Abstract
Although the number of studies using tandem high-dose chemotherapy and autologous stem cell transplantation (HDCT/autoSCT) for the treatment of high-risk pediatric solid tumors has been increasing, documentation of hematologic recovery after tandem HDCT/autoSCT is very limited. For this reason, we retrospectively analyzed the hematologic recovery of 236 children with high-risk solid tumors who underwent tandem HDCT/autoSCT. The median numbers of CD34+ cells transplanted during the first and second HDCT/autoSCT were 4.3 × 106/kg (range 0.6-220.2) and 4.1 × 106/kg (range 0.9-157.6), respectively (P = 0.664). While there was no difference in neutrophil recovery between the first and second HDCT/autoSCT, platelet and RBC recoveries were significantly delayed in the second HDCT/autoSCT (P < 0.001 and P < 0.001, respectively). Delayed recovery in the second HDCT/autoSCT was more prominent when the number of transplanted CD34+ cells was lower, especially if it was < 2 × 106/kg. A lower CD34+ cell count was also associated with increased RBC transfusion requirements and a higher serum ferritin level after tandem HDCT/autoSCT. More CD34+ cells need to be transplanted during the second HDCT/autoSCT in order to achieve the same hematologic recovery as the first HDCT/autoSCT.
Keywords: High-Dose Chemotherapy, Autologous Stem Cell Transplantation, CD34+ Cells, Hematologic Recovery, Iron Overload
INTRODUCTION
Several subsets of pediatric solid tumors have a poor prognosis despite an initially good response to conventional chemotherapy. In children with these high-risk or recurrent solid tumors, a treatment strategy using high-dose chemotherapy and autologous stem cell transplantation (HDCT/autoSCT) has emerged and shown the potential to improve outcomes (1-3). However, the outcome is still unsatisfactory, and the main cause of failure after a single HDCT/autoSCT is relapse or progression of tumor rather than treatment-related mortality (TRM). For this reason, investigators have explored the efficacy of tandem HDCT/autoSCT and results suggest that further dose-escalation may improve the event-free survival (EFS) of high-risk patients (4-8). Recently, the number of studies using tandem HDCT/autoSCT for the treatment of high-risk pediatric solid tumors has been increasing.
When treating with tandem HDCT/autoSCT, half of the stem cells that are collected during the hematologic recovery phase after conventional chemotherapy are infused for bone marrow (BM) rescue during each HDCT/autoSCT session. Therefore, the number of CD34+ cells transplanted during each session of tandem HDCT/autoSCT may be lower than the number of CD34+ cells transplanted during only a single HDCT/autoSCT. Consequently, a longer time may be needed for hematologic recovery when using tandem HDCT/autoSCT compared to single HDCT/autoSCT. Furthermore, probable BM damage during the first HDCT/autoSCT may result in delayed hematologic recovery in the second HDCT/autoSCT (9). However, documentation of hematologic recovery after tandem HDCT/autoSCT and possible differences in hematologic recovery between the first and second HDCT/autoSCT is very limited to date. For this reason, we retrospectively analyzed the hematologic recovery of 236 children with high-risk solid tumors who were treated with tandem HDCT/autoSCT.
MATERIALS AND METHODS
Patients
All children with high-risk solid tumors who were treated with tandem HDCT/autoSCT at Samsung Medical Center between April 1998 and June 2011 were eligible for the study. Patients who underwent the first HDCT/autoSCT but were unable to proceed to the second HDCT/autoSCT due to TRM during the first HDCT/autoSCT, tumor progression after the first HDCT/autoSCT, or parental refusal of the second HDCT/autoSCT were excluded from the analysis.
Stem cell collection
Treatment prior to HDCT/autoSCT was determined by the diagnosis, biology, extent, and status of the tumor. In most patients, peripheral blood stem cells (PBSCs) were collected during the hematologic recovery phase after conventional chemotherapy. Patients received 2-10 µg/kg of granulocyte-colony stimulating factor (G-CSF) daily if their absolute neutrophil count (ANC) fell below 500/µL after conventional chemotherapy, and G-CSF was continued until the completion of leukapheresis. In six patients, G-CSF (5-10 µg/kg) was administered without a prior chemotherapy and PBSCs were collected from the fourth day of GCSF treatment. PBSCs were collected for at least two days per leukapheresis round. The aim was to collect a minimum of 2 × 106 CD34+ cells/kg in total, with an optimal collection of > 5 × 106/kg, to be used for BM rescue during tandem HDCT/autoSCT. Ideally, all PBSCs were collected during a single round of leukapheresis. However, if < 2 × 106 CD34+ cells/kg were collected during the first round of leukapheresis, a second round was performed during the next chemotherapy cycle in order to infuse > 1 × 106 CD34+ cells/kg for each HDCT/autoSCT. Collected PBSCs were cryopreserved in a liquid nitrogen tank until later use.
Tandem HDCT/autoSCT
Various HDCT regimens were used according to the treatment era, diagnosis, and tumor status (Table 1). G-CSF (2-10 µg/kg) was administered beginning on the day of stem cell infusion. Generally, the doses of G-CSF for a patient were the same during the first and second HDCT/autoSCT. Generally, 6 units/m2 of irradiated platelet concentrates (PCs) were transfused when the platelet count fell below 20,000/µL and 10 mL/kg of irradiated RBCs were transfused when the hemoglobin level fell below 8.0 g/dL. We tried to maintain a hemoglobin level greater than 10.0 g/dL if the patient underwent surgery, developed a high fever (≥ 38℃), or required oxygen supplementation for any reason.
Table 1.
Patient characteristics
HDCT, high-dose chemotherapy; CEC, carboplatin + etoposide + cyclophosphamide; CTE, carboplatin + thiotepa + etoposide; CEM, carboplatin + etoposide + melphalan; CEM-TBI, CEM + total-body irradiation; CM, cyclophosphamide + melphalan; BM, busulfan + melphalan; TM, thiotepa + melphalan; CTM, carboplatin + TM; MIBG-TM, 131I-metaiodobenzylguanidine treatment + TM.
Definition of hematologic recovery
The timing of neutrophil recovery was defined as the first day when ANC exceeded 500/µL for three consecutive days. Platelet recovery was defined as the first day when the platelet count exceeded 20,000/µL without a transfusion for the previous seven days. RBC recovery was defined as the first day when the hemoglobin level exceeded 8.0 g/dL without a transfusion for the previous 28 days.
Serum ferritin and iron chelation treatment
Serum ferritin levels were measured within two weeks prior to each cycle of HDCT/autoSCT and one year after the second HDCT/autoSCT. From July 2007, in order to reduce toxicities during HDCT/autoSCT, patients over two years old received iron chelation treatment with deferasirox if their serum ferritin level exceeded 1,000 ng/mL prior to HDCT/autoSCT (10). However, iron chelation treatment was not given between the first and second HDCT/autoSCT or after the second HDCT/autoSCT.
Statistics
Patients were categorized into three groups according to the number of transplanted CD34+ cells. Differences in the proportions of patients in each group between the first and second HDCT/autoSCT were analyzed using a chi-squared test. Differences in continuous variables between groups during each cycle of HDCT/autoSCT were analyzed using the Kruskal-Wallis test. Differences in continuous variables between the first and second HDCT/autoSCT were analyzed using the Mann-Whitney U test. Multivariate analysis comprising clinical characteristics with hematologic recovery after tandem HDCT/autoSCT was performed using the Cox regression analysis. P values < 0.05 were considered statistically significant.
Ethics Statement
This study has been reviewed and approved by the Institutional Review Board of Samsung Medical Center (No. 2012-01-071). Informed consent was waived by the board.
RESULTS
Patient characteristics
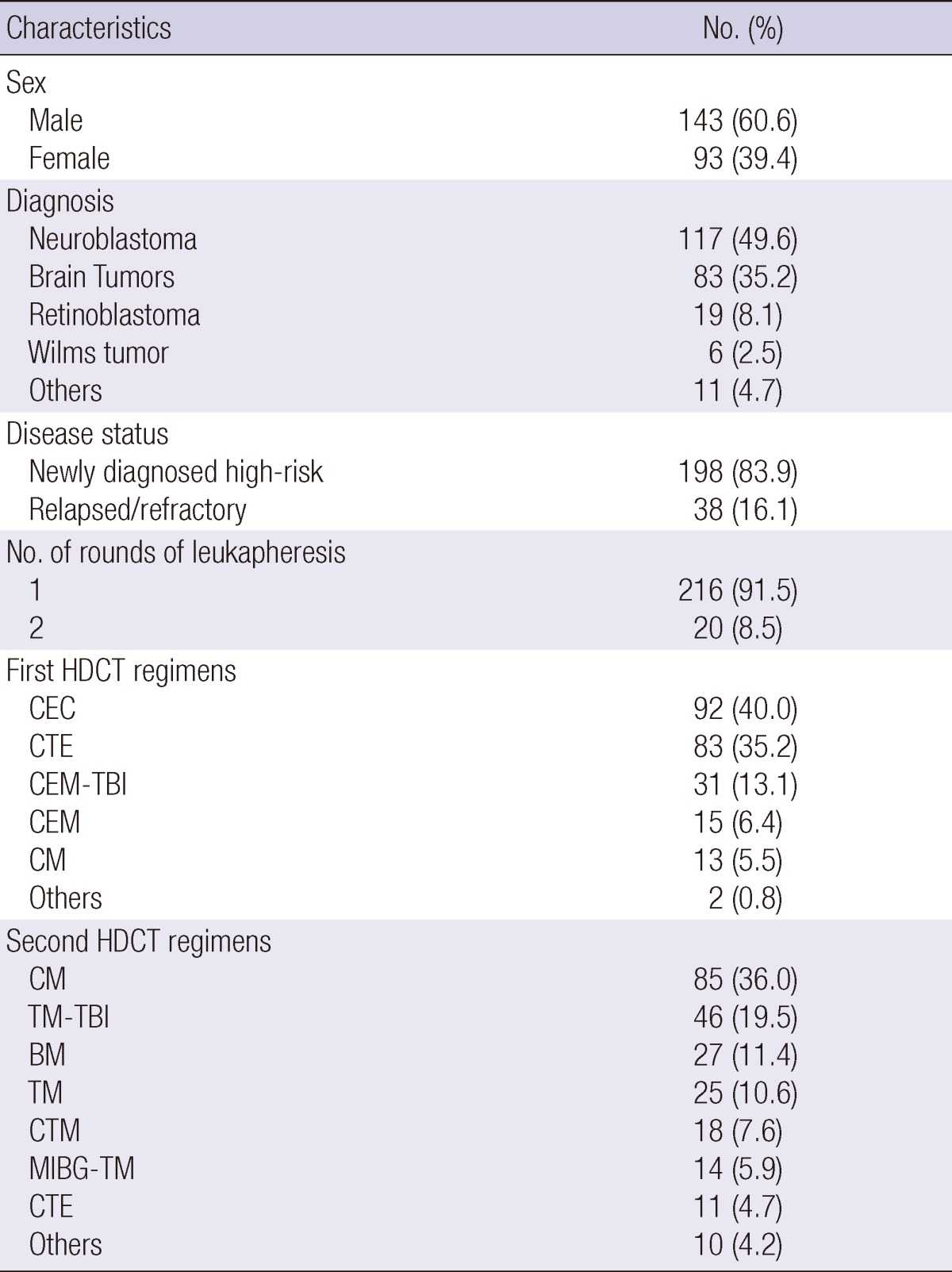
From April 1998 to June 2011, a total of 236 children with high-risk solid tumors underwent tandem HDCT/autoSCT. Table 1 lists patient characteristics. One hundred ninety-eight patients had newly diagnosed high-risk tumors and the remaining 38 patients had recurrent tumors. Neuroblastoma was the most frequent diagnosis, followed by brain tumors. The median age at the time of the first HDCT/autoSCT was 47 months (range 7-299). The median time interval between diagnosis and the first HDCT/autoSCT was 9 months (range 2-84). The median time interval from day 0 of the first HDCT/autoSCT to the day 0 of the second HDCT/autoSCT was 91 days (range 46-341). In 10 patients, conventional chemotherapy was given between the first and second HDCT/autoSCT because the patients were in partial response. When these 10 patients were not included in the analysis, the median time interval between the first and second HDCT/autoSCT was 91 days (range 46-188).
Stem cell collection
A median of 9.9×106 CD34+ cells/kg (range 0.9-338.1) were collected during the first round of leukapheresis. The median number of leukapheresis events during the first round was 4 (range 2-12). Twenty (8.5%) patients required a second round of leukapheresis to collect more CD34+ cells. A median of 2.0×106 CD34+ cells/kg (range 0.7-62.6) were collected during the second round of leukapheresis, and the median number of leukapheresis events during the second round was 4 (range 2-12). In total, we were able to collect a median of 9.9×106 CD34+ cells/kg (range 1.9-338.1) with a median of 4 leukapheresis events (range 2-19). It was possible to collect >10.0×106 CD34+ cells/kg in 116 (49.2%) patients and >4.0×106 CD34+ cells/kg in 196 (83.1%) patients.
Hematologic recovery after tandem HDCT/autoSCT
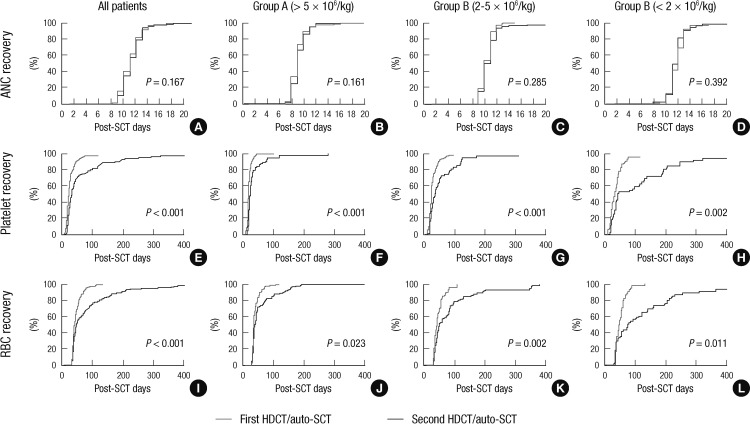
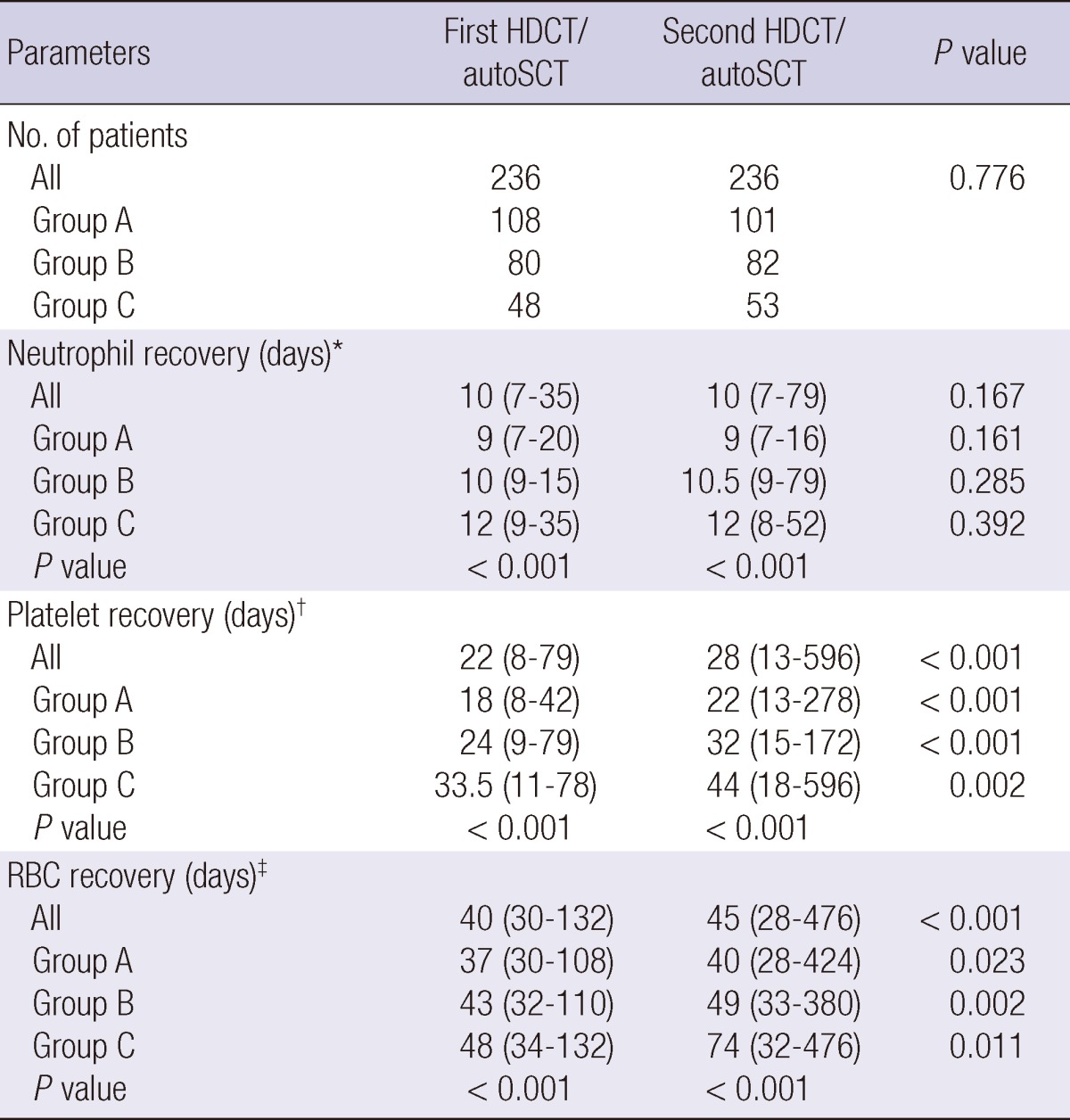
Table 2 lists the hematologic recovery after the first and second HDCT/autoSCT. The median number of CD34+ cells transplanted during the first HDCT/autoSCT was 4.3×106/kg (range 0.6-220.2), and during the second HDCT/autoSCT it was 4.1×106/kg (range 0.9-157.6) (P=0.664). Patients were divided into three groups according to the number of transplanted CD34+ cells (group A: >5×106/kg, group B: 2-5×106/kg, and group C: <2×106/kg). One hundred eighty-nine (80.1%) patients stayed in the same group during the second HDCT/autoSCT as they were in their original group during the first HDCT/autoSCT. The proportion of patients in each of the three groups was not significantly different between the first and second HDCT/autoSCT (P=0.776). Neutrophil, platelet, and RBC recoveries were significantly delayed when the number of transplanted CD34+ cells was low, especially if it was <2×106/kg both in the first and second HDCT/autoSCT.
Table 2.
Hematologic recovery by number of transplanted CD34+ cells
The number of CD34+ cells transplanted was > 5 × 106/kg in group A, 2-5 × 106/kg in group B, and < 2 × 106/kg in group C. *The first day when the absolute neutrophil count exceeded 500/µL for three consecutive days; †The first day when the platelet count exceeded 20,000/µL without a transfusion for the previous seven days; ‡The first day when the hemoglobin level exceeded 8.0 g/dL without a transfusion for the previous 28 days; HDCT/autoSCT, high-dose chemotherapy and autologous stem cell transplantation.
There was no difference in the neutrophil recovery between the first and second HDCT/autoSCT (median 10 vs 10 days, P=0.167). However, platelet and RBC recoveries took longer in the second HDCT/autoSCT compared to the first HDCT/autoSCT (Table 2 and Fig. 1). The time to reach a platelet count of 20,000/µL without a transfusion for the previous seven days was longer in the second HDCT/autoSCT than in the first HDCT/autoSCT (median 22 vs 28 days, P<0.001). The time to reach a hemoglobin level of 8.0 g/dL without a transfusion for the previous 28 days was also longer in the second HDCT/autoSCT than in the first HDCT/autoSCT (median 40 vs 45 days, P<0.001). Here again, the delay in the platelet and RBC recoveries after the second HDCT/auto-SCT was more prominent when the number of transplanted CD34+ cells was lower (P=0.001 and P=0.003, respectively).
Fig. 1.
Hematologic recovery based on the number of transplanted CD34+ cells. There are no differences in neutrophil recovery between the first and second HDCT/autoSCT (A-D). However, platelet recovery (E-H) and RBC recovery (I-L) are significantly delayed in the second HDCT/autoSCT compared to the first HDCT/autoSCT. The delays in the platelet and RBC recoveries after the second HDCT/autoSCT are more prominent when the number of transplanted CD34+ cells was lower, particularly if it was < 2 × 106/kg (H and L).
In the multivariate analysis for hematologic recovery after tandem HDCT/autoSCT, lower CD34+ cell dose was a significant risk factor for delayed neutrophil, platelet, and RBC recoveries. Second HDCT/autoSCT and total-body irradiation in the conditioning were also significant factors for delayed platelet and RBC recoveries, but not for neutrophil recovery (Table 3). There was no difference in neutrophil, platelet, and RBC recoveries according to the interval (<91 days versus ≥91 days) between the first and second HDCT/autoSCT (P=0.869, P=0.308, and P=0.864, respectively).
Table 3.
Multivariate analysis for hematologic recovery after tandem HDCT/autoSCT
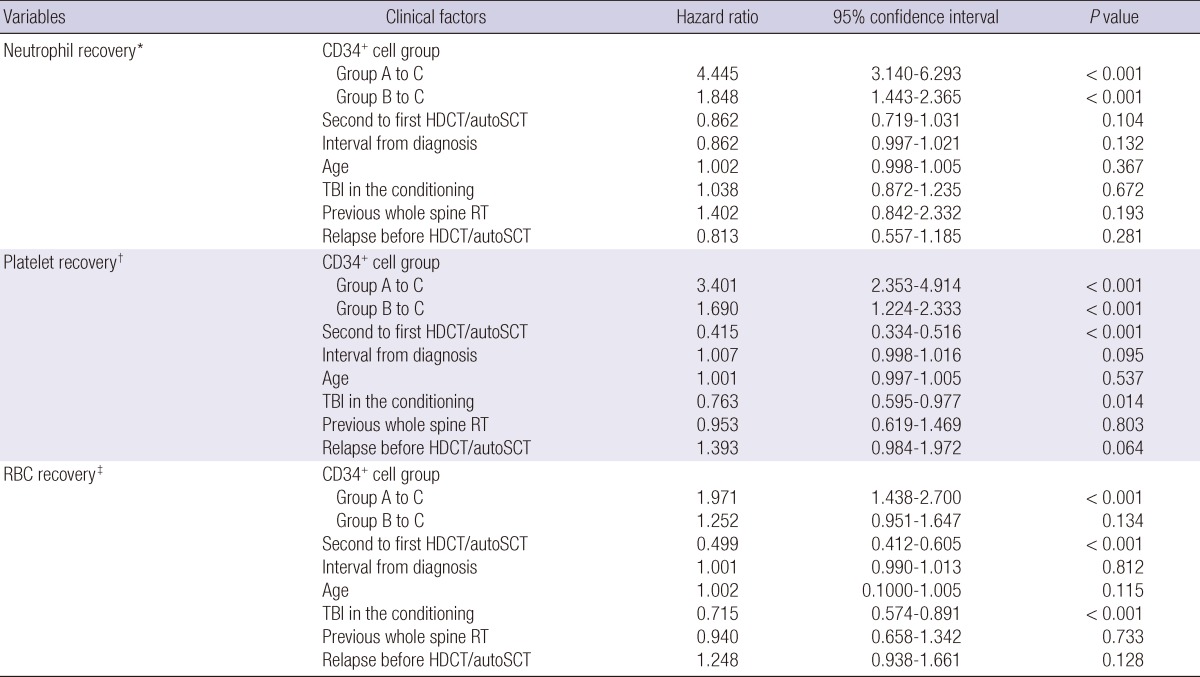
The number of CD34+ cells transplanted was >5×106/kg in group A, 2-5×106/kg in group B, and <2×106/kg in group C. *The first day when the absolute neutrophil count exceeded 500/µL for three consecutive days; †The first day when the platelet count exceeded 20,000/µL without a transfusion for the previous seven days; ‡The first day when the hemoglobin level exceeded 8.0 g/dL without a transfusion for the previous 28 days. HDCT/autoSCT, high-dose chemotherapy and autologous stem cell transplantation; TBI, total body irradiation; RT, radiotherapy.
Transfusion requirement and serum ferritin level
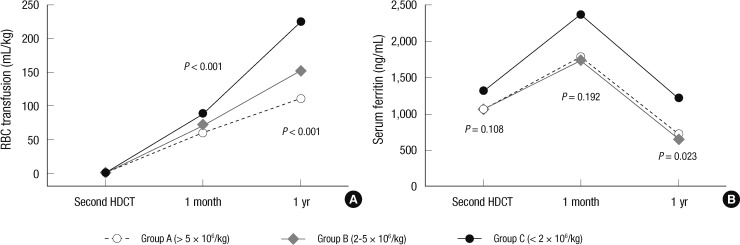
For the 163 patients who remained event free for at least one year after the second HDCT/autoSCT, Table 4 shows their one-year transfusion requirements and serum ferritin levels before the second HDCT/autoSCT and one year after the second HDCT/autoSCT (Fig. 2). The number of PC and RBC transfusions during the first year after the second HDCT/autoSCT was higher if the number of CD34+ cells transplanted in the second HDCT/autoSCT was lower, particularly if it was <2×106/kg (P<0.001 and P<0.001, respectively). Similarly, the serum ferritin level one year after the second HDCT/autoSCT was higher when the number of CD34+ cells transplanted in the second HDCT/autoSCT was lower (P=0.023) while there is no difference in serum ferritin level before the second HDCT/autoSCT (P=0.108).
Table 4.
Transfusions and ferritin levels during first year after the second HDCT/autoSCT
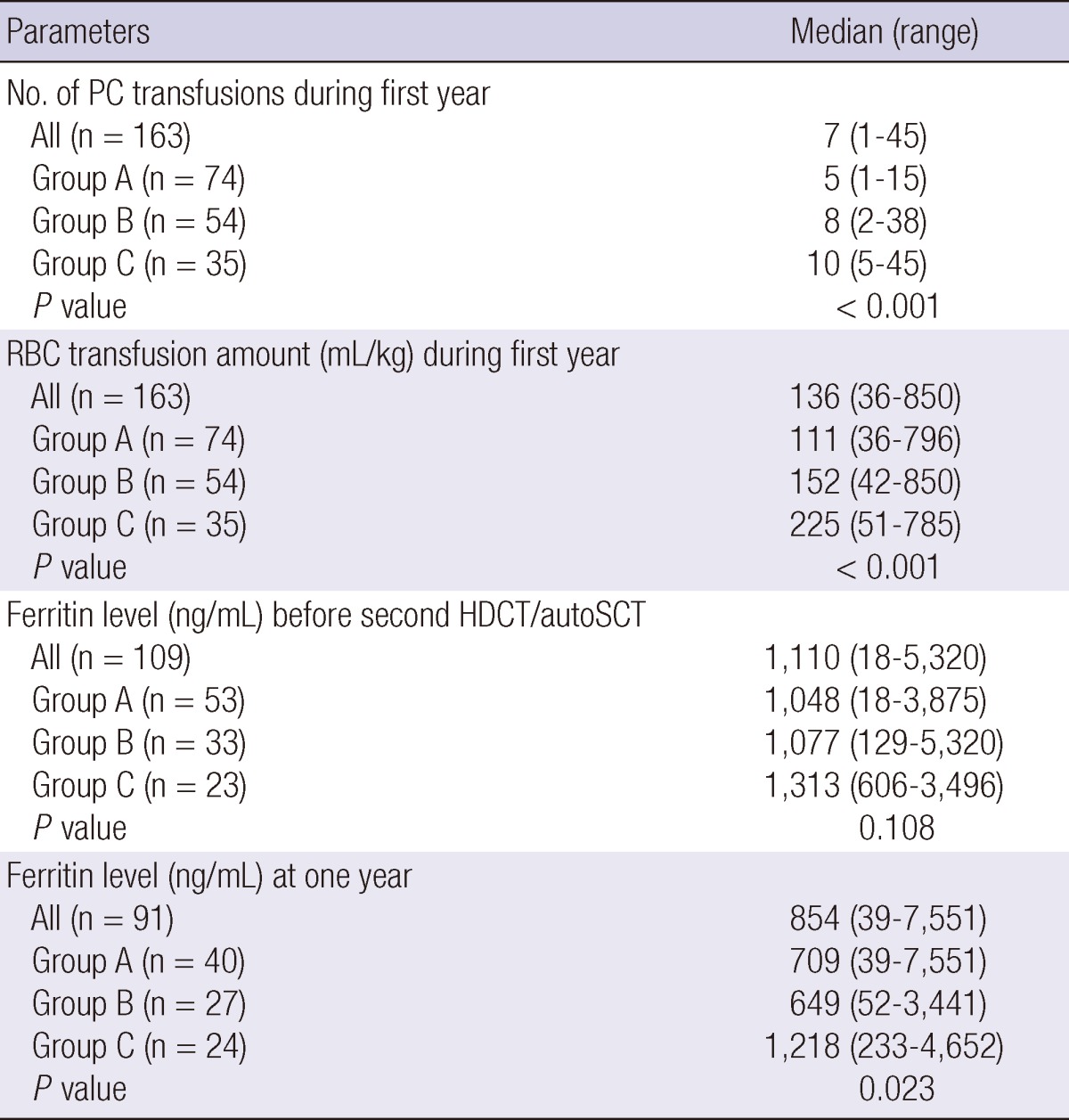
The number of CD34+ cells transplanted in the second HDCT/autoSCT was >5×106/kg in group A, 2-5×106/kg in group B, and <2×106/kg in group C. PC, platelet concentrate.
Fig. 2.
RBC transfusion amount (mL/kg) and serum ferritin level (ng/mL) after the second HDCT/autoSCT based on the number of transplanted CD34+ cells. (A) The RBC transfusion amount during the first year after the second HDCT/autoSCT is higher when the number of transplanted CD34+ cells was lower, particularly if it was < 2 × 106/kg. (B) The serum ferritin level one year after the second HDCT/autoSCT is higher when the number of CD34+ cells transplanted in the second HDCT/autoSCT was lower.
DISCUSSION
Although the number of studies using tandem HDCT/autoSCT for the treatment of high-risk pediatric solid tumors is increasing, documentation of hematologic recovery after tandem HDCT/autoSCT is very limited. This is the first study that has demonstrated that platelet and RBC recoveries, but not neutrophil recovery, in the second HDCT/autoSCT are significantly delayed compared to those in the first HDCT/autoSCT.
As most studies on hematologic recovery after SCT have reported (11-13), the present study confirms that a higher CD34+ cell count is associated with a faster hematologic recovery in both the first and second HDCT/autoSCT. Although it is difficult to define the minimum number of stem cells needed for successful hematologic recovery after SCT, a substantial number of patients in the present study were transplanted with <2×106 CD34+ cells/kg and consequently needed prolonged transfusion support. Although they eventually achieved hematologic recovery without the need for further transfusions, the number of transfusions and serum ferritin level were higher in these patients than in patients transplanted with a higher number of CD34+ cells.
In the present study, there was no difference in the number of CD34+ cells transplanted in the first and second HDCT/autoSCT, and there was no difference in neutrophil recovery between the first and second HDCT/autoSCT. However, platelet and RBC recoveries were significantly delayed in the second HDCT/autoSCT compared to the first HDCT/autoSCT. The delay in platelet and RBC recoveries was more prominent when the number of CD34+ cells transplanted in the second HDCT/autoSCT was lower. Delayed platelet and RBC recoveries resulted in prolongation of the need for transfusion support and significant iron overload following completion of tandem HDCT/autoSCT. The delayed recovery in the second HDCT/autoSCT may be related to BM damage during the first HDCT/autoSCT (9). Therefore, our findings suggest that more CD34+ cells need to be transplanted in the second HDCT/autoSCT in order to achieve the same hematologic recovery as in the first HDCT/autoSCT.
Although our results demonstrated that a higher CD34+ cell count is associated with a faster hematologic recovery, it was difficult to collect sufficient number of stem cells in many patients. It was possible to collect >10×106 CD34+ cells/kg only in about half of patients and the number of CD34+ cells collected was <4×106/kg in 40 (16.9%) patients. For such poor mobilizers, plerixafor might be helpful in collecting more CD34+ cells. Recent studies demonstrated that more CD34+ cells can be collected with G-CSF + plerixafor than with G-CSF alone (14, 15). However, its efficacy and toxicity have not been validated in children with solid tumors.
Infection and bleeding are severe complications that can occur during the relatively early period after SCT. Therefore, prompt neutrophil and platelet recoveries have been prerequisites for successful SCT and most previous studies have used the times to reach an ANC of 500/µL or a platelet count of 20,000/µL as parameters of hematologic recovery (11-13). Also, as the number of long-term survivors after SCT increases, the clinical importance of RBC recovery is increasing. Iron overload from multiple transfusions is generally unavoidable after SCT, and sustained iron overload can result in various late adverse effects including abnormal liver function tests, hepatic fibrosis/cirrhosis, cardiac dysfunction, and increased susceptibility to infection (16-21). Furthermore, sustained iron overload may potentiate late adverse effects from chemo-radiotherapy. Iron overload and its late adverse effects may be more prominent following tandem HDCT/autoSCT compared to single HDCT/autoSCT. However, a parameter defining RBC recovery after SCT has not yet been proposed. For this reason, we developed a parameter to define RBC recovery after SCT. Although the transfusion-free duration (28 days) prior to reaching a hemoglobin level of 8.0 g/dL was arbitrarily determined in the present study, our metric was associated with the number of transplanted CD34+ cells and serum ferritin level one year after the second HDCT/autoSCT. To our knowledge, this is the first study to propose a parameter for RBC recovery after SCT.
In summary, platelet and RBC recoveries are significantly delayed in the second HDCT/autoSCT compared to the first HDCT/autoSCT. Therefore, our findings suggest that more CD34+ cells need to be transplanted in the second HDCT/autoSCT in order to achieve the same hematologic recovery as in the first HDCT/autoSCT. Also, the novel parameter we used to assess RBC recovery is associated with the number of transplanted CD34+ cells, RBC transfusion requirements, and post-SCT serum ferritin level. Further studies are needed to define the optimal parameter to assess RBC recovery after SCT.
ACKNOWLEDGMENTS
We would like to thank Miss So Yeon Lee for her assistance with data collection. The authors have no conflicts of interest to disclose.
References
- 1.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, et al. Children's Cancer Group. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 2.Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, Klingebiel T, Kremens B, Schilling FH, Schrappe M, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomized controlled trial. Lancet Oncol. 2005;6:649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 3.Pritchard J, Cotterill SJ, Germond SM, Imeson J, de Kraker J, Jones DR. High dose melphalan in the treatment of advanced neuroblastoma: results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatr Blood Cancer. 2005;44:348–357. doi: 10.1002/pbc.20219. [DOI] [PubMed] [Google Scholar]
- 4.George RE, Li S, Medeiros-Nancarrow C, Neuberg D, Marcus K, Shamberger RC, Pulsipher M, Grupp SA, Diller L. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006;24:2891–2896. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 5.Kletzel M, Katzenstein HM, Haut PR, Yu AL, Morgan E, Reynolds M, Geissler G, Marymount MH, Liu D, Kalapurakal JA, et al. Treatment of high-risk neuroblastoma with triple-tandem high-dose therapy and stem-cell rescue: results of the Chicago Pilot II Study. J Clin Oncol. 2002;20:2284–2292. doi: 10.1200/JCO.2002.06.060. [DOI] [PubMed] [Google Scholar]
- 6.Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 7.Sung KW, Lee SH, Yoo KH, Jung HL, Cho EJ, Koo HH, Lee SK, Kim J, Lim DH, Suh YL, et al. Tandem high-dose chemotherapy and autologous stem cell rescue in patients over 1 year of age with stage 4 neuroblastoma. Bone Marrow Transplant. 2007;40:37–45. doi: 10.1038/sj.bmt.1705691. [DOI] [PubMed] [Google Scholar]
- 8.Sung KW, Yoo KH, Cho EJ, Koo HH, Lim DH, Shin HJ, Ahn SD, Ra YS, Choi ES, Ghim TT. High-dose chemotherapy and autologous stem cell rescue in children with newly diagnosed high-risk or relapsed medulloblastoma or supratentorial primitive neuroectodermal tumor. Pediatr Blood Cancer. 2007;48:408–415. doi: 10.1002/pbc.21064. [DOI] [PubMed] [Google Scholar]
- 9.Banfi A, Bianchi G, Galotto M, Cancedda R, Quarto R. Bone marrow stromal damage after chemo/radiotherapy: occurrence, consequences and possibilities of treatment. Leuk Lymphoma. 2001;42:863–870. doi: 10.3109/10428190109097705. [DOI] [PubMed] [Google Scholar]
- 10.Chueh HW, Sung KW, Lee SH, Yoo KH, Koo HH, Kim JY, Cho EJ. Iron chelation treatment with deferasirox prior to high-dose chemotherapy and autologous stem cell transplantation may reduce the risk of hepatic veno-occlusive disease in children with high-risk solid tumors. Pediatr Blood Cancer. 2011;58:441–447. doi: 10.1002/pbc.23198. [DOI] [PubMed] [Google Scholar]
- 11.Siena S, Schiavo R, Pedrazzoli P, Carlo-Stella C. Therapeutic relevance off CD34 cell dose in blood cell transplantation for cancer therapy. J Clin Oncol. 2000;18:1360–1377. doi: 10.1200/JCO.2000.18.6.1360. [DOI] [PubMed] [Google Scholar]
- 12.Klaus J, Herrmann D, Breitkreutz I, Hegenbart U, Mazitschek U, Egerer G, Cremer FW, Lowenthal RM, Huesing J, Fruehauf S, et al. Effect of CD34 cell dose on hematopoietic reconstitution and outcome in 508 patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Eur J Haematol. 2007;78:21–28. doi: 10.1111/j.0902-4441.2006.t01-1-EJH2895.x. [DOI] [PubMed] [Google Scholar]
- 13.Mavroudis D, Read E, Cottler-Fox M, Couriel D, Molldrem J, Carter C, Yu M, Dunbar C, Barrett J. CD34+ cell dose predicts survival, posttransplant morbidity, and rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies. Blood. 1996;88:3223–3229. [PubMed] [Google Scholar]
- 14.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, Nademanee A, McCarty J, Bridger G, Calandra G 3101 Investigators. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27:4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 15.Nademanee AP, Dipersio JF, Maziarz RT, Stadtmauer EA, Micallef IN, Stiff PJ, Hsu FJ, Bridger G, Bolwell BJ. Plerixafor plus granulocyte colony-stimulating factor versus placebo plus granulocyte colony-stimulating factor for mobilization of CD34+ hematopoietic stem cells in patients with multiple myeloma and low peripheral blood CD34+ cell count: results of a subset analysis of a randomized trial. Biol Blood Marrow Transplant. 2012;18:1564–1572. doi: 10.1016/j.bbmt.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Shander A, Cappellini MD, Goodnough LT. Iron overload and toxicity: the hidden risk of multiple blood transfusions. Vox Sang. 2009;97:185–197. doi: 10.1111/j.1423-0410.2009.01207.x. [DOI] [PubMed] [Google Scholar]
- 17.Fraga CG, Oteiza PI. Iron toxicity and antioxidant nutrients. Toxicology. 2002;180:23–32. doi: 10.1016/s0300-483x(02)00379-7. [DOI] [PubMed] [Google Scholar]
- 18.Borgna-Pignatti C, Cappellini MD, De Stefano P, Del Vecchio GC, Forni GL, Gamberini MR, Ghilardi R, Origa R, Piga A, Romeo MA, et al. Survival and complications in thalassemia. Ann N Y Acad Sci. 2005;1054:40–47. doi: 10.1196/annals.1345.006. [DOI] [PubMed] [Google Scholar]
- 19.Harmatz P, Butensky E, Quirolo K, Williams R, Ferrell L, Moyer T, Golden D, Neumayr L, Vichinsky E. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood. 2000;96:76–79. [PubMed] [Google Scholar]
- 20.Fenaux P, Rose C. Impact of iron overload in myelodysplastic syndromes. Blood Rev. 2009;23:S15–S19. doi: 10.1016/S0268-960X(09)70005-0. [DOI] [PubMed] [Google Scholar]
- 21.Bae SJ, Kang C, Sung KW, Chueh HW, Son MH, Lee SH, Yoo KH, Koo HH. Iron overload during follow-up after tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk neuroblastoma. J Korean Med Sci. 2012;27:363–369. doi: 10.3346/jkms.2012.27.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]