Abstract
Background
Biased causal attribution is a critical factor in the cognitive model of depression. Whereas depressed patients interpret events negatively, healthy people show a self-serving bias (internal attribution of positive events and external attribution of negative events).
Methods
Using fMRI, depressed patients (n=15) and healthy controls (n=15) were confronted with positive and negative social events and made causal attributions (internal vs. external). Functional data were analyzed using a mixed effects model.
Results
Behaviourally, controls showed a self-serving bias, whereas patients demonstrated a balanced attributional pattern. Analysis of functional data revealed a significant group difference in a fronto-temporal network. Higher activation of this network was associated with non self-serving attributions in controls but self-serving attributions in patients. Applying a psycho-physiological interaction analysis, we observed reduced coupling between a dorsomedial PFC seed region and limbic areas during self-serving attributions in patients compared to controls.
Limitations
Results of the PPI analysis are preliminary given the liberal statistical threshold.
Conclusions
The association of the behaviourally less frequent attributional pattern with activation in a fronto-temporal network suggests that non self-serving responses may produce a self-related response conflict in controls, while self-serving responses produce this conflict in patients. Moreover, attribution-modulated coupling between the dorsomedial PFC and limbic regions was weaker in patients than controls. This preliminary finding suggests that depression may be associated with disturbances in fronto-limbic coupling during attributional decisions. Our results implicate that treatment of major depression may benefit from approaches that facilitate reinterpretation of emotional events in a more positive, more self-serving way.
Keywords: locus of control, emotions, emotional disturbances, affective disorders, reappraisal, cognitive control
Introduction
Beck’s cognitive model of depression (Beck, 2008) postulates that negatively biased information processing is critical in the pathogenesis and maintenance of depressive symptoms. A critical element in this model is a change in causal attribution, i.e., the inference one draws regarding the cause of an event. One can feel responsible (internal attribution) for an event or one can attribute the cause of an event to other people or the situation (external attribution). Healthy people display a so-called “self-serving” bias: they feel responsible for positive events, but assign external causes to negative events (Snyder and Uranowitz, 1978). Although this self-serving bias often may not reflect reality accurately, it is thought to serve a psychologically protective function (Chandler et al., 1997). In contrast, depressed patients show a so-called “depressive realism” (Moritz et al., 2007) or a “non self-serving” bias (Diez-Alegria et al., 2006). The non self-serving bias is characterized by internal attribution of negative events in combination with external attribution of positive events. Despite the critical role this attributional bias is thought to play in major depression, the neural correlates of this cognitive process have not been examined.
In a previous fMRI study (Seidel et al., 2010) healthy subjects viewed short sentences describing positive and negative social events. They were asked to imagine the event happening, decide the main cause and assign it to one of two categories: themselves (internal) or another person/situation (external). Here, the self-serving tendency was associated with activation in the dorsal part of the striatum, suggesting that self-serving attributions have a rewarding effect (e.g., Hikosaka et al., 2008).
Based on these findings and behavioural studies in depression (Moritz et al., 2007; Diez-Alegria et al., 2006), in the current experiment we expected depressed patients to show less “self-serving” attributions on the behavioural level. On the brain level, we hypothesized that a reduced activity of the reward system in depressed patients (e.g., Pizzagalli et al., 2009) is associated with the frequency of “self-serving” attributional decisions in depressed patients. Since self-related processes are required by the task, we predicted, based on previous evidence (Lemogne et al., 2009; Grimm et al., 2009), that depressed patients will show altered neural responses in brain regions associated with self-processing, such as the medial PFC (Andrews-Hanna et al., 2010; Northoff et al., 2006).
Finally, we used an exploratory psycho-physiological interaction (PPI) analysis (Friston et al., 1997) to examine aberrant interactions among fronto-limbic brain regions. Here, we hypothesize fronto-limbic neural circuits to be altered in depressed patients (Johnstone et al., 2007; Sheline et al., 2009).
Methods and Materials
Participants
Fifteen depressed patients (9 females) meeting DSM-IV criteria for major depression and 15 healthy controls (9 females) matched for gender, age and education participated. All subjects were native German speakers and right-handed as assessed by the Edinburgh Inventory (Oldfield, 1971). The study was approved by the local Institutional Review Board and conducted according to the Declaration of Helsinki (1989). Written informed consent was obtained and all subjects were paid for participation (€30).
Patients were recruited from the inpatient units of the Department of Psychiatry and Psychotherapy, University Hospital Aachen. They had no substance abuse for the last six months and no co-morbid psychiatric (axis I or II) or neurological illness, as assessed by the German version of the Structured Clinical Interview for DSM (SCID; Wittchen et al., 1998). None of these patients experienced psychotic symptoms during the current or previous episodes. Severity of affective symptoms was assessed with the Beck Depression Inventory (mean=25.6, SD=6.05; BDI; Beck et al., 1961) and the 17-item Hamilton Depression Rating Scale (mean=19.87, SD=7.30; HAMD; Hamilton, 1960). The mean age of onset was 29.47 years (SD=10.81), with mean illness duration of 4.67 years (SD=7.12) and mean number of previous episodes 1.39 (SD=0.65). All but two of the depressed patients were taking psychotropic medication at the time of testing (SSRI [n=2], SNRI [n=5], SSRI+SNRI [n=4], SNRI+quetiapine [n=2]).
The non-psychiatric control group consisted of 15 healthy adults with no history of psychiatric or neurological illness (including head trauma with loss of consciousness) in themselves and in their first degree relatives. They were recruited via advertisements.
All participants completed a neuropsychological test battery assessing executive functions (TMT-A/B; Reitan, 1956), and working memory (digit span, WAIS III; Von Aster et al., 2006). To estimate the subjects’ self-esteem we applied the German version (Ferring and Filipp, 1996) of the Rosenberg Self-Esteem Scale (RSES; (Rosenberg, 1965). For demographic and neuropsychological data see Table 1.
Table 1.
Demographic and neuropsychological data of depressed patients (DP) and healthy controls (HC). Standard deviations are given in parentheses
| DP (n=15) | HC (n=15) | t-values | p-values | |
|---|---|---|---|---|
| Gender (M:F) | 6:9 | 6:9 | - | - |
| Age (range) | 34.13 (11.95) | 32.87 (10.93) | 0.303 | 0.764 |
| Years of education | 16.13 (3.72) | 17.00 (4.00) | 0.614 | 0.544 |
|
| ||||
| TMT-A (seconds) | 19.19 (3.76) | 20.61 (6.54) | 0.716 | 0.480 |
| TMT-B (seconds) | 40.05 (13.54) | 38.29 (11.28) | 0.370 | 0.714 |
| Digit span (raw score) | 15.60 (4.24) | 15.62 (4.52) | 0.009 | 0.993 |
|
| ||||
| Self-esteem | 12.20 (4.57) | 23.47 (3.60) | 7.498 | < 0.001 |
Task
The experimental paradigm has been validated and described in detail in our previous study (Seidel et al., 2010). Briefly, participants were presented with 80 sentences describing 40 positive (e.g., “A friend sent you a postcard.”) and 40 negative (e.g., “A friend ignored you.”) social events. Participants were asked to imagine the event happening to them and select the most likely cause with a button press: self (internal), another person / situation (external). In a response triggered design, these stimuli, together with the response categories, were presented maximally 10 seconds or until a response was given, which was on average after around 4 seconds. Each trial was followed by a jittered inter-stimulus interval (1.9 – 4.3s) of a fixation cross.
Behavioural data analysis
Behavioural statistical analyses were performed using SPSS 16.0 (Statistical Packages for the Social Sciences, Version 16.0, SPSS Inc., USA) with a level of significance of p=0.05. We performed 2 (diagnosis) × 2 (valence) × 2 (attribution) repeated measures ANOVAs on the percentage of self-serving and non self-serving attributions and on the reaction times (RT). Planned post-hoc group comparisons regarding attributional biases and reaction times (RT) were performed by between-group independent t-tests comparing both groups as well as paired-samples t-tests comparing percentages of self-serving and non self-serving attributions. Within-group correlation analyses between attributional data and BDI, HAM-D, and RSES were performed using the Pearson product-moment correlation coefficient.
Image acquisition and preprocessing
Functional MR images were acquired on a Siemens Trio 3 Tesla system (Erlangen, Germany) using a standard echoplanar sequence. fMRI data were quality controlled, preprocessed, and analysed using FEAT (fMRI Expert Analysis Tool) Version 5.9, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl) with standard fMRI settings (for details on image acquisition and pre-processing see supplementary methods).
Subject level analysis was carried out using FILM (FMRIB’s Improved General Linear Model). Analysis of functional data was dependent on the individual response given by a particular subject which determined the type of event. The general linear model (GLM) included four response-based event variables (external attribution of positive events, internal attribution of positive events, external attribution of negative events, internal attribution of negative events) that were convolved with a canonical hemodynamic response function. We have chosen the onset of the stimulus (sentence and response categories) as the onset time point and the RT as the duration of the event. Temporal derivatives and six rigid body movement parameters were included as nuisance variables.
Statistical image analysis
Whole-brain mixed effects analyses were performed with FLAME 2 (FMRIB’s local analysis of mixed effects). Subject level contrast maps were entered into group-level one and two-sample t-tests. As in our previous study (Seidel et al., 2010) the main contrast of interest compared self-serving vs. non self-serving attributions. This contrast was composed of: [positive situation, internal attribution + negative situation, external attribution] vs. [positive situation, external attribution + negative situation, internal attribution], and is equivalent to a within-subject valence-attribution interaction effect. We evaluated this bidirectional contrast on a within-group and between-group basis. For completeness, other evaluated contrasts included task (all conditions vs. resting baseline), valence (positive vs. negative) and attribution (internal vs. external) and are presented in the online supplement.
We calculated correlations between neural activation (“self-serving” / “non self-serving” contrasts) and symptom severity in depressed patients as well as self-esteem in the whole group by including mean centred rating scale / questionnaire values as additional covariates of interest in the same model.
Based on previous evidence (Johnstone et al., 2007; Ochsner and Gross, 2005) the dorsomedial PFC seems to be a critical node involved in the appraisal of emotional events and regulation of affective responses. In order to explore interactions between the dorsomedial PFC and other brain regions, we conducted a PPI analysis (Friston et al., 1997) examining differential connectivity during self-serving vs. non self-serving attributions. This seed was a 4mm radius sphere defined around a local maximum (−6, 38, 24) of activation across groups in the contrast of interest (non self-serving > self-serving). More details on the PPI analysis are given in the online supplement.
Our prior results (Seidel et al., 2010) indicated neural activation in the dorsal striatum associated with self-serving attributions. Based on previous evidence in depression (Epstein et al., 2006); (Pizzagalli et al., 2009) showing reduced reward-related responses in both the dorsal and ventral striatum, we decided to select the whole striatum as our a priori ROI. These two regions were anatomically defined using the Harvard-Oxford subcortical atlas at a threshold of 0.75 probability. Within these ROIs, we corrected for multiple comparisons using a small volume correction (p<0.05, FWE corrected; (Friston, 1997) to identify clusters of at least 5 contiguous voxels.
For whole-brain analyses we corrected for multiple comparisons using a Monte Carlo method with AFNI AlphaSim (R. W. Cox, National Institutes of Health) at a z threshold of 2.33 and a probability of spatial extent at a p value <0.05. For the PPI analysis, we decided to apply a secondary more liberal threshold of p<0.001 uncorrected to increase sensitivity. This more liberal threshold reduces risk of type two error, while increasing risk of type one error, therefore we interpreted the findings based on this threshold as tentative and preliminary. Identified clusters were then labelled according to anatomical regions using the Harvard-Oxford atlas. Coordinates are reported in MNI space. Images are presented using Mango (J.L. Lancaster and J. Martinez, San Antonio, Texas).
Results
Behavioural data
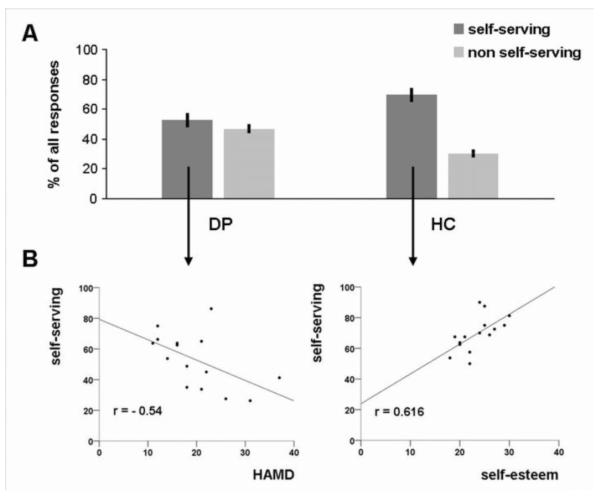
The repeated measures ANOVA of attributional decisions showed a significant diagnosis x valence x attribution interaction (F(1,28)=8.998, p=0.006). To disentangle this interaction, we performed planned post-hoc comparisons to examine group differences regarding attributional biases. This revealed that controls show significantly (t(28)=3.021, p=0.005) more self-serving attributions (mean=69.5%, SD=11.39) compared to depressed patients (mean=52.91%, SD=17.05). Directly comparing the percentage of self-serving vs. non self-serving responses within each group revealed that controls are more self-serving (t(14)=6.633, p<0.001) whereas patients show a balanced attributional style (t(14)=0.645, p=0.529). These effects are illustrated in Figure 1 A. The repeated measures ANOVA on the RT showed no significant main effect of diagnosis (F(1,28)=1.317, p=0.261) indicating both groups responded equally fast. Also all interactions with the factor diagnosis (diagnosis by valence, diagnosis by attribution) remained non-significant. (For ANOVA main effects and interactions without the diagnosis factor see the online supplement).
Figure 1.
Behavioural results: Panel A displays biased causal attribution in healthy controls (HC; N=15) and balanced causal attribution in depressed patients (DP; N=15). Panel B shows that the percentage of self-serving responses in healthy controls is associated with higher self-esteem and in patients is associated with lower depression severity (Hamilton Depression Rating Scale, HAMD).
In the within-group correlation analyses, we observed a positive correlation of self-serving attributions with self-esteem in controls (r=0.616, p=0.015, corr. p=0.03) but not patients (r=0.077, p=0.784). In patients, symptom severity on the HAMD was negatively correlated with the percentage of self-serving attributions (r=−0.54, p=0.038, corr. p=0.076). These correlations are illustrated in Figure 1 B. There were no significant correlations with RT data.
Imaging data
Self-serving vs. non self-serving
Analysing within and between group effects of the self-serving vs. non self-serving contrast did not reveal significant activation within our a priori ROI but did show significant effects in the whole-brain analysis (Table 2).
Table 2.
Results of within and between group (depressed patients=DP, healthy controls=HC) analyses of attributional biases, including MNI coordinates and z-values of the peak as well as the cluster size (k)
| Cluster | MNI | z-value | k | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| HC self-serving | - | - | - | - | - | - |
| HC non self-serving | L. dorsomedial PFC | −4 | 58 | 14 | 3.5 | 457 |
| R. ventrolateral PFC | 40 | 26 | −2 | 3.73 | 290 | |
| DP self-serving | L. middle cingulate | −6 | 16 | 40 | 3.74 | 239 |
| DP non self-serving | - | - | - | - | - | - |
| HC (non self-serving) vs. DP (self-serving) | ||||||
| L. temporal pole | −32 | 8 | −22 | 3.52 | 498 | |
| L. dorsomedial PFC | −2 | 54 | 16 | 3.42 | 341 | |
| R. ventrolateral PFC | 44 | 22 | −2 | 3.54 | 283 | |
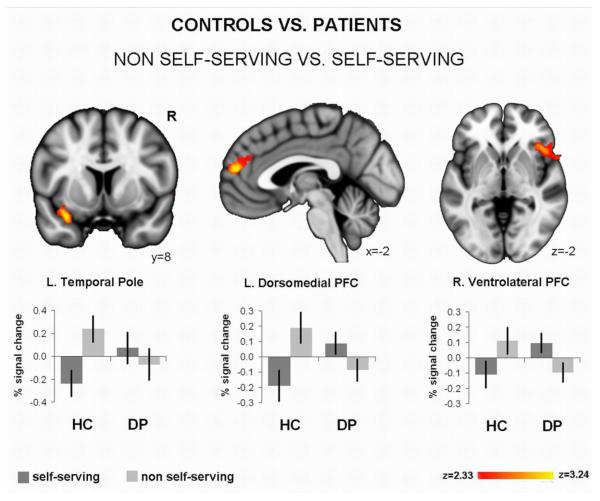
In healthy controls, we observed that neural activation in a left dorsomedial and a right ventrolateral PFC cluster is more associated with “non self-serving” than “self-serving” responses (see Table 2). The reverse t-contrast (“self-serving” > “non self-serving”) did not reveal any significant clusters. In depressed patients, however, neural activation in a middle cingulate cluster was stronger for “self-serving” than “non self-serving” responses (see Table 2). Here, the “non self-serving” > “self-serving” t-contrast did not reveal significant activation.
The group comparison regarding “self-serving” and “non self-serving” (i.e. the diagnosis × valence × attribution interaction) showed a significant interaction effect in the left temporal pole, the left dorsomedial and the right ventrolateral PFC (see Table 2). This interaction was driven by stronger activation for “non self-serving” judgments in healthy controls and “self-serving” ones in depressed patients. Figure 2 illustrates this interaction effect.
Figure 2.
Imaging results: Illustration of the group difference regarding non self-serving vs. self-serving attributions. The bar graphs (% signal change per condition) show that neural activation in a fronto-temporal network relates to non self-serving attributions in healthy controls (HC; N=15) whereas in depressed patients (DP; N=15) this activation is associated with self-serving attributions.
Group differences in the general task contrast, internal vs. external attribution and positive vs. negative valence can be found in the supplementary online material.
Correlation analysis
Correlation analysis on the whole brain level and in our a priori ROI did not reveal any significant associations of BOLD response with symptom severity or self-esteem values regarding the “self-serving” or “non self-serving” contrasts.
Connectivity analysis
In healthy controls the PPI analysis revealed four significant clusters in the right amygdala, the left caudate and the bilateral insula (see Table 3). All of these brain regions showed stronger coupling with activity in the dorsomedial PFC seed region during “self-serving” than during “non self-serving” judgements (see Figure 3). In depressed patients two clusters with negative z-values – in the right frontal pole and the right precentral gyrus – did not survive the whole-brain correction but did surpass an uncorrected threshold of z=3.09 (see Table 3). These two brain regions showed a stronger coupling with activity in the dorsomedial PFC seed during “non self-serving” than during “self-serving”.
Table 3.
PPI analysis: Clusters showing positive or negative PPI with the dorsomedial PFC during “self-serving” compared to “non self-serving” (depressed patients=DP, healthy controls=HC). MNI coordinates of the peak, cluster size (k), z-values and p-values (cluster level corrected, p<0.05) are given. Only the highest peak is listed in the case of several confluent peaks
| Cluster | MNI | z-value | k | p-value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| HC | ||||||
| R. amygdala | 20 | −6 | −16 | 5.54 | 1317 | < 0.001 |
| L. caudate | −16 | 14 | 16 | 3.52 | 498 | < 0.001 |
| L. insula | −50 | 2 | 6 | 4.67 | 468 | < 0.001 |
| R. insula | 56 | 8 | 6 | 4.84 | 265 | 0.029 |
| DP | ||||||
| R. frontal pole | 28 | 60 | 6 | −4.4 | 165 | 0.001 (uncorr.) |
| R. precentral gyrus | 16 | −16 | 78 | −3.74 | 114 | 0.001 (uncorr.) |
| HC > DP | ||||||
| R. insula | 32 | 6 | 10 | 3.88 | 203 | 0.001 (uncorr.) |
| L. temporal pole | −34 | 14 | −34 | 4.14 | 117 | 0.001 (uncorr.) |
| L. inferior temporal gyrus | −38 | −8 | −40 | 4.16 | 111 | 0.001 (uncorr.) |
| L. insula | −36 | 2 | 16 | 3.88 | 89 | 0.001 (uncorr.) |
| R. amygdala | 18 | −6 | −16 | 3.85 | 73 | 0.001 (uncorr.) |
| L. medial OFC | −2 | 24 | −20 | 3.82 | 73 | 0.001 (uncorr.) |
Figure 3.
PPI analysis: Illustration of the task (self-serving > non self-serving) modulated coupling between the dorsomedial PFC seed (top) and limbic regions in healthy controls (HC; N=15), which was not present in depressed patients.
Directly comparing healthy controls and depressed patients did not reveal significant differences at a whole-brain corrected threshold, but at an uncorrected threshold of z=3.09 several clusters remained significant (see Table 3). This group difference was driven by more positive z-values in healthy controls. In healthy controls compared to depressed patients, the bilateral insula, the left temporal pole, the left inferior temporal gyrus, the right amygdala and the left medial OFC showed increased coupling with activity in the dorsomedial PFC seed during “self-serving” compared to “non self-serving” and vice versa.
Discussion
Despite the critical role cognitive biases play in the pathogenesis of depression, the neural correlates remain sparsely investigated. This study used fMRI to investigate behavioural and neural correlates of causal attribution in major depression. We presented positive and negative social scenarios to a sample of controls and depressed patients and asked them to make causal attributions. On the behavioural level, controls displayed a pronounced self-serving bias (i.e. internal attribution of positive events and external attribution of negative events) whereas the attributional style of depressed patients was balanced. Imaging revealed an interesting interaction effect of group and attributional bias in a fronto-temporal network. This group difference was driven by non self-serving attributions in controls and self-serving attribution in depressed patients. Preliminary evidence from an exploratory PPI analysis showed an attribution-modulated coupling between the dorsomedial PFC seed and limbic regions, which was weaker in in depressed patients than controls.
Depressive realism vs. self-serving bias
In controls, causal attribution was biased in a self-serving manner. Depressed patients showed a more balanced attribution pattern consistent with depressive realism (Moritz et al., 2007). This group difference showed that depressed patients do not see the world through the proverbial “rose-coloured glasses” associated with self-serving tendencies in controls. Indeed, those with the greatest symptom severity exhibited a negative attribution bias. The negative correlation of the percentage of self-serving attributions and depressive symptom severity shows that with increasing symptom severity, depressive realism turns into a frankly non self-serving tendency. In contrast, controls are happily self-serving and those with more self-serving attributions also reported the greatest self-esteem. However, these correlations do not allow us to determine whether one or both causal directions are operating.
Conflictive attributions activate a fronto-temporal network in controls and depressed patients
Functional imaging revealed neural group differences that paralleled the behavioural differences in attributional style. This group difference in activation of the dorsomedial PFC, the ventrolateral PFC and the temporal pole was driven by non self-serving attributions in controls and self-serving attributions in depressed patients. It is notable that in each group, the less frequent attributional pattern was associated with greater activation within this network. This may reflect that in controls non self-serving and in depressed patients self-serving attributions are in greater conflict with the prevailing self-concept. This conflict may require higher degrees of cognitive control (Krusemark et al., 2008) to inhibit the prepotent tendency toward either self-serving or non self-serving responses, leading to greater recruitment of fronto-temporal brain regions.
Such an interpretation is consistent with prior literature regarding the functions associated with these regions. The dorsomedial PFC has been associated with self-relatedness (Andrews-Hanna et al., 2010; Northoff et al., 2006), and introspective self-referential processing (Gallagher and Frith, 2003; Schmitz et al., 2004). In depressed patients this brain region has been implicated in mediating abnormal self-relatedness and the characteristically negative self-concept (Lemogne et al., 2009; Grimm et al., 2009). In addition to its role in self-related processes, the dorsomedial PFC is involved in conflict monitoring and cognitive control (e.g., Amodio and Frith, 2006; Etkin et al., 2006). Similarly, the ventrolateral PFC has also been implicated in control-related processes such as response inhibition when a prepotent response is withheld (e.g., Rubia et al., 2003; Aron et al., 2004). Analogously, activation in the temporal pole may reflect higher effort in the retrieval of socially-relevant semantic knowledge (Zahn et al., 2007) when a less automated attributional decision is made. When taken together with prior results, our current findings suggest that depressed patients show a different pattern of activation in a fronto-temporal network during causal attributions due to the emotional context of their own negative self-concept, resulting in greater conflict when a self-serving judgment is made.
Prefrontal-limbic connectivity is modulated by attribution
The exploratory PPI analysis revealed a group difference in fronto-limbic connectivity modulated by attributional decisions. However, this finding should be considered preliminary given the liberal statistical threshold. Compared to controls, depressed patients showed decreased connectivity between the dorsomedial PFC seed and limbic regions including the amygdala during self-serving attributions. During non self-serving attributions, however, the PFC-limbic coupling was increased in patients.
We speculate that causal attribution may serve a regulatory function influencing emotional reactions to positive and negative events (Terbeck et al., 2008). In response to positive events internal attribution may help induce a positive emotional reaction, whereas in response to negative events external attribution could prevent a negative emotional reaction (also called distancing). Therefore, the decreased PFC-limbic coupling during non self-serving attributions, which was stronger in controls compared to patients, might reflect a stronger suppression of limbic activation by the PFC to enable distancing. This speculative interpretation is consistent with previous research suggesting that the PFC exerts a regulatory influence on limbic structures, such as the amygdala (Quirk and Beer, 2006; Urry et al., 2006). Specifically, the dorsomedial PFC has been implicated in self-focused cognitive reappraisal (Ochsner et al., 2004; for a review see Ochsner and Gross, 2008).
This modulation of connectivity was weaker in patients than controls, suggesting a disturbance of fronto-limbic regulation during the attribution process in depressed patients. This result parallels previously reported abnormalities in the suppression of limbic responses during the reappraisal of negative pictures in depressed patients (Johnstone et al., 2007; Sheline et al., 2009). However, this result is based on a rather liberal statistical threshold and therefore is tentative pending replication in larger samples.
Limitations and future prospects
This study has several limitations that should be noted. First, we were primarily interested in examining group differences in attributional biases; we therefore combined individual valence-attribution conditions into self-serving and non self-serving categories. We did not separately examine the effects of each individual condition, which would have been difficult given the small number of events in particular conditions for some of our subjects. Second, we did not replicate the finding from our previous study (Seidel et al., 2010) of activations in the striatum in healthy subjects associated with self-serving responses. Type II error may contribute to the lack of striatal effects in the current study: the current control sample was half the size of the previous sample. However, the current finding of an association between front-temporal activation and non-self serving attributions was not observed in the previous study. Differences in the control samples of the two studies may contribute to the difference in results. The first study included only college students, while the current study used a community control sample which was significantly older. Compared to the previous student sample, the current community sample also showed a more pronounced self-serving bias, associated with greater self-esteem. Future studies with larger samples of heterogeneous healthy subjects should seek to identify significant individual difference variables. Third, this study was not designed to evaluate effects of antidepressant medication on the processes studied. However, future neuroimaging studies should highlight how and where antidepressants influence the neural correlates of causal attribution.
Notwithstanding these limitations, the current study provides novel data on the neural correlates of causal attribution in major depression. Our results suggest that the attributional style of depressive realism seen in patients is associated with abnormalities in fronto-temporal brain regions. Specifically, increased activation to self-serving judgments in these regions may reflect conflict associated with such attributions; this stands in sharp contrast to the increased responses of these same brain regions to non self-serving judgments in controls. Furthermore, as suggested by the preliminary results of the exploratory PPI analysis, these changes may be related to a alterations of fronto-limbic connectivity.
These results have implications for treatments of major depression, such as cognitive therapy, designed to reduce negatively biased causal attributions by facilitating reinterpretation of emotional events in a more positive way. Investigating the effects of psychotherapy and pharmacotherapy on fronto-limbic abnormalities elicited by causal attribution in depressed patients will be an important avenue for future studies.
Supplementary Material
Acknowledgments
This study was funded by the Medical Faculty of the RWTH Aachen University (START 690811). EMS was supported by the Interdisciplinary Centre for Clinical Research (IZKF, NWW11-SP3) within the Faculty of Medicine at the RWTH Aachen University. TDS was supported by NIMH grants MH 19112 and 5R25MH60490. SBE was supported by the Human Brain Project (R01-MH074457-01A1) and the Helmholtz-Initiative on Systems Biology (“Human Brain Model”). R.C.G. was supported by the NIMH grant MH 60722. TDS and DHW are supported by NARSAD and APIRE. UH and BD were supported by the German Research Foundation (DFG, IRTG 1328).
Role of funding source: This study was funded by the Medical Faculty of the RWTH Aachen University (START 690811). EMS was supported by the Interdisciplinary Centre for Clinical Research (IZKF, NWW11-SP3) within the Faculty of Medicine at the RWTH Aachen University. TDS was supported by NIMH grants MH 19112 and 5R25MH60490. SBE was supported by the Human Brain Project (R01-MH074457-01A1) and the Helmholtz-Initiative on Systems Biology (“Human Brain Model”). R.C.G. was supported by the NIMH grant MH 60722. TDS and DHW are supported by NARSAD and APIRE. UH and BD were supported by the German Research Foundation (DFG, IRTG 1328).
All these funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest. Each author declares that they have no potential conflict of interest.
Contributors. Authors EMS, BD, SBE and UH designed the study and wrote the protocol. EMS collected the data and EMS, TDS, DHW and SBE undertook the statistical analysis. EMS and BD wrote the first draft of the manuscript. TDS, DHW, SBE, FS, RCG and UH contributed to the discussion and interpretation of the results. All authors contributed to and have approved the final manuscript.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am. J. Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beck AT, Erbaugh J, Ward CH, Mock J, Mendelsohn M. An Inventory for Measuring Depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Chandler TA, Lee MS, Pengilly JW. Self-esteem and causal attributions. Genet. Soc. Gen. Psychol. Monogr. 1997;123:479–491. [PubMed] [Google Scholar]
- Diez-Alegria C, Vazquez C, Nieto-Moreno M, Valiente C, Fuentenebro F. Personalizing and externalizing biases in deluded and depressed patients: Are attributional biases a stable and specific characteristic of delusions? Br. J. Clin. Psychol. 2006;45:531–544. doi: 10.1348/014466505X86681. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang YH, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am. J. Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Ferring D, Filipp S-H. Messung des Selbstwertgefühls: Befunde zur Reliabilität, Validität und Stabilität der Rosenberg-Skala. Diagnostica. 1996;42:284–292. [Google Scholar]
- Friston KJ. Testing for anatomically specified regional effects. Hum. Brain Mapp. 1997;5:133–136. doi: 10.1002/(sici)1097-0193(1997)5:2<133::aid-hbm7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn. Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Hell D, Boeker H, Northoff G. Increased Self-Focus in Major Depressive Disorder Is Related to Neural Abnormalities in Subcortical-Cortical Midline Structures. Hum. Brain Mapp. 2009;30:2617–2627. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A Rating Scale for Depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Bromberg-Martin E, Hong S, Matsumoto M. New insights on the subcortical representation of reward. Curr. Opin. Neurobiol. 2008;18:203–208. doi: 10.1016/j.conb.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J. Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusemark EA, Campbell WK, Clementz BA. Attributions, deception, and event related potentials: An investigation of the self-serving bias. Psychophysiology. 2008;45:511–515. doi: 10.1111/j.1469-8986.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- Lemogne C, le Bastard G, Mayberg H, Volle E, Bergouignan L, Lehericy S, Allilaire JF, Fossati P. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Soc. Cogn. Affect. Neurosci. 2009;4:305–312. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz S, Woodward TS, Burlon M, Braus DF, Andresen B. Attributional style in schizophrenia: Evidence for a decreased sense of self-causation in currently paranoid patients. Cogn. Ther. Res. 2007;31:371–383. [Google Scholar]
- Northoff G, Heinzel A, Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain - A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Curr. Dir. Psychol. Sci. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced Caudate and Nucleus Accumbens Response to Rewards in Unmedicated Individuals With Major Depressive Disorder. Am. J. Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr. Opin. Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Trail Making Test: manual for administration, scoring and interpretation. Indiana University Press; Indianapolis: 1956. [Google Scholar]
- Rosenberg M. Society and the adolescent self-image. Princeton University Press; Princeton, NJ: 1965. [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22:941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Seidel E-M, Eickhoff SB, Kellermann T, Schneider F, Gur RC, Habel U, Derntl B. Who is to blame? Neural correlates of causal attribution in social situations. Soc. Neurosci. 2010;5:335–350. doi: 10.1080/17470911003615997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M, Uranowitz SW. Reconstructing Past - Some Cognitive Consequences of Person Perception. J. Pers. Soc. Psychol. 1978;36:941–950. [Google Scholar]
- Terbeck S, Chesterman P, Fischmeister FPS, Leodolter U, Bauer H. Attribution and Social Cognitive Neuroscience: A new approach for the “online-assessment” of causality ascriptions and their emotional consequences. J. Neurosci. Methods. 2008;173:13–19. doi: 10.1016/j.jneumeth.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Aster M, Neubauer A, Horn R. Hamburg-Wechsler-Intelligenz-Test für Erwachsene III. Harcourt; Frankfurt: 2006. [Google Scholar]
- Wittchen H-U, Zaudig M, Fydrich T. Strukturiertes Klinisches Interview für DSM-IV. Hogrefe; Göttingen: 1998. [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc. Natl. Acad. Sci. U. S. A. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.