Abstract
The purpose of this study was to assess the feasibility and efficacy of stereotactic ablative radiotherapy (SABR) for liver tumor in patients with Barcelona Clinic Liver Cancer (BCLC)-C stage hepatocellular carcinoma (HCC). We retrospectively reviewed the medical records of 35 patients between 2003 and 2011. Vascular invasion was diagnosed in 32 patients, extrahepatic metastases in 11 and both in 8. Thirty-two patients were categorized under Child-Pugh (CP) class A and 3 patients with CP class B. The median SABR dose was 45 Gy (range, 30-60 Gy) in 3-5 fractions. The median survival time was 14 months. The 1- and 3-yr overall survival (OS) rate was 52% and 21%, respectively. On univariate analysis, CP class A and biologically equivalent dose ≥ 80 Gy10 were significant determinants of better OS. Severe toxicity above grade 3, requiring prompt therapeutic intervention, was observed in 5 patients. In conclusion, SABR for BCLC-C stage HCC showed 1-yr OS rate of 52% but treatment related toxicity was moderate. We suggest that patients with CP class A are the best candidate and at least SABR dose of 80 Gy10 is required for BCLC-C stage.
Keywords: Carcinoma, Hepatocellular, Neoplasm Staging, Radiotherapy, Stereotactic Techniques
INTRODUCTION
Hepatocellular carcinoma (HCC) is a highly prevalent disease in many Asian countries including Korea, accounting for 80% of the cases worldwide (1). Screening programs improve the detection of early HCC and have a positive impact on survival, but the majority of HCC patients in Asia still present with advanced stage disease (2). However, there is no consensus guideline in Asia; and most Asian countries follow their own HCC staging system. In Korea, the Clinical Practice Guidelines for HCC management, introduced in 2003 and revised in 2009, are followed (3). Although the Asian Pacific Association for the Study of the Liver proposed a treatment algorithm for HCC to develop a consensus management strategy, this algorithm is not thoroughly validated (4). On the other hand, the Barcelona Clinic Liver Cancer (BCLC) staging system has been externally validated in the USA, Europe, and Taiwan, and is endorsed by both the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver (5-9).
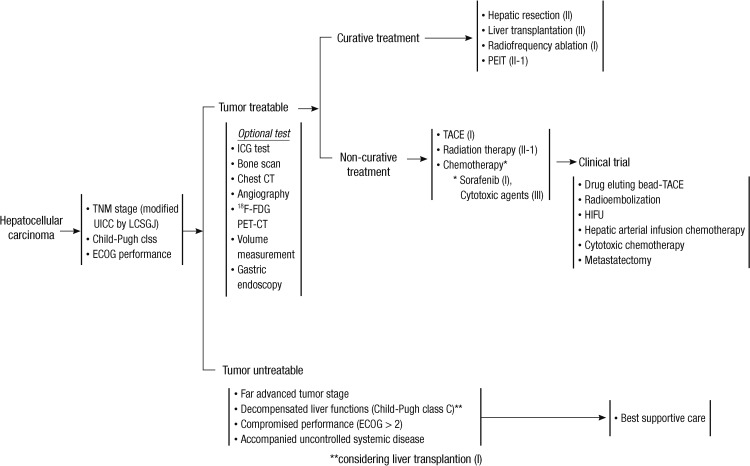
According to the BCLC system, sorafenib, which is the first targeted agent with survival benefits proven by 2 large, randomized clinical studies, is the standard treatment for patients with BCLC-C stage (10, 11). However, sorafenib for BCLC-C stage is still not easy for Asian physicians to prescribe due to high cost (2). In Korea, sorafenib has been subsidized by the National Insurance Scheme since 2011. Among patients with advanced HCC, those unsuitable for local treatment or with inoperable lesions are eligible for 1 yr. Therefore, according to the treatment algorithm of Korea, not only sorafenib, but also other therapeutic options, including surgical resection, transarterial chemoembolization (TACE) or external beam radiotherapy (EBRT) could be indicated for treating advanced HCC (Fig. 1).
Fig. 1.
Treatment algorithm of hepatocellular carcinoma in Korea, proposed by Korean Liver Cancer Study Group and National Cancer Center.
Previously, the role of EBRT for HCC has been limited because of the low tolerance of the whole liver to RT and the risks of radiation-induced liver disease (12). However, further radiotherapeutic developments have gradually expanded EBRT indication from a palliative to a curative modality, whereby high dose can be delivered to the tumor more safely, without adversely affecting liver function (13). Stereotactic ablative radiotherapy (SABR) is an emerging treatment method that enables high precision and high dose to the tumor using a small number of fractions. The Korea Cancer Center Hospital has used SABR to treat patients with HCC since 2003. Recently we have reported the promising outcomes of SABR in patients with inoperable HCC previously treated with incomplete TACE (14, 15). In this study, we assessed the feasibility and efficacy of SABR for liver tumor in patients with BCLC-C stage HCC.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of patients with HCC treated with SABR between 2003 and 2011 at the Korea Cancer Center Hospital. We found 35 patients with BCLC-C stage HCC, who were treated with SABR for liver lesion. All patients presented Eastern Cooperative Oncology Group (ECOG) performance status of 1 or 2 and had either vascular invasion (VI) or extrahepatic metastases (EHM) confirmed by computed tomography (CT), magnetic resonance imaging (MRI) or positron emission tomography/computed tomography (PET/CT).
SABR technique
Planning CT with free breathing for SABR was performed with each patient in the supine position with both arms raised above the head. Thin-slice CT scanning was performed with a slice thickness of 2 mm, and the data were entered into the CyberKnife planning system (Accuray Inc., Sunnyvale, CA, USA) in 12 patients or the Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA, USA) in 23 patients. Entire visible lesions were included for radiation target in 18 patients (curative field). Otherwise, part of the visible lesions was included in 17 patients (palliative field): VI only (8 patients); primary HCC only (6 patients); primary HCC and EHM (2 patients); and VI and EHM (1 patient). Gross tumor volume (GTV) was identified and contoured on the axial CT images. Sometimes, MRI and PET/CT scan were used to delineate the GTVs accurately. GTV was considered to be equal to the clinical target volume (CTV). Planning target volume (PTV) was defined as CTV plus 4 mm in the longitudinal direction and CTV plus 2 mm in all other directions. Radiation doses were prescribed at 56%-83% isodose line of the maximum dose in CyberKnife and at 91%-100% in RapidArc to cover at least 95% of the PTVs.
We adopted the following constraints: 1) at least 700 mL of the normal liver (entire liver minus PTV) had to receive a total dose of < 17 Gy in 3 fractions; 2) the maximum dose to the spinal cord should not exceed 22 Gy in 3 fractions and 0.25 mL or less of irradiated volume of the spinal cord > 18 Gy in 3 fractions. Besides, although other normal tissue constraints were not considered, dosages to the kidney, colon, and stomach were restricted to the lowest levels as possible.
Various fractionation schedules were used considering the expected morbidity based on the target volume, the patients' performance, and normal tissue constraints. Total SABR doses were converted into biologically equivalent dose (BED) for the comparison purpose, assuming that α/β was 10 Gy.
Follow-up after SABR
All patients were regularly followed up at 1 month after the completion of SABR and then at 2-3 month intervals with CT or PET/CT. Radiographic in-field tumor responses were evaluated by CT performed 1 month after SABR. Complete response (CR) was defined as the disappearance of contrast enhancement in the tumor during the arterial phase according to modified Response Evaluation Criteria for Solid Tumor. A partial response (PR) was defined as a decrease in volume of an enhancing area by > 30% of the initial tumor size. Stable disease (SD) was defined as a decrease in initial tumor volume < 30%, and progressive disease (PD) as defined as an increase in tumor volume ≥ 20%. As various target volumes (curative or palliative field) were applied, the further recurrences in RT field following SABR were classified in-field failure. Treatment related toxicity was scored according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
Statistical analysis
Survival rates were estimated by the Kaplan-Meier method and the groups were compared using the log-rank test. Multivariate analysis was performed to assess the relationships between the overall survival (OS) and the possible prognostic variables using the Cox proportional hazards model. All calculations were performed using the Statistical Package for the Social Sciences software version 13.0 (SPSS Inc., Chicago, IL, USA), and P values < 0.05 were considered statistically significant.
Ethics statement
This study was approved of permission by the institutional review board of Korea Institute of Radiological & Medical Sciences (IRB No. K-1206-002-029). Informed consent was waived by the board.
RESULTS
Patients' characteristics
Thirty-five patients, treated with SABR for liver lesion of BCLC-C stage HCC between 2003 and 2011, were retrospectively reviewed. Thirty-three patients received various courses (range, 1-9 courses) of previous treatments, including surgery, radiofrequency ablation, TACE and SABR. The age range of the patients was 34 to 67 yr (median age, 56 yr). There were 30 males (86%) and 5 females (14%). Twenty-six patients (74%) presented ECOG performance status of 1 and 9 (26%) presented of 2. Twenty-four patients (69%) had VI, 3 (8%) had EHM and 8 (23%) had both VI and EHM. Among the patients with EHM, 8 had regional lymph node (LN) metastases, 2 had lung metastases and 1 had adrenal metastases. Before SABR, 2 patients with lung metastasis received chemotherapy or sorafenib. At SABR, 5 patients with LN metastases and 1 patient with adrenal metastases also was treated with SABR for the metastatic lesion. On the other hand, 3 patients did not receive local treatment for LN metastases; 2 patients treated with further TACE and 1 patients treated with sorafenib after SABR. According to the Child-Pugh (CP) class, 32 patients (91%) had class A and the remaining 3 (9%) class B (CP score of 7 in 2 patients; 8 in 1). Liver cirrhosis was present in 23 patients (66%). The alpha-feto protein levels ranged from 1 to 338100 IU/mL (median, 183 IU/mL). Main portal vein tumor thrombosis (PVTT) was observed in 8 patients (23%). The median PTV was 131 mL (range, 21-2,189 mL). The median SABR dose was 45 Gy (range, 30-60 Gy). The number of fraction was 3 in 21 (60%), 4 in 11 (31%) and 5 in 3 patients (9%). When the total SABR doses were converted into BED, the median BED was 101 Gy10 (range, 58-180 Gy10). Twenty patients (57%) received further treatment after SABR.
Radiographic in-field tumor response after SABR
One patient (3%) achieved CR 1 month after SABR. Ten patients (28%) had a PR, 21 (60%) had SD and 3 (9%) had PD. The objective response rate (defined as CR plus PR) was 31%. During follow-up, 10 patients (29%) experienced in-field failure. The infield failure free survival rates at 1- and 3-yr were 69% and 51%, respectively.
Overall survival and prognostic factors
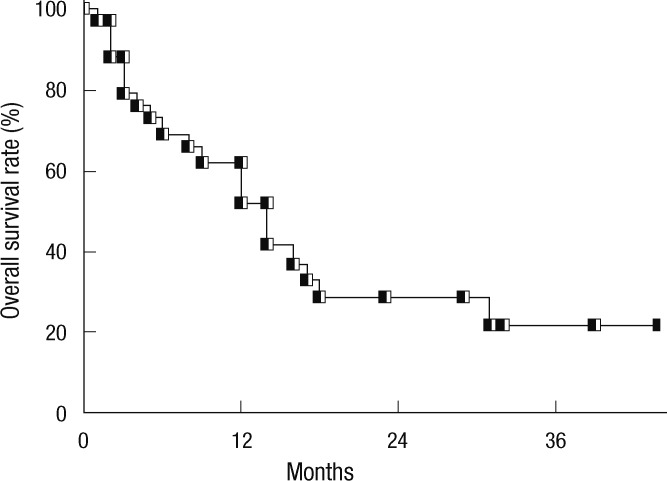
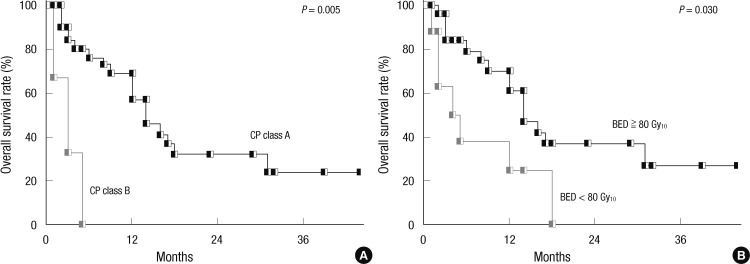
The median follow-up period after SABR was 14 months (range, 1-44 months). The median survival time was 14 months. The OS rates at 1- and 3-yr were 52% and 21%, respectively (Fig. 2). On univariate analysis, CP class and BED were significant determinants of OS (Table 1). Fig. 3 shows OS according to CP class and BED. On multivariate analysis, CP class (hazard ratio = 8.26, P = 0.042) was the only significant prognostic factor for OS.
Fig. 2.
Overall survival rate of all patients with Barcelona Clinic Liver Cancer-C stage hepatocellular carcinoma treated with stereotactic ablative radiotherapy.
Table 1.
Univariate analysis for overall survival (OS)
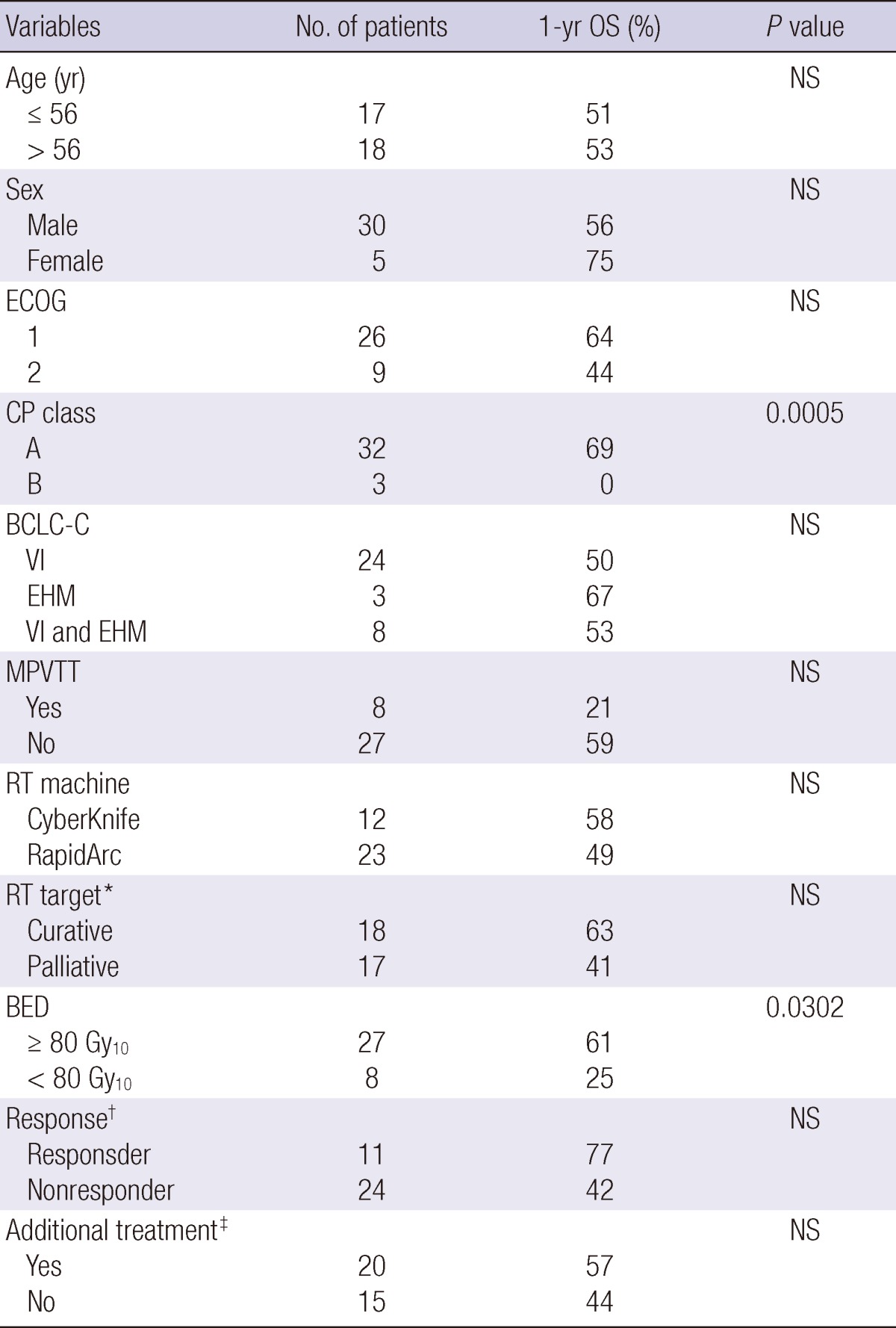
*RT target is curative when all grossly visible lesions are included or palliative when part of the visible lesions are included; †Response was evaluated 1 month after SABR using modified RECIST criteria. Responder means complete response and partial response. Nonresponder means stable disease and progressive disease; ‡Twenty patients received additional treatment after SABR. ECOG, Eastern Cooperative Oncology Group; CP, Child-Pugh; BCLC, Barcelona Clinic Liver Cancer; VI, vascular invasion; EHM, extrahepatic metastases; MPVTT, main portal vein tumor thrombosis; BED, biologically equivalent dose.
Fig. 3.
Overall survival rates according to Child-Pugh (CP) class (A) and Biologically equivalent dose (BED), assuming that α/β was 10 Gy (B).
Death was observed in 22 patients. The deterioration of hepatic function associated with intrahepatic progression was a major cause of death with 15 cases. One patient died of a combination of SABR-related toxicity and intrahepatic progression. One patient died of complication of underlying liver cirrhosis without disease progression and 1 patient died of pneumonia associated with lung metastases. One patient died of aortic dissection without disease progression, while no information was available for 3 patients.
Treatment-related toxicity
Eight patients (23%) experienced toxicity of CTCAE grade ≥3. Three patients had grade 3 elevation of aspartate aminotransferase (AST). Among these, 1 patient also had grade 3 hyperbilirubinemia. However, all patients had pre-existing grade 2 elevation of AST or hyperbilirubinemia and experienced progression of intrahepatic HCC. There were 5 patients (14%) with severe toxicity, requiring prompt therapeutic intervention (Table 2). Grade 3 hepatic failure without progression of intrahepatic HCC, and colonic ulcer were developed in each 1 patient 1 month after SABR. One patient experienced grade 4 myelitis 18 months after SABR. One patient suffered from panperitonitis due to grade 4 gastric perforation but he refused operation and received conservative management at the hospital. One patient experienced 2 severe toxicities. He underwent hemicolectomy due to grade 4 colonic perforation at 3 months and received transfusion due to duodenal ulcer bleeding at 5 months. He eventually died from hypovolemic shock. However, it was difficult to conclude that the cause of death was absolutely attributable to the treatment related toxicity because he had progression of intrahepatic HCC and also suffered from aspiration pneumonia.
Table 2.
Adverse events requiring prompt therapeutic intervention
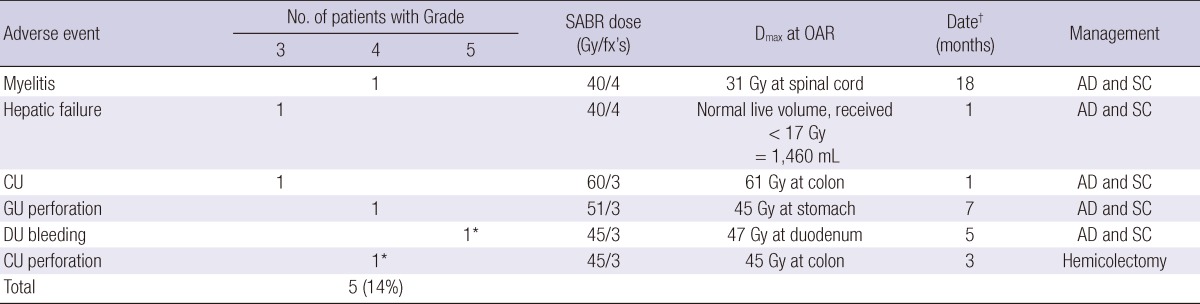
*One patient experienced grade 5 duodenal ulcer and grade 4 colonic perforation; †Date means the time of development of severe toxicity after SABR. Dmax, maximal point dose; OAR, organ at risk; CU, colonic ulcer; GU, gastric ulcer; DU, duodenal ulcer; AD and SC, admission and supportive care; fx, fraction.
DISCUSSION
This is the first study to evaluate the efficacy of SABR for BCLC-C stage HCC with the largest population. Table 3 shows recently published reports of various treatment modalities for BCLC-C stage (9-11, 16-20). The median survival time of BCLC-C stage was 2-28 months. One-year and 3-yr OS rates were 6%-70% and 1%-41%, respectively. The best treatment outcome was associated with surgery. However, surgery is indicated in highly selected patients among BCLC-C stage. Vitale et al. (16) reported that liver transplantation could result in survival benefit for patients with HCC and advanced liver cirrhosis (BCLC-D stage) and in those with intermediate tumors (BCLC-B/C stage), regardless of the nodule number-size criteria, provided that macroscopic VI and EHM were absent. Yang et al. (17) evaluated long-term outcomes of surgical resection in patients with BCLC-C stage. In this study, 314 patients (61%) were diagnosed with BCLC-C stage not because of the presence of VI or EHM but because of ECOG performance status of 1-2. One hundred ninety-seven patients with VI and/or EHM had a lower 1- and 3-yr OS rate than the other 314 patients (48% and 17% vs 84% and 56%). Recently, improvement in radiotherapy technique has allowed RT to be used as an alternative treatment option for primary HCC. With regard to BCLC-C stage, RT for PVTT, in particular, shows good responses with the promising outcomes (13, 18, 19). Our study showed the median survival time of 14 months, and 1- and 3-yr OS rates of 52% and 21%, respectively. This result is comparable with other published reports, especially those that employed sorafenib: a multitarget tyrosine kinase inhibitor (10, 11). Therefore, SABR would be considered a treatment option for BCLC-C stage, especially in Asian countries, where there is a practical limitation on the use of sorafenib.
Table 3.
Selected published series of BCLC-C stage HCC
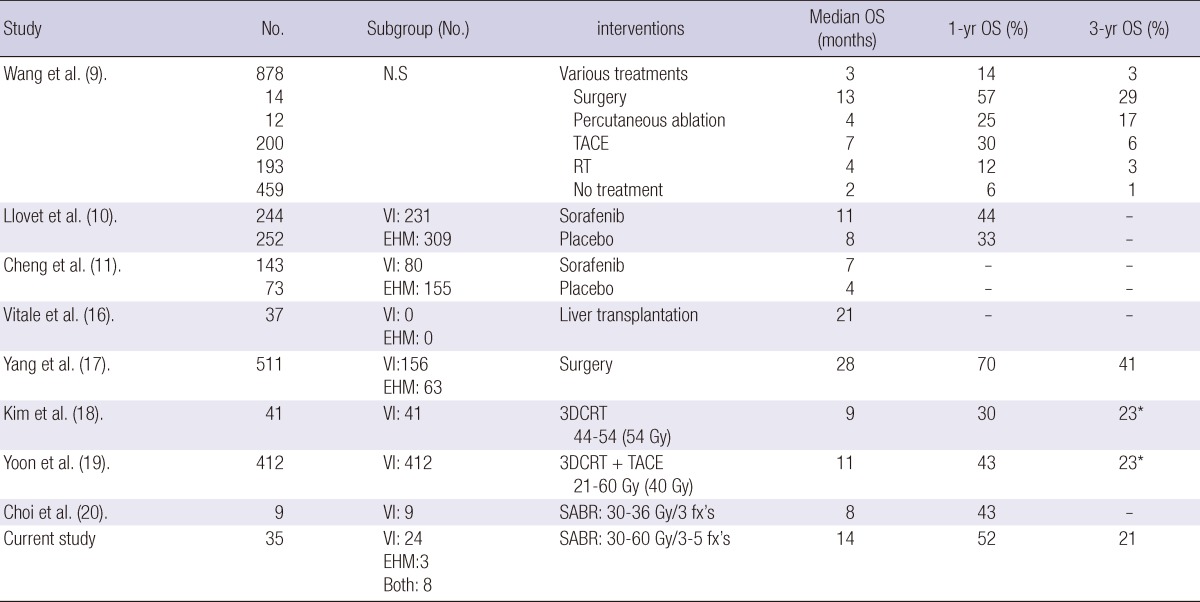
*This means overall survival rate at 2 yr. OS, overall survival; N.S, not specified; VI, vascular invasion; EHM, extrahepatic metastases; TACE, transarterial chemo-embolization; RT, radiotherapy; 3D-CRT, 3-dimensioanl conformal radiotherapy; SABR, stereotactic ablative radiotherapy; fx, fraction.
Our study showed that CP class was the most significant prognostic factor for OS on univariate and multivariate analysis. Child-Pugh class is generally considered as one of the most important prognostic factors, because HCC is a complex neoplasm inserted on a preneoplastic cirrhotic liver and thus variables of both diseases leading to death should be taken into account (21). In very early, early or intermediate stages, the best candidates for curative treatment are patients with CP class A. In intermediate stage, patients with hepatic decompensation or failure (CP class B or C) are not considered as the candidate for TACE because ischemic insults can lead to severe adverse events in these cases (22). In current study, 3 patients with CP class B received SABR. They eventually died from hepatic decompensation accompanied by infection or progression of intrahepatic HCC at 1, 3, and 5 months. Therefore, we suggest CP class A is the best candidate for SABR in patients with BCLC-C stage.
While several studies have showed promising results using various SABR doses, there is no standard SABR dose for optimal tumor control in HCC. Kwon et al. (23) reported a 2-yr local control rate of 72% with a median dose of 33 Gy (range, 30-39 Gy) in 3 fractions for primary HCC treated with SABR. Andolino et al. (24) reported a 2-yr local control rate of 87% with a median dose of 40 Gy (range, 24-48 Gy) for CP class A and 44 Gy (range, 30-48 Gy) for CP class B in 3-5 fractions. On the other hand, in our hospital, SABR dose for HCC was escalated from 33 to 57 Gy in 3 fractions and 2-yr local control rate was 66% (14). Dose of SABR ≥42 Gy was the only significant prognostic factor for OS. Because we observed several local recurrences at 51 or 54 Gy, we escalated SABR dose to 60 Gy in 3 fractions and recently published results of a phase 2 clinical trial (15). The 2-yr local control rate was 95% with median dose of 57 Gy (range, 42-60 Gy) in 3 fractions. In current study, SABR dose of BED ≥80 Gy10 was a significant prognostic factor for OS on univariate analysis. Based on our experience, a higher SABR dose would be needed to achieve optimal treatment outcomes. However, there are limitations on an application of higher SABR dose in BCLC-C stage because of the proximity of tumor in the gastrointestinal track, the larger extent of disease and the smaller normal liver volume. Based on aforementioned and current studies, SABR dose of at least 80 Gy10 would be required to achieve a considerable treatment outcome for BCLC-C stage and higher dose ≥80 Gy10 would be indicated in selected patients according to tumor site and normal tissue constraints.
There are no guidelines to indicate whether to target visible lesions entirely or partially for BCLC-C stage because most patients have huge and infiltrative HCC. Kim et al. (18) reported outcomes of 3-dimentional conformal RT (3D-CRT) in 70 patients with unresectable HCC for whom TACE was ineffective or not indicated. Portal vein tumor thrombosis was present in 41 patients. All gross lesions (PVTT plus HCC) were included within radiation field. The median survival time was 9 months in HCC patients with PVTT. Yoon et al. (19) reported 3D-CRT plus TACE in 412 patients with HCC invading PVTT. Only PVTT was included within radiation field in 343 patients (83%). The median survival time was 11 months. The former with 3D-CRT alone for entire visible lesion and the latter with combination of limited field RT and TACE showed the similar promising result. In our study, there was no difference in survival between patients with curative field and patients with palliative field. There are similar limitations on applications of curative field as discussed for SABR dose. Therefore, curative field would be applied to selected patients, who can be treated with SABR dose of BED ≥80 Gy10 for entire visible lesion. If this approach is impossible, palliative field would be applied with at least SABR dose of 80 Gy10 and should consider additional treatments, including TACE or sorafenib. A multidisciplinary approach would overcome the limitation of an application of SABR for BCLC-C stage and have better survival.
In our study, 8 patients (23%) experienced toxicity of CTCAE grade ≥3. Among these, 5 (14%) required prompt therapeutic intervention. Severe gastrointestinal toxicity of grade 3-5 developed in 3 patients. Because there are no definite constraints regarding gastrointestinal tract for SABR, we restricted the dosages to stomach, duodenum, and colon to the lowest levels as possible until last year. Recently, we published the analysis for predictor of severe gastroduodenal toxicity after SABR with 3 fractions from our experience (25). The maximum point dose of 35 Gy and 38 Gy in the gastroduodenum were respectively associated with a 5% and 10% probability of developing severe gastroduodenal toxicity. This severe gastroduodenal toxicity would be reduced, applying this dose constraint strictly. One patient experienced myelitis of grade 4. Last year, we had published the corresponding case report (26). After experience of this severe toxicity, we have maintained faithfully constraints of spinal cord. One patient had grade 3 hepatic failure. He received SABR for the right hepatic vein and inferior vena cava tumor thrombosis. Although the normal liver volume received <17 Gy was 1,460 mL, hepatic encephalopathy of grade 3 developed 1 month later. He had unchanged liver function after SABR, while reporting a history of constipation 2 days before onset of hepatic encephalopathy, which rapidly subsided by lactulose enema the following day. We think his hepatic encephalopathy may have been due to constipation-induced excessive nitrogen load and not due to SABR related toxicity.
There were some limitations in current study. First, this study was a single-institutional retrospective analysis. Therefore patients with a better prognosis among BCLC-C patients might be selected previously to receive SABR. Second, this study had a small sample size. Because of the limited number of patients, we could not form a definite conclusion. To confirm the efficacy of SABR in BCLC-C stage HCC, further prospective trials should be needed.
In conclusion, SABR for BCLC-C stage HCC showed 1- and 3-yr OS rates of 52% and 21%, respectively. SABR would be considered a treatment option for BCLC-C stage, especially in Asian countries. However, treatment related toxicity requiring prompt therapeutic intervention was moderate. We suggest that CP class A is the best candidate for SABR in patients with BCLC-C stage. In addition, SABR dose of at least 80 Gy10 would be required to achieve a considerable treatment outcome. If the entire visible lesion dose not included in SABR target, we suggest a multidisciplinary approach of palliative field with BED ≥80 Gy10 and additional treatments, including TACE or sorafenib.
ACKNOWLEDGMENTS
The authors have no conflicts of interest to disclose.
Footnotes
This work was supported by the National Nuclear R & D program of the Ministry of Education, Science and Technology, Republic of Korea (2012).
References
- 1.Kudo M, Han KH, Kokudo N, Cheng AL, Choi BI, Furuse J, Izumi N, Park JW, Poon RT, Sakamoto M. Liver cancer working group report. Jpn J Clin Oncol. 2010;40:i19–i27. doi: 10.1093/jjco/hyq123. [DOI] [PubMed] [Google Scholar]
- 2.Han KH, Kudo M, Ye SL, Choi JY, Poon RT, Seong J, Park JW, Ichida T, Chung JW, Chow P, et al. Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology. 2011;81:158–164. doi: 10.1159/000333280. [DOI] [PubMed] [Google Scholar]
- 3.Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 4.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, et al. Asian Pacific association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrero JA, Kudo M, Bronowicki JP. The challenge of prognosis and staging for hepatocellular carcinoma. Oncologist. 2010;15:23–33. doi: 10.1634/theoncologist.2010-S4-23. [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, Lok AS. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an american cohort. Hepatology. 2005;41:707–716. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 7.Cillo U, Bassanello M, Vitale A, Grigoletto FA, Burra P, Fagiuoli S, D'Amico F, Ciarleglio FA, Boccagni P, Brolese A, et al. The critical issue of hepatocellular carcinoma prognostic classification: which is the best tool available? J Hepatol. 2004;40:124–131. doi: 10.1016/j.jhep.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D'Amico F, et al. Prospective validation of the Barcelona Clinic Liver Cancer Staging System. J Hepatol. 2006;44:723–731. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Wang JH, Changchien CS, Hu TH, Lee CM, Kee KM, Lin CY, Chen CL, Chen TY, Huang YJ, Lu SN. The efficacy of treatment schedules according to Barcelona Clinic Liver Cancer Staging for hepatocellular carcinoma - survival analysis of 3892 patients. Eur J Cancer. 2008;44:1000–1006. doi: 10.1016/j.ejca.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 11.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 12.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Chung SM, Choi BO, Kay CS. Hepatocellular carcinoma with portal vein tumor thrombosis: improved treatment outcomes with external beam radiation therapy. Hepatol Res. 2011;41:813–824. doi: 10.1111/j.1872-034X.2011.00826.x. [DOI] [PubMed] [Google Scholar]
- 14.Seo YS, Kim MS, Yoo SY, Cho CK, Choi CW, Kim JH, Han CJ, Park SC, Lee BH, Kim YH, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol. 2010;102:209–214. doi: 10.1002/jso.21593. [DOI] [PubMed] [Google Scholar]
- 15.Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, Bae SH, Jung DH, Kim KB, Lee DH, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424–5431. doi: 10.1002/cncr.27533. [DOI] [PubMed] [Google Scholar]
- 16.Vitale A, Morales RR, Zanus G, Farinati F, Burra P, Angeli P, Frigo AC, Del Poggio P, Rapaccini G, Di Nolfo MA, et al. Barcelona clinic liver cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: a multicentre, cohort study. Lancet Oncol. 2011;12:654–662. doi: 10.1016/S1470-2045(11)70144-9. [DOI] [PubMed] [Google Scholar]
- 17.Yang T, Lin C, Zhai J, Shi S, Zhu M, Zhu N, Lu JH, Yang GS, Wu MC. Surgical resection for advanced hepatocellular carcinoma according to Barcelona Clinic Liver Cancer (BCLC) Staging. J Cancer Res Clin Oncol. 2012;138:1121–1129. doi: 10.1007/s00432-012-1188-0. [DOI] [PubMed] [Google Scholar]
- 18.Kim TH, Kim DY, Park JW, Kim YI, Kim SH, Park HS, Lee WJ, Park SJ, Hong EK, Kim CM. Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am J Clin Oncol. 2006;29:568–575. doi: 10.1097/01.coc.0000239147.60196.11. [DOI] [PubMed] [Google Scholar]
- 19.Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, Chung YH, Lee YS, Lee SG, Park JH, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–2011. doi: 10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Choi BO, Choi IB, Jang HS, Kang YN, Jang JS, Bae SH, Yoon SK, Chai GY, Kang KM. Stereotactic body radiation therapy with or without transarterial chemoembolization for patients with primary hepatocellular carcinoma: preliminary analysis. BMC Cancer. 2008;8:351. doi: 10.1186/1471-2407-8-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225–235. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 22.Groupe d'etude et de traitement du carcinome hepatocellulaire. A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med. 1995;332:1256–1261. doi: 10.1056/NEJM199505113321903. [DOI] [PubMed] [Google Scholar]
- 23.Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, Choi JY, Yoon SK, Chung KW. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. doi: 10.1186/1471-2407-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, Johnstone PA, Cardenes HR. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Bae SH, Kim MS, Cho CK, Kang JK, Lee SY, Lee KN, Lee DH, Han CJ, Yang KY, Kim SB. Predictor of severe gastroduodenal toxicity after stereotactic body radiotherapy for abdomino-pelvic malignancies. Int J Radiat Oncol Biol Phys. 2012;84:e469–e474. doi: 10.1016/j.ijrobp.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Noh G, Han C, Kim Y, Yang K, Park S, Kim J, Kim Y, Kim M. A case of paraplegia after successful treatment of cyberknife for hepatocellular carcinoma. J Korean Liver Cancer Study Group. 2011;11:165–171. [Google Scholar]