Abstract
Background:
Bariatric surgery results in bone loss at weight-bearing sites, the mechanism of which is unknown.
Methods:
Twenty-two women (mean body mass index 44 kg/m2; aged 45 years) who underwent Roux-en-Y gastric bypass (n = 14) and restrictive procedures (n = 8) had measurements of areal bone mineral density by dual-energy x-ray absorptiometry at the lumbar spine, total hip (TH), femoral neck (FN), and one third radius and trabecular and cortical volumetric bone mineral density and microstructure at the distal radius and tibia by high-resolution peripheral quantitative computed tomography (HR-pQCT) at baseline and 12 months postoperatively.
Results:
Mean weight loss was 28 ± 3 kg (P < .0001). PTH rose 23% (P < .02) and 25-hydroxyvitamin D was stable. C-telopeptide increased by 144% (P < .001). Bone-specific alkaline phosphatase did not change. Areal bone mineral density declined at TH (−5.2%; P < .005) and FN (−4.5%; P < .005). By HR-pQCT, trabecular parameters were stable, whereas cortical bone deteriorated, particularly at the tibia: cortical area (−2.7%; P < .01); cortical thickness (−2.1%; P < .01); total density (−1.3%; P = .059); cortical density (−1.7%; P < .01). In multivariate regression, bone loss at the TH and FN were predicted by weight loss. In contrast, only PTH increase predicted cortical deterioration at the tibia. Roux-en-Y gastric bypass patients lost more weight, had more bone loss by dual-energy x-ray absorptiometry and HR-pQCT than those with restrictive procedures, and had declines in cortical load share estimated by finite element analysis.
Conclusions:
After bariatric surgery, hip bone loss reflects skeletal unloading and cortical bone loss reflects secondary hyperparathyroidism. This study highlights deterioration of cortical bone loss as a novel mechanism for bone loss after bariatric surgery.
Bariatric surgery results in significant, sustained weight loss (1), reverses many complications of obesity (2–4), and decreases mortality (5, 6). In addition to these salutary outcomes, however, these procedures result in potentially important skeletal abnormalities, in particular bone loss at weight-bearing sites, specifically, the total hip and femoral neck (7–10).
Several possible mechanisms have been proposed to explain this bone loss. The strong association between the amount of bone loss and weight loss (7) suggests an adaptation to skeletal unloading. Longstanding vitamin D deficiency, common in obese patients (11–13), may result in metabolic and skeletal abnormalities that antedate but are only detected after surgery. Furthermore, the most effective bariatric procedures, including gastric bypass, reduce the intestinal surface area available for caloric absorption, leading to malabsorption of minerals and fat-soluble vitamins, including calcium and vitamin D (9, 10), and subsequent secondary hyperparathyroidism and bone loss (7, 14, 15).
Some of the bone loss measured by dual-energy x-ray absorptiometry (DXA) may be artifactual because extreme obesity and changes in fat mass may compromise the accuracy of DXA measurements (16, 17). Nor can DXA assess the extent to which cortical and trabecular bone are differentially affected after bariatric surgery. The availability of a novel technology, high-resolution peripheral quantitative computed tomography (HR-pQCT) has allowed us to address some of these issues. HR-pQCT measures volumetric BMD (vBMD) of the distal radius and tibia. It can distinguish between cortical and trabecular bone and visualize details of trabecular microarchitecture. HR-pQCT scans can be computationally modeled by microstructural finite element analysis to assess bone mechanical competence (stiffness), a surrogate measure of bone strength. This technique can also discriminate fracture status and has been used to evaluate the structural basis of fragility in several different studies of normal weight populations (18–21). These peripheral measures are associated with measurements of microarchitecture and strength at central sites (22). The goal of this study was to use HR-pQCT to examine the relationship between weight loss after bariatric surgery and changes in microarchitecture. We hypothesized that weight loss would be associated with microarchitectural change just as it had been associated with loss of areal BMD at the hip in our previous study. We further postulated that microarchitectural changes would be most pronounced in those who lost the most weight and that that there would be more pronounced changes at the tibia than the radius, mirroring the effects on weight-bearing sites seen by DXA.
Patients and Methods
We prospectively evaluated women who underwent bariatric surgery between May 2009 and February 2011 prior to and 1 year after surgery. Subjects were recruited from the Columbia University Medical Center (CUMC) Obesity Surgery Center. Exclusion criteria included conditions/medications affecting bone or mineral metabolism; weight greater than 300 pounds (our DXA machine limit). Of 101 women screened, 22 were eligible and enrolled. Common reasons for exclusion included patient preference (48%), and weight over 300 pounds (19%). All patients undergoing surgery were instructed to start a multivitamin. Subjects undergoing Roux-en-Y gastric bypass (RYGB) were prescribed 50 000 IU of vitamin D per week and daily calcium citrate 1500 mg (19 and 50 years) or 1800 mg (over 50 years). Subjects undergoing restrictive procedures were prescribed 1000 IU of vitamin D per day and calcium citrate 1000 mg (19 and 50 years) or 1200 mg (over 50 years). The CUMC Institutional Review Board approved this study. All subjects gave written informed consent. At study visits, comorbidities were assessed by self-report and confirmed by chart review.
RYGB procedures at CUMC include a 20-mL gastric pouch, a 150-cm Roux-limb, and a 75-cm biliopancreatic limb. For the adjustable gastric band, an implant (Lap Bands [Allergan, Irvine, California] or Realize bands [Ethicon, Somerville, New Jersey]) around the proximal stomach created a 30-mL pouch. Sleeve gastrectomy involved a gastric volume reduction of 75%–80% by resecting the stomach alongside a 40-French bougie beginning 4–6 cm proximal to the pylorus and ending at the angle of His.
Biochemistries
Serum calcium, albumin, and creatinine were measured using automated techniques. 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 were measured by ultraperformance liquid chromatography combined with tandem mass spectrometry using a 1290 ultraperformance liquid chromatography and a 6410 tandem mass spectrometer (Agilent, Santa Clara, California). The interassay coefficient of variation (CV) is 2.9% for 25-hydroxyvitamin D2 and 5.4% for 25-hydroxyvitamin D3. Intact PTH was measured by immunoradiometric assay (Scantibodies Laboratories, Santee, California; CV 6.8%). Serum C-telopeptide (CTX) was measured by ELISA (Immunodiagnostics Systems, Scottsdale, Arizona; CV < 10%). Bone-specific alkaline phosphatase was measured by ELISA (Quidel Corp, San Diego, California; CV 7.6%). Serum was archived at −80°C and analyzed in 1 batch after all subjects had completed their 1-year follow-up visit.
Areal bone mineral density (aBMD) and body composition
aBMD was measured by DXA (QDR-4500; Hologic Inc, Waltham, Massachusetts) of the lumbar spine (LS), total hip (TH), femoral neck (FN), and one third radius. Z-scores compared subjects and controls with age-matched populations of the same race and sex, as provided by the manufacturer. The total fat mass, truncal fat, and lean mass were measured by DXA.
HR-pQCT and structural finite element analysis of the distal radius and tibia
HR-pQCT (XtremeCT; Scanco Medical AG, Brüttisellen, Switzerland) was performed on the nondominant forearm and ipsilateral distal tibia as described (20). The region of interest was defined by manual placement of a reference line at the endplate of the radius or tibia, with the first slice 9.5 and 22.5 mm proximal to the reference line at the radius and tibia, respectively. A 3-dimensional image of approximately 9 mm in the axial direction was acquired from a stack of 110 parallel computed tomography slices using an effective energy of 40 keV, image matrix size 1024 × 1024, with a nominal voxel size of 82 μm. Attenuation data were converted to equivalent hydroxyapatite (HA) densities. The European Forearm Phantom was scanned daily for quality control. The analytic methods have been described, validated, and applied in recent clinical studies (18, 20, 23). HR-pQCT data were used to calculate whole bone stiffness, a surrogate measure of bone's resistance to force, as we have previously described (18). Trabecular bone stiffness and cortical load share were calculated after isolating each compartment.
Statistical analysis
Descriptive data are presented as mean ± SD for continuous measures or count (percentage of total) for categorical variables. Group comparisons are presented as mean ± SEM. Paired t tests were used to assess changes from baseline in raw units, whereas assessments of percent change from baseline were estimated with a t test against the null hypothesis of zero percent change. Changes in proportions of comorbidities were tested with Fisher's exact test. Pearson's correlation was used to measure the association between change in weight and PTH with change in bone parameters. Simple linear regression was used to identify univariate associations between baseline age, menopausal status, CTX, and bone-specific alkaline phosphatase (BSAP), and the 1-year change in weight, PTH, and 25-hydroxyvitamin D (25OHD) level, with change in bone parameters. Multiple regression analysis was used to determine the joint contribution of age, change in weight, 25OHD, and PTH to change in bone. One subject whose 25OHD increased by greater than 70 ng/mL after surgery was excluded from analyses that included 25OHD. Analyses were performed using SAS software (SAS Institute Inc, Cary, North Carolina). Two-sided P ≤ .05 values were considered significant.
Because there were no data on HR-pQCT changes after bariatric surgery, sample size calculations were based on the changes in aBMD reported by Fleischer et al (7). Assuming 90% power and a type 1 error rate of 1% calculations indicated that we would require at least 18 subjects to detect significant differences in aBMD at the TH and FN. Because we were planning to enroll subjects undergoing both RYGB and restrictive surgery and anticipated that some subjects would drop out, we increased this number to 22.
Results
Twenty-two women (aged 45 ± 10 years; mean ± SD) were followed up prospectively before and 12 months after bariatric surgery. The majority underwent RYGB (n = 14) and the remainder restrictive procedures (6 gastric band and 2 sleeve gastrectomy). Subjects had a mean body mass index (BMI) of 44 ± 5 kg/m2. The majority were Latina (59%) and Caucasian (23%). Half were premenopausal (45%). Comorbidities related to obesity were common (36% of the subjects had diabetes mellitus, 64% had hypertension, 36% had hyperlipidemia, 41% had obstructive sleep apnea, and 36% had osteoarthritis). Before surgery, all subjects met with a nutritionist and were started on high-dose supplementation.
Supplementation varied according to surgical type. Among the RYGB subjects, 9 were taking ergocalciferol in doses of 50 000 IU weekly (mean duration of use 13 weeks), 2 were receiving vitamin D in a multivitamin (as cholecalciferol; mean dose 550 IU/d, mean duration of use 18 months), whereas 3 subjects were not using any vitamin D supplementation. Of those who had restrictive procedures, 3 were taking ergocalciferol in doses of 50 000 IU weekly (mean duration of use 5 wk), 4 were receiving cholecalciferol as part of a multivitamin, and 1 subject was not using any supplementation prior to surgery. Mean daily calcium intake was 918 mg for a duration of 9 months among RYGB candidates and 767 mg over a duration of 9 months for restrictive surgery candidates. At baseline, mean 25OHD levels were in the normal range 33 ± 13 ng/mL, as were serum calcium, PTH, creatinine, albumin, CTX, and BSAP (Table 1).
Table 1.
Baseline and 1-Year Body Composition and Calciotropic Indices (Mean ± SE)
| Baseline | 1 y | P Value | |
|---|---|---|---|
| Body composition | |||
| Weight, kg | 115 ± 3 | 87 ± 3 | <.0001 |
| Lean body mass, % | 52.11 ± 0.65 | 59.82 ± 1.54 | <.0001 |
| Truncal fat, % | 48.70 ± 0.70 | 38.99 ± 1.72 | <.0001 |
| Fat, % (subtotal) | 49.26 ± 0.68 | 41.36 ± 1.59 | <.0001 |
| Lean body mass, g | 57824 ± 1298 | 50399 ± 1303 | <.0001 |
| Truncal fat, g | 26795 ± 796 | 16359 ± 1243 | <.0001 |
| Fat (subtotal), g | 52189 ± 1450 | 34084 ± 2392 | <.0001 |
| Calciotropic indices | |||
| Calcium intake, mg/d | 875 ± 89 | 1443 ± 205 | .02 |
| Vitamin D intake, IU/d | 5440 ± 1242 | 5852 ± 1810 | .80 |
| Corrected calciuma (8.6–10.2 mg/dL)b | 9.37 ± 0.01 | 9.17 ± 0.06 | <.02 |
| PTH (14–66 pg/mL) | 37 ± 3 | 47 ± 5 | <.05 |
| Serum 25OHD (30–80 ng/mL) | 33.0 ± 3.1 | 35.8 ± 3.6 | .07 |
| Serum CTX (0.112–0.738 ng/mL) | 0.236 ± 0.026 | 0.562 ± 0.071 | <.0001 |
| BSAP (11.6–42.7 U/L) | 31.9 ± 2.1 | 35.9 ± 2.9 | .32 |
Corrected for serum albumin.
Normal range and units for biochemistries in parentheses.
Subjects lost substantial weight (28 ± 3 kg, P < .0001) over the 12 months. Mean BMI decreased by 11 kg/m2, to 33 ± 1 kg/m2 (P < .0001); mean percentage excess BMI loss [(BMI initial − BMI final)/(BMI initial − 25) × 100] was 59 ± 5%. Body fat declined by 35 ± 4%, whereas truncal fat declined by 39 ± 4%, and lean mass decreased by 13 ± 1% (P < .0001 for all).
After surgery, comorbidities improved or resolved as follows: diabetes in 100%, hypertension in 71%, hyperlipidemia in 63%, sleep apnea in 89%, and osteoarthritis in 50%. After surgery, falls increased in 2 subjects. There were no fractures.
Despite an 86 ± 28% increase in calcium intake (P < .01; Table 1), there was a small but significant 2 ± 1% decline in serum calcium corrected for albumin (P < .02). Vitamin D intake and serum 25OHD remained stable. PTH increased substantially, by 23 ± 8% (P < .02). Two subjects (1 bypass and 1 band) developed frank hyperparathyroidism, with levels greater than 66 pg/mL. Bone resorption increased after surgery, with a rise in serum CTX of 144 ± 32% (P < .001), whereas bone formation, measured by BSAP, did not change.
Changes in aBMD and bone microarchitecture
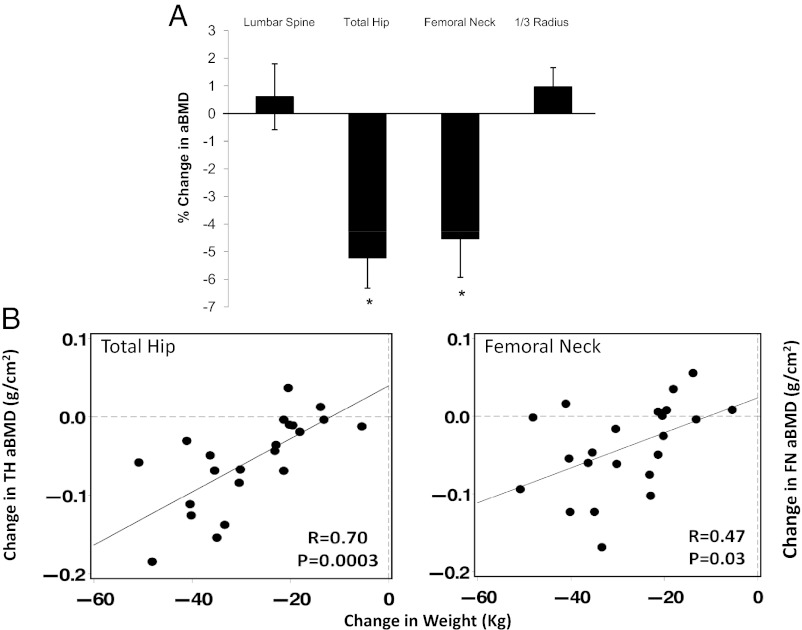
Baseline Z-scores were normal (LS Z-score: 0.51 ± 0.97; TH: 0.93 ± 0.88; FN: 0.60 ± 0.91; 1/3 radius: 0.71 ± 0.95). One year after bariatric surgery, aBMD declined (Fig. 1A) by 5.2 ± 1.1% at the total hip (P < .001) and by 4.5 ± 1.5% at the femoral neck (P < .005). In contrast, aBMD did not change at the spine or the forearm.
Figure 1.
A, Percent change in aBMD by DXA 1 year after bariatric surgery presented as mean with SE. *P < .05 compared with baseline. B, Association between weight loss and bone loss at the total hip and femoral neck.
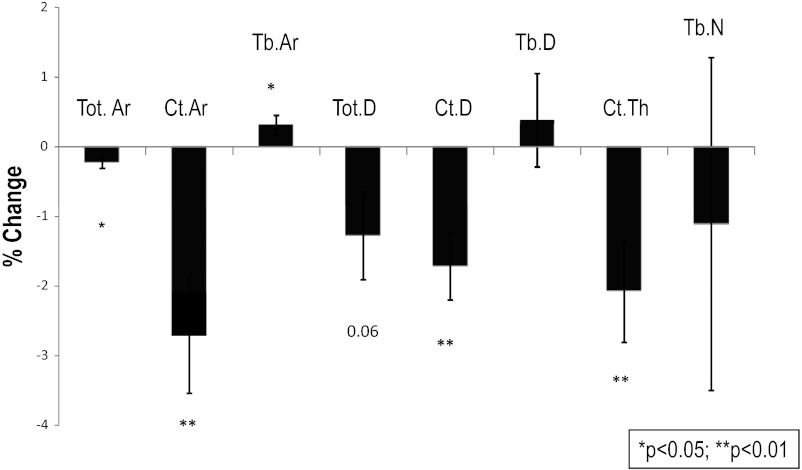
Cortical bone deteriorated significantly after surgery. At the radius, cortical and total area declined and there were nonsignificant reductions in total density, cortical thickness, and cortical density (Table 2). More pronounced changes were seen at the tibia, in which total area and cortical area decreased, whereas trabecular area increased. Total density, cortical density, and cortical thickness also declined (see Fig. 2). Trabecular vBMD and microarchitectural indices by HR-pQCT were stable at both the radius and tibia. Finite element analysis of both radius and tibia failed to show change in whole bone stiffness, trabecular bone stiffness, or cortical load share.
Table 2.
Baseline and 1-Year Microarchitectural and Microstructural Finite Element Analysis Parameters (Mean ± SE)
| Baseline | 1 Year | P Value | |
|---|---|---|---|
| Radius | |||
| Radius Ct area, mm2 | 57.3 ± 2.5 | 56.0 ± 2.7 | .05 |
| Radius Tb area, mm2 | 179.6 ± 12.4 | 180.3 ± 12.5 | .15 |
| Radius Total area, mm2 | 236.8 ± 11.6 | 236.3 ± 11.5 | .04 |
| Radius Ct density, mg HA/cm3 | 915.0 ± 14.9 | 907.4 ± 17.2 | .12 |
| Radius Ct thickness, μm | 890 ± 50 | 878 ± 50 | .14 |
| Radius Tb density, mg HA/cm3 | 147.3 ± 8.5 | 147.9 ± 8.1 | .51 |
| Radius Tb number, 1/mm | 1.90 ± 0.07 | 1.92 ± 0.06 | .73 |
| Radius total density, mg HA/cm3 | 352.0 ± 16.2 | 348.6 ± 17.1 | .18 |
| Radius Tb bone stiffness, kN/mm | 14.9 ± 2.7 | 16.1 ± 2.6 | .67 |
| Radius whole bone stiffness, kN/mm | 87.7 ± 0.8 | 88.3 ± 4.5 | .65 |
| Radius cortical load share distal | 51 ± 2 | 41 ± 8 | .23 |
| Radius cortical load share proximal | 91 ± 2 | 91 ± 2 | .70 |
| Tibia | |||
| Tibia Ct area, mm2 | 124.7 ± 4.9 | 121.6 ± 5.1 | .005 |
| Tibia Tb area, mm2 | 589.7 ± 34.1 | 591.2 ± 34.0 | .052 |
| Tibia Total area, mm2 | 714.4 ± 31.7 | 712.7 ± 31.6 | .006 |
| Tibia Ct density, mg HA/cm3 | 875.2 ± 13.1 | 860.6 ± 14.9 | .002 |
| Tibia Ct thickness, μm | 1190 ± 60 | 1170 ± 60 | .02 |
| Tibia Tb density, mg HA/cm3 | 164.0 ± 6.6 | 164.3 ± 6.4 | .77 |
| Tibia Tb number, 1/mm | 1.98 ± 0.06 | 1.95 ± 0.07 | .62 |
| Tibia total density, mg HA/cm3 | 298.9 ± 12.7 | 294.9 ± 12.4 | .053 |
| Tibia Tb bone stiffness, kN/mm | 90.3 ± 6.0 | 95.0 ± 8.0 | .48 |
| Tibia whole bone stiffness, kN/mm | 262.9 ± 7.4 | 261.9 ± 8.5 | .51 |
| Tibia cortical load share distal, % | 39 ± 2 | 38 ± 2 | .18 |
| Tibia cortical load share proximal, % | 74 ± 2 | 73 ± 2 | .15 |
Abbreviations: Ct, cortical; TB, trabecular.
Values of P < .05 were considered significant and are shown in boldface.
Figure 2.
Percent change in vBMD and microarchitecture by HR-pQCT 1 year after bariatric surgery presented as mean with SE. *P < .05 compared with baseline. Ct.Ar, cortical area; Tb. Ar, trabecular area; Tot. Ar, total area; Tot.D, total density; Ct.D cortical density; Tb.D, trabecular density; CT.Th cortical thickness; Tb.N, trabecular number.
Comparison of changes in DXA and HR-pQCT according to menopausal status revealed that women who were postmenopausal had greater declines in aBMD at the one third radius. Changes in other parameters did not differ according to menopausal status.
Predictors of skeletal changes after bariatric surgery
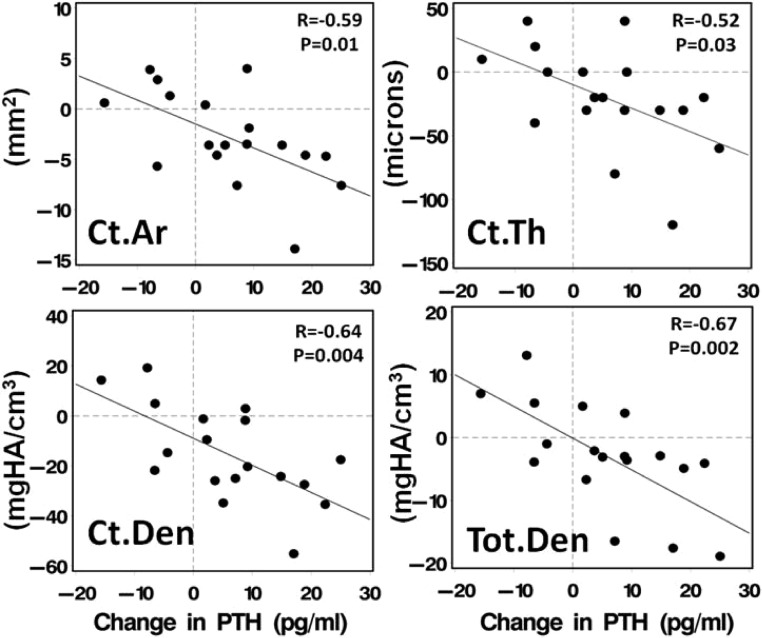
Weight loss was associated with bone loss at the total hip (r = 0.70, P < .0003) and femoral neck (r = 0.47, P = .03; Fig. 1B). In contrast, weight change did not predict any of the changes in tibial vBMD or microarchitecture after bariatric surgery. In an effort to determine whether bone metabolism-related indices other than weight predicted the changes observed in DXA at the hip and HR-pQCT at the tibia, univariate regression of baseline age, menopausal status, and change in weight (kilograms), 25OHD, and PTH were performed for each bone parameter. Increases in PTH were associated with declines in cortical area (r = −0.59, P = .01); cortical thickness (r = −0.52, P = .03), cortical density (r = −0.64, P = .004), total density (r = −0.67, P = .002) (Figure 3), and total area (r = −0.72, P < .001). Advanced age was associated with greater declines in cortical area (r = −0.62, P < .03) and cortical density (r = −0.50, P < .02). Change in 25OHD was associated with change in femoral neck BMD (r = 0.51; P < .04), total density (r = 0.51, P < .04), and cortical density (r = 0.51; P < .04). Menopausal status was not associated with the observed bone changes. A multivariate regression model was created that included those variables associated with change in bone density at the hip or vBMD and microarchitecture at the tibia (weight change, age, change in PTH or 25OHD). In this model, weight loss was the only predictor of change in BMD at the TH. Weight loss and change in 25OHD predicted bone loss at the FN. Only increased PTH predicted bone loss at the tibia (Table 3).
Figure 3.
Association between change in PTH and cortical area, cortical thickness, cortical density, and total density by HR-pQCT at the tibia. Ct.Ar, cortical area; Ct.Den, cortical density; Ct.Th, cortical thickness; Tot.D, total density.
Table 3.
Multiple Regression Models for Prediction of 1-Year Change in Skeletal Index by Weight Lost, Age, and 1-Year Change in 25OHD and PTH
| Skeletal Index (1 y Δ) | Modela | Δ Weight (per 10 kg) | Age (per 10 y) | Δ 25OHD (per 10 ng/mL) | Δ PTH (per 10 pg/mL) |
|---|---|---|---|---|---|
| TH BMD, g/cm2 | 0.43 (0.03) | 0.036–0.74 (0.002) | 0.0017–0.04 (0.90) | 0.016–0.3 (0.30) | 0.0023–0.06 (0.84) |
| FN BMD, g/cm2 | 0.64 (0.002) | 0.029–0.74 (0.002) | 0.0031–0.08 (0.78) | 0.044–0.73 (0.003) | −0.014–0.41 (0.14) |
| Tibia Ct.Th., μm | 0.16 (0.20) | 0.005–0.17 (0.53) | −0.0081–0.21 (0.47) | 0.012–0.28 (0.34) | −0.013–0.37 (0.19) |
| Tibia Ct.Ar., μm2 | 0.29 (0.09) | 0.641–0.23 (0.44) | −1.323–0.32 (0.27) | 1.171–0.26 (0.38) | −1.649–0.44 (0.11) |
| Tibia Ct.Dn., mg/μm3 | 0.52 (0.01) | 2.603–0.27 (0.34) | −5.074–0.36 (0.20) | 7.932–0.48 (0.08) | −7.415–0.56 (0.04) |
| Tibia Tot.Ar., μm3 | 0.51 (0.02) | 0.377–0.29 (0.31) | −0.714–0.37 (0.19) | 0.525–0.26 (0.37) | −1.177–0.62 (0.02) |
| Tibia Tot.Dn., mg/μm3 | 0.44 (0.03) | −0.126–0.03 (0.92) | −0.797–0.12 (0.68) | 3.511–0.43 (0.12) | −4.13–0.60 (0.02) |
Abbreviations: Ct.Ar., cortical area; Ct.Dn., cortical density; Ct.Th., cortical thickness; Tot Ar., total area; Tot.Dn., total density.
Data are shown as β-correlation R (P value).
Model R2 (P value).
Roux-en-Y gastric bypass
The 14 patients who underwent RYGB were analyzed separately. Mean weight loss among these subjects was 35 ± 9 kg (P < .0001), substantially greater than the weight loss that occurred in the band (15 ± 6 kg) or sleeve (20 ± 2 kg) subjects. BMI among RYGB decreased by 30 ± 8%. Fat mass decreased by 45 ± 3%, truncal fat by 49 ± 4%, and lean mass by 15 ± 1% (P < .0001 for all). Among RYGB subjects, PTH increased by 32 ± 12% (P < .04), whereas corrected calcium tended to decrease (2.3 ± 1.1%, P = .07) and 25OHD was stable. Markers of bone resorption and formation increased [CTX by 212 ± 47% (P < .001) and BSAP by 20 ± 7% (P < .02)].
Changes in aBMD were more pronounced than in the cohort as a whole. Total hip aBMD declined by 8.1 ± 1.1% (P < .0001) and FN by 7.9 ± 1.5% (P < .003), whereas LS and one third radius BMD did not change. By HR-pQCT at the tibia, there was a reduction in cortical area by 4 ± 1% (P < .004) and a 0.5 ± 0.2% increase in trabecular area (P < .04), cortical density (2 ± 1%; P < .006), and cortical thickness (3 ± 1%; P < .01) decreased. At the radius, the pattern was similar [cortical area declined (3 ± 1%; P < .03); trabecular area increased (0.8 ± 0.3%; P < .03); cortical thickness also decreased (3 ± 1%; P = .05)]. By finite element analysis, whole bone stiffness and trabecular stiffness did not change. However, there were significant declines in proximal (2 ± 1%; P < .03) and distal cortical load share (7 ± 2%; P < .01) at the tibia. In comparison with the RYGB group, subjects who underwent restrictive procedures did not have significant changes in DXA or microarchitecture at 1 year.
Discussion
Bariatric surgery may offer the best opportunity to treat not only morbid obesity but also its many comorbidities. However, exploration of its potential deleterious skeletal effects is only beginning. Studies to date show bone loss, but findings have been restricted by limitations of the analytic tool (DXA). To our knowledge, this is the first study to evaluate changes in vBMD and microstructure after bariatric surgery. Using novel HR-pQCT technology, we confirmed bone loss and found not only that it is cortical bone that is primarily affected but also that cortical changes were associated with increased PTH levels. In contrast, bone loss at the hip was primarily associated with weight loss. Fewer changes occurred at non-weight-bearing sites.
Our study confirmed the uniform finding of decreased hip aBMD after bariatric surgery. Preferential loss of bone at the hip, a weight-bearing site, suggests a response to unloading of the skeleton, a hypothesis supported by the strong association between extent of weight loss and amount of bone loss in this and other studies. Hip bone loss has been documented in individuals who lose even small amounts of weight from caloric restriction (24–26). The change in mechanical stress leads to increased bone turnover (we documented increased bone resorption markers) and subsequent reductions in BMD. We also observed an association between change in 25OHD and bone loss at the FN. Subjects with increased or stable 25OHD had less bone loss than those whose 25OHD declined, suggesting that postoperative maintenance of 25OHD stores is important to bone preservation at this site. Artifact related to changes in the amount of overlying fat may have also influenced the observed changes at the hip.
We found that spine aBMD did not decline, similar to some (7, 14, 27) but not all studies (8–10). Although it is possible that the variable findings at the spine are artifactual, it is also possible that the absence of bone loss at the lumbar spine may be related to an effect of PTH. Although chronically elevated PTH has catabolic effects on cortical bone, mild increases in PTH have anabolic effects on cancellous bone, which predominates at the spine (28), as is seen in mild primary hyperparathyroidism or treatment of osteoporosis. Lumbar spine aBMD did not decline in this or other studies in which postoperative PTH levels increased (7, 14, 27).
In contrast to prior reports in which most patients undergoing bariatric surgery had low 25OHD levels (8, 14, 15, 29), most of our patients went to surgery vitamin D replete due to extremely high daily supplementation doses. Despite these doses, 25OHD levels were in the low normal range, lower than would be anticipated in a normal-weight population (30). This suggests decreased absorption of vitamin D or increased distribution to the adipose tissue, which remained well above normal despite surgery. In a recent randomized study, RYGB patients who received 50 000 IU/wk achieved 25OHD levels similar to ours (31). These data speak against the theory that vitamin D stores are mobilized from adipose tissue after bariatric surgery (32). Despite normal vitamin D levels and a doubling of calcium intake, we found a substantial secondary hyperparathyroid response. Many (7, 14, 33) but not all (8, 34) studies have documented hyperparathyroidism after bariatric surgery. These disparities may have related to differences in preoperative vitamin D status, surgical techniques, calcium and vitamin D repletion protocols, or varying length of follow-up.
HR-pQCT revealed novel information on the mechanism for bone loss after bariatric surgery, namely deteriorating cortical microstructure. Bariatric surgery led to a reduction in total area, driven by a decline in cortical area, whereas trabecular area tended to increase. These changes were most strongly predicted by the rise in PTH and suggest endocortical bone resorption. At the weight-bearing tibia, we also observed reduced total density, cortical density, and cortical thickness. It is possible that greater variability in trabecular measures may have precluded our ability to observe changes in that compartment, given the small sample size of this study. Although the observed cortical changes were small, they were of similar magnitude to those observed after initiation of osteoporosis treatment (35, 36). The significance of these HR-pQCT changes is supported by numerous studies relating abnormalities in cortical and trabecular volumetric BMD, microarchitecture and stiffness with fragility fractures (18–21).
It is not possible to distinguish on the basis of DXA measurements whether hip bone loss was cortical or trabecular in nature. Central quantitative computed tomography measurements would be helpful in making this determination, nor is it possible to rule out some role for weight loss in the bone loss at the tibia. Our finding of pronounced changes at the tibia but not the radius suggests that there may be an interaction between PTH and weight bearing. The fact that hip carries a greater load (approximately 2–3 times body weight compared with the tibia, which carries 1 time body weight) may explain a different magnitude of the association between weight loss and bone loss at the 2 sites (37, 38). It is possible that we would have detected such an association at the tibia in a larger study.
As expected, our findings were magnified in the RYGB patients, who had the most weight loss and greatest increase in PTH. RYGB bypasses the duodenum, the primary site of calcium absorption (39), which likely contributed to secondary hyperparathyroidism in these patients. These patients had a greater increase in markers of bone turnover and more hip bone loss. HR-pQCT revealed not only cortical bone loss but also reduced proximal and distal cortical load share. It is possible that this finding is indicative of decreased cortical bone strength, a hypothesis that merits further exploration. If there is an associated reduction in cortical strength, RYGB may be associated with an increased fracture risk, particularly in those patients in whom weight loss and hyperparathyroidism are most pronounced.
Our study has important strengths and limitations. The use of HR-pQCT allowed us to avoid DXA artifacts associated with central obesity (16, 17) and bone size in the bariatric population. There is recent evidence that HR-pQCT measures may be affected by surrounding fat, albeit to a lesser extent than DXA. In a small study, lower amounts of fat surrounding bone were associated with increased HR-pQCT measurements (40), suggesting that the actual declines in vBMD and cortical microarchitecture may actually be more pronounced than those we reported. Although most studies in this field had DXA data only on the subset of participants who met weight criteria for central DXA; we included only subjects below our DXA weight threshold and therefore had complete data on our entire cohort. We further limited the heterogeneity of our results by restricting our sample to women. A significant limitation of this work is our inclusion of women who underwent RYGB as well as restrictive procedures, which introduced some variability into our findings. However, this design enabled us to evaluate microarchitectural changes over a wide range of weight loss, which was our primary goal. Although we did not have sufficient power to perform a comparison of bone structural change between subjects who underwent different types of bariatric surgery, this is an important question that needs to be addressed in larger studies in the future. Our findings, although compelling, are largely exploratory and lay the foundation for further work on this topic. Thresholding was based on the standard Scanco software which may be subject to artifact particularly regarding cortical measures. Other limitations of the study include small sample size, similar to other prospective studies in this field. Ethical considerations prevented us from including a randomized control group. Finally, we did not collect data on physical activity, which may have played a role in the changes we observed at the hip and tibia.
The important question of whether bone loss after bariatric surgery is associated with increased fractures remains unanswered. Whereas obese individuals were previously thought to be protected from fracture compared with their normal-weight and underweight counterparts, recent studies report increased fracture rates in obese patients, particularly at peripheral sites (41, 42). Our findings raise concern that bone loss after bariatric surgery may further increase skeletal fragility and fracture susceptibility among these individuals.
In conclusion, our results suggest at least 2 mechanisms for bone loss after bariatric surgery: hip bone loss due to skeletal unloading and deterioration of cortical microstructure due to secondary hyperparathyroidism. This study highlights deterioration of cortical bone as a novel mechanism for bone loss after bariatric surgery. Larger, long-term studies are needed to further explore these mechanisms and the association between the observed skeletal changes and fracture risk.
Acknowledgments
We thank Dr Elizabeth Shane for her thoughtful review of the manuscript.
This work was supported by Grant K24 DK074457 and The Thomas L. Kempner and Katheryn C. Patterson Foundation.
Disclosure Summary: The authors have no conflicts of interest.
Footnotes
- aBMD
- Areal bone mineral density
- BMI
- body mass index
- BSAP
- bone-specific alkaline phosphatase
- CV
- coefficient of variation
- CTX
- C-telopeptide
- CUMC
- Columbia University Medical Center
- DXA
- dual-energy x-ray absorptiometry
- FN
- femoral neck
- HA
- hydroxyapatite
- HR-pQCT
- high-resolution peripheral quantitative computed tomography
- LS
- lumbar spine
- 25OHD
- 25-hydroxyvitamin D
- RYGB
- Roux-en-Y gastric bypass
- TH
- total hip
- vBMD
- volumetric BMD.
References
- 1. Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–2796 [DOI] [PubMed] [Google Scholar]
- 2. Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 3. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–1585 [DOI] [PubMed] [Google Scholar]
- 5. Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 6. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761 [DOI] [PubMed] [Google Scholar]
- 7. Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93:3735–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061–1065 [DOI] [PubMed] [Google Scholar]
- 9. Vilarrasa N, San Jose P, Garcia I, et al. Evaluation of bone mineral density loss in morbidly obese women after gastric bypass: 3-year follow-up. Obes Surg. 2011;21:465–472 [DOI] [PubMed] [Google Scholar]
- 10. Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19:41–46 [DOI] [PubMed] [Google Scholar]
- 11. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693 [DOI] [PubMed] [Google Scholar]
- 12. Grethen E, McClintock R, Gupta CE, et al. Vitamin D and hyperparathyroidism in obesity. J Clin Endocrinol Metab. 2011;96:1320–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stein EM, Strain G, Sinha N, et al. Vitamin D insufficiency prior to bariatric surgery: risk factors and a pilot treatment study. Clin Endocrinol (Oxf). 2009;71:176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goode LR, Brolin RE, Chowdhury HA, Shapses SA. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12:40–47 [DOI] [PubMed] [Google Scholar]
- 15. Avgerinos DV, Leitman IM, Martinez RE, Liao EP. Evaluation of markers for calcium homeostasis in a population of obese adults undergoing gastric bypass operations. J Am Coll Surg. 2007;205:294–297 [DOI] [PubMed] [Google Scholar]
- 16. Binkley N, Krueger D, Vallarta-Ast N. An overlying fat panniculus affects femur bone mass measurement. J Clin Densitom. 2003;6:199–204 [DOI] [PubMed] [Google Scholar]
- 17. Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012;27 (Suppl 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stein EM, Liu XS, Nickolas TL, et al. Abnormal microarchitecture and stiffness in postmenopausal women with ankle fractures. J Clin Endocrinol Metab. 2011;96:2041–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23:392–399 [DOI] [PubMed] [Google Scholar]
- 20. Stein EM, Liu XS, Nickolas TL, et al. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res. 2010;25:2572–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Melton LJ, 3rd, Christen D, Riggs BL, Achenbach SJ, et al. Assessing forearm fracture risk in postmenopausal women. Osteoporos Int. 2010;21:1161–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu XS, Cohen A, Shane E, et al. Bone density, geometry, microstructure, and stiffness: Relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res. 2010;25:2229–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu XS, Zhang XH, Sekhon KK, et al. High-resolution peripheral quantitative computed tomography can assess microstructural and mechanical properties of human distal tibial bone. J Bone Miner Res. 2010;25:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salamone LM, Cauley JA, Black DM, et al. Effect of a lifestyle intervention on bone mineral density in premenopausal women: a randomized trial. Am J Clin Nutr. 1999;70:97–103 [DOI] [PubMed] [Google Scholar]
- 25. Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res. 2005;20:455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwartz AV, Johnson KC, Kahn SE, et al. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res. 2012;27:619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ott MT, Fanti P, Malluche HH, et al. Biochemical evidence of metabolic bone disease in women following Roux-Y gastric bypass for morbid obesity. Obes Surg. 1992;2:341–348 [DOI] [PubMed] [Google Scholar]
- 28. Silverberg SJ, Shane E, de la Cruz L, et al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4:283–291 [DOI] [PubMed] [Google Scholar]
- 29. Sinha N, Shieh A, Stein EM, et al. Increased PTH and 1.25(OH)(2)D levels associated with increased markers of bone turnover following bariatric surgery. Obesity (Silver Spring). 2011;19:2388–2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210 [DOI] [PubMed] [Google Scholar]
- 31. Carlin AM, Rao DS, Yager KM, Parikh NJ, Kapke A. Treatment of vitamin D depletion after Roux-en-Y gastric bypass: a randomized prospective clinical trial. Surg Obes Relat Dis. 2009;5:444–449 [DOI] [PubMed] [Google Scholar]
- 32. Lin E, Armstrong-Moore D, Liang Z, et al. Contribution of adipose tissue to plasma 25-hydroxyvitamin D concentrations during weight loss following gastric bypass surgery. Obesity (Silver Spring). 2011;19:588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Prisco C, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci. 2005;329:57–61 [DOI] [PubMed] [Google Scholar]
- 34. Bruno C, Fulford AD, Potts JR, et al. Serum markers of bone turnover are increased at six and 18 months after Roux-en-Y bariatric surgery: correlation with the reduction in leptin. J Clin Endocrinol Metab. 2010;95:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seeman E, Delmas PD, Hanley DA, et al. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res. 2010;25:1886–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S. A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res. 2010;25:2558–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bergmann G, Deuretzbacher G, Heller M, Graichen F, Rohlmann A, Strauss J, Duda GN. Hip contact forces and gait patterns from routine activities. J Biomech. 2001;34:859–871 [DOI] [PubMed] [Google Scholar]
- 38. Nilsson J, Thorstensson A. Ground reaction forces at different speeds of human walking and running. Acta Physiol Scand. 1989;136:217–227 [DOI] [PubMed] [Google Scholar]
- 39. Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91:4223–4231 [DOI] [PubMed] [Google Scholar]
- 40. Colt E, Akram M, Javed F, Shane E, Boutroy S. Comparison of the effect of surrounding fat on measurements of BMD by DXA and high resolution quantitative computerized tomography. J Bone Miner Res. 2011;26(suppl 1). http://www.abstracts2view.com/asbmr/view.php?nu=ASBMR11L_A11007757-89 Accessed August 16, 2012 [Google Scholar]
- 41. Gnudi S, Sitta E, Lisi L. Relationship of body mass index with main limb fragility fractures in postmenopausal women. J Bone Miner Metab. 2009;27:479–484 [DOI] [PubMed] [Google Scholar]
- 42. Compston JE, Watts NB, Chapurlat R, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124:1043–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]