Abstract
There is growing evidence that obesity represents a risk for enhanced gray matter (GM) density changes comparable to those demonstrated for mild cognitive impairment in the elderly. However, it is not clear what mechanisms underlie this apparent alteration in brain structure of overweight subjects and to what extent these changes can already occur in the adolescent human brain. In the present volumetric magnetic resonance imaging study, we investigated GM changes and serum levels of neuron-specific enolase (NSE), a marker for neuronal injury, in a set of overweight/obese subjects and controls. We report a negative correlation for overweight and obese subjects between serum NSE and GM density in hippocampal and cerebellar regions. To validate our neuroimaging findings, we complement these data with NSE gene expression information obtained from the Allen Brain atlas. GM density changes were localized in brain areas that mediate cognitive function—the hippocampus associated with memory performance, and the cognitive cerebellum (lateral posterior lobes) associated with executive, spatial and linguistic processing. The data of our present study highlight the importance of extending current research on cognitive function and brain plasticity in the elderly in the context of obesity to young adult subjects and include serum biomarkers to validate imaging findings generally.
Keywords: hippocampus, magnetic resonance imaging, neuronal injury, neuron-specific enolase, obesity
Introduction
Obesity, a medical condition with increasing prevalence, compromises health and personal well-being. Obesity has been discussed in the neurobiological framework of addiction, because it might result from food addiction that strongly resembles addiction to drugs.1, 2 Cognitive abnormalities, specifically learning and memory deficits, have been reported for obesity and typical comorbid disorders, such as hypertension3 and diabetes type 2.4 Even more alarmingly, compelling evidence is growing for cognitive dysfunction to be associated with obesity in otherwise healthy adults (for review see Sellbom and Gunstad5). Based on this evidence, obesity has been discussed as a risk factor for neurodegenerative disorders, such as Alzheimer's disease, a disorder characterized by learning and memory deficits.6 Neuroimaging studies have identified regional cerebral atrophy paralleling high body mass index (BMI) using computed tomography7 and magnetic resonance imaging (MRI).8, 9, 10 However, the mechanisms underlying this apparent alteration in brain structure of obese subjects are yet unclear.
We combine the neuroimaging method MRI and the assessment of a biochemical marker for neuronal damage to study the effects of higher BMI on brain plasticity. Specifically, we investigate cerebral gray matter (GM) density in brain regions implicated in learning and memory, such as the hippocampus. In order to clarify whether obesity-associated brain atrophy could be explained by a putative brain injury, we include measurements of neuron-specific enolase (NSE) serum levels. NSE is primarily localized in the cytoplasm of neurons and not secreted. Thus, this enzyme can be used as reliable marker for structural neuronal damage and increased serum NSE concentrations have been observed in patients suffering from traumatic brain injury11 and neurodegenerative disease.12 In the present study, we hypothesize a higher BMI to be associated with increased NSE serum levels and decreased hippocampal GM. To validate our results we include an analysis of NSE gene expression data in the whole human brain. We propose NSE expression to be increased in brain regions that reveal the most prominent GM density changes in the neuroimaging.
Materials and methods
Subjects
A total of 43 (27 overweight/obese: 15 female, 12 male; and 16 controls: 6 female, 10 male) young adult subjects (aged 20–41) without cognitive impairment were included in the study. Demographic details are shown in Table 1. The study was approved by the ethics committee of the University of Leipzig adhering to the Declaration of Helsinki. All subjects provided written informed consent.
Table 1. Demographics for subjects included in the MRI-scanning protocol.
| Control subjects (BMI<25) | Overweight and obese subjects (BMI>25) | Control versus overweight/obese subjects P-values | |
|---|---|---|---|
| N | 16 (6 female/10 male) | 27 (15 female/12 male) | |
| BMI (kg m−2; mean±s.d.; range) | 22.5±2.0 (18.5–24.9) | 33.0±6.4 (25.3–50.7) | P<0.000001 |
| age (years; mean±s.d.; range) | 24.8±3.0 (20–34) | 26.4±5.4 (19–41) | NS (P=0.28) |
| NSE (μg l−1; mean±s.d.; range) | 8.4±1.7 (5.3–11.3) | 9.1±1.4 (6.9–13.6) | NS (P=0.14) |
Abbreviatons: BMI, body mass index; MRI, magnetic resonance imaging; NSE, neuron-specific enolase; NS, not significant.
Measurements
MRI: Scanning was performed with a 3-Tesla TIM Trio Scanner (Siemens, Erlangen, Germany). High-resolution anatomical images were acquired using a T1-weighted 3D magnetization-prepared rapid gradient echo (MPRAGE) sequence with selective water excitation and linear phase encoding.13 Scanning was done using a sagittal slice orientation with the following imaging parameters: TI=650 ms; TR=1300 ms; TE=3.5 ms; alpha=10° bandwidth=190 Hz per pixel; image matrix=256 × 240; FOV=256 mm × 240 mm; spatial resolution=1 mm × 1 mm × 1 mm; 2 acquisitions.
Serum NSE: Blood samples were collected on the day of MR scanning and stored at −80 °C until assayed. Serum NSE levels were determined by monoclonal 2-site immunoluminometric assays, respectively, in one batch as described in more detail previously.14, 15, 16
NSE gene expression: Gene expression was investigated in the The Allen Human Brain Atlas, a publicly available online resource of gene expression (www.brain-map.org). It characterizes gene expression in human brain tissue with genome-wide microarray-based gene expression profiles including over 62 000 gene probes for 500 samples from each hemisphere covering the whole brain. Microarray analysis data, normalized across each individual brain, are included in the Allen Human Brain Atlas data set and illustrated in heat map format as Z scores. Z scores represent individual regional gene expression normalized to whole brain expression of that gene. To date (search conducted on the 17 July 2012), three subjects without a history of neuropsychiatric or neurological conditions are contained in the database (age 57, 24 and 39 years; all male). Note that these subjects were not included in our imaging study. NSE is coded in the Allen Human Brain Atlas as enolase 2 (gamma, neuronal) (ENO2), which is a synonym and, hence, describes the same enzyme. Gene expression of NSE was analyzed in two probes in the Allen Human Brain Atlas. We report and analyzed both probes to ensure high validity of the data—described as NSE-1/probe 1 or NSE-2/probe 2 in Figure 4 (Probe 1 A_24_P236091, sequence TTCATTCATCCCATTAATCATTTCCCCATAACTCAATGGCCTAAAACTGGCCTGACTTGG; Probe 2 CUST_15875_PI416261804, sequence ATCGTGATGGCAAATATGACTTGGACTTCAAGTCTCCCACTGATCCTTCCCGATACATCA). Detailed information for subjects included and analysis methods is available at www.brain-map.org. To calculate mean values of normalized expression in brain regions of interest we extracted normalized Z scores from the database for each individual and region of interest. Thereafter, expression values were compared using two-tailed Student's t-tests against 0 to investigate significant changes in expression compared with whole brain and thereafter tested with paired Student's t-tests to compare expression between different brain regions.
Analysis
Structural T1-weighted images were processed with the VBM8 toolbox (dbm.neuro.uni-jena.de/vbm/) using SPM8 (Wellcome Trust Center for Neuroimaging, UCL, London, UK) and Matlab 7 (Mathworks, Sherborn, MA, USA). Pre-processing included bias-field correction, segmentation and normalization to the standard MNI (Montreal Neurological Institute) space including modulation to account for local compression and expansion during transformation. GM density was obtained using the normalized and modulated GM segmentations. Subsequently, images were smoothed with a Gaussian kernel of 8 mm full width at half maximum (FWHM). GM density values and serum NSE concentrations were correlated using age, total GM volume and the serum soluble leptin receptor (sObR) level representing sensitivity of tissue to leptin, a hormone closely associated with obesity1, 9, 17 as covariates in the general linear model. In a second approach, we repeated the analysis without sObR as a covariate. We further assessed the interaction between BMI and NSE using both groups of subjects within a full-factorial design. Clusters were obtained using a voxel-threshold of P<0.005, and the anatomical localization of significant clusters (P<0.05, false discovery rate (FDR)-corrected) was investigated with the SPM Anatomy toolbox. To calculate mean values of normalized gene expression of NSE in brain regions of interest we extracted normalized Z scores from the Allen Human Brain Atlas database for each individual and region of interest. Thereafter, expression values were compared with two-tailed Student's t-tests.16
Results
In the group of overweight and obese subjects, we found a negative correlation between GM density values and serum NSE concentrations in the left and right cerebellum (Figure 1, middle row). In this region, an increased body weight was associated with decreased GM density values accompanied with elevated serum NSE concentrations. Note that NSE serum levels were in the normal range in all of our subjects (Table 1) as the upper limit of the normal range was determined as 18.3 μg l−1 in our test system in agreement with the literature.18
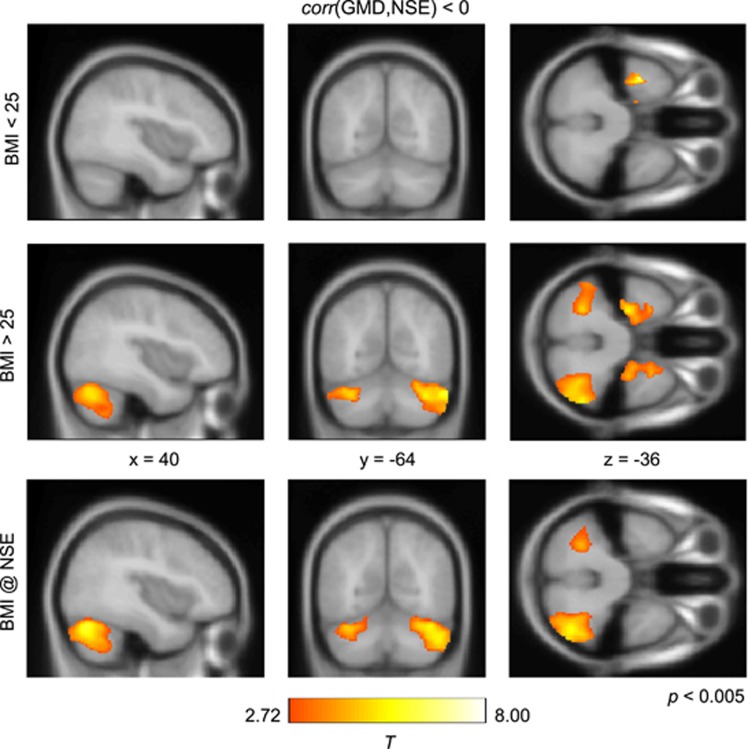
Figure 1.
Negative correlation between GM density and serum NSE detected in a set of lean (BMI<25) and overweight and obese (BMI>25) participants, color coded in red/yellow, using a voxel threshold of P<0.005 and a cluster threshold of P<0.05, FDR-corrected. In the group of 27 overweight and obese subjects, we found a significant negative correlation between GM density and serum NSE concentration in the left and right cerebellum (middle row). Such a negative correlation was also detected bilaterally in the medial temporal lobe (see middle row, axial view). For the control group we were not able to show a negative correlation between GM density and NSE levels in the cerebellum (top row). The interaction contrast between both factors of body weight and NSE shows the difference between lean and non-lean subjects with respect to the negative correlation between GM density and NSE (bottom row). In that interaction contrast, both cerebellar clusters remained significant showing the negative correlation between GM density and NSE specific to overweight and obese subjects.
Using the SPM Anatomy toolbox, local maxima of both cerebellar clusters were assigned to lobule VIIa Crus I (Hem) (for details, see Figure 2, top row and Table 2). We also observed a negative correlation between GM density and serum NSE concentration in the left and right medial temporal lobe, which were assigned to the entorhinal cortex (Figure 2, bottom row and Table 2). In the control group of lean subjects, we detected a negative correlation between GM density and serum NSE concentration in the temporal lobe in the left hemisphere (see Figure 1, top row). However, the maxima within that cluster were not assigned to a specific homogenous anatomical region within the SPM Anatomy toolbox.
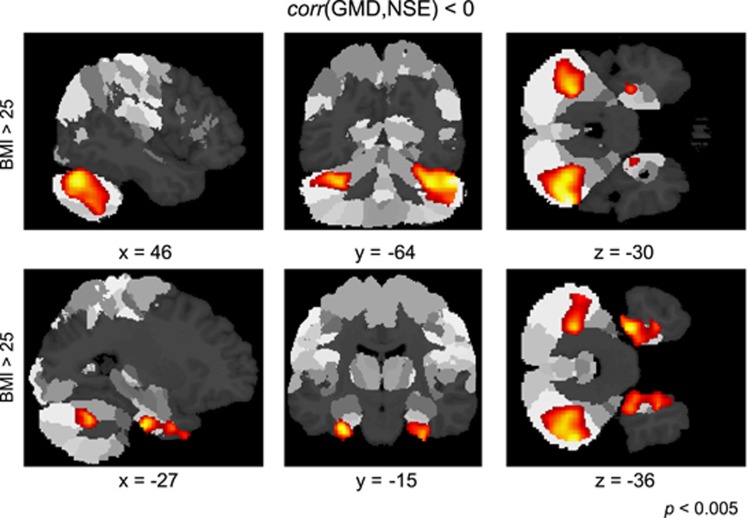
Figure 2.
Anatomical localization of GM density decrease accompanied with an increase of serum NSE concentration in overweight and obese subjects. Significant negative correlation between GM density and NSE was detected in hippocampal and cerebellar regions using a voxel threshold of P<0.005 and a cluster threshold of P<0.05, FDR-corrected, color coded in red/yellow. Using the SPM Anatomy toolbox, both cerebellar clusters were assigned to Lobule VIIa Crus I (Hem) (top row, color coded in red/yellow, see also Table 2). Both clusters located in the medial temporal lobe were assigned to the left and right entorhinal cortex (bottom row).
Table 2. Spatial localization of significant clusters showing a negative correlation between GM density and serum NSE concentration for the group of 27 overweight and obese subjects (BMI>25) with a voxel threshold of P<0.005 and a cluster threshold of P<0.05, FDR-corrected.
| PFDR | x | y | z | Hem | T | Assigned to | Probability (%) | Range (%) |
|---|---|---|---|---|---|---|---|---|
| 0.017 | −28 | −16 | −36 | L | 5.27 | Hippocampus (EC) | 80 | (50–100) |
| −28 | 3 | −38 | L | 4.19 | Hippocampus (EC) | 40 | (20–60) | |
| −27 | 14 | −45 | L | 3.67 | — | — | — | |
| 0.033 | 30 | −16 | −39 | R | 4.49 | — | — | — |
| 27 | 4 | −38 | R | 4.28 | Hippocampus (EC) | 90 | (60–100) | |
| 24 | −17 | −28 | R | 4.22 | Hippocampus (EC) | 100 | (80–100) | |
| 0.003 | −32 | −60 | −33 | L | 5.29 | Cerebellum, Lobule VIIa Crus I (Hem) | 75 | (59–86) |
| −52 | −54 | −38 | L | 3.79 | Cerebellum, Lobule VIIa Crus I (Hem) | 94 | (91–100) | |
| −45 | −46 | −44 | L | 3.40 | Cerebellum, Lobule VIIa Crus I (Hem) | 56 | (56–78) | |
| 0.000 | 56 | −63 | −32 | R | 6.01 | Cerebellum, Lobule VIIa Crus I (Hem) | 86 | (61–100) |
| 54 | −60 | −47 | R | 5.99 | Cerebellum, Lobule VIIa Crus I (Hem) | 74 | (73–83) | |
| 36 | −64 | −33 | R | 5.20 | Cerebellum, Lobule VIIa Crus I (Hem) | 100 | (91–100) |
Abbreviations: BMI, body mass index; FDR, false discovery rate; GM, gray matter; Hem, Hemisphere; L, left; MNI, Montreal Neurological Institute; NSE, neuron-specific enolase; PFDR, cluster-level FDR-corrected P-value; R, right; x,y,z, MNI coordinates.
Anatomical localization was obtained using the SPM Anatomy toolbox. Right columns show the probability of finding the assigned anatomical structure with the local cluster maximum. Range shows the range of those probabilities including the surrounding voxels.
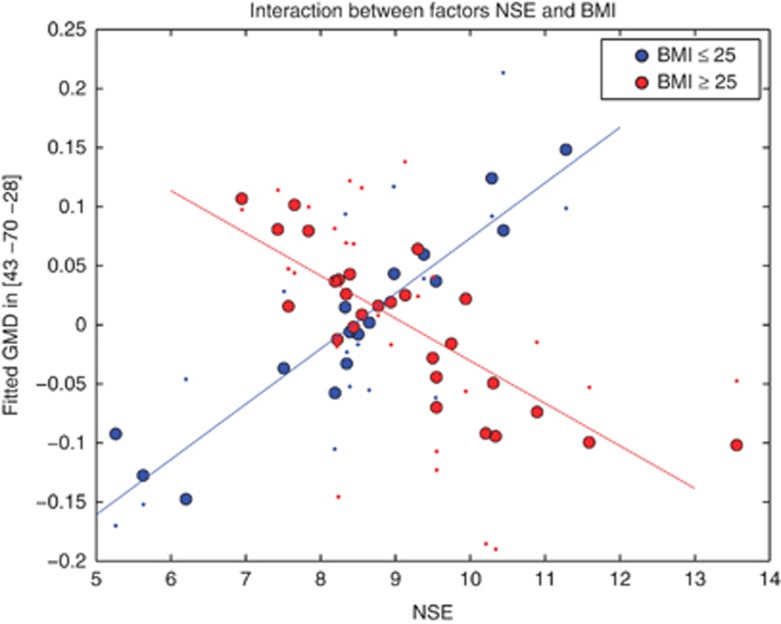
The interaction contrast between both factors of body weight and NSE showed the difference between lean and non-lean subjects with respect to the negative correlation between GM density and NSE concentration: in that interaction contrast, both cerebellar clusters remained significant showing the negative correlation between GM density and NSE specific for overweight and obese subjects (Figure 1, bottom row). Figure 3 depicts this interaction between BMI and NSE concentration within the cerebellum as a dot-plot, revealing the relationship between GM density and NSE concentration to differ with BMI category.
Figure 3.
Interaction between both factors of NSE and BMI in the right cerebellum. Filled circles (red: BMI>25; blue: BMI<25) show fitted GM density representing the interaction between NSE and BMI, while dots show fitted GM density including the error term of the general linear model. Thus, the dots show original GM density values adjusted for the confounds of age and total GM volume. For the spatial localization of the NSE and BMI interaction, see Figure 1, bottom row.
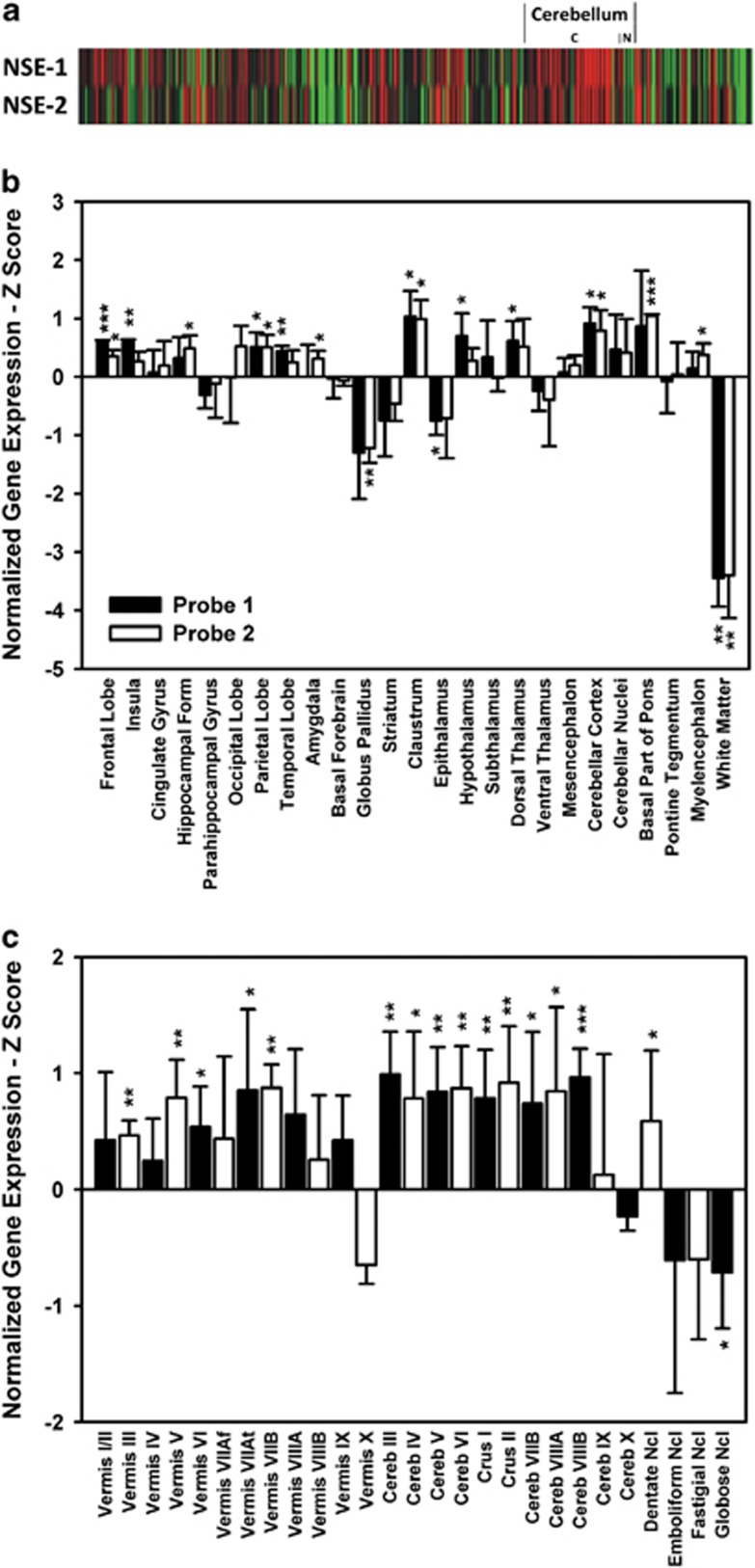
Figure 4 illustrates normalized Z scores for gene expression of NSE in the whole human brain. To identify brain regions with increased expression in comparison to whole brain, we calculated mean values for each region across the three subjects in the database (see top bar chart in Figure 4) and compared these values against 0 with a Student's t-test. One-tailed P is reported, because we expected higher expression in the cerebellum according to Herculano-Houzel.19 Moreover, we report only regions that showed significant changes for both NSE probes to guarantee a high consistency.
Figure 4.
Expression of NSE in the human brain. Individually normalized gene expression is shown in Z scores normalized to whole human brain expression. (a) Heat map across the whole human brain and for each of the three subjects beside each other, where green indicates low and red high expression. For the cerebellum cortex (C) and nuclei (N) are separated. Bars represent mean normalized gene expression±s.d. across three male subjects for (b) the whole brain and (c) cerebellar sub-regions. In (a), NSE-1/NSE-2, and in (b), probe 1/probe 2 (white and black bars, respectively) represent data for the two NSE probes analyzed in the Allen Human Brain Atlas (see Materials and methods section). In (c), probes 1 and 2 are pooled. Accordingly, white and black color is only used to contrast adjacent bars here. ***P<0.001, **P<0.01, *P<0.05 one-tailed Student's t-test against 0. Cereb: cerebellum, Form: formation, Ncl: nucleus.
Brain regions with significantly increased normalized gene expression included the frontal and parietal lobes, the claustrum and cerebellar cortex, whereas gene expression of NSE was significantly reduced in the white matter (Figure 4). For these regions gene expression was highest in the claustrum followed by the cerebellar cortex in probe 1 (1.03±0.44 and 0.92±0.28) and probe 2 (0.99±0.33 and 0.79±0.35) without significant differences for gene expression between both brain regions (P=0.40, P=0.53 for probe 1 and 2, respectively; paired two-tailed Student's t-test). Cerebellar gene expression of NSE was higher than in the white matter (P=0.003/P=0.014), without significant differences to gene expression in the frontal or parietal lobe (P=0.14, 0.31/P=0.22, 0.47 again for probe 1 and 2).
Finally, we analyzed gene expression of NSE in cerebellar sub-regions (bottom bar chart in Figure 4; data were from both NSE probes, because data were not available for some sub-regions in single subjects): gene expression was significantly increased in vermis (lobules III, V, VI, VIIA and VIIB) and cerebellar lobules III, IV, V, VI, VIIB, VIIIA, VIIIB, Crus I and II, dentate nuclei and significantly decreased in globose nuclei.
Discussion
In summary, we found that in overweight and obese young adult subjects, reduced GM correlates with increased serum NSE levels. The brain regions revealing GM density to correlate with serum NSE concentration in the overweight/obese group include bilateral hippocampal areas (Figure 1, middle row), as well as bilateral cerebellar areas (Figure 1, bottom row). In the control group, we found a negative correlation between GM density and NSE in the temporal lobe for the left hemisphere (Figure 1, top row). The interaction between body weight and NSE revealed a significant difference between groups for both hippocampal and cerebellar clusters (Figure 2). In the right cerebellum, we found the relationship between GM density and NSE to be determined by weight category (Figure 3). Interestingly, whole brain gene expression of NSE was highest in the cerebellum in healthy subjects of another cohort (Figure 4), although this finding has to be replicated if a higher number of subjects is available in the Allen Human Brain Atlas database.
This is the first study to examine indicators of brain atrophy in a combination of blood biomarkers with a neuroimaging design. One other structural MRI study has investigated the relationship between heightened BMI and brain atrophy in adults; in a voxel based morphometric (VBM) MRI study, Pannacciulli et al.,10 showed a negative association between BMI and GM density of the left post-central gyrus in obese subjects. However, they also found higher BMI to correlate with increased GM density in some brain regions, and concluded that the exploratory nature of their study design did not allow for valid interpretation regarding the nature of these differences of brain morphology in obesity. This limitation can be partly overcome by detection of serum markers specific for neuronal damage; the negative correlation between GM density and serum NSE concentration we found in overweight and obese subjects suggests a potential structural damage of neuronal brain cells.
In rodents, it has been demonstrated that the induction of an obesity phenotype can lead to impaired structural integrity of the hippocampus via downregulation of hypothalamic insulin receptors.20 This model of deficits in hippocampal plasticity in combination with compromised leptin signaling following symptoms of a metabolic syndrome20 may provide a theoretic framework for the preclinical cognitive impairment observed in obesity. Our data extend results from a cohort study revealing greater waist-hip ratio to negatively correlate with hippocampal volume in a sample of advanced age that could not be sufficiently accounted for by vascular factors, such as comorbid hypertension and diabetes.21 Given that human preclinical cognitive impairment has been shown to correlate with smaller hippocampus size,22, 23, 24 and that the hippocampus exhibits signs of atrophy in Alzheimer's disease,25, 26 our finding of decreased hippocampal GM to correlate with heightened NSE levels in obese subjects is the first evidence in young adults to link obesity with a specific indicator for neuronal injury in a brain region of particular sensitivity to neurodegeneration and cognitive decline. Interestingly, transgenic mouse models overexpressing tau protein solely in the entorhinal cortex suggest that neurofibrillary tangles spread to other connected areas such as the dentate gyrus, Cornu Ammonis (CA) fields of the hippocampus and cingulate cortex.27
We further found the decrease of GM density to reveal a significant difference between groups for the interaction between body weight and NSE levels in the bilateral cerebellum. We acknowledge the limitation that this finding could reflect a higher signal-to-noise ratio rather than a true signal due to the cerebellum containing the majority of all brain neurons regardless of brain size (for review, see Herculano-Houzel19). However, gene expression data obtained from the Allen Brain Atlas clearly identify the cerebellum as a brain structure where NSE is highly expressed (Figure 4) and, thus highlight the potential heightened sensitivity of the cerebellum to indicate early processes of subtle axonal injury, regeneration or re-innervation. Second, in line with the model of compromised neural plasticity in combination with impaired leptin signaling, GM in the cerebellum has been observed to correlate with leptin levels in elderly overweight and obese subjects.28 Interestingly, structural changes in the cerebellum were also shown in childhood obesity leading to reduced cerebellar volume.29 Our findings can thus be interpreted as a confirmation of the vulnerability of the cerebellum to neuronal injury linked with obesity in young adulthood.
Specifying the clinical consequences of cerebellar injury in overweight/obesity, sub-regional information for the cerebellum is of particular interest. Our analysis identified two sub-regions as mainly impaired—Lobule VIIa and Crus I. As discussed recently by Stoodley and Schmahmann30 these lateral structures are part of the neocerebellum that has expanded massively through evolution together with cerebral association areas. Of particular interest to the interpretation of our current findings, lobule VII and Crus I represent parts of the cognitive cerebellum, whereas areas assigned to the sensorimotor and limbic (or emotional) cerebellum did not reveal any GM density changes in our data set. Lesions of the cognitive cerebellum lead to cognitive impairments via disruption of cerebellar modulation of cognitive loops with cerebral association areas. Impairments include a range of executive, spatial and linguistic deficits. Interpreting these cerebellar GM density changes in the context of the hippocampal findings, one might speculate that overweight/obesity in young age could contribute to the heightened vulnerability brain regions, that host cognitive function, exhibit to neurodegeneration in later life, such as observed in Alzheimer's disease.
A very recent study has identified aerobic exercise as a significant beneficial factor for maintenance of cognitive function and integrity of brain structure vulnerable to obesity-related decline in older adults.31 The data of our present study highlight the importance of extending this line of work in young overweight and obese adults to maximize the potential benefits of such a fitness regimen on brain plasticity and to further explore this promising strategy to maintain optimal cognitive function. Our study suggests the inclusion of serum biomarkers to validate imaging findings in future studies.
Acknowledgments
We thank Dr Terri Gilbert from the Allen Institute for Brain Sciences for support in data analyses. This research received project and salary support from LIFE—Leipzig Research Center for Civilization Diseases at the University of Leipzig—funded by European Union, European Regional Development Fund and by Free State of Saxony within the framework of the excellence initiative to KA, AV and MLS; from the German Consortium for Frontotemporal Lobar Degeneration, funded by the German Federal Ministry of Education and Research to MLS; from the Alexander von Humboldt Foundation (AvH) and from the Society in Science Branco Weiss fellowship to JS.
The authors declare no conflict of interest.
References
- Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12:638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model. Nat Rev Neurosci. 2012;13:279–286. doi: 10.1038/nrn3212. [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- Sellbom KS, Gunstad J. Cognitive function and decline in obesity. J Alzheimers Dis. 2012;30:S89–S95. doi: 10.3233/JAD-2011-111073. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog IA. 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63:1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- Horstmann A, Busse FP, Mathar D, Muller K, Lepsien J, Schlogl H, et al. Obesity-Related Differences between Women and Men in Brain Structure and Goal-Directed Behavior. Front Hum Neurosci. 2011;5:58. doi: 10.3389/fnhum.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Anwander A, Moller HE, Horstmann A, Lepsien J, Busse F, et al. Sex-dependent influences of obesity on cerebral white matter investigated by diffusion-tensor imaging. PLoS One. 2011;6:e18544. doi: 10.1371/journal.pone.0018544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Guzel A, Er U, Tatli M, Aluclu U, Ozkan U, Duzenli Y, et al. Serum neuron-specific enolase as a predictor of short-term outcome and its correlation with Glasgow Coma Scale in traumatic brain injury. Neurosurg Rev. 2008;31:439–445. doi: 10.1007/s10143-008-0148-2. [DOI] [PubMed] [Google Scholar]
- Chaves ML, Camozzato AL, Ferreira ED, Piazenski I, Kochhann R, Dall'Igna O, et al. Serum levels of S100B and NSE proteins in Alzheimer's disease patients. J Neuroinflammation. 2010;7:6. doi: 10.1186/1742-2094-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugler JP, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE) Magn Reson Med. 1990;15:152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Abdul-Khaliq H, Krebs M, Diefenbacher A, Blasig IE. Serum markers support disease-specific glial pathology in major depression. J Affect Disord. 2008;111:271–280. doi: 10.1016/j.jad.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Abdul-Khaliq H, Krebs M, Diefenbacher A, Blasig IE. Neuron-specific enolase is unaltered whereas S100B is elevated in serum of patients with schizophrenia--original research and meta-analysis. Psychiatry Res. 2009;167:66–72. doi: 10.1016/j.psychres.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Streitbürger DJ, Arelin K, Kratzsch J, Thiery J, Steiner J, Villringer A, et al. Validating serum S100B and neuron-specific enolase as biomarkers for the human brain—A combined serum, gene expression and MRI study. PLoS One. 2012;7:e43284. doi: 10.1371/journal.pone.0043284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuo K, Straznicky NE, Lambert GW, Katsuya T, Sugimoto K, Rakugi H, et al. Leptin-receptor polymorphisms relate to obesity through blunted leptin-mediated sympathetic nerve activation in a Caucasian male population. Hypertens Res. 2008;31:1093–1100. doi: 10.1291/hypres.31.1093. [DOI] [PubMed] [Google Scholar]
- Burghuber OC, Worofka B, Schernthaner G, Vetter N, Neumann M, Dudczak R, et al. Serum neuron-specific enolase is a useful tumor marker for small cell lung cancer. Cancer. 1990;65:1386–1390. doi: 10.1002/1097-0142(19900315)65:6<1386::aid-cncr2820650623>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S. Not all brains are made the same: new views on brain scaling in evolution. Brain Behav Evol. 2011;78:22–36. doi: 10.1159/000327318. [DOI] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Junor L, Wilson SP, Mott DD, Wilson MA, et al. Obesity/hyperleptinemic phenotype impairs structural and functional plasticity in the rat hippocampus. Physiol Behav. 2011;105:138–144. doi: 10.1016/j.physbeh.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. 2005;62:1545–1548. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- Convit A, de Leon MJ, Tarshish C, De Santi S, Kluger A, Rusinek H, et al. Hippocampal volume losses in minimally impaired elderly. Lancet. 1995;345:266. doi: 10.1016/s0140-6736(95)90265-1. [DOI] [PubMed] [Google Scholar]
- de Toledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer's disease: in vivo detection of entorhinal cortex atrophy. Ann N Y Acad Sci. 2000;911:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22:529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Stein T, Maslowski N, Neumann J. Neural correlates of Alzheimer's disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. Neuroimage. 2009;47:1196–1206. doi: 10.1016/j.neuroimage.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suárez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, et al. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K, Kosaka H, Okazawa H, Murata T, Wada Y. Relationship between plasma leptin level and brain structure in elderly: a voxel-based morphometric study. Biol Psychiatry. 2009;65:992–994. doi: 10.1016/j.biopsych.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Miller JL, Couch J, Schwenk K, Long M, Towler S, Theriaque DW, et al. Early childhood obesity is associated with compromised cerebellar development. Dev Neuropsychol. 2009;34:272–283. doi: 10.1080/87565640802530961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg JM, Shah K, Villareal DT, Head D. Cognitive and neural correlates of aerobic fitness in obese older adults. Exp Aging Res. 2012;38:131–145. doi: 10.1080/0361073X.2012.659995. [DOI] [PMC free article] [PubMed] [Google Scholar]