Abstract
Purpose
Studies have shown an association between socioeconomic status (SES) and quality of oncology care, but less is known about the impact of patient SES on clinical trial participation.
Patients and Methods
We assessed clinical trial participation patterns according to important SES (income, education) and demographic factors in a large sample of patients surveyed via an Internet-based treatment decision tool. Logistic regression, conditioning on type of cancer, was used. Attitudes toward clinical trials were assessed using prespecified items about treatment, treatment tolerability, convenience, and cost.
Results
From 2007 to 2011, 5,499 patients were successfully surveyed. Forty percent discussed clinical trials with their physician, 45% of discussions led to physician offers of clinical trial participation, and 51% of offers led to clinical trial participation. The overall clinical trial participation rate was 9%. In univariate models, older patients (P = .002) and patients with lower income (P = .001) and education (P = .02) were less likely to participate in clinical trials. In a multivariable model, income remained a statistically significant predictor of clinical trial participation (odds ratio, 0.73; 95% CI, 0.57 to 0.94; P = .01). Even in patients age ≥ 65 years, who have universal access to Medicare, lower income predicted lower trial participation. Cost concerns were much more evident among lower-income patients (P < .001).
Conclusion
Lower-income patients were less likely to participate in clinical trials, even when considering age group. A better understanding of why income is a barrier may help identify ways to make clinical trials better available to all patients and would increase the generalizability of clinical trial results across all income levels.
INTRODUCTION
Clinical trials represent the final, critical step in evaluating the efficacy of new treatments for cancer. Continued access to and participation in cancer clinical trials are essential for reducing cancer mortality. However, only a small proportion of adult patients with cancer participate in clinical trials.1,2
The decision about what cancer treatment to undergo is complex and personal. Even if patients are eligible for a trial, they often refuse to participate.3,4 Patients express dislike of randomized treatment assignment and may already have a particular treatment in mind.4–7 Access factors (eg, transportation, cost), disease status, and physicians' attitudes toward patient participation are also important.3,4,7,8
The generalizability of clinical trial results depends on how representative the trial population is of the cancer population. For instance, comorbid conditions may discourage older patients from trial participation or may render patients clinically ineligible.9 In 1999, investigators at SWOG, a national clinical trials consortium, showed that older patients were substantially underrepresented in cancer trials.10 In 2000, Medicare changed its policy to cover the routine care costs of trial participation. A later SWOG study suggested that this policy change improved enrollment of older patients, but only for those with supplemental insurance to Medicare.11
Heightened awareness of socioeconomic (SES) disparities in access to medical, economic, or other resources is an important and current public policy issue and provided the motivation to investigate potential SES barriers to clinical trial participation, which might also inform the age disparity. Because patient-specific SES data are rarely collected for patients with cancer enrolled onto national clinical trials, this topic has not been previously well studied.12 In addition, participation in clinical trials is strongly influenced by the presence of comorbid illnesses; however, studies of clinical trial participation rarely include comorbidity data.12 Therefore, to address the research question, SWOG collaborated with a provider of online treatment decision tools to assess patterns of clinical trial participation according to patient-level SES and demographic variables, accounting for comorbid illnesses.
PATIENTS AND METHODS
Administration
Investigators from SWOG collaborated with NexCura (The Woodlands, TX), a provider of proprietary Internet-based cancer treatment decision tools.13 Patients were accessed through their registration to the NexCura treatment decision tool, which was embedded in multiple major cancer-oriented Web sites (eg, American Cancer Society). An e-mail was generated 3 months after each new registration (to allow patients time to make a treatment decision), offering the patient the opportunity to participate in the survey and providing a link to the survey Web site. The survey was completed online.
Patients were notified that information they provided would be reported in aggregate only. Because no individual identifiers were provided to researchers, the study was deemed institutional review board exempt.
Eligibility
To be eligible, patients had to be adults (age ≥ 18 years) living in the United States. Patients must have been investigating treatment options for a first diagnosis of breast, colorectal, lung, or prostate cancer.
Design
All patients who agreed to participate were asked questions pertaining to their demographics, staging, and comorbid illnesses. Patients were asked whether they had made a treatment decision within the prior 3 months. Patients who answered no were asked to indicate why. These patients were then finished with the survey.
Patients who answered yes represented the evaluable patient cohort. These patients were asked whether they had discussed with their physician possible participation in a clinical trial. A brief description of a clinical trial was provided. Patients who answered either yes but were not offered a clinical trial, or no, were asked whether they thought clinical trials: 1 represented better treatment, 2 were more difficult to tolerate, 3 represented a gamble, 4 were inconvenient, and 5 were more difficult to pay for. These patients were then finished with the survey.
Patients who discussed a clinical trial with their physician and were offered trial participation were asked whether they participated in a clinical trial. Patients who answered yes were asked about their attitudes toward clinical trials (as noted in the previous paragraph) and were then finished with the survey. Patients who answered no were asked about their reasons for declining trial participation based on 24 prespecified questions from a prior study of barriers to clinical trial participation.7 These patients were then finished with the survey. The Data Supplement provides a full survey description.
Statistical Considerations
The primary objective was to assess, by SES and demographic factors: 1) rates of clinical trial enrollment, 2) patterns of enrollment onto trials, 3) attitudes toward clinical trials, and 4) reasons for nonparticipation in clinical trials. Conditional logistic regression was used to assess the association between SES or demographic variables and a given outcome, conditioning on cancer type. All evaluable patients of any stage were included. Multivariable logistic regression was used to assess multiple factors associated with clinical trial participation simultaneously.
SES, Demographic, and Comorbidity Variables
The demographic factors were: age (< 65 v ≥ 65 years), sex, and race (African American v white/other). Yearly household income was categorized as < $20,000 versus $20,000 to $34,999 versus $35,000 to $49,999 versus $50,000 to $99,999 versus ≥ $100,000 versus “don't know” versus “prefer not to answer.” For the primary analysis, income was categorized as < $50,000 versus ≥ $50,000 per year (the nearest approximation to the median), and education was categorized as < 2-year versus ≥ 2-year college degree. Binary indicator variables were used for consistency across factors and to aid interpretation. Additional analyses explored other binary categorizations of income. Income analyses were limited to patients with a reported income value.
A comorbidity score was derived from data on 18 comorbid conditions.14 Total travel distance to the clinic (a surrogate for convenience) was also obtained. Both factors were included as covariates in multivariable regression models, split at the median (comorbidity, zero to one v ≥ two; travel distance, < 13 v ≥ 13 miles). In addition, each multivariable model included the SES and demographic variables.
RESULTS
Cohort Profile
A total of 77,752 survey invitations were sent; 6,259 were returned, for a response rate of 8%, approximately twice that of a prior survey using the NexCura treatment decision tool.13 Most patients (5,499; 88%) had made a treatment decision in the prior 3 months, representing the evaluable patient cohort; responses by cancer type included 2,894 breast (53%), 1,546 prostate (28%), 651 lung (12%), and 408 colorectal (7%) cancers.
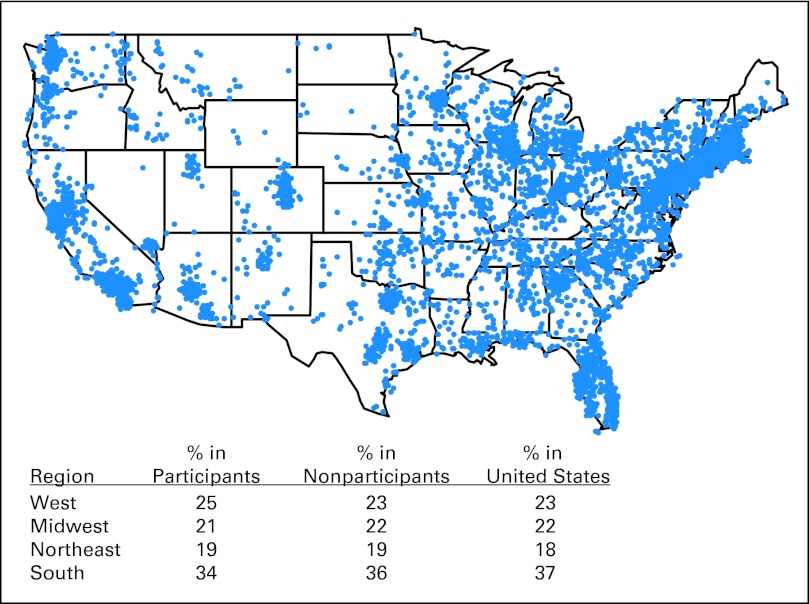
Twenty-two percent of patients were age ≥ 65 years, 2.5% were African American, and 62% were women (Table 1). In comparison, the US adult cancer population with a similar cancer distribution was estimated to be older (58% age ≥ 65 years) and to have more African Americans (10%) but the same proportion of women (62%).11 There was good geographic representativeness compared with the US adult population (Fig 1). Survey participants were similar to nonparticipants with respect to sex (female, 62% v 63%), income (> national median, 64% v 63%), and geographic region (Fig 1) but were more likely to have breast (52% v 42%) or prostate cancer (28% v 20%) and less likely to have lung cancer (10% v 23%).
Table 1.
Characteristics of the Survey Sample (N = 5,499)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| < 65 | 4,300 | 78 |
| ≥ 65 | 1,199 | 22 |
| Sex | ||
| Female | 3,420 | 62 |
| Male | 2,079 | 38 |
| Race | ||
| White | 5,192 | 94.4 |
| African American | 135 | 2.5 |
| Asian/Pacific Islander | 62 | 1.1 |
| Native American | 20 | 0.4 |
| Other | 90 | 1.6 |
| Annual income* | ||
| < $20,000 | 342 | 7 |
| $20,000-$34,999 | 483 | 11 |
| $35,000-$49,999 | 631 | 14 |
| $50,000-$99,999 | 1,679 | 37 |
| ≥ $100,000 | 1,444 | 32 |
| Do not know | 24 | |
| Prefer not to answer | 896 | |
| Education | ||
| ≤ High school degree | 660 | 12 |
| Some college | 1,223 | 22 |
| 2-year college degree | 627 | 11 |
| 4-year college degree | 1,288 | 23 |
| Graduate school | 1,701 | 31 |
| Comborbidity score | ||
| 0-1 | 3,629 | 59 |
| ≥ 2 | 2,230 | 41 |
| Distance to clinic, miles | ||
| < 13 | 2,726 | 50 |
| ≥ 13 | 2,727 | 50 |
| Cancer type | ||
| Breast | 2,894 | 53 |
| Colorectal | 408 | 7 |
| Lung | 651 | 12 |
| Prostate | 1,546 | 28 |
| Geographic region | ||
| West | 1,388 | 25 |
| Midwest | 1,151 | 21 |
| Northeast | 1,063 | 19 |
| South | 1,893 | 34 |
Percentages are based on the total of 4,579 respondents (83% of evaluable patients) who reported an income value.
Fig 1.
Geographic distribution of survey participants. The legend shows the proportion of survey participants by geographic region compared with proportions in nonparticipants and in the US adult population.
Income was reported by 83% of patients, of whom 32% made < $50,000 per year. Education level was obtained for all patients; 34% reported < a 2-year college degree.
Patterns of Decision Making
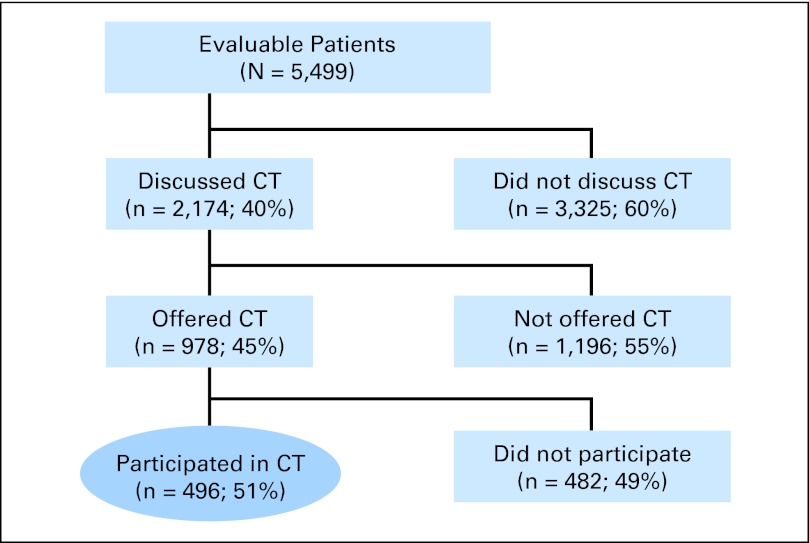
Of the 5,499 evaluable patients, 2,174 (40%) reported discussing clinical trial participation with their physician; among these, 978 (45%) were offered a trial, and among these, 496 (51%) participated in a clinical trial, and 482 (49%) did not (Fig 2). There were statistically significant differences in the proportion of patients discussing trial participation in multivariable analysis by age (42% < 65 years v 29% ≥ 65 years; P < .001), income (36% < $50,000 per year v 42% ≥ $50,000 year; P < .001), and education (35% < 2-year college degree v 42% ≥ 2-year college degree; P < .001). Given clinical trial participation was discussed, African Americans were more likely (57% v 45%; P = .03) and lower-income patients less likely (40% v 47%; P = .05) to be offered a clinical trial. No other statistically significant differences by SES or demographic factors were found (Data Supplement).
Fig 2.
Patient flow diagram indicating decision-making patterns about clinical trial (CT) participation. A total of 77,752 survey invitations were sent, and 6,259 were returned; 760 respondents had not made a treatment decision within the 3 months since their initial registration on the NexCura Web site, leaving 5,499 evaluable patients. For the 760 respondents who had not made a treatment decision within the 3 months since their initial registration, most had made a treatment decision earlier than 3 months before (65%) or were recently diagnosed but still undecided about treatment (15%).
Univariate Estimation of Clinical Trial Participation
The overall rate of clinical trial participation among evaluable patients was 9.0% (496 of 5,499 patients; Fig 2). Conditioning on cancer type, clinical trial participation was less likely in older patients (5.4% v 10.0%; P = .002) and patients with lower income (7.6% v 10.0%; P = .001) and lower education (7.9% v 9.6%; P = .02; Table 2). There were no statistically significant differences in trial participation by race or sex. Higher comorbidity scores and shorter distance to clinic were also associated with lower participation.
Table 2.
Rates of Clinical Trial Enrollment
| Factor | Enrolled Onto Clinical Trial (%) | OR | 95% CI | P |
|---|---|---|---|---|
| Univariate model results | ||||
| Age, years | 0.64 | 0.48 to 0.85 | .002 | |
| < 65* | 10.0 | |||
| ≥ 65† | 5.4 | |||
| Sex‡ | 0.97 | 0.63 to 1.48 | .88 | |
| Male* | 5.6 | |||
| Female† | 11.1 | |||
| Race | 1.26 | 0.73 to 2.19 | .40 | |
| White/other* | 9.0 | |||
| African American† | 11.1 | |||
| Annual income | 0.68 | 0.54 to 0.86 | .001 | |
| ≥ $50,000* | 10.0 | |||
| < $50,000† | 7.6 | |||
| Education | 0.78 | 0.64 to 0.96 | .02 | |
| ≥ College* | 9.6 | |||
| < College† | 7.9 | |||
| Comborbidity score | 0.78 | 0.64 to 0.95 | .01 | |
| 0-1* | 10.1 | |||
| ≥ 2† | 7.5 | |||
| Distance to clinic, miles | 0.66 | 0.55 to 0.80 | < .001 | |
| ≥ 13* | 10.5 | |||
| < 13† | 7.6 | |||
| Multivariable model results | ||||
| Age | 0.79 | 0.58 to 1.08 | .14 | |
| Sex | 0.93 | 0.58 to 1.49 | .75 | |
| Race | 1.31 | 0.74 to 2.33 | .35 | |
| Income | 0.73 | 0.57 to 0.94 | .01 | |
| Education | 0.92 | 0.73 to 1.16 | .49 | |
| Comorbidity score | 0.81 | 0.65 to 1.02 | .07 | |
| Distance to clinic | 0.66 | 0.54 to 0.81 | < .001 | |
| Multivariable model results for different annual income cut points | ||||
| $20,000 | 0.56 | 0.35 to 0.91 | .02 | |
| $35,000 | 0.73 | 0.54 to 0.98 | .04 | |
| $50,000 | 0.73 | 0.57 to 0.94 | .01 | |
| $100,000 | 0.79 | 0.63 to 0.98 | .04 |
Abbreviation: OR, odds ratio.
Coded as 1.
Coded as 0.
Although the observed participation rate for women was almost twice that for men (11.1% v 5.6%), the difference was not statistically significant after conditioning on cancer type because of the relatively high rate of enrollment among patients with breast cancer. In non–sex-specific diseases only (lung and colorectal cancers), the rates were 8.4% for men and 8.0% for women.
Multivariable Estimation of Clinical Trial Participation
In a multivariable conditional logistic regression model including all SES and demographic factors as well as the covariates comorbidity status and distance to clinic, income was the only SES or demographic factor that was statistically significantly associated with clinical trial participation (odds ratio [OR], 0.73; P = .01; Table 2). Patients with lower income had lower clinical trial participation regardless of income cut point (Table 2). For instance, patients with income < $20,000 per year showed 44% lower odds of participation.
Association of Income and Clinical Trial Participation in Subcategories
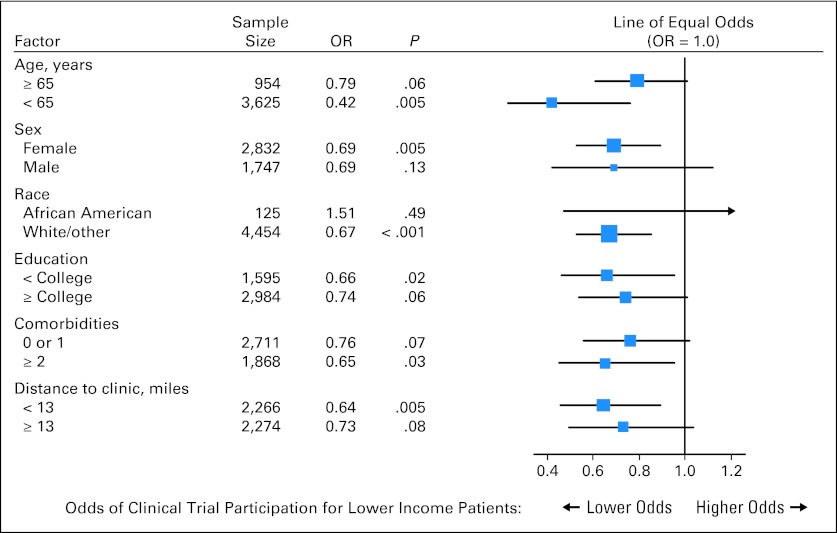
We explored the association of income with trial participation within subgroups of respondents using a forest plot (Fig 3). The odds of clinical trial participation were consistently lower in nearly all subcategories. Importantly, although there was a trend toward a stronger association of income with trial participation among those age < 65 years (test for interaction of age and income, P = .06), income was associated with participation in older patients as well.
Fig 3.
Forest plot of the association of income and clinical trial participation by each study factor. Each square represents an odds ratio (OR), and each horizontal line is the 95% CI. The vertical line is the line of equal odds. For lower-income individuals, the odds of clinical trial participation were consistently lower (that is, to the left of the line of equal odds) within nearly all subgroups of all the factors included in this analysis, indicating that the association between income and participation was independent of subgroup membership.
Attitudes Toward Clinical Trials
Patient attitudes toward clinical trials were similar for patients who did not discuss a trial (n = 3,325) versus those who discussed but were not offered a trial (n = 1,196; data not shown). These subsets were combined. Concern about how to pay for clinical trial participation was higher in lower-income patients (OR, 1.79; 95% CI, 1.53 to 2.10; P < .001) and lower-education patients (OR, 1.50; 95% CI, 1.29 to 1.75; P < .001) but was lower in older patients (OR, 0.54; 95% CI, 0.44 to 0.65; P < .001), likely because of Medicare access. Rates of concern about how to pay for trial participation were 53% for those with income < $20,000, 46% for income from $20,000 to $34,999, 43% for income from $35,000 to $49,999, 36% for income from $50,000 to $99,999, and 24% for income ≥ $100,000, indicating an inverse linear relationship (P < .001).
Among patients who participated in a clinical trial (n = 496), older patients were more likely to consider trial participation a gamble (OR, 2.51; 95% CI, 1.28 to 4.93; P = .008), lower-education patients were less likely to think a clinical trial involved extra inconvenience (OR, 0.56; 95% CI, 0.35 to 0.88; P = .01), and women (OR, 4.02; 95% CI, 1.26 to 12.82; P = .02) and lower-income patients (OR, 2.63; 95% CI, 1.53 to 4.51; P < .001) were more likely to worry about how clinical trial participation would be paid for. A strong linear association between income level and concern about how to pay for clinical trial participation was also evident in patients who participated in a clinical trial (P < .001; rates not shown).
Reasons for Nonparticipation in Clinical Trials
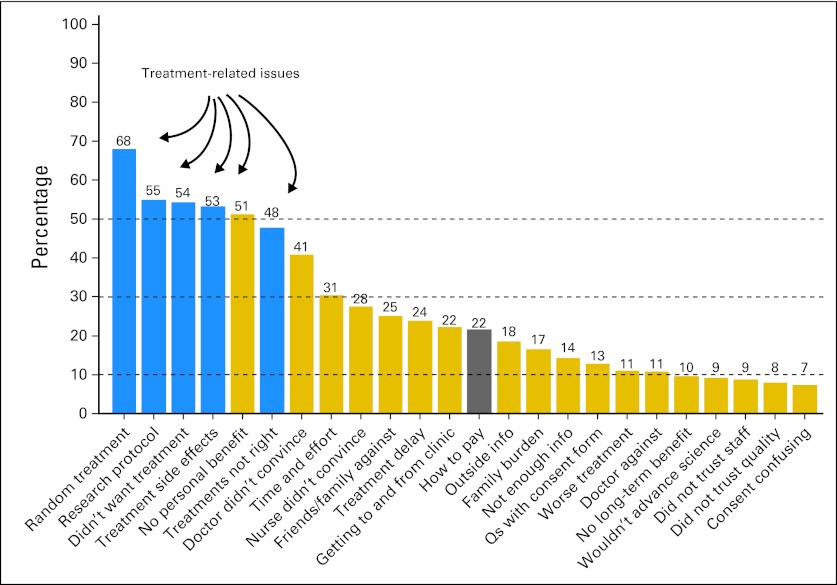
Among those patients who were offered a clinical trial but declined (n = 482), treatment-related concerns, especially dislike of randomized treatment (68%), were the most frequently cited reasons for nonparticipation (Fig 4). Each of the 24 questions in Figure 4 was analyzed in multivariable regression. Lower income was strongly associated with more concern about how to pay for clinical trial treatment (P = .01) and less concern that a research protocol would determine choice of treatment (P = .01). Other differences are shown in Figure 4. A strong linear association between income level and concern about how to pay for clinical trial participation was also evident in patients who were offered a clinical trial but declined (P < .001; rates not shown).
Fig 4.
Reasons for declining to participate in clinical trials. Among patients who were offered a clinical trial but did not participate (n = 482), the most common reasons cited for nonparticipation were treatment-related concerns (ie, fear of random assignment, did not want the specified protocol treatment, and so on). Other reasons that differed by socioeconomic or demographic factors included the following: age: older patients were less concerned about travel related to clinical trial participation (P = .02), less concerned about how to pay for clinical trial participation (P = .04), and less concerned about time and effort related to clinical trial participation (P = .04); sex: women were less likely to have family and friends opposed to their clinical trial participation (P = .03); income: lower-income patients were more concerned about how to pay for clinical trial participation (P = .01), less concerned that a research protocol would determine their treatment choice (P = .01), less likely to have family or friends opposed to their clinical trial participation (P = .03), and less concerned about random treatment assignment in a clinical trial (P = .04); and education: lower-education patients were less concerned about clinical trial adverse effects (P = .05).
DISCUSSION
In this large national survey, patients with lower income were less likely to participate in a clinical trial, even accounting for comorbidity and education. Simultaneously, lower-income patients were more likely than higher-income patients to report concern about how to pay for clinical trial participation.
Proportional minority and female enrollment onto cancer clinical trials and inadequate older patient enrollment have been observed in many studies.6,10,11,15–17 Although distance to clinic has been cited as a reason for nonparticipation in clinical trials, in this study, trial participation was higher among patients who traveled a greater distance.4 This may be the result of the willingness of patients seeking trials to travel farther. Such patients may also be healthier, which could partly explain why travel distance has been found to be positively associated with improved survival in clinical trials, even after accounting for cancer stage and performance status.18
Few studies have evaluated the association between income and clinical trial enrollment. Instead, some studies have used area-level surrogates for SES variables through census data.19–21 The associations found in these studies were sometimes not retained in multivariable modeling, perhaps because of a lack of patient-level data and corresponding loss of power.20,21 A large study using a telephone survey to assess clinical trial recruitment patterns found that middle-income individuals ($15,000 to $50,000 per year) were less likely to participate.22 However, this study was limited to Maryland only, was not cancer specific, and did not collect comorbidity data. Some studies have found associations of lower SES with surrogate outcomes for trial participation, such as willingness to participate in or awareness of clinical trials.23,24 Other studies have found no SES association with trial participation, although these studies had small sample sizes25,26 and high levels of missingness for SES factors.27 In contrast, our study had patient-level SES data, assessed clinical trial enrollment (by patient report) rather than willingness to participate in or awareness of clinical trials, and had a large number of patients.
One explanation for our study results might be that higher-income individuals are better insured. Although insurance status was not collected in this survey, we observed that lower income predicted lower clinical trial participation in patients age ≥ 65 years, indicating an income disparity even in a population with universal access to Medicare. We also found no evidence that the association of lower income with trial enrollment differed between states with and without insurance mandates for coverage of clinical trials (interaction P = .32). Finally, lower income may be a selection factor for hospitals that are less likely to offer clinical trial participation. However, we observed that lower-income patients were less likely to be offered a trial given a trial was discussed, suggesting an income disparity even when a clinical trial was explicitly under consideration.
This study also provides strong evidence that lower-income patients are more likely than higher-income patients to be concerned about how to pay for clinical trial participation. However, the National Cancer Institute, in summarizing the literature, has stated that “patient care costs for clinical trials are not appreciably higher than costs for patients not enrolled in trials.”28 The National Cancer Institute cites in particular the Costs of Cancer Treatment study by Goldman et al29 at RAND, which found an overall small, nonstatistically significant increase (6.5%) in trial versus nontrial costs and no increase in prescription out-of-pocket costs.30 Other studies have found similar results.31–34
One limitation of this study was the inability to assess concerns about cancer treatment costs in general. But even if treatment cost concerns are a general issue, public policy changes could reduce these concerns in the clinical trial setting specifically, with the potential to reduce the disparity in trial participation rates. One approach would be to cover excess costs for trial participation for all patients. Prior evidence suggests that even in an insured population, copays and coinsurance may deter clinical trial participation.11 Similarly, payment to individuals to encourage clinical trial participation has been explored.35–37 Such approaches would require careful calibration of the size of any monetary incentive attached to an offer of trial participation38 to avoid undue influence, as indicated in the US Common Rule for the Protection of Human Subjects. A different strategy could be direct-to-consumer advertising (DTCA) targeted to lower-income populations. DTCA has increased in recent years, and awareness of oncology-related DTCA is generally high among patients with cancer (> 80%).39,40 Finally, lower-income patients may be more sensitive to indirect costs, such as having to take time off work for extra clinic visits. Certain low-cost considerations for patients related to time, convenience, or transportation, such as parking passes or bus fare, could improve participation among lower-income patients in particular.41 Interventions to provide more-comprehensive information to patients about costs and benefits associated with clinical trial participation could also be useful. Jacobsen et al42 recently reported that patients randomly assigned to receive printed educational materials plus a multimedia DVD intervention had more positive attitudes about clinical trials and more willingness to participate in trials than patients who received printed educational materials alone.
Patient self-report may limit the reliability of certain types of data (eg, comorbidity status). On the other hand, self-report is preferable for patient attitudes and SES variables. Also, although all P values ≤ .05 are reported, those cases where 0.01 ≤ P ≤ .05 should be regarded with some suspicion because of the number of tests performed, whereas highly statistically significant P values (P ≤ .01) are less likely to be significant by chance alone. Furthermore, inferences pertaining to African American patients were limited by the small proportion of African Americans in the survey. Finally, the survey was conducted among Web-based users to obtain a national sample of patients with patient-level SES data. Although the survey response rate was low, it was similar to rates reported in other Internet-based surveys.13,43,44 The participant cohort was younger and less diverse than the general cancer population but was comparable to nonparticipants for several factors examined, including income. Nonetheless, the most important issue is the extent to which the inference about the association between income and clinical trial participation is generalizable beyond this Web-based cohort.45 Because income may be found to have a different impact on clinical trial participation in different populations, such as those without Internet access or nonrespondents, the validity of this finding requires confirmation in different study settings.
Improving participation of lower-income patients in clinical trials is important from multiple perspectives. Because clinical trials offer the newest cancer treatments, equal access to this important resource for patients of all income groups is essential. Also, improved lower-income participation would allow clinical trials to be conducted more quickly and would better ensure the applicability of trial results to all income levels (ie, generalizability). Understanding that income is related to clinical trial participation could help guide policy decisions aimed at increasing access for lower-income patients.
Supplementary Material
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Supported in part by Grant No. CA037429 from the National Cancer Institute.
Presented at the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012; 18th Annual National Research Services Award Conference, Orlando, FL, June 23, 2012; and AcademyHealth Annual Research Meeting, Orlando, FL, June 23-26, 2012.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a "U" are those for which no compensation was received; those relationships marked with a "C" were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy,please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Kenda Burg, NexCura (C) Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Joseph M. Unger, Kathy S. Albain, Carol M. Moinpour, Judith A. Petersen, John J. Crowley
Provision of study materials or patients: Judith A. Petersen
Collection and assembly of data: Joseph M. Unger, Judith A. Petersen, Kenda Burg
Data analysis and interpretation: Joseph M. Unger, Dawn L. Hershman, Kathy S. Albain, Carol M. Moinpour, Judith A. Petersen, John J. Crowley
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 2.Tejeda HA, Green SB, Trimble EL, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996;88:812–816. doi: 10.1093/jnci/88.12.812. [DOI] [PubMed] [Google Scholar]
- 3.Comis RL, Miller JD, Aldigé CR, et al. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21:830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 4.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 5.Hunter CP, Frelick RW, Feldman AR, et al. Selection factors in clinical trials: Results from the Community Clinical Oncology Program Physician's Patient Log. Cancer Treat Rep. 1987;71:559–565. [PubMed] [Google Scholar]
- 6.Klabunde CN, Springer BC, Butler B, et al. Factors influencing enrollment in clinical trials for cancer treatment. South Med J. 1999;92:1189–1193. doi: 10.1097/00007611-199912000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Javid SH, Unger JM, Gralow JR, et al. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment to breast cancer clinical trials (SWOG S0316) Oncologist. 2012;17:1180–1190. doi: 10.1634/theoncologist.2011-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross S, Grant A, Counsell C, et al. Barriers to participation in randomised controlled trials: A systematic review. J Clin Epidemiol. 1999;52:1143–1156. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 9.Bergman L, Dekker G, van Kerhoff EH, et al. Influence of age and comorbidity on treatment choice and survival in elderly patients with breast cancer. Breast Cancer Res Treat. 1991;18:189–198. doi: 10.1007/BF01990035. [DOI] [PubMed] [Google Scholar]
- 10.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 11.Unger JM, Coltman CA, Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006;24:141–144. doi: 10.1200/JCO.2005.02.8928. [DOI] [PubMed] [Google Scholar]
- 12.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer. 2008;112:228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 13.Markman M, Luce R. Impact of the cost of cancer treatment: An Internet-based survey. J Oncol Pract. 2010;6:69–73. doi: 10.1200/JOP.091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Trimble EL, Carter CL, Cain D, et al. Representation of older patients in cancer treatment trials. Cancer. 1994;74(suppl):2208–2214. doi: 10.1002/1097-0142(19941001)74:7+<2208::aid-cncr2820741737>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 18.Lamont EB, Hayreh D, Pickett KE, et al. Is patient travel distance associated with survival on phase II clinical trials in oncology? J Natl Cancer Inst. 2003;95:1370–1375. doi: 10.1093/jnci/djg035. [DOI] [PubMed] [Google Scholar]
- 19.Gross CP, Filardo G, Mayne ST, et al. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103:483–491. doi: 10.1002/cncr.20792. [DOI] [PubMed] [Google Scholar]
- 20.Du W, Gadgeel SM, Simon MS. Predictors of enrollment in lung cancer clinical trials. Cancer. 2006;106:420–425. doi: 10.1002/cncr.21638. [DOI] [PubMed] [Google Scholar]
- 21.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20:2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 22.Baquet CR, Commiskey P, Daniel Mullins C, et al. Recruitment and participation in clinical trials: Socio-demographic, rural/urban, and health care access predictors. Cancer Detect Prev. 2006;30:24–33. doi: 10.1016/j.cdp.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown DR, Topcu M. Willingness to participate in clinical treatment research among older African Americans and whites. Gerontologist. 2003;43:62–72. doi: 10.1093/geront/43.1.62. [DOI] [PubMed] [Google Scholar]
- 24.Lara PN, Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23:9282–9289. doi: 10.1200/JCO.2005.02.6245. [DOI] [PubMed] [Google Scholar]
- 25.Avis NE, Smith KW, Link CL, et al. Factors associated with participation in breast cancer treatment clinical trials. J Clin Oncol. 2006;24:1860–1867. doi: 10.1200/JCO.2005.03.8976. [DOI] [PubMed] [Google Scholar]
- 26.Kemeny MM, Peterson BL, Kornblith AB, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003;21:2268–2275. doi: 10.1200/JCO.2003.09.124. [DOI] [PubMed] [Google Scholar]
- 27.Chang SM, Barker FG 2nd, Schmidt MH, et al. Clinical trial participation among patients enrolled in the Glioma Outcomes Project. Cancer. 2002;94:2681–2687. doi: 10.1002/cncr.10536. [DOI] [PubMed] [Google Scholar]
- 28.National Cancer Institute: Conducting clinical trials. uri http://www.cancer.gov/clinicaltrials/conducting/cost.
- 29.Goldman DP, Berry SH, McCabe MS, et al. Incremental treatment costs in national cancer institute-sponsored clinical trials. JAMA. 2003;289:2970–2977. doi: 10.1001/jama.289.22.2970. [DOI] [PubMed] [Google Scholar]
- 30.Kilgore ML, Goldman DP. Drug costs and out-of-pocket spending in cancer clinical trials. Contemp Clin Trials. 2008;29:1–8. doi: 10.1016/j.cct.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Barlow W, Taplin S, Seger D, et al. Medical care costs of cancer patients on protocol. Presented at a National Cancer Institute meeting; July 7, 1998; Bethesda, MD. [Google Scholar]
- 32.Bennett CL, Stinson TJ, Vogel V, et al. Evaluating the financial impact of clinical trials in oncology: Results from a pilot study from the association of American Cancer Institutes/Northwestern University Clinical Trials Costs and Charges Project. J Clin Oncol. 2000;18:2805–2810. doi: 10.1200/JCO.2000.18.15.2805. [DOI] [PubMed] [Google Scholar]
- 33.Fireman BH, Fehrenbacher L, Gruskin EP, et al. Cost of care for patients in clinical trials. J Natl Cancer Inst. 2000;92:136–142. doi: 10.1093/jnci/92.2.136. [DOI] [PubMed] [Google Scholar]
- 34.Wagner JL, Alberts SR, Sloan JA, et al. Incremental costs of enrolling cancer patients in clinical trials: A population-based study. J Natl Cancer Inst. 1999;91:847–853. doi: 10.1093/jnci/91.10.847. [DOI] [PubMed] [Google Scholar]
- 35.Macklin R. On paying money to research subjects: “Due” and “undue” inducements. IRB. 1981;3:1–6. [PubMed] [Google Scholar]
- 36.McNeill P. Paying people to participate in research: Why not? A response to Wilkinson and Moore. Bioethics. 1997;11:390–396. doi: 10.1111/1467-8519.00079. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson M, Moore A. Inducement in research. Bioethics. 1997;11:373–389. doi: 10.1111/1467-8519.00078. [DOI] [PubMed] [Google Scholar]
- 38.Viens AM. Socio-economic status and inducement to participate. Am J Bioeth. 2001;1:1f–2f. [Google Scholar]
- 39.Donohue JM, Cevasco M, Rosenthal MB. A decade of direct-to-consumer advertising of prescription drugs. N Engl J Med. 2007;357:673–681. doi: 10.1056/NEJMsa070502. [DOI] [PubMed] [Google Scholar]
- 40.Abel GA, Burstein HJ, Hevelone ND, et al. Cancer-related direct-to-consumer advertising: Awareness, perceptions, and reported impact among patients undergoing active cancer treatment. J Clin Oncol. 2009;27:4182–4187. doi: 10.1200/JCO.2008.20.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCabe MS, Varricchio CG, Padberg RM. Efforts to recruit the economically disadvantaged to national clinical trials. Semin Oncol Nurs. 1994;10:123–129. doi: 10.1016/s0749-2081(05)80066-x. [DOI] [PubMed] [Google Scholar]
- 42.Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: A multicenter randomized controlled trial. J Clin Oncol. 2012;30:2516–2521. doi: 10.1200/JCO.2011.39.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollowell CM, Patel RV, Bales GT, et al. Internet and postal survey of endourologic practice patterns among American urologists. J Urol. 2000;163:1779–1782. [PubMed] [Google Scholar]
- 44.Kim HL, Gerber GS, Patel RV, et al. Practice patterns in the treatment of female urinary incontinence: A postal and internet survey. Urology. 2001;57:45–48. doi: 10.1016/s0090-4295(00)00885-2. [DOI] [PubMed] [Google Scholar]
- 45.Krosnick JA. Survey research. Annu Rev Psychol. 1999;50:537–567. doi: 10.1146/annurev.psych.50.1.537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.