziz R, Baskin LS, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deodhar AA, Woolf AD. Ehlers Danlos syndrome and osteoporosis. Ann Rheum Dis. 1994;53:841–842 [DOI] [PMC free article] [PubMed] [Google Scholar]