Abstract
Context:
Hypoglycemia due to congenital hyperinsulinism (HI) is caused by mutations in 9 genes.
Objective:
Our objective was to correlate genotype with phenotype in 417 children with HI.
Methods:
Mutation analysis was carried out for the ATP-sensitive potassium (KATP) channel genes (ABCC8 and KCNJ11), GLUD1, and GCK with supplemental screening of rarer genes, HADH, UCP2, HNF4A, HNF1A, and SLC16A1.
Results:
Mutations were identified in 91% (272 of 298) of diazoxide-unresponsive probands (ABCC8, KCNJ11, and GCK), and in 47% (56 of 118) of diazoxide-responsive probands (ABCC8, KCNJ11, GLUD1, HADH, UCP2, HNF4A, and HNF1A). In diazoxide-unresponsive diffuse probands, 89% (109 of 122) carried KATP mutations; 2% (2 of 122) had GCK mutations. In mutation-positive diazoxide-responsive probands, 42% were GLUD1, 41% were dominant KATP mutations, and 16% were in rare genes (HADH, UCP2, HNF4A, and HNF1A). Of the 183 unique KATP mutations, 70% were novel at the time of identification. Focal HI accounted for 53% (149 of 282) of diazoxide-unresponsive probands; monoallelic recessive KATP mutations were detectable in 97% (145 of 149) of these cases (maternal transmission excluded in all cases tested). The presence of a monoallelic recessive KATP mutation predicted focal HI with 97% sensitivity and 90% specificity.
Conclusions:
Genotype to phenotype correlations were most successful in children with GLUD1, GCK, and recessive KATP mutations. Correlations were complicated by the high frequency of novel missense KATP mutations that were uncharacterized, because such defects might be either recessive or dominant and, if dominant, be either responsive or unresponsive to diazoxide. Accurate and timely prediction of phenotype based on genotype is critical to limit exposure to persistent hypoglycemia in infants and children with congenital HI.
Congenital hyperinsulinism (HI) is the most frequent cause of persistent hypoglycemia in infants and children. The disorder represents a heterogeneous group of diseases of pancreatic insulin regulation that differ with regard to responsiveness to medical treatment with diazoxide, requirement for surgery, histopathology, and molecular etiology (1). HI is most commonly associated with inactivating mutations in one of two adjacent genes located on chromosome 11p15.1: ABCC8 and KCNJ11, encoding the sulfonylurea receptor 1 and Kir6.2 proteins, which together form the ATP-sensitive plasma membrane potassium (KATP) channel in pancreatic β-cells (2–5). The loss of KATP channel activity leads to persistent membrane depolarization and insulin release, regardless of plasma glucose levels. Because treatment with diazoxide, a KATP channel agonist, is often ineffective in children with KATP mutations, many require near-total pancreatectomy to control hypoglycemia. Some KATP mutations act recessively, whereas others act in a dominant fashion (6, 7). Recessive KATP mutations result in subunit proteins that do not traffic to the cell surface and can cause either diffuse HI or focal HI. Diffuse HI involving the entire pancreas is caused by biallelic recessive mutations. Focal HI represents a localized area of islet cell adenomatosis and is caused by paternal transmission of a monoallelic recessive mutation followed by an embryonic somatic loss of maternal 11p15.1 (8). Accurate diagnosis of focal HI is important because surgery may be curative in such cases. Dominant KATP mutations produce subunit proteins that traffic normally and form KATP channel complexes at the cell surface that have impaired opening in response to MgADP and diazoxide. Children with dominant KATP mutations may be responsive or nonresponsive to diazoxide, depending on the degree of residual channel activity (6, 7).
The second most common form of congenital HI results from dominant activating mutations in glutamate dehydrogenase, encoded by GLUD1 on chromosome 10, which cause the hyperinsulinemia/hyperammonemia syndrome (9). These mutations impair regulation of amino-acid–stimulated insulin secretion leading to fasting hypoglycemia, as well as leucine sensitive hypoglycemia. Patients with GLUD1 mutations are responsive to diazoxide treatment. Dominant, activating mutations in glucokinase, encoded by GCK on chromosome 7, are the third most common genetic cause of HI (10). These mutations lower the glucose threshold for insulin release and are frequently severe enough to interfere with responsiveness to treatment with diazoxide (11).
Mutations in 5 additional genetic loci are rarer causes of diazoxide-responsive HI. Recessive, inactivating mutations of short-chain, 3-hydroxy acyl-coenzyme A dehydrogenase, a mitochondrial fatty acid β-oxidation enzyme encoded by HADH on chromosome 4, cause HI by a mechanism that has been shown to result from loss of an inhibitory protein interaction of short-chain, 3-hydroxy acyl-coenzyme A dehydrogenase on glutamate dehydrogenase activity (12–15). Dominant, inactivating mutations of a mitochondrial membrane protein, UCP2 on chromosome 11, have been identified in a small number of HI cases (16). A persistent form of HI has been described in neonates who are heterozygous for dominant, inactivating mutations of the HNF4A transcription factor, which are also responsible for the maturity onset diabetes of the young, type 1 form of monogenic diabetes (17–19). Recently, mutations of the HNF1A transcription factor, which cause maturity onset diabetes of the young, type 3, have also been reported in two children with persistent HI (20, 21). Finally, in three families, dominant, activating mutations of a plasma membrane pyruvate transporter, MCT1, encoded by SLC16A1 on chromosome 1, have been shown to cause exercise-induced HI (22).
The aim of this study was to identify genotype-phenotype correlations, specifically diazoxide responsiveness and potential for surgical cure, to provide guidance in the clinical management of congenital HI. We report the mutations found in HI-related genes and their associated clinical features in a large series of 417 children with congenital HI treated at the Hyperinsulinism Center at The Children's Hospital of Philadelphia (CHOP).
Materials and Methods
Probands
The cases included are probands who were referred to CHOP between 1997 and 2010 and diagnosed with HI based on previously described criteria: fasting hypoglycemia accompanied by inadequate suppression of plasma insulin, inappropriately low plasma free fatty acid and β-hydroxybutyrate concentrations, and an inappropriate glycemic response to glucagon injection at the time of hypoglycemia (23, 24). Children were defined as being responsive to diazoxide if evidence of fasting hyperinsulinism could be completely controlled by treatment with diazoxide at doses <15 mg/kg/d, as demonstrated by maintaining plasma glucose concentration >70 mg/dL for 12–18 hours of fasting or developing appropriate hyperketonemia (β-hydroxybutyrate > 2 mmol/L) before plasma glucose dropped to 50 mg/dL. Most diazoxide-unresponsive cases required surgical pancreatectomy and were diagnosed as focal or diffuse based on pancreatic histology of surgical specimens. Diazoxide-unresponsive cases that did not have surgery were managed with combinations of octreotide and tube feedings. Affected family members of probands were not included in this series. Children with transient HI that resolved before 1 year of age were also excluded.
DNA isolation
Genomic DNA was isolated from peripheral blood (5 PRIME, Gaithersburg, Maryland) or from saliva (Oragene DNA self-collection kit; DNA Genotek, Kanata, Ontario, Canada). DNA from surgical pancreatic specimens was extracted using the DNA/RNA Allprep kit (QIAGEN, Valencia, California).
Mutation analysis
Coding sequences and intron/exon splice junctions were amplified and directly sequenced on an ABI 3730 capillary DNA analyzer (Applied Biosystems, Carlsbad, California). The nucleotides of ABCC8 and corresponding sulfonylurea receptor 1 amino acids were numbered according to the sequence reported by Nestorowicz et al (5) that includes the alternatively spliced exon 17 sequence (NCBI accession no. L78224). The functional consequences of novel, missense mutations were predicted with bioinformatics software, SIFT (25) and PolyPhen (26). Genetic variants were searched for against the Single Nucleotide Polymorphism Database (dbSNP), the 1000 Genomes Project, and the NHLBI Exome Sequencing Project [Exome Variant Server, NHLBI GO Exome Sequencing Project (ESP), Seattle, WA; http://evs.gs.washington.edu/EVS/] (27, 29). Intronic variants were analyzed with GenSCAN (http://genes.mit.edu/GENSCAN.html) to determine whether the consensus sequence of any splice site was altered. Beginning in 2006, mutation analysis for ABCC8, KCNJ11, GCK, and GLUD1 genes was performed in commercial laboratories for a subset of children with HI.
Exonic dosage assays
TaqMan copy number assays (Applied Biosystems/Life Technologies, Carlsbad, California) for individual exons of ABCC8 were used to test for intragenic deletions/insertions that are missed by direct sequencing. A subset of cases of focal or diffuse HI but no detectable mutations and diffuse cases with only a monoallelic recessive KATP mutation were screened where ample DNA was available.
Functional analysis of mutant channels
The functional properties of selected missense KATP mutations were analyzed as previously described (30).
Consent
Written informed consent was obtained from parents of the probands included in this study. The study was reviewed and approved by CHOP's Institutional Review Board.
Results
Mutation screening was completed in 417 children with congenital HI seen at CHOP between 1997 and 2010 (Tables 1 and 2). Not included in this series were 14 children with syndromic HI (6 with Beckwith-Weidemann syndrome, 6 with Turner syndrome, 1 with Kabuki syndrome, and 1 with congenital disorder of glycosylation) and 11 children with acquired HI (10 with insulinoma and 1 with autoimmune HI).
Table 1.
Mutation Analysis in 298 Diazoxide-Unresponsive Children With Congenital HI
| No. of Probands | |
|---|---|
| Surgery | 282 |
| Focal HI | 149 |
| KATP, monoallelic recessive, paternal | 120 |
| KATP, monoallelic recessive, nonmaternala | 9 |
| KATP, monoallelic recessive, de novob | 5 |
| KATP, monoallelic recessive, parent of origin unknownc | 11 |
| No mutation found | 4 |
| Diffuse HI | 122 |
| KATP, biallelic recessived | 84 |
| KATP, monoallelic dominant | 16 |
| KATP, monoallelic recessive | 9e |
| GCK | 2f |
| No mutation found | 11 |
| LINE HI (no mutation found) | 7 |
| Normal histology (no mutation found) | 4 |
| No surgery | 16 |
| GCK | 5 |
| KATP, monoallelic recessive, paternal | 6 |
| KATP, biallelic recessive | 4 |
| KATP, monoallelic dominant | 1 |
Only maternal sample available for testing and was negative.
Mutation was not identified in either the paternal or maternal DNA.
Neither parent sample was available for testing.
There were 73% confirmed compound heterozygous, 18% homozygous, 2% unknown (parental samples unavailable), 7% paternal sample unavailable.
Probable misdiagnosed focal cases.
One patient has a postzygotic mutation in GCK.
Table 2.
Mutation Analysis in 118 Diazoxide-Responsive Children With Congenital HI
| No. of Probands | |
|---|---|
| Mutation positive | 56 (47%) |
| GLUD1 | 24a (42%) |
| Dominant KATP | 23b (41%) |
| ABCC8, monoallelic dominant (n = 19) | |
| KCNJ11, monoallelic dominant (n = 4) | |
| HADH | 2c (4%) |
| UCP2 | 2 (4%) |
| HNF4A | 2 (4%) |
| HNF1A | 3 (5%) |
| Mutation negative | |
| Negative for ABCC8, KCNJ11, GCK, and GLUD1 | 62d (53%) |
| Also negative for HADH, UCP2, and HNF4A | 55 (89%) |
One patient has a postzygotic mutation of GLUD1.
One patient possesses the ABCC8 c.3992–9 G→A on the maternal allele along with a dominant mutation (ABCC8 p.K1337N) on the paternal allele and was diazoxide-responsive.
One patient has a confirmed mutation in HADH along with a second suspected disease-causing mutation.
One patient has a presumed postzygotic mutation of GLUD1.
Genotypes in diazoxide-unresponsive congenital HI
As shown in Table 1, 72% (298 of 417) of cases were diazoxide-unresponsive; 282 underwent surgery that revealed focal HI in 53% (149 of 282) and diffuse HI in 43% (122 of 282). Histology that could not be classified as either focal or diffuse was observed in 4% (11 of 282), including 7 children diagnosed as having localized islet nuclear enlargement (LINE) HI (31).
Focal HI
In 97% (145 of 149) of focal HI cases, monoallelic recessive KATP mutations were identified. Paternal inheritance was confirmed in 83% (120 of 145), and nonmaternal inheritance was demonstrated in an additional 6% (9 of 145). Putative de novo mutations were found in 3% (5 of 145) of focal HI cases, but nonpaternity was not excluded. Parental samples were unavailable in 8% (11 of 145) of the cases. There were no instances in which a maternally derived mutation was associated with focal HI.
Diffuse HI
Mutations of the KATP genes were identified in 89% (109 of 122) of children with diffuse HI; another 2% (2 of 122) had GCK mutations. Most these cases were biallelic for recessive KATP mutations (69%, 84 of 122). However, 13% (16 of 122) had monoallelic dominant mutations of ABCC8 (all shown to produce normally trafficking channels but with reduced activities) (7). Monoallelic recessive mutations (8 null and 1 missense) were identified in 9 HI cases classified initially as diffuse, but on re-review may have had focal HI that escaped detection. Paternal transmission was confirmed in 7 of these 9 cases; 1 case was de novo, and parental samples were unavailable in the remaining case. In 4 of the 9 cases, exonic dosage assays showed that none had cryptic ABCC8 mutations (intragenic indels or large rearrangements). Re-examination of the surgical histology from 5 of these cases showed that 4 had regions with localized expansion of endocrine tissue centrally located within a lobule. Although these did not fit the usual size criteria for a focal lesion, they appeared much larger than islets in adjacent areas of the pancreas. In addition, in 3 of the latter 4 cases, immunohistochemical staining for p57 was weak or absent in the areas of increased proliferation, consistent with focal HI and maternal loss of heterozygosity for 11p15.1 (8).
Two probands who required subtotal pancreatectomy to control hypoglycemia were found to have diffuse HI and subsequently discovered to have mutations of GCK. In one of these, the mutation was not found in peripheral blood DNA, but pancreatic DNA revealed a postzygotic mosaic GCK mutation (c.1361_1363dupCGG/p.Ala454dup). An additional 5 probands had germline GCK mutations but were managed medically (see below).
Atypical histology
Normal pancreatic histology was observed in 4 surgical cases, whereas atypical diffuse disease (LINE HI) was observed in 7 others. In these 11 cases, no mutations could be detected in the KATP or GCK genes in DNA from either peripheral blood or pancreatic tissue.
Medically managed diazoxide-unresponsive congenital HI
In 16 children who did not respond adequately to diazoxide, hypoglycemia was managed with various combinations of octreotide and tube feedings, rather than surgery, due to parental preference (Table 1). In this group, 5 had mutations in GCK and 11 carried mutations in the KATP genes. Of the cases with KATP mutations, 55% (6 of 11) had a monoallelic paternal recessive mutation, consistent with possible focal HI; the remainder had genotypes consistent with diffuse HI, 36% (4 of 11) with biallelic recessive mutations and 9% (1 of 11) with a monoallelic dominant diazoxide-unresponsive mutation.
Genotypes in diazoxide-responsive HI cases
As shown in Table 2, mutations were identified in only 47% (56 of 118) of the diazoxide-responsive cases. Of these, 42% (24 of 56) were GLUD1 mutations, 41% (23 of 56) were KATP channel mutations, and 16% (9 of 56) were in rarer genes (HADH, UCP2, HNF4A, and HNF1A).
GLUD1 mutations
Dominant mutations of GLUD1 were identified (n = 23) or suspected (n = 2) in 25 diazoxide-responsive patients. The latter 2 cases were negative for mutations in peripheral blood but had elevated serum ammonia and were sensitive to leucine and protein, consistent with the hyperinsulinemia/hyperammonemia syndrome. One of these elected to have a pancreatectomy, despite being controllable on diazoxide, and was found to be heterozygous for a postzygotic mosaic GLUD1 mutation (c.1493c→t/p.Ser445Leu) in pancreatic DNA.
KATP mutations
Monoallelic missense KATP mutations were identified in 20% (23 of 118) of the diazoxide-responsive patients: 19 in ABCC8 and 4 in KCNJ11. All of these mutations were found to traffic normally by expression studies, consistent with a dominant defect (unpublished data) (6).
Other loci
Additional mutation analysis for rarer HI genes was done in diazoxide-responsive cases who were negative for mutations in ABCC8, KCNJ11, GCK, and GLUD1. Two cases had dominant mutations in HNF4A, 3 had dominant mutations in HNF1A, 2 had dominant UCP2 mutations, and 2 were biallelic for recessive HADH mutations. One case, not treated with diazoxide and therefore not shown in Table 1 or Table 2, was screened for mutations in the SLC16A1 (monocarboxylate transporter 1) promoter based on features suggestive of exercise-induced HI; no mutation was detected.
Nature of congenital HI mutations
A total of 213 unique mutations were identified in these 417 cases: 160 in ABCC8, 23 in KCNJ11, 6 in GCK, 14 in GLUD1, 3 in HADH, 2 in UCP2, 2 in HNF4A, and 3 in HNF1A. Of these, 76 mutations are reported for the first time in the present series. Details about these mutations are shown in the Supplemental Appendix (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
ABCC8 mutations
Of the 160 ABCC8 mutations, 91 affected a single amino acid (missense or in-frame deletion/insertion). Expression studies on 50 of these 91 demonstrated that 20 were nontrafficking recessive mutations, whereas 30 were normal-trafficking dominant mutations (6, 7). The dominant ABCC8 mutations were associated with either diazoxide-unresponsive (n = 13) or diazoxide-responsive (n = 17) HI. With the possible exception of 1 putative intronic mutation that may alter splicing (c.3402+13 G→A; see Supplemental Appendix), all other types of mutations were exclusively recessive, including one mutation involving a duplication of four exons.
As shown in Figure 1, mutations occurred throughout ABCC8, with the exception of exon 17, which contains only 35 nucleotides and has also not been affected in other series (32–34). The location of missense ABCC8 mutations did not predict whether the mutation was dominant or recessive. In some instances, different missense mutations at the same residue resulted in either recessive or dominant defects (p.D310V/N; p.R1353P/H; p.G1384R/E).
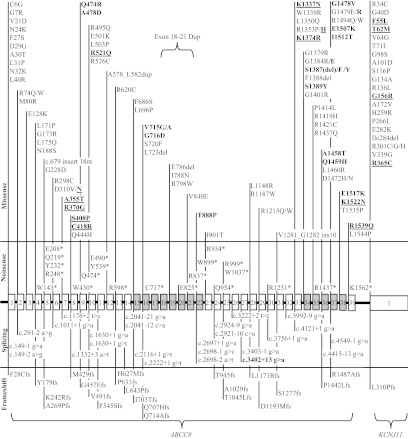
Figure 1.
Locations of 160 KATP HI Mutations in ABCC8 and KCNJ11. Shown are the 39 exons of ABCC8 and the single exon of the adjacent KCNJ11 gene. Shaded exons encode the two nucleotide-binding regions of ABCC8. Plain type indicates recessive mutations (114 missense, 21 nonsense, 25 splicing, and 23 frameshift). Bold type indicates 33 dominant missense mutations; underscored bold indicates 16 diazoxide-responsive missense mutations (all other dominant and recessive mutations were diazoxide-unresponsive). Note one large indel mutation (exon 18–21 duplication); as noted in Results, disease association of c.3402+13g→a splice-site mutation is uncertain.
KCNJ11 mutations
Of the 23 mutations in KCNJ11, 22 affected a single amino acid (missense or in-frame deletion/insertion) and 1 was a frame-shift mutation; 19 were recessive and 4 were dominant (confirmed by expression studies to traffic normally) (6, 7). Unlike the dominant ABCC8 mutations that were either diazoxide-unresponsive or diazoxide-responsive, the 4 dominant KCNJ11 mutations were exclusively associated with diazoxide-responsive HI.
GLUD1 and GCK mutations
Mutations identified in both GLUD1 and GCK were all dominant missense mutations (Supplemental Appendix). Mutations arose de novo in 52% of cases (3 of 7 in GCK and 13 of 24 GLUD1). Only 23% (2 of 7 in GCK and 5 of 24 in GLUD1) were inherited. In the remaining cases, parental samples were unavailable.
HADH, UCP2, HNF4A, and HNF1A
The 2 mutations in HNF4A included 1 missense and 1 frameshift mutation. The 3 mutations in HNF1A included 1 nonsense and 2 missense mutations. Both mutations identified in UCP2 were missense mutations. The 3 mutations in HADH included 1 missense, 1 frameshift, and 1 intronic splicing mutation.
Discussion
In this group of 417 children with congenital HI, disease-causing mutations were identified in 79% (328 of 417). Identification was most successful in diazoxide-unresponsive cases, in which 89% (265 of 298) carried KATP mutations and 2% (7 of 298) had GCK defects. Identification was less successful in diazoxide-responsive cases where defects could be identified in only 47% (56 of 118) of children.
Children with GLUD1, HADH, HNF4A, HNF1A, and UCP2 mutations were exclusively diazoxide-responsive, whereas children with GCK mutations and recessive KATP mutations were solely diazoxide-unresponsive. However, children with dominant mutations of the KATP genes were either diazoxide-responsive or diazoxide-unresponsive. This complex relationship between genotype and phenotype observed with mutations of the KATP channel genes is discussed below.
Although 25% of children with KATP mutations were found to carry common founder mutations in ABCC8 (49 had c.3992–9 g→a, 11 had p.Phe1388del, and 11 had p.Arg837*) (3, 5, 35), approximately 70% of the KATP mutations were novel at the time of identification, ie, never seen previously at CHOP or reported elsewhere. Predicting the clinical phenotype of such novel defects was not problematic for null mutations, such as nonsense defects, because these could only act in a recessive manner. However, novel missense mutations created major concern because they could potentially be either recessive or dominant. A parental history of hypoglycemia was sometimes helpful in ascertaining dominant mutations. However, adult carriers of dominant KATP mutations are often asymptomatic unless challenged with fasting or protein tolerance tests (6, 7). In general, the clinical phenotypes of specific dominant mutations were consistent; ie, all affected individuals were either diazoxide-responsive or nonresponsive. The only exception encountered was ABCC8 p.delS1387, which is usually associated with diazoxide-responsive HI (6), but also occurred in two diazoxide-unresponsive probands; whether these cases had a second genetic defect (eg, postzygotic) seems unlikely but cannot be excluded.
As shown in the Supplemental Appendix, SIFT and PolyPhen correctly predicted 79% (83 of 105) of the disease-associated missense KATP mutations to be damaging, gave conflicting predictions in 17% (18 of 105), and incorrectly predicted that 4% (4 of 105) would be benign. The accuracy of these prediction programs was similar with dominant vs recessive KATP missense mutations and diazoxide-unresponsive compared with diazoxide-responsive dominant ABCC8 mutations.
Predicting the consequences of novel missense KATP mutations is further complicated if the mutation occurs in combination with a second mutant allele. For example, one child in our series with diazoxide-responsive hyperinsulinism had a paternal novel missense mutation (ABCC8 p.K1337N) and a maternal known recessive mutation (ABCC8 c.3992–9 g→a). Although expression studies of the K1337N variant failed to demonstrate abnormalities of channel activity, it seems likely that the K1337N is a mild dominant diazoxide-responsive mutation whose effect was unmasked when combined with a second null allele. Muzyamba et al (36) have reported two cases with two missense mutations of ABCC8 on the same allele in which detailed expression studies were required to determine which variants were disease-causing. Reports of disease-associated mutations may also occasionally be misleading if not confirmed by expression studies or multiple affected cases. For example, in the present series, one child with focal HI had a paternally derived mutation in KCNJ11 (p.R34C) that was previously reported to be associated with transient neonatal diabetes (37). The carrier father was unaffected by either HI or diabetes. Expression studies revealed a defect in trafficking, consistent with R34C being a recessive loss-of-function HI mutation rather than an activating diabetes defect.
Preoperative diagnosis of focal HI is of great importance for clinical decision-making in patients with HI. In this series, the sensitivity of mutation analysis for predicting focal HI based on finding a monoallelic recessive KATP mutation was 97% (Table 3). Demonstration of paternal origin provided only a slight increase in specificity. The accuracy of diagnosing focal HI might actually be higher, because some of our cases with monoallelic paternal recessive mutations, who had been classified as diffuse HI, may have actually had focal HI that was not detected. Other reported series have also noted an excess of paternal-only KATP mutations in cases diagnosed as diffuse HI; however, these diagnoses were based solely on radiologic methods, such as [18F] fluoro DOPA positron emission tomography scan (33, 34, 38). Given the fact that approximately 15%–25% of focal lesions are not detected by such methods, it seems likely that many of these cases also represent unrecognized focal lesions. Although mutation analysis appears to be more sensitive than imaging methods for diagnosis of focal HI, [18F]DOPA positron emission tomography scan remains the only accurate method for localizing focal HI lesions prior to surgery.
Table 3.
Use of Genetic Testing to Predict Focal KATP HI Lesion
| Focal HI | Diffuse HI | |
|---|---|---|
| Single recessive KATP mutation | 145 | 9 |
| Paternal single recessive KATP mutation | 120 | 7 |
| No single recessive KATP mutations | 4 | 84 |
For prediction of focal HI based on a single recessive KATP mutation, sensitivity = 97%, specificity = 90%, positive predictive value = 94%, and negative predictive value = 95%. For prediction of focal HI based on a single paternal recessive KATP mutation, sensitivity = 97%, specificity = 92%, positive predictive value = 94%, and negative predictive value = 95%.
Glaser et al (39) have calculated the risk of focal HI in a fetus carrying a paternal recessive KATP mutation to be 1:270. Using similar analysis, we can compute the carrier frequency of a recessive KATP mutation in the CHOP population (similar to general population of the United States) to be ∼1:231 (see Supplemental Appendix for calculations). This is lower than the estimated frequency in Israeli Ashkenazi where two ABCC8 founder mutations are frequent (1:52) (39). Based on the current series, we can compute the prevalence of HI disease due to recessive KATP mutations (focal and diffuse) in the U.S. population of between 1:61 349 and 1:79 365. This compares to the previous estimates of the frequency of HI in Northern European populations of approximately 1:30 000 to 1:40 000 births (40).
Only a small fraction of diazoxide-unresponsive cases in our series had no identifiable mutation (9%, 26 of 298), consistent with reports of other series (Table 1) (32–34). This might, in part, be due to the failure of direct sequencing to detect cryptic mutations in the usual diazoxide-unresponsive genes (ABCC8, KCNJ11, and GCK). Examples of such mutations in ABCC8 identified in the present series included 1 case with a large deletion/insertion found by exonic dosage assay and 4 cases with intronic ABCC8 mutations not identified by commercial laboratory testing. In addition, some cases with missing mutations might have postzygotic mutations of dominant HI genes, such as the case in the present series with a postzygotic GCK mutation detected in pancreas. The possibility of a novel locus for diazoxide-unresponsive HI also may be considered.
In contrast to diazoxide-unresponsive HI cases, the proportion of diazoxide-responsive children lacking mutations in any of the known HI loci was quite large (53%, 62 of 118). Although some of these could represent undetected postzygotic mutations in 1 of the 6 known dominant HI genes, it is also possible that the large size of this group implies that other diazoxide-responsive HI loci remain to be discovered. For example, HI associated with dominant mutations of HNF1A has only recently been described (20). In the current series, HNF4A and HADH mutations accounted for <4% of diazoxide-responsive cases. However, other groups have identified mutations in HNF4A and HADH more frequently in diazoxide-responsive HI (19% and 10%) (19, 28). These differences might reflect differences in case selection or referral patterns between centers.
In conclusion, this large series of cases demonstrates the utility of genotype-phenotype correlations in children with congenital HI, especially those with GCK and GLUD1 mutations or those with recessive KATP mutations. Identification of a monoallelic recessive KATP mutation appears to predict focal HI with high sensitivity and specificity. Thus, mutation analysis, including parent-of-origin testing, should be considered in children with HI who cannot be controlled on diazoxide and might be candidates for surgery. For the large number of novel missense KATP mutations, it is currently not possible, without additional clinical or functional testing information, to predict whether the mutation will be dominant or recessive and, if dominant, whether responsive to diazoxide. Accurate and timely prediction of phenotype based on genotype is crucial for limiting exposure to persistent hypoglycemia and reducing the risk of seizures and permanent brain damage in infants and children with congenital HI.
Acknowledgments
We thank the nurses and research staff of The Hyperinsulinism Center and the Clinical Translational Research Center Core Laboratory.
This work was supported in part by grants from the National Institutes of Health (R37 DK056268 and R01 DK53012 to C.A.S., R01 DK057699 and R01 DK066485 to S.-L.S., and UL1 RR 024134) as well as by The Clifford and Katherine Goldsmith Philanthropic Fund.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CHOP
- The Children's Hospital of Philadelphia
- HI
- hyperinsulinism
- KATP
- ATP-sensitive plasma membrane potassium
- LINE
- localized islet nuclear enlargement.
References
- 1. Stanley CA, DeLeon DD, eds. 2012. Monogenic Hyperinsulinemic Hypoglycemia Disorders. Basel, Switzerland: Karger [Google Scholar]
- 2. Aguilar-Bryan L, Nichols CG, Wechsler SW, et al. Cloning of the β cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426 [DOI] [PubMed] [Google Scholar]
- 3. Thomas PM, Cote GJ, Wohllk N, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–429 [DOI] [PubMed] [Google Scholar]
- 4. Nestorowicz A, Inagaki N, Gonoi T, et al. A nonsense mutation in the inward rectifier potassium channel gene, Kir6.2, is associated with familial hyperinsulinism. Diabetes. 1997;46:1743–1748 [DOI] [PubMed] [Google Scholar]
- 5. Nestorowicz A, Wilson BA, Schoor KP, et al. Mutations in the sulonylurea receptor gene are associated with familial hyperinsulinism in Ashkenazi Jews. Hum Mol Genet. 1996;5:1813–1822 [DOI] [PubMed] [Google Scholar]
- 6. Pinney SE, MacMullen C, Becker S, et al. Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest. 2008;118:2877–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Macmullen CM, Zhou Q, Snider KE, et al. Diazoxide-unresponsive congenital hyperinsulinism in children with dominant mutations of the β-cell sulfonylurea receptor SUR1. Diabetes. 2011;60:1797–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Lonlay P, Fournet JC, Rahier J, et al. Somatic deletion of the imprinted 11p15 region in sporadic persistent hyperinsulinemic hypoglycemia of infancy is specific of focal adenomatous hyperplasia and endorses partial pancreatectomy. J Clin Invest. 1997;100:802–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stanley CA, Lieu YK, Hsu BY, et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med. 1998;338:1352–1357 [DOI] [PubMed] [Google Scholar]
- 10. Glaser B, Kesavan P, Heyman M, et al. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med. 1998;338:226–230 [DOI] [PubMed] [Google Scholar]
- 11. Sayed S, Langdon DR, Odili S, et al. Extremes of clinical and enzymatic phenotypes in children with hyperinsulinism caused by glucokinase activating mutations. Diabetes. 2009;58:1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li C, Chen P, Palladino A, et al. Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J Biol Chem. 2010;285:31806–31818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molven A, Matre GE, Duran M, et al. Familial hyperinsulinemic hypoglycemia caused by a defect in the SCHAD enzyme of mitochondrial fatty acid oxidation. Diabetes. 2004;53:221–227 [DOI] [PubMed] [Google Scholar]
- 14. Clayton PT, Eaton S, Aynsley-Green A, et al. Hyperinsulinism in short-chain l-3-hydroxyacyl-CoA dehydrogenase deficiency reveals the importance of β-oxidation in insulin secretion. J Clin Invest. 2001;108:457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heslegrave AJ, Kapoor RR, Eaton S, et al. Leucine-sensitive hyperinsulinaemic hypoglycaemia in patients with loss of function mutations in 3-hydroxyacyl-CoA dehydrogenase. Orphanet J Rare Dis. 2012;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonzalez-Barroso MM, Giurgea I, Bouillaud F, et al. Mutations in UCP2 in congenital hyperinsulinism reveal a role for regulation of insulin secretion. PLoS One. 2008;3:e3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pingul MM, Hughes N, Wu A, Stanley CA, Gruppuso PA. Hepatocyte nuclear factor 4α gene mutation associated with familial neonatal hyperinsulinism and maturity-onset diabetes of the young. J Pediatr. 2011;158:852–854 [DOI] [PubMed] [Google Scholar]
- 18. Pearson ER, Boj SF, Steele AM, et al. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med. 2007;4:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flanagan SE, Kapoor RR, Mali G, et al. Diazoxide-responsive hyperinsulinemic hypoglycemia caused by HNF4A gene mutations. Eur J Endocrinol. 2010;162:987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stanescu DE, Hughes N, Kaplan B, Stanley CA, De Leon DD. Novel presentations of congenital hyperinsulinism due to mutations in the MODY genes: HNF1A and HNF4A. J Clin Endocrinol Metab. 2012;97:E2026–E2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dusatkova P, Pruhova S, Sumnik Z, et al. HNF1A mutation presenting with fetal macrosomia and hypoglycemia in childhood prior to onset of overt diabetes. J Pediatr Endocrinol Metab. 2011;24:377–379 [PubMed] [Google Scholar]
- 22. Otonkoski T, Kaminen N, Ustinov J, et al. Physical exercise-induced hyperinsulinemic hypoglycemia is an autosomal-dominant trait characterized by abnormal pyruvate-induced insulin release. Diabetes. 2003;52:199–204 [DOI] [PubMed] [Google Scholar]
- 23. Stanley CA, Baker L. Hyperinsulinism in infancy: diagnosis by demonstration of abnormal response to fasting hypoglycemia. Pediatrics. 1976;57:702–711 [PubMed] [Google Scholar]
- 24. Finegold DN, Stanley CA, Baker L. Glycemic response to glucagon during fasting hypoglycemia: an aid in the diagnosis of hyperinsulinism. J Pediatr. 1980;96:257–259 [DOI] [PubMed] [Google Scholar]
- 25. Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081 [DOI] [PubMed] [Google Scholar]
- 26. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. 1000 Genomes Project Consortium A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flanagan SE, Patch AM, Locke JM, et al. Genome-wide homozygosity analysis reveals HADH mutations as a common cause of diazoxide-responsive hyperinsulinemic-hypoglycemia in consanguineous pedigrees. J Clin Endocrinol Metab. 2011;96:E498–E502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sherry ST, Ward MH, Kholodov M, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan FF, Lin YW, MacMullen C, Ganguly A, Stanley CA, Shyng SL. Congenital hyperinsulinism associated ABCC8 mutations that cause defective trafficking of ATP-sensitive K+ channels: identification and rescue. Diabetes. 2007;56:2339–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ernst LE, Suchi M, Stanley CA, Adzick NS, MacMullen C, Ruchelli ED. 2007 Localized islet cell nuclear enlargement in congenital hyperinsulinism: a distinct clinicopathologic entity. Abstract presented at: Society for Pediatric Pathology Spring Meeting; 2007; San Diego, CA [Google Scholar]
- 32. Flanagan SE, Clauin S, Bellanne-Chantelot C, et al. Update of mutations in the genes encoding the pancreatic β-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2009;30:170–180 [DOI] [PubMed] [Google Scholar]
- 33. Fernandez-Marmiesse A, Salas A, Vega A, Fernandez-Lorenzo JR, Barreiro J, Carracedo A. Mutation spectra of ABCC8 gene in Spanish patients with hyperinsulinism of infancy (HI). Hum Mutat. 2006;27:214. [DOI] [PubMed] [Google Scholar]
- 34. Bellanne-Chantelot C, Saint-Martin C, Ribeiro MJ, et al. ABCC8 and KCNJ11 molecular spectrum of 109 patients with diazoxide-unresponsive congenital hyperinsulinism. J Med Genet. 2010;47:752–759 [DOI] [PubMed] [Google Scholar]
- 35. Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev. 1999;20:101–135 [DOI] [PubMed] [Google Scholar]
- 36. Muzyamba M, Farzaneh T, Behe P, et al. Complex ABCC8 DNA variations in congenital hyperinsulinism: lessons from functional studies. Clin Endocrinol (Oxf). 2007;67:115–124 [DOI] [PubMed] [Google Scholar]
- 37. Flanagan SE, Patch AM, Mackay DJ, et al. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56:1930–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yorifuji T, Kawakita R, Nagai S, et al. Molecular and clinical analysis of Japanese patients with persistent congenital hyperinsulinism: predominance of paternally inherited monoallelic mutations in the KATP channel genes. J Clin Endocrinol Metab. 2011;96:E141–E145 [DOI] [PubMed] [Google Scholar]
- 39. Glaser B, Blech I, Krakinovsky Y, et al. ABCC8 mutation allele frequency in the Ashkenazi Jewish population and risk of focal hyperinsulinemic hypoglycemia. Genet Med. 2011;13:891–894 [DOI] [PubMed] [Google Scholar]
- 40. Otonkoski T, Ammala C, Huopio H, et al. A point mutation inactivating the sulfonylurea receptor causes the severe form of persistent hyperinsulinemic hypoglycemia of infancy in Finland. Diabetes. 1999;48:408–415 [DOI] [PubMed] [Google Scholar]