Abstract
Context:
Although variation in radioactive iodine (RAI) use for thyroid cancer has been demonstrated, the role of region and nonclinical correlates of use within risk groups has not been investigated.
Objective:
The objective of the study was to determine the correlates of RAI use within risk groups.
Design/Setting/Patients:
Use of RAI was evaluated across 9 US regions in 85 948 patients with well-differentiated thyroid cancer diagnosed between 2004 and 2008 at 986 hospitals associated with the US National Cancer Database. Cancers were then categorized as low risk (tumor size ≤1 cm and American Joint Committee on Cancer stage I disease), medium risk (neither low nor high-risk), and high risk (American Joint Committee on Cancer stage III or IV). Within each risk stratum, the role of region and nonclinical correlates of RAI use were evaluated using hierarchical logistic regression.
Main Outcome Measure:
Use of RAI was measured.
Results:
Rates of RAI use varied across geographic regions from 49% to 66%. Regional differences persisted after controlling for patient and hospital characteristics and evaluating less vs more intensive regions within low-risk [odds ratio (OR) 0.36 (95% confidence interval [CI] 0.25–0.53)], medium-risk [OR 0.23 (95% CI 0.16–0.34)], and high-risk cancers [OR 0.30 (95% CI 0.19–0.49)]. Patterns of RAI use were similar in medium- and high-risk patients. The most nonclinical correlates of use were in low-risk patients.
Conclusion:
Similar treatment patterns for the heterogeneous medium-risk thyroid cancer patients compared with the high-risk patients suggest more intensive management in patients with medium-risk disease. The large number of nonclinical correlates of RAI use, including region, imply controversy over indications for RAI.
There is large variation in radioactive iodine (RAI) use for thyroid cancer with much of the variation attributed to the hospital at which care is received (1). The general trend has been an increase in RAI use over time, both for small and large tumors (1, 2). These treatment trends have implications for patients and for the health care system. RAI is associated with damage to other iodine avid tissues and, rarely, second primary malignancies (3, 4). In addition, the administration of RAI in patients with low-risk disease is more costly (5).
Although variation in the use of RAI has been documented, patterns of use within risk categories remain unknown. We hypothesized that there would be both regional variation in RAI use, most likely due to unmeasured differences in provider knowledge, attitudes, and beliefs and that the correlates of RAI use would differ between low-, medium-, and high-risk patients. To test these hypotheses, we evaluated correlates of RAI use, including region of the United States, across risk strata in a large cohort of patients treated at 986 hospitals associated with the US National Cancer Database between 2004 and 2008.
Materials and Methods
Data source and study population
The National Cancer Database is a joint project of the American College of Surgeons Commission on Cancer and the American Cancer Society. Close to 85% of all thyroid cancers in the United States are included in this database (6). We selected the 85 948 patients diagnosed with well-differentiated thyroid cancer between 2004 and 2008 who underwent total thyroidectomy. The cohort of patients was selected from hospitals affiliated with the US National Cancer Database four of the 5 specified years. After a patient was diagnosed and treated at a Commission on Cancer-accredited cancer program, the hospital registrar was responsible for documenting care, even when the patient was transferred to another facility (7). The data were then coded and reported according to nationally established protocols coordinated by the North American Association of Central Cancer Registries (8). Because no patient, physician, or hospital identifiers were examined, the University of Michigan Institutional Review Board granted exemption for this study.
Measures
Hospitals were assigned to their corresponding US census region. States included in the New England region were Connecticut, Massachusetts, Maine, New Hampshire, Rhode Island, and Vermont. The mid-Atlantic region consisted of New York, New Jersey, and Pennsylvania. The South Atlantic region included Washington, DC; Maryland; Delaware; Florida; Georgia; North Carolina; South Carolina; Virginia; and West Virginia. The East North Central region involved Illinois, Indiana, Michigan, Ohio, and Wisconsin. The East South Central region included Alabama, Kentucky, Mississippi, and Tennessee. The West North Central region included Iowa, Kansas, Minnesota, Missouri, North Dakota, Nebraska, and South Dakota. The West South Central region consisted of Arkansas, Louisiana, Oklahoma, and Texas. The Mountain region encompassed Arizona, Colorado, Idaho, Montana, New Mexico, Nevada, Utah, and Wyoming. The Pacific region included Alaska, California, Oregon, Washington, and Hawaii.
We created 3 disease severity categories for subgroup analysis. Because recent clinical guidelines do not recommend RAI in tumors 1 cm or less unless there are additional high-risk features, tumors that are 1 cm or less and still American Joint Committee on Cancer (AJCC) stage I were included in the low-risk category. Because RAI is typically recommended for iodine-avid advanced stage disease, AJCC stage III or IV cancers were categorized as high risk. The medium-risk category was a heterogeneous population that did not belong in the low or high-risk categories. In many cases medium-risk thyroid cancer would be associated with a selective-use recommendation in the recent guidelines (9).
As previously described (1), hospital case volume categories were created by computing a weighted average of the annual thyroid cancer case volume at each reporting cancer program for the years 2004–2008 and dividing the distribution into equal-sized quintiles of hospitals. We then combined the lowest 2 and then the middle 2 quintiles to create 3 volume categories: low (≤ 11 cases per year), medium (12–34 cases per year), and high (≥ 35 cases per year). Patient age was stratified into 4 biologically relevant groups: age less than 20, 20–44, 45–59, and 60 years and older. Patient race/ethnicity was categorized by the National Cancer Database as non-Hispanic white, black, Hispanic, Asian/ Pacific Islanders, and Native American. Due to smaller numbers, Hispanic, Asian/Pacific Islanders, and Native American were collapsed into the category, other. Race/ethnicity was included in the analysis because race/ethnicity has been shown to influence cancer treatment (10). With data drawn from the 2000 Census, we assigned insurance type and rural-urban continuum. We used the Charlson-Deyo Index to identify comorbid conditions within the cohort (11, 12). Tumor size was categorized according to the definitions used by the AJCC (13). Tumor histology was limited to the International Classification of Diseases for Oncology classification codes for papillary, follicular, and Hurthle cell cancer types (14). Lymph node status was classified as no lymph node metastases (N0), lymph node metastases (N1), or unknown lymph node status (NX). Distant metastases were either present or absent.
Statistical analyses
Our analytical cohort was comprised of patients diagnosed with well-differentiated thyroid cancer between 2004 and 2008 who underwent total thyroidectomy. To assess the role of region and nonclinical correlates of RAI use among low-, medium-, and high-risk patients, we performed stratified analyses. Within each disease severity category, hierarchical logistic regression models (15, 16) were fitted to obtain unadjusted and adjusted odds ratios (OR) for the use of radioactive iodine corresponding to region, hospital case volume, and patient clinical and nonclinical covariates. To account for the clustering of thyroid cancer patients within hospitals, we included a random hospital-specific intercept.
We let Yij = 1, if the jth patient seen at the ith hospital used radioactive iodine, and Yij = 0 otherwise. The probability of radioactive iodine use by the jth patient seen at the ith hospital can then be modeled as follows:
Level 1 was between patients (within hospitals), logit [P (Yij =1)] = μ0i + θ′Xij;
Level 2 was between hospitals: μ0i = β00 + β0i + γ′Zi;
Combined model was logit [P (Yij =1)] = β00 + β0i + γ′Zi + θ′Xij
where β00 is the population-averaged log odds of radioactive iodine use, and β0i is the hospital-specific random effect that captures the heterogeneity across hospitals. The random intercept β0i is assumed to follow a normal distribution with mean 0 and variance σ2hosp, Xij is the matrix of patient clinical and nonclinical covariates, θ is the corresponding vector of fixed effects representing changes in the log odds of radioactive iodine use corresponding to each unit change in the covariate values, Zi represents the vector of hospital-level covariates (hospital region and case volume) for the ith hospital, and γ is the corresponding vector of coefficients. We obtained the model estimates using the likelihood-based approach in SAS PROC GLIMMIX (SAS version 9.3; SAS Institute, Cary, North Carolina).
All statistical analyses were performed using SAS software (SAS version 9.3; SAS Institute). We calculated 95% confidence intervals (CI), and exclusion of the null value (1) was considered statistically significant.
Results
Region and RAI use
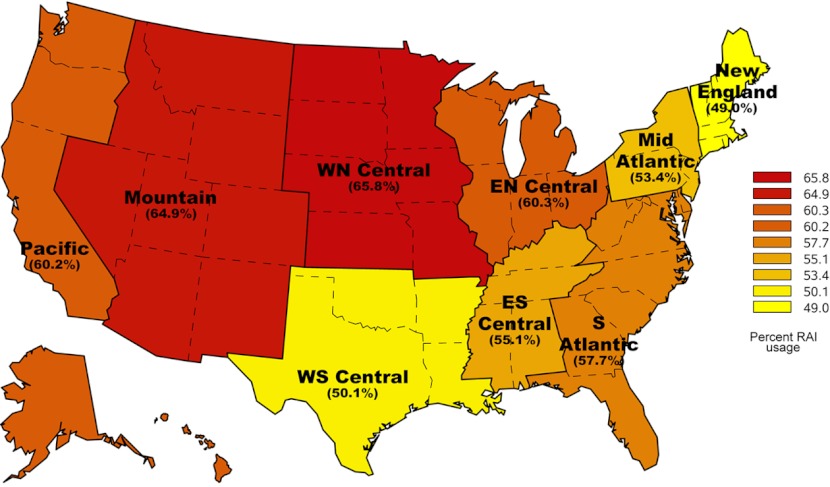
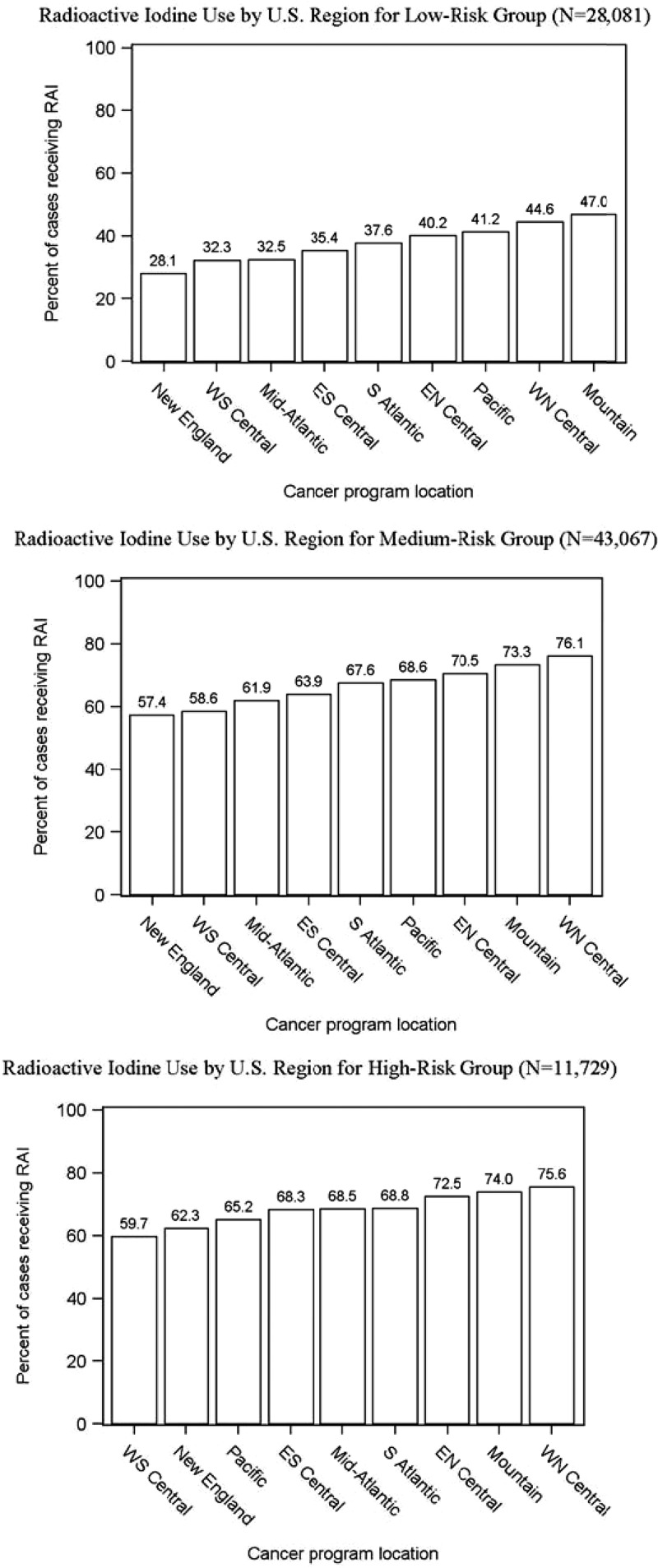
Figure 1 demonstrates the large regional variation in RAI use for thyroid cancer with the New England region treating 49% of patients and the West North Central region treating 66% of patients. As shown in Fig. 2, although the proportion of patients treated varies by disease severity category, regional variation is present in all 3 risk groups. The proportion of patients receiving RAI ranged from 28% to 47% of low-risk patients, 57% to 76% of medium-risk patients, and 60% to 76% of high-risk patients.
Figure 1.
Use of RAI by region of the United States.
Figure 2.
RAI use by region within low-, medium-, and high-risk patients.
Low-risk thyroid cancer
Table 1 shows the correlates of RAI use in low-risk patients. There are significant differences in RAI use based on the region in which treatment is received. Relative to the West North Central region, New England is significantly less likely to treat low-risk patients with RAI [OR 0.36 (95% CI 0.25–0.53)] after controlling for hospital case volume, patient sex, patient age, comorbidity, race, insurance, rural-urban continuum, tumor histology, and lymph node involvement. The low-volume hospitals were slightly less likely to treat low-risk patients with RAI than the high-volume hospitals. Nonclinical patient correlates of less RAI use in low-risk patients included: age 60 years or older, 1 or more comorbidities, black race, Medicaid/uninsured, and Medicare insurance. Papillary thyroid cancer patients were less likely to receive RAI than follicular/Hurthle cell patients. Those without lymph node metastases or with unknown metastases were less likely to receive RAI than those with lymph node metastases.
Table 1.
RAI Use for Low-Risk Thyroid Cancer
| Patients, n, % |
OR (95% CI) |
|||
|---|---|---|---|---|
| Overall | Treated With RAI | Unadjusted | Adjusted | |
| Hospital characteristics | ||||
| Hospital region | ||||
| EN Central | 4519 (16.1) | 1816 (40.2) | 0.75 (0.56–1.00) | 0.75 (0.55–1.01) |
| ES Central | 1688 (6.0) | 597 (35.4) | 0.60 (0.42–0.85) | 0.64 (0.44–0.92) |
| Mid-Atlantic | 4807 (17.1) | 1562 (32.5) | 0.47 (0.35–0.64) | 0.48 (0.35–0.66) |
| Mountain | 1930 (6.9) | 907 (47.0) | 1.13 (0.78–1.63) | 1.02 (0.69–1.51) |
| New England | 1798 (6.4) | 505 (28.1) | 0.39 (0.27–0.55) | 0.36 (0.25–0.53) |
| Pacific | 3345 (11.9) | 1379 (41.2) | 0.84 (0.62–1.14) | 0.75 (0.55–1.03) |
| S Atlantic | 5597 (19.9) | 2106 (37.6) | 0.66 (0.49–0.88) | 0.68 (0.50–0.91) |
| WS Central | 2267 (8.1) | 733 (32.3) | 0.58 (0.42–0.81) | 0.58 (0.41–0.83) |
| WN Central | 2130 (7.6) | 950 (44.6) | 1.0 (Reference) | 1.0 (Reference) |
| Case volume (cases/y) | ||||
| Low (≤11) | 2480 (8.8) | 801 (32.3) | 0.77 (0.63–0.93) | 0.81 (0.66–0.98) |
| Medium (12–34) | 9423 (33.6) | 3679 (39.0) | 1.03 (0.87–1.21) | 1.04 (0.87–1.23) |
| High (≥35) | 16 178 (57.6) | 6075 (37.6) | 1.0 (Reference) | 1.0 (Reference) |
| Patient characteristics | ||||
| Sex | ||||
| Female | 23 392 (83.3) | 8703 (37.2) | 0.89 (0.83–0.95) | 0.94 (0.87–1.02) |
| Male | 4689 (16.7) | 1852 (39.5) | 1.0 (Reference) | 1.0 (Reference) |
| Age (y) | ||||
| <20 | 259 (0.9) | 113 (43.6) | 1.84 (1.41–2.41) | 1.06 (0.78–1.43) |
| 20–44 | 9982 (35.5) | 4361 (43.7) | 1.85 (1.72–1.98) | 1.25 (1.14–1.37) |
| 45–59 | 10 841 (38.6) | 3960 (36.5) | 1.34 (1.25–1.43) | 1.17 (1.07–1.28) |
| ≥60 | 6999 (24.9) | 2121 (30.3) | 1.0 (Reference) | 1.0 (Reference) |
| Charlson-Deyo comorbidity index score | ||||
| 0 | 23 855 (85.0) | 9156 (38.4) | 1.31 (1.22–1.42) | 1.13 (1.04–1.23) |
| ≥1 | 4226 (15.0) | 1399 (33.1) | 1.0 (Reference) | 1.0 (Reference) |
| Race/ethnicity | ||||
| Black | 1827 (6.5) | 510 (27.9) | 0.61 (0.55–0.69) | 0.66 (0.58–0.75) |
| Other | 3561 (12.7) | 1412 (39.7) | 1.08 (0.99–1.17) | 1.00 (0.91–1.10) |
| White | 22 693 (80.8) | 8633 (38.0) | 1.0 (Reference) | 1.0 (Reference) |
| Insurance | ||||
| Insurance, NOS | 4302 (15.6) | 1661 (38.6) | 0.97 (0.89–1.05) | 0.96 (0.88–1.05) |
| Medicaid/uninsured | 1586 (5.7) | 556 (35.1) | 0.81 (0.72–0.91) | 0.78 (0.69–0.89) |
| Medicare | 4614 (16.7) | 1325 (28.7) | 0.60 (0.55–0.64) | 0.74 (0.67–0.82) |
| Private/government | 17 125 (62.0) | 6898 (40.3) | 1.0 (Reference) | 1.0 (Reference) |
| Rural-urban continuum | ||||
| Metro population | 22 248 (85.9) | 8363 (37.6) | 1.05 (0.96–1.15) | 1.04 (0.95–1.14) |
| Other | 3663 (14.1) | 1377 (37.6) | 1.0 (Reference) | 1.0 (Reference) |
| Tumor characteristics | ||||
| Tumor histology | ||||
| Papillary | 27 614 (98.3) | 10 313 (37.3) | 0.51 (0.42–0.62) | 0.47 (0.38–0.58) |
| Follicular/Hurthle cell | 467 (1.7) | 242 (51.8) | 1.0 (Reference) | 1.0 (Reference) |
| Lymph node involvement | ||||
| NX | 195 (0.7) | 86 (44.1) | 0.32 (0.23–0.46) | 0.39 (0.27–0.58) |
| N0 | 25 763 (91.7) | 8949 (34.7) | 0.17 (0.15–0.19) | 0.19 (0.17–0.21) |
| N1 | 2123 (7.6) | 1520 (71.6) | 1.0 (Reference) | 1.0 (Reference) |
Abbreviations: EN, East North; ES, East South; NOS, not otherwise specified; S, south; WN, West North; WS, West South.
Medium-risk thyroid cancer
Table 2 shows correlates of RAI use in medium-risk patients. Although a larger proportion of patients with medium-risk disease receive RAI, similar to low-risk patients, region, low hospital case volume, Medicaid/uninsured, Medicare insurance, and absence of lymph node metastases were associated with less RAI use. In the adjusted model, there was a significant difference in RAI use based on region, with the greatest difference seen between the mid-Atlantic and West North Central regions [OR 0.23 (95% CI 0.16–0.34)]. Similar to the other risk categories, RAI use in New England was still low.
Table 2.
RAI Use for Medium-Risk Thyroid Cancer
| Patients, n, % |
OR (95% CI) |
|||
|---|---|---|---|---|
| Overall | Treated With RAI | Unadjusted | Adjusted | |
| Hospital characteristics | ||||
| Hospital region | ||||
| EN Central | 6845 (15.9) | 4826 (70.5) | 0.52 (0.36–0.75) | 0.55 (0.38–0.80) |
| ES Central | 2672 (6.2) | 1707 (63.9) | 0.44 (0.28–0.70) | 0.46 (0.29–0.72) |
| Mid-Atlantic | 7250 (16.8) | 4489 (61.9) | 0.24 (0.16–0.36) | 0.23 (0.16–0.34) |
| Mountain | 2811 (6.5) | 2060 (73.3) | 0.85 (0.51–1.40) | 0.73 (0.44–1.19) |
| New England | 2958 (6.9) | 1699 (57.4) | 0.23 (0.15–0.36) | 0.24 (0.15–0.38) |
| Pacific | 5971 (13.9) | 4094 (68.6) | 0.55 (0.37–0.82) | 0.50 (0.34–0.74) |
| S Atlantic | 8040 (18.7) | 5438 (67.6) | 0.44 (0.30–0.64) | 0.43 (0.30–0.63) |
| WS Central | 3125 (7.3) | 1831 (58.6) | 0.35 (0.23–0.54) | 0.33 (0.22–0.51) |
| WN Central | 3395 (7.9) | 2585 (76.1) | 1.0 (Reference) | 1.0 (Reference) |
| Case volume (cases/y) | ||||
| Low (≤11) | 4460 (10.4) | 2438 (54.7) | 0.46 (0.37–0.59) | 0.49 (0.39–0.62) |
| Medium (12–34) | 14 980 (34.8) | 10 007 (66.8) | 0.87 (0.69–1.10) | 0.85 (0.68–1.07) |
| High (≥35) | 23 627 (54.9) | 16 284 (68.9) | 1.0 (Reference) | 1.0 (Reference) |
| Patient characteristics | ||||
| Sex | ||||
| Female | 33 541 (77.9) | 22 288 (66.5) | 0.95 (0.90–1.01) | 0.96 (0.91–1.02) |
| Male | 9526 (22.1) | 6441 (67.6) | 1.0 (Reference) | 1.0 (Reference) |
| Age (y) | ||||
| <20 | 1144 (2.7) | 822 (71.9) | 1.50 (1.29–1.75) | 1.10 (0.92–1.31) |
| 20–44 | 21 553 (50.0) | 14 697 (68.2) | 1.30 (1.22–1.38) | 1.05 (0.96–1.14) |
| 45–59 | 12 046 (28.0) | 7938 (65.9) | 1.17 (1.09–1.25) | 1.08 (0.99–1.18) |
| ≥60 | 8324 (19.3) | 5272 (63.3) | 1.0 (Reference) | 1.0 (Reference) |
| Charlson-Deyo comorbidity index score | ||||
| 0 | 37 853 (87.9) | 25 324 (66.9) | 1.13 (1.06–1.22) | 1.08 (1.00–1.16) |
| ≥1 | 5214 (12.1) | 3405 (65.3) | 1.0 (Reference) | 1.0 (Reference) |
| Race/ethnicity | ||||
| Black | 2787 (6.5) | 1748 (62.7) | 0.92 (0.83–1.01) | 1.01 (0.91–1.11) |
| Other | 6994 (16.2) | 4547 (65.0) | 1.02 (0.95–1.10) | 0.99 (0.92–1.07) |
| White | 33 286 (77.3) | 22 434 (67.4) | 1 (Reference) | 1 (Reference) |
| Insurance | ||||
| Insurance, NOS | 6957 (16.4) | 4611 (66.3) | 0.94 (0.87–1.01) | 0.93 (0.87–1.01) |
| Medicaid/uninsured | 3354 (7.9) | 2124 (63.3) | 0.80 (0.73–0.88) | 0.80 (0.73–0.88) |
| Medicare | 5845 (13.8) | 3632 (62.1) | 0.73 (0.69–0.79) | 0.84 (0.76–0.92) |
| Private/government | 26 157 (61.8) | 17 990 (68.8) | 1 (Reference) | 1 (Reference) |
| Rural-urban continuum | ||||
| Metro population | 34 081 (86.0) | 22 614 (66.4) | 1.08 (1.00–1.17) | 1.06 (0.98–1.16) |
| Other | 5528 (14.0) | 3770 (68.2) | 1 (Reference) | 1 (Reference) |
| Tumor characteristics | ||||
| Tumor histology | ||||
| Papillary | 38 358 (89.1) | 25 681 (67.0) | 1.18 (1.10–1.27) | 1.07 (0.99–1.16) |
| Follicular/Hurthle cell | 4709 (10.9) | 3048 (64.7) | 1 (Reference) | 1 (Reference) |
| Lymph node involvement | ||||
| NX | 355 (0.8) | 252 (71.0) | 1.08 (0.81–1.45) | 1.13 (0.83–1.55) |
| N0 | 34 687 (80.5) | 22 521 (64.9) | 0.58 (0.54–0.61) | 0.59 (0.55–0.63) |
| N1 | 8025 (18.6) | 5956 (74.2) | 1 (Reference) | 1 (Reference) |
Abbreviations: EN, East North; ES, East South; NOS, not otherwise specified; S, south; WN, West North; WS, West South.
High-risk thyroid cancer
Table 3 shows the factors that correlate with RAI use in high-risk patients. Similar to other risk categories, region and low hospital case volume still correlate with less RAI use. The maximum regional difference is between the New England and West North Central regions [OR 0.30 (95% CI 0.19–0.49)]. Nonclinical patient correlates of less use for high-risk patients include age 60 years or older and Medicare insurance. Both age and insurance status were independent predictors of RAI use. In contrast to low-risk disease, patients with papillary thyroid cancer are more likely to receive RAI than those with follicular/Hurthle cell cancer. Similar to low- and medium-risk patients, not having any lymph node metastases is associated with less RAI use.
Table 3.
RAI Use for High-Risk Thyroid Cancer
| Patients, n, % |
OR (95% CI) |
|||
|---|---|---|---|---|
| Overall | Treated With RAI | Unadjusted | Adjusted | |
| Hospital characteristics | ||||
| Hospital region | ||||
| EN Central | 1774 (15.1) | 1287 (72.5) | 0.57 (0.38–0.84) | 0.60 (0.41–0.89) |
| ES Central | 624 (5.3) | 426 (68.3) | 0.47 (0.29–0.76) | 0.48 (0.30–0.77) |
| Mid-Atlantic | 2057 (17.5) | 1410 (68.5) | 0.31 (0.20–0.46) | 0.31 (0.21–0.47) |
| Mountain | 669 (5.7) | 495 (74.0) | 0.78 (0.47–1.30) | 0.70 (0.43–1.17) |
| New England | 816 (7.0) | 508 (62.3) | 0.27 (0.17–0.44) | 0.30 (0.19–0.49) |
| Pacific | 1738 (14.8) | 1133 (65.2) | 0.45 (0.30–0.68) | 0.43 (0.29–0.64) |
| S Atlantic | 2154 (18.4) | 1481 (68.8) | 0.43 (0.29–0.63) | 0.42 (0.28–0.62) |
| WS Central | 861 (7.3) | 514 (59.7) | 0.30 (0.19–0.46) | 0.31 (0.20–0.48) |
| WN Central | 1036 (8.8) | 783 (75.6) | 1.0 (Reference) | 1.0 (Reference) |
| Case volume, cases/y | ||||
| Low (≤11) | 1266 (10.8) | 740 (58.5) | 0.48 (0.38–0.61) | 0.53 (0.41–0.67) |
| Medium (12–34) | 3916 (33.4) | 2586 (66.0) | 0.74 (0.60–0.92) | 0.77 (0.62–0.96) |
| High (≥35) | 6547 (55.8) | 4711 (72.0) | 1.0 (Reference) | 1.0 (Reference) |
| Patient characteristics | ||||
| Sex | ||||
| Female | 6995 (59.6) | 4790 (68.5) | 1.05 (0.96–1.15) | 1.06 (0.97–1.17) |
| Male | 4734 (40.4) | 3247 (68.6) | 1.0 (Reference) | 1.0 (Reference) |
| Age, y | ||||
| 45–59 | 6497 (55.4) | 4658 (71.7) | 1.45 (1.33–1.59) | 1.22 (1.09–1.38) |
| ≥60 | 5232 (44.6) | 3379 (64.6) | 1.0 (Reference) | 1.0 (Reference) |
| Charlson-Deyo comorbidity index score | ||||
| 0 | 9434 (80.4) | 6504 (68.9) | 1.17 (1.05–1.31) | 1.06 (0.94–1.19) |
| ≥1 | 2295 (19.6) | 1533 (66.8) | 1.0 (Reference) | 1.0 (Reference) |
| Race/ethnicity | ||||
| Black | 713 (6.1) | 431 (60.4) | 0.79 (0.66–0.96) | 0.92 (0.76–1.12) |
| Other | 1773 (15.1) | 1146 (64.6) | 0.98 (0.86–1.12) | 0.96 (0.83–1.12) |
| White | 9243 (78.8) | 6460 (69.9) | 1.0 (Reference) | 1.0 (Reference) |
| Insurance | ||||
| Insurance, NOS | 1452 (12.6) | 1011 (69.6) | 0.87 (0.74–1.01) | 0.86 (0.73–1.00) |
| Medicaid/uninsured | 731 (6.4) | 471 (64.4) | 0.82 (0.67–0.99) | 0.86 (0.70–1.06) |
| Medicare | 3498 (30.4) | 2214 (63.3) | 0.63 (0.57–0.70) | 0.76 (0.67–0.87) |
| Private/government | 5822 (50.6) | 4235 (72.7) | 1.0 (Reference) | 1 (Reference) |
| Rural-urban continuum | ||||
| Metro population | 9114 (83.8) | 6198 (68.0) | 0.94 (0.81–1.08) | 0.94 (0.81–1.09) |
| Other | 1758 (16.2) | 1250 (71.1) | 1.0 (Reference) | 1.0 (Reference) |
| Tumor characteristics | ||||
| Tumor histology | ||||
| Papillary | 10 001 (85.3) | 6992 (69.9) | 1.60 (1.42–1.80) | 1.21 (1.04–1.40) |
| Follicular/Hurthle cell | 1728 (14.7) | 1045 (60.5) | 1.0 (Reference) | 1.0 (Reference) |
| Lymph node involvement | ||||
| NX | 58 (0.5) | 39 (67.2) | 0.94 (0.50–1.77) | 1.13 (0.56–2.28) |
| N0 | 3749 (32.0) | 2352 (62.7) | 0.65 (0.59–0.71) | 0.78 (0.70–0.88) |
| N1 | 7922 (67.5) | 5646 (71.3) | 1.0 (Reference) | 1.0 (Reference) |
| Distant metastases | ||||
| M0 | 11 283 (96.2) | 7769 (68.9) | 1.42 (1.14–1.77) | 1.18 (0.93–1.49) |
| M1 | 446 (3.8) | 268 (60.1) | 1.0 (Reference) | 1.0 (Reference) |
Abbreviations: EN, East North; ES, East South; M1, distant metastases present; M0, distant metastases absent; NOS, not otherwise specified; S, south; WN, West North; WS, West South.
Discussion
The proportion of patients across US regions receiving RAI was similar in medium- and high-risk patients. Regional treatment variation was seen in all risk categories, with a greater number of nonclinical correlates of RAI use in low-risk patients compared with medium- and high-risk patients.
It is known that some providers prefer more intensive management of thyroid cancer (17–19), and a previous survey study suggests that these treatment preferences differ by region (20). These physician-driven differences in treatment intensity may explain the regional variation seen in the management of low- and medium-risk disease. The persistence of this regional variation in high-risk patients was less expected. One contributing factor to regional variation in high-risk patients may be differences in provider beliefs regarding prophylactic central lymph node dissections and pretreatment scans, which can influence the perceived indications for RAI (21). For example, prophylactic central lymph node dissection can reveal occult lymph node metastases, which would subsequently upstage as many as one third of patients over age 45 years to AJCC stage III (22, 23). Because some regions may be less likely to perform prophylactic central lymph node dissections, they may have fewer patients with occult micrometastases in their stage III cohort, thus potentially influencing the proportion of patients treated with RAI. In addition, assuming no extrathyroidal extension, some providers would assume surgical cure after prophylactic lymph node dissection, whereas others would treat with RAI (22, 24, 25). In addition to differences in physician treatment intensity and variable use of interventions that expose more disease, there is a large body of work in medicine correlating regional variation in use to health care spending, with greater use seen in high-spending regions (26, 27). It is possible that similar to other diseases, health care spending correlates with thyroid cancer treatment intensity.
The medium-risk thyroid cancer cohort is very heterogeneous because it can include patients under age 45 years with distant metastases and patients over the age of 45 years with 1.1–2 cm unifocal, intrathyroidal tumors. In contrast, the high-risk cohort is more homogeneous. Assuming iodine avidity, RAI would typically be recommended for the high-risk cohort (9). Yet, as shown in Fig. 2, the proportion receiving RAI across regions is very similar in the medium and high-risk cohorts. This suggests a lower threshold for RAI administration than what is advocated in the most recent guidelines, with a tendency for more aggressive management when treatment choices are left to provider discretion.
The clinical correlates of use we identified were consistent with our expectations. For example, it is probable that there is less RAI use for low-risk papillary thyroid cancers vs low-risk follicular/Hurthle cell cancers because these small, potentially indolent papillary thyroid cancers are often incidental discoveries in postoperative pathology. These occult papillary thyroid cancers are seen in 4%–10% of surgical pathology specimens when surgery is performed for benign thyroid disease (28, 29), and because close to one third of people have occult papillary thyroid cancers on thorough autopsy (30), there may be no clinical consequence to their discovery (31). In contrast, we found that for high-risk patients, those with papillary thyroid cancer are more likely to receive RAI than those with follicular/Hurthle cell cancer. Hurthle cell cancers are known to be less iodine avid than papillary cancer, thus potentially partially explaining this finding (32). Similarly, it is not surprising that the presence of lymph node metastases is associated with greater RAI use in all risk categories because many physicians use lymph node status as an indication for RAI (21, 22). There are 2 reasons that RAI use may not differ in patients with distant metastases vs those without. One possibility is that the threshold for RAI use is lower (ie, the threshold is based on the tumor size and/or the presence or absence of lymph node metastases). The other possible explanation is that more patients with distant metastases have noniodine-avid tumors.
In contrast to the predictability of the clinical correlates, many of the nonclinical correlates of RAI use we identified were less expected. Although the relationship between low hospital case volume and less RAI has been demonstrated in previous work (1), it is not clear why patients with Medicare insurance are less likely to receive RAI, regardless of disease severity. One possibility is that older patients are not being referred to high-volume facilities at the same rate as younger patients (33), thus also explaining the relationship between age 60 years and older and less RAI use in both low- and high-risk patients. Alternatively, because patient preference cannot be assessed with this data set, it is possible that for low-risk disease in particular, patient preference may be different in younger vs older patients. Finally, it is possible that there is age disparity in physician treatment recommendations. Sosa and colleagues (34, 35) previously documented both age and race disparities in the management of thyroid cancer. In our data set, the relationship between both black race and Medicaid/uninsured status with less RAI use may suggest health disparity; however, the fact that this difference is not seen in the highest-risk patients is somewhat reassuring.
The greater number of nonclinical correlates of RAI use we identified for low- vs high-risk patients is supported by previous research showing that nonclinical correlates of use are more likely when there is discretionary decision making (36). The reliance on discretionary decision making in thyroid cancer guidelines is related to the fact that compared with other malignancies, such as breast cancer, there are fewer concrete data to support recommendations. It is well known that when clinical guidelines are not supported by strong evidence, physicians are less likely to provide guideline-concordant care (37), and thus, one would expect more treatment variation and potentially differences in use based on nonclinical factors such as age, race, and insurance status. Although RAI has been recommended for high-risk, iodine-avid patients for many years, the pendulum has only recently swung toward less RAI use in the lowest-risk patients (9, 38). However, interestingly, despite being a cohort in which even the most recent clinical guidelines still leave use of RAI to physician discretion in a large proportion of patients (9), the number of nonclinical correlates of use for the medium-risk cohort was similar and even lower than that of high-risk patients.
Strengths of this study include the large cohort of recently treated thyroid cancer patients, the exhaustive set of variables included in the analyses, and the representation of patients treated across the United States. Limitations include a lack of detail on use of interventions that can change perceived indications for RAI, such as pretreatment scans and prophylactic central lymph node dissections, and a lack of pathological details that may influence physician decision making, such as micro- vs macrolymph node metastases and information on extrathyroidal extension. In addition, we cannot distinguish the role of physician vs patient preference.
Previously a rare cancer and still a cancer with a low mortality rate, it is no surprise that there are currently no randomized controlled trials evaluating patient outcome with and without RAI. Secondary to the lack of randomized controlled trials and conflicting observational studies (39–41), the clinical guidelines leave much of thyroid cancer management to provider discretion (9). However, because thyroid cancer is becoming an increasingly common cancer (42), there is now a need now to reevaluate the risks and benefits of RAI and the impact of current practice patterns on population health. Understanding the current correlates of RAI use is critical to recognize areas of controversy in thyroid cancer management and improve patient care. In the future, it is important that disease severity and extent of treatment benefit, not region, age, race, or insurance status, drive the use of RAI for thyroid cancer.
Acknowledgments
This work was supported by the National Cancer Institute at the National Institutes of Health (Grant K07CA154595-02, to M.R.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AJCC
- American Joint Committee on Cancer
- CI
- confidence interval
- N0
- no lymph node metastases
- N1
- lymph node metastases
- NX
- unknown lymph node status
- OR
- odds ratio
- RAI
- radioactive iodine.
References
- 1. Haymart MR, Banerjee M, Stewart AK, Koenig RJ, Birkmeyer JD, Griggs JJ. Use of radioactive iodine for thyroid cancer. JAMA. 2011;306:721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117:4439–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Solans R, Bosch JA, Galofre P, et al. Salivary and lacrimal gland dysfunction (Sicca syndrome) after radioiodine therapy. J Nucl Med. 2001;42:738–743 [PubMed] [Google Scholar]
- 4. Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93:504–515 [DOI] [PubMed] [Google Scholar]
- 5. Pace-Asciak PZ, Payne RJ, Eski SJ, Walfish P, Damani M, Freeman JL. Cost savings of patients with a MACIS score lower than 6 when radioactive iodine is not given. Arch Otolaryngol Head Neck Surg. 2007;133:870–873 [DOI] [PubMed] [Google Scholar]
- 6. Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488–490 [DOI] [PubMed] [Google Scholar]
- 8. Phillips JK, Stewart AK, ed. Facility Oncology Registry Data Standards. Chicago: Commission on Cancer; 2011 [Google Scholar]
- 9. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 10. Smedley BD, Stith AY, Nelson AR, ed. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: The National Academies Press; 2003 [PubMed] [Google Scholar]
- 11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 12. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619 [DOI] [PubMed] [Google Scholar]
- 13. Greene FL, ed. AJCC Cancer Staging Manual. 6th ed New York: Springer; 2002 [Google Scholar]
- 14. Fritz A, Jack A, Parkin DM, et al. ICD-O: International Classification of Diseases for Oncology. 3rd ed Geneva, Switzerland: World Health Organization; 2000 [Google Scholar]
- 15. Snijders TAB, Bosker RJ. Multilevel Analysis. Thousand Oaks, CA: Sage; 1999 [Google Scholar]
- 16. Subramanian S, Jones K, Duncan C. Multilevel Methods for Public Health Research. New York, NY: Oxford University Press; 2003 [Google Scholar]
- 17. Haymart MR, Banerjee M, Yang D, et al. The relationship between extent of thyroid cancer surgery and use of radioactive iodine [published online ahead of print September 20, 2012]. Ann Surg. doi:10.1097/SLA.0b013e31826c8915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haymart MR, Banerjee M, Yang D, Stewart AK, Koenig RJ, Griggs JJ. The role of clinicians in determining radioactive iodine use for low-risk thyroid cancer [published online ahead of print June 28, 2012]. Cancer. doi: 10.1002/cncr. 27721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solomon BL, Wartofsky L, Burman KD. Current trends in the management of well differentiated papillary thyroid carcinoma. J Clin Endocrinol Metab. 1996;81:333–339 [DOI] [PubMed] [Google Scholar]
- 20. Sawka AM, Rotstein L, Brierley JD, et al. Regional differences in opinions on adjuvant radioactive iodine treatment of thyroid carcinoma within Canada and the United States. Thyroid. 2007;17:1235–1242 [DOI] [PubMed] [Google Scholar]
- 21. Bonnet S, Hartl D, Leboulleux S, et al. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implications for radioiodine treatment. J Clin Endocrinol Metab. 2009;94:1162–1167 [DOI] [PubMed] [Google Scholar]
- 22. Hughes DT, White ML, Miller BS, Gauger PG, Burney RE, Doherty GM. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery. 2010;148:1100–1106; discussion 1106–1107 [DOI] [PubMed] [Google Scholar]
- 23. Teixeira G, Teixeira T, Gubert F, Chikota H, Tufano R. The incidence of central neck micrometastatic disease in patients with papillary thyroid cancer staged preoperatively and intraoperatively as N0. Surgery. 2011;150:1161–1167 [DOI] [PubMed] [Google Scholar]
- 24. Cooper DS, Tufano RP. Prophylactic central neck dissection in differentiated thyroid cancer: a procedure in search of an indication. Thyroid. 2012;22:341–343 [DOI] [PubMed] [Google Scholar]
- 25. Moreno MA, Edeiken-Monroe BS, Siegel ER, Sherman SI, Clayman GL. In papillary thyroid cancer, preoperative central neck ultrasound detects only macroscopic surgical disease, but negative findings predict excellent long-term regional control and survival. Thyroid. 2012;22:347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–287 [DOI] [PubMed] [Google Scholar]
- 27. Sirovich BE, Gottlieb DJ, Welch HG, Fisher ES. Regional variations in health care intensity and physician perceptions of quality of care. Ann Intern Med. 2006;144:641–649 [DOI] [PubMed] [Google Scholar]
- 28. Roti E, Rossi R, Trasforini G, et al. Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab. 2006;91:2171–2178 [DOI] [PubMed] [Google Scholar]
- 29. Miccoli P, Minuto MN, Galleri D, et al. Incidental thyroid carcinoma in a large series of consecutive patients operated on for benign thyroid disease. ANZ J Surg. 2006;76:123–126 [DOI] [PubMed] [Google Scholar]
- 30. Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 1985;56:531–538 [DOI] [PubMed] [Google Scholar]
- 31. Neuhold N, Schultheis A, Hermann M, Krotla G, Koperek O, Birner P. Incidental papillary microcarcinoma of the thyroid—further evidence of a very low malignant potential: a retrospective clinicopathological study with up to 30 years of follow-up. Ann Surg Oncol. 2011;18:3430–3436 [DOI] [PubMed] [Google Scholar]
- 32. Cooper DS, Schneyer CR. Follicular and Hurthle cell carcinoma of the thyroid. Endocrinol Metab Clin North Am. 1990;19:577–591 [PubMed] [Google Scholar]
- 33. Machens A, Dralle H. Age disparities in referrals to specialist surgical care for papillary thyroid cancer. Eur J Surg Oncol. 2009;35:1312–1317 [DOI] [PubMed] [Google Scholar]
- 34. Park HS, Roman SA, Sosa JA. Treatment patterns of aging Americans with differentiated thyroid cancer. Cancer. 2010;116:20–30 [DOI] [PubMed] [Google Scholar]
- 35. Sosa JA, Mehta PJ, Wang TS, Yeo HL, Roman SA. Racial disparities in clinical and economic outcomes from thyroidectomy. Ann Surg. 2007;246:1083–1091 [DOI] [PubMed] [Google Scholar]
- 36. Griggs JJ, Sorbero ME, Ahrendt GM, et al. The pen and the scalpel: effect of diffusion of information on nonclinical variations in surgical treatment. Med Care. 2009;47:749–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Greenberg CC, Lipsitz SR, Neville B, et al. Receipt of appropriate surgical care for Medicare beneficiaries with cancer. Arch Surg. 2011;146:1128–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142 [DOI] [PubMed] [Google Scholar]
- 39. Schvartz C, Bonnetain F, Dabakuyo S, et al. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J Clin Endocrinol Metab. 2012;97:1526–1535 [DOI] [PubMed] [Google Scholar]
- 40. Jonklaas J, Cooper DS, Ain KB, et al. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 2010;20:1423–1424 [DOI] [PubMed] [Google Scholar]
- 41. Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992;75:714–720 [DOI] [PubMed] [Google Scholar]
- 42. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–2167 [DOI] [PubMed] [Google Scholar]