Abstract
Background: The areas of the bed nucleus of the stria terminalis (BST) with a high density of estrogen receptors are involved in cardiovascular regulation and send axonal projections to the rostroventrolateral medulla (RVLM). We aimed to find the contribution of the RVLM to cardiovascular responses elicited by glutamate microinjection into the BST.
Methods: Experiments were done in α-chloralose anesthetized ovariectomized (OVX) or OVX estrogen treated (OVX+E) female Wistar rats. Drugs were microinjected into the BST and RVLM. The average changes in mean arterial pressure (MAP) and heart rate (HR) were compared between the case and control groups using t test and with the pre-injection values using paired t test.
Results: Unilateral microinjection of glutamate (0.25 M/50 nl) into the BST decreased MAP and HR, in the OVX+E and OVX rats. These cardiovascular responses were reversibly attenuated 10 minutes after microinjection of synaptic blocker cobalt chloride (CoCl2, 5 mM/50 nl) into the ipsilateral RVLM. Re-stimulation of the BST 60 min after CoCl2 injection elicited cardiovascular responses that were not different from the control values. Ipsilateral microinjection of GABAA antagonist bicuculline (1.0 mM/50 nl) into the RVLM caused a 50% attenuation of glutamate induced depressor and bradycardic responses in both groups. Ipsilateral microinjection of GABAB antagonist, phaclophen (5.0 mM/50 nl), into the RVLM did not affect the depressor and bradycardic responses due to re-stimulation of the BST by glutamate.
Conclusion: The RVLM sympathetic premotor neurons contain GABAA receptors that mediate in part the sympathoinhibitory responses to stimulation of the BST in the OVX animals.
Key Words: Bed nucleus of the stria terminalis, Gamma-aminobutyric acid, Estrogen
Introduction
Epidemiological studies have shown that cardiovascular diseases rarely affect women before menopause, suggesting that the decrease in the level of circulating estrogen (17β estradiol) might be a risk factor for the development of hypertension. Experimental studies have shown that estrogen plays an important role in the maintenance of the baroreceptor reflex.1,2
The bed nucleus of stria terminalis (BST) is a limbic forebrain structure that concentrate estrogen,3,4 and aromatase enzyme,5 which locally convert testosterone to estrogen. The BST is known to be influenced by circulating gonadal hormones altering immunoreactivity of the neuropeptides vasopressin, substance P, and cholecystokinin.6,7 The BST is also one of the major sites for integrating steroid hormones and olfactory information for sexual behavior.8 The cell number in the BST is controlled by estrogen receptor subtypes.9 This finding suggests that gonadal steroid hormones may play an important role in the regulation of the BST function.
An important function of the BST is cardiovascular regulation. Microinjection of glutamate into the BST decreased arterial pressure (AP) and heart rate (HR).10,11 A recent study has also indicated that the cholinergic system of the BST is involved in baroreflex activity and cardiovascular response.12 Moreover, the GABAergic system of the BST mediates cardiovascular effect via the sympathetic system and vasopressin release.13 The areas within the BST that elicit cardiovascular responses contain high densities of estrogen receptors.3,10 In addition, the neurons within these areas that concentrate estrogen send axonal projections to medullary regions; the caudal ventrolateral medulla (CVLM),14 and the rostroventrolateral medulla (RVLM),15 which are both directly involved in autonomic output to the heart and vasculature.16 Therefore, it is hypothesized that estrogen can act on neurons within the BST to alter cardiovascular function. On the other hand, major outputs from the BST go to the RVLM and this medullary region may mediate the BST cardiovascular responses to the heart and vasculature.15,16
The RVLM contain many neurotransmitters and neuromodulators, for example GABA is one of the major inhibitory neurotransmitters. Nitric oxide (NO) in the RVLM increases the release of GABA and glutamate in conscious rats.17-19 The inhibition of GABAA receptor by microinjection of bicuculline into the RVLM caused a marked increase in mean arterial pressure (MAP) in normotensive (but not hypertensive) rats.20
This study was performed to find the contribution of the RVLM to cardiovascular responses elicited by glutamate microinjection into the BST. We studied ovariectomized (OVX) and OVX estrogen treated (OVX+E) anesthetized rats to assess the following:
-The effect of circulatory estrogen on cardiovascular responses of the BST,
-The effect of reversible ablation of the RVLM on the BST cardiovascular responses, and
-The effect of blockade of the GABA system of RVLM on the BST cardiovascular responses.
Materials and Methods
General Procedures
Experiments were performed on 53 female Wistar rats (200-250 g) in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC). The rats were anesthetized with equithesin (0.3 ml/100g, intraperitoneally [i.p.]). Ovariectomy was performed using sterile procedures, by making a 1 cm incision on both sides of the back to expose the ovaries. The ovaries were clamped and removed with the fallopian tubes being ligated, and the skin was then sutured.
Then, a Silastic capsule (internal diameter: 1.57 mm, outer diameter: 3.17 mm, length: 5.0 mm) was implanted between the shoulder blades subcutaneously which either contained cholesterol (for OVX rats, n=27) or 17 β estradiol (for OVX+E rats, n=26). The estrogen containing capsule produced a plasma level estrogen concentration of approximately 30 pg/ml of blood which mimics the proestrus stage of the estrous cycle in rats. On the other hand, one study showed that the estrogen level is not detectable (<1 pg/ml) in OVX animals implanted with a cholesterol capsule.21
The animals were given postoperative care for two days. For a period of 10-20 days the animals were housed under controlled condition with 12 h light/dark cycle with food and water available ad libitum.
On the day of experiments, the animals were anesthetized again with equithesin (i.p) for surgical procedure and for the rest of the experiments with alpha chloralose (60 mg/kg I.V). Supplementary doses (30 mg/kg) were given as required. The paw pinch reflex was used to assess the depth of anesthesia. The trachea was cannulated and the animals were artificially ventilated using a small rodent ventilator (Harvard Apparatus Inc., U.S, model 683) with a mixture of room air and 95% O2. Body temperature was maintained at 37.0±0.2 using a heating pad controller (model 73; Yellow Spring Instrument, Yellow Spring, Ohio). The femoral vein was cannulated for systemic injections. The femoral artery was cannulated with polyethylene catheter (PE-50, Stoleting, USA) filled with heparinized saline and connected to a Statham P23XL pressure transducer and HR was monitored with a 7P4DEF Grass tachograph (USA) triggered by the AP pulse. AP and HR were continuously recorded by a Grass 79D polygraph. In some of the experiments AP and HR were continuously recorded by both a Harvard polygraph and a computer program written in our laboratory.22
The animals were placed in prone position in a stereotaxic frame (Stoelting, USA) and two bilateral small holes were drilled through the parietal bone over the BST and through the occipital bone over the RVLM.
Drug Microinjection
Microinjection of the drugs were performed by two single barreled micropipettes with an internal diameter of 35-45 mm. Micropipette tips were positioned in the BST and RVLM according to a stereotaxic atlas of the brain.23 The stereotaxic coordinates of the BST were explored -0.2 to -0.4 mm caudal, 2 mm lateral, and 5.5-7.5 mm ventral from the bregma. The RVLM was explored -11.6 to -12 caudal, 2 mm lateral, and 9.6-10.8 mm ventral from the bregma. The injection sites were 200 µm apart, and 1-6 injections were made in each animal on both sides. Microinjection was performed by a pressurized nitrogen pulse controlled by a picospritzer (General Valve, Fairfield, NJ). The injection volume was measured by direct observation of the fluid meniscus in the micropipette with a microscope fitted with an ocular micrometer. The injection volume of glutamate (Glu) into the BST and RVLM were 20 and 50 nl, respectively. All drugs were dissolved in saline and injected unilaterally.
Experimental Groups
The experiments were designed for studying the neuronal connectivity between BST and RVLM in relation to cardiovascular responses. The experiments were done on different groups of OVX and OVX+E female rats, as follows:
-Microinjection of saline into the BST (the control group [n=6], 20 nl; OVX [n=3], 13 injections; and OVX+E [n=3], 14 injections)
-Microinjection of L-glutamate into the BST (0.25 M/20 nl; Sigma, OVX [n=24], 81 injections; and OVX+E [n=23], 76 injections).
-To find the neuronal connectivity between the BST and the RVLM, L-glutamate was initially injected into the BST (control), then after arterial pressure and HR returned to the baseline, reversible synaptic blocker cobalt chloride (CoCl2 5 mM/50 nl, Sigma) was injected into the RVLM. The BST was re-stimulated at 10, 20, 40, and 60 minutes after the injection of CoCl2 into the RVLM of the OVX (n=6, 23 injections) and OVX+E (n=4, 19 injections) rats.
-To investigate the effect of inhibition of GABAA receptors of the RVLM on cardiovascular responses of the BST, first L-glutamate was injected into the BST (control) and after the arterial pressure and HR returned to the baseline, a GABAA antagonist, bicuculline (1 mM/50 nl, Sigma) was injected into the RVLM and the BST was re-stimulated at 10, 20, 40, and 60 minutes after the injection of bicuculline into the RVLM of the OVX (n=6, 36 injections) and OVX+E (n=7, 41 injections) rats.
-To find the effect of inhibition of GABAB receptors of the RVLM on cardiovascular responses of the BST, first L-glutamate was injected into the BST (control) and after the AP and HR returned to the baseline, a GABAB antagonist phaclophen (5 mM/50 nl, Sigma) was injected into the RVLM and the BST was re-stimulated at 10, 20, 40, and 60 minutes after the injection of phaclophen into the RVLM of the OVX (n=6, 36 injections) and OVX+E (n=7, 41 injections) rats.
Histology
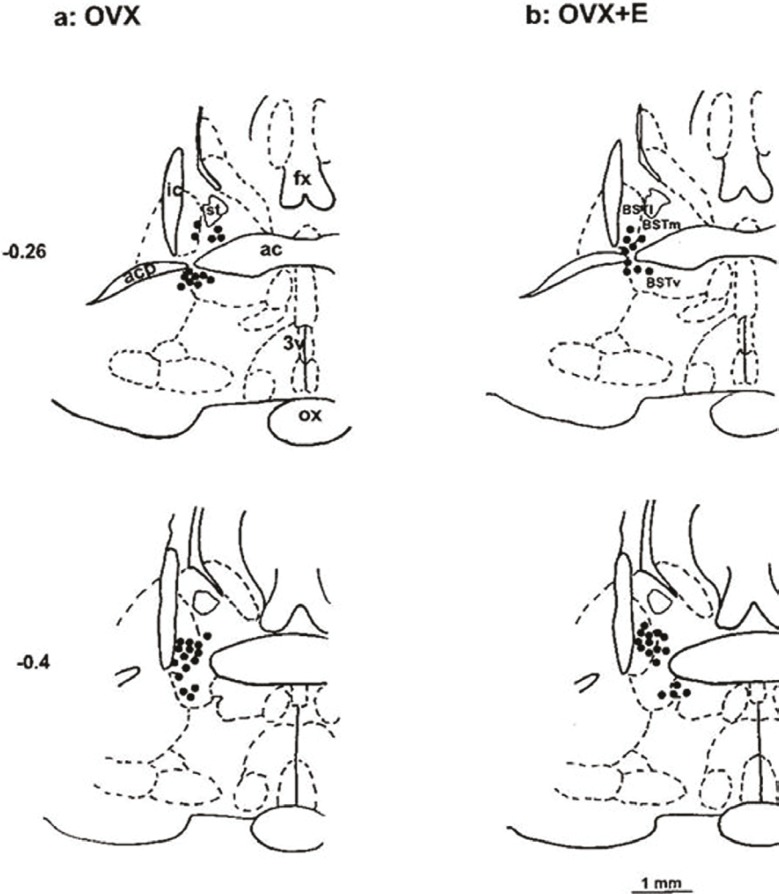
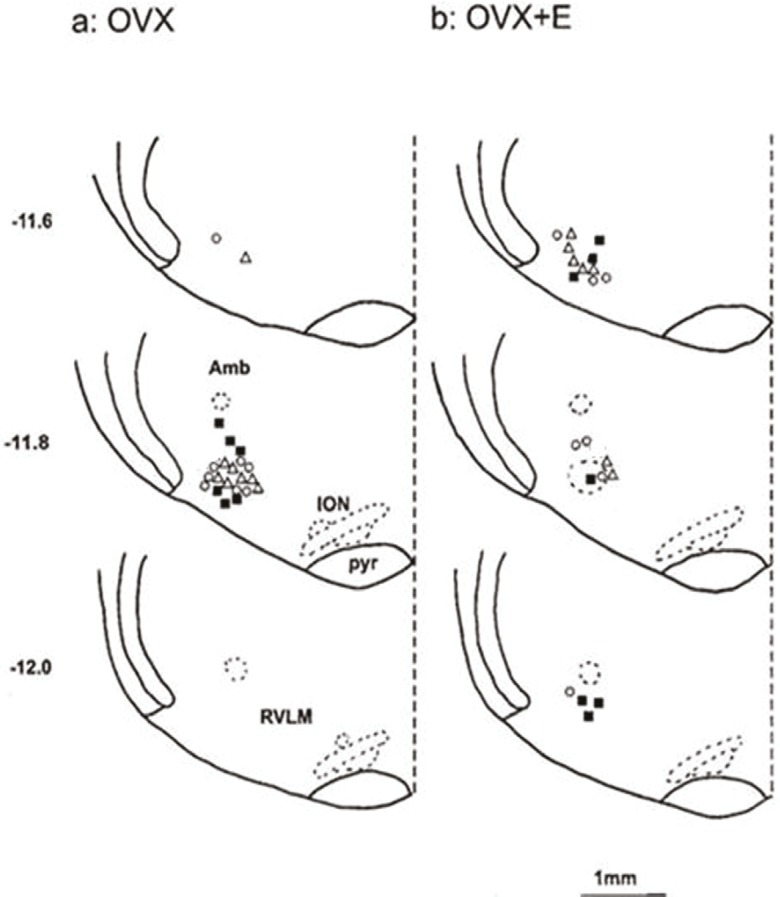
At the end of each experiment, 20 and 50 nl pontamine sky blue (Sigma, USA) were microinjected into the BST and the RVLM, respectively, then the animal was sacrificed by a high dose of the anesthetic, and perfused transcardially with100 ml of 0.9% saline followed by 100 ml of 10% saline formalin. The brain was removed and stored in 10% formalin for at least 24 hours. Frozen serial transverse sections (50 μm) of the regions of the BST and RVLM were cut and stained with neutral red (Merk, Germany). The injection sites were determined according to a rat brain atlas,21 under the light microscope (Nikon, Japan) (figures 1 and 2).
Figure 1.
Schematic coronal sections of rat brain adopted from an atlas,23 show the injection of glutamate (●) into the BST sites which decrease blood pressure and heart rate. Ac: anterior commissure; acp: anterior commissure posterior part; BSTm: bed nucleus stria terminals medial part; ic: inferior colliculus; fx: fornix; OX: optic chiasma; 3v: third ventricle
Figure 2.
Schematic coronal sections of rat brain adopted from an atlas,23 show the injection sites of cobalt chloride (∆), bicuculline (█), and phaclophen (O) into the RVLM. Amb: ambiggus; ION: inferior olive nucleus; Pyr: pyramid tract
Data Analysis
The results are expressed as mean±standard error of mean (SEM). First a normality test, Kolmogorov Smirnov, was performed on all data. All data were normal except for MAP after CoCl2 injection in the RVLM, for which the Wilcoxon analysis was used. The maximum changes of heart rate (ΔHR) and the maximum changes of MAP (ΔMAP) were compared between OVX, OVX+E, and saline groups using independent t test. Data were also compared with the pre-injection value using paired t test. P value<0.05 was considered as statistically significant.
Results
Cardiovascular Responses of Regional Controls
In the anaesthetized female OVX and OVX+E rats, microinjection of 20 nl of glutamate in the regions around the BST did not affect the pressure (ΔMAP=2.6±3.6 mmHg) and the HR (ΔHR=-3.2±2.3 beats/min; 43 injections).
Cardiovascular Responses to Vehicle Microinjection into the BST
To test whether cardiovascular response of BST was related to the volume of injection or mechanical torsion, 20 nl saline was microinjected into the BST of OVX and OVX+E animals. The changes in MAP (ΔMAP=0.7±0.6 mmHg) and HR (ΔHR=0.57±0.2 beats/min) were not significantly different from the pre-injection values.
Cardiovascular Responses to Glutamate Microinjection into the BST
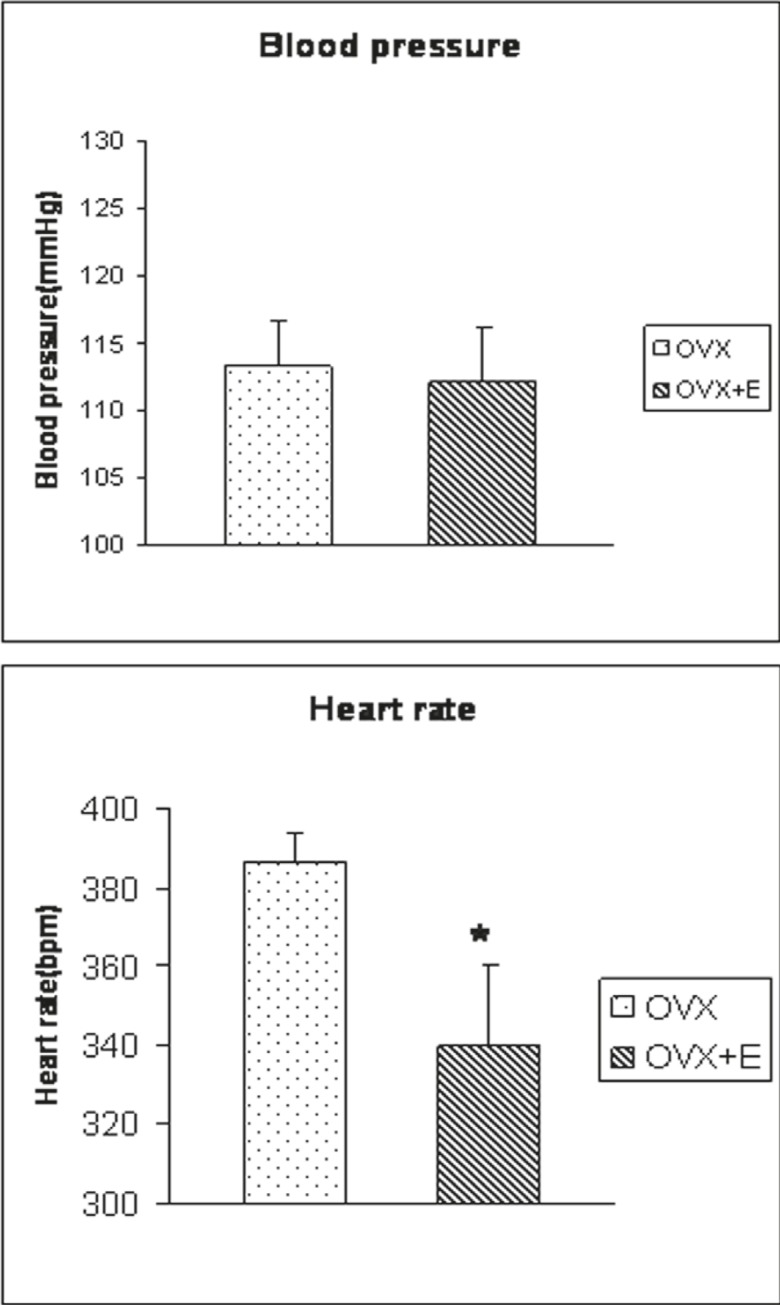
To determine the effect of glutamate on the MAP and HR, it was microinjected into the BST (0.25 M/20 nl). The baseline mean (±SE) AP and HR were 113.3 (±3.23) mmHg and 386.4 (±7.6) bpm in OVX rats and 112.1 (±3.98) mmHg and 339.9 (±20.2) bpm in OVX+E rats, respectively. The changes of the HR in the OVX+E rats was lower than the OVX rats (P<0.01, figure 3). Microinjection of glutamate into the dorsolateral and ventral subnuclei of the BST complex in both OVX and OVX+E rats showed decreases in HR and MAP (OVX: ΔMAP: -25.7±0.4 mmHg and ΔHR: -14.3±0.3 bpm; OVX+E: ΔMAP: -25.5±0.3 mmHg and ΔHR; -12.6±0.5 bpm). The changes were significantly different from the saline group (P<0.01) and the pre-injection values (P<0.01). However, the magnitude of bradycardia and depressor response was not significantly different between OVX and OVX+E rats.
Figure 3.
This bar chart show the magnitude of blood pressure and heart rate in the OVX and OVX+E rats. *Significant difference between OVX and OVX+E groups, t test, P<0.01
Cardiovascular Response Elicited by Glutamate Injection into the BST after the Injection of Synaptic Blocker in the RVLM
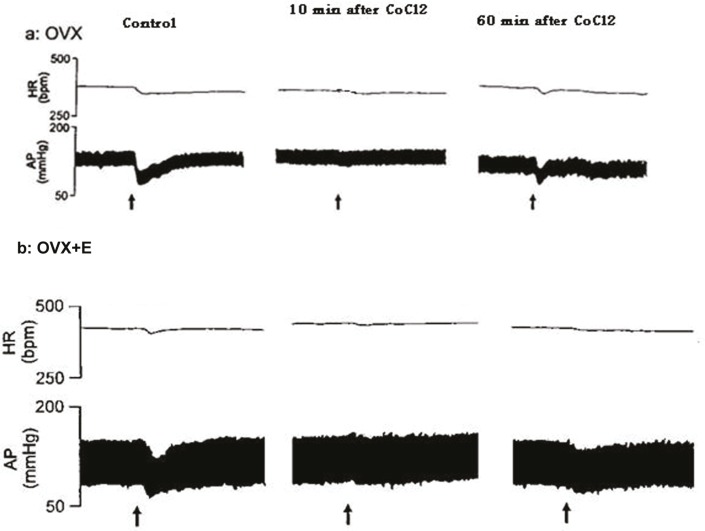
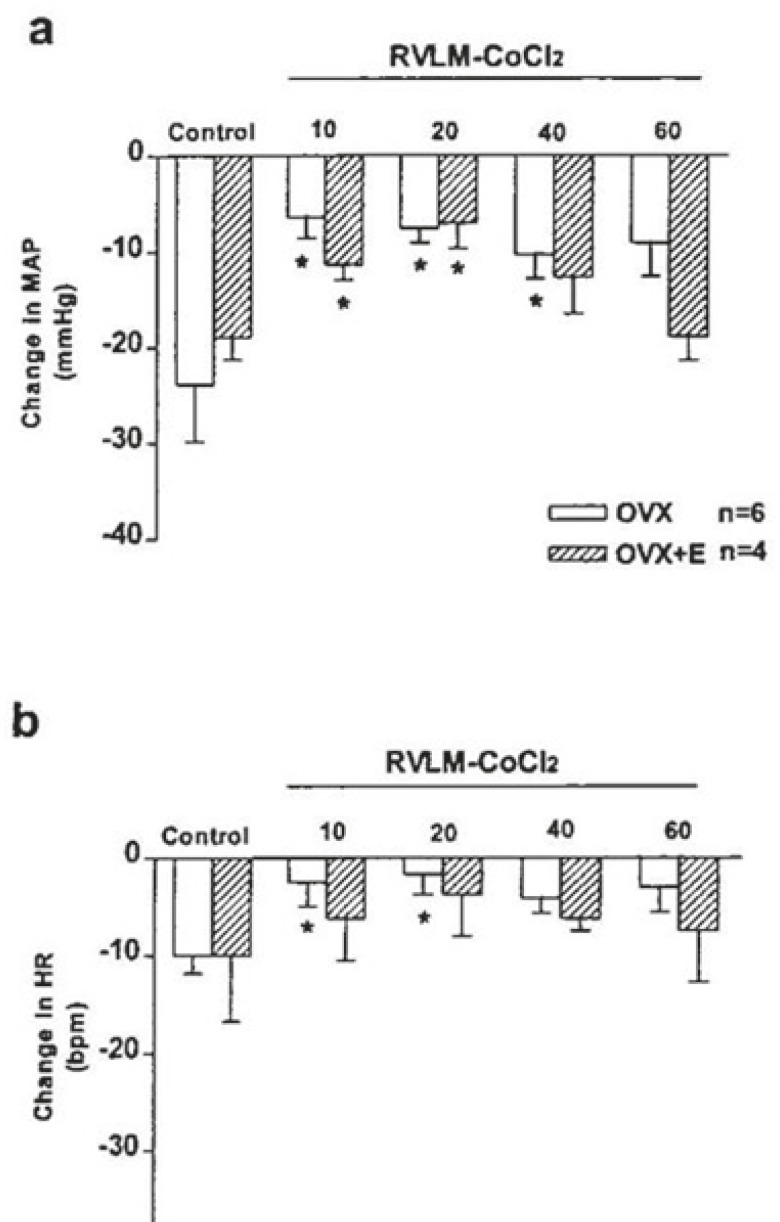
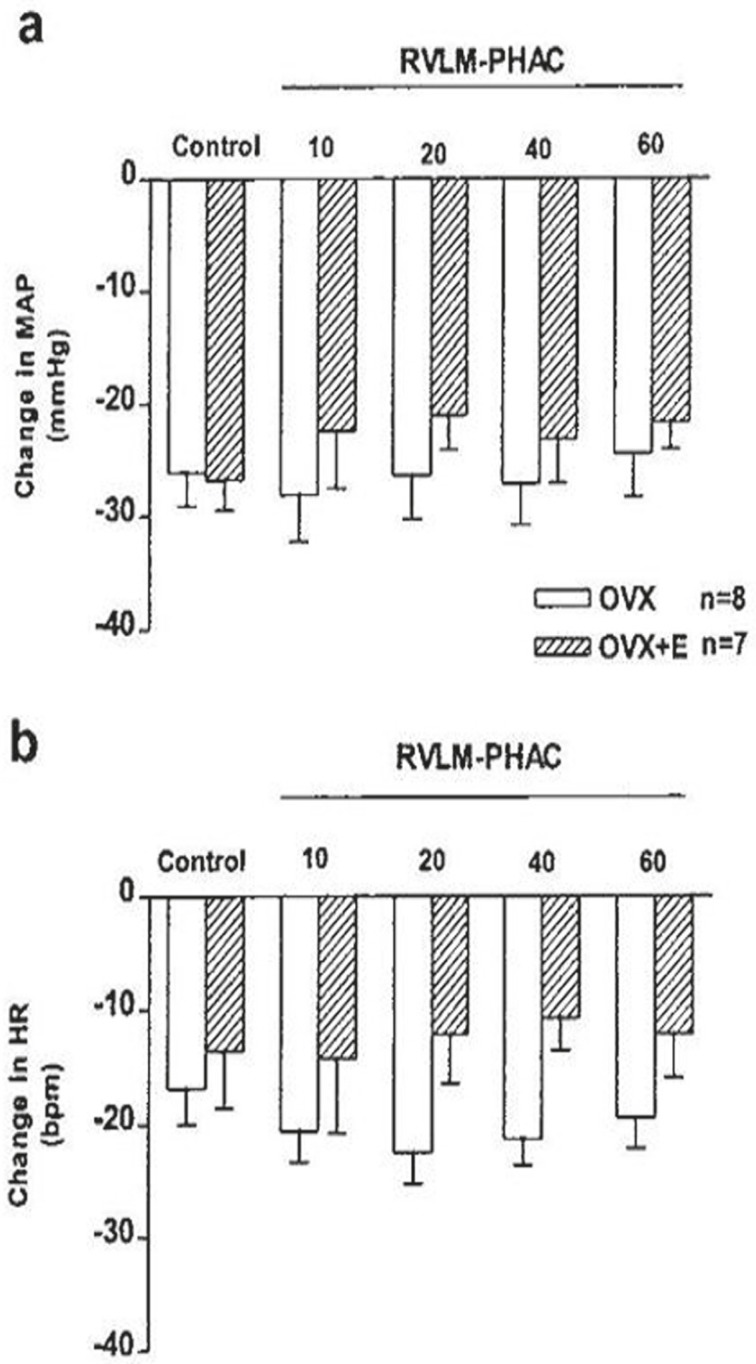
To investigate the possibility that the BST cardiovascular responses were mediated by the projection to RVLM, glutamate was first injected into the BST of the OVX and OVX+E rats. The depressor and bradycardic responses were similar in the magnitude in the pervious experiments (OVX: ΔMAP: -23.8±5.97 mmHg and ΔHR: -10.0±2.5 bpm; OVX+E: ΔMAP: -19.0±2.3 mmHg and ΔHR: -10.0±6.7 bpm, P<0.01), then CoCl2, was injected into the RVLM. Microinjection of CoCl2 into the RVLM of OVX and OVX+E rats had no significant effect on the baseline values of MAP and HR compared with the pre-injection values (OVX: ΔMAP: -0.2±0.2 mmHg and ΔHR: -4.0±2.3 bpm; OVX+E: ΔMAP: -1.0±0.2 mmHg and ΔHR: 0.8±0.1 bpm). Re-stimulation of the BST, 10 minutes after microinjection of CoCl2 into the cardiovascular site of RVLM significantly attenuated the depressor and bradycardic responses of the same site of the BST.
The magnitude of depressor response during stimulation of BST 10 minutes after CoCl2 microinjection into the RVLM was significantly different from the pre-injection values (OVX: ΔMAP: -6.3±2.1 mmHg and ΔHR: -2.5±2.5 bpm [P<0.01]; OVX+E: ΔMAP: -11.3±1.6.3 mmHg and ΔHR: -6.2±4.2 bpm [P<0.05]). The magnitude of depressor and bradycardic responses by re-stimulation of BST, 60 min after CoCl2 microinjection into the RVLM (OVX: ΔMAP: -19.8±3. 7 mmHg and ΔHR: -10.0±4.5 bpm; OVX+E: ΔMAP: -18.9±2.5 mmHg and ΔHR: -8.5.0±5.2 bpm) did not yield different results from the control values indicating that the effect of CoCl2 was abolished (figures 4 and 5).
Figure 4.
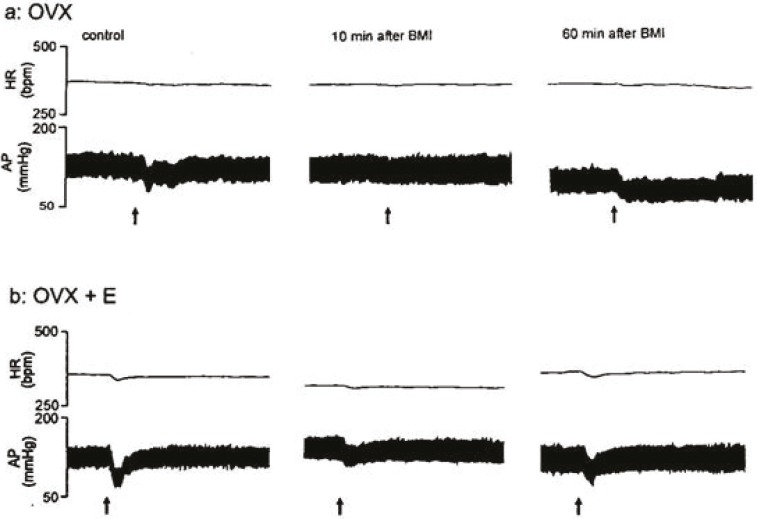
This figure shows tracings of blood pressure and heart rate responses elicited by microinjection of glutamate into the BST before (control) and after injection of CoCl2 into the RVLM and re-stimulation of BST at 10 and 60 minutes after injection of CoCl2 in RVLM in OVX and OVX+E rats, arrows indicate the injection times. Scale bar: 1 min
Figure 5.
This figure shows the cardiovascular effect of glutamate (0.25 M/20 nl) injection into the BST before (control) and 10, 20, 40, and 60 min after injection of CoCl2 (5 mM/50 nl) into the RVLM in OVX and OVX+E rats. *Significant different compared with pre-injection values (control), paired t test, P<0.01
Cardiovascular Responses Elicited by Glutamate Injection into the BST after the Injection of Bicuculline in the RVLM
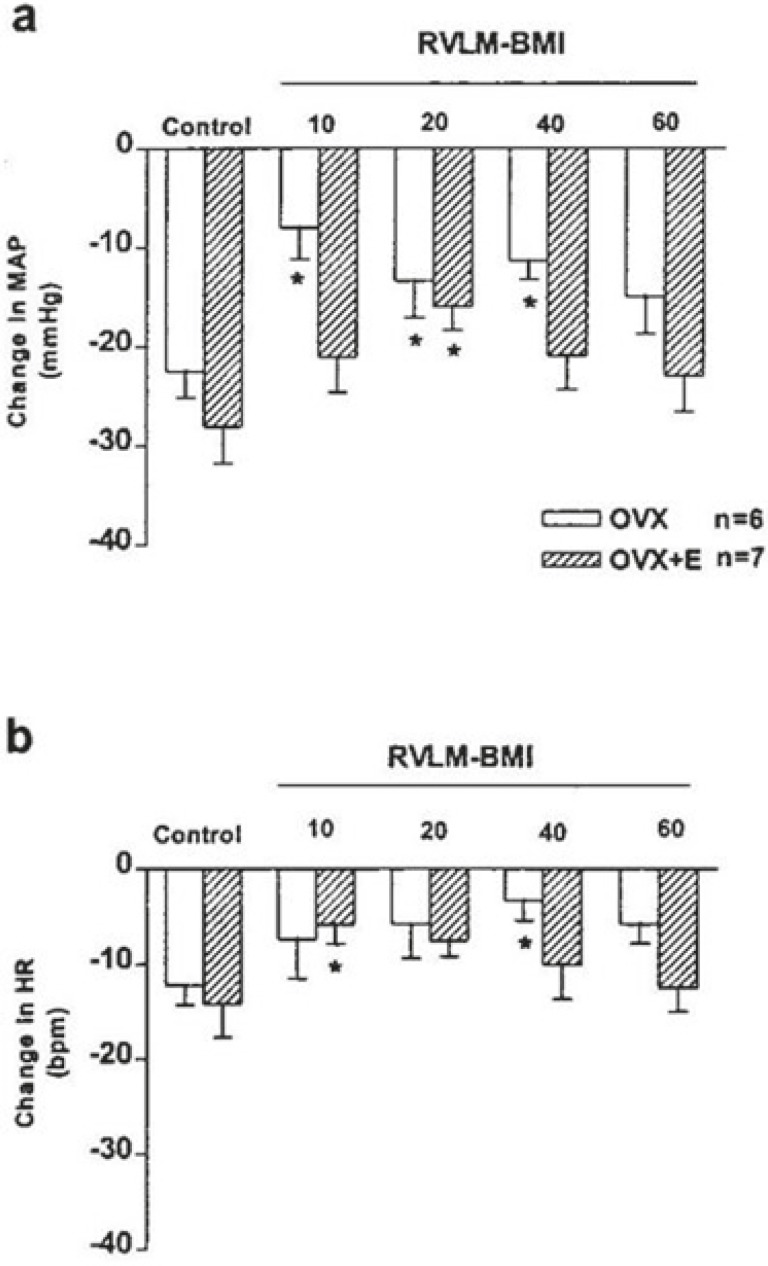
To test the possible inhibition of cardiovascular responses of the BST by GABAergic neurons of the RVLM, first glutamate was injected into the BST of the OVX and OVX+E rats, then a GABAA antagonist bicuculline, was injected into the RVLM. Microinjection of bicuculline into the RVLM of the OVX and OVX+E rats led to a significant increase on the MAP with no significant effect on HR compared with their pre-injection value (OVX: ΔMAP: 30.4±5.2 mmHg and ΔHR: 12.2.0±8.3 bpm; OVX+E: ΔMAP: 35.95±4.4 mmHg and ΔHR: 9.2±6.11 bpm, P<0.01). Similar to pervious experiments, 10 minutes later, glutamate was injected into the BST of the OVX and OVX+E rats. The depressor and bradycardic responses caused by stimulation of the BST were similar to those of the pervious experiments (OVX: ΔMAP: -22.5±2.68 mmHg and ΔHR: -12.2±2.1 bpm; OVX+E: ΔMAP: -28.1±3.7 mmHg and ΔHR: -14.2±3.5 bpm, P<0.01, t test).
The magnitude of depressor response during stimulation of the BST 10 minutes after bicuculline microinjection into the RVLM were reduced to almost 50% of their control value (OVX: ΔMAP: -11.9±3.3 mmHg and ΔHR: -10.0±2.5 bpm; OVX+E: ΔMAP: -16.3±2.4 mmHg and ΔHR: -7.5±1.7 bpm, P<0.01).
60 min after the microinjection of bicuculline into the RVLM, the magnitude of depressor and bradycardic responses by re-stimulation of the BST approximately returned to their control value (OVX: ΔMAP: -15.8±3.7 mmHg and ΔHR: -9.0±4.5 bpm; OVX+E: ΔMAP: -23.0±3.6 mmHg and ΔHR: -12.5±2.5 bpm, figures 6 and 7).
Figure 6.
This figure shows tracings of blood pressure and heart rate responses elicited by microinjection of glutamate into the BST before (control) and after injection of bicuculline (1 mM/50 nl) into the RVLM and re-stimulation of BST at 10 and 60 minutes after injection of bicuculline into the RVLM in OVX and OVX+E rats, arrows indicate the injection times. Scale bar: 1 min
Figure 7.
This figure shows the cardiovascular effect of glutamate (0.25 M/20 nl) injection into the BST before (control) and 10, 20, 40, and 60 min after injection of bicuculline into the RVLM in OVX and OVX+E rats. *Significant difference with pre-injection values (control), paired t test, P<0.01
Cardiovascular Response Elicited by Glutamate Injected into the BST after the Injection of Phaclophen in the RVLM
To find the possible effects of GABAB receptors of the RVLM on the cardiovascular responses of the BST, phaclophen, a GABAB antagonist, was microinjected into the RVLM of the OVX and OVX+E rats. No significant difference was found in both MAP and HR compared with their pre-injection values (OVX: ΔMAP: 4.3±1.1 mmHg and ΔHR: 3.3±2.1 bpm; OVX+E: ΔMAP: 6.9±1.4 mmHg and ΔHR: 8.7±3.2 bpm). Similar to pervious experiments, microinjection of glutamate into the BST elicited bradycardic and depressor responses (OVX: ΔMAP: -26.1±3.0 mmHg and ΔHR: -16.9±3.1 bpm; OVX+E: ΔMAP: -26.8±2.62 mmHg and ΔHR: -13.6±5 bpm, P<0.01, t test). Unlike the effect of bicuculline, microinjection of phaclophen did not alter the magnitude of depressor and bradycardic responses by re-stimulation of the BST, 10, 20, 40, and 60 minutes after microinjection of phaclophen into the cardiovascular site of RVLM (figures 8 and 9).
Figure 8.
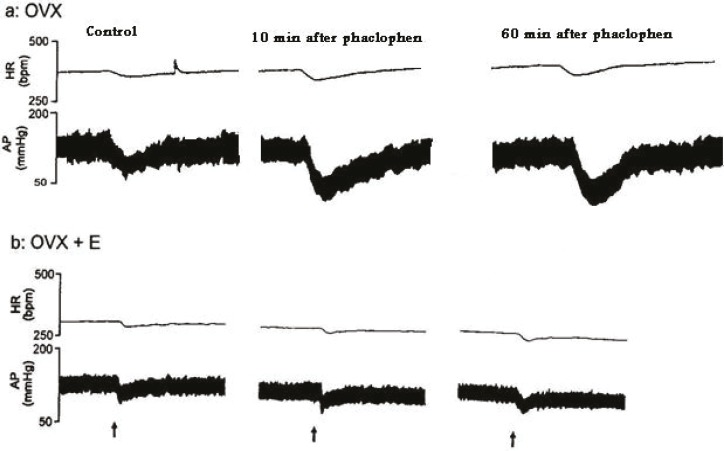
This figure shows tracings of blood pressure and heart rate responses elicited by microinjection of glutamate into the BST before (control) and after injection of phaclophen into the RVLM and re-stimulation of BST at 10 and 60 minutes after the injection of phaclophen into the RVLM in OVX and OVX+E rats, arrows indicate the injection times. Scale bar: 1 min
Figure 9.
This figure shows the cardiovascular effect of glutamate (0.25 M/20 nl) injection into the BST before (control) and 10, 20, 40, and 60 min after injection of phaclophen (5 mM/50 nl) into the RVLM in OVX and OVX+E rats.
Discussion
We found that estrogen decreased HR significantly, circulatory estrogen did not alter the cardiovascular depressor response evoked by BST stimulation, RVLM neurons in the medulla mediated the BST cardiovascular responses, and the depressor response of the BST was partly mediated by the activation of GABA neurons of the RVLM.
Circulating estrogen within the physiological range affects HR, since the baseline HR in the OVX+E rats was lower than in the OVX rats. Our finding supports the previous report that estrogen directly affects the heart and alters cardiac output.24 Estrogen facilitates parasympathetic activity of the heart and potentiates the bradycardic component of the barorereflex.2 Estrogen facilitates bradycardic response evoked by intravenous phenylephrine injection in ovariectomized female mice. The effect of estrogen on resting vascular tone was mediated by the estrogen beta receptor subtype, whereas its effect on the heart rate was mediated by the alpha subtype.25 The role of endogenous estrogen in the protection of the heart from ischemic heart disease has recently been suggested.26 Estrogens also stimulate NO generation and cause NO dependent vasodilatation which may increase myocardial blood flow.26 Microinjection of estrogen analogue in the RVLM reportedly produced a depressor effect through a decrease in sympathetic nerve activity,27 and activation of inducible nitric oxide synthase (iNOS)-derived NO in rats.28
We investigated the role of estrogen in the cardiovascular responses of the BST for the first time. Microinjection of glutamate into the BST of OVX and OVX+E female anesthetized rats elicited depressor and bradycardic responses in both groups (figures 3-9). The magnitude of depressor and bradycardic responses in OVX and OVX+E rats were similar, suggesting that the circulatory level of estrogen did not affect the BST cardiovascular responses, even though the BST contains a high density of estrogen receptors that affect some physiological functions such as sexual behavior,29 social affiliation,30 sexual dimorphism, and masculinization of the BST neurons.31 The depressor and bradycardic responses to the BST stimulation by glutamate are similar to the findings of the pervious experiments in male anesthetized rat.10,11 The depressor effect is caused by the inhibition of sympathetic effect on the vasculature and heart.10 In other areas of the central nervous system, the regulatory effect of estrogen on the cardiovascular system has been shown. For example circulatory estrogen potentiates the bradycardic response to stimulation of nucleus tractus solitarius by microinjection of hypocretine-1;21 however, we found that the estrogen receptor of the BST had no significant effect on its cardiovascular effects.
For the first time, our study showed that the decrease in MAP and HR in response to the BST stimulation was partly mediated by a pathway from the BST to the RVLM. This is based on the finding that microinjection of the reversible synaptic blocker CoCl2 into the RVLM of OVX and OVX+E animals reduced the depressor and bradycardic responses elicited by the BST stimulation for approximately 20 minutes. The responses to re-stimulation of the BST 60 min after the injection of CoCl2 into the RVLM returned to the control values, suggesting that previous BST stimulation of the same site did not produce neuronal damage. The injection of CoCl2 into the RVLM disrupt temporary neuronal transmission because of reversible inactivation of calcium channel on presynaptic terminals.32,33
In another part of this study we showed that GABAergic neurons in the RVLM are involved in the cardiovascular responses elicited by activation of the BST. Microinjection of bicuculline, a GABAA antagonist, significantly attenuated the BST cardiovascular responses. But injection of phaclophen, a GABAB antagonist, did not alter the BST cardiovascular responses. The alternation of the BST responses by the injection of bicuculline suggests that the cardiovascular output neurons of the BST might stimulate the RVLM neurons. It is probable that the activation of GABAergic neuron of the RVLM in part might be under the influence of the BST neurons. A previous study showed that GABA neurons in the RVLM were located caudal to the end pole of the facial nucleus,34 which is the same site that we injected CoCl2 and GABA antagonists. Taken together, these data suggest that there is a direct projection from the BST to the RVLM. However, this possibility cannot be considered that the BST innervate other forebrain and brainstem area,15,35 that all are involved in cardiovascular regulation and which in turn project to the RVLM.14,36 The RVLM is a critical site in the tonic and reflex control of blood pressure and monosynaptically connected to sympathetic preganglionic neurons in intermediolateral horn of the spinal cord. The spinal cord mediates the sympathetic effect of the forebrain and brain stem nuclei to vasculature and heart.37
Conclusion
We demonstrated that circulatory estrogen at the physiological level did not alter the depressor and bradycardic responses to the BST stimulation by glutamate. For the first time we showed that these responses are mediated partly by pathways that terminate at RVLM neurons. The present result also indicates that the GABAA receptors of the RVLM are in part involved in the BST cardiovascular responses.
Acknowledgment
The authors wish to thank Professor John Ciriello. This study was started in the Department of Physiology, University of Western Ontario and supported by the Heart and Stroke Foundation of Ontario and Medical Research Council of Canada and completed by a grant from the Vice–Chancellor for Research Affairs, Hormozgan University of Medical Sciences, Iran.
Conflict of Interest: None declared
References
- 1.Saleh MC, Connell BJ, Saleh TM. Autonomic and cardiovascular reflex responses to central estrogen injection in ovariectomized female rats. Brain Res. 2000;879:105–14. doi: 10.1016/s0006-8993(00)02757-8. doi: 10.1016/S0006-8993(00)02757-8. PubMed PMID: 11011011. [DOI] [PubMed] [Google Scholar]
- 2.Saleh TM, Connell BJ, Saleh MC. Acute injection of 17beta-estradiol enhances cardiovascular reflexes and autonomic tone in ovariectomized female rats. Auton Neurosci. 2000;84:78–88. doi: 10.1016/s1566-0702(00)00196-x. PubMed PMID: 11109992. [DOI] [PubMed] [Google Scholar]
- 3.Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–58. doi: 10.1002/cne.901510204. doi: 10.1002/cne.901510204. PubMed PMID: 4744471. [DOI] [PubMed] [Google Scholar]
- 4.Simerly RB, Young BJ. Regulation of estrogen receptor messenger ribonucleic acid in rat hypothalamus by sex steroid hormones. Mol Endocrinol. 1991;5:424–32. doi: 10.1210/mend-5-3-424. doi: 10.1210/mend-5-3-424. PubMed PMID: 1890991. [DOI] [PubMed] [Google Scholar]
- 5.Roselli CE, Resko JA. The distribution and regulation of aromatase activity in the central nervous system. Steroids. 1987;50:495–508. doi: 10.1016/0039-128x(87)90034-1. doi: 10.1016/0039-128X(87)90034-1. PubMed PMID: 3332938. [DOI] [PubMed] [Google Scholar]
- 6.Malsbury CW, McKay K. A sex difference in the pattern of substance P-like immunoreactivity in the bed nucleus of the stria terminalis. Brain Res. 1987;420:365–70. doi: 10.1016/0006-8993(87)91258-3. doi: 10.1016/0006-8993(87)91258-3. PubMed PMID: 2445435. [DOI] [PubMed] [Google Scholar]
- 7.Micevych P, Akesson T, Elde R. Distribution of cholecystokinin-immunoreactive cell bodies in the male and female rat: II. Bed nucleus of the stria terminalis and amygdala. J Comp Neurol. 1988;269:381–91. doi: 10.1002/cne.902690306. doi: 10.1002/cne.902690306. PubMed PMID: 3372720. [DOI] [PubMed] [Google Scholar]
- 8.Cavanaugh BL, Lonstein JS. Androgenic and oestrogenic influences on tyrosine hydroxylase-immunoreactive cells of the prairie vole medial amygdala and bed nucleus of the stria terminalis. J Neuroendocrinol. 2010;22:217–25. doi: 10.1111/j.1365-2826.2010.01958.x. doi: 10.1111/j.1365-2826.2010.01958.x. PubMed PMID: 20136687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hisasue S, Seney ML, Immerman E, Forger NG. Control of cell number in the bed nucleus of the stria terminalis of mice: role of testosterone metabolites and estrogen receptor subtypes. J Sex Med. 2010;7:1401–9. doi: 10.1111/j.1743-6109.2009.01669.x. doi: 10.1111/j.1743-6109.2009.01669.x. PubMed PMID: 20102443. [DOI] [PubMed] [Google Scholar]
- 10.Ciriello J, Janssen SA. Effect of glutamate stimulation of bed nucleus of the stria terminalis on arterial pressure and heart rate. Am J Physiol. 1993;265:H1516–22. doi: 10.1152/ajpheart.1993.265.5.H1516. PubMed PMID: 7902013. [DOI] [PubMed] [Google Scholar]
- 11.Hatam M, Nasimi A. Glutamatergic systems in the bed nucleus of the stria terminalis, effects on cardiovascular system. Exp Brain Res. 2007;178:394–401. doi: 10.1007/s00221-006-0748-4. doi: 10.1007/s00221-006-0748-4. PubMed PMID: 17136533. [DOI] [PubMed] [Google Scholar]
- 12.Nasimi A, Hatam M. The role of the cholinergic system of the bed nucleus of the stria terminalis on the cardiovascular responses and the baroreflex modulation in rats. Brain Res. 2011;1386:81–8. doi: 10.1016/j.brainres.2011.02.056. doi: 10.1016/j.brainres.2011.02.056. PubMed PMID: 21354114. [DOI] [PubMed] [Google Scholar]
- 13.Hatam M, Kharazmi F, Nasimi A. Vasopressin and sympathetic systems mediate the cardiovascular effects of the GABAergic system in the bed nucleus of the stria terminalis. Neurosci Res. 2009;65:347–52. doi: 10.1016/j.neures.2009.08.011. doi: 10.1016/j.neures.2009.08.011. PubMed PMID: 19716851. [DOI] [PubMed] [Google Scholar]
- 14.Giancola SB, Roder S, Ciriello J. Contribution of caudal ventrolateral medulla to the cardiovascular responses elicited by activation of bed nucleus of the stria terminalis. Brain Res. 1993;606:162–6. doi: 10.1016/0006-8993(93)91585-g. doi: 10.1016/0006-8993(93)91585-G. PubMed PMID: 8461997. [DOI] [PubMed] [Google Scholar]
- 15.Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res. 1985;58:379–91. doi: 10.1007/BF00235319. doi: 10.1007/BF00235319. PubMed PMID: 3996501. [DOI] [PubMed] [Google Scholar]
- 16.Corodimas KP, Morrell JI. Estradiol-concentrating forebrain and midbrain neurons project directly to the medulla. J Comp Neurol. 1990;291:609–20. doi: 10.1002/cne.902910408. doi: 10.1002/cne.902910408. PubMed PMID: 2329192. [DOI] [PubMed] [Google Scholar]
- 17.Dampney RA, Tagawa T, Horiuchi J, Potts PD, Fontes M, Polson JW. What drives the tonic activity of presympathetic neurons in the rostral ventrolateral medulla. Clin Exp Pharmacol Physiol. 2000;27:1049–53. doi: 10.1046/j.1440-1681.2000.03375.x. doi: 10.1046/j.1440-1681.2000.03375.x. PubMed PMID: 11117229. [DOI] [PubMed] [Google Scholar]
- 18.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46. doi: 10.1038/nrn1902. doi: 10.1038/nrn1902. PubMed PMID: 16760914. [DOI] [PubMed] [Google Scholar]
- 19.Kishi T, Hirooka Y, Sakai K, Shigematsu H, Shimokawa H, Takeshita A. Overexpression of eNOS in the RVLM causes hypotension and bradycardia via GABA release. Hypertension. 2001;38:896–901. PubMed PMID: 11641305. [PubMed] [Google Scholar]
- 20.Smith JK, Barron KW. GABAergic responses in ventrolateral medulla in spontaneously hypertensive rats. Am J Physiol. 1990;258:R450–6. doi: 10.1152/ajpregu.1990.258.2.R450. PubMed PMID: 1968724. [DOI] [PubMed] [Google Scholar]
- 21.de OliveiraCV, Rosas-Arellano MP, Solano-Flores LP, Babic T, Li Z, Ciriello J. Estrogen alters the bradycardia response to hypocretin-1 in the nucleus tractus solitarius of the ovariectomized female. Brain Res. 2003;978:14–23. doi: 10.1016/s0006-8993(03)02724-0. doi: 10.1016/S0006-8993(03)02724-0. PubMed PMID: 12834893. [DOI] [PubMed] [Google Scholar]
- 22.Hatam M, Nasimi A. Interaction of GABA and glutamate in the horizontal limb of diagonal band of Broca (hDB): role in cardiovascular responses. Brain Res. 2005;1042:37–43. doi: 10.1016/j.brainres.2005.02.016. doi: 10.1016/j.brainres.2005.02.016. PubMed PMID: 15823251. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. San Diego, California: Academic Press; 1997. [Google Scholar]
- 24.Magness RR, Rosenfeld CR. Local and systemic estradiol-17 beta: effects on uterine and systemic vasodilation. Am J Physiol. 1989;256:E536–42. doi: 10.1152/ajpendo.1989.256.4.E536. PubMed PMID: 2650565. [DOI] [PubMed] [Google Scholar]
- 25.Pamidimukkala J, Xue B, Newton LG, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen facilitation of baroreflex heart rate responses in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H1063–70. doi: 10.1152/ajpheart.01163.2003. PubMed PMID: 15550515. [DOI] [PubMed] [Google Scholar]
- 26.Deschamps AM, Murphy E, Sun J. Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends Cardiovasc Med. 2010;20:73–8. doi: 10.1016/j.tcm.2010.05.001. doi: 10.1016/j.tcm.2010.05.001. PubMed PMID: 21130949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma HJ, Cao YK, Liu YX, Wang R, Wu YM. Microinjection of resveratrol into rostral ventrolateral medulla decreases sympathetic vasomotor tone through nitric oxide and intracellular Ca2+ in anesthetized male rats. Acta Pharmacol Sin. 2008;29:906–12. doi: 10.1111/j.1745-7254.2008.00827.x. PubMed PMID: 18664323. [DOI] [PubMed] [Google Scholar]
- 28.Shih CD. Activation of estrogen receptor beta-dependent nitric oxide signaling mediates the hypotensive effects of estrogen in the rostral ventrolateral medulla of anesthetized rats. J Biomed Sci. 2009;16:60. doi: 10.1186/1423-0127-16-60. PubMed PMID: 19583861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hnatczuk OC, Lisciotto CA, DonCarlos LL, Carter CS, Morrell JI. Estrogen receptor immunoreactivity in specific brain areas of the prairie vole (Microtus ochrogaster) is altered by sexual receptivity and genetic sex. J Neuroendocrinol. 1994;6:89–100. doi: 10.1111/j.1365-2826.1994.tb00558.x. doi: 10.1111/j.1365-2826.1994.tb00558.x. PubMed PMID: 7517750. [DOI] [PubMed] [Google Scholar]
- 30.Lei K, Cushing BS, Musatov S, Ogawa S, Kramer KM. Estrogen receptor-alpha in the bed nucleus of the stria terminalis regulates social affiliation in male prairie voles (Microtus ochrogaster) PLoS One. 2010;5 doi: 10.1371/journal.pone.0008931. PubMed PMID: 20111713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsukahara S, Tsuda MC, Kurihara R, Kato Y, Kuroda Y, Nakata M, et al. Effects of aromatase or estrogen receptor gene deletion on masculinization of the principal nucleus of the bed nucleus of the stria terminalis of mice. Neuroendocrinology. 2011;94:137–47. doi: 10.1159/000327541. doi: 10.1159/000327541. PubMed PMID: 21525731. [DOI] [PubMed] [Google Scholar]
- 32.Malpeli JG, Burch BD. Cobalt destroys neurons without destroying fibers of passage in the lateral geniculate nucleus of the cat. Neurosci Lett. 1982;32:29–34. doi: 10.1016/0304-3940(82)90224-5. doi: 10.1016/0304-3940(82)90224-5. PubMed PMID: 6183623. [DOI] [PubMed] [Google Scholar]
- 33.Masuda N, Terui N, Koshiya N, Kumada M. Neurons in the caudal ventrolateral medulla mediate the arterial baroreceptor reflex by inhibiting barosensitive reticulospinal neurons in the rostral ventrolateral medulla in rabbits. J Auton Nerv Syst. 1991;34:103–17. doi: 10.1016/0165-1838(91)90077-g. doi: 10.1016/0165-1838(91)90077-G. PubMed PMID: 1680889. [DOI] [PubMed] [Google Scholar]
- 34.Campos RR, Carillo BA, Oliveira-Sales EB, Silva AM, Silva NF, Futuro NetoHA, et al. Role of the caudal pressor area in the regulation of sympathetic vasomotor tone. Braz J Med Biol Res. 2008;41:557–62. doi: 10.1590/s0100-879x2008000700002. doi: 10.1590/S0100-879X2008000700002. PubMed PMID: 18719736. [DOI] [PubMed] [Google Scholar]
- 35.Almeida A, Cobos A, Tavares I, Lima D. Brain afferents to the medullary dorsal reticular nucleus: a retrograde and anterograde tracing study in the rat. Eur J Neurosci. 2002;16:81–95. doi: 10.1046/j.1460-9568.2002.02058.x. doi: 10.1046/j.1460-9568.2002.02058.x. PubMed PMID: 12153533. [DOI] [PubMed] [Google Scholar]
- 36.Roder S, Ciriello J. Contribution of bed nucleus of the stria terminalis to the cardiovascular responses elicited by stimulation of the amygdala. J Auton Nerv Syst. 1993;45:61–75. doi: 10.1016/0165-1838(93)90362-x. doi: 10.1016/0165-1838(93)90362-X. PubMed PMID: 8227965. [DOI] [PubMed] [Google Scholar]
- 37.Oshima N, McMullan S, Goodchild AK, Pilowsky PM. A monosynaptic connection between baroinhibited neurons in the RVLM and IML in Sprague-Dawley rats. Brain Res. 2006;1089:153–61. doi: 10.1016/j.brainres.2006.03.024. doi: 10.1016/j.brainres.2006.03.024. PubMed PMID: 16650389. [DOI] [PubMed] [Google Scholar]