Abstract
Medtronic’s INFUSE Bone Graft provides surgeons with a potent tool for stimulating bone formation. Current delivery vehicles that rely on Absorbable Collagen Sponges (ACS) require excessive quantities of the active ingredient in INFUSE, recombinant human Bone Morphogenic Protein-2 (rhBMP2), to achieve physiologically relevant concentrations of the growth factor, driving up the cost of the product and increasing the likelihood of undesirable side effects in neighboring tissues. We demonstrate that a simple light-mediated, thiol-ene chemistry can be used to create an effective polymer delivery vehicle for rhBMP2, eliminating the use of xenografic materials and reducing the dose of rhBMP2 required to achieve therapeutic effects. Comprised entirely of synthetic components, this system entraps rhBMP2 within a biocompatible hydrogel scaffold that is degraded by naturally occurring remodeling enzymes, clearing the way for new tissue formation. When tested side-by-side with ACS in a critical-sized bone defect model in rats, this polymeric delivery system significantly increased bone formation over ACS controls.
Keywords: INFUSE Bone Graft, recombinant human Bone Morphogenic Protein-2 (rhBMP2), Absorbable Collagen Sponge (ACS), delivery vehicle, hydrogel
Introduction
Medtronic’s INFUSE Bone Graft products have revolutionized the medical field’s approach to targeted bone mass regeneration.1, 2 The active ingredient in INFUSE, recombinant human Bone Morphogenic Protein-2 (rhBMP2), is a naturally occurring growth factor that induces osteogenic differentiation and bone formation.3 The result is an aggressive activation of bone formation even in areas of the body where bone does not normally grow. This property has made rhBMP2 an excellent choice for procedures involving spinal fusions, where the growth factor is used to stimulate osteogenesis between two adjacent vertebrae.4 In addition, FDA approval has been granted for the use of INFUSE Bone Graft materials for tibial fractures as well as sinus and alveolar ridge augmentations. INFUSE kits include an Absorbable Collagen Sponge (ACS) made from collagen purified from bovine cartilage to serve as the delivery vehicle for the rhBMP2 solution. rhBMP2, resuspended with sterile water, is added dropwise to the ACS, which soaks up the protein solution, providing a construct that can be easily packed into bone defect areas during surgical procedures. For spinal fusion applications, the protein-loaded ACS is then packed into metal cages that immobilize the vertebrae while the rhBMP2 stimulates bone growth in the surrounding area, fusing the bones around the cage.
Although ACS has proven to be a viable delivery vehicle for rhBMP2, improved materials could reduce the adverse effects that are associated with xenographic ACS, enhance the potency of rhBMP2, and reduce clinical costs associated with the product.5, 6 Purified from bovine tissue, ACS naturally supports bone formation and can be degraded by the body as new tissue is formed. Due to its xenographic origin, however, bovine-derived ACS poses an inherent risk of eliciting adverse immunogenic responses that can complicate or delay bone formation in some patients. Additionally, delivery of rhBMP2 with ACS requires high concentrations of the growth factor in order to provide a therapeutic dose. Because rhBMP2 is an extremely potent growth factor that acts on a wide range of cell types, unintended side effects have been observed systemically and in surrounding tissues when the biological effects of the rhBMP2 extend beyond the desired defect sites.7, 8 The necessity to deliver high doses of rhBMP2 also significantly increases the cost of the INFUSE product, making it prohibitively expensive for many clinical applications.
In the work presented here, we demonstrate that a simple synthetic extracellular matrix mimic can be used to create an effective polymer delivery vehicle for rhBMP2. Using a light-initiated chemistry, peptide functionalized poly(ethylene glycol) networks can be created to provide a versatile alternative to xenografic materials. Furthermore, these networks have the additional benefit of reducing the rhBMP2 dose required to achieve therapeutic effects. Comprised entirely of synthetic components, this system entraps rhBMP2 upon exposure to 365nm light within a biocompatible hydrogel matrix that can be placed within bone defects. By using peptide crosslinkers that are be cleaved by naturally occurring matrix metalloproteinases, the polymer is easily degraded and cleared the body. When tested side-by-side with ACS in a critical-sized bone defect model in rats, this polymeric delivery system resulted in significantly increased bone formation over ACS controls. In fact, thiol-ene hydrogels delivering 0.2µg rhBMP2 showed roughly equivalent bone formation as ACS loaded with 2.0µg rhBMP2, a ten-fold decrease in the therapeutic range of the INFUSE product.
Methods
INFUSE preparation and calculations
An XX-small INFUSE kit was purchased from Medtronics. As per the manufacturer’s instructions, 0.9ml of sterile water was used to resuspend the lyophilized rhBMP2, making a 1.5mg/ml stock solution. According to the clinical instructions, 0.7ml of this solution is then to be applied to an Absorbable Collagen Sponge (ACS) having a top surface area of 6.24cm2 based on its rectangular dimensions of 1.3cm (width) X 4.8cm (length). An 8mm diameter circular calvaria defect would have a surface area of 0.50cm2 (area = πr2), requiring 56µl of the rhBMP2 solution based on the ratio of 0.7ml/6.24cm2 (above) used for the clinical applications. According to the FDA filings by Medtronic4, the therapeutic dose for the INFUSE Bone Graft product in rodents was determined to be 0.025–0.05mg/ml, which equates to 1.4–2.8µg of INFUSE per 8mm calvaria defect (56µl X 0.025–0.05mg/ml = 1.4–2.8µg). To fit within this therapeutic range, we chose to deliver 2.0µg as the highest dose.
ACS preparation and use
An 8mm biopsy punch was used to create discs from of the ACS supplied in the INFUSE kit. Based on the manufacturer’s instructions (see above), 56µl of the rhBMP2 solution was applied to the ASC disc 10–15 minutes before transferring the soaked disc into the defect. The stock solution of INFUSE was diluted with the supplied sterile water such that either 2.0µg, 0.2µg, or 0µg of rhBMP2 would be delivered to the defect site with the ACS.
Hydrogel preparation and use
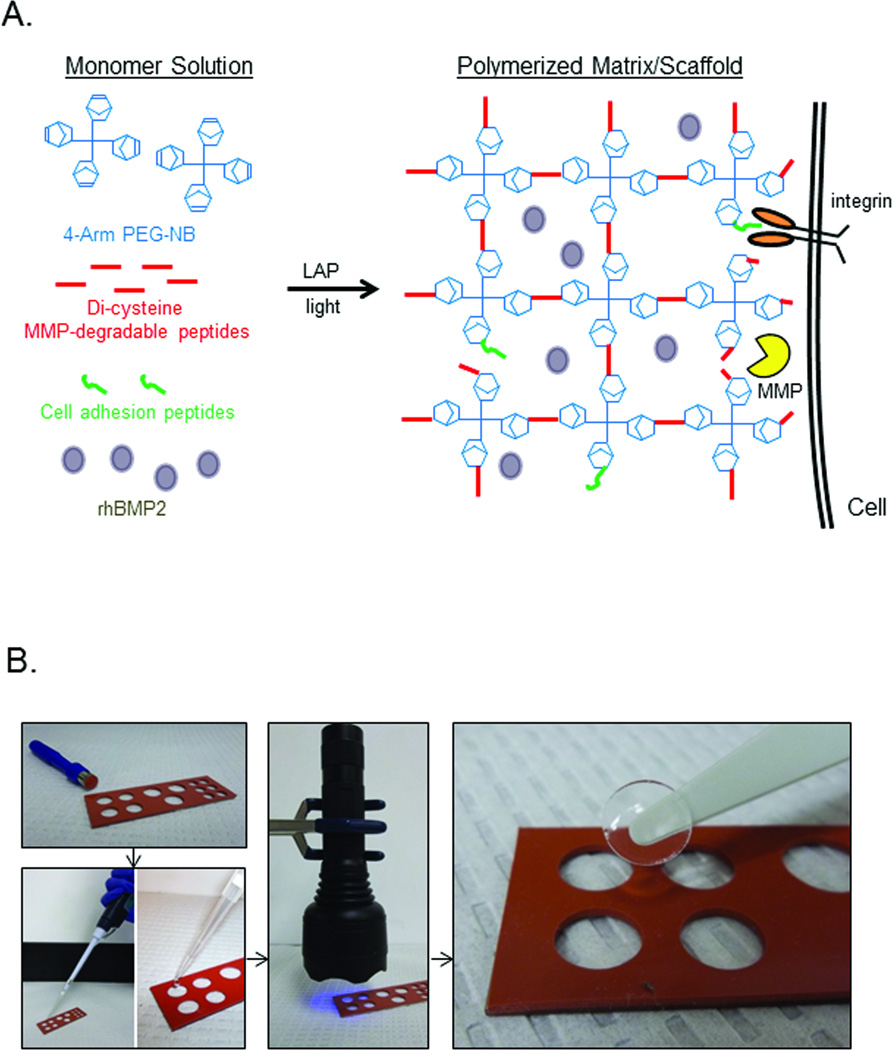
Monomer solutions were prepared in PBS containing 6%wt/vol 20K 4-arm poly(ethylene glycol)-norbornene (PEG-NB), 1mM adhesion peptide (CRGDS), 5.5mM di-cysteine MMP-degradable crosslinker peptide (KCGPQGIAGQCK), and 0.01% of the photoinitiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP), and 15%wt/vol glycerol as previously described.9 The solution was then passed through a 0.2µm sterile filter. To create each hydrogel disc, 70µl of the monomer solution (a roughly equivalent volume as 56µl INFUSE solution + ACS) was placed into a mold and placed on a clean glass slide (Figure 1B). Polymerization of the hydrogels was accomplished by shining a handheld LED flashlight over the solution for 1 minute. For hydrogels carrying rhBMP2, the INFUSE solution was added to the monomer mix, vortexed to achieve an even distribution of the growth factor at 2.0µg, 0.2µg, or 0µg of rhBMP2, and then transferred to the mold (Figure 1B). Upon exposure to light, the hydrogel polymerizes around the rhBMP2, effectively encapsulating the growth factor within the synthetic matrix.
Figure 1.
Synthetic hydrogel delivery system. (A) Monomer solutions containing three basic polymer components (4-arm PEG backbone functionalized with norbornene groups, MMP-degradable peptide containing, and short cell adhesion peptides) are polymerized by a photoinitiated reaction to create a synthetic hydrogel matrix. For the studies described in this work, rhBMP2 was added to the monomer solution before polymerization. (B) Hydrogel discs implanted within the calvaria defects were created by polymerizing the monomer solutions in molds created with an 8mm biopsy punch. Monomer solutions with different concentrations of rhBMP2 were applied as a liquid to the molds; 385nm light was used to polymerize the discs, which were then removed from the molds and placed within the defect site.
Rat cranial surgery
All work performed with animals was done with the approval and guidance of the University of Colorado Denver IACUC. 8–10 week-old male Sprague-Dawley rats were anesthetized by intraperitoneal injection of ketamine hydrochloride (40 mg/kg) and xylazine (10 mg/kg). After shaving the hair covering the top of the head, an incision was made along the sagittal suture. The periosteum was then elevated from the skull, and an 8 mm circular calvaria bone defect was created with a 4mm coarse diamond bur (Stryker). The defect was then filled with either ACS or hydrogel discs followed by closure of the periosteum and skin with resorbable sutures. In untreated control animals, the periosteum and skin were closed without applying materials to the calvaria defect. Six animals per treatment group were used, and microCT data for each animal is presented in Supplemental Figure 2.
microCT and Image Processing
To non-invasively assess new bone formation over the time course of the study, micro-Computed Tomography (micro-CT) was used. Each animal was scanned within 4 days of the initial surgery to establish a baseline defect area (Week 0). Each animal was re-scanned after 2 and 6 weeks. Segmentation and analysis were guided by a 3D rat skull template (Supplemental Figure 1), created from Gaussian smoothed (3D, 1mm FWHM) and aligned coronal microCT data at a resolution of .16×.16×.16 mm3. Rough alignment with the template was achieved using array shifting in x, y and z to translate the data, followed by evaluation of a mean squared error cost function to assess alignment. Refined co-registration with the template was achieved through affine transformations (translation and rotation), with a cross-correlation cost function used to assess alignment. A 3×3×3 voxel median filter was then applied to the dataset to reduce noise while preserving edges. Interior skull voxels near the location of bone defect were identified and used as points to form a 3D spline surface spanning regions of missing bone. A threshold of approximately 918 HU (filtered data) was used to identify the voxels, based on visual inspection of prior datasets and bone thresholds. The lack of bone regeneration in the skull area was calculated based on the spline surface and absence of bone in the direction of the dorsal surface of the cranium. Histogram information (unfiltered data) was obtained from a volume of interest (VOI) surrounding the bone removal site, approximately 15.5 × 12 × 20 mm3. The histogram was binned in units of 10 HU and extends from −1000 HU to 3500 HU. Mean, variance, skewness and kurtosis were also calculated.
Statistical analysis
Data presented for defect area closure and bone volume measurements was performed using a two-way ANOVA. Post hoc multiple comparisons at each time point were made using a Fisher PLSD test. p values for critical comparisons are described in the text or figure legends.
Tissue processing and staining
Rat skull caps were dissected and placed into 10% Neutral buffered formalin (NBF; Fisher Scientific) for a minimum of 7 days before gross dissection. Each skull was decalcified with Poly-NoCal solution (Polysciences). The tissues were then placed into fresh NBF for an additional 24 hours post decalcification to complete the fixation and processed into paraffin (Richard Allen #9, Fisher) utilizing a Tissue Tek VIP processor (Sakura). The modified overnight schedule was performed in the following sequence: NBF; 30 minutes, 70% ethanol; 10 minutes, 95% ethanol; 30 minutes X 3, 100% ethanol; 30 minutes, 45 minutes, 60 minutes, Xylene; 60 minutes X 2, and Paraffin; 60 minutes X 4. Five micron thick sections were cut on a rotary microtome (Model RM2025, Leica) and mounted onto plus charged slides. After baking for 1 hour at 60°C, slides were stained with Masson’s trichrome.10 Slides were scanned at 40X with the ScanScope XT (Aperio, Vista, CA) and digital images were generated using Aperio ImageScope software.
Results
Rational design of hydrogel vehicle
As diagramed in Figure 1A, 3-dimensional hydrogel tissue scaffolds used in these studies were constructed with three basic components: a norbornene-functionalized PEG backbone (PEG-NB), a degradable peptide crosslinker containing two cysteine functional groups (KCGPQGIAGQCK), and a cell adhesion peptide sequence (CRGDS). Formation of the network is accomplished by a light-initiated reaction, allowing the user to dictate precisely when and where the polymerization occurs (Figure 1B). For these studies , the peptide crosslinker was designed to be susceptible to enzymatic cleavage by cell-secreted matrix metaloproteinases (MMPs)11, allowing for invading cells to degrade the matrix and solubilize the scaffold as new matrix is deposited. Short, bioactive peptides containing a fibronectin mimic, CRGDS, were also incorporated into the polymer matrices to promote both cell spreading and motility.12 As the matrix is degraded, PEG-peptide fragments are generally cleared by the body and do not appear to persist within the defect site.13, 14
Hydrogel discs (8mm diameter, 1.4mm thick) were pre-polymerized in molds as illustrated in Figure 1B. Immediately following polymerization, the hydrogel discs were removed from the molds and placed within the calvaria defects. For INFUSE controls, 8mm biopsy punches were used to punch discs out of the supplied ACS, to which the rhBMP2 solution was applied 10–15 minutes before transferring the soaked discs into the defects.
INFUSE delivered from synthetic hydrogels increases bone defect closure
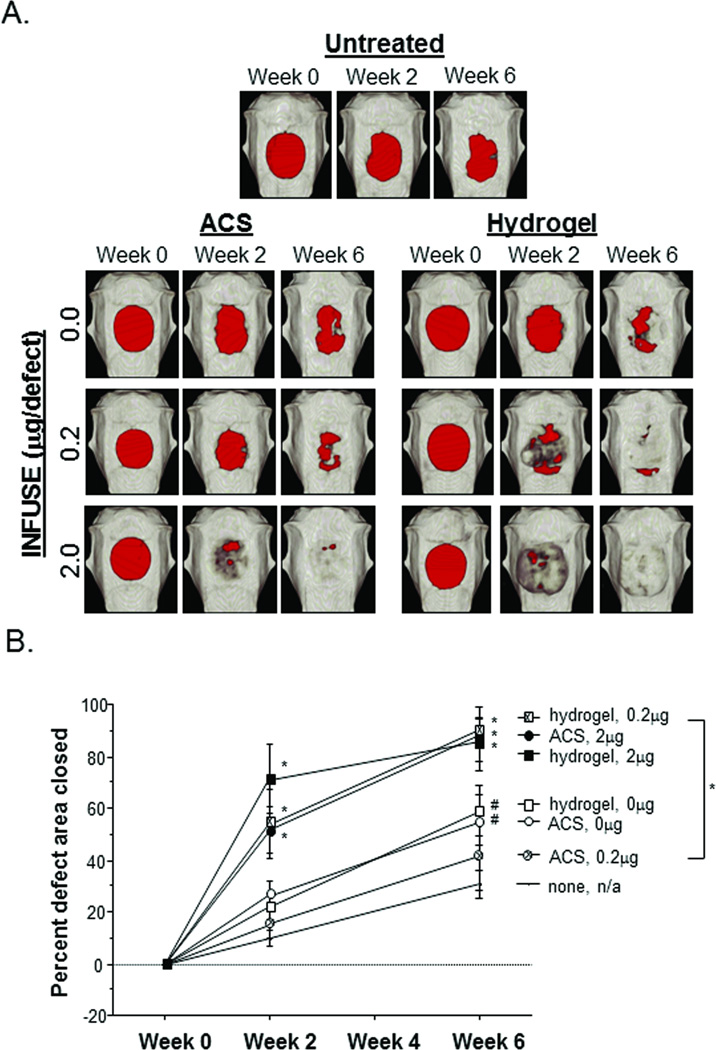
Representative scans shown in Figure 2A clearly identify the original bone defects (red areas) created in the calvaria of each animal. Some obvious difference can be seen in the size and shape of the original defects, but each of the defects was of sufficient area to be considered critical-sized (i.e., too large for complete bone regeneration if left untreated). Reconstructions from the remaining animals are presented in Supplemental Figure 2, and quantification of the defect area over time is presented in Figure 2B.
Figure 2.
Calvaria defect closure. (A) Representative images from each experimental group are presented to illustrate defect closure over the 6 week study. Areas that do not meet threshold levels of optical density consistent with bone are represented in red. (B) Week 0 defect area measurement was considered the original defect size, providing a reference for percent defect area closed. Statistical comparisons were made with the untreated controls, and error bars represent 95% Confidence Intervals; # p ≤ 0.05, * p ≤ 0.01.
As can be easily observed in Figure 2, defects treated with MMP-degradable hydrogels containing rhBMP2 had significantly faster rates of closure when compared to defects treated with hydrogels alone. While these data do not provide quantitative evidence of the percentage of rhBMP2 that remains active through the encapsulation and delivery with the hydrogels, there were clearly sufficient levels of active protein to have a physiological effect and stimulate bone growth within the defect area. Moreover, hydrogel delivery of the rhBMP2 resulted in significantly faster defect closure at a ten-fold lower INFUSE dose, as shown in defects treated with hydrogel + 0.2µg rhBMP2 closing at a similar rate to defects treated with ACS + 2.0µg rhBMP2.
Hydrogels result in more bone volume regenerated per dosage of rhBMP2
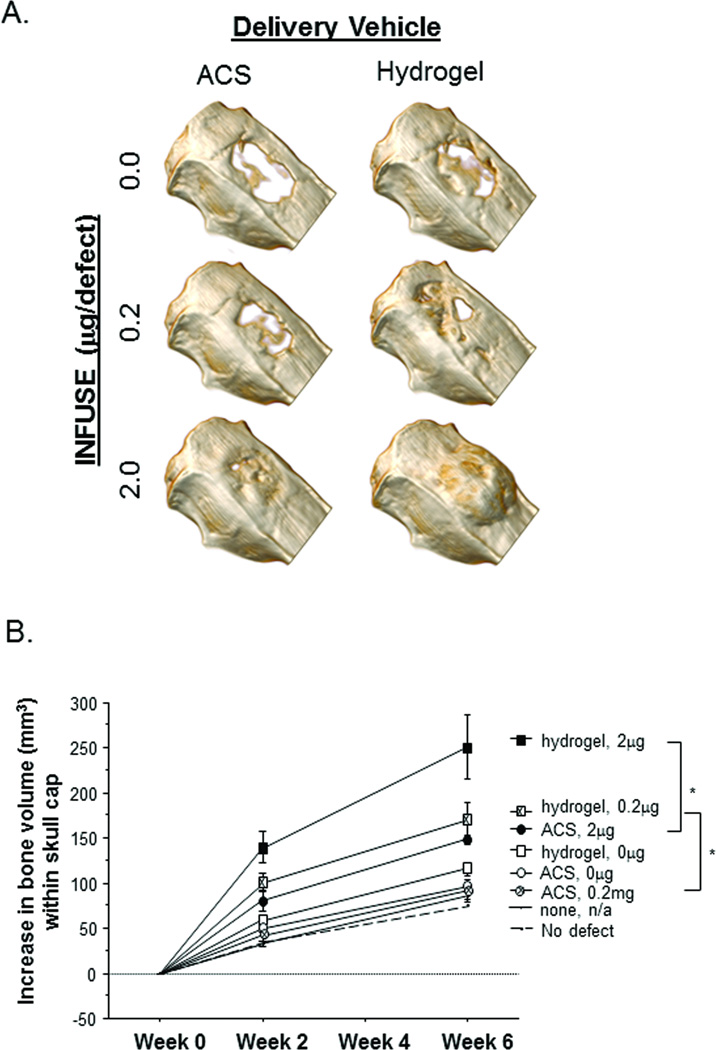
The top-down images presented in Figure 2A provide additional insight into the increased efficacy of hydrogel-delivered rhBMP2 on bone formation. In the images of the defect treated with 2.0µg rhBMP2 via hydrogel, bone growth appears to extend well beyond the original defect area, suggesting that excessive bone formation occurs. This effect is better observed in 3D CT renderings displaying a different vantage point (Figure 3A), in which a side view provides a clear picture of the excessive bone mass that was generated in the animals receiving 2.0µg of hydrogel-delivered rhBMP2.
Figure 3.
Skull cap bone volume. (A) Representative 3D rendering at Week 6 from each experimental group are presented to illustrate bone formation at the defect site. Extraneous bone generation is observed in animals treated with hydrogels containing 2.0µg rhBMP2. (B) Quantification of new bone volume within skull cap sections. Week 0 skull cap bone volume is subtracted from the Week 2 and Week 6 measurements to represent new bone formation. Statistical comparisons were made with the untreated controls, and error bars represent 95% Confidence Intervals; * p ≤ 0.01.
Whereas rates of defect closure resulting from a dosage of 2.0µg of rhBMP2 were comparable between hydrogel and ACS delivery vehicles, this metric does not take into account the quantity of bone formation that occurs within the 3-dimensional space. By quantifying the volume of bone within the each skull cap, significant differences were observed between hydrogel and ACS delivery of rhBMP2 over the 6 week study (Figure 3B). Increased bone volume resulting from hydrogel delivery was also observed at the 0.2µg dose, with similar levels to ACS + 2.0µg rhBMP2.
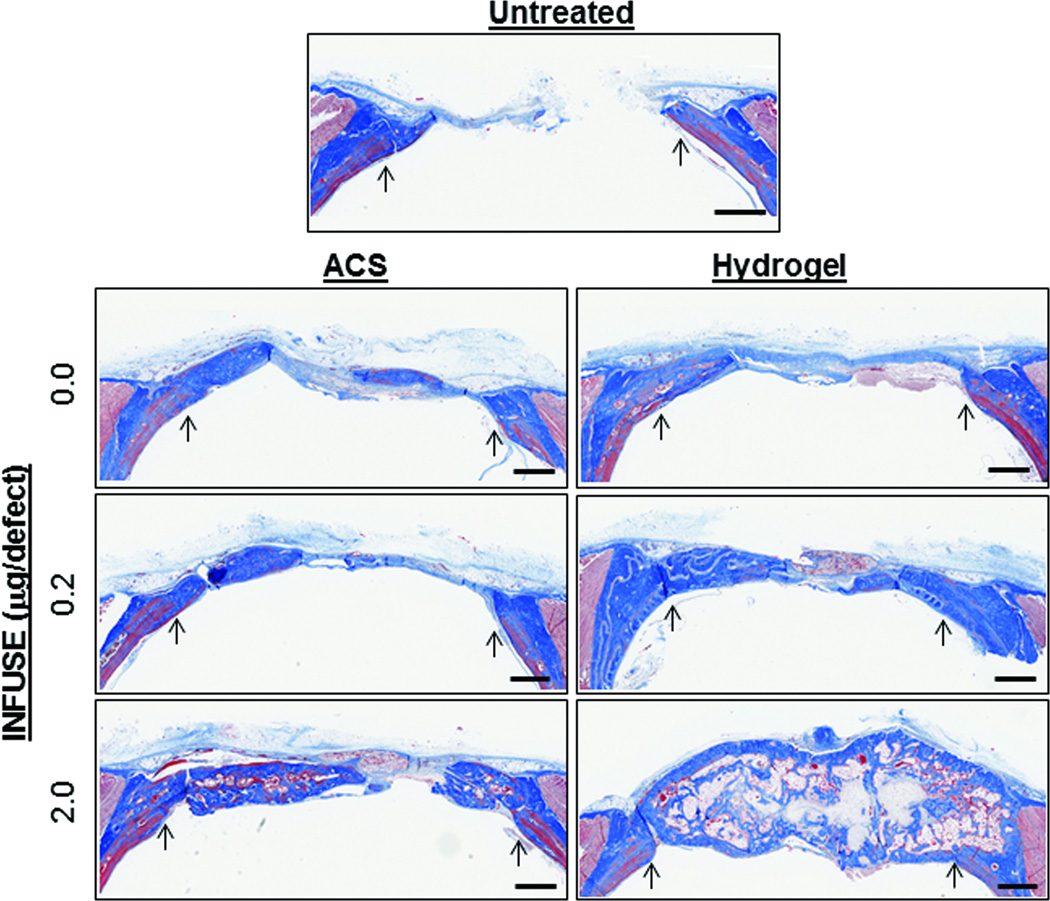
Hydrogel replaced by new tissue
While the CT images presented in Figures 2 and 3 suggest that bone tissue had replaced the ACS and hydrogel carriers with new tissue, histological sections were prepared to evaluate the quality of the bone tissue that had filled the defect sites. As shown in Figure 4, cross sections of decalcified skulls show significant collagen deposition (stained blue) within the defect sites that appeared to correspond to mineralized tissue when looking at calcified sections (Supplemental Figure 3A). Distinct differences in the organization of the collagen fibers can be observed between old and new tissues, suggesting that the newly formed bone does not fully reproduce the natural state and, thus, may not reach an equal strength. However, the appearance of newly formed bone in the animals treated with 2.0µg rhBMP2 + ACS was similar to that of the animals treated with 0.2µg rhBMP2 + hydgrogel (Supplemental Figure 3B), suggesting that the use of the synthetic hydrogel to deliver rhBMP2 did not have a deleterious effect on tissue formation when compared to FDA-approved ACS.
Figure 4.
Histological evaluation of bone regeneration. Coronal tissue sections through bone defect sites were prepared from de-calcified skulls taken upon completion of the study. Representative sections stained with Masson’s Trichrome are shown with arrows pointing to original defect edge. Additional histology images are presented in Supplemental Figure 3. Scale bars represent 1mm.
For animals treated with the hydrogels carrying 2.0µg rhBMP2, the large bone masses that grew beyond the normal skull area appeared to contain a dense cortical layer of bone along the interior and exterior surfaces, with cancellous bone filling the volume in between. Furthermore, there appeared to be small pockets of sparse cellularity toward the center of the bone mass which may represent residual hydrogel material that has not been fully degraded. Even if some of the material remains in the newly formed bone, most of the hydrogel disc is no longer intact, indicating that it has been cleared as new mineralized matrix is formed. No evidence of hydrogel material was observed in in histological sections from animals treated with hydrogels containing no rhBMP2 or 0.2µg rhBMP2, suggesting that higher doses of the growth factor may alter the progression of tissue formation, perhaps impeding rapid degradation of the matrix.
Discussion
The incredible simplicity of the thiol-ene polymerization hydrogel system makes it a versatile platform for designing tissue scaffolds that release rhBMP2 to promote bone regeneration. As shown in the work presented here, the mild reaction conditions used to polymerize the matrices do not significantly affect the ability of the encapsulated growth factor to induce a potent tissue response. Although we did not directly measure the biological activity of the rhBMP2 encapsulated within the matrices, the fact that increased bone formation was observed when hydrogels were used to deliver the growth factor in vivo suggests that growth factor activity remains and that any incremental loss in activity was clearly overcome by the superior delivery vehicle. This is further supported by similar results encapsulating active proteins using the thiol-ene chemistry.15
Initial studies performed by our group to examine these scaffolds as delivery vehicles for rhBMP2 did not show an appreciable effect on bone growth.13 The quantities of rhBMP2 used in these studies (5ng/defect), however, were well below therapeutic doses reported in preclinical studies by Medtronic, which would explain the lack of bone formation observed in rhBMP2-treated defects. According to the FDA filings by Medtronic 4, the therapeutic dose for the INFUSE Bone Graft product in rodents was determined to be 0.025–0.05mg/ml, which equates to 1.4–2.8µg of INFUSE per 8mm calvaria defect, roughly 400 times the amount used in our first set of experiments. In order to fit within this dosing range, we chose to deliver 2.0µg of rhBMP2 per defect for the work presented here. In addition, we purchased an INFUSE kit from Medtronic instead of research grade material from R&D Systems, allowing us to test a clinically available source of rhBMP2, as well as compare our polymer system to a clinically relevant delivery vehicle in a side-by-side experiments. As the data clearly demonstrate, our polymer delivery system is an effective vehicle for rhBMP2. Defects receiving rhBMP2 encapsulated in our matrices showed significant increases in bone formation within the defect area.
While the ability of our polymer system to effectively deliver rhBMP2 was expected, the significant increase in bone formation over ACS vehicle controls was not. Previous work from the Hubbell Group showed that similar polymer matrices could be used to deliver active rhBMP2 to critical-sized calvaria defects in rats, but little difference was observed between synthetic matrices and the ACS controls delivering the same quantity of BMP-2 (5µg/defect).16 In the work presented here, significant increases in bone volume were measured in rats receiving hydrogels encapsulating only 0.2 µg rhBMP2, and the hydrogel carrier clearly outperformed ACS controls. A shown in Figure 3, abnormal boney masses formed at the defect sites that received 2.0µg of rhBMP2 delivered with the synthetic matrix, suggesting that the 2.0µg dose was well above the therapeutic range. For ACS controls, however, a dose of 2.0µg/defect appeared to be within therapeutic range as would be expected from Medtronic’s preclinical data.
It remains unclear why our peptide-functionalized hydrogels formed via thiol-ene polymerization appear to have an increased efficacy over hydrogels of very similar composition. Although no direct comparisons were made between our hydrogels and other PEG-based polymers, our thiol-ene system resulted in increased biological activity of the rhBMP2 over ACS controls, an effect not observed in a similar system.16 One obvious difference between these polymer systems is the chemical mechanism used to initiate polymerization. While our thiol-ene hydrogels are photopolymerized using a free-radical mechanism, the hydrogels used in previous studies were created with a Michael-Type addition reaction. It is possible that some portion of the BMP is rendered inactive though unintended side reactions that occur during Michael-Type reactions that do not occur during the free radical reactions employed to create the hydrogels used in this study. If these side reactions involve the BMP, a drop in biologically active growth factor might be expected. It should be noted, of course, that deleterious side reactions may also occur in the free radical system, but the frequency of these reactions must be relatively low and/or do not completely offset the apparent benefits that the hydrogel system has since bone formation is actually increased over ACS controls.
Although we were encouraged by the results showing that our materials can reduce the therapeutic range of rhBMP by 10-fold, the extraneous bone formation observed in animals receiving the 2.0µg dose of rhBMP2 the indicates that continued development of this hydrogel system will be necessary to ensure that bone formation remains localized to desired locations within the body. One distinct benefit of this polymer system is its ability to be easily modified and tuned via systematic variations in the building-block components that are used to make the final matrices. As such, the versatile nature of this polymer system provides a broad platform for the development of unique delivery devices of varying complexities.
Supplementary Material
Acknowledgements
This work was supported by funding from the Howard Hughes Medical Institute and the NIH (5R01DE016523, 5R21AR057904).
Footnotes
The authors have no conflicts of interest to disclose regarding this work.
References
- 1.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) International orthopaedics. 2007;31(6):729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szpalski M, Gunzburg R. Recombinant human bone morphogenetic protein-2: a novel osteoinductive alternative to autogenous bone graft? Acta orthopaedica Belgica. 2005;71(2):133–148. [PubMed] [Google Scholar]
- 3.Valdes MA, Thakur NA, Namdari S, Ciombor DM, Palumbo M. Recombinant bone morphogenic protein-2 in orthopaedic surgery: a review. Archives of orthopaedic and trauma surgery. 2009;129(12):1651–1657. doi: 10.1007/s00402-009-0850-8. [DOI] [PubMed] [Google Scholar]
- 4.Summary of Safety and Efficacy Data: Pre-Market Approval of InFUSE Bone Graft/LT-CAGE™ Lumbar Tapered Fusion Device. Food and Drug Administration; 2002. [Google Scholar]
- 5.King GN. The importance of drug delivery to optimize the effects of bone morphogenetic proteins during periodontal regeneration. Current pharmaceutical biotechnology. 2001;2(2):131–142. doi: 10.2174/1389201013378716. [DOI] [PubMed] [Google Scholar]
- 6.Schmidmaier G, Schwabe P, Strobel C, Wildemann B. Carrier systems and application of growth factors in orthopaedics. Injury. 2008;39(Suppl 2):S37–S43. doi: 10.1016/S0020-1383(08)70014-7. [DOI] [PubMed] [Google Scholar]
- 7.Robin BN, Chaput CD, Zeitouni S, Rahm MD, Zerris VA, Sampson HW. Cytokine-mediated inflammatory reaction following posterior cervical decompression and fusion associated with recombinant human bone morphogenetic protein-2: a case study. Spine. 2010;35(23):E1350–E1354. doi: 10.1097/BRS.0b013e3181e85756. [DOI] [PubMed] [Google Scholar]
- 8.Latzman JM, Kong L, Liu C, Samadani U. Administration of human recombinant bone morphogenetic protein-2 for spine fusion may be associated with transient postoperative renal insufficiency. Spine. 2010;35(7):E231–E237. doi: 10.1097/BRS.0b013e3181c71447. [DOI] [PubMed] [Google Scholar]
- 9.Fairbanks BD, Schwartz MP, Bowman CN, Anseth KS. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials. 2009;30(35):6702–6707. doi: 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carson FL. Histotechnology: A Self-Instructional Text. Second Edition. Chicago: ASCP Press; 1997. [Google Scholar]
- 11.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31(30):7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 12.Rezania A, Healy KE. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnology progress. 1999;15(1):19–32. doi: 10.1021/bp980083b. [DOI] [PubMed] [Google Scholar]
- 13.Terella A, Mariner P, Brown N, Anseth K, Streubel SO. Repair of a calvarial defect with biofactor and stem cell-embedded polyethylene glycol scaffold. Archives of facial plastic surgery : official publication for the American Academy of Facial Plastic and Reconstructive Surgery, Inc. and the International Federation of Facial Plastic Surgery Societies. 2010;12(3):166–171. doi: 10.1001/archfacial.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webster R, Didier E, Harris P, Siegel N, Stadler J, Tilbury L, Smith D. PEGylated proteins: evaluation of their safety in the absence of definitive metabolism studies. Drug metabolism and disposition: the biological fate of chemicals. 2007;35(1):9–16. doi: 10.1124/dmd.106.012419. [DOI] [PubMed] [Google Scholar]
- 15.Aimetti AA, Machen AJ, Anseth KS. Poly(ethylene glycol) hydrogels formed by thiol-ene photopolymerization for enzyme-responsive protein delivery. Biomaterials. 2009;30(30):6048–6054. doi: 10.1016/j.biomaterials.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nature biotechnology. 2003;21(5):513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.