Abstract
Context:
In the United States, generic substitution of levothyroxine (l-T4) by pharmacists is permitted if the formulations are deemed to be bioequivalent by the Federal Drug Administration, but there is widespread concern that the pharmacokinetic standard used is too insensitive.
Objective:
We aimed to evaluate the bioequivalence of a brand-name l-T4 (Synthroid) and an AB-rated generic formulation (Sandoz, Princeton, NJ) in children with severe hypothyroidism.
Design:
This was a prospective randomized crossover study in which patients received 8 weeks of one l-T4 formulation followed by 8 weeks of the other.
Setting:
The setting was an academic medical center.
Patients:
Of 31 children with an initial serum TSH concentration >100 mU/L, 20 had congenital hypothyroidism (CH), and 11 had autoimmune thyroiditis.
Main Outcome Measures:
The primary endpoint was the serum TSH concentration. Secondary endpoints were the free T4 and total T3 concentrations.
Results:
The serum TSH concentration was significantly lower after 8 weeks of Synthroid than after generic drug (P = .002), but thyroid hormone levels did not differ significantly. Subgroup analysis revealed that the difference in TSH was restricted to patients with CH (P = .0005). Patients with CH required a higher l-T4 dose (P < .0004) and were younger (P = .003) but were not resistant to thyroid hormone; 15 of 16 CH patients had severe thyroid dysgenesis or agenesis on imaging. The response to generic vs brand-name preparation remained significant when adjusted for age.
Conclusions:
Synthroid and an AB-rated generic l-T4 are not bioequivalent for patients with severe hypothyroidism due to CH, probably because of diminished thyroid reserve. It would therefore seem prudent not to substitute l-T4 formulations in patients with severe CH, particularly in those <3 yr of age. Our results may have important implications for other severely hypothyroid patients in whom precise titration of l-T4 is necessary.
Hypothyroidism has been estimated to affect >10 million Americans over the age of 12 years (1). Although the condition is less common in the young, infants and children comprise a particularly vulnerable population because of the critical role of thyroid hormone in growth and brain development (2). Like in adult hypothyroid patients in whom even minor deviations in thyroid hormone equilibrium have been associated with adverse effects on a variety of target organs (3), including bone (4, 5), lipids (6–10), the heart (9, 11) and neurocognition (12, 13), precise titration of levothyroxine (l-T4) replacement is important in infants and children. For example, in babies with severe congenital hypothyroidism (CH), an increase in the recommended starting l-T4 dose from 6–8 μg/kg/d to 10–15 μg/kg/d has been associated with a significant improvement in cognitive outcome (14–16), and even mild maternal thyroid dysfunction during pregnancy has been related to a decrement in intellectual and motor development of the offspring (17, 18). As a result, in clinical practice, often the dose is adjusted by increments of 10% or less to keep the serum TSH concentration within the therapeutic range.
In the United States, multiple branded and generic formulations of l-T4 are available for use, and whether or not they can be substituted without retitration is a matter of great controversy (19–24). The Federal Drug Administration (FDA) determines bioequivalence on the basis of pharmacokinetic parameters (area under the curve and maximum T4 concentration) in euthyroid adult volunteers who are administered a supraphysiological dose (600 μg) of the test l-T4 formulation. Results are compared with 1 of 4 reference preparations (Unithroid, Levoxyl, Synthroid, or Levothroid). If the 90% confidence interval of the difference in area under the curve and maximum T4 concentration falls between 80% and 125% of the reference formulation, products are considered to be bioequivalent and therefore therapeutically equivalent and interchangeable (AB rated). Although this pharmacokinetic paradigm may be appropriate for comparing formulations of many classes of drugs, a large body of evidence suggests that it is too insensitive to assess therapeutic equivalence for l-T4 because it ignores effects on serum TSH concentration and the contribution of endogenous T4 production. The relation between the serum concentration of T4 and TSH is log-linear such that small changes in the serum free T4 concentration result in much larger changes in the TSH level (25), and in hypothyroid adults, the administration of just 25 μg l-T4 less than optimal results in a significant elevation in the serum TSH concentration in most subjects but no change in either the circulating free T4 or free T3 level (26). Similarly, in a pharmacokinetic study of 36 normal volunteers, Blakesley et al (27) have demonstrated that when current FDA methodology is used and no baseline correction is made for the endogenous T4 pool, a difference as large as 25%–33% of the administered dose would not be detected, and even after baseline correction, a difference of 12.5% would not be identified.
Despite widespread concern about the validity of current FDA methodology in determining l-T4 bioequivalence, there is a paucity of clinical data in which this question has been assessed directly in patients, and those results that have been obtained are conflicting and controversial. A commonly cited pharmacokinetic study that purported to demonstrate bioequivalence between generic and branded preparations (28) has been soundly criticized because of multiple flaws in both methodology and execution (29). Similarly, in another study of 31 hypothyroid adult patients, the severity of the initial hypothyroidism was not described (30). Thus, the failure to demonstrate a difference between a single branded and a single generic preparation might have been due to the contribution of endogenous l-T4. In contrast, in a recent pharmacovigilance study conducted by the American Thyroid Association, The Endocrine Society, and the American Association of Clinical Endocrinology, 177 adverse events attributable to changes in TSH values were reported in patients after their l-T4 products were switched from brand to generic formulations, often by pharmacists without the clinicians' knowledge (31). There have been no prospective randomized controlled trials in patients with little endogenous thyroid hormone production and none in small children, a particularly vulnerable population. The present study was performed to compare the bioequivalence of a single branded l-T4 product (Synthroid) and a generic formulation (Sandoz, Princeton, NJ) that is considered by the FDA to be interchangeable in a group of children with severe hypothyroidism.
Patients and Methods
Patients
We identified children and adolescents with severe hypothyroidism seen in the Endocrine Program at Boston Children's Hospital between November 2006 and March 2010 by reviewing International Classification of Disease codes for congenital and acquired hypothyroidism and by referral from other endocrinologists. The study was also listed on ClinicalTrials.gov (NCT00403390). For inclusion into the study, patients had to fulfill the following criteria: (1) age 3 to 18 years, (2) serum TSH >100 mU/L at the time of diagnosis, (3) normal serum TSH concentration for age within 4 weeks of the initial study visit, and (4) willingness and ability of family to comply with study design. Subjects were excluded if they had gastrointestinal disease that might affect l-T4 absorption or were being treated with a medication known to interfere with l-T4 absorption or action (eg, glucocorticoids or anticonvulsants). Of 34 patients who were initially randomized, 1 patient did not attend any clinic visits, and so no data were available for him. Two additional randomized patients were excluded from the analysis because their serum TSH concentration at study entry was elevated (9.4 and 20 mU/L, respectively), and thus they did not meet entry criteria. Subsequent noncompliance (2 patients) or incomplete data (2 patients) was not considered to be exclusion criteria according to the intention-to-treat model. Of the 31 patients whose data were analyzed, 20 children had CH and 11 patients had acquired hypothyroidism. The study was approved by the Boston Children's Hospital Committee on Clinical Investigation; informed consent was obtained in all cases from the parent, and assent was obtained from all children >7 years of age.
Study design
This was a 16-week, open-label, randomized, controlled, crossover trial in which subjects were randomly assigned to receive their usual l-T4 replacement dose as either Synthroid or generic (Sandoz) for 8 weeks sequentially. To assign treatment arms, we used permutated block randomization with random block sizes of 2, 4, or 6. Sixteen patients were randomized to receive brand-name l-T4 first, while 18 subjects were randomized to receive generic first. All 3 excluded patients had been randomized to the former group, leaving 13 subjects (7 CH, 6 acquired hypothyroidism) who received Synthroid first and 18 (13 CH, 5 acquired hypothyroidism) who received generic first. Patients were seen at baseline (week 0, visit 1), 8 weeks (visit 2), and 16 weeks (visit 3) in the Clinical and Translational Studies Unit at Boston Children's Hospital (Figure 1). Patients were instructed to take their l-T4 with water in the morning in a fasting state and to have nothing to eat for 30 minutes. If they missed a dose, they were told to double their dose the next day. All other medications and supplements were to be given at a different time of the day with particular emphasis on avoidance of soy and iron, because these are known to affect thyroid hormone absorption. To minimize the potential influence of diurnal variation in serum TSH concentration, blood was obtained at the same time of day ±2 hours, corresponding to within 2 to 8 hours after the daily dose of hormone. All medication was dispensed through the research pharmacy in 25-μg tablets. Because of the time required to recruit an adequate sample of patients, a total of 12 lots of the Sandoz formulation and 7 lots of Synthroid were used to avoid expiration. Compliance was assessed by pill counts and patient/parent recall at each visit. In addition, parents were contacted either by phone or email between visits to review progress and answer questions.
Figure 1.
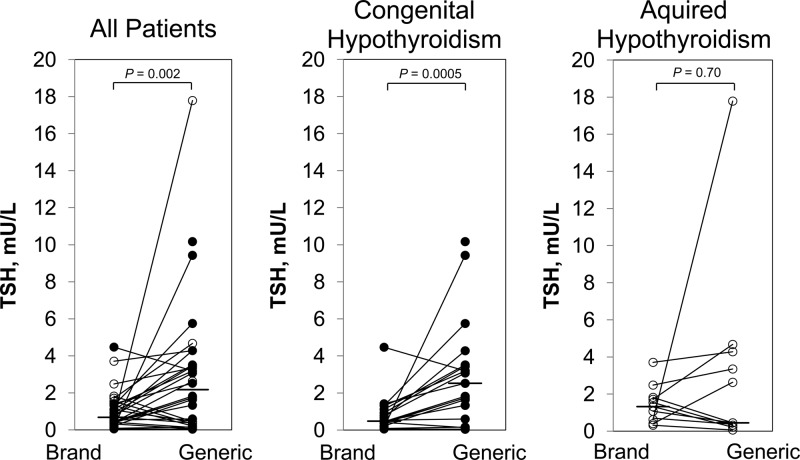
Serum TSH concentration after 8 weeks of brand-name l-T4 vs generic formulation. Closed circles denote patients with congenital hypothyroidism; open circles indicate patients with acquired hypothyroidism. Lines connect paired data. The horizontal bar indicates the median value. The serum TSH concentration was significantly greater after generic vs brand-name product, but subgroup analysis revealed that this difference was seen only in patients with CH.
The primary endpoint was a difference in the serum TSH concentration after each 8-week treatment period with branded vs generic formulation. Secondary endpoints were the serum concentration of free T4 and total T3.
Hormone assays
Samples were stored at −20°C until study completion, and all assays were performed without knowledge of the patient group in duplicate in the same batch. TSH was measured by a third-generation chemiluminescent immunoassay (Access, hypersensitive human TSH; Beckman Coulter, Fullerton, CA). The functional sensitivity of this assay is 0.015 mU/L; the intra-assay coefficient of variation (CV) is 3.1% at 1 mU/L and 2.5% at 28.6 mU/L. The nonparametric range of human TSH concentration with this assay in presumably normal adult patients is 0.34 to 5.6 mU/L. Free T4 and total T3 levels were measured by electrochemiluminescence immunoassays (Roche Diagnostics, Mannheim, Germany). The analytical sensitivity of the free T4 assay is 0.023 ng/dL with an intra-assay CV of 1.8% at 1.3 ng/dL and 2.0% at 2.7 ng/dL; the analytical sensitivity of the total T3 assay is 1.95 ng/dL with an intra-assay CV of 3.6% at 7.9 ng/dL and 4.2% at 18.7 ng/dL. The normal range for free T4 in adult subjects with this assay is 0.93 to 1.7 ng/dL; the normal range for total T3 in adult subjects with this assay is 80 to 200 ng/dL. Normal ranges for these analytes vary with age and are provided in Tables 1 and 2.
Table 1.
Clinical Characteristics of Patients at Diagnosis
| Diagnosis | n | M:F | Age | T4, μg/dLa (Mean ± SEM) | TSH, mU/Lb (Median ± Range) |
|---|---|---|---|---|---|
| CH | 20 | 6:14 | 4.3 ± 0.6 d | 6.2 ± 0.9c,d | >200 (100–563)d |
| Acquired hypothyroidism | 11 | 1:10 | 10.0 ± 0.8 y | 1.4 ± 0.3e | 254 (100–1000) |
Abbreviations: F, female; M, male. SI conversion factor: To convert T4 to nmol/L, multiply by 12.8717.
Age-related normal ranges: 1–4 d, 14.0–28.4 μg/dL (42); 2–20 wk, 7.2–15.7 μg/dL (42); 6–10 y, 5.8–12 μg/dL (43).
Age-related normal range: 1–4 d, 1.0–39.0 mU/L (42); 2–20 wk, 1.7–9.1 mU/L (42); 6–10 y 0.6–5.1 mU/L (43).
n = 15.
Serum or filter paper value at the time of newborn screening. In most cases, the precise value for TSH was not further quantitated.
n = 9.
Table 2.
Clinical Characteristics of Randomized Groups
| Brand First | Generic First | P Value | |
|---|---|---|---|
| n | 13 | 18 | |
| M:F | 4:9 | 3:15 | .413 |
| Congenital/acquired hypothyroidism | 7/6 | 13/5 | .449 |
| Age at study entry, ya | 10.0 ± 1.2 | 10.0 ± 1.0 | .975 |
| Free T4 at study entry, μg/dLa | 1.41 ± 0.05 | 1.09 ± 0.42 | .52 |
| TSH at study entry, mU/Lb | 1.63 (0.24–4.55) | 1.72 (0.3–5.3) | .603 |
| l-T4 dose at study entry, μg/kgb | 2.5 ± 0.2 | 2.7 ± 0.2 | .638 |
Abbreviations: F, female; M, male.
Mean ± SEM.
Median (range).
Statistical analysis
The primary analysis included all 31 randomized patients whose baseline assessment was normal, based on an intention-to-treat principle. For normally distributed variables, we used a two-tailed Student's t test, paired or unpaired as appropriate, to assess the significance of the difference between groups; results are expressed as means ± SEM. For analysis of the serum TSH concentration, which exhibited a skewed distribution, we used the Wilcoxon signed rank test for paired comparisons and the rank sum test for unpaired comparisons; results are reported as median (minimum-maximum). Subjects with incomplete data were excluded from paired analyses. We employed Pearson's r to assess the correlation between variables and regression analysis to adjust the paired t test for covariates. We used Fisher's exact test and Pearson's χ2 test to assess distribution of categorical variables with 2 and 3 possible values across randomization groups. A P value <.05 was considered to be significant. All statistical analyses were performed using IBM SPSS version 19.0 (IBM, Armonk, NY) and SAS version 9.2 (Cary, NC).
Power analysis, based on pilot data in patients taking l-T4 for thyroiditis, indicated that 23 subjects would provide 80% power to detect a 40% difference between TSH levels on the two drugs, using a critical significance level of P = .05. In the pilot data, a 40% difference corresponded to 1 mU/L.
Results
Clinical characteristics at diagnosis
The clinical characteristics of the 31 patients at diagnosis are summarized in Table 1. The patients with CH, detected by newborn screening, were diagnosed at 4.3 ± 0.6 days of life, and the male to female ratio in them was 1:1.2. Their serum or filter paper total T4 concentration was 6.2 ± 0.9 μg/dL (mean ± SEM), and their median serum or filter paper TSH concentration was >200 mU/L (range 100–563 mU/L), although in many cases, the precise concentration was not determined. Of the 20 children with CH, 16 had thyroid imaging (ultrasonography or 123I uptake and scan) performed either at the time of diagnosis or at study entry. In 15 patients, the etiology of the CH was thyroid dysgenesis (12 agenesis, 2 dysgenesis with ectopia, 1 severe hypoplasia with unilateral agenesis), and in 1 subject, a normal-appearing thyroid gland was found. In the remaining 4 CH patients, the etiology was not determined.
The patients with acquired hypothyroidism were diagnosed at 10 ± 0.8 years of age and the male to female ratio in them was 1:10. Their total T4 concentration at diagnosis was 1.4 ± 0.3 μg/dL (mean ± SEM), and their median serum TSH concentration was 254 mU/L (range 100-1000 mU/L). All patients with acquired hypothyroidism had positive thyroid peroxidase and/or thyroglobulin antibodies, consistent with a diagnosis of chronic lymphocytic thyroiditis.
Clinical characteristics of randomized groups
Patients randomized to receive brand-name vs generic l-T4 first were well matched in terms of gender, diagnosis, age at study, free T4, TSH, and l-T4 dose (Table 2).
Clinical characteristics at study entry
At study entry (visit 1), all patients were well controlled on their current dose of thyroid replacement (TSH concentration 1.71 mU/L [range 0.2–5.3 mU/L], free T4 concentration 1.5 ± 0.04 ng/dL, total T3 139.2 ± 5.2 ng/dL) and, as indicated in Table 3, there was no significant difference between patients with CH and acquired hypothyroidism. Sixteen patients were receiving Synthroid while the remaining 15 patients were on another l-T4 product (6 Levoxyl, 9 generic). Patients with CH were significantly younger than those with acquired hypothyroidism (8.4 ± 0.8 vs 12.9 ± 1.1 years, P = .003) and required a higher l-T4 dose to control their hypothyroidism (3.0 ± 2.0 vs 2.0 ± 0. 2 μg/kg/d, P = .0004). There was a significant negative correlation between age and l-T4 dose (r = −0.70, P < .0001) irrespective of the diagnosis.
Table 3.
Clinical Characteristics of Patients at Study Entry
| Diagnosis | Age, y | Free T4,a ng/dL | Total T3,b ng/dL | TSH,c mU/L | l-T4 Dose, μg/kg |
|---|---|---|---|---|---|
| CH | 8.4 ± 0.8 | 1.5 ± 0.1 | 141.4 ± 6.0 | 2.4 (0.2–4.6) | 3.0 ± 0.2 |
| Acquired hypothyroidism | 12.9 ± 1.1 | 1.4 ± 0.1 | 135.5 ± 10.1 | 1.6 (0.3–5.3) | 2.0 ± 0.2 |
| Difference | P = .003 | P = .113 | P = .59 | P = .549 | P < .0005 |
Free T4 and total T3 are shown as mean ± SEM; TSH is shown as median (range).
SI conversion factors: To convert free T4 to nmol/L, multiply by 12.8717. To convert total T3 to pmol/L, multiply by 15.361.
Age-related normal range (43): 6–10 y, 0.64–2.64 ng/dL; 11–15 y, 0.69–2.44 ng/dL.
Age-related normal range (43): 6–10 y, 105–203 ng/dL; 11–15 y, 71–192 ng/dL.
Age-related normal range (43): 6–10 y, 0.6–5.1 mU/L; 11–15 y, 0.5–4.4 mU/L.
Thyrotropin
Paired serum TSH concentrations obtained 8 weeks after Synthroid or a generic (Sandoz) l-T4 formulation are summarized in Figure 1. The serum TSH concentration was significantly lower in patients after 8 weeks of Synthroid (0.7 [0.05–4.5] mU/L) as compared with generic l-T4 (1.8 [0.04–17.8] mU/L), P = .002). Subgroup analysis indicated that the significant within-subject difference was confined to the CH patients (1.4 [1.3–9.3] mU/L, P = .0005) and was not observed in those with acquired hypothyroidism (−0.3 [−1.4 to 17.2] mU/L, P = .70). In 2 patients, documented by pill count to be noncompliant while on generic l-T4, the serum TSH concentration was 17.8 and 9.4 mU/L. Reanalysis of the data excluding these 2 noncompliant patients yielded similar results: median within-subject difference in the serum TSH on brand-name vs generic formulation was 0.9 (−1.4 to 4.4) mU/L (P = .006). Again, the difference was significant in CH patients (P = .001) but not in those with acquired hypothyroidism (P > .99). The 16 patients who had been treated previously with Synthroid showed no significant difference in the serum TSH concentration between study entry (1.6 [0.3–3.8] mU/L) and after 8 weeks of Synthroid (0.9 [0.05–4.5] mU/L; the median within-subject difference was −0.5 [−3.3 to 2.5] mU/L, P = .08). The significant within-subject difference in TSH concentration on brand-name as compared with generic formulation was corroborated by paired t test (1.8 ± 0.7 mU/L, P = .015), and covariate adjustment indicated that the difference was not explained by age (P = .76) or order of administration of Synthroid vs generic formulation (P = −.54).
Free T4 and total T3
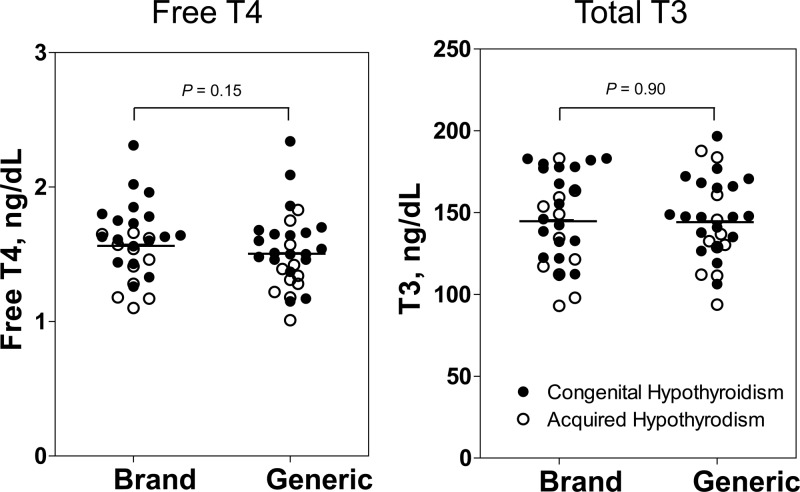
The serum free T4 concentration was slightly higher after 8 weeks of Synthroid (1.6 ± 0.1 ng/dL) as compared with generic l-T4 in the group overall (1.5 ± 0.1 ng/dL), but the mean within-subject difference was not statistically significant (−0.07 ± 0.05 ng/dL, P = .15; Figure 2). Similar results were observed when CH patients and those with acquired hypothyroidism were analyzed separately. There was no difference in the mean total T3 concentration after brand-name (143 ± 5 ng/dL) vs generic formulation (142 ± 5 ng/dL); the mean within-subject difference was −1 ± 4 ng/dL (P = .90).
Figure 2.
Serum free T4 and total T3 concentration after 8 weeks of brand l-T4 vs generic formulation. Closed circles denote patients with congenital hypothyroidism; open circles indicate patients with acquired hypothyroidism. Paired analysis showed no significant difference between groups; pairing of data is not illustrated.
Free T4:TSH and total T3:TSH
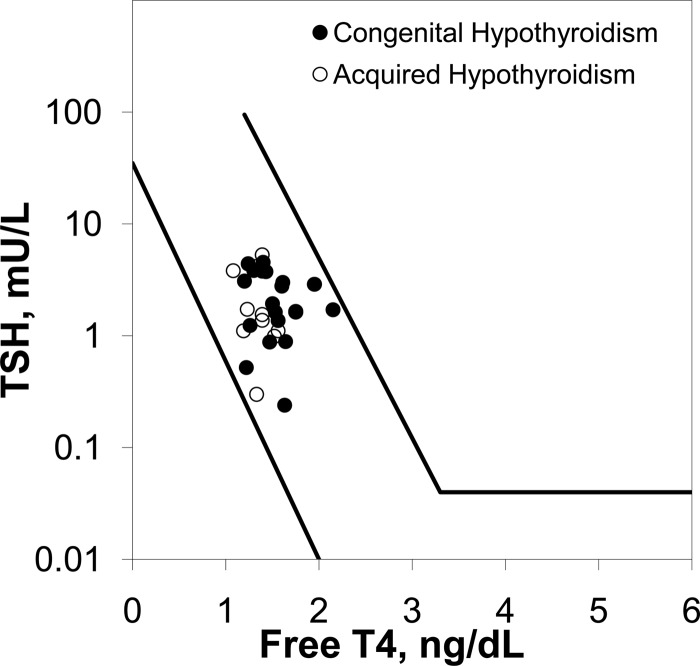
There was no difference in either the free T4:TSH ratio (Figure 3) or the free T3:TSH ratio (not pictured) between patients with CH and acquired hypothyroidism at study entry.
Figure 3.
Serum TSH vs free T4 concentration in patients with CH (closed circles) vs acquired hypothyroidism (open circles). Diagonal lines represent the normal range (35).
Discussion
Despite widespread criticism that the pharmacokinetic endpoint used by the FDA might be too insensitive to detect small but significant differences between branded and generic formulations of l-T4, no randomized controlled studies to date have substantiated this concern. The importance of the present study is the demonstration that, in children with severe hypothyroidism who have little if any thyroid hormone reserve, there is a significant difference in the serum TSH concentration after 8 weeks of a brand-name vs a generic l-T4 formulation that is considered by the FDA to be bioequivalent. We purposely compared Synthroid and the generic levothyroxine produced by Sandoz because these formulations have an AB rating, ie, are considered by the FDA to be interchangeable. The difference in circulating thyroid hormone and TSH might have been even greater had we compared BX-rated generic formulations (ie, those in which bioequivalence has not been demonstrated), which are commonly substituted by pharmacists. It is also likely that the difference between brand-name and generic formulations is greater in the clinical setting than under highly controlled research conditions such as those employed in the present study, particularly in infants with CH who are the most at risk. Because infants feed frequently, it is often not possible to administer l-T4 in a fasting state (32), thereby magnifying potential differences in dissolution properties between l-T4 formulations (22).
It was of interest that the patients with CH required significantly more l-T4 to control their hypothyroidism. We considered the possibility that this difference might have been due to their younger age, because younger children have a higher metabolic rate and are known to require a relatively higher l-T4 dose per kilogram body weight (2). Indeed, we observed a relation between age and l-T4 dose, but there was no relation between age and serum TSH concentration after brand-name vs generic l-T4. We next considered the possibility that the reason for the differential response to l-T4 formulation in patients with CH vs acquired hypothyroidism might have been relative pituitary resistance to thyroid hormone, a phenomenon that has been described in some patients with CH (33–35). However, the free T4:TSH and total T3:TSH ratios were normal in the CH patients (Figure 3) and there was no difference between groups. Also, thyroid hormone resistance in CH patients tends to be self-limited and is not usually observed at the age of our patients (35).
A third and more likely reason for our results was therefore diminished thyroid hormone reserve in the patients with CH (36). In support of this possibility, most of the patients with CH had athyreosis or severe thyroid dysgenesis on imaging, and as noted above, patients with CH required a higher l-T4 dose for control of their hypothyroidism, consistent with findings in adults with severe hypothyroidism (36). Unfortunately, we could not compare the severity of the initial hypothyroidism directly because patients with CH were diagnosed within the first week of life, a time at which a significant fraction of the measured hormone is maternal in origin (37). In addition, the serum TSH concentration was not quantitated by serial dilution in many patients at the time of newborn screening, limiting our ability to determine more accurately the severity of the initial hypothyroidism. If our thesis is correct, however, our results may have important implications for severely hypothyroid patients of all ages particularly those in whom precise titration of the serum TSH concentration is required, such as individuals with thyroid carcinoma after thyroid ablation (38, 39) and the elderly (11). In contrast, for patients with milder forms of disease and more thyroid reserve, it may be less important whether brand-name or generic is prescribed.
We could not study infants and children <3 yr of age, the most vulnerable population in childhood, for ethical reasons, but one might anticipate that the difference in serum TSH concentration between l-T4 formulations might be even greater in them because their TSH:T4 ratio is higher than in older children and adults (35). Because of the critical role of thyroid hormone in brain development in the first 3 years of life, frequent monitoring is recommended for precise titration of the serum T4 and TSH concentrations (40, 41). The present study demonstrates that substitution of the l-T4 formulation may be a further cause of variability in results and may contribute to the need for even more frequent testing. Additional studies are needed to determine the pathophysiological consequences of these changes.
Our study had several limitations. Because of the duration of time required to recruit a sufficient number of subjects, several different lots of both Synthroid and generic formulations were used to avoid expiration. The validity of our results is supported by our finding that in patients who had been treated previously with Synthroid, there was no difference in the serum TSH concentration at study entry and after 8 weeks of Synthroid, even though the Synthroid was presumably obtained from a different lot. Also, it was not possible to schedule study visits early in the morning in some patients for logistical reasons. Therefore, to minimize the effects of diurnal variation of TSH and time of l-T4 administration, we scheduled all study visits for any one child within 2 hours of the same time of day. Although this resulted in some patients being sampled at the nadir and others at peak free T4 levels, the serum T4 concentration varies relatively little (13%) after a single daily dose, and each patient served as his/her own control, thereby minimizing this source of variability. A third potential limitation was the unequal randomization to brand-name vs generic formulation first. This, too, is unlikely to have affected results because the groups were well matched. Also, it should be noted that, because of the crossover design, all patients received both l-T4 formulations and carryover effects would be expected to be negligible by design (>8 half-lives of drug). Indeed, we found no difference in results whether generic or brand-name was administered first.
In summary, we have demonstrated that a brand-name l-T4 and an AB-rated generic formulation are not bioequivalent in patients with severe CH, although the difference appeared to be less important for patients with acquired hypothyroidism, probably because they had more thyroid reserve. Until the pathophysiological consequences of these differences are known, it would seem prudent not to substitute l-T4 formulations in patients with severe CH, particularly those <3 yr of age in view of the critical role of thyroid hormone on brain development at this age. Our findings may have implications for other vulnerable populations, particularly athyreotic patients in whom precise titration of l-T4 is important, such as patients with thyroid carcinoma after thyroid ablation and the elderly.
Acknowledgments
We are indebted to Dr. Henry Feldman for helpful statistical advice and to Dr. Joseph Majzoub for reviewing the manuscript.
This project was funded in part by Grant MO1-RR02172 from the National Center for Research Resources, National Institutes of Health (NIH), to the Children's Hospital Boston General Clinical Research Center and support from Harvard Catalyst/The Harvard Clinical and Translational Science Center (NIH Award UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). J.M.C. was supported in part by NIH Training Grant 5T32DK007699-23.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the NIH.
Disclosure Summary: The authors have nothing to declare.
For editorial see page 511
- CH
- Congenital hypothyroidism
- CV
- coefficient of variation
- l-T4
- levothyroxine.
References
- 1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489–499 [DOI] [PubMed] [Google Scholar]
- 2. Brown RS. The Thyroid. In: Brook CGD, Clayton PE, Brown RS, eds. Brook's Clinical Pediatric Endocrinology. 6th ed Oxford, UK: Wiley-Blackwell; 2009:250–282 [Google Scholar]
- 3. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142–1154 [DOI] [PubMed] [Google Scholar]
- 4. Ross DS, Neer RM, Ridgway EC, Daniels GH. Subclinical hyperthyroidism and reduced bone density as a possible result of prolonged suppression of the pituitary-thyroid axis with l-thyroxine. Am J Med. 1987;82:1167–1170 [DOI] [PubMed] [Google Scholar]
- 5. Jodar E, Begona Lopez M, et al. Bone changes in pre- and postmenopausal women with thyroid cancer on levothyroxine therapy: evolution of axial and appendicular bone mass. Osteoporos Int. 1998;8:311–316 [DOI] [PubMed] [Google Scholar]
- 6. Ayala AR, Danese MD, Ladenson PW. When to treat mild hypothyroidism. Endocrinol Metab Clin North Am. 2000;29:399–415 [DOI] [PubMed] [Google Scholar]
- 7. Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88:2438–2444 [DOI] [PubMed] [Google Scholar]
- 8. Monzani F, Caraccio N, Kozakowa M, et al. Effect of levothyroxine replacement on lipid profile and intima-media thickness in subclinical hypothyroidism: a double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2004;89:2099–2106 [DOI] [PubMed] [Google Scholar]
- 9. Biondi B, Klein I. Hypothyroidism as a risk factor for cardiovascular disease. Endocrine. 2004;24:1–13 [DOI] [PubMed] [Google Scholar]
- 10. Biondi B, Kahaly GJ. Cardiovascular involvement in patients with different causes of hyperthyroidism. Nat Rev Endocrinol. 2010;6:431–443 [DOI] [PubMed] [Google Scholar]
- 11. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–1252 [DOI] [PubMed] [Google Scholar]
- 12. Baldini IM, Vita A, Mauri MC, et al. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:925–935 [DOI] [PubMed] [Google Scholar]
- 13. Kalmijn S, Mehta KM, Pols HA, Hofman A, Drexhage HA, Breteler MM. Subclinical hyperthyroidism and the risk of dementia. The Rotterdam study. Clin Endocrinol (Oxf). 2000;53:733–737 [DOI] [PubMed] [Google Scholar]
- 14. Dubuis JM, Glorieux J, Richer F, Deal CL, Dussault JH, Van Vliet G. Outcome of severe congenital hypothyroidism: closing the developmental gap with early high dose levothyroxine treatment. J Clin Endocrinol Metab. 1996;81:222–227 [DOI] [PubMed] [Google Scholar]
- 15. Bongers-Schokking JJ, Koot HM, Wiersma D, Verkerk PH, de Muinck Keizer-Schrama SM. Influence of timing and dose of thyroid hormone replacement on development in infants with congenital hypothyroidism. J Pediatr. 2000;136:292–297 [DOI] [PubMed] [Google Scholar]
- 16. Salerno M, Militerni R, Bravaccio C, et al. Effect of different starting doses of levothyroxine on growth and intellectual outcome at four years of age in congenital hypothyroidism. Thyroid. 2002;12:45–52 [DOI] [PubMed] [Google Scholar]
- 17. Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol (Oxf). 2010;72:825–829 [DOI] [PubMed] [Google Scholar]
- 18. Williams F, Watson J, Ogston S, Hume R, Willatts P, Visser T. Mild maternal thyroid dysfunction at delivery of infants born ≤34 weeks and neurodevelopmental outcome at 5.5 years. J Clin Endocrinol Metab. 2012;97:1977–1985 [DOI] [PubMed] [Google Scholar]
- 19. Oppenheimer JH, Braverman LE, Toft A, Jackson IM, Ladenson PW. A therapeutic controversy. Thyroid hormone treatment: when and what? J Clin Endocrinol Metab. 1995;80:2873–2883 [DOI] [PubMed] [Google Scholar]
- 20. Baskin HJ, Cobin RH, Duick DS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8:457–469 [PubMed] [Google Scholar]
- 21. Hennessey JV. Levothyroxine a new drug? Since when? How could that be? Thyroid. 2003;13:279–282 [DOI] [PubMed] [Google Scholar]
- 22. Klein I, Danzi S. Evaluation of the therapeutic efficacy of different levothyroxine preparations in the treatment of human thyroid disease. Thyroid. 2003;13:1127–1132 [DOI] [PubMed] [Google Scholar]
- 23. Stockigt J. Testing the bioavailability of oral l-thyroxine by studying its absorption: smoke or mirrors? Thyroid. 2004;14:167–168 [DOI] [PubMed] [Google Scholar]
- 24. American Thyroid Association; Endocrine Society; American Association of Clinical Endocrinologists Joint statement on the U. S. Food and Drug Administration's decision regarding bioequivalence of levothyroxine sodium. Thyroid. 2004;14:486. [DOI] [PubMed] [Google Scholar]
- 25. Spencer CA, LoPresti JS, Patel A, et al. Applications of a new chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endocrinol Metab. 1990;70:453–460 [DOI] [PubMed] [Google Scholar]
- 26. Carr D, McLeod DT, Parry G, Thornes HM. Fine adjustment of thyroxine replacement dosage: comparison of the thyrotrophin releasing hormone test using a sensitive thyrotrophin assay with measurement of free thyroid hormones and clinical assessment. Clin Endocrinol (Oxf). 1988;28:325–333 [DOI] [PubMed] [Google Scholar]
- 27. Blakesley V, Awni W, Locke C, Ludden T, Granneman GR, Braverman LE. Are bioequivalence studies of levothyroxine sodium formulations in euthyroid volunteers reliable? Thyroid. 2004;14:191–200 [DOI] [PubMed] [Google Scholar]
- 28. Dong BJ, Hauck WW, Gambertoglio JG, et al. Bioequivalence of generic and brand-name levothyroxine products in the treatment of hypothyroidism. JAMA. 1997;277:1205–1213 [PubMed] [Google Scholar]
- 29. Mayor GH, Orlando T, Kurtz NM. Limitations of Levothyroxine Bioequivalence Evaluation: Analysis of An Attempted Study. Am J Ther. 1995;2:417–432 [PubMed] [Google Scholar]
- 30. Escalante DA, Arem N, Arem R. Assessment of interchangeability of two brands of levothyroxine preparations with a third-generation TSH assay. Am J Med. 1995;98:374–378 [DOI] [PubMed] [Google Scholar]
- 31. Hennessey JV, Malabanan AO, Haugen BR, Levy EG. Adverse event reporting in patients treated with levothyroxine: results of the Pharmacovigilance Task Force survey of the American Thyroid Association, American Association of Clinical Endocrinologists, and The Endocrine Society. Endocr Pract. 2010;16:357–370 [DOI] [PubMed] [Google Scholar]
- 32. Zeitler P, Solberg P. Food and levothyroxine administration in infants and children. J Pediatr. 2010;157:13–14e11 [DOI] [PubMed] [Google Scholar]
- 33. Sato T, Suzuki Y, Taketani T, Ishiguro K, Nakajima H. Age-related change in pituitary threshold for TSH release during thyroxine replacement therapy for cretinism. J Clin Endocrinol Metab. 1977;44:553–559 [DOI] [PubMed] [Google Scholar]
- 34. Sack J, Shafrir Y, Urbach D, Amado O. Thyroid-stimulating hormone, prolactin, and growth hormone response to thyrotropin-releasing hormone in treated children with congenital hypothyroidism. Pediatr Res. 1985;19:1037–1039 [DOI] [PubMed] [Google Scholar]
- 35. Fisher DA, Schoen EJ, La Franchi S, et al. The hypothalamic-pituitary-thyroid negative feedback control axis in children with treated congenital hypothyroidism. J Clin Endocrinol Metab. 2000;85:2722–2727 [DOI] [PubMed] [Google Scholar]
- 36. Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764–770 [DOI] [PubMed] [Google Scholar]
- 37. Vulsma T, Gons MH, de Vijlder JJ. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med. 1989;321:13–16 [DOI] [PubMed] [Google Scholar]
- 38. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737–744 [DOI] [PubMed] [Google Scholar]
- 39. Diessl S, Holzberger B, Mader U, et al. Impact of moderate vs stringent TSH suppression on survival in advanced differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 2012;76:586–592 [DOI] [PubMed] [Google Scholar]
- 40. Rose SR, Brown RS, Foley T, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117:2290–2303 [DOI] [PubMed] [Google Scholar]
- 41. Balhara B, Misra M, Levitsky LL. Clinical monitoring guidelines for congenital hypothyroidism: laboratory outcome data in the first year of life. J Pediatr. 2011;158:532–537 [DOI] [PubMed] [Google Scholar]
- 42. DeBoer MD, Lafranchi SH. Pediatric thyroid testing issues. Pediatr Endocrinol Rev. 2007;1(5)(suppl):570–577 [PubMed] [Google Scholar]
- 43. Zurakowski D, Di Canzio J, Majzoub JA. Pediatric reference intervals for serum thyroxine, triiodothyronine, thyrotropin, and free thyroxine. Clin Chem. 1999;45:1087–1091 [PubMed] [Google Scholar]