Abstract
Purpose
Randomized data suggest that single-fraction or short-course palliative radiation therapy (RT) is sufficient in the majority of patients with metastatic cancer. We investigated population-based patterns in the use of palliative RT among patients with metastatic non–small-cell lung cancer (NSCLC).
Patients and Methods
From patients diagnosed with lung cancer from 2003 to 2005 at a participating geographic or organizational site and who consented to the Cancer Care Outcomes Research and Surveillance Consortium study, we identified patients with metastatic NSCLC who had complete medical records abstractions. Patient characteristics and clinical factors associated with receipt of palliative RT and RT intensity (total dose and number of treatments) were evaluated with multivariable regression.
Results
Of 1,574 patients with metastatic NSCLC, 780 (50%) received at least one course of RT, and 21% and 12% received RT to the chest and bone, respectively. Use of palliative RT was associated with younger age at diagnosis and receipt of chemotherapy and surgery to metastatic sites. Among patients receiving palliative bone RT, only 6% received single-fraction treatment. Among patients receiving palliative chest RT, 42% received more than 20 fractions. Patients treated in integrated networks were more likely to receive lower doses and fewer fractions to the bone and chest.
Conclusion
When palliative RT is used in patients with metastatic NSCLC, a substantial proportion of patients receive a greater number of treatments and higher doses than supported by current evidence, suggesting an opportunity to improve care delivery.
INTRODUCTION
About half of patients with non–small-cell lung cancer (NSCLC) have metastatic disease at diagnosis. Because the life expectancy of most patients with metastatic NSCLC is measured in months,1–3 symptom management and quality of life are important treatment goals. Radiation therapy (RT) in this setting is not curative and is frequently used to palliate symptoms from both thoracic and distant metastatic disease. When palliative RT is recommended, radiation oncologists must decide on a total dose and dose per treatment (dose per fraction). This determines the total number of treatments (number of fractions) and consequently the number of patient visits needed. For RT delivered with curative intent, the goal is to deliver a high total dose to the tumor while limiting long-term toxicity to normal tissues, achieved by dividing the treatment into a high number of low-dose fractions. This results in a long overall treatment course, requiring up to 7 or 8 weeks of daily treatment. In contrast, for patients receiving palliative RT, shorter overall treatment times are desirable because they offer lower costs and greater convenience, with fewer daily trips for RT, a particular burden in the face of short life expectancy.4
The optimal strategy for delivery of palliative RT to bony metastases has been evaluated in multiple randomized trials.5–7 Several meta-analyses, including up to 16 randomized studies, have shown no significant difference in pain relief between single-fraction (total dose 5 to 15 Gy) and multifraction (total dose 15 to 30 Gy in three to 10 fractions) RT for painful bony metastases, although reirradiation rates were higher (20% v 8%) with single-fraction RT, which may suggest poorer durability.8–10 Furthermore, quality of the randomized studies was variable, comparison arms were heterogeneous, and follow-up was relatively short.
For palliative RT to the chest, the data are more nuanced. Several meta-analyses, including up to 14 randomized studies, have found that shorter, lower-dose RT courses (10 to 35 Gy in one to 10 daily or 16 twice-daily fractions) result in equivalent palliation of specific symptoms with less treatment-related dysphagia, although higher doses (17 to 60 Gy in two to 30 fractions) were associated with a small improvement (27% v 22%) in 1-year survival.11–15 Because of heterogeneity in doses and treatment technique, the optimal dose is unknown, although sensitivity analysis suggested a benefit with a biologically effective dose (BED) of 35 (roughly 30 Gy in 10 treatments) compared with lower doses.13
Despite this extensive literature, controversy persists, and surveys of radiation oncologists that used clinical vignettes suggest that, particularly for bone metastases, providers may prefer higher doses and a greater number of fractions than those supported by data from randomized studies.16 However, there are scarce data on actual treatment practice. We therefore sought to characterize palliative RT dose and fractionation in a large, national population-based cohort of patients with metastatic NSCLC to understand how efficacy studies have been translated into practice.
PATIENTS AND METHODS
Sources of Data
The Cancer Care Outcomes Research and Surveillance Consortium (CanCORS) study prospectively enrolled 5,528 patients age 21 years or older who were diagnosed with lung cancer in Northern California, Los Angeles County, North Carolina, Iowa, or Alabama, or who received care in one of 10 Veterans Administration sites or one of five large health maintenance organizations (HMOs) from 2003 to 2005. Patients were identified within 3 months of diagnosis from population-based cancer registries at geographic sites and from pathology and cytology records at organizational sites.
Patients or their surrogates (for patients too ill to respond or deceased) completed an initial phone survey 4 to 7 months after diagnosis. Medical records (MRs) from all physicians involved in the patients' cancer care from 3 months before through 15 months after diagnosis were abstracted, including information on cancer-related diagnostic and staging procedures, surgery, chemotherapy, RT, tumor stage, comorbid illnesses, and test results. Among patients receiving RT, treatment dates, site of RT, type of RT, total dose, and fractionation were recorded. All patients gave written informed consent to participate in the study.17,18 The demographics of the CanCORS population have been shown to correspond well to those of the Surveillance, Epidemiology, and End Results (SEER) population, although the age distribution is slightly younger.19
Study Cohort
We identified patients who had complete MR abstraction and presented with stage IV NSCLC or developed metastatic recurrence after initial earlier-stage disease. Metastatic recurrence was defined when first tumor recurrence occurred only in an extrathoracic site.
RT
Use of palliative RT.
Radiation oncology consultation and treatment with external beam RT (excluding stereotactic RT) were identified from the MR abstraction. RT site was identified from MR abstraction, and only radiation courses occurring after the date of stage IV diagnosis or metastatic recurrence were included in the analysis.
Radiation dose and fractionation.
We focused on dose and fractionation to chest and bony sites of disease. Because we did not have data with sufficient granularity on brain metastases to determine appropriate treatment options, dose and fractionation to the brain were not assessed. For descriptive analyses, radiation dose was grouped as ≤10, more than 10 to 20, more than 20 to 30, more than 30 to 40, more than 40 to 50, and more than 50 Gy. Numbers of fractions were grouped as less than 6, 6 to 10, 11 to 15, 16 to 20, and more than 30, with five fractions corresponding to roughly 1 week of treatment. Total dose was missing for 9% and 6% of chest and bone RT patients, respectively. When data on number of fractions were incomplete in the MRs, we calculated missing values when possible by using the following equation: total dose = dose per fraction × number of fractions. Data on number of fractions were missing for 22% and 14% of chest and bone RT patients, respectively. After using calculated values, 15% and 11% of chest and bone RT patients, respectively, had missing values.
Because total dose may not always reflect potency, we also calculated BED, a measure of potency that accounts for dose and fractionation. In our cohort, BED (without time adjustment) was highly correlated with total dose (Pearson correlation of 0.99 for chest RT and 0.98 for bone RT). Therefore only total dose data were reported.
Covariates
Demographic variables.
Sociodemographic characteristics including sex, marital status, race, and insurance were obtained from the initial survey. For patients with missing information in the survey, data from the MR abstractions were used when available. All variables were grouped into mutually exclusive categories as listed in Table 1. Patients with any Medicaid coverage were classified in the Medicaid group.
Table 1.
Baseline Characteristics Among Patients With Metastatic NSCLC
| Characteristic | No. of Patients(N = 1,574) | % |
|---|---|---|
| Demographic characteristics | ||
| Age at diagnosis, years | ||
| 21-54 | 205 | 13 |
| 55-64 | 389 | 25 |
| 65-74 | 539 | 34 |
| 75+ | 441 | 28 |
| Sex | ||
| Male | 1,022 | 65 |
| Female | 552 | 35 |
| Marital status | ||
| Married/living as married | 933 | 59 |
| Not married | 623 | 40 |
| Unknown | 18 | 1 |
| Race | ||
| White | 1,122 | 71 |
| Hispanic/Latino | 74 | 5 |
| African American | 222 | 14 |
| Asian/Pacific Islander | 78 | 5 |
| Other | 72 | 5 |
| Unknown | 6 | 0.4 |
| Insurance | ||
| Medicare | 263 | 17 |
| Medicaid | 158 | 10 |
| Medicare plus private | 539 | 34 |
| Private | 387 | 25 |
| Other | 212 | 13 |
| Unknown | 15 | 1 |
| Clinical characteristics | ||
| Comorbidity score* | ||
| None | 326 | 21 |
| Mild | 581 | 37 |
| Moderate | 335 | 21 |
| Severe | 332 | 21 |
| Chemotherapy after metastasis | ||
| No | 770 | 49 |
| Yes | 804 | 51 |
| Primary lung cancer–directed surgery after metastasis | ||
| No | 1,474 | 94 |
| Yes | 100 | 6 |
| Metastatic site surgery† | ||
| No | 1,480 | 94 |
| Yes | 94 | 6 |
| Provider characteristics | ||
| Integrated network | ||
| No | 919 | 58 |
| Yes | 655 | 42 |
| PDCR site | ||
| Cancer Research Network | 218 | 14 |
| Northern California | 322 | 20 |
| Alabama | 192 | 12 |
| Los Angeles | 235 | 15 |
| Iowa | 336 | 21 |
| Veterans Administration | 271 | 17 |
Abbreviation: NSCLC, non–small-cell lung cancer; PDCR, Primary Data Collection and Research.
Defined by using the Adult Comorbidity Evaluation 27, a validated medical record-based system that assigns each patient a four-category comorbidity score (none, mild, moderate, or severe) based on severity noted across multiple body systems, from 3 months prior to diagnosis to initial treatment.
Includes surgery to brain, bone, liver, or adrenal glands.
Clinical variables.
Clinical variables were obtained from MR abstraction and included comorbidity as well as use of chemotherapy and surgery. Comorbidity was classified as none, mild, moderate, or severe according to the Adult Comorbidity Evaluation.20,21 Receipt of chemotherapy was identified at any time after metastatic diagnosis or recurrence. Surgery after metastatic diagnosis or recurrence was classified according to whether it was directed at the primary site (wedge/segmental resection, lobectomy, or pneumonectomy) or to a metastatic site (brain, bone, liver, or adrenal glands).
Provider variables.
Patients were categorized by CanCORS Primary Data Collection and Research (PDCR) site and whether treatment was received within an integrated network. Patients were considered to be treated in an integrated health system network if they were enrolled in a staff model health maintenance organization (HMO) or received their care through the Veterans Administration.
Statistical Analysis
We used univariable logistic regression to assess associations between demographic, clinical, and provider variables and the use of palliative RT. All a priori variables of interest, regardless of statistical significance, were included in a multivariable logistic regression model to identify variables independently associated with use of RT. Univariable and multivariable linear regression analyses were also performed to assess associations between the covariates and total dose and number of fractions of palliative chest and bone RT.
Item nonresponse was minimal (< 2% for variables of interest), and statistical analyses were conducted on a multiply-imputed data set by using standard statistical methods.22,23 Because there was significant collinearity between the PDCR site and care in an integrated network, final models included only integrated networks. Alternative models that included only PDCR site or both PDCR site and integrated network were also evaluated. P values were two-sided, and values less than .05 were considered statistically significant. Statistical analyses were conducted by using SAS, version 9.2 (SAS Institute, Cary, NC) and STATA, version 11.1 (STATA, College Station, TX).
RESULTS
Patients
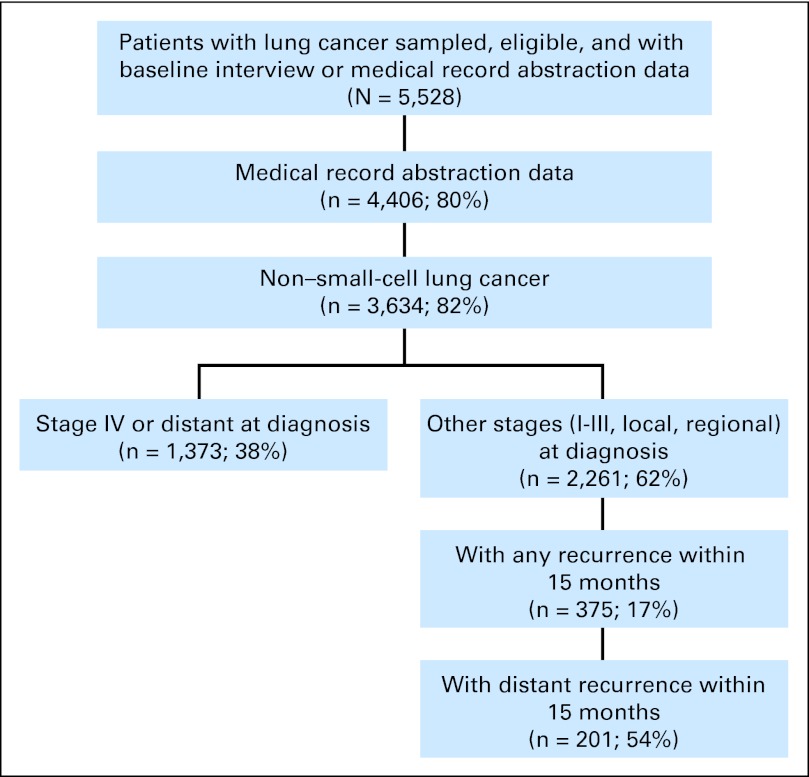
Of 5,528 patients with a diagnosis of lung cancer enrolled onto CanCORS, 1,574 satisfied our inclusion criteria and had metastatic disease at diagnosis (n = 1,373) or distant first recurrence (n = 201) within 15 months of diagnosis (Fig 1). Median survival following metastatic diagnosis was 4.7 months; 20% were alive at 15 months, the end of our observation period.
Fig 1.
Study cohort
The median age of our cohort was 68 years, and 35% were female. Overall, 895 patients (57%) had at least one visit with a radiation oncologist following the date of metastatic diagnosis, and of these, 780 (87%) received at least one course of RT. Fifty-one percent received at least one course of chemotherapy after metastatic diagnosis, 6% had surgery directed at the primary tumor, and 6% had surgery to a metastatic site. Table 1 lists the baseline demographic and clinical characteristics of our cohort, as well as attributes of their providers.
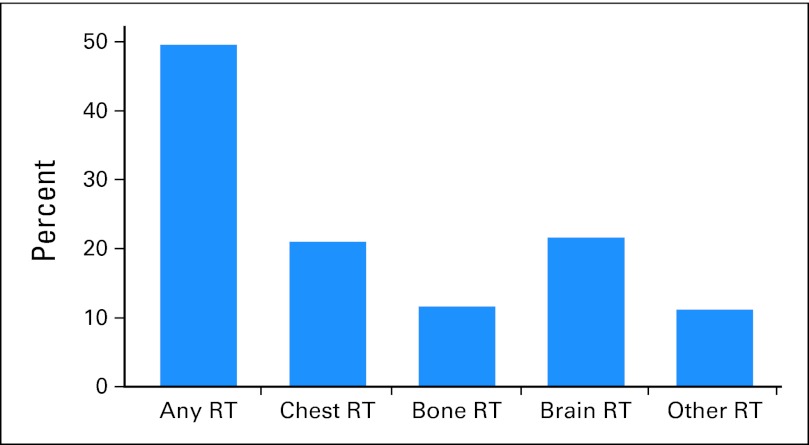
Among patients who received palliative RT, 67% had one course of treatment, 25% had two courses, and 8% had more than two courses during the 15 month follow-up period. The most common sites of treatment were the chest, bone, and brain, with 21%, 12%, and 22% of patients in our cohort receiving at least one course of RT to these sites, respectively (Fig 2).
Fig 2.
Use of palliative radiation by treatment site. Each patient was counted once for each treatment site. RT, radiation therapy.
Factors Associated With Use of RT
The only demographic or provider characteristic statistically significantly associated with use of RT in multivariable analysis was patient age. Patients older than age 80 years had only half the odds of receiving RT as those younger than age 55 years (39% v 61%; adjusted odds ratio [OR], 0.47; overall P = .003). There was no significant association with sex, marital status, race, comorbidity, or insurance type.
Patients treated with systemic chemotherapy were more likely to receive RT (56% v 42%; adjusted OR, 1.66; P < .001). Only 6% of patients had surgery directed at the primary tumor, but those who did were less likely to receive RT (36% v 50%; adjusted OR, 0.41; P < .001). In contrast, those with surgery directed at metastatic sites (bone, brain, liver, adrenal glands) were more likely to receive RT (65% v 49%; adjusted OR, 1.90; P = .006; Table 2).
Table 2.
Demographic, Provider, and Clinical Factors Associated With Use of RT
| Predictor | % of Patients Who Received RT | Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Age at diagnosis, years | < .001 | .003 | |||||
| 21-54 | 61 | Reference | Reference | ||||
| 55-69 | 56 | 0.81 | 0.57 to 1.14 | 0.89 | 0.62 to 1.28 | ||
| 70-79 | 49 | 0.61 | 0.44 to 0.85 | 0.63 | 0.41 to 0.95 | ||
| 80+ | 39 | 0.42 | 0.30 to 0.59 | 0.47 | 0.30 to 0.73 | ||
| Sex | .63 | .93 | |||||
| Male | 50 | Reference | Reference | ||||
| Female | 49 | 0.95 | 0.77 to 1.17 | 0.99 | 0.78 to 1.25 | ||
| Marital status | .02 | .13 | |||||
| Married/living as married | 52 | Reference | Reference | ||||
| Not married | 46 | 0.78 | 0.64 to 0.96 | 0.84 | 0.67 to 1.05 | ||
| Race | .20 | .29 | |||||
| White | 49 | Reference | Reference | ||||
| Hispanic/Latino | 53 | 1.18 | 0.73 to 1.88 | 1.10 | 0.67 to 1.79 | ||
| African American | 48 | 0.96 | 0.72 to 1.29 | 0.93 | 0.68 to 1.28 | ||
| Asian/Pacific Islander | 53 | 1.17 | 0.74 to 1.85 | 1.01 | 0.62 to 1.63 | ||
| Other | 63 | 1.76 | 1.08 to 2.87 | 1.75 | 1.04 to 2.94 | ||
| Insurance | .04 | .65 | |||||
| Medicare | 49 | Reference | Reference | ||||
| Medicaid | 47 | 0.95 | 0.64 to 1.42 | 0.80 | 0.52 to 1.23 | ||
| Medicare plus private | 45 | 0.88 | 0.65 to 1.18 | 0.81 | 0.59 to 1.12 | ||
| Private | 55 | 1.30 | 0.95 to 1.78 | 0.75 | 0.50 to 1.12 | ||
| Other | 54 | 1.21 | 0.85 to 1.74 | 0.81 | 0.52 to 1.25 | ||
| Comorbidity score | .14 | .34 | |||||
| None | 49 | Reference | Reference | ||||
| Mild | 53 | 1.19 | 0.91 to 1.57 | 1.23 | 0.92 to 1.64 | ||
| Moderate | 48 | 0.96 | 0.71 to 1.30 | 1.04 | 0.75 to 1.44 | ||
| Severe | 46 | 0.89 | 0.65 to 1.20 | 0.99 | 0.71 to 1.38 | ||
| Chemotherapy after metastasis | < .001 | < .001 | |||||
| No | 42 | Reference | Reference | ||||
| Yes | 56 | 1.77 | 1.45 to 2.16 | 1.66 | 1.34 to 2.05 | ||
| Primary lung cancer–directed surgery after metastasis | .006 | < .001 | |||||
| No | 50 | Reference | Reference | ||||
| Yes | 36 | 0.55 | 0.36 to 0.84 | 0.41 | 0.26 to 0.63 | ||
| Metastatic site surgery | .003 | .006 | |||||
| No | 49 | Reference | Reference | ||||
| Yes | 65 | 1.96 | 1.27 to 3.02 | 1.90 | 1.20 to 3.00 | ||
| Integrated network | .02 | .07 | |||||
| No | 52 | Reference | Reference | ||||
| Yes | 46 | 0.79 | 0.65 to 0.96 | 0.82 | 0.66 to 1.02 | ||
Abbreviations: OR, odds ratio; RT, radiation therapy.
Radiation Dose and Fractionation
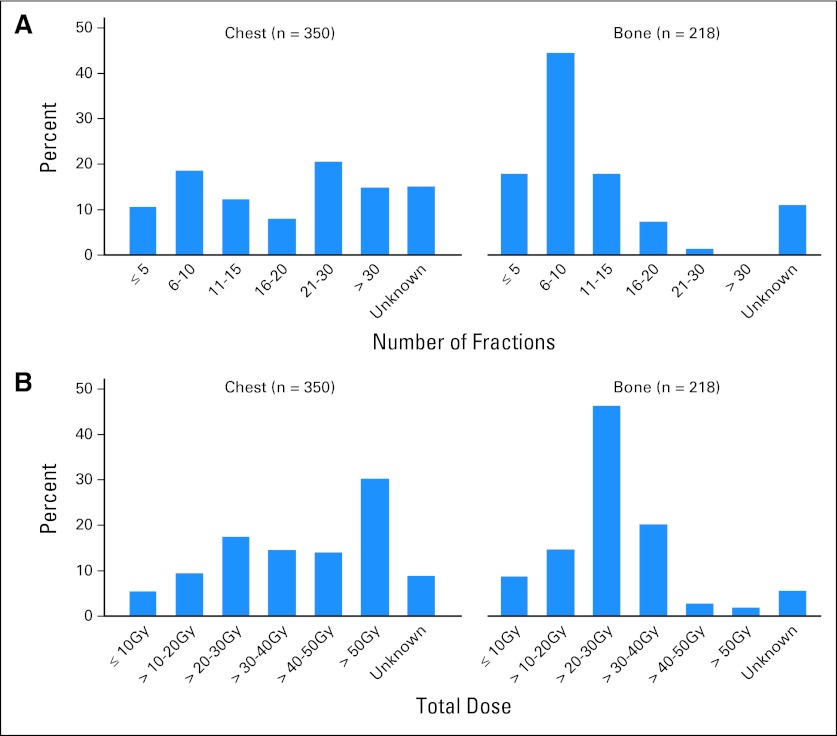
Among 194 patients who received palliative RT to the bone with known number of fractions, 50% of patients received six to 10 treatments, 20% received five fractions or fewer, and 6% received a single fraction. Among 206 patients with known dose, 49% received between 21 and 30 Gy.
Among 297 patients who received palliative RT to the chest with known number of fractions, 42% received more than 20 fractions, corresponding to about 4 weeks of daily treatment. Among 319 patients with known dose, 65% received in excess of 30 Gy, and 33% received more than 50 Gy. Figures 3A and 3B show the distribution of number of fractions and dose among patients receiving RT to the chest or bone.
Fig 3.
(A) Distribution of number of palliative radiation fractions (chest, bone); 568 courses for 472 patients. (B) Distribution of palliative radiation total dose by site (chest, bone); 568 courses for 472 patients.
On multivariable analysis, we found that patients treated in integrated networks received on average 3.4 fewer fractions (P = .001) and 4.0 Gy less dose (P = .049) when treated with palliative RT to the bone. They received, on average, 2.9 fewer fractions (P = .047) and 4.8 Gy less dose (P = .04) when treated with palliative RT to the chest. Those with greater comorbidity scores tended to receive higher total doses to the bone (overall P = .005), although the number of fractions did not differ significantly (overall P = .10). Those receiving chemotherapy after metastatic diagnosis, on average, received 7.0 more fractions and 11.3 Gy more dose to the chest (both P < .001). None of the other covariates were significantly associated with total RT dose or number of fractions to the chest or bone.
DISCUSSION
We found that 50% of patients with metastatic NSCLC received at least one course of palliative RT within 15 months of diagnosis, consistent with prior estimates of palliative RT use in patients with NSCLC.24,25 In addition, our finding that palliative RT was more likely to be used in younger patients who received chemotherapy and in patients treated with surgery to metastatic disease corroborates prior work showing that patients who were younger and had higher performance status were more likely to receive intensive treatment for NSCLC.26
Prior studies evaluating RT use in population-based cohorts have relied on registry-based data such as that from SEER-Medicare, which do not permit ascertainment of how treatment was delivered or dose and fractionation.24 Because CanCORS included comprehensive MR abstraction, we had a unique opportunity to measure not only whether but also how palliative RT was delivered. Indeed, our most striking finding is that when patients received palliative RT, they often had higher doses delivered in more fractions than the randomized data suggest is necessary. This occurred despite the fact that patients in our cohort had shorter median survival than most of the populations included the randomized studies of RT dose and fractionation.5–7,11,12
Although there is ample evidence that single-fraction RT for bone metastases is effective in most patients, we found that only 6% of patients treated for bone metastases received single-fraction RT. Since the 1980s, multiple randomized studies have compared single-fraction to multifraction RT, and most have failed to demonstrate a significant difference in symptomatic pain relief.5–7 Meta-analyses in 2003 and 2007 also did not find any differences in pain relief between single-fraction and multifraction bone RT.8,9 Although reirradiation rates were higher in the lower-dose arms, it is unclear whether this reflected a true difference in outcome, or simply increased willingness of providers to retreat after single-fraction versus multifraction RT.9 Notwithstanding this body of evidence, international surveys of practicing radiation oncologists have suggested a reluctance to recommend single-fraction regimens, which is especially pronounced in the United States.16,27
Despite data suggesting a small survival benefit in patients receiving modestly higher palliative doses to the chest (approximately 30 Gy in 10 fractions), there is no strong evidence of additional benefit from even higher doses. Meta-analyses published between 2003 and 2008 concluded that shorter, lower-dose RT to the chest results in equivalent control of most symptoms, with fewer treatment-related adverse effects. The most recent analysis found a 4.8% improvement in 1-year survival among patients receiving higher doses,13 although only one randomized study evaluated a dose in excess of 50 Gy.28 In our study, we found that 65% of patients treated with palliative chest RT received more than 30 Gy, and 33% received more than 50 Gy, which exceeds the dose levels evaluated in nearly all of the randomized studies. Some may believe that the small differences in dose potency evaluated in randomized studies were not high enough to observe a dose-response effect. However, this would argue for additional studies, rather than wide adoption of untested dose levels.
One possible explanation is that, despite evidence to the contrary, radiation oncologists continue to believe that higher total doses and fractionation schema are preferred. Providers may believe that higher doses can be delivered with minimal toxicity and may fail to adequately weigh the time and monetary costs incurred by patients and the health care system. Prior studies have shown that providers may also be overly optimistic about or have difficulty communicating a patient's prognosis.29–32 There are scarce data on patient preferences in the United States, but small studies from other countries suggest that patients can be involved in the decision-making process and that, although preferences vary,27,33–35 many may prefer shorter palliative RT courses when informed about alternatives. Longer treatment schedules are reimbursed at higher levels by insurance providers, raising the possibility that financial incentives could also influence practice patterns. Indeed, our observation that patients treated in integrated networks (HMO and Veterans Administration providers) had similar rates of palliative RT but received shorter treatments when RT was given, raises provocative questions about the role that physicians' financial incentives may play in decision making.36,37 Alternatively, the ability of integrated networks to shape care delivery may also influence RT treatment patterns.
Recent practice guidelines from the American Society of Radiation Oncology (ASTRO), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN) are supportive of shorter palliative RT courses.38–42 Although such guidelines have the potential to improve radiation oncology practice by better educating practitioners, our observation that practice may vary according to reimbursement structure suggests that realignment of financial incentives for treatment may also be warranted.
There are several limitations to our study. Notwithstanding the extensive MR abstractions, there was limited clinical information on symptoms or events leading to palliative RT, the specific anatomic site of RT, or response to treatment. More importantly, we could not ascertain physicians' thought processes or rationale for selecting a particular dose and fractionation. In addition, improved survival among patients with oligometastic disease or among patients receiving molecular agents, such as epidermal growth factor inhibitors, could alter how the costs and benefits of palliative RT are weighed. Most patients in our study were also enrolled before publication of the largest US bone metastases study,6 and practitioners could be slower to adopt data from foreign studies. Although our analysis found shorter treatment courses in integrated network settings, we cannot rule out the possibility that our integrated network variable could also be capturing regional effects, since these patients were concentrated near the Northern California PDCR site. However, the magnitude of the integrated network effect on dose and number of fractions persisted in sensitivity analyses that controlled for PDCR site.
In conclusion, palliative RT is frequently used in patients with metastatic NSCLC and has clearly demonstrated ability to improve quality of life in those patients. However, treatment can incur significant time and monetary costs for patients with limited life expectancy. We found that a substantial proportion of patients treated to the bone or chest receive higher doses and more fractions than clinical trial data supports. Our observation that patients treated in integrated networks receive lower total doses and fewer fractions suggests that provider characteristics, organizational structures and processes, and/or financial incentives may influence clinical practice. However, further study is necessary to clarify the reasons for the extent of overly intensive care and to develop strategies for bringing evidence and practice into better alignment.
Footnotes
Supported by Mentored Research Scholar Grant No. MRSG-10-170-01-PCSM (A.B.C.) from the American Cancer Society, by Grants No. U01 CA093332 (J.C.W.), U01 CA093324, U01 CA093348, U01 CA093329, U01 CA093339, U01 CA093326, and U01 CA093344 from the National Cancer Institute, and by Grant No. CRS 02-164 from the Department of Veterans Affairs (VA) to the Durham VA Medical Center.
Presented at the 53rd Annual Meeting of the American Society of Radiation Oncology, Miami Beach, FL, October 2-6, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Jennifer Malin, WellPoint (C) Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Aileen B. Chen, Jane C. Weeks,Deborah Schrag
Financial support: Aileen B. Chen, Jane C. Weeks
Provision of study materials or patients: Jane C. Weeks
Collection and assembly of data: Angel Cronin, Jane C. Weeks
Data analysis and interpretation: Aileen B. Chen, Angel Cronin, Jane C. Weeks, Elizabeth A. Chrischilles, Jennifer Malin, James A. Hayman, Deborah Schrag
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Edge SB, American Joint Committee on Cancer, American Cancer Society . AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual (ed 7) New York, NY: Springer; 2010. http://www.springer.com/medicine/surgery/book/978-0-387-88442-4. [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Konski A, James J, Hartsell W, et al. Economic analysis of radiation therapy oncology group 97-14: Multiple versus single fraction radiation treatment of patients with bone metastases. Am J Clin Oncol. 2009;32:423–428. doi: 10.1097/COC.0b013e31818da9f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: Randomised comparison with a multifraction schedule over 12 months of patient follow-up—Bone Pain Trial Working Party. Radiother Oncol. 1999;52:111–121. No authors listed. [PubMed] [Google Scholar]
- 6.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 7.Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52:101–109. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 8.Chow E, Harris K, Fan G, et al. Palliative radiotherapy trials for bone metastases: A systematic review. J Clin Oncol. 2007;25:1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 9.Wu JS, Wong R, Johnston M, et al. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003;55:594–605. doi: 10.1016/s0360-3016(02)04147-0. [DOI] [PubMed] [Google Scholar]
- 10.Sze WM, Shelley MD, Held I, et al. Palliation of metastatic bone pain: Single fraction versus multifraction radiotherapy—A systematic review of randomised trials. Clin Oncol (R Coll Radiol) 2003;15:345–352. doi: 10.1016/s0936-6555(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 11.Macbeth FR, Bolger JJ, Hopwood P, et al. Randomized trial of palliative two-fraction versus more intensive 13-fraction radiotherapy for patients with inoperable non-small cell lung cancer and good performance status: Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol) 1996;8:167–175. doi: 10.1016/s0936-6555(96)80041-0. [DOI] [PubMed] [Google Scholar]
- 12.Sundstrøm S, Bremnes R, Aasebø U, et al. Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: A national phase III trial. J Clin Oncol. 2004;22:801–810. doi: 10.1200/JCO.2004.06.123. [DOI] [PubMed] [Google Scholar]
- 13.Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: A systematic review. J Clin Oncol. 2008;26:4001–4011. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 14.Toy E, Macbeth F, Coles B, et al. Palliative thoracic radiotherapy for non-small-cell lung cancer: A systematic review. Am J Clin Oncol. 2003;26:112–120. doi: 10.1097/00000421-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Lester JF, Macbeth FR, Toy E, et al. Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev. 2006;4:CD002143. doi: 10.1002/14651858.CD002143.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: Evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75:1501–1510. doi: 10.1016/j.ijrobp.2008.12.084. [DOI] [PubMed] [Google Scholar]
- 17.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Malin JL, Ko C, Ayanian JZ, et al. Understanding cancer patients' experience and outcomes: Development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Support Care Cancer. 2006;14:837–848. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 19.Catalano PJ, Ayanian JZ, Weeks JC, et al. Representativeness of Participants in the Cancer Care Outcomes Research and Surveillance Consortium Relative to the Surveillance, Epidemiology, and End Results Program. Med Care. doi: 10.1097/MLR.0b013e318222a711. [epub ahead of print on March 7, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 21.Piccirillo JF, Costas I, Claybour P, et al. The measurement of comorbidity by cancer registries. J Regist Manage. 2003;30:8–14. [Google Scholar]
- 22.He Y, Zaslavsky AM, Landrum MB, et al. Multiple imputation in a large-scale complex survey: A practical guide. Stat Methods Med Res. 2010;19:653–670. doi: 10.1177/0962280208101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y. Missing data analysis using multiple imputation: Getting to the heart of the matter. Circ Cardiovasc Qual Outcomes. 2010;3:98–105. doi: 10.1161/CIRCOUTCOMES.109.875658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayman JA, Abrahamse PH, Lakhani I, et al. Use of palliative radiotherapy among patients with metastatic non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;69:1001–1007. doi: 10.1016/j.ijrobp.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 25.Fry WA, Phillips JL, Menck HR. Ten-year survey of lung cancer treatment and survival in hospitals in the United States: A national cancer data base report. Cancer. 1999;86:1867–1876. doi: 10.1002/(sici)1097-0142(19991101)86:9<1867::aid-cncr31>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Potosky AL, Saxman S, Wallace RB, et al. Population variations in the initial treatment of non-small-cell lung cancer. J Clin Oncol. 2004;22:3261–3268. doi: 10.1200/JCO.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 27.Bradley NM, Husted J, Sey MS, et al. Review of patterns of practice and patients' preferences in the treatment of bone metastases with palliative radiotherapy. Support Care Cancer. 2007;15:373–385. doi: 10.1007/s00520-006-0161-3. [DOI] [PubMed] [Google Scholar]
- 28.Nestle U, Nieder C, Walter K, et al. A palliative accelerated irradiation regimen for advanced non-small-cell lung cancer vs. conventionally fractionated 60 GY: Results of a randomized equivalence study. Int J Radiat Oncol Biol Phys. 2000;48:95–103. doi: 10.1016/s0360-3016(00)00607-6. [DOI] [PubMed] [Google Scholar]
- 29.Glare P, Virik K, Jones M, et al. A systematic review of physicians' survival predictions in terminally ill cancer patients. BMJ. 2003;327:195–198. doi: 10.1136/bmj.327.7408.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gripp S, Moeller S, Bölke E, et al. Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol. 2007;25:3313–3320. doi: 10.1200/JCO.2006.10.5411. [DOI] [PubMed] [Google Scholar]
- 31.Timmermans LM, van der Maazen RW, Leer JW, et al. Palliative or curative treatment intent affects communication in radiation therapy consultations. Psychooncology. 2006;15:713–725. doi: 10.1002/pon.1008. [DOI] [PubMed] [Google Scholar]
- 32.Lamont EB, Christakis NA. Prognostic disclosure to patients with cancer near the end of life. Ann Intern Med. 2001;134:1096–1105. doi: 10.7326/0003-4819-134-12-200106190-00009. [DOI] [PubMed] [Google Scholar]
- 33.Shakespeare TP, Lu JJ, Back MF, et al. Patient preference for radiotherapy fractionation schedule in the palliation of painful bone metastases. J Clin Oncol. 2003;21:2156–2162. doi: 10.1200/JCO.2003.10.112. [DOI] [PubMed] [Google Scholar]
- 34.Barton MB, Dawson R, Jacob S, et al. Palliative radiotherapy of bone metastases: An evaluation of outcome measures. J Eval Clin Pract. 2001;7:47–64. doi: 10.1046/j.1365-2753.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 35.Szumacher E, Llewellyn-Thomas H, Franssen E, et al. Treatment of bone metastases with palliative radiotherapy: Patients' treatment preferences. Int J Radiat Oncol Biol Phys. 2005;61:1473–1481. doi: 10.1016/j.ijrobp.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 36.Lievens Y, Van den Bogaert W, Rijnders A, et al. Palliative radiotherapy practice within Western European countries: Impact of the radiotherapy financing system? Radiother Oncol. 2000;56:289–295. doi: 10.1016/s0167-8140(00)00214-0. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson M, O'Malley AJ, Earle CC, et al. Does reimbursement influence chemotherapy treatment for cancer patients? Health Aff (Millwood) 2006;25:437–443. doi: 10.1377/hlthaff.25.2.437. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1:60–71. doi: 10.1016/j.prro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: An ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 40.American College of Radiology Appropriateness Criteria. Bone metastases. http://www.acr.org/Quality-Safety/Appropriateness-Criteria/Oncology/Bone-Metastases.
- 41.Janjan N, Lutz ST, Bedwinek JM, et al. Therapeutic guidelines for the treatment of bone metastasis: A report from the American College of Radiology Appropriateness Criteria Expert Panel on Radiation Oncology. J Palliat Med. 2009;12:417–426. doi: 10.1089/jpm.2009.9633. [DOI] [PubMed] [Google Scholar]
- 42.National Comprehensive Cancer Network. Clinical Practice Guidelines: Non-Small Cell Lung Cancer (v.2.2010) http://www.nccn.org. [Google Scholar]