Abstract
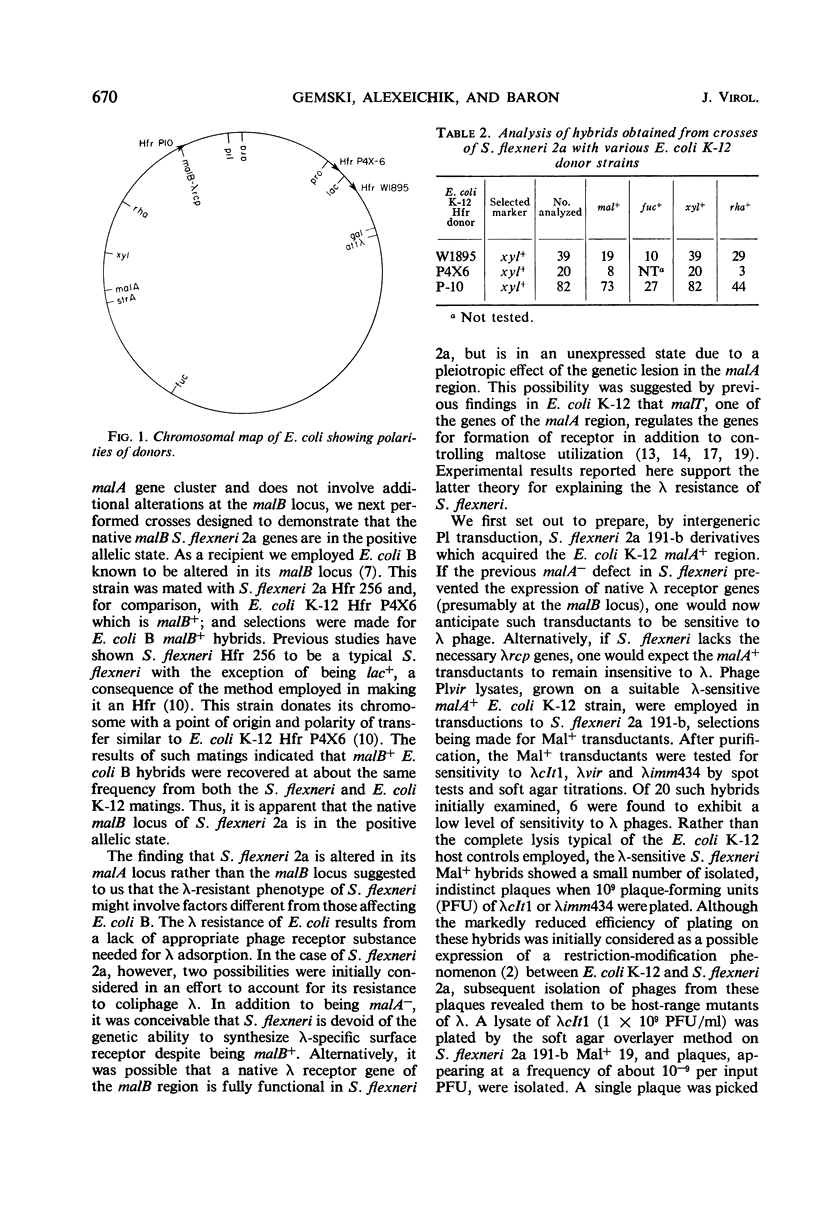
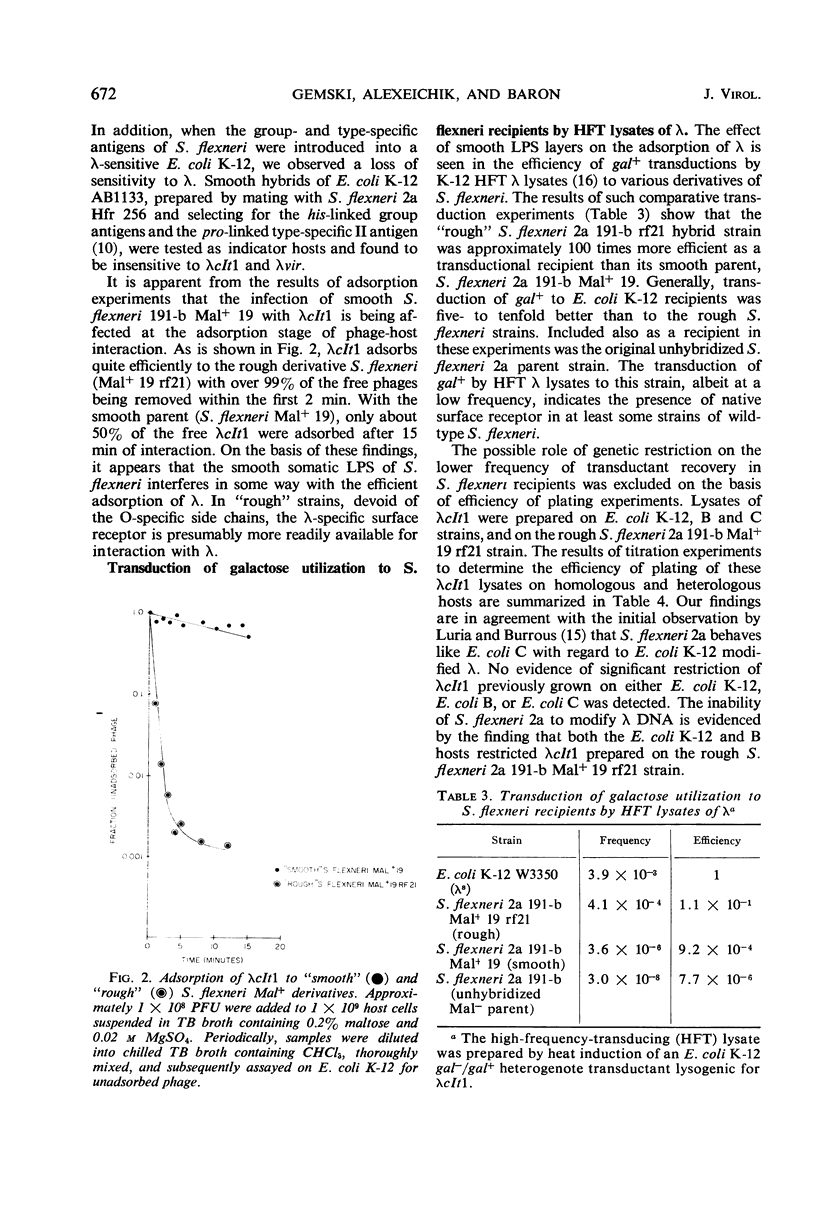
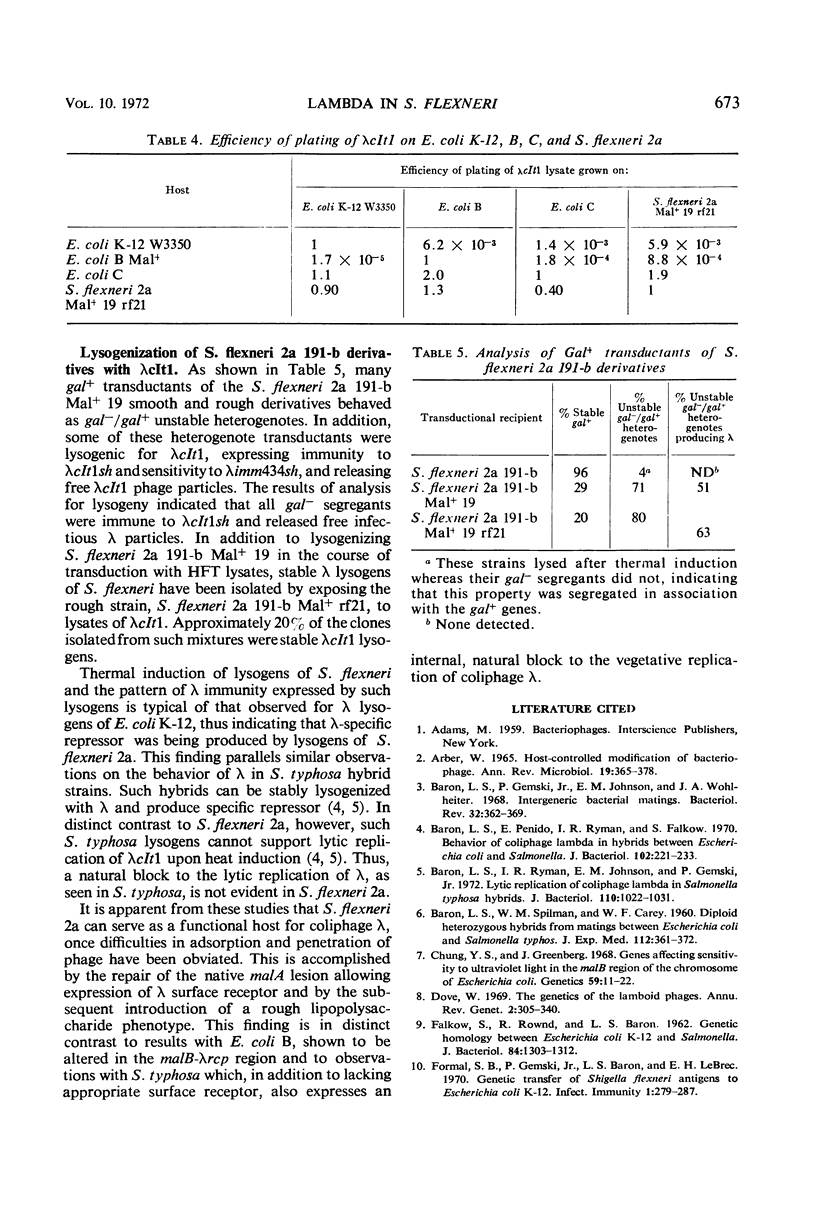
The insensitivity of wild-type Shigella flexneri 2a to coliphage λ is a consequence of its native genetic defect in the malA gene cluster. The “smooth” S. flexneri 2a lipopolysaccharide layer affects the efficient adsorption of λ. Derivatives, capable of serving as functional hosts for λ, were obtained by repairing the malA lesion, enabling the expression of the malB-λrcp region of S. flexneri. Introduction of a mutation into S. flexneri causing a “rough” lipopolysaccharide character resulted in more efficient adsorption of λ. Such S. flexneri hosts can be stably lysogenized and upon induction yield gal+-transducing lysates. Lambda propagated on a malA+ rough S. flexneri host was restricted by Escherichia coli K-12 and E. coli B, but not by E. coli C. This S. flexneri host did not restrict λ grown on these E. coli strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W. Host-controlled modification of bacteriophage. Annu Rev Microbiol. 1965;19:365–378. doi: 10.1146/annurev.mi.19.100165.002053. [DOI] [PubMed] [Google Scholar]
- BARON L. S., SPILMAN W. M., CAREY W. F. Diploid heterozygous hybrids from matings between Escherichia coli and Salmonella typhosa. J Exp Med. 1960 Aug 1;112:361–372. doi: 10.1084/jem.112.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron L. S., Gemski P., Johnson E. M., Wohlhieter J. A. Intergeneric bacterial matings. Bacteriol Rev. 1968 Dec;32(4 Pt 1):362–369. [PMC free article] [PubMed] [Google Scholar]
- Baron L. S., Penido E., Ryman I. R., Falkow S. Behavior of coliphage lambda in hybrids between Escherichia coli and Salmonella. J Bacteriol. 1970 Apr;102(1):221–233. doi: 10.1128/jb.102.1.221-233.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron L. S., Ryman I. R., Johnson E. M., Gemski P., Jr Lytic replication of coliphage lambda in Salmonella typhosa hybrids. J Bacteriol. 1972 Jun;110(3):1022–1031. doi: 10.1128/jb.110.3.1022-1031.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y. S., Greenberg J. Genes affecting sensitivity to ultraviolet light in the malB region of the chromosome of Escherichia coli. Genetics. 1968 May;59(1):11–22. doi: 10.1093/genetics/59.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Rownd R., Baron L. S. GENETIC HOMOLOGY BETWEEN ESCHERICHIA COLI K-12 AND SALMONELLA. J Bacteriol. 1962 Dec;84(6):1303–1312. doi: 10.1128/jb.84.6.1303-1312.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Gemski P., Baron L. S., Labrec E. H. A Chromosomal Locus Which Controls the Ability of Shigella flexneri to Evoke Keratoconjunctivitis. Infect Immun. 1971 Jan;3(1):73–79. doi: 10.1128/iai.3.1.73-79.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Gemski P., Baron L. S., Labrec E. H. Genetic Transfer of Shigella flexneri Antigens to Escherichia coli K-12. Infect Immun. 1970 Mar;1(3):279–287. doi: 10.1128/iai.1.3.279-287.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Jr, Stocker B. A. Transduction by bacteriophage P22 in nonsmooth mutants of Salmonella typhimurium. J Bacteriol. 1967 May;93(5):1588–1597. doi: 10.1128/jb.93.5.1588-1597.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D., Hofnung M., Schwartz M. Nonsense mutations in the maltose A region of the genetic map of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1311–1315. doi: 10.1128/jb.100.3.1311-1315.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofnung M., Schwartz M. Mutations allowing growth on maltose of Escherichia coli K 12 strains with a deleted malT gene. Mol Gen Genet. 1971;112(2):117–132. doi: 10.1007/BF00267490. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M L, Lederberg E M, Lederberg J. Transductional Heterogenotes in Escherichia Coli. Genetics. 1956 Sep;41(5):758–779. doi: 10.1093/genetics/41.5.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen A., Raanan-Ashkenazi O. Temperature sensitivity of maltose utilization and lambda resistance in Escherichia coli B. J Bacteriol. 1971 Jun;106(3):791–796. doi: 10.1128/jb.106.3.791-796.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Location of the maltose A and B loci on the genetic map of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1083–1089. doi: 10.1128/jb.92.4.1083-1089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Sur l'existence chez Escherichia coli K 12 d'une régulation commune à la biosynthèse des récepteurs du bactériophage et au métabolisme du maltose. Ann Inst Pasteur (Paris) 1967 Nov;113(5):685–704. [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



