Abstract
The association between microalbuminuria (MAU) and the indices of macrovascular complication in patients with newly diagnosed type 2 diabetes (D) or essential hypertension (H) was evaluated. Total 446 patients were classified into four groups according to the urinary albumin-to-creatinine ratio: MAU-D (n = 104), normoalbuminuria (NAU)-D (n = 114), MAU-H (n = 116), and NAU-H (n = 112). The indices of macrovascular complication including arterial stiffness evaluated by pulse-wave-velocity (PWV), carotid intima-media thickness (IMT), and vascular inflammation marked by high-sensitivity C-reactive protein (hsCRP) were assessed. PWV, IMT, and hsCRP were higher in patients with MAU than in those with NAU in both diabetes and hypertension groups. In both MAU-D and MAU-H groups, PWV and hsCRP levels were positively correlated with MAU level (MAU-D: r = 0.47, 0.41, MAU-H: r = 0.36, 0.62, respectively, P < 0.05). Additionally, PWV and hsCRP were independent factors predicting MAU (diabetes group: OR 1.85, 1.54, hypertension group: OR 1.38, 1.51, respectively, P < 0.001), but not IMT. MAU is independently associated with arterial stiffness and vascular inflammation but not with IMT in patients with newly diagnosed type 2 diabetes or essential hypertension, which emphasizes the importance of proactive clinical investigations for atherosclerotic complications in patients with MAU, even in newly diagnosed diabetes or hypertension.
Keywords: Albuminuria, Vascular Stiffness, Carotid Intima-Media Thickness, Inflammation
INTRODUCTION
Patients with type 2 diabetes or essential hypertension generally have a substantial burden of the macrovascular complication including atherosclerotic cardiovascular disease (CVD) (1, 2). Despite the successful adoption of evidence-based strategies, a greater number of patients are not appropriately identified before their first attack, or they continue to experience cardiovascular events despite optimal managements (1, 2). Thus, there has been mounting interest in the early assessment of the risk for macrovascular atherosclerotic complications in patients with diabetes or hypertension (1-3).
Several indices including arterial stiffness, vascular inflammatory markers, and carotid intima-media thickness (IMT) have been applied to estimate the macrovascular complication (4, 5). Arterial stiffness plays an important role in the occurrence of atherosclerotic CVD (3, 4). The measurement of pulse wave velocity (PWV) has been generally accepted as the gold standard for determining arterial stiffness (5, 6). Moreover, this surrogate index of large artery compliance has been demonstrated to predict cardiovascular morbidity and mortality in a variety of populations (3-7). Additionally, increased IMT has also been reported as a risk factor for future cardiovascular events in patients with diabetes or hypertension (8).
Meanwhile, microalbuminuria (MAU) is regarded as an early index of generalized microvascular impairment including endothelial dysfunction, which reflects subclinical vascular abnormalities of renal glomeruli (4, 9). The detection of MAU is the main clue for the early recognition and treatment of clinically evident microvascular complications in diabetic or hypertensive patients (2, 10). Furthermore, MAU is also highly associated with an increased risk of atherosclerotic CVD in patients with type 2 diabetes or essential hypertension (5, 9).
The positive, independent associations between MAU, a marker of microvascular dysfunction, and the indices of macrovascular complication such as PWV, IMT, and serum high-sensitivity C-reactive protein (hsCRP) in patients with type 2 diabetes or essential hypertension have been reported in several previous studies (3, 5, 11-13). However, some other studies showed conflicting results, especially for the association between MAU and IMT (6, 14). Additionally, many previous studies included the patients with longstanding diabetes or hypertension or with unclear duration of diseases as study subjects. Thus, it is yet to be determined whether the marker of microvascular dysfunction is positively associated with all the indices of macrovascular complications, especially in patients with early stage or newly diagnosed type 2 diabetes or essential hypertension. Consequently, we investigated the association between the presence of MAU and the indices of macrovascular complication such as PWV, IMT, and hsCRP in patients with newly diagnosed type 2 diabetes or essential hypertension who had never been treated.
MATERIALS AND METHODS
Study population
Of the patients who had been receiving regular medical checkups at the health promotion centers of Incheon St. Mary's Hospital and Seoul St. Mary's Hospital from April 2009 to June 2012, only those newly diagnosed with type 2 diabetes or essential hypertension within the prior 12 months were enrolled consecutively in this study. They had never received medical treatments for diabetes or hypertension. All medical documents of the patients including the health check-up records were reviewed. Through this process, we excluded those who have had diabetes or hypertension more than 12 months as well as those with unclear duration of diseases due to absence or uncertainty of medical records. Type 2 diabetes was diagnosed according to the level of fasting blood glucose (FBG) or the result of additional 75 g oral glucose tolerance test (OGTT) (15). For the patients with FBG levels ≥ 126 mg/dL on their medical check-ups, an additional check of FBG or a 75 g OGTT was performed. The patients who had additionally met the following criteria of rechecked FBG ≥ 126 mg/dL or 2-hr postload glucose ≥ 200 mg/dL in OGTT were diagnosed as diabetes. We did not use the diagnostic criteria according to the level of hemoglobin A1c. Essential hypertension was diagnosed according to the criteria of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (16). Patients with type 1 diabetes or secondary hypertension were excluded based on the clinical and laboratory findings. Patients who manifested diabetes and hypertension simultaneously at the time of inclusion were also excluded. Other exclusion criteria were age < 18 or > 65 yr; evidence of clinically evident nephropathy (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2 or overt proteinuria) (17); previous therapy for hypertension or diabetes; electrocardiographic or echocardiographic left ventricular hypertrophy (LVH); and positive history or clinical signs of atherosclerotic CVD, heart failure, infection, and neoplastic or inflammatory diseases.
Baseline biochemical assays
In all the subjects, a fasting venous blood sample was taken to estimate the routine blood chemistry including the level of hsCRP. The serum hsCRP was measured via the commercially available enzyme-linked immunosorbent assay (ELISA) (5). From all subjects enrolled who were newly diagnosed as type 2 diabetes or essential hypertension, the first morning spot urine samples were collected for 3 consecutive days before the initial medical therapy to determine the urinary albumin-to-creatinine ratio (ACR). ACR for each subject was determined as the mean value for 3 days. The urinary albumin concentration was measured by subjecting a spot urine sample to a latex agglutination immunoassay on an auto-analyzer (LX-6000®, Eiken Kagaku Co., Tokyo, Japan). The urinary creatinine concentration was measured through an enzymatic method. To define MAU, we used the ACR cut-off values of 30-300 mg/g for both men and women (4). The ACR of 30-300 mg/g was defined as MAU. Normoalbuminuria (NAU) was defined as the ACR of less than 30 mg/g and overt proteinuria (or macroalbuminuria) was defined as the ACR of more than 300 mg/g (4). The baseline renal function was estimated based on the eGFR, using the Cockcroft-Gault formula (18).
Classification of the study groups
According to the criteria of normo- and microalbuminuria, all the subjects were classified into four groups: diabetes with microalbuminuria (MAU-D), hypertension with microalbuminuria (MAU-H), diabetes with normoalbuminuria (NAU-D), and hypertension with normoalbuminuria (NAU-H).
Measurement of PWV
A volume-plethysmographic apparatus (VP2000, Colin Co. Ltd., Komaki, Japan) was used to measure the blood pressure (BP), electrocardiogram, heart sounds, and bilateral PWV. The bilateral brachial-ankle PWV was measured in all study subjects using the previously validated method (4, 14, 19) on the first day of taking a urine sample. The subjects were examined in the supine position. A microphone for detecting heart sounds was placed on the left edge of the sternum, and electrocardiogram electrodes were placed on both forearms. Doppler sensors were placed on the right carotid artery and right femoral artery. Cuffs were wrapped on both the brachials and ankles. Both were connected to a plethysmographic sensor to determine the pulse volume waveforms, with an oscillometric pressure sensor measuring the BP. The PWV sample acquisition frequency was set at 1,200 Hz. The volume waveforms for the bilateral brachial and ankle arteries were stored. Repeated samples were taken at 10-second intervals via an automatic gain analysis and quality adjustment. Finally, the mean values of the right and left PWV were calculated for the final analysis.
Measurement of IMT
Ultrasonographic scanning was also performed simultaneously with PWV to determine the carotid IMT and other routine echocardiographic parameters, all of which were analyzed in the core laboratory by the same examiner. Carotid duplex scanning was performed via high-resolution ultrasonography (Vivid 7®, GE Medical Systems, Milwaukee, WI, USA), using a 12 MHz inline scanner to estimate the IMT in all the subjects. The carotid arteries were scanned bilaterally in longitudinal and transverse projections. The site of the greatest distance between the lumenintimal interface and the media-adventitial interface (IMT) was measured in the right and left carotid arteries. IMT measurement was performed on at least three standard sites on the far wall of the common carotid artery (CCA) and carotid bulb (6, 8, 19). The first site was measured from the take-off of the CCA to a point 2 cm proximal to the carotid bulb, and the second was from 2 cm proximal to the carotid bulb to the beginning of the carotid bulb. The third measurement was performed from the beginning of the carotid bulb to the bifurcation of the internal and external carotid arteries. The selected images for these sites were printed for the measurement of the mean values of the right and left carotid IMT. The mean values of the right and left carotid IMT were used for the final analysis. Apparently, the focally extruded plaque was excluded in the estimation.
Statistical analysis
Continuous variables were expressed as means ± SD. The statistical differences were compared using an unpaired Student's t-test. The levels of triglyceride, hsCRP, and ACR were expressed as median (range) and a Mann-Whitney U test was used to determine the statistical differences. Categorical variables were presented as frequency counts, and the results of the intergroup comparisons were analyzed through a chi-square test. The degrees of correlation between the variables were assessed using the Pearson's correlation coefficient analysis (r). Multivariate logistic regression analysis was performed for the identification of the independent factors for MAU. Triglyceride, hsCRP, and ACR were natural logarithmically transformed to permit application of normal distribution statistics. Variables for univariate analysis were selected based on the previous reports having similar issues (5, 6, 14). Univariate variables with a P < 0.20 were entered into multivariate logistic models. The odds ratio and 95% confidence intervals were also calculated. The statistical significance was set at P < 0.05. All the statistical analyses were performed using the SPSS statistical software, version 18.0 (Chicago, IL, USA).
Ethics statement
The study protocol of this two-center observational study was reviewed and approved by the institutional review board of the Catholic University of Korea (IRB No. XC11EIMI0022K). All of the participants had submitted their written informed consent.
RESULTS
A total of 558 patients newly diagnosed as type 2 diabetes or essential hypertension were enrolled during the study period (the duration of diseases < 12 months). Out of these patients, 112 patients were excluded because they manifested diabetes and hypertension simultaneously (n = 56), lacked fully available medical information that included previous laboratory findings (n = 31), had clinically evident nephropathy (n = 20) and met other exclusion criteria (n = 5). Therefore, the final analysis was performed on a total of 446 patients (79.9%). MAU was observed in 220 (49.3%) patients (104 in MAU-D group and 116 in MAU-H group), and 226 demonstrated NAU (114 in NAU-D group and 112 in NAU-H group). Table 1 shows the baseline characteristics of both MAU and NAU groups. In most patients, the duration of the disease was less than six months (58.2% in MAU group and 70.8% in NAU group). No significant difference was observed between the two groups in terms of the baseline characteristics, except for the frequency of current smoking and the levels of systolic blood pressure, fasting blood glucose and hemoglobin A1c (Table 1). However, the levels of PWV, IMT, and hsCRP were significantly higher in MAU group than in NAU group (P < 0.001, Table 1).
Table 1.
Baseline characteristics of MAU and NAU groups
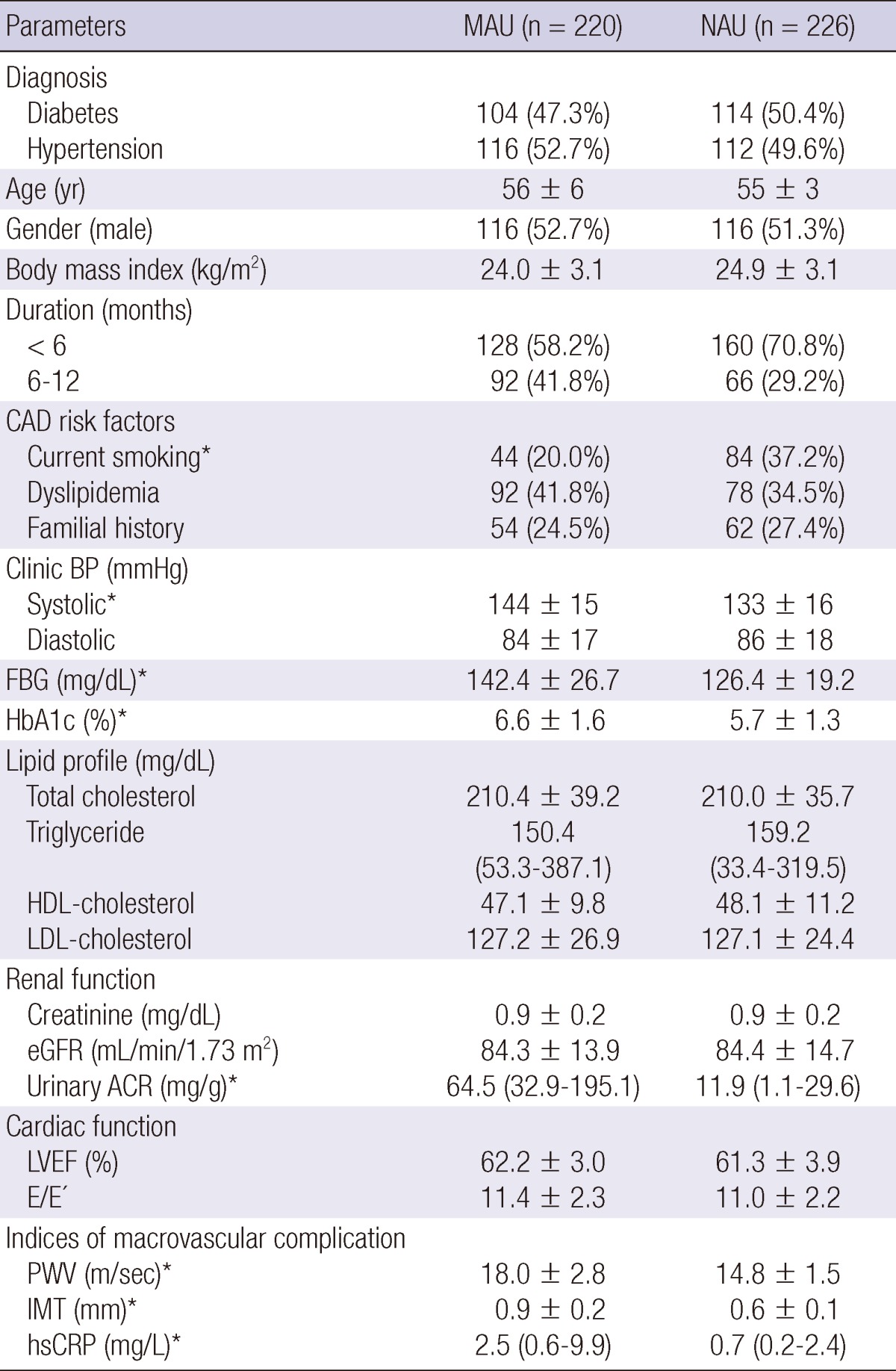
*Significant difference between MAU and NAU groups (P < 0.05). MAU, microalbuminuria; NAU, normoalbuminuria; CAD, coronary artery disease; BP, blood pressure; FBG, fasting blood sugar; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; ACR, albumin-to-creatinine ratio; LVEF, left ventricle ejection fraction; PWV, brachial-ankle pulse wave velocity; IMT, carotid intima-media thickness; hsCRP, high-sensitivity C-reactive protein.
In comparing MAU-D and NAU-D groups, no significant differences were seen in most of the baseline characteristics, except for the frequency of coronary artery disease familial history and current smoking. However, MAU-D group had the higher levels of fasting blood glucose and hemoglobin A1c than NAU-D group (Table 2). The mean PWVs were 18.8 ± 2.8 m/sec in MAU-D group and 15.5 ± 1.4 m/sec in NAU-D group (P < 0.001). The carotid IMT and hsCRP levels were also significantly higher in MAU-D group than in NAU-D group (0.9 ± 0.2 mm vs 0.6 ± 0.1 mm and 3.2 (1.1-4.4) mg/L vs 0.9 (0.5-1.9) mg/L, respectively; P < 0.001, Table 2).
Table 2.
Baseline characteristics of MAU-D, NAU-D, MAU-H and NAU-H groups
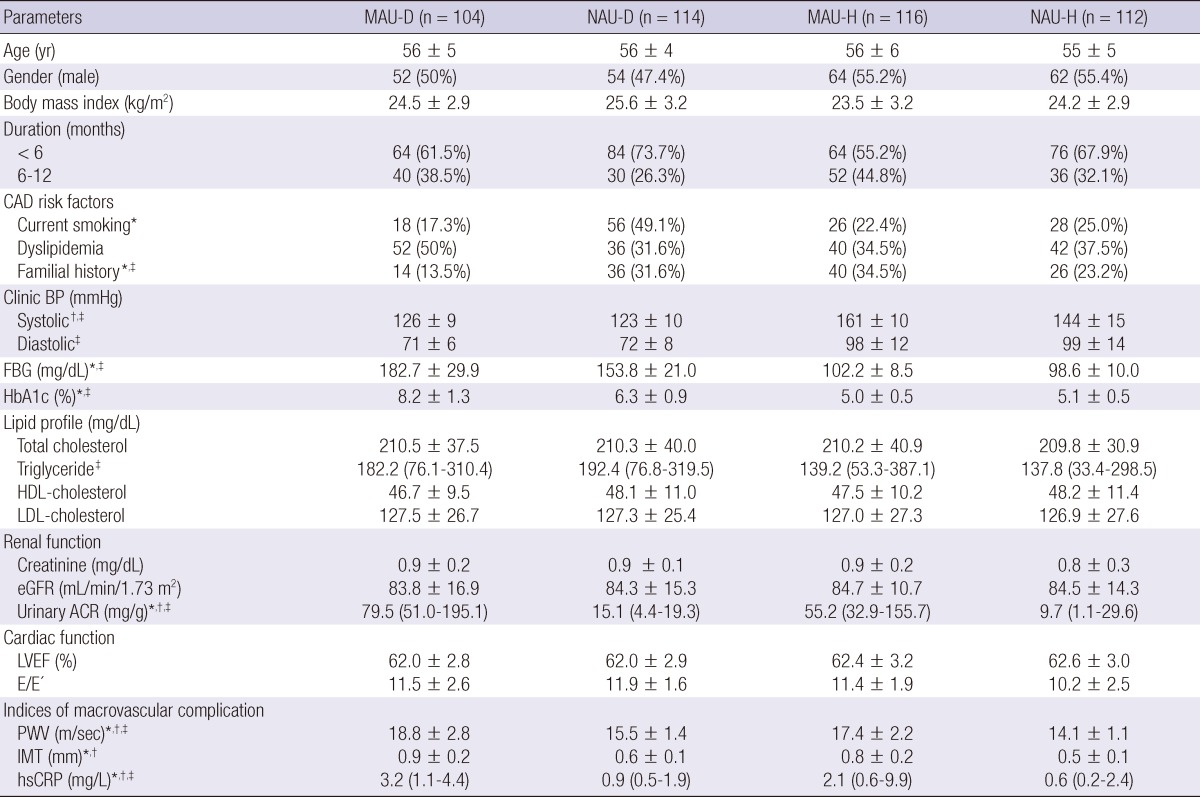
*Significant difference between MAU-D and NAU-D groups (P < 0.05); †Significant difference between MAU-H and NAU-H groups (P < 0.05); ‡Significant difference between MAU-D and MAU-H groups (P < 0.05). MAU-D, diabetes with microalbuminuria; NAU-D, diabetes with normoalbuminuria; MAU-H, hypertension with microalbuminuria; NAU-H, hypertension with normoalbuminuria; CAD, coronary artery disease; BP, blood pressure; FBG, fasting blood sugar; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; ACR, albumin-to-creatinine ratio; LVEF, left ventricle ejection fraction; PWV, brachial-ankle pulse wave velocity; IMT, carotid intima-media thickness; hsCRP, high-sensitivity C-reactive protein.
In comparison between MAU-H and NAU-H groups, the level of systolic blood pressure was higher in MAU-H group than in NAU-H group (Table 2). MAU-H group demonstrated higher levels of PWV, IMT, and hsCRP than NAU-H group (17.4 ± 2.2 m/sec vs 14.1 ± 1.1 m/sec, 0.8 ± 0.2 mm vs 0.5 ± 0.1 mm, and 2.1 (0.6-9.9) mg/L vs 0.6 (0.2-2.4) mg/L, respectively; P < 0.001, Table 2).
To estimate the differences in the association of MAU with the indices of macrovascular complications between the diabetic and hypertensive groups, MAU-D group and MAU-H group were compared (Table 2). The IMT level was not statistically different between the two groups (P = 0.18). However, MAU-D group had significantly higher levels of PWV and hsCRP than MAU-H group (PWV: P = 0.02, hsCRP: P = 0.03).
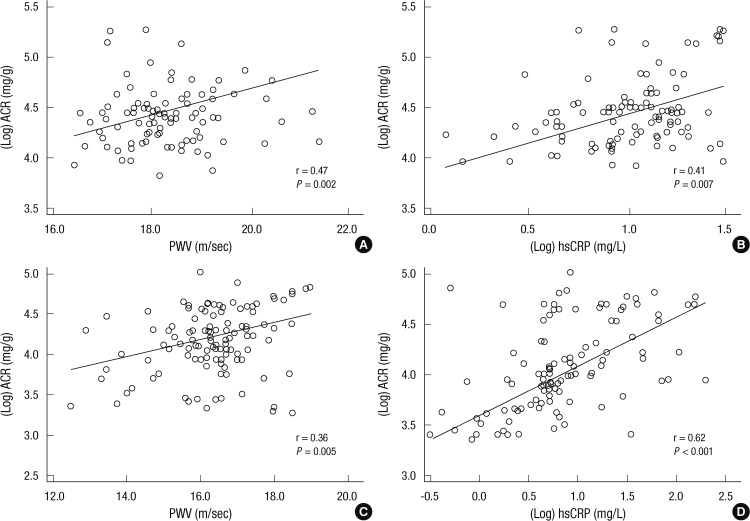
Table 3 and Fig. 1 demonstrate the correlation between the MAU level (Log ACR) and the levels of PWV, IMT, and hsCRP in MAU-D and MAU-H groups. In MAU-D group, the MAU level showed a positive correlation with the PWV and hsCRP levels (PWV: r = 0.47; P = 0.002, hsCRP: r = 0.41; P = 0.007, respectively) but not with the IMT level (r = 0.22; P = 0.18). In MAU-H group, the MAU level was also shown to be positively correlated with the PWV and hsCRP levels (PWV: r = 0.36; P = 0.005, hsCRP: r = 0.62, P < 0.001, respectively) but not with the IMT level (r = 0.17; P = 0.19).
Table 3.
Correlation between the level of MAU and the levels of other predictors
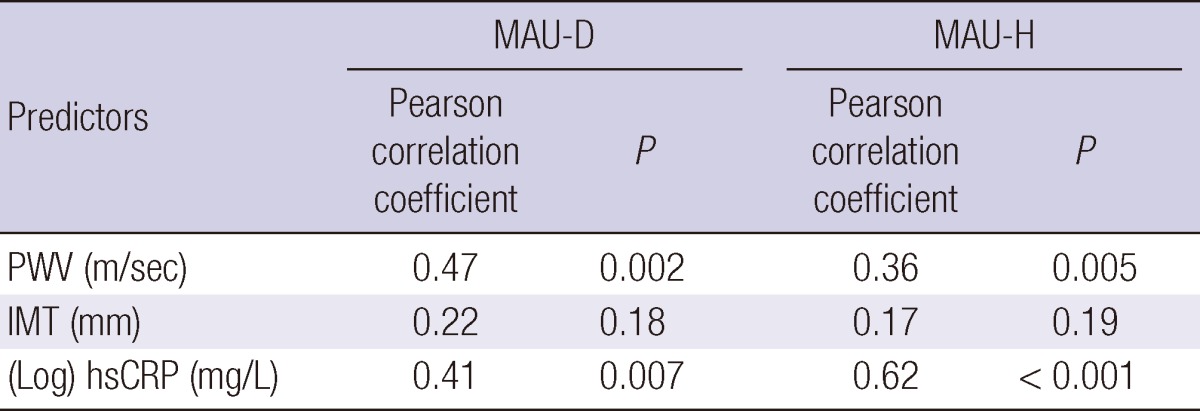
The level of MAU = (Log) ACR (mg/g). MAU-D, diabetes with microalbuminuria; NAU-D, diabetes with normoalbuminuria; MAU-H, hypertension with microalbuminuria; NAU-H, hypertension with normoalbuminuria; PWV, brachial-ankle pulse wave velocity; IMT, carotid intima-media thickness; hsCRP, high-sensitivity C-reactive protein; ACR, albumin-to-creatinine ratio.
Fig. 1.
Correlations between the level of MAU and the levels of PWV and hsCRP. In both MAU-D (A, B) and MAU-H (C, D) groups, the MAU level (Log ACR) was positively correlated with the levels of PWV and hsCRP. MAU-D, diabetes with microalbuminuria; MAU-H, hypertension with microalbuminuria; ACR, albumin-to-creatinine ratio; PWV, brachial-ankle pulse wave velocity; hsCRP, high-sensitivity C-reactive protein.
Multivariate logistic regression analysis was performed to determine the independent factors related to MAU (Table 4). The analysis demonstrated that PWV and hsCRP were the independent factors related to MAU both in the diabetes group (OR 1.85, 1.54, respectively; P < 0.001) and in the hypertensive group (OR 1.38, 1.51, respectively; P < 0.001). Serum fasting blood glucose in the diabetes group and systolic BP in the hypertensive group were also revealed as the independent factors related to MAU (OR 1.21; P = 0.04, OR 1.26; P = 0.03, respectively), but IMT was not an independent factor.
Table 4.
Independent factors related to MAU
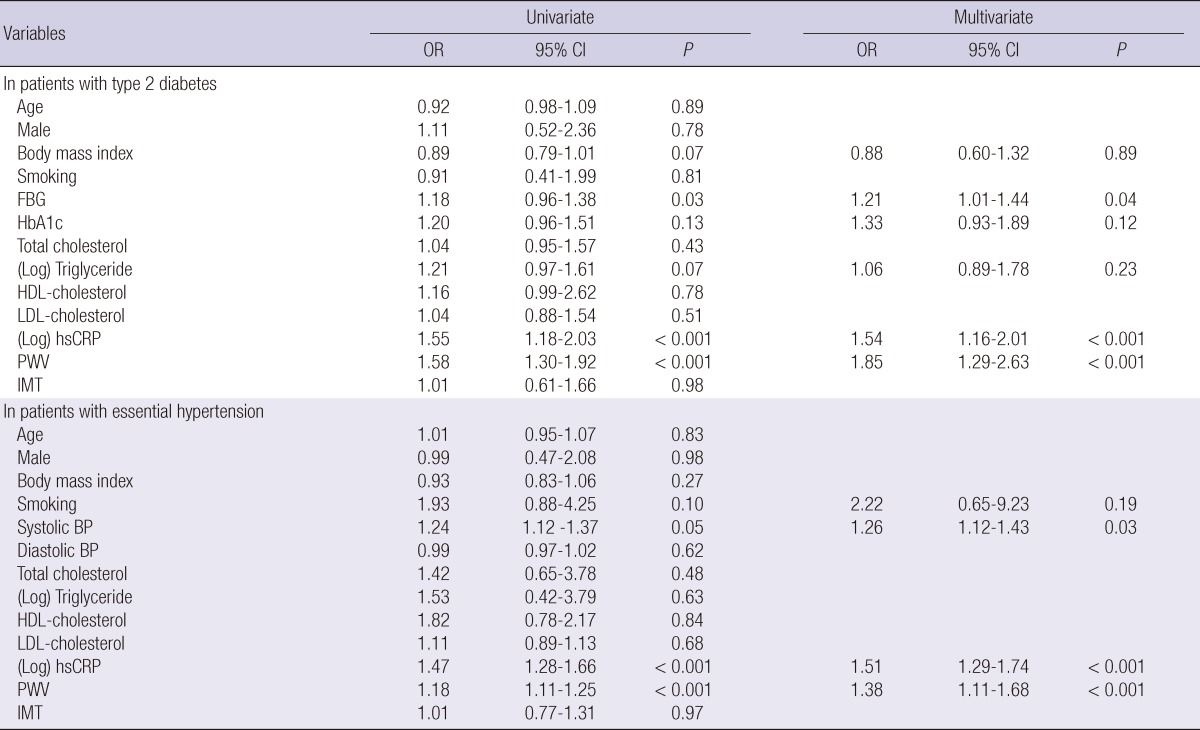
OR, odds ratio; CI, confidence interval; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; PWV, brachial-ankle pulse wave velocity; IMT, carotid intima-media thickness; BP, blood pressure.
DISCUSSION
In the present study, the most important finding was that MAU was independently associated with arterial stiffness and vascular inflammation even in the patients with newly diagnosed type 2 diabetes or essential hypertension. However, MAU did not show an independent association with IMT in both diabetes and hypertension groups. Additionally, in comparison between diabetic patients with MAU and hypertensive patients with MAU, the levels of PWV and hsCRP were significantly higher in the diabetic group than in the hypertensive group, while the level of IMT was similar between both of the groups. Although the disease duration was not significantly different between the 2 groups, the level of MAU (ACR) was significant higher in the diabetic group. Based on our result which demonstrated the independent association of MAU with PWV and hsCRP, the higher level of MAU could account for the higher level of PWV and hsCRP in the diabetic group than in the hypertensive group. Moreover, the result could suggest that endothelial dysfunction more rapidly progress in the diabetic group than in the hypertensive group since MAU and PWV were considered as markers of vascular endothelial dysfunction and an inflammatory stimulus also may trigger endothelial dysfunction (4, 5, 22).
While several cross-sectional studies have demonstrated a relationship between MAU and arterial stiffness in various populations (3-6, 11, 14), there have been few reports on the association between MAU and arterial stiffness in patients with untreated, newly diagnosed type 2 diabetes or essential hypertension. Recently, Mulè et al. (3) have shown a correlation between albumin excretion rate and aortic stiffness in untreated essential hypertensive patients diagnosed within the past 2 yr. However, they enrolled only 19 microalbuminuric hypertensive patients. Also, they did not evaluate the association between MAU and other macrovascular indices such as IMT and hsCRP. Other previous studies which revealed the independent association between MAU and arterial stiffness have enrolled patients with relatively long duration of diseases (6, 14), or patients with unclear duration of diseases (5), or general populations (4, 11) as study subjects. Although atherosclerotic cardiovascular risk has been reported to be elevated at the earliest stages of diabetes or hypertension (17, 21), it is not clear whether this association based on the results from patients with advanced stage of diabetes and hypertension can be extrapolated to the patients on early stage of the diseases. In this regard, this study has a merit of recruiting only patients with untreated, newly diagnosed diabetes or hypertension, whose duration of diseases was clear (less than 12 months), as study subjects.
Although both PWV and IMT have been regarded as the indices of subclinical or early atherosclerosis (4, 22), the present study has shown that MAU was independently associated with only arterial stiffness but not with IMT. While IMT was higher in patients with MAU than in those with NAU, the level of IMT was neither positively correlated with MAU level nor revealed as an independent factor for MAU. This finding of the preferential association of MAU with increased arterial stiffness over IMT coincides with the results of previous reports. Ishimura et al. demonstrated that urinary albumin excretion was significantly associated with arterial wall stiffness rather than IMT in type 2 diabetic patients (6). In addition, Mulè et al. also reported that MAU was independently associated with increased aortic stiffness in patients with essential hypertension (5). Meanwhile, arterial stiffness has been closely linked with microvascular complications, such as diabetic neuropathy and retinopathy (23, 24). These findings are consistent with our results, which demonstrated a close correlation between the markers of microvascular dysfunction and large artery stiffening. On the other hand, conflicting results about the relationship between MAU and IMT have been reported in some previous studies. Keech et al. (13) indicated that IMT in patients with type 2 diabetes was independently related to urine albumin levels. Furthermore, Huang et al. (12) showed that even low-grade albuminuria, which was below the cutoff point of MAU, was associated with high IMT in type 2 diabetic patients. Whereas, some other studies reported that MAU was not independently associated with IMT, which could be in line with our results (6, 14). Especially, Choi et al. (14) studied in total 673 Korean type 2 diabetic patients and indicated that albuminuria was significantly associated with PWV but not with carotid plaque or IMT. However, their study subjects also had relatively long duration of diabetes; mean 10.1 yr (6) and mean 8.9 yr (14). Thus, in the present study, we tried to determine the association between MAU and IMT in only patients with the newly diagnosed diseases.
Although the precise reasons why IMT did not have statistically independent correlation with MAU in the present study still remains elusive, there might be several possible explanations. First, the short duration of diseases in the present study could account for the result. Interestingly, it has been reported that the duration of diseases was independently correlated to IMT in patients with type 2 diabetes (13) and those with essential hypertension (25). In most of our study subjects, the disease duration was only less than 6 months. Secondly, because the formation and progression of carotid plaque commonly occur at place of non-laminar turbulent flow such as the proximal internal carotid artery or bifurcating segments (14), the assessment of IMT within common carotid artery in the present study might not necessarily reflect an atherosclerotic process. Finally, a small number of patients were included in the analysis. Although the association between MAU and IMT was not independent in the present study, the mean level of IMT was higher in patients with MAU than in patients with NAU in both diabetes and hypertension groups, which could be consistent with the result of the previous study (6). Thus, a larger-scale study might show a significant association.
The result of the preferential association between MAU and PWV suggests that functional large artery stiffening may share similar pathophysiological relevance to MAU, as compared with anatomical large artery thickening marked by IMT, especially in newly diagnosed diabetes or hypertension. Although the accurate mechanisms were not completely revealed, endothelial dysfunction may be responsible for a common underlying mechanism. MAU has been considered an early marker of systemic microvascular damage and resulted from the vascular endothelial dysfunction (4, 20, 21). Endothelial dysfunction may be one of the mechanisms of increased arterial stiffness on PWV (4, 26). Considering we only included patients with untreated, newly diagnosed diseases, vascular endothelial dysfunction in the early stage of diabetes or hypertension could play a potential role in the genesis of MAU and increased arterial stiffness. Vascular endothelial dysfunction could induce the remodeling of arterial wall through structural and functional changes in target vessels, leading to increased arterial stiffness, and also affect the glomerular basement membrane leading to the alteration of glomerular barrier permeability and increased albuminuria (4, 20).
In all phases of the atherosclerotic process, inflammation plays an important role (5). MAU was found to be associated with inflammatory markers such as hsCRP in previous studies (5, 27, 28). One study found a relationship between hsCRP and albuminuria in a newly diagnosed hypertensive group, similar to the subjects in the present study (27). More recently, one multivariate analysis study demonstrated that a one-milligram-perliter increase in CRP had a significant association with 2% increased odds of MAU (28). These facts are in agreement with the result of the present study, demonstrating that MAU was strongly associated with hsCRP in both the diabetic and hypertensive groups.
Several limitations of the present study should be noted. First, the follow-up values of the indices after diabetes or hypertension treatments were not present. The present study only focused on the association between MAU and the indices of macrovascular complication before medical treatments. A serial measurement of MAU in this cohort of patients would be more informative. Additionally, because the present study was a cross-sectional study, only the association between MAU and the indices of macrovascular complication was demonstrated without an investigation of cause and effect inferences. To address these issues, a long-term, large-scale prospective study is needed. Secondly, we could not explain why the rate of patients with MAU (49.3%) was higher in the present study than in other previous reports. MAU is a frequent finding in patients with diabetes or hypertension and about 20%-30% of people with newly diagnosed type 2 diabetes show MAU (29). While the exact reasons remain unclear, 47.7% of type 2 diabetic patients showed MAU in the present study. Finally, although medical histories of patients were carefully considered and all the available medical records including those of the annual heath check-up were reviewed, it is still possible that patients who had diseases longer than 12 months were enrolled into the present study. In general, estimating the precise duration of type 2 diabetes and essential hypertension is difficult. However, we tried to exclude patients whose diseases had an uncertain duration.
In conclusion, MAU is independently associated with arterial stiffness and vascular inflammation but not with IMT in patients with newly diagnosed type 2 diabetes or essential hypertension, which emphasizes the importance of proactive clinical investigations for atherosclerotic complications in patients with MAU, even in newly diagnosed diabetes or hypertension.
ACKNOWLEDGMENTS
The authors have no conflicts of interest to disclose.
References
- 1.Rosenson RS, Fioretto P, Dodson PM. Does microvascular disease predict macrovascular events in type 2 diabetes? Atherosclerosis. 2011;218:13–18. doi: 10.1016/j.atherosclerosis.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Kim DJ, Jang HC, Choi SH. Epidemiology of micro- and macrovascular complications of type 2 diabetes in Korea. Diabetes Metab J. 2011;35:571–577. doi: 10.4093/dmj.2011.35.6.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulè G, Cottone S, Vadalà A, Volpe V, Mezzatesta G, Mongiovì R, Piazza G, Nardi E, Andronico G, Cerasola G. Relationship between albumin excretion rate and aortic stiffness in untreated essential hypertensive patients. J Intern Med. 2004;256:22–29. doi: 10.1111/j.1365-2796.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim BJ, Lee HA, Kim NH, Kim MW, Kim BS, Kang JH. The association of albuminuria, arterial stiffness, and blood pressure status in nondiabetic, nonhypertensive individuals. J Hypertens. 2011;29:2091–2098. doi: 10.1097/HJH.0b013e32834b5627. [DOI] [PubMed] [Google Scholar]
- 5.Mulè G, Cottone S, Cusimano P, Riccobene R, Palermo A, Geraci C, Nardi E, Bellavia T, Foraci AC, Cerasola G. The association of microalbuminuria with aortic stiffness is independent of C-reactive protein in essential hypertension. Am J Hypertens. 2009;22:1041–1047. doi: 10.1038/ajh.2009.132. [DOI] [PubMed] [Google Scholar]
- 6.Ishimura E, Taniwaki H, Tsuchida T, Obatake N, Emoto M, Shoji T, Shioi A, Inaba M, Nishizawa Y. Urinary albumin excretion associated with arterial wall stiffness rather than thickness in type 2 diabetic patients. J Nephrol. 2007;20:204–211. [PubMed] [Google Scholar]
- 7.Jia EZ, An FH, Liu P, Li F, Mao HW, Cui WJ, Xu HY. Relationship between brachial-ankle pulse wave velocity and cardiovascular risk factors: a multi-ethnic study. Intern Med. 2012;51:537–543. doi: 10.2169/internalmedicine.51.6480. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso CR, Marques CE, Leite NC, Salles GF. Factors associated with carotid intima-media thickness and carotid plaques in type 2 diabetic patients. J Hypertens. 2012;30:940–947. doi: 10.1097/HJH.0b013e328352aba6. [DOI] [PubMed] [Google Scholar]
- 9.Karalliedde J, Viberti G. Microalbuminuria and cardiovascular risk. Am J Hypertens. 2004;17:986–993. doi: 10.1016/j.amjhyper.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Chae HW, Shin JI, Kwon AR, Kim HS, Kim DH. Spot urine albumin to creatinine ratio and serum cystatin C are effective for detection of diabetic nephropathy in childhood diabetic patients. J Korean Med Sci. 2012;27:784–787. doi: 10.3346/jkms.2012.27.7.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa T, Hashimoto J, Morito RH, Hanazawa T, Aikawa T, Hara A, Shintani Y, Metoki H, Inoue R, Asayama K, et al. Association of microalbuminuria with brachial-ankle pulse wave velocity: the Ohasama study. Am J Hypertens. 2008;21:413–418. doi: 10.1038/ajh.2007.77. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Chen Y, Xu M, Gu W, Bi Y, Li X, Ning G. Low-grade albuminuria is associated with carotid intima-media thickness in Chinese type 2 diabetic patients. J Clin Endocrinol Metab. 2010;95:5122–5128. doi: 10.1210/jc.2010-0544. [DOI] [PubMed] [Google Scholar]
- 13.Keech AC, Grieve SM, Patel A, Griffiths K, Skilton M, Watts GF, Marwick TH, Groshens M, Celermajer DS. Urinary albumin levels in the normal range determine arterial wall thickness in adults with type 2 diabetes: a FIELD substudy. Diabet Med. 2005;22:1558–1565. doi: 10.1111/j.1464-5491.2005.01688.x. [DOI] [PubMed] [Google Scholar]
- 14.Choi SW, Yun WJ, Kim HY, Lee YH, Kweon SS, Rhee JA, Choi JS, Shin MH. Association between albuminuria, carotid atherosclerosis, arterial stiffness, and peripheral arterial disease in Korean type 2 diabetic patients. Kidney Blood Press Res. 2010;33:111–118. doi: 10.1159/000313594. [DOI] [PubMed] [Google Scholar]
- 15.Gavin JR, Alberti KGMM, Davidson MB, DeFronzo RA, Drash A, Gabbe SG, Genuth S, Harris MI, Kahn R, Keen H, et al. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 17.An HR, Park S, Yoo TH, Kang SW, Ryu JH, Lee YK, Yu M, Ryu DR, Kim SJ, Kang DH, et al. Non-dipper status and left ventricular hypertrophy as predictors of incident chronic kidney disease. J Korean Med Sci. 2011;26:1185–1190. doi: 10.3346/jkms.2011.26.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 19.Ahn MS, Kim JY, Youn YJ, Kim SY, Koh SB, Lee K, Yoo BS, Lee SH, Yoon J, Park JK, et al. Cardiovascular parameters correlated with metabolic syndrome in a rural community cohort of Korea: the ARIRANG Study. J Korean Med Sci. 2010;25:1045–1052. doi: 10.3346/jkms.2010.25.7.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelhafiz AH, Ahmed S, El Nahas M. Microalbuminuria: marker or maker of cardiovascular disease. Nephron Exp Nephrol. 2011;119:e6–e10. doi: 10.1159/000328015. [DOI] [PubMed] [Google Scholar]
- 21.Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214–2219. doi: 10.1046/j.1523-1755.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama H, Sone H, Saito K, Yamada D, Honjo J, Haneda M. Flow-mediated dilation is associated with microalbuminuria independent of cardiovascular risk factors in type 2 diabetes: interrelations with arterial thickness and stiffness. J Atheroscler Thromb. 2011;18:744–752. doi: 10.5551/jat.7526. [DOI] [PubMed] [Google Scholar]
- 23.Kim ES, Moon SD, Kim HS, Lim DJ, Cho JH, Kwon HS, Ahn CW, Yoon KH, Kang MI, Cha BY, et al. Diabetic peripheral neuropathy is associated with increased arterial stiffness without changes in carotid intima-media thickness in type 2 diabetes. Diabetes Care. 2011;34:1403–1405. doi: 10.2337/dc10-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung N, Wang JJ, Rogers SL, Brancati F, Klein R, Sharrett AR, Wong TY ARIC (Atherosclerosis Risk In Communities) Study Investigators. Diabetic retinopathy and risk of heart failure. J Am Coll Cardiol. 2008;51:1573–1578. doi: 10.1016/j.jacc.2007.11.076. [DOI] [PubMed] [Google Scholar]
- 25.Zizek B, Poredos P. Dependence of morphological changes of the carotid arteries on essential hypertension and accompanying risk factors. Int Angiol. 2002;21:70–77. [PubMed] [Google Scholar]
- 26.Tomiyama H, Yamashina A. Non-invasive vascular function tests: their pathophysiological background and clinical application. Circ J. 2010;74:24–33. doi: 10.1253/circj.cj-09-0534. [DOI] [PubMed] [Google Scholar]
- 27.Tsioufis C, Dimitriadis K, Taxiarchou E, Vasiliadou C, Chartzoulakis G, Tousoulis D, Manolis A, Stefanadis C, Kallikazaros I. Diverse associations of microalbuminuria with C-reactive protein, interleukin-18, and soluble CD 40 ligand in male essential hypertensive subjects. Am J Hypertens. 2006;19:462–466. doi: 10.1016/j.amjhyper.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 28.Kshirsagar AV, Bomback AS, Bang H, Gerber LM, Vupputuri S, Shoham DA, Mazumdar M, Ballantyne CM, Paparello JJ, Klemmer PJ. Association of C-reactive protein and microalbuminuria (from the National Health and Nutrition Examination Survey, 1999 to 2004) Am J Cardiol. 2008;101:401–406. doi: 10.1016/j.amjcard.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruilope L, Izzo J, Haller H, Waeber B, Oparil S, Weber M, Bakris G, Sowers J. Prevention of microalbuminuria in patients with type 2 diabetes: what do we know? J Clin Hypertens (Greenwich) 2010;12:422–430. doi: 10.1111/j.1751-7176.2010.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]