Abstract
Contents:
Ghrelin is implicated in meal initiation because circulating levels increase before and fall after meal consumption. In rodents, ghrelin stimulates food intake via hypothalamic circuits expressing the melanocortin 4 receptor (MC4R).
Objective:
The aim of the study was to investigate the impact of central melanocortinergic tone on ghrelin secretion in humans.
Design/Setting/Patients/Main Outcome Measure:
We measured plasma total ghrelin before and after 3 standardized meals in patients with MC4R mutations and obese and lean controls. Fasting total ghrelin, area under the curve, and early (30-min) and intermeal postprandial total ghrelin suppression (the percentage difference between the premeal and the 30-min postprandial or intermeal nadir total ghrelin concentration) were calculated.
Results:
Fasting total ghrelin concentrations and area under the curve for total ghrelin concentrations were significantly decreased in both obese groups compared to lean controls (fasting total ghrelin: lean controls, 1083 ± 203 pg/ml; and obese controls, 696 ± 81 pg/ml; MC4R: 609 ± 99 pg/ml, P < .05). Early and intermeal postprandial total ghrelin suppression was attenuated in MC4R-deficient patients compared to lean controls (P < .05); a similar pattern was observed for early postprandial suppression in comparison with obese controls, but this difference did not reach statistical significance (P = .09).
Conclusions:
These findings are consistent with a role for central melanocortinergic signaling in modulating meal-related total ghrelin suppression.
Genetic and pharmacological studies have shown that the central melanocortin system plays a key role in the regulation of food intake in rodents and humans (1, 2). In addition to interacting with long-term regulators of energy homeostasis such as leptin, the central melanocortin system also responds to gut-derived peptides involved in mediating meal-induced satiety (3).
Ghrelin is an acylated peptide that is released primarily by oxyntic cells in the fundus of the stomach and the proximal small intestine and potently increases food intake in rodents (4) and humans (5). Circulating ghrelin levels increase shortly before meals and are suppressed by ingested nutrients (carbohydrates being more effective than proteins, which are more effective than lipids). Ghrelin stimulates food intake in part by activating the orexigenic hypothalamic neurons that secrete neuropeptide Y and agouti-related peptide. The stimulatory effects of ghrelin on these orexigenic neurons are complemented by inhibition of anorexigenic neurons that express pro-opiomelanocortin. Pro-opiomelanocortin-derived peptides are agonists, and agouti-related peptide is a competitive antagonist at the melanocortin 4 receptor (MC4R). Ghrelin does not stimulate food intake in mc4r null mice, suggesting that the effects of ghrelin are partly mediated by the hypothalamic melanocortinergic system (6).
To investigate the impact of central melanocortinergic tone on ghrelin secretion in humans, we studied the pre- and postprandial secretion of total ghrelin in patients with genetic disruption of MC4R. These patients develop severe obesity from a young age due to hyperphagia and impaired satiety (7). We studied 6 obese patients with heterozygous complete loss of function mutations in MC4R and compared data to 6 equally obese subjects and 6 lean controls in whom MC4R mutations had been excluded.
Subjects and Methods
Total ghrelin concentrations were obtained in 6 lean controls (4 women), 6 obese subjects (6 women), and 6 patients with MC4R deficiency (4 women) during a 12-hour sampling period with 3 meals standardized for macronutrient and caloric content after a 10-hour overnight fast. All subjects had normal levels of lipids, glucose, and thyroid hormones; none of the participants had been diagnosed with diabetes mellitus or gastrointestinal disease; subjects were not taking any medications. In addition, obese controls had a normal MC4R genotype and no history suggestive of other known monogenic obesity syndromes. The groups were matched for age, and ages ranged from 20 to 71 years, which was not different between the 3 groups. Mean body mass index (BMI) was 44.1 ± 1.1 kg/m2 in patients with MC4R deficiency, 41.2 ± 1.4 kg/m2 in obese controls, and 24.6 ± 1.1 kg/m2 in lean controls. The study was approved by the Local Regional Ethics Committee in Cambridge. All subjects provided written informed consent.
Meals were provided at 8 am, noon, and 5:30 pm. Blood was drawn every half hour from 7:30 am to 8:30 pm. Visual analog scales, which require respondents to specify their level of hunger or fullness indicating a position along a continuous line between two end-points (not hungry, most hungry you have ever been; not full, most full you have ever been) were used to assess hunger and fullness every half hour starting at 7:30 am. Meals were calculated to provide 20, 35, and 35% of daily individual energy requirements (breakfast, lunch, and dinner, respectively) using the Schofield formula (8); the macronutrient content of the meals was 20% protein, 30% fat, and 50% carbohydrates.
Blood for plasma glucose was collected from an indwelling iv catheter in a fluoride oxalate tube, for insulin in tubes containing lithium-heparin, and for ghrelin in lithium-heparin tubes containing of 100 μL aprotinin. Blood was spun immediately at 4°C, and plasma was frozen and stored at −80°C until analysis. Total ghrelin immunoreactivity was measured using a RIA by Linco Research Inc. (Minneapolis, Minnesota) with a sensitivity of 93 pg/ml and an interassay variability of 14.7% at 1000 pg/ml and 16.7% at 3000 pg/ml. Plasma glucose was assayed on the same day by using the glucose oxidase method. Insulin was quantified using a commercially available immunoassay (AutoDELFIA Insulin Kit; PerkinElmer, Wellesley, Massachusetts), which has an intraassay coefficient of variation of 3.5–4.5%.
Total ghrelin suppression and insulin secretion were calculated as the percentage difference between the average premeal value and the average values at 30 minutes after the meal and at the intermeal nadir of the combined nadir values after breakfast and lunch. Area under the curve (AUC) was estimated using the trapezoidal method. The homeostasis model estimate insulin resistance (HOMA IR) and quantitative insulin-sensitivity check index (QUICKI) were calculated and compared using ANOVA (9). Data are reported as mean ± SEM. The nonparametric Kruskal-Wallis test was used to compare insulin and total ghrelin suppression between the 3 groups, and post hoc comparisons were performed with the nonparametric Mann-Whitney test. Average, maximal, minimal, fasting, and AUC of total ghrelin and insulin concentrations were log-transformed before comparison using ANOVA with post hoc comparisons. Pearson's correlation coefficients were calculated for total ghrelin secretion, insulin, and BMI. Significance levels were assumed at 5% (P = .05). All analyses were performed in SPSS 18 (SPSS Inc., Chicago, Illinois).
Results
Fasting total ghrelin was 36% lower in obese controls and 44% lower in patients with MC4R deficiency compared to lean controls (1083 ± 203 pg/ml in lean controls, 696 ± 81 pg/ml in obese controls, and 609 ± 99 pg/ml in MC4R deficiency; P < .05 compared to lean controls). Average total ghrelin concentrations were also 33 and 37% lower, respectively, as were AUC values for obese controls and MC4R deficiency. Maximal total ghrelin concentrations were higher in lean controls (1433 ± 246 pg/ml) compared to obese controls (846 ± 123 pg/ml; P = .034) and patients with MC4R deficiency (842 ± 135 pg/ml; P = .032). In addition, fasting, mean, maximal, and minimal total ghrelin concentrations and AUC were all significantly, negatively related to BMI when considering all subjects in a combined group (R = −.599 [P = .009], R = −.619 [P = .006], R = −.630 [P = .005], R = −.597 [P = .009], and R = −.629 [P = .005], respectively).
Total ghrelin levels decreased postprandially in all groups. However, compared to lean controls, this decrease was less marked at 30 minutes (−8 ± 2% in MC4R deficiency and −6 ± 4% in lean controls; P = .009) and at the intermeal nadir (−13 ± 3% in MC4R deficiency and −26 ± 4% in lean controls; P = .041) in patients with MC4R deficiency. A similar pattern was found compared to obese controls (−3% ± 4% at 30 min, P = .093; and −20 ± 2% at intermeal nadir, P = .180) although this difference did not reach statistical significance (Figure 1). There was no difference in postprandial total ghrelin suppression between lean and obese volunteers (P = .662 for 30 min and P = .394 for intermeal nadir suppression).
Figure 1.
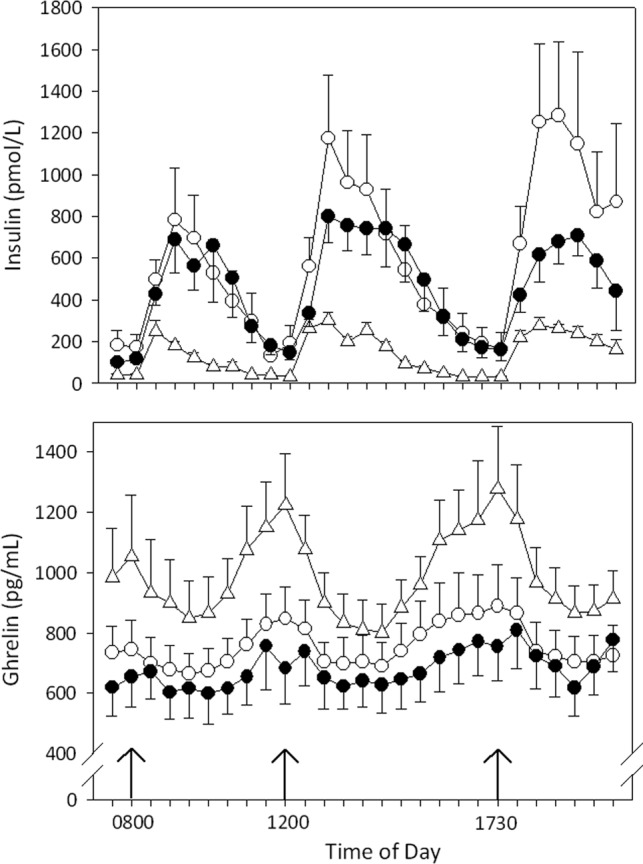
Insulin (upper graph) and total ghrelin (lower graph) concentrations in 6 lean controls (triangles), 6 obese subjects (open circles), and 6 obese patients with MC4R deficiency (closed circles) during a 12-h sampling period with 3 meals standardized for macronutrient and caloric content. Meals were provided at 8 am, noon, and 5:30 pm (arrows). Samples were taken every 30 min. Results are expressed as mean ± SE.
Postprandial insulin secretion was slightly lower in MC4R-deficient patients compared to lean and obese controls, but this difference did not reach statistical significance (292 ± 101 pmol/L in MC4R vs 594 ± 243 pmol/L in lean and 432 ± 132 pmol/L in obese controls; P = .244). Postprandial insulin has been shown to be inversely correlated with postprandial total ghrelin suppression. We also found a negative correlation in the whole study group (Pearson R = −.37; P = .003; Figure 2) and in MC4R-deficient patients (Pearson R = −.837; P = .038). In addition, linear regression analysis did not show any differences between groups in the relationship between postprandial total ghrelin suppression and postprandial insulin secretion. HOMA IR scores were .7 ± .1 in lean controls, 3.5 ± 1.4 in obese controls, and 1.9 ± .3 in MC4R deficiency (P = .104). The QUICKI was .38 ± .03 in lean controls, .32 ± .02 in obese controls, and .33 ± .01 in MC4R deficiency (P = .122). Neither HOMA IR nor QUICKI correlated with mean total ghrelin or 30-minute or intermeal total ghrelin suppression. Hunger and fullness scores did not differ between the 3 groups and did not correlate with total ghrelin concentrations at any time point (data not shown).
Figure 2.
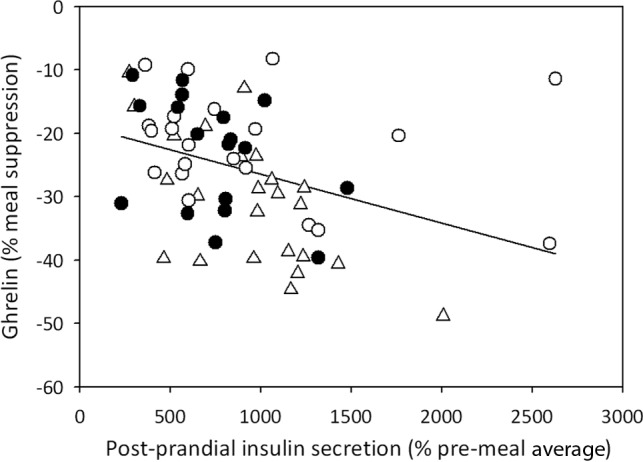
Correlation between percentage of postmeal insulin secretion and percentage of total ghrelin suppression in lean controls (triangles), obese controls (open circles), and obese subjects with MC4R deficiency (closed circles). Each data point represents a different meal (n = 63). Pearson R = −.37; P = .003.
Discussion
We found an attenuated postprandial total ghrelin suppression in MC4R deficiency compared to lean controls. A similar pattern was observed compared to obese controls, but this difference did not reach statistical significance. Previous studies have implied that ghrelin is not suppressed by meal intake in obese people, but most of these studies only measured the ghrelin response at one meal, typically breakfast. In this study we looked at the effect of multiple meals on total ghrelin secretion. When we looked at the individual meals, we also found that there was no significant total ghrelin suppression with breakfast in obese people, but we did find evidence of significant postprandial total ghrelin suppression with the other meals. These differences could be explained by the energy content and volume of food delivered for the 3 meals in our study (20% energy requirement for breakfast; 35% energy requirement for lunch and dinner).
Our study has a number of limitations related to the small number of subjects studied and the genetic and phenotypic heterogeneity of obesity. Although we excluded subjects with hyperlipidemia, type 2 diabetes, and gastrointestinal disease from the study, other differences between the groups may have influenced our findings. Of note, we have previously shown using the hyperinsulinemic euglycemic clamp, that when matched for BMI, obese individuals with MC4R deficiency are as insulin sensitive as equally obese controls with a normal MC4R genotype (10) and that body fat content, fat distribution, and serum leptin do not differ between obese MC4R-deficient patients and obese controls (10).
The exact mechanisms mediating the postprandial decrease in ghrelin levels are unknown but are likely to involve peripheral as well as central pathways. Postprandial ghrelin suppression does not require luminal nutrient delivery to the stomach in humans because direct intraduodenal delivery of glucose suppresses ghrelin secretion (11). In addition, although vagotomy alters fasting ghrelin concentrations, it does not alter postprandial ghrelin suppression in rodents, suggesting that the specific postprandial effects might be mediated by other neurally transmitted intestinal signals (12) and may, according to some studies, be augmented by insulin.
Moreover, the central mechanisms involved in responding to changes in ghrelin levels remain to be elucidated. The nucleus tractus solitarius integrates visceral sensory signals with information from the forebrain to regulate food intake (13, 14). Brainstem administration of MC4R ligands modulates meal size but not frequency, suggesting the enhancement of gut-derived satiety signals (15). Somatostatin has been shown to suppress ghrelin secretion in humans and rodents (16, 17). By studying GH secretory patterns in adults with MC4R deficiency, we have previously demonstrated that impaired melanocortin signaling is associated with reduced somatostatinergic tone (18). As such, attenuated somatostatin-mediated suppression of ghrelin could contribute to the differences in meal-related ghrelin suppression observed in MC4R deficiency. We found a trend toward lower postprandial insulin secretion in patients with MC4R deficiency compared to lean and obese controls, which might contribute to the attenuated postprandial total ghrelin suppression. We have previously shown that compared to age- and BMI-matched controls, children with MC4R deficiency have higher insulin levels that decline with age, presumably due to β-cell failure. Further studies in patients of different ages may be needed to more clearly investigate the potential relationship between insulin secretion and postprandial ghrelin suppression in MC4R deficiency and to study the effects of aging and gender on ghrelin secretion in MC4R deficiency. The assay we used measured total ghrelin, which includes active, acetylated, and inactive, desacyl ghrelin. Future studies determining the effects of a test meal on active and inactive ghrelin in patients with MC4R deficiency would be valuable (19).
In conclusion, in this study we show that the postprandial suppression of total ghrelin is attenuated in patients with MC4R deficiency compared to lean controls. There was no statistically significant difference between patients with MC4R deficiency and obese controls with a normal MC4R genotype.
Our study suggests that the regulation of postprandial ghrelin suppression in humans may involve central melanocortin signaling. Further studies will be needed to investigate the mechanisms underlying our observations. Understanding how central melanocortinergic pathways receive and integrate information about short-term satiety signals from the gut may inform the development of therapeutic strategies in obesity.
Acknowledgments
I.S.F. is supported by the Wellcome Trust, Medical Research Council Centre for Obesity and Related Metabolic Disorders, National Institute of Health Research, Biochemical Research Centre. J.Q.P. is supported by National Institutes of Health Grant R01 DK071161. A.M.H. is supported by the Canadian Institutes of Health Research, the Foundation for Prader-Willi Research, the Alberta Diabetes Institute, and Women & Children's Health Research Institute, University of Alberta.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Area under the curve
- BMI
- body mass index
- HOMA IR
- homeostasis model estimate insulin resistance
- MC4R
- melanocortin 4 receptor
- QUICKI
- quantitative insulin-sensitivity check index.
References
- 1. Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749 [DOI] [PubMed] [Google Scholar]
- 2. Williams DL, Schwartz MW. The melanocortin system as a central integrator of direct and indirect controls of food intake. Am J Physiol Regul Integr Comp Physiol. 2005;289:R2–R3 [DOI] [PubMed] [Google Scholar]
- 3. Ellacott KL, Halatchev IG, Cone RD. Interactions between gut peptides and the central melanocortin system in the regulation of energy homeostasis. Peptides. 2006;27:340–349 [DOI] [PubMed] [Google Scholar]
- 4. Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–2130 [DOI] [PubMed] [Google Scholar]
- 5. Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen HY, Trumbauer ME, Chen AS, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612 [DOI] [PubMed] [Google Scholar]
- 7. Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095 [DOI] [PubMed] [Google Scholar]
- 8. Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(suppl 1):5–41 [PubMed] [Google Scholar]
- 9. Mather KJ, Hunt AE, Steinberg HO, et al. Repeatability characteristics of simple indices of insulin resistance: implications for research applications. J Clin Endocrinol Metab. 2001;86:5457–5464 [DOI] [PubMed] [Google Scholar]
- 10. Greenfield JR, Miller JW, Keogh JM, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2008;360:44–52 [DOI] [PubMed] [Google Scholar]
- 11. Parker BA, Doran S, Wishart J, Horowitz M, Chapman IM. Effects of small intestinal and gastric glucose administration on the suppression of plasma ghrelin concentrations in healthy older men and women. Clin Endocrinol (Oxf). 2005;62:539–546 [DOI] [PubMed] [Google Scholar]
- 12. Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology. 2003;144:5184–5187 [DOI] [PubMed] [Google Scholar]
- 13. Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428 [DOI] [PubMed] [Google Scholar]
- 14. Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol. 2002;23:2–40 [DOI] [PubMed] [Google Scholar]
- 15. Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol. 2005;289:R247–R258 [DOI] [PubMed] [Google Scholar]
- 16. Barkan AL, Dimaraki EV, Jessup SK, Symons KV, Ermolenko M, Jaffe CA. Ghrelin secretion in humans is sexually dimorphic, suppressed by somatostatin, and not affected by the ambient growth hormone levels. J Clin Endocrinol Metab. 2003;88:2180–2184 [DOI] [PubMed] [Google Scholar]
- 17. Shimada M, Date Y, Mondal MS, Toshinai K, Shimbara T, Fukunaga K. Somatostatin suppresses ghrelin secretion from the rat stomach. Biochem Biophys Res Commun. 2003;302:520–525 [DOI] [PubMed] [Google Scholar]
- 18. Martinelli CE, Keogh JM, Greenfield JR, et al. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J Clin Endocrinol Metab. 2011;96:E181–E188 [DOI] [PubMed] [Google Scholar]
- 19. Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84 [DOI] [PubMed] [Google Scholar]


