Abstract
Failure to extinguish fear can lead to persevering anxiety and has been postulated as an important mechanism in the pathogenesis of human anxiety disorders. In animals, it is well documented that the endogenous cannabinoid system has a pivotal role in the successful extinction of fear, most importantly through the cannabinoid receptor 1. However, no human studies have reported a translation of this preclinical evidence yet. Healthy medication-free human subjects (N=150) underwent a fear conditioning and extinction procedure in a virtual reality environment. Fear potentiation of the eyeblink startle reflex was measured to assess fear-conditioned responding, and subjective fear ratings were collected. Participants were genotyped for two polymorphisms located within the promoter region (rs2180619) and the coding region (rs1049353) of cannabinoid receptor 1. As predicted from the preclinical literature, acquisition and expression of conditioned fear did not differ between genotypes. Crucially, whereas both homozygote (G/G, N=23) and heterozygote (A/G, N=68) G-allele carriers of rs2180619 displayed robust extinction of fear, extinction of fear-potentiated startle was absent in A/A homozygotes (N=51). Additionally, this resistance to extinguish fear left A/A carriers of rs2180619 with significantly higher levels of fear-potentiated startle at the end of the extinction training. No effects of rs1049353 genotype were observed regarding fear acquisition and extinction. These results suggest for the first time involvement of the human endocannabinoid system in fear extinction. Implications are that genetic variability in this system may underlie individual differences in anxiety, rendering cannabinoid receptor 1 a potential target for novel pharmacological treatments of anxiety disorders.
Keywords: cannabinoid receptor 1, CB1/CNR1, fear extinction, fear-potentiated startle
Introduction
From an evolutionary perspective, the acquisition of fear responses enables organisms to respond appropriately to predictors of aversive events.1, 2 Yet, it is equally important to extinguish fear when cues that previously predicted danger are no longer followed by an aversive event.3 Failure to do so can lead to persevering fear and has been postulated as an important mechanism in the pathogenesis of human anxiety disorders.4, 5 Exposure-based psychotherapy aims to counteract the previously acquired conditioned fear responses by repeatedly presenting the learned predictor of the aversive event (conditioned stimulus, CS) in absence of its negative consequences.3, 4, 6 A better understanding of the factors that impede fear extinction is likely to increase the efficacy of therapy for anxiety disorders and could also stimulate the development of new treatments.7
Several neurotransmitter systems that constitute targets for the pharmacological facilitation of fear extinction have been proposed recently.3, 4, 6, 8 One of these is the endogenous cannabinoid system (ECS). A vast amount of research has been conducted in animals, where the pivotal role of the ECS in the extinction of fear is well documented (see Lafenêtre et al.9 for a review). These effects have been shown for both cue-specific conditioned responses (suggested to model phobic fear10, 11) as well as context-conditioned responses (suggested to model more diffuse anxiety10, 12). Most actions of cannabinoids in the central nervous system are mediated by cannabinoid receptor 1 (CB1), one of the most abundant G-protein-coupled receptors in the mammalian brain.13, 14, 15 CB1 is highly expressed in brain regions essential for fear processing, such as the amygdala, the hippocampus and the medial prefrontal cortex.16, 17, 18, 19 In rodents, it has been shown that both genetic deletion and pharmacological blockade of CB1 prevent the successful extinction of fear,20 whereas enhancing cannabinoid neurotransmission by anandamide reuptake inhibitors11, 21 or a direct CB1 agonist22 augments fear extinction. Given the vast amount of preclinical evidence, cannabinoid-based pharmacotherapy in humans has been proposed frequently as a promising avenue for the development of new treatments for anxiety.6, 23, 24, 25, 26 However, there is as of yet a great paucity in research regarding mechanisms through which the ECS may regulate fear states in humans, such as through fear extinction. This impedes utilization of the ECS in development of novel human treatments. Narrowing the gap between preclinical and human studies is therefore highly desirable and could enable novel pharmacological applications that exploit the ECS.
In anxiety research, an established tool for the assessment of fear-conditioned responses is fear potentiation of the startle reflex (FPS). It serves as a physiological index of the defensive state of an organism evoked by threat,27, 28, 29, 30, 31, 32 and is widely used in both animal and human research on cue-specific fear and contextual anxiety.27, 29 This includes preclinical studies on the role of CB1,11, 33 rendering it an excellent tool for translational research.27, 34 Thus far, however, only a single published study investigated the role of cannabinoids in human fear extinction.35 In this study, the CB1 agonist Δ9-THC was administered before extinction that followed a classical fear conditioning procedure, but no long-lasting effects on fear extinction were observed. This may be due to relatively little room for improvement of this process in healthy human subjects, as observed with studies on augmentation of extinction in conditioning paradigms vs patients using 𝒟-cycloserine.35, 36, 37, 38 Also, the lack of selective CB1 (ant)agonists available for use in humans may underlie the paucity of human pharmacological studies.
An alternative to the pharmacological approach is to study the effects of genetic variability in human endocannabinoid signaling, in analogy to the animal work with knock-out mice.20, 39, 40 This is a potentially fruitful approach as anxiety disorders show strong heritability,41 and evidence indicates that both fear acquisition42 and fear extinction43 are modulated by genetic factors (see Lonsdorf and Kalisch44 for an overview). Many polymorphic sites have been identified within the human gene that encodes for cannabinoid receptor 1 (ref. 45) (often referred to as CNR1). Consequences at the molecular level have not been determined for most of these polymorphisms, however functional significance is suggested by studies in which significant associations with a behavioral phenotype were reported.
For this study, two single-nucleotide polymorphisms (SNP) that have been associated with individual differences in anxiety were selected as candidates. The first SNP within the promoter region of CNR1, rs2180619,16 was associated with individual differences in trait anxiety in healthy humans in interaction with the 5-HTTLPR polymorphism.46 In silico analysis suggest possible phenotypic effects of rs2180619 through allele-specific transcription factor-binding sites46 and its location within a regulatory region of CNR1.16 A second SNP located within the coding region of CB1, rs1049353, was associated with post-traumatic stress disorder as part of a CB1 haplotype.47 Moreover, this SNP was associated with reduced anti-depressant treatment response in subjects with major depressive disorder, particularly in females with high comorbid anxiety.48
In sum, the aim of this paper is to investigate if preclinical evidence regarding the involvement of CB1 in fear extinction can be translated to humans. To this end, 150 healthy human subjects (90 women, 60 men) were subjected to a well-established fear conditioning and extinction paradigm49, 50 in a virtual reality environment in which both cue-specific and contextual conditioned responses were assessed. Eyeblink startle reflex was recorded as physiological index of defensive state, and subjective fear ratings were gathered at regular intervals during the experiment. To assess possible effects of the ECS on these processes, subjects were genotyped for the selected CNR1 SNPs (rs2180619 and rs1049353).
Materials and methods
The ethical institutional review board of the University Medical Center Utrecht approved this study, and all subjects gave written informed consent.
Participants
A total of 150 subjects (90 females, 60 males; mean age=21.6, s.d.=2.4) were recruited via advertisements at Utrecht University. Participants filled out screening forms in which they reported to be free of any current or previous psychiatric or neurological disorder, drug or alcohol dependence, current psychoactive medication, hearing problems and color blindness. Of the 150 subjects, 148 were of Caucasian ethnicity, the remaining 2 reported to be of Asian ancestry. Participants received € 30 for their participation in the experiment. Eight subjects were excluded from the final sample owing to incomplete recordings of startle data (n=4), artifacts yielding unreliable startle measurements (n=2) and insufficient quality of isolated DNA (n=2). The final sample therefore comprised 142 subjects between 18 and 28 years of age (84 females, 58 males; mean age=21.7, s.d=2.4).
Experimental paradigm
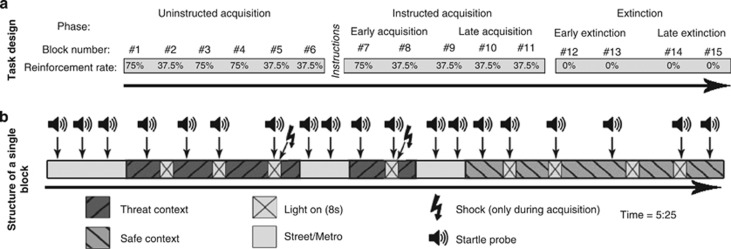
All subjects completed a well-established fear-potentiated startle (FPS)-conditioning paradigm in a virtual reality environment adapted from Baas et al.49, 50, 51 to assess fear extinction. Subjects were presented with two virtual environments. These were an apartment in a high-rise in a downtown area and a house in a suburban area. For each subject, one of the contexts was defined as the threat context where shocks were administered (CXT+), whereas the other represented the safe context without shock reinforcement (CXT−). Assignment of the threat context and order of visits to the contexts was counterbalanced across subjects. Similar to animal studies,20 an increase in background illumination (light on, 8 s duration) signaled when shocks could be administered in the threat context. Light-on presentations in the safe context were never followed by shock and were originally implemented to assess generalization of fear. As this phenomenon was not the focus of the present study and as there was no significant effect of CB1 genotype on fear generalization in any phase of the experiment, data from this condition will be omitted for sake of brevity. Pictures from both contexts during light off and light on as well as procedural details of this paradigm can be found elsewhere.50, 51 Subjects were presented with the virtual environments in blocks lasting 5.25 min during which both contexts were visited. The beginning of each block and transitions between contexts consisted of virtual transits through a metro station during which startle probes were presented to maintain startle habituation.50, 51
The experiment was divided into three phases (see Figure 1 for an illustration). The paradigm started with six uninstructed acquisition blocks to assess the development of contingency awareness (uninstructed acquisition). During this phase, training blocks with a relatively high reinforcement rate of 75% to facilitate learning were alternated with testing blocks. Relatively low reinforcement rates in testing blocks (37.5%) prevented contamination of the assessment of physiological responding due to shock sensitization (see also Baas et al.50, 51). The uninstructed acquisition phase was followed by explicit verbal and written instructions about the contingency between threat context, threat cue and shock reinforcements to ensure contingency learning in all participants. These instructions were followed by one training block to reinforce the instructions and four testing blocks to assess instructed fear acquisition. The primary focus of this study was to investigate CB1/CNR1 effects on fear extinction. Analyses of CB1/CNR1 genotype effects during acquisition are reported succinctly to rule out confounds due to possible differences before the extinction phase. Note, that the fear acquisition phase was split up into uninstructed and instructed acquisition to investigate the effect of individual differences on the spontaneous acquisition of cue-dependent and context-dependent fear (not the topic of this manuscript), while ensuring fear learning for all subjects by means of explicit instructions. This approach allows for strong and homogeneous fear acquisition against which extinction can be tested robustly.
Figure 1.
(a) Outline of the design of the virtual reality fear conditioning/extinction task delineating number of blocks and reinforcement rates per experimental phase. (b) An example of the composition of a single acquisition block of the virtual reality fear conditioning task is given. During extinction blocks, no shocks were administered. Note that reinforcement rates were 37.5% within the acquisition blocks during which physiological data was recorded. The blocks with a reinforcement rate of 75% were included to ensure learning. Physiological data from these blocks were omitted during analyses as shock administration contaminates startle measurements.49, 50
Immediately following the instructed acquisition phase, without an additional pause or additional instructions, the extinction phase followed comprising four more blocks that were identical to the blocks used during acquisition but without shock reinforcements.
For analyses purposes, the extinction phase was divided into early extinction (first two extinction blocks) and late extinction (last two extinction blocks). These were compared with the last two blocks of the acquisition phase (referred to as late acquisition). This way, data from the three phases that were used to measure fear extinction (late acquisition, early extinction, late extinction) contain an identical number of blocks. An illustration of the experimental design is provided in Figure 1.
Throughout the experiment, startle probes were presented during three out of four light-on presentations in both contexts. In addition, three startles probes were presented in absence of the light cue in each context. These are further referred to as the light-off condition. As a result, each block contained three startles measurements per condition (light-on/CXT+ light-off/CXT+ light-on/CXT− (omitted for sake of brevity, see above); light-off/CXT−). To increase signal-to-noise ratio, startle data from two subsequent testing blocks were averaged, resulting in an average computed across six startles per condition for final analyses.
Shock administration and workup
Electrical shocks were administered with a constant current generator (Digitimer DS7A, Digitimer, Letchworth Garden City, UK) via tin cup electrodes located approximately over the medial nerve on the inner left wrist. Before the experiment started, subjects completed a shock workup to determine individual shock intensities as described elsewhere.52, 53 Intensities were adjusted per subject so that they corresponded to a level of 4 out of 5, representing ‘quite annoying/ painful'.
Startle probe presentation, data recording and processing
Recording and amplification of the eyeblink startle reflex was performed via electromyography of the right orbicularis oculi muscle using a Biosemi ActiveTwo system (BioSemi Instrumentation, Amsterdam, The Netherlands). Startle probes comprised 50-ms, 105-dB white-noise bursts with instantaneous rise time and were delivered through headphones (Sennheiser Electronic HD202, Wennebostel, Germany). Processing of startle data was performed using Brain Vision Analyzer software (Brain Products, Gilching, Germany) according to published guidelines54 and previous studies.52, 53 After segmentation of trials, artifacts were rejected and null responses identified as in previous publications.52, 53, 55 Participants were only included in the final analysis if at least one artifact-free startle trial for each condition and each phase was recorded. Two participants were excluded from the analyses due to not meeting this criterion. According to guidelines, startle data were Z-transformed per subject based on individual trial amplitudes from all startles recorded during the experiment to remove between-subjects variance in baseline startle amplitude. All statistical analyses involving startle data were conducted on Z-scores.
Subjective measures
Before the experiment, subjects filled out Spielberger's Trait Anxiety Inventory (Dutch translation,56) and the neuroticism subscale of the NEO-PI-R questionnaire (Dutch translation,57). Between blocks, subjects rated their subjective fearfulness during the experimental conditions on a visual analog scale displayed on the computer screen while seeing screenshots from the pre-recorded videos representative for each condition. See Baas et al.50 for examples of screenshots. The question was ‘How fearful do you feel in this situation?' with the anchors: ‘Not at all fearful of shock' (0) and ‘Very fearful of shock' (100). Two screenshots per condition were presented after each block, and an average was computed. Further analysis of the data was congruent to our approach of the startle data.
Genotyping
DNA was harvested by collecting buccal swaps frozen immediately at −40 °C for later genotyping. Genomic DNA was extracted and purified using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). rs2180619 and rs1049353 were genotyped using Taqman SNP Genotyping assays (ASSAY ID's: C__15841551_10 and C___1652590_10, respectively, Applied Biosystems, Foster City, CA, USA). Subjects were classified through endpoint analysis performed on an ABI Prism 7000 (Applied Biosystems) as either A/A homozygotes, A/G heterozygotes or G/G homozygotes. In contrast to the original studies on rs218061946 and rs104935347, 48 that did not use a priori grouping of genotypes, we here collapsed the smallest homozygote group of each polymorphism with the heterozygotes to maximize statistical power, as is common in the field (see Lonsdorf and Kalisch44 for an overview of genetic association studies on human fear conditioning and extinction). For rs2180619, G-allele carriers were grouped against A/A homozygotes, and for rs1049353, A-allele carriers were grouped against G/G homozygotes. Genotyping was performed in duplicate for ∼80% of the samples without deviations. Descriptive statistics of both CB1/CNR1 polymorphisms are presented in Table 1. We did not perform gene × gene interaction analysis as minimal cell sizes did not exceed n=30, thus insufficient for calculation of gene × gene interaction effects according to statistical guidelines.58
Table 1. Frequencies and statistics (Hardy–Weinberg equilibrium, linkage equilibrium and sex distribution) of the genetic polymorphisms under study.
| Polymorphism | Hardy–Weinberg equilibrium P-value | Lowest linkage equilibrium P-value | N's | % females | x2 (gender × genotype) | P (gender × genotype) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| rs2180619 | 0.97 | 0.38 | G/G | A/G | A/A | G/G | A/G | A/A | <1 | 0.60 |
| 23 | 68 | 51 | 52% | 63% | 57% | |||||
| rs1049353 | 0.19 | 0.38 | G/G | A/G | A/A | G/G | A/G | A/A | 6.3 | 0.04 |
| 73 | 53 | 16 | 49% | 68% | 75% | |||||
Statistical analyses
Because of our a priori hypotheses and for clarity, planned comparisons rather than a full factorial design are reported. Absolute potentiation of the startle reflex (light-on/CXT+ vs light-off/CXT−) was used as our primary outcome measure because animal literature has shown that CB1 exerts its effects on fear extinction of both cue-specific fear11, 20 and contextual responses.21, 22 Subsequently, in case of a significant CB1 effect on this summed score, additional analyses were performed to determine if CB1 modulates cue-dependent fear extinction and context-dependent fear extinction specifically.
The repeated measures ANOVA for total fear (cue and context summed) included within-subject factors condition (contrast: light-on/CXT+ vs light-off/CXT−) and phase (last acquisition vs late extinction). CNR1 genotypes were included as between-subjects' factors with two levels (A/A homozygotes vs G-allele carriers for rs2180619, and G/G homozygotes vs A-allele carriers for rs1049353). In case of significant CB1/CNR1 genotype × fear extinction interactions for the summed cue and context measure, three sets of follow-up tests were conducted. First, we tested whether there was a significant reduction of fear responding within both genotype groups separately. Second, between-genotypes differences in fear responding at the end of extinction were assessed to ascertain a statistically significant difference in remaining fear responding at the end of the extinction phase between genotypes. Finally, genotype differences in extinction were tested separately for cue-specific responses (contrast: light-on/CXT+ vs light-off/CXT+) and context-conditioned responses (contrast: light-off/CXT+ vs light-off/CXT−) to assess if one of the two domains was affected specifically. Sex and age were included as covariates in the analyses to account for possible confounding effects (statistical test outcomes were identical with and without inclusion of covariates).
Results
Genetics and descriptive statistics
Both polymorphisms under study were in Hardy–Weinberg equilibrium and in linkage equilibrium (see Table 1). Furthermore, sex was evenly distributed across rs2180619 genotypes but unevenly distributed across rs1049353 genotypes (see Table 1 for frequencies and statistics). We compared the CNR1 genotypes with regard to trait anxiety, neuroticism, shock intensities and baseline startle amplitude, which did not reveal significant differences (see Table 2). Hence, CNR1 genotype groups did not differ on parameters that may be regarded as potential confounds in the fear conditioning/extinction task, such as personality or basic defensive response levels.
Table 2. Descriptive statistics.
| rs2180619 | rs1049353 | |||||||
|---|---|---|---|---|---|---|---|---|
|
Measurement |
Genotype |
M |
s.d. |
Statistics |
Genotype |
M |
s.d. |
Statistics |
| STAI-T | G/G | 36.83 | 8.85 | F<1 | G/G | 35.58 | 8.57 | F<1 |
| A/G | 35.99 | 8.45 | A/G | 36.73 | 8.17 | |||
| A/A | 35.63 | 6.99 | A/A | 36.56 | 6.25 | |||
| NEO neuroticism | G/G | 132.90 | 24.80 | F<1 | G/G | 130.20 | 22.77 | F<1 |
| A/G | 133.30 | 22.40 | A/G | 134.27 | 23.05 | |||
| A/A | 130.50 | 21.90 | A/A | 132.31 | 15.94 | |||
| Baseline startle amplitude (μV) | G/G | 96.46 | 72.41 | F<1.1 | G/G | 91.10 | 42.07 | F<1 |
| A/G | 79.88 | 50.51 | A/G | 88.21 | 60.55 | |||
| A/A | 94.01 | 62.95 | A/A | 86.07 | 61.70 | |||
| Final shock intensity (mA) | G/G | 1.62 | 0.66 | F<1.4 | G/G | 1.79 | 0.87 | F<1 |
| A/G | 1.70 | 0.88 | A/G | 1.82 | 0.97 | |||
| A/A | 1.93 | 0.99 | A/A | 1.48 | 0.69 | |||
Means and s.d. for trait anxiety score, neuroticism, baseline startle amplitude and shock intensity reported as a function of rs2180619 and rs1049353. Statistics are reported for the comparison between A/A homozygotes and G-allele carriers of rs2180619, and G/G homozygotes vs A-allele carriers of rs1049353.
Startle results—fear acquisition
During both the uninstructed acquisition phase and after the instructions, significant potentiation of the eyeblink startle reflex (FPS) was observed (contrast: light-on/CXT+ vs light-off/CXT− uninstructed acquisition: F1,141=158, P<0.001, η2=0.51; instructed acquisition: F1,141=488, P<0.001, η2=0.77). To rule out the possibility that CB1/CNR1-dependent differences during the acquisition phases confound later differences during fear extinction, we tested if genetic variability in the polymorphisms under study was associated with FPS during both uninstructed acquisition and instructed acquisition. In both phases, FPS was unrelated to rs2180619 and rs1049353 genotype (F's<1). We used FPS data of the last two blocks of the acquisition phase (further on called ‘late acquisition') as a baseline to evaluate the magnitude of fear extinction (see Materials and Methods for rationale). The robust FPS effect in these blocks (F1,141=402, P<0.001, η2=0.74) was again independent of rs2180619 and rs1049353 genotype (F's<1).
Startle results—fear extinction
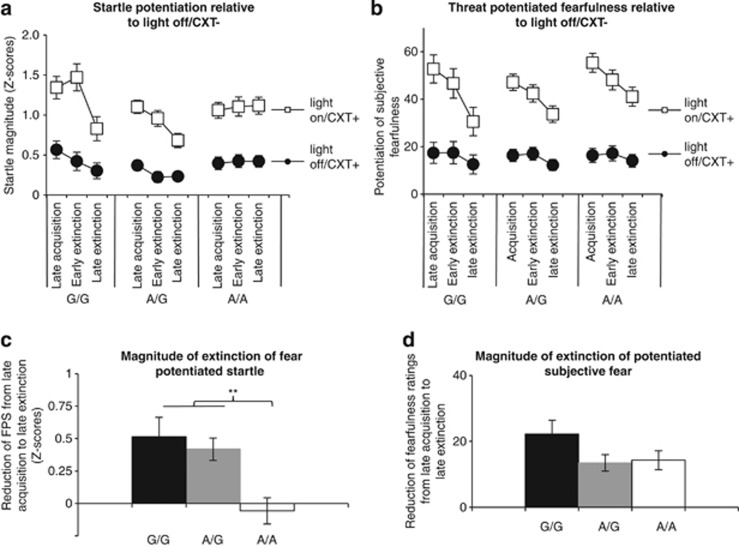
The extinction phase followed acquisition without instructions or an additional pause. Fear extinction, defined as decrease of FPS from the late acquisition to late extinction (the last two blocks of extinction), was strongly modulated by rs2180619 genotype (late acquisition FPS vs late extinction FPS × rs2180619 genotype (A/A vs G-carriers); F1,138=14.3, P<0.001, η2=0.09, Figures 2a and c). Notably, post-hoc tests revealed that A/A homozygotes of rs2180619 differed significantly from both G/G homozygotes (P=0.003) and A/G heterozygotes (P=0.001) with regard to fear extinction (late acquisition FPS vs late extinction FPS), whereas G/G homozygotes and A/G heterozygotes did not differ from each other (P=0.60). Follow-up tests revealed that the extinction procedure did not result in reduction of FPS in the group of 51 A/A carriers (F1,50<1), whereas G-allele carriers of rs2180619 displayed robust extinction of fear (F1,90=43.9, P<0.001, η2=0.33). Crucially, failure to extinguish fear resulted in significantly higher levels of FPS for A/A homozygotes compared with G-allele carriers at the end of extinction (F1,138=10.6, P=0.001, η2=0.07). Note, that the three genotype groups are displayed separately in Figure 2 to illustrate the data as per subgroup for sake of completeness, whereas the respective G-carrier vs A/A data is displayed in Supplementary Figure 1.
Figure 2.
Extinction of fear-potentiated startle (FPS) is dependent on allelic variation in the human cannabinoid receptor 1 gene. Subjects were classified as G/G (N=23), A/G (N=68) or A/A (N=51) allele carriers of rs2180619. Note that for purpose of statistical testing, A/A homozygotes were grouped against G-allele carriers to assure sufficient group sizes. Top. Fear conditioning and extinction data of the startle reflex (a) and of subjective fear ratings (b). White squares denote fear potentiation to the light cue (light on) in the threat context (CXT+), black circles reflect potentiation during the threat context (CXT+) in absence of the light cue (light off). Both potentiation measures were computed relative to the absence of the light cue in the safe context (light-off/CXT−). Bottom. Magnitude of extinction defined as reduction of FPS (c) and threat-potentiated fearfulness (d) for the contrast light-on/CXT+ vs light-off/CXT− from late acquisition to late extinction. Greater values indicate stronger fear extinction. Startle magnitudes were standardized within subjects (Z-transformation) and averaged per phase (six startles per condition per phase). Error bars display ±1 s.e.m. **P<0.01.
There were no significant effects of the other CB1/CNR1 polymorphism (rs1049353) on fear extinction as measured by decrease of FPS from acquisition to late extinction (F<1) or higher levels of FPS at the end of extinction (F<1). Note, that different grouping of the rs1039353 genotypes (A/A vs G-carriers) did not change the results. Therefore, no follow-up tests were performed for rs1049353.
Cue/context-specific startle results
As planned, significant genotype effects on summed potentiation of the startle reflex (light-on/CXT+ vs light-off/CXT−) were followed up by testing if these effects were specifically dependent on differences in potentiation to the threat cue or the threat context. During late acquisition, there was significant potentiation to the threat cue (cue FPS: F1,141=182, P<0.001, η2=0.56) as measured by contrasting startles during light-on/CXT+ compared with light-off/CXT+. In addition, there was significant potentiation to the threat context defined as light-off/CXT+ vs light-off/CXT− (context FPS: F1,141=88, P<0.001, η2=0.38). Both cue and context FPS were independent of rs2180619 genotype during all phases of acquisition (F's<1). There was significant reduction from late acquisition to late extinction of potentiation to the threat cue (cue FPS: F1,141=6.9, P=0.009, η2=0.05) and the threat context (context FPS: F1,141=4.2, P=0.04, η2=0.03). This reduction was significantly modulated by rs2180619 genotype regarding the threat cue (cue FPS: F1,138=4.79, P=0.03, η2=0.03) and marginally significant for the threat context (context FPS: F1,138=3.1, P=0.08, η2=0.02). See Figure 2a for the time courses and Supplementary Figures 2 and 3 for the magnitude of extinction of cue and context FPS separately. Failure to extinguish fear resulted in significantly higher cued FPS (F1,138=4.80, P=0.03, η2=0.03) and contextual FPS (F1,138=4.85, P=0.03, η2=0.03) in A/A homozygotes compared with G-allele carriers at the end of extinction.
Subjective fear ratings
During the acquisition phase, significant potentiation of subjective fear ratings (light-on/CXT+ vs light-off/CXT−) was observed (uninstructed acquisition: F1,141=302, P<0.001, η2=0.68; instructed acquisition: F1,141=488, P<0.001, η2=0.78; late acquisition: F1,141=456, P<0.001, η2=0.76), which was unrelated to rs2180619 and rs1049353 genotype (F's<1.3). On average, subjects showed significant extinction of threat-potentiated subjective fear (F1,141=78, P<0.001, η2=0.36). However, no significant effects of rs2180619 or rs1049353 genotype on fear extinction of threat-potentiated subjective fear were observed (F's<1; Figures 2b and d). As in the startle data, A/A homozygotes of rs2180619 showed higher threat-potentiated subjective fear at the end of extinction, but these differences did not reach significance (F1,138=2.0, P=0.16). Furthermore, no statistically significant effects of the genetic polymorphisms under study on cue-specific or context-specific potentiation of subjective fear ratings were observed.
Discussion
In this study, we tested if genetic variability in the human cannabinoid receptor 1 is associated with the extinction of fear. This hypothesis was directly derived from preclinical studies,11, 20 where converging evidence demonstrates CB1 as a major determinant of fear extinction (see Lafenêtre et al.9 for a review). To translate these findings to humans, healthy subjects (N=150) were conditioned using threat of electrical shock in a virtual reality environment and subsequently underwent an extinction procedure. Fear-conditioned responses were measured by potentiation of the eyeblink startle reflex, which serves as an objective index of basic defensive states.27, 29, 32 In addition, subjects rated their subjective fearfulness during the task.
The present data indicate that genetic variability in rs2180619, a SNP located in the promoter region of CB1, is strongly associated with the extinction of fear in humans. This translates previous animal work into the human realm for the first time. While G-allele carriers of rs2180619 (N=91) displayed robust reductions of basic defensive state during the course of extinction, such a reduction was completely absent in A/A homozygotes (N=51), which is particularly noteworthy given this group size and the fact that participants were selected owing to self-reported mental health. Moreover, this effect was accompanied by higher levels of FPS at the end of the extinction procedure in A/A homozygotes. Of note, both rs2180619 genotype groups showed solid and indifferent fear-conditioned responding during the acquisition phase and during baseline startle measurements that preceded the experimental paradigm. This suggests that differences in fear extinction can be ascribed to extinction rather than differences that were present during acquisition of fear.
These findings suggest central human endocannabinoid signaling as an important factor in regulation of the basic defensive state when previously acquired fear is no longer adaptive. Moreover, our results demonstrate that after exposure to threat, CNR1-dependent deficits in fear regulation as observed in A/A homozygotes of rs2180619 may lead to both sustained cue-specific and context-specific fear. Deficient fear extinction has been suggested to model a mechanism towards clinical anxiety59 and may hence constitute a risk factor for the development of anxiety disorders.5, 6, 60 During life, every individual is inevitably exposed to a variety of adverse events, ranging from simple everyday stressors to traumatic experiences. Extinguishing fear associations once they no longer lead to the expected negative consequences hereby serves as a crucial mechanism to adapt to the environment.3, 60 Even though our subjects were selected for good mental health, the insufficient extinction of fear in A/A carriers of rs2180619 might predispose individuals with this particular genetic CB1/CNR1 makeup to develop anxiety disorders.
We did not find a significant association between rs1049353 located in the coding region of the CB1 gene and fear extinction. Detailed studies on the molecular pathways by which the candidate SNPs in this study affect CB1 functioning are currently lacking, which complicates the interpretation of our findings. Additional research on the molecular pathways by which genetic polymorphisms in CB1/CNR1 affect receptor functioning is thus warranted.
Neuropharmacologically, cannabinoids have a major regulatory role for neurotransmitters important in anxiety such as GABA, glutamate and serotonin.61, 62 Recently, several theoretical frameworks based on preclinical work have been published6, 9, 19 that posit how this may affect fear and anxiety. A working hypothesis9 describes CB1 effects on fear extinction as a process centered on the amygdala that modulates both associative and non-associative (that is, habituation-like) learning processes. During acquisition, coupling of the conditioned stimulus (CS) and the unconditioned stimulus (US) potentiates fear circuits, resulting in a fear-conditioned response (the ‘fear pathway'). During extinction, presentation of the CS in absence of the US leads to activation of a ‘no-fear pathway'. This means that a new inhibitory association is formed between the CS and the lack of the US that competes with activation of the fear pathway for control over behavior.4, 8 Concurrently, non-associative processes occur that decrease the organisms' general fear state when the US no longer is presented.8, 63 According to Lafenêtre et al.,9 the ECS acts on both these processes. First, the ECS inhibits GABAergic neurotransmission during extinction, resulting in disinhibition of the ‘no-fear pathway' and facilitation of the new associative learning component formed during extinction. Second, retrograde activation of the ECS onto glutamatergic neurons leads to decreased glutamatergic neurotransmission, therefore contributing to the depotentiation of the fear pathway (often called ‘habituation' or non-associative learning). Notably, recent studies support this view by showing that CB1-dependent neurotransmission modulates within session extinction.64
In contrast to our startle results, subjective ratings of fear during extinction were not significantly modulated by CB1 genotype. Absence of an effect on subjective ratings while startle potentiation does differentiate has been reported earlier, and various factors might contribute to a reduced sensitivity of subjective measures, including individual differences in the interpretation of questionnaires, intentional distortion and demand characteristics (for example, see Hermans et al.,7 Lonsdorf et al.65 and Schinka et al.66). Furthermore, explicit knowledge about the CS–US contingencies and corresponding subjective states may not be so dynamically altered by adaptation in limbic structures that regulate defensive responding, such as the amygdala. In all, subjective ratings might not be as sensitive to differences in CB1/CNR1 as physiological data.
The present study was based on the assumption that the genetic polymorphisms under study modulate CB1 expression. On this assumption, the present findings implicate cannabinergic neurotransmission in human fear extinction, thereby pointing towards a potential new target for treatment of anxiety disorders. Because both the preclinical literature and our findings explicitly suggest an interaction between the cannabinoid system and extinction training, CB1 compounds may be applicable as augmentation of exposure therapy, similar to 𝒟-cycloserine, the partial agonist at the glycine recognition site of the glutamatergic N-methyl-𝒟-aspartate receptor.67 However, recent studies demonstrated anxiolytic effects of cannabinoid compounds, such as cannabidiol68, 69 or nabilone70 in absence of extinction training. Together with the theoretical model based on preclinical findings discussed above,9 these preliminary human treatment studies suggest that cannabinoid pharmacotherapy may serve a broader goal of improving adaption of fear responses, without being specific to exposure training. Cannabinoid-based pharmacotherapy for anxiety disorder could potentially be greatly facilitated by additional studies of the specific fear- and anxiety-related processes that may be affected by existent pharmacological agents and the development of more selective agents, as CB1 agonists appear to have rather diffuse effects.
In conclusion, the present study provides one of the first attempts of translating preclinical evidence regarding the involvement of the ECS in fear extinction to humans. Our data suggest that endocannabinoid signaling is involved in regulation of the basic defensive state when previously acquired fear is no longer adaptive. Human CB1 functioning may hence modulate maintenance of fear responses, which supports the possibility of novel treatments of human anxiety by targeting this receptor. However, further development of cannabinoid-dependent pharmacological treatments for anxiety must await additional experimental and pharmacological research. Molecular evidence that rs2180619 indeed modulates CB1 expression may provide the missing link to conclude that CB1 is a promising target for the treatment of anxiety in humans. This may provide an important breakthrough given the high prevalence of anxiety disorders71 and the limited efficacy of current treatments.72, 73, 74, 75
Acknowledgments
We thank Ioannis Lois, Joris van Oosterhout and Lucas Koolen for help during data collection and processing, Siarhei Uzunbajakau for technical assistance and Erik Hendriksen for technical help during genotyping. This work was supported by a grant from the Brain Foundation of the Netherlands (Hersenstichting Nederland).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Mineka S, Zinbarg R. Conditioning and ethological models of anxiety disorders: stress-in-dynamic-context anxiety models. Nebr Symp Motiv. 1996;43:135–210. [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol. 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Hofmann SG. Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clin Psychol Rev. 2008;28:199–210. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Heinrichs SC, Carey RJ. Treatment of addiction and anxiety using extinction approaches: neural mechanisms and their treatment implications. Pharmacol Biochem Behav. 2011;97:619–625. doi: 10.1016/j.pbb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Hermans D, Craske MG, Mineka S, Lovibond PF. Extinction in human fear conditioning. Biol Psychiatry. 2006;60:361–368. doi: 10.1016/j.biopsych.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Lafenêtre P, Chaouloff F, Marsicano G. The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol Res. 2007;56:367–381. doi: 10.1016/j.phrs.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Bitencourt RM, Takahashi RN. Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiol Learn Mem. 2008;90:290–293. doi: 10.1016/j.nlm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC. The CB1 cannabinoid receptor in the brain. Neurobiol Dis. 1998;5:405–416. doi: 10.1006/nbdi.1998.0215. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Bidaut-Russell M, Devane WA, Melvin LS, Johnson MR, Herkenham M. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13:420–423. doi: 10.1016/0166-2236(90)90124-s. [DOI] [PubMed] [Google Scholar]
- Zhang P-W, Ishiguro H, Ohtsuki T, Hess J, Carillo F, Walther D, et al. Human cannabinoid receptor 1: 5' exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol Psychiatry. 2004;9:916–931. doi: 10.1038/sj.mp.4001560. [DOI] [PubMed] [Google Scholar]
- Davies SN, Pertwee RG, Riedel G. Functions of cannabinoid receptors in the hippocampus. Neuropharmacology. 2002;42:993–1007. doi: 10.1016/s0028-3908(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126 (Pt 6:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Resstel LB, Lisboa SF, Campos AC, Gomes FV, et al. Neuroanatomical substrates involved in cannabinoid modulation of defensive responses. J Psychopharmacol. 2012;26:40–55. doi: 10.1177/0269881111400651. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol. 2008;18:849–859. doi: 10.1016/j.euroneuro.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Prediger RD, Pandolfo P, Takahashi RN. The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology. 2006;188:641–649. doi: 10.1007/s00213-006-0514-0. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Ressler KJ. Modulation of fear and anxiety by the endogenous cannabinoid system. CNS Spectr. 2007;12:211–220. doi: 10.1017/s1092852900020939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. The study of fear extinction: implications for anxiety disorders. Am J Psychiatry. 2011;168:1255–1265. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Zwanzger P. GABAergic and endocannabinoid dysfunction in anxiety - future therapeutic targets. Curr Pharm Des. 2008;14:3508–3517. doi: 10.2174/138161208786848784. [DOI] [PubMed] [Google Scholar]
- Lutz B. The endocannabinoid system and extinction learning. Mol Neurobiol. 2007;36:92–101. doi: 10.1007/s12035-007-8004-x. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Moulder B, Lang PJ. When good things go bad: the reflex physiology of defense. Psychol Sci. 2005;16:468–473. doi: 10.1111/j.0956-7976.2005.01558.x. [DOI] [PubMed] [Google Scholar]
- Klumpers F, Heitland I, Oosting RS, Kenemans JL, Baas JM. Genetic variation in serotonin transporter function affects human fear expression indexed by fear-potentiated startle. Biol Psychol. 2011;89:277–282. doi: 10.1016/j.biopsycho.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Chen PS, Gean PW. Chronic cannabinoid administration in vivo compromises extinction of fear memory. Learn Mem. 2008;15:876–884. doi: 10.1101/lm.1081908. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, et al. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learn Mem. 2006;13:681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Denys D, Kenemans JL, Grillon C, van der Aart J, Baas JM. Testing the effects of Δ9-THC and d-cycloserine on extinction of conditioned fear in humans. J Psychopharmacol. 2012;26:471–478. doi: 10.1177/0269881111431624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet B. Learning and memory in conditioned fear extinction: effects of D-cycloserine. Acta Psychol. 2008;127:601–613. doi: 10.1016/j.actpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson RA. randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2007;41:466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Mem. 2004;11:625–632. doi: 10.1101/lm.77904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, et al. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Annas P, Neale MC, Kendler KS, Fredrikson M. A twin study of the genetics of fear conditioning. Arch Gen Psychiatry. 2003;60:702–708. doi: 10.1001/archpsyc.60.7.702. [DOI] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Kalisch R. A review on experimental and clinical genetic associations studies on fear conditioning, extinction and cognitive-behavioral treatment. Transl Psychiatry. 2011;1:e41. doi: 10.1038/tp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PW, Ishiguro H, Ohtsuki T, Hess J, Carillo F, Walther D, et al. Human cannabinoid receptor 1: 5' exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol Psychiatry. 2004;9:916–931. doi: 10.1038/sj.mp.4001560. [DOI] [PubMed] [Google Scholar]
- Lazary J, Lazary A, Gonda X, Benko A, Molnar E, Hunyady L, et al. Promoter variants of the cannabinoid receptor 1 gene (CNR1) in interaction with 5-HTTLPR affect the anxious phenotype. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1118–1127. doi: 10.1002/ajmg.b.31024. [DOI] [PubMed] [Google Scholar]
- Lu AT, Ogdie MN, Jarvelin MR, Moilanen IK, Loo SK, McCracken JT, et al. Association of the cannabinoid receptor gene (CNR1) with ADHD and post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1488–1494. doi: 10.1002/ajmg.b.30693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U, Ohrmann P, Lawford B, Bauer J, Kugel H, et al. Cannabinoid receptor 1 (CNR1) gene: impact on antidepressant treatment response and emotion processing in major depression. Eur Neuropsychopharmacol. 2008;18:751–759. doi: 10.1016/j.euroneuro.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Baas JM, Nugent M, Lissek S, Pine DS, Grillon C. Fear conditioning in virtual reality contexts: a new tool for the study of anxiety. Biol Psychiatry. 2004;55:1056–1060. doi: 10.1016/j.biopsych.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Baas JM, van Ooijen L, Goudriaan A, Kenemans JL. Failure to condition to a cue is associated with sustained contextual fear. Acta Psychol. 2008;127:581–592. doi: 10.1016/j.actpsy.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Baas JMP.Individual differences in predicting aversive events and modulating contextual anxiety in a context and cue conditioning paradigm Biol Psychol 2012. e-pub ahead of print. [DOI] [PubMed]
- Klumpers F, Raemaekers MA, Ruigrok AN, Hermans EJ, Kenemans JL, Baas JM. Prefrontal mechanisms of fear reduction after threat offset. Biol Psychiatry. 2010;68:1031–1038. doi: 10.1016/j.biopsych.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Klumpers F, van Gerven JM, Prinssen EP, Niklson I, Roesch F, Riedel WJ, et al. Method development studies for repeatedly measuring anxiolytic drug effects in healthy humans. J Psychopharmacol. 2010;24:657–666. doi: 10.1177/0269881109103115. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bocker KB, Baas JM, Kenemans JL, Verbaten MN. Differences in startle modulation during instructed threat and selective attention. Biol Psychol. 2004;67:343–358. doi: 10.1016/j.biopsycho.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Lukkes J, Vuong S, Scholl J, Oliver H, Forster G. Corticotropin-releasing factor receptor antagonism within the dorsal raphe nucleus reduces social anxiety-like behavior after early-life social isolation. J Neurosci. 2009;29:9955–9960. doi: 10.1523/JNEUROSCI.0854-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR.NEO-PI-R Professional Manual Psychological Assessment Resources; Odessa, FL1992 [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Erlbaum: Hillsdale, NJ; 1988. [Google Scholar]
- Grillon C, Davis M. Fear-potentiated startle conditioning in humans: explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol Disord Drug Targets. 2009;8:451–458. doi: 10.2174/187152709789824624. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Wotjak CT. Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learn Mem. 2004;11:770–786. doi: 10.1101/lm.86104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plendl W, Wotjak CT. Dissociation of within- and between-session extinction of conditioned fear. J Neurosci. 2010;30:4990–4998. doi: 10.1523/JNEUROSCI.6038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Ohman A. Genetic gating of human fear learning and extinction: possible implications for gene-environment interaction in anxiety disorder. Psychol Sci. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- Hofmann SG. Enhancing exposure-based therapy from a translational research perspective. Behav Res Ther. 2007;45:1987–2001. doi: 10.1016/j.brat.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology. 2011;36:1219–1226. doi: 10.1038/npp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25:121–130. doi: 10.1177/0269881110379283. [DOI] [PubMed] [Google Scholar]
- Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD) CNS Neurosci Ther. 2009;15:84–88. doi: 10.1111/j.1755-5949.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, et al. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004. pp. 21–27. [DOI] [PubMed]
- Deacon BJ, Abramowitz JS. Cognitive and behavioral treatments for anxiety disorders: a review of meta-analytic findings. J Clin Psychol. 2004;60:429–441. doi: 10.1002/jclp.10255. [DOI] [PubMed] [Google Scholar]
- Mitte K, Noack P, Steil R, Hautzinger M. A meta-analytic review of the efficacy of drug treatment in generalized anxiety disorder. J Clin Psychopharmacol. 2005;25:141–150. doi: 10.1097/01.jcp.0000155821.74832.f9. [DOI] [PubMed] [Google Scholar]
- Schruers K, Koning K, Luermans J, Haack MJ, Griez E. Obsessive-compulsive disorder: a critical review of therapeutic perspectives. Acta Psychiatr Scand. 2005;111:261–271. doi: 10.1111/j.1600-0447.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- Westen D, Morrison K. A multidimensional meta-analysis of treatments for depression, panic, and generalized anxiety disorder: an empirical examination of the status of empirically supported therapies. J Consult Clin Psychol. 2001;69:875–899. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.