Abstract
Genetic microcephaly and lissencephaly are two of the most common brain malformations. Each of them is a heterogeneous group of disorders caused by mutations of many different genes. They are a significant cause of neurological morbidity in children worldwide, responsible for many cases of mental retardation, cerebral palsy and epilepsy. Recent advances in molecular genetics have led to the identification of several genes causing these disorders, and thus accurate molecular diagnosis and improved genetic counseling has become available for many patients and their families. More recently identified genes include STIL, causing primary autosomal recessive microcephaly (microcephaly vera), and TUBA1A, causing lissencephaly. Numerous other disease genes are likely still to be identified. Functional studies of genes that cause microcephaly and lissencephaly have provided valuable insight into the molecular mechanisms of human brain development.
Introduction
Brain malformations are a significant cause of neurological morbidity in children worldwide. They may be associated with mental retardation, cerebral palsy, and/or epilepsy. Since brain malformations encompass a wide range of disorders, many attempts have been made to classify them. In recent years, a classification system based on the stage of brain development that is affected has been widely accepted and used.1 This classification system divides brain malformations into disorders of cell proliferation, neuronal migration and cortical organization. Within this classification scheme, microcephaly accounts for a large proportion of the disorders of defective cell proliferation, though some microcephaly syndromes can involve other stages of development. Lissencephaly forms a major group within the disorders of neuronal migration. These two group of disorders, microcephaly and lissencephaly, are discussed below.
Microcephaly
Microcephaly (“small head”) is a condition in which the brain fails to achieve normal growth. It is a clinical observation, rather than a specific diagnosis. Many different genetic causes or environmental insults to the brain during prenatal, perinatal, or early postnatal period can lead to microcephaly. It is the genetic forms of microcephaly that have been under particularly intense study in recent years. There are several ways of classifying genetic microcephaly syndromes and these include: identifying the presence or absence of microcephaly at birth (congenital versus postnatal microcephaly), determining if the brain architecture is normal or disturbed, and discerning if there is an associated non-CNS phenotype.2 The prototype microcephaly syndrome is called microcephaly vera (“true” microcephaly) or primary autosomal recessive microcephaly and it is characterized by congenital microcephaly with relatively preserved brain artchitecture and absent non-CNS phenotypes.
Clinical and genetic aspects of microcephaly vera
The clinical entity of microcephaly vera has been known for more than a century. The term “microcephalia vera” was coined by Giacomini in 18853, and the condition he described probably represents what we refer to as microcephaly vera today. The diagnostic criteria for microcephaly vera is still evolving as we continue to learn more about its genetic basis. The currently accepted criteria, however, are: microcephaly of at least -3 standard deviations below age and sex means, mental retardation of varying degrees, and the absence of other neurological or growth disorders.4-6 Specifically, microcephaly is present at the time of birth (congenital microcephaly), and the head circumference may be as small as -12 standard deviations below the mean.7 Epilepsy is quite uncommon, though it has been reported in some patients, and does not exclude the diagnosis of microcephaly vera.5,8
Microcephaly vera has been known for a long time to be inherited as an autosomal recessive trait, but it is only during the last decade that its genetic basis has begun to be understood. Despite a relatively homogeneous clinical picture, microcephaly vera shows remarkable genetic heterogeneity. There are at least seven loci that are associated with this condition, and these loci are named MCPH1 through MCPH7.9-15 Among these loci, five genes have been identified thus far: MCPH1 (or microcephalin) for MCPH116, CDK5RAP2 for MCPH317, ASPM for MCPH518, CENPJ (or CPAP) for MCPH617, and STIL for MCPH7.12 No clear genotype-phenotype correlation has yet emerged, and therefore it is usually not possible to determine from the clinical findings which gene is responsible for the condition in a patient. Extensive radiological studies of microcephaly vera have not been performed, but the brain architecture is generally normal, with mild simplification in the gyral pattern reported in some patients with ASPM and MCPH1 mutations.19-21(Figure 1) Periventricular nodular heterotopia have been reported in one family with an MCPH1 mutation,21 though it is not clear if this feature is unique to MCPH1.
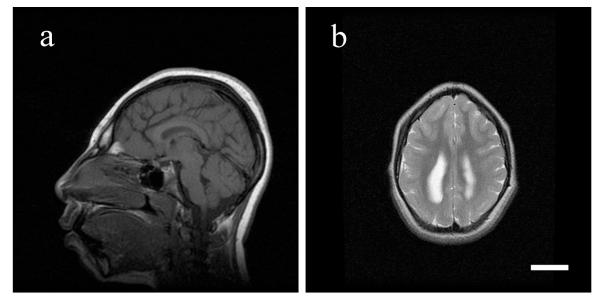
Figure 1. MRI findings of microcephaly vera due to an ASPM muation.
T1-weighted sagittal (a) and T2-weighted axial (b) images of a patient with microcephaly vera due to an ASPM mutation are shown. The sagittal image shows a severe reduction of the cerebral volume, but a relatively normal midline structure. The axial image shows a simplified gyral pattern. Scale bar = 3cm.
Of these loci and genes, ASPM (MCPH5) is the most common cause of microcephaly vera. It has been shown to account for about 40% of cases in patients with Pakistani or Arab and European origin.5,6 To date, more than 50 ASPM mutations have been reported, and their presence seems to be limited to patients who fulfill the diagnostic criteria for microcephaly vera as described above.5 Thus, the clinical spectrum of ASPM mutations may be relatively narrow. Only a small number of independent mutations have been reported for the other known microcephaly vera genes, and their clinical spectra are less well defined.
Biology of microcephaly vera
The microcephaly vera genes described to date have all been implicated in cell division and cell cycle regulation. Among them, ASPM, CDK5RAP2, CENPJ and STIL encode proteins that localize to the centrosome.12,17,19 Despite their similar subcellular localization, the biological functions of these genes may be varied however. ASPM is the homolog of the Drosophila gene asp (abnormal spindle), which encodes a protein essential for maintaining the integrity of mitotic centrosome and mitotic spindle.22 The CDK5RAP2 protein is implicated in maintaining centrosome cohesion,23 and the CENPJ protein has been shown to be an essential component of centriole biogenesis.24 Exactly how defects in these centrosomal proteins lead to a small brain is not clear. The ASPM protein has been shown to be important in maintaining a proper orientation of the cell division cleavage plane in neural progenitors,25 which is thought to be an important factor in determining the fate of their daughter cells.26 Therefore it is possible that disruption of the cleavage plane leads to early depletion of progenitor cells, leading to insufficient production of neurons and resulting microcephaly.
The MCPH1 protein is implicated in DNA repair27,28 and chromosome condensation21 and may therefore be involved in a different biological pathway. Interestingly, MCPH1, at least partially, belongs to the same signaling pathway as ATR (ataxia-telangiectasia and RAD3-related), which is mutated in some cases of Seckel syndrome, a microcephaly syndrome with unique facial features and severe short stature.29 Additionally, it has been suggested that MCPH1 also localizes to centrosome,30 and that it functions in centrosome duplication. 31 Further studies to elucidate the role of MCPH1 in centrosomal function are needed.
One of the most obvious structural characteristics of the human brain, when compared to that of other animals, is its large size. Since the microcephaly genes, when mutated, cause significant reduction in brain size without affecting other organs, it has been speculated that the molecular evolution of these genes may have been a driving force for the size increase of the brain during human evolution. Though it is quite difficult to test this hypothesis experimentally, there are data to support it. For example, there is strong evidence of positive selection for evolutionary changes in the amino acid sequence of ASPM in human lineage.32-34 Similar evidence of positive selection for MCPH1,35 and CDK5RAP2 is also reported in human lineage.36
Lissencephaly
Lissencephaly is a group of disorders that is characterized by an abnormally smooth surface of the cerebral cortex. It has generally been divided into two categories: classic lissencephaly (also known as type 1 lissencephaly) and cobblestone complex (also known as type 2 lissencephaly). Though both groups can be associated with a smooth-appearing cerebral cortex, they are quite different from each other in terms of pathogenesis. The underlying biochemical defect in cobblestone complex is thought to be defective O-glycosylation of α-dystroglycan.37 This leads to a compromise in the integrity of the pial surface, and subsequent overmigration of cortical neurons through the pial surface. These heterotopic (abnormally positioned) neurons form cobblestone-like nodules on the surface of the brain; hence the term cobblestone complex. Cobblestone complex is often seen as part of multisystem disorders including Walker-Warburg syndrome, muscle-eye-brain disease, and Fukuyama-type congenital muscular dystrophy. In classic lissencephaly the integrity of the pial surface is intact, but the cerebral cortex is abnormally thick, and the normal six-layered structure of the cortex is seriously impaired. Classic lissencephaly, the main focus of this article, is discussed below.
Clinical and genetic aspects of lissencephaly
The first gene identified to cause lissencephaly was LIS1.38 LIS1 localizes to chromosome 17p13.3 and mutations in this gene are associated with two clinical entities: Miller-Dieker syndrome (MDS) and isolated lissencephaly sequence (ILS). MDS is characterized by classic lissencephaly and unique facial features (prominent forehead, bitemporal hollowing, short nose with upturned nares, protuberant upper lip, thin vermilion border, and small jaw).39 When a patient with classic lissencephaly lacks the characteristic facial features of MDS, their condition is called ILS. ILS is caused by a mutation in or a small deletion involving LIS1, and MDS is caused by a larger deletion encompassing LIS1 and neighboring genes.40 Lissencephaly due to mutations or deletions of LIS1 is a dominant trait. The majority of LIS1 mutations are de novo (not inherited from a parent) and therefore the recurrence risk is generally low. However, in some cases a parent harbors a balanced translocation involving the LIS1 gene,41,42 and so their risk of recurrence could be much higher.
DCX (also known as doublecortin) is a gene located on the X chromosome, and its mutation in males (in a hemizygous state) cause lissencephaly.43,44 The mutation of DCX in one of a female’s two X chromosomes (in a heterozygous state) typically leads to a much milder brain phenotype, including subcortical band heterotopias (SBH). SBH is also known as “double cortex” syndrome, since a band of heterotopic neurons is found within the cerebral white matter between a normal appearing cortex and the ventricular surface. These females may present with seizures, but often have only mild cognitive delay. Therefore, mutations in DCX may be inherited from a mother with SBH to her son, causing lissencephaly, or to her daughter, causing SBH. If the mother of a male child with lissencephaly has an unexplained seizure disorder or cognitive problems, obtaining a brain MRI of the mother to investigate for SBH is often useful.
More recently, the TUBA1A gene has been found to be mutated in some patients with classic lissencephaly.45 Congenital microcephaly, spastic diplegia or quadriplegia, and mental retardation are common clinical features seen in patients with TUBA1A mutations. However, some patients have a milder lissencephaly phenotype on MRI and they develop some expressive language and also the ability to walk. 46,47 Lissencephaly due to TUBA1A mutations manifests as a dominant trait, and therefore the mutations are generally de novo, as seen with LIS1. 46,47
LIS1 and DCX collectively account for about three quarters of isolated classic lissencephaly 48, and TUBA1A is estimated to account for about 4% of cases. 49 Though LIS1, DCX and TUBA1A can cause similar lissencephaly phenotypes, radiological findings sometimes help distinguish these causative genes. LIS1 mutations tend to cause lissencephaly that is more severe posteriorly (Figure 2), whereas DCX mutations cause lissencephaly that is more severe anteriorly50. TUBA1A may show posterior-predominant lissencephaly like LIS1, but it is also often associated with perisylvian pachygyria.46 Additionally, dysgenesis of the anterior limb of the internal capsule has also been suggested as a unique finding in TUBA1A mutations. 46
Figure 2. MRI findings typical of LIS1 mutations.
T2-weighted axial MRI image of a patient with classic lissencephaly is shown. There is almost complete agyria posteriorly, but some rudimentary gyri are seen anteriorly. This pattern of a more severely affected posterior cerebral cortex is typical of LIS1 mutations.
Other genes that are associated with classic lissencephaly include RELN, VLDLR, and ARX. However, lissencephaly associated with these genes are pathologically and radiologically quite distinct from lissencephaly due to LIS1, DCX and TUBA1A. Therefore, it has recently been suggested to separate lissencephaly due to RELN, VLDLR, and ARX from classic lissencephaly (due to LIS1, DCX and TUBA1A) by categorizing it as “variant lissencephaly.”51,52 Mutations in the human RELN (reelin) gene were identified in patients with lissencephaly and cerebellar hypoplasia.53 Though only a few patients have been described to date, generalized pachygyria, severe cerebellar hypoplasia and hippocampal abnormalities seem to be common features (Figure 3). Very low density lipoprotein receptor gene (VLDLR) mutations cause similar abnormalities to RELN with severe cerebellar hypoplasia, but the simplification of gyri may be milder.54 ARX was initially identified as the causative gene for X-linked lissencephaly with abnormal genitalia. 55 Radiological features of lissencephaly due to ARX mutations include agyria or pachygyria with posterior to anterior gradient of severity, abnormally thick cortex (less thick than lissencephaly due to LIS1 or DCX, however), agenesis of corpus callosum, abnormal white matter signals and poorly delineated basal ganglia.56,57 It has since been shown that the neurological spectrum of ARX mutations is quite broad, and ranges from non-syndromic mental retardation58 to hydranencephaly.57 Epilepsy syndromes without a brain malformation, such as early infantile epileptic encephalopathy (Otahara syndrome) have also been reported to be associated with ARX mutations. 59
Figure 3. MRI findings of a RELN mutation.
T1-weighted coronal MRI image of a patient with a RELN mutation is shown. There are a reduced number of gyri, which are also abnormally thick (pachygyria). The posterior fossa is empty due to an almost complete absence of the cerebellar hemispheres.
Biology of lissencephaly
Functions of some lissencephaly genes (LIS1, DCX and TUBA1A) are closely related to microtubules. Microtubules are a component of the cytoskeleton, and play important roles in cellular processes such as mitosis and cytokinesis. LIS1 and DCX are known to be critical regulators of microtubule function in neurons. Migrating neurons first extend a leading process along the scaffold of radial glia, and then the centrosome and nucleus are pulled toward the leading process. The network of microtubules and molecular motor, dynein, are critical to this movement of the centrosome and nucleus in migrating neurons. The LIS1 protein interacts with dynein60, and along with its biding partner NDEL, it regulates this microtubule-based molecular motor.61-63 The DCX protein binds to microtubules and stabilizes their polymerization. 64-66 TUBA1A, on the other hand, is a structural component of microtubules themselves. Microtubules consist of dimers of α- and β-tubulin, and TUBA1A is one of the α-tubulin isoforms, and is highly expressed in the developing brain. 47
Proteins encoded by the “variant lissencephaly” genes (RELN, VLDLR and ARX) are associated with different biological pathways. RELN is a human homolog of the mouse gene responsible for the spontaneous mouse mutant called “reeler”.67 Reeler mice are characterized by severe lamination defects of the cerebral cortex, cerebellar hypoplasia, and a “reeling” gait.68,69 The RELN protein is secreted by Cajal-Retzius cells on the molecular layer (layer I) of the cerebral cortex and has been shown to inhibit neuronal migration, perhaps by acting as a stop signal for migrating cortical neurons.70 The product of VLDLR gene, which is associated with similar phenotype to RELN, belongs to the same biological pathway as RELN. VLDLR, along with apolipoprotein E receptor 2 (APOER2), acts as a receptor for the RELN protein in migrating neurons, and transmits extracellular RELN signal to the intracellular signaling pathway.71,72 There is also notable evidence of interaction between RELN signaling and LIS1.73,74
In some forms of classic lissencephaly, defects in GABAergic inhibitory interneurons have been suggested. GABAergic interneurons of the cerebral cortex are derived in the ganglionic eminence (which develops into basal ganglia), and migrate tangentially into the cerebral cortex. The best known example of defects in GABAergic interneuons is due to ARX mutations. ARX encodes Aristaless-related homeobox protein, and mice lacking this protein show defective differentiation and migration of GABAergic interneuons. 55 The mouse homolog of LIS1 has also been shown to be essential in tangential migration of interneurons in the brain. 75 Additionally, the number of inhibitory interneurons is greatly diminished in the brain of patients with LIS1 mutations, Miller-Dieker syndrome.76 Lissencephaly is often associated with severe, intractable epilepsy, and defects in interneurons, in addition to abnormal cortical lamination, may be in part responsible for this. Interestingly, mutations in TUBA1A seem to be associated with a lower incidence of epilepsy compared to LIS1, though still only a small number of patients have been reported. For example, in a series of 11 patients, 6 (ages ranging from 2 to 7 years) had not been diagnosed with epilepsy.46 It is not yet known whether or not there are similar defects in interneurons in the brains of patients with TUBA1A mutations.
Studies correlating genetic defects with neuropathological findings are still few, but it has been suggested that some lissencephaly genes are associated with specific neuropathology of the cerebral cortex. Mutations in LIS1 and DCX generally lead to a four-layered cerebral cortex, and DCX mutations, compared to LIS1, tend to cause a more varied layer structure with an irregular gray-white junction.51 ARX mutations are associated with a three-layered cerebral cortex, with hypercellularity of the molecular layer as a unique feature.51
Classic lissencephaly is often considered the prototype of “neuronal migration disorders.” As evident from neuropathological findings, neuronal migration is severely affected in these conditions; however, at least in some lissencephaly syndromes, proliferation of neural precursor cells may also be impaired. In a human postmortem study of neural precursor cells from a fetus with a LIS1 deletion, the rate of cell proliferation was shown to be diminished.77 Also the mouse homolog of LIS1 has been shown to be essential for cell fate determination of neural progenitor cells.78 Thus, the pathogenesis of classic lissencephaly is unlikely to be due to defective neuronal migration alone, but may include an aspect of abnormal proliferation.
Conclusions
As new genes for human disease are identified, clinical entities are redefined, and novel classifications emerge. Classic lissencephaly and microcephaly vera are no exception. Both were initially speculated to be relatively homogeneous clinical conditions, but as genetic research has progressed, remarkable genetic heterogeneity emerged. Identification of causative genes for these disorders and subsequent studies of their functions have led to a better understanding of the development of the human brain. However, our knowledge of molecular mechanisms of brain development is far from complete. It is clear that there are genes associated with lissencephaly and microcephaly that have yet to be identified. Further genetic and neurobiological studies are essential to our understanding, diagnosis and treatment of these devastating neurological disorders.
Acknowledgements
The author is supported in part by grants from NIH/NINDS (R37NS35129), the Dubai Harvard Foundation for Medical Research, and the Manton Center for Orphan Disease Research. The author thanks Ms. Jennifer Partlow for critical reading of the manuscript, and Ms. Brenda Barry, Prof. Mustafa A. M. Salih and Dr. Kalpathy S. Krishnamoorthy for the help in preparation of the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. A developmental and genetic classification for malformations of cortical development. Neurology. 2005;65(12):1873–87. doi: 10.1212/01.wnl.0000183747.05269.2d. [DOI] [PubMed] [Google Scholar]
- 2.Mochida GH, Walsh CA. Molecular genetics of human microcephaly. Curr Opin Neurol. 2001;14(2):151–6. doi: 10.1097/00019052-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Friede RL. Developmental neuropathology. 2nd rev. and expanded edn Springer-Verlag; Berlin: 1989. Disturbances in bulk growth: megalencephaly, micrencephaly, atelencephaly and others; pp. 296–308. [Google Scholar]
- 4.Cox J, Jackson AP, Bond J, Woods CG. What primary microcephaly can tell us about brain growth. Trends Mol Med. 2006;12(8):358–66. doi: 10.1016/j.molmed.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Nicholas AK, Swanson EA, Cox JJ, Karbani G, Malik S, Springell K, Hampshire D, Ahmed M, Bond J, Di Benedetto D. The molecular landscape of ASPM mutations in primary microcephaly. J Med Genet. 2009;46(4):249–53. doi: 10.1136/jmg.2008.062380. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts E, Hampshire DJ, Pattison L, Springell K, Jafri H, Corry P, Mannon J, Rashid Y, Crow Y, Bond J. Autosomal recessive primary microcephaly: an analysis of locus heterogeneity and phenotypic variation. J Med Genet. 2002;39(10):718–21. doi: 10.1136/jmg.39.10.718. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woods CG. Human microcephaly. Curr Opin Neurobiol. 2004;14(1):112–7. doi: 10.1016/j.conb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Shen J, Eyaid W, Mochida GH, Al-Moayyad, Bodell A, Woods CG, Walsh CA. ASPM mutations identified in patients with primary microcephaly and seizures. J Med Genet. 2005 doi: 10.1136/jmg.2004.027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson AP, McHale DP, Campbell DA, Jafri H, Rashid Y, Mannan J, Karbani G, Corry P, Levene MI, Mueller RF. Primary autosomal recessive microcephaly (MCPH1) maps to chromosome 8p22-pter. Am J Hum Genet. 1998;63(2):541–6. doi: 10.1086/301966. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamieson CR, Fryns JP, Jacobs J, Matthijs G, Abramowicz MJ. Primary autosomal recessive microcephaly: MCPH5 maps to 1q25-q32. Am J Hum Genet. 2000;67(6):1575–7. doi: 10.1086/316909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamieson CR, Govaerts C, Abramowicz MJ. Primary autosomal recessive microcephaly: homozygosity mapping of MCPH4 to chromosome 15. Am J Hum Genet. 1999;65(5):1465–9. doi: 10.1086/302640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar A, Girimaji SC, Duvvari MR, Blanton SH. Mutations in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly. Am J Hum Genet. 2009;84(2):286–90. doi: 10.1016/j.ajhg.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moynihan L, Jackson AP, Roberts E, Karbani G, Lewis I, Corry P, Turner G, Mueller RF, Lench NJ, Woods CG. A third novel locus for primary autosomal recessive microcephaly maps to chromosome 9q34. Am J Hum Genet. 2000;66(2):724–7. doi: 10.1086/302777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pattison L, Crow YJ, Deeble VJ, Jackson AP, Jafri H, Rashid Y, Roberts E, Woods CG. A fifth locus for primary autosomal recessive microcephaly maps to chromosome 1q31. Am J Hum Genet. 2000;67(6):1578–80. doi: 10.1086/316910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts E, Jackson AP, Carradice AC, Deeble VJ, Mannan J, Rashid Y, Jafri H, McHale DP, Markham AF, Lench NJ. The second locus for autosomal recessive primary microcephaly (MCPH2) maps to chromosome 19q13.1-13.2. Eur J Hum Genet. 1999;7(7):815–20. doi: 10.1038/sj.ejhg.5200385. others. [DOI] [PubMed] [Google Scholar]
- 16.Jackson AP, Eastwood H, Bell SM, Adu J, Toomes C, Carr IM, Roberts E, Hampshire DJ, Crow YJ, Mighell AJ. Identification of microcephalin, a protein implicated in determining the size of the human brain. Am J Hum Genet. 2002;71(1):136–42. doi: 10.1086/341283. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bond J, Roberts E, Springell K, Lizarraga S, Scott S, Higgins J, Hampshire DJ, Morrison EE, Leal GF, Silva EO. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat Genet. 2005;37(4):353–5. doi: 10.1038/ng1539. others. [DOI] [PubMed] [Google Scholar]
- 18.Bond J, Scott S, Hampshire DJ, Springell K, Corry P, Abramowicz MJ, Mochida GH, Hennekam RC, Maher ER, Fryns JP. Protein-truncating mutations in ASPM cause variable reduction in brain size. Am J Hum Genet. 2003;73(5):1170–7. doi: 10.1086/379085. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bond J, Roberts E, Mochida GH, Hampshire DJ, Scott S, Askham JM, Springell K, Mahadevan M, Crow YJ, Markham AF. ASPM is a major determinant of cerebral cortical size. Nat Genet. 2002;32(2):316–20. doi: 10.1038/ng995. others. [DOI] [PubMed] [Google Scholar]
- 20.Desir J, Cassart M, David P, Van Bogaert P, Abramowicz M. Primary microcephaly with ASPM mutation shows simplified cortical gyration with antero-posterior gradient pre- and post-natally. Am J Med Genet A. 2008;146A(11):1439–43. doi: 10.1002/ajmg.a.32312. [DOI] [PubMed] [Google Scholar]
- 21.Trimborn M, Bell SM, Felix C, Rashid Y, Jafri H, Griffiths PD, Neumann LM, Krebs A, Reis A, Sperling K. Mutations in microcephalin cause aberrant regulation of chromosome condensation. Am J Hum Genet. 2004;75(2):261–6. doi: 10.1086/422855. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.do Carmo Avides M, Glover DM. Abnormal spindle protein, Asp, and the integrity of mitotic centrosomal microtubule organizing centers. Science. 1999;283(5408):1733–5. doi: 10.1126/science.283.5408.1733. [DOI] [PubMed] [Google Scholar]
- 23.Graser S, Stierhof YD, Nigg EA. Cep68 and Cep215 (Cdk5rap2) are required for centrosome cohesion. J Cell Sci. 2007;120(Pt 24):4321–31. doi: 10.1242/jcs.020248. [DOI] [PubMed] [Google Scholar]
- 24.Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA. Plk4-induced centriole biogenesis in human cells. Dev Cell. 2007;13(2):190–202. doi: 10.1016/j.devcel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Fish JL, Kosodo Y, Enard W, Paabo S, Huttner WB. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc Natl Acad Sci U S A. 2006;103(27):10438–43. doi: 10.1073/pnas.0604066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82(4):631–41. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 27.Lin SY, Rai R, Li K, Xu ZX, Elledge SJ. BRIT1/MCPH1 is a DNA damage responsive protein that regulates the Brca1-Chk1 pathway, implicating checkpoint dysfunction in microcephaly. Proc Natl Acad Sci U S A. 2005;102(42):15105–9. doi: 10.1073/pnas.0507722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Lee J, Stern DF. Microcephalin is a DNA damage response protein involved in regulation of CHK1 and BRCA1. J Biol Chem. 2004;279(33):34091–4. doi: 10.1074/jbc.C400139200. [DOI] [PubMed] [Google Scholar]
- 29.Alderton GK, Galbiati L, Griffith E, Surinya KH, Neitzel H, Jackson AP, Jeggo PA, O’Driscoll M. Regulation of mitotic entry by microcephalin and its overlap with ATR signalling. Nat Cell Biol. 2006;8(7):725–33. doi: 10.1038/ncb1431. [DOI] [PubMed] [Google Scholar]
- 30.Zhong X, Pfeifer GP, Xu X. Microcephalin encodes a centrosomal protein. Cell Cycle. 2006;5(4):457–8. doi: 10.4161/cc.5.4.2481. [DOI] [PubMed] [Google Scholar]
- 31.Rai R, Phadnis A, Haralkar S, Badwe RA, Dai H, Li K, Lin SY. Differential regulation of centrosome integrity by DNA damage response proteins. Cell Cycle. 2008;7(14):2225–33. doi: 10.4161/cc.7.14.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans PD, Anderson JR, Vallender EJ, Gilbert SL, Malcom CM, Dorus S, Lahn BT. Adaptive evolution of ASPM, a major determinant of cerebral cortical size in humans. Hum Mol Genet. 2004;13(5):489–94. doi: 10.1093/hmg/ddh055. [DOI] [PubMed] [Google Scholar]
- 33.Kouprina N, Pavlicek A, Mochida GH, Solomon G, Gersch W, Yoon YH, Collura R, Ruvolo M, Barret JC, Woods CG. Accelerated evolution of the ASPM gene controlling brain size begins prior to human brain expansion. PLoS Biology. 2004 doi: 10.1371/journal.pbio.0020126. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J. Evolution of the human ASPM gene, a major determinant of brain size. Genetics. 2003;165(4):2063–70. doi: 10.1093/genetics/165.4.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang YQ, Su B. Molecular evolution of microcephalin, a gene determining human brain size. Hum Mol Genet. 2004;13(11):1131–7. doi: 10.1093/hmg/ddh127. [DOI] [PubMed] [Google Scholar]
- 36.Evans PD, Vallender EJ, Lahn BT. Molecular evolution of the brain size regulator genes CDK5RAP2 and CENPJ. Gene. 2006;375:75–9. doi: 10.1016/j.gene.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Muntoni F, Brockington M, Blake DJ, Torelli S, Brown SC. Defective glycosylation in muscular dystrophy. Lancet. 2002;360(9343):1419–21. doi: 10.1016/S0140-6736(02)11397-3. [DOI] [PubMed] [Google Scholar]
- 38.Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, Caskey CT, Ledbetter DH. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364(6439):717–21. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- 39.Dobyns WB, Curry CJ, Hoyme HE, Turlington L, Ledbetter DH. Clinical and molecular diagnosis of Miller-Dieker syndrome. Am J Hum Genet. 1991;48(3):584–94. [PMC free article] [PubMed] [Google Scholar]
- 40.Cardoso C, Leventer RJ, Ward HL, Toyo-Oka K, Chung J, Gross A, Martin CL, Allanson J, Pilz DT, Olney AH. Refinement of a 400-kb critical region allows genotypic differentiation between isolated lissencephaly, Miller-Dieker syndrome, and other phenotypes secondary to deletions of 17p13.3. Am J Hum Genet. 2003;72(4):918–30. doi: 10.1086/374320. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dobyns WB, Reiner O, Carrozzo R, Ledbetter DH. Lissencephaly. A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. Jama. 1993;270(23):2838–42. doi: 10.1001/jama.270.23.2838. [DOI] [PubMed] [Google Scholar]
- 42.Kuwano A, Ledbetter SA, Dobyns WB, Emanuel BS, Ledbetter DH. Detection of deletions and cryptic translocations in Miller-Dieker syndrome by in situ hybridization. Am J Hum Genet. 1991;49(4):707–14. [PMC free article] [PubMed] [Google Scholar]
- 43.des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, Gelot A, Dupuis E, Motte J, Berwald-Netter Y. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92(1):51–61. doi: 10.1016/s0092-8674(00)80898-3. others. [DOI] [PubMed] [Google Scholar]
- 44.Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92(1):63–72. doi: 10.1016/s0092-8674(00)80899-5. others. [DOI] [PubMed] [Google Scholar]
- 45.Keays DA, Tian G, Poirier K, Huang GJ, Siebold C, Cleak J, Oliver PL, Fray M, Harvey RJ, Molnar Z. Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell. 2007;128(1):45–57. doi: 10.1016/j.cell.2006.12.017. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bahi-Buisson N, Poirier K, Boddaert N, Saillour Y, Castelnau L, Philip N, Buyse G, Villard L, Joriot S, Marret S. Refinement of cortical dysgeneses spectrum associated with TUBA1A mutations. J Med Genet. 2008;45(10):647–53. doi: 10.1136/jmg.2008.058073. others. [DOI] [PubMed] [Google Scholar]
- 47.Poirier K, Keays DA, Francis F, Saillour Y, Bahi N, Manouvrier S, Fallet-Bianco C, Pasquier L, Toutain A, Tuy FP. Large spectrum of lissencephaly and pachygyria phenotypes resulting from de novo missense mutations in tubulin alpha 1A (TUBA1A) Hum Mutat. 2007;28(11):1055–64. doi: 10.1002/humu.20572. others. [DOI] [PubMed] [Google Scholar]
- 48.Pilz DT, Matsumoto N, Minnerath S, Mills P, Gleeson JG, Allen KM, Walsh CA, Barkovich AJ, Dobyns WB, Ledbetter DH. LIS1 and XLIS (DCX) mutations cause most classical lissencephaly, but different patterns of malformation. Hum Mol Genet. 1998;7(13):2029–37. doi: 10.1093/hmg/7.13.2029. others. [DOI] [PubMed] [Google Scholar]
- 49.Morris-Rosendahl DJ, Najm J, Lachmeijer AM, Sztriha L, Martins M, Kuechler A, Haug V, Zeschnigk C, Martin P, Santos M. Refining the phenotype of alpha-1a Tubulin (TUBA1A) mutation in patients with classical lissencephaly. Clin Genet. 2008;74(5):425–33. doi: 10.1111/j.1399-0004.2008.01093.x. others. [DOI] [PubMed] [Google Scholar]
- 50.Dobyns WB, Truwit CL, Ross ME, Matsumoto N, Pilz DT, Ledbetter DH, Gleeson JG, Walsh CA, Barkovich AJ. Differences in the gyral pattern distinguish chromosome 17-linked and X-linked lissencephaly. Neurology. 1999;53(2):270–7. doi: 10.1212/wnl.53.2.270. [DOI] [PubMed] [Google Scholar]
- 51.Forman MS, Squier W, Dobyns WB, Golden JA. Genotypically defined lissencephalies show distinct pathologies. J Neuropathol Exp Neurol. 2005;64(10):847–57. doi: 10.1097/01.jnen.0000182978.56612.41. [DOI] [PubMed] [Google Scholar]
- 52.Jissendi-Tchofo P, Kara S, Barkovich AJ. Midbrain-hindbrain involvement in lissencephalies. Neurology. 2009;72(5):410–8. doi: 10.1212/01.wnl.0000333256.74903.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martin ND, Walsh CA. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 2000;26(1):93–6. doi: 10.1038/79246. [DOI] [PubMed] [Google Scholar]
- 54.Boycott KM, Flavelle S, Bureau A, Glass HC, Fujiwara TM, Wirrell E, Davey K, Chudley AE, Scott JN, McLeod DR. Homozygous deletion of the very low density lipoprotein receptor gene causes autosomal recessive cerebellar hypoplasia with cerebral gyral simplification. Am J Hum Genet. 2005;77(3):477–83. doi: 10.1086/444400. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32(3):359–69. doi: 10.1038/ng1009. others. [DOI] [PubMed] [Google Scholar]
- 56.Bonneau D, Toutain A, Laquerriere A, Marret S, Saugier-Veber P, Barthez MA, Radi S, Biran-Mucignat V, Rodriguez D, Gelot A. X-linked lissencephaly with absent corpus callosum and ambiguous genitalia (XLAG): clinical, magnetic resonance imaging, and neuropathological findings. Ann Neurol. 2002;51(3):340–9. doi: 10.1002/ana.10119. [DOI] [PubMed] [Google Scholar]
- 57.Kato M, Das S, Petras K, Kitamura K, Morohashi K, Abuelo DN, Barr M, Bonneau D, Brady AF, Carpenter NJ. Mutations of ARX are associated with striking pleiotropy and consistent genotype-phenotype correlation. Hum Mutat. 2004;23(2):147–59. doi: 10.1002/humu.10310. others. [DOI] [PubMed] [Google Scholar]
- 58.Stromme P, Mangelsdorf ME, Shaw MA, Lower KM, Lewis SM, Bruyere H, Lutcherath V, Gedeon AK, Wallace RH, Scheffer IE. Mutations in the human ortholog of Aristaless cause X-linked mental retardation and epilepsy. Nat Genet. 2002;30(4):441–5. doi: 10.1038/ng862. others. [DOI] [PubMed] [Google Scholar]
- 59.Kato M, Saitoh S, Kamei A, Shiraishi H, Ueda Y, Akasaka M, Tohyama J, Akasaka N, Hayasaka K. A longer polyalanine expansion mutation in the ARX gene causes early infantile epileptic encephalopathy with suppression-burst pattern (Ohtahara syndrome) Am J Hum Genet. 2007;81(2):361–6. doi: 10.1086/518903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith DS, Niethammer M, Ayala R, Zhou Y, Gambello MJ, Wynshaw-Boris A, Tsai LH. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat Cell Biol. 2000;2(11):767–75. doi: 10.1038/35041000. [DOI] [PubMed] [Google Scholar]
- 61.Shu T, Ayala R, Nguyen MD, Xie Z, Gleeson JG, Tsai LH. Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron. 2004;44(2):263–77. doi: 10.1016/j.neuron.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka T, Serneo FF, Higgins C, Gambello MJ, Wynshaw-Boris A, Gleeson JG. Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J Cell Biol. 2004;165(5):709–21. doi: 10.1083/jcb.200309025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat Neurosci. 2007;10(8):970–9. doi: 10.1038/nn1934. [DOI] [PubMed] [Google Scholar]
- 64.Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23(2):247–56. doi: 10.1016/s0896-6273(00)80777-1. others. [DOI] [PubMed] [Google Scholar]
- 65.Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23(2):257–71. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 66.Horesh D, Sapir T, Francis F, Wolf SG, Caspi M, Elbaum M, Chelly J, Reiner O. Doublecortin, a stabilizer of microtubules. Hum Mol Genet. 1999;8(9):1599–610. doi: 10.1093/hmg/8.9.1599. [DOI] [PubMed] [Google Scholar]
- 67.D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374(6524):719–23. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 68.Caviness VS, Jr., Sidman RL. Time of origin or corresponding cell classes in the cerebral cortex of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol. 1973;148(2):141–51. doi: 10.1002/cne.901480202. [DOI] [PubMed] [Google Scholar]
- 69.Falconer D. 2 new mutants, trembler and reeler, with neurological actions in the house mouse (Mus musculus L.) J Genet. 1951;50:192–201. doi: 10.1007/BF02996215. [DOI] [PubMed] [Google Scholar]
- 70.Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, Kreidberg JA, Anton ES. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron. 2000;27(1):33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 71.Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24(2):481–9. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 72.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97(6):689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 73.Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, Quattrocchi CC, Antalffy BA, Sheldon M, Armstrong DD. Interaction of reelin signaling and Lis1 in brain development. Nat Genet. 2003;35(3):270–6. doi: 10.1038/ng1257. others. [DOI] [PubMed] [Google Scholar]
- 74.Zhang G, Assadi AH, McNeil RS, Beffert U, Wynshaw-Boris A, Herz J, Clark GD, D’Arcangelo G. The Pafah1b complex interacts with the Reelin receptor VLDLR. PLoS ONE. 2007;2(2):e252. doi: 10.1371/journal.pone.0000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McManus MF, Nasrallah IM, Pancoast MM, Wynshaw-Boris A, Golden JA. Lis1 is necessary for normal non-radial migration of inhibitory interneurons. Am J Pathol. 2004;165(3):775–84. doi: 10.1016/S0002-9440(10)63340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pancoast M, Dobyns W, Golden JA. Interneuron deficits in patients with the Miller-Dieker syndrome. Acta Neuropathol. 2005;109(4):400–4. doi: 10.1007/s00401-004-0979-z. [DOI] [PubMed] [Google Scholar]
- 77.Sheen VL, Ferland RJ, Harney M, Hill RS, Neal J, Banham AH, Brown P, Chenn A, Corbo J, Hecht J. Impaired proliferation and migration in human Miller-Dieker neural precursors. Ann Neurol. 2006;60(1):137–44. doi: 10.1002/ana.20843. others. [DOI] [PubMed] [Google Scholar]
- 78.Pawlisz AS, Mutch C, Wynshaw-Boris A, Chenn A, Walsh CA, Feng Y. Lis1-Nde1-dependent neuronal fate control determines cerebral cortical size and lamination. Hum Mol Genet. 2008;17(16):2441–55. doi: 10.1093/hmg/ddn144. [DOI] [PMC free article] [PubMed] [Google Scholar]