Abstract
Participants’ eye movements were monitored in an experiment that manipulated the frequency of target words (high vs. low) as well as their availability for parafoveal processing during fixations on the pre-target word (valid vs. invalid preview). The influence of the word-frequency by preview validity manipulation on the distributions of first fixation duration was examined by using ex-Gaussian fitting as well as a novel survival analysis technique which provided precise estimates of the timing of the first discernible influence of word frequency on first fixation duration. Using this technique, we found a significant influence of word frequency on fixation duration in normal reading (valid preview) as early as 145 ms from the start of fixation. We also demonstrated an equally rapid non-lexical influence on first fixation duration as a function of initial landing position (location) on target words. The time-course of frequency effects, but not location effects was strongly influenced by preview validity, demonstrating the crucial role of parafoveal processing in enabling direct lexical control of reading fixation times. Implications for models of eye-movement control are discussed.
Keywords: Eye movements, Reading, Lexical processing, Word frequency, Parafoveal preview, Direct control, Initial landing position, Fixation location, Fixation duration
1. Introduction
The human visual system compensates for its lack of high resolution outside of the fovea by making eye and head movements. During the performance of complex visual tasks such as reading, high-velocity saccadic eye movements, during which vision is largely suppressed (Matin, 1974), serve to align the fovea with the part of the text that is being encoded by the reader. Reading saccades, which occur at an average rate of 3–4 per second, are separated by fixations during which the eyes are relatively still and perceptual information is extracted (see Rayner (1998, 2009a)) for reviews).
Given the vital role of eye movements in selecting the location of the text to be foveated (i.e., fixation position within words) as well as delimiting the duration of each foveation (i.e., fixation duration), researchers have examined the factors that influence when the eyes move as well as where they move. Over the past three decades, this investigation of the nature of eye-movement control in reading generated a substantial body of findings as well as considerable controversy (see Rayner (1998, 2009a)) and Starr and Rayner (2001) for reviews). Early models of eye movement control in reading differed dramatically with respect to the hypothesized role of ongoing lexical and comprehension processes in controlling eye movements. Oculomotor theories (e.g., McConkie, Kerr, Reddix, & Zola, 1988; McConkie, Kerr, Reddix, Zola, & Jacobs, 1989; O’Regan, 1990, 1992) assumed that non-lexical, low-level visual information and oculomotor constraints jointly determine eye-movement control in reading. In contrast, processing theories (e.g., Henderson & Ferreira, 1990; Just & Carpenter, 1980; Kennison & Clifton, 1995; Morrison, 1984; Rayner & Pollatsek, 1989) advocated a critical role for lexical and attentional processes.
In addition to the lexical vs. non-lexical dichotomy, models of eye-movement control of fixation times in reading often refer to the distinction between direct vs. indirect control. Direct control refers to the assumption that the processing of the properties of the fixated word (wordn) influences the timing of the saccade terminating that fixation, regardless of whether this processing was initiated while wordn was foveated or when it was parafoveally processed during fixations on the pre-target word (wordn−1). Consequently, direct control is by definition an immediate fixation-by-fixation adjustment based on the properties of the local stimulus (i.e., wordn). Direct control of fixation times is often contrasted with a variety of indirect control mechanisms that are assumed to cause the eyes to continue to move forward at a rate that (on average) allows enough time to encode the text. Importantly, if the triggering of the saccade that terminates a fixation on wordn was caused by indirect control, then the duration of that fixation is assumed not to be influenced by any lexical or non-lexical properties of wordn. Thus, in order to allow for optimal encoding of the text, indirect control is often postulated to involve a delayed adjustment (i.e., non-real-time) of fixation times that is typically based on the average processing difficulty encountered by the reader and other contextual factors (i.e., global control). Thus, in the context of the eye-movement control literature, the direct/indirect dichotomy often incorporates the immediate/delayed and local/global distinctions. It is important to note that the above use of the terms “direct” and “indirect” is not consistent with the more colloquial use of the terms to refer to whether or not some behavior is mediated by intervening stages or mechanisms. Given that there is an unknown number of synaptic junctions between the cortical systems that support cognition and the brainstem circuitry that is ultimately responsible for moving the eyes, our usage of the direct/indirect distinction reflects our decision to remain agnostic about the degree to which the mechanisms that control eye movements in reading are direct or indirect in the more colloquial sense (i.e., non-mediated vs. mediated).
The nature of the debate concerning the eye-movement control of fixation times in reading has become more complex in recent years with several models incorporating a combination of direct and indirect control mechanisms (sometimes referred to as mixed control models; e.g., Rayner & Pollatsek, 1981). Furthermore, although it is now generally accepted that fixation times are influenced by lexical and/or linguistic variables such as word frequency (Inhoff & Rayner, 1986; Rayner & Duffy, 1986; see White (2008) for a review), contextual constraint or predictability (Ehrlich & Rayner, 1981; Rayner, Ashby, Pollatsek, & Reichle, 2004; Rayner & Well, 1996), lexical ambiguity (e.g., Duffy, Morris, & Rayner, 1988; Rayner & Duffy, 1986; Sheridan, Reingold, & Daneman, 2009; see Duffy, Kambe, and Rayner (2001) for a review) and age of acquisition (e.g., Juhasz & Rayner, 2006), there remains an intense disagreement as a result of differing assumptions concerning both the time course of lexical influences and the proportion of reading fixations that are impacted by lexical processing. Specifically, recent models advocating primarily visual/oculomotor control of fixation times in reading (e.g., Deubel, O’Regan, & Radach, 2000; Feng, 2006; McConkie & Yang, 2003; Yang, 2006; Yang & McConkie, 2001) assume that lexical effects are limited to a small subset of long fixations and that the vast majority of reading fixations are unaffected by these variables. In marked contrast, other recent models suggest that lexical and linguistic processes play a non-trivial role in controlling fixation times in reading (e.g., Engbert, Longtin, & Kliegl, 2002; Engbert, Nuthmann, Richter, & Kliegl, 2005; Just & Carpenter, 1980, 1987; Kliegl & Engbert, 2003; McDonald, Carpenter, & Shillcock, 2005; Morrison, 1984; Pollatsek, Reichle, & Rayner, 2006; Rayner, Liversedge, White, & Vergilino-Perez, 2003; Reichle, Pollatsek, Fisher, & Rayner, 1998; Reichle, Pollatsek, & Rayner, 2006; Reichle, Rayner, & Pollatsek, 1999, 2003; Reilly & Radach, 2003, 2006; Reingold, Yang, & Rayner, 2010; Richter, Engbert, & Kliegl, 2006; Thibadeau, Just, & Carpenter, 1982; White, 2008). Thus, conflicting assumptions concerning the time-course of the influence of lexical variables on fixation times constitute a key differentiator between various theories of eye-movement control in reading.
A comprehensive review of models of eye-movement control in reading is beyond the scope of the present manuscript (see the 2006 special issue of Cognitive Systems Research). However, in order to illustrate the complexity of current models, Table 1 introduces a 2 × 2 classification of eye-movement control mechanisms based on the type of control that is assumed (direct vs. indirect) and the type of underlying information mediating the control (lexical vs. non-lexical). It is important to emphasize that this taxonomy provides a basis for classifying the mechanisms that control eye movements during reading, and not the models themselves, which as Table 1 shows, often incorporate more than a single mechanism. As discussed above, the ongoing controversy concerning eye-movements control in reading is primarily focused on one of the four cells shown in Table 1, namely the hypothesized category of direct lexical mechanisms. Accordingly, the primary goal of the present study was to provide a strong test for the validity of the direct lexical control hypothesis (e.g., Rayner & Pollatsek, 1981; Rayner, Sereno, & Raney, 1996; see Rayner (1998, 2009b) for reviews), which states that the influence of lexical variables such as word frequency is rapid enough to impact the duration of most fixations. In addition, we explored the potential role of parafoveal processing in enabling direct lexical control despite the tight temporal constraints caused by neural delays in the perceptual and oculomotor systems. Accordingly, we begin by briefly reviewing two types of direct control mechanisms that are incorporated into current models of eye-movement control in reading. We then consider the temporal constraints that direct control models must satisfy in order to be viable. Next, we outline the rationale of present methodology and report on the findings from an experiment that manipulated the frequency of the target words as well as the availability of the target words for parafoveal processing during fixations on the pre-target word. Finally, we explore the implications of our results for models of eye-movement control of fixation times in reading.
Table 1.
Brief descriptions of proposed eye-movement control mechanisms, arranged along two dimensions that determine when saccadic programming is initiated. The type of control mechanism can be direct or indirect, and the type of information mediating control can be lexical or non-lexical.
| Control type |
Information type |
Examples |
|---|---|---|
| Direct | Lexical | • Completion of some stage of lexical processing initiates saccade (e.g., Just & Carpenter, 1980; Morrison, 1984; Rayner & Pollatsek, 1989; Reichle et al., 1998; Reilly, 1993; Salvucci, 2001) |
| • Lexical processing difficulty inhibits saccade initiation (e.g., Engbert et al., 2005; Reilly & Radach, 2006; Yang, 2006) | ||
| Non-lexical | • Completion of pre-lexical visual word encoding initiates saccade (e.g., McDonald et al., 2005) | |
| • Visual encoding difficulty inhibits saccade initiation (e.g., Engbert et al., 2005; Reilly & Radach, 2006) | ||
| • An autonomous and automatic low-level visual routine that was acquired based on reading experience influences saccade initiation (Vitu et al., 2001, 2007) | ||
| • Efference copy of a saccade program initiates rapid “corrective” saccade (e.g., Engbert et al., 2005; McDonald et al., 2005; Reichle et al., 2009) | ||
| Indirect | Lexical | • Saccades are initiated to maintain overall rate of lexical processing (e.g., Bouma & DeVoogd, 1974; O’Regan, 1990; Yang, 2006) |
| Non-lexical | • Random timer initiates saccade (e.g., Engbert et al., 2005) | |
| • Low activity of “maintain fixation” mechanism initiates saccade (Reilly & Radach, 2006; Yang, 2006) | ||
| • Saccade initiated if fixation duration exceeds deadline (e.g., Henderson & Ferreira, 1990; Reilly & Radach, 2006) | ||
| • Successive fixation durations are correlated (McDonald et al., 2005) |
Note The above descriptions are meant to illustrate our classification scheme, but do not provide a complete description of the cited models or an exhaustive list of all proposed control mechanisms. Furthermore, this scheme refers to the mechanisms of control, not the models per se, as indicated by the fact that most of the models include two or more mechanisms (e.g., SWIFT includes both direct lexical and indirect non-lexical mechanisms).
1.1. Mechanisms of direct lexical control
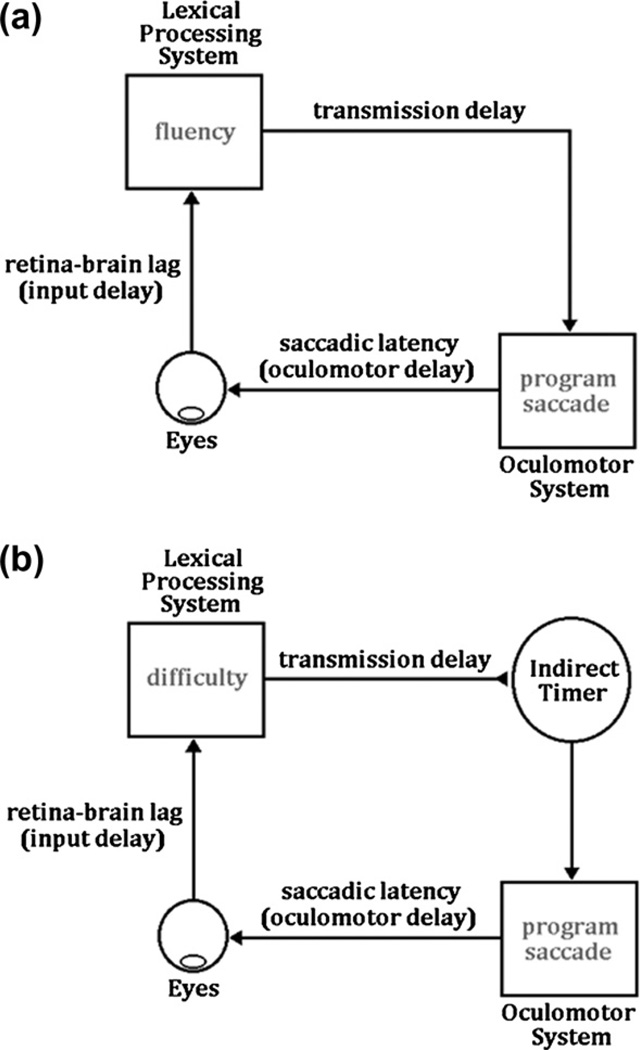
The conventional use of first-pass fixation times on target words as measures of the efficiency of the lexical processing of those words is implicitly or explicitly based on an assumption of some sort of direct lexical control. As indicated, although it is possible to conceive of numerous specific direct lexical control mechanisms, logically, there are only two non-mutually exclusive general types of possible mechanisms, which assume either that: (1) the fluency in lexical processing triggers saccadic programming (henceforth referred to as the triggering mechanism), or that (2) regardless of the nature of the mechanism that triggers reading saccades, difficulty in lexical processing produces delays in the initiation or the execution of the saccade terminating the fixation (henceforth referred to as the interference mechanism). Fig. 1 schematically illustrates the triggering mechanism (Panel a) and the interference mechanism (Panel b).
Fig. 1.
Two mechanisms for implementing direct control of fixation durations during reading. Panel a: Lexical processing fluency triggers the initiation of saccadic programming. Panel b: Lexical processing difficulty inhibits saccadic programming that is initiated by an indirect timer mechanism.
Reading models that incorporate a triggering mechanism attempt to explain the normal progression of the eyes through the text as follows: after completing some amount of cognitive processing of wordn, a system that monitors this processing sends a signal to the oculomotor system so that it can start programming a saccade to move the eyes to wordn+1. Because the cognitive “events” that trigger the eye movements are assumed to be sequential in nature (i.e., the processing of wordn causes the eye to move to wordn+1, which is then processed, causing the eyes to move to wordn+2, and so on), the strongest versions of the triggering mechanism have been associated with the corollary assumption that words are processed one at a time, in a strictly serial manner (Reichle, 2011; see also Reichle, Liversedge, Pollatsek, & Rayner, 2009). Perhaps because of the simplicity of the triggering mechanism, the notion that the serial lexical processing of words is the “engine” that drives eye movements has frequently been used in models of eye-movement control.
The earliest example of such a model was the Reader model (Just & Carpenter, 1980, 1987; Thibadeau et al., 1982). This model assumed that wordn has to be completely processed before a saccadic program to move the eyes to wordn+1 can be initiated. This processing was assumed to entail both the encoding and identification of a word, along with whatever syntactic and semantic processing is required to integrate the word’s meaning into the overall meaning of the text being constructed by the reader. Although this hypothesis was elegant and would have facilitated the task of interpreting fixation durations during reading (i.e., a fixation would indicate the time needed to encode, identify, and integrate the meaning of a word), the hypothesis was demonstrated to be implausible because of the temporal constraints imposed by word identification and saccadic programming. More recently, advocates of the triggering mechanism have adopted a more modest view about how much word processing must actually be completed to trigger saccadic programming. For example, the core assumption of the EMMA model is that the encoding of wordn (but not its subsequent processing) triggers the programming of the saccade to move the eyes to wordn+1 (Salvucci, 2001). Similarly, in the E-Z Reader model (Reichle et al., 1998; for a review, see Reichle, 2011), the core assumption is that a superficial stage of lexical processing triggers saccadic programming. This early stage of processing is labeled L1 to differentiate it from a subsequent stage of processing that causes attention to shift to the next word, labeled L2. The L1 stage has been variously described as corresponding to a rapid recognition response that reflects the word’s familiarity (Reichle & Perfetti, 2003) or an early stage of orthographic processing (Reichle, Tokowicz, Liu, & Perfetti, 2011). By either interpretation, the completion of the L1 stage indicates that lexical access to wordn is imminent, so that the oculomotor system can begin programming a saccade to move the eyes to wordn+1 (Reingold & Rayner, 2006). The amount of cognitive processing that is assumed to be necessary to initiate saccadic programming is reduced even further in two recent models. Specifically, the SERIF model (McDonald et al., 2005) and the Glenmore model (Reilly & Radach, 2006) include the assumption that visual word encoding results in the triggering of saccades.
In contrast to the triggering mechanism, the interference mechanism assumes a very different implementation of direct lexical control of fixation durations. The most complete instantiation of such a mechanism is incorporated into the SWIFT model (Engbert et al., 2005). According to this model, saccades are triggered by an autonomous random timer (which is an exemplar of an indirect control mechanism), and not by the completion of some cognitive process. Importantly, lexical processing difficulty can modulate fixation durations by actively inhibiting the timer so that it cannot initiate new saccadic programs. The assumption here is that, by preventing the initiation of saccadic programming, fixations will be lengthened, allowing additional time for lexical processing. Although the SWIFT model also assumes that two or more words are normally processed in parallel, this inhibition due to processing difficulty is limited to the processing difficulty of the word being fixated. Furthermore, because this inhibition is itself delayed,1 any difficulty that is specifically associated with the processing of wordn usually results in a longer fixation on wordn+1. Finally, although the fundamental hypothesis of the interference mechanism that lexical processing difficulty can delay the initiation or execution of saccades has been incorporated into several other models of eye-movement control (e.g., Feng, 2006; Findlay & Walker, 1999; Yang, 2006), none of these models have been developed to the same degree as the SWIFT model.
1.2. Temporal constraints for direct lexical control
In order to evaluate the feasibility of direct lexical control it is instructive to briefly consider the impact of temporal constraints caused by perceptual and oculomotor neural delays on the control of fixation durations in reading. In their seminal analysis of this issue, Sereno, Rayner, and Posner (1998) and Sereno and Rayner (2003) integrated findings from eye movements studies and event-related potentials (ERP) studies to derive estimates of lexical processing latencies. Specifically, due to delays in the visual system involved in the transduction of light into neural signals and the transmission of these signals for cortical processing, images of the foveated text that are formed on the retina at the beginning of the fixation do not instantaneously reach the lexical processing system (henceforth referred to as the retina-brain lag). Sereno and colleagues estimated a minimum input delay of 60 ms for information about the fixated word to reach the cortical systems where lexical processing begins. Most importantly, Sereno and colleagues documented ERP evidence demonstrating word frequency effects (Sereno et al., 1998) and an influence of predictability (Sereno, Brewer, & O’Donnell, 2003) as early as 132 ms post-stimulus onset. Another important factor considered by Sereno and colleagues is that, given the minimum oculomotor latency required to program an eye movement (i.e., the interval between the initiation and the execution of saccades), lexical processing occurring during the final 100–150 ms of a given fixation can have no impact on the timing of the saccade that ends that fixation. Consequently, in order for lexical processing to impact the timing of the saccade that terminates a fixation, it must exert at least part of its influence prior to this “dead time” at the end of fixation, during which the saccade that has been initiated is still being programmed. Thus, delays of oculomotor output constitute another important temporal constraint on the mechanisms of eye-movement control in reading.
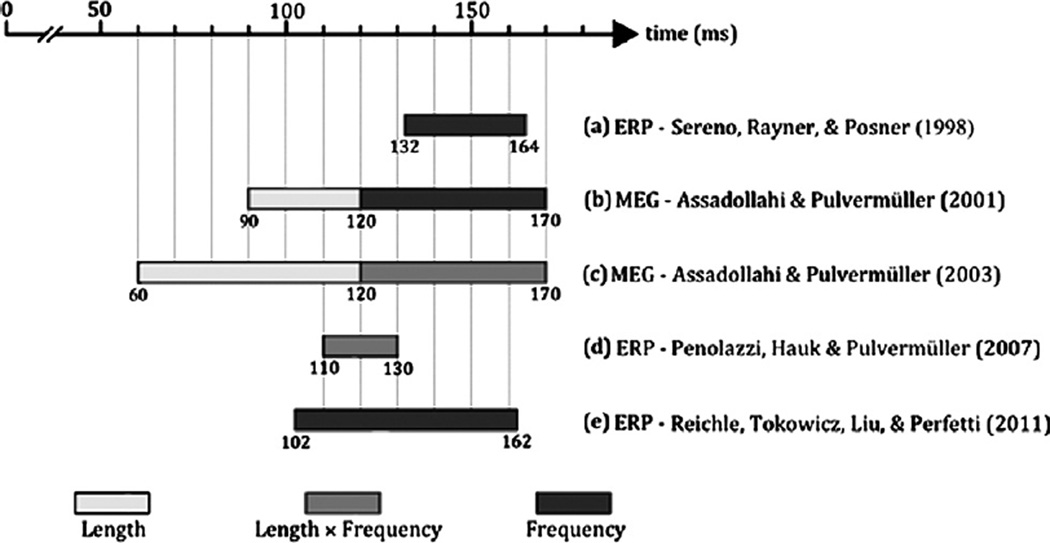
It is also important to emphasize that these estimated temporal constraints have been independently corroborated. For example, several experiments have independently corroborated 50–60 ms estimates of the retina-brain lag (Clark, Fan, & Hillyard, 1995; Foxe & Simpson, 2002; Mouchetant-Rostaing, Giard, Bentin, Aguera, & Pernier, 2000; Van Rullen & Thorpe, 2001). Similarly, as shown in Fig. 2, recent experiments using ERP have demonstrated effects of word frequency as early as 102 ms post-stimulus onset (Reichle et al., 2011), as well as interactions between word length and frequency as early as 110 ms post-stimulus onset (Penolazzi, Hauk, & Pulvermuller, 2007). Furthermore, experiments using magnetoencephalography (MEG) have demonstrated very early (i.e., 60–90 ms poststimulus onset) effects of word length in conjunction with rapid (i.e., 120 ms post-stimulus onset) effects of word frequency (Assadollahi & Pulvermuller, 2001, 2003). Together, these results suggest that information propagates from the eye to the brain in as little as 50 ms, and that whatever lexical processing is differentially affected by variables like word frequency is well enough under way by 120 ms to be detected using electrophysiological methods.2
Fig. 2.
Summary of results from studies using electrophysiological methods to investigate the time-course of brain differential responding to variation in word length and word frequency. Together, these results suggest that differentiation based on lexical variables such as word frequency is clearly evident 120 ms post-stimulus.
Critiques of direct lexical control often argue that given input (visual) delays and output (oculomotor) delays, there is simply not enough time in the average reading fixation that lasts approximately 250 milliseconds to perceptually encode and lexically process the fixated word, and to then use this information in real time to influence the initiation of the saccade that ends the fixation. However, what such criticism often ignores is the fact that lexical processing of a target word (wordn) is typically initiated when this word is parafoveally processed during fixations on the pre-target word (wordn−1). The processing of information acquired during this parafoveal preview period then continues during the wordn−1-to-wordn saccade, as well as during the retina-brain lag at the start of the first fixation on wordn.
Finally, less is known about some of the temporal constraints that might be relevant for evaluating the feasibility of an interference mechanism of direct control. Although some temporal constraints such as the retina-brain lag remain constant regardless of the mechanism type, other temporal constraints might differ between an interference and a triggering mechanism. Specifically, it is unclear how the duration required for establishing lexical difficulty compares to the time required for detecting lexical fluency. Consequently, it is difficult to estimate the timing parameters for the signal to inhibit or delay the saccades in the case of the lexical difficulty. Nevertheless, the feasibility of an interference mechanism is clearly supported by evidence suggesting that oculomotor neural delays associated with the inhibition of saccades in reading are even shorter than those involved in the programming of reading saccades (Reingold & Stampe, 1999, 2000, 2002, 2003, 2004). Thus, although specific implementations of direct control mechanisms might fail to satisfy the temporal constraints resulting from neural delays in visual and oculomotor systems (e.g., early triggering models), in principle, both a triggering and an interference mechanism appear consistent with the available behavioral and neurophysiological evidence.
1.3. The present study
A primary motivation for the present study was our hypothesis that a substantial component of lexical effects that are observed on the very first fixation on a word (first fixation duration) reflect differential lexical processing of target words that was initiated during the parafoveal preview of the words. Given that word frequency effects constitute a primary empirical marker for lexical processing (see Rayner, 1998, 2009a), we manipulated the frequency of target words as well as the availability of the target words for parafoveal processing during fixations on the pre-target word (i.e., valid vs. invalid preview). Specifically, in the invalid preview trials used in the present study, an unrelated letter string occupied the position of the target word and was replaced with the target word during the saccade that crossed an invisible boundary located just to the left of that word (see Rayner, 1975, for a description of this boundary technique). Consequently, in contrast to valid preview trials in which the sentence was read normally, in invalid preview trials the target word was not available for parafoveal processing during fixations on the pre-target word. Accordingly, in the present study we contrasted the distributions of first fixation durations on high frequency (HF) and low frequency (LF) target words in both the valid and the invalid preview condition. By comparing the nature and time-course of word frequency effects as a function of preview validity, we hoped to shed light on the influence of parafoveal lexical processing on the control of fixation times in reading.
Several prior studies included a manipulation of both word frequency and preview validity (e.g., Inhoff & Rayner, 1986; Kennison & Clifton, 1995; Rayner, Liversedge, & White, 2006; Schroyens, Vitu, Brysbaert, & d’Ydewalle, 1999; Sereno & Rayner, 2000; Vitu, 1991). The results from these studies were somewhat mixed, and integrating findings across these investigations is hindered by several methodological differences between studies (see Sereno and Rayner (2000) for a discussion of this issue). Nevertheless, when examining first-pass fixation time data, one finding that was demonstrated relatively consistently across studies was larger preview benefits (i.e., the facilitation of processing of the target word in the valid as compared to the invalid preview condition) for HF than LF targets. As pointed out by Inhoff and Rayner (1986), this pattern of findings provides support for the influence of parafoveally initiated lexical processing on fixation durations in reading.
The present study was designed to extend these prior investigations by primarily focusing on analyzing the differences in the distributions of first fixation duration across the word frequency by preview validity conditions. Note that distributional analyses are inherently more suitable than the analysis of mean fixation durations for determining the time-course of the influence of lexical variables. Accordingly we explored several methods of distributional analyses in an attempt to test conflicting predictions concerning the earliest discernable lexical influence on first fixation duration. The most common method for comparing distributions across conditions involves the use of normalized histograms which plot the proportion of fixations that fall into consecutive, non-overlapping fixation duration intervals (i.e., time bins). However, due to the normalizing of histograms (i.e., the area under each histogram summing to one), a late influence of a variable that produces a difference across conditions in the tail of the distribution would also result in a separation between the histograms in earlier parts of the distribution. Consequently, such a pattern of differences between normalized histograms might be erroneously interpreted to indicate that the variable being studied exerts a more rapid influence on fixation duration than is actually the case. A more sophisticated approach that avoids this issue involves modeling the fixation duration distribution using the ex-Gaussian distribution. Specifically, Staub, White, Drieghe, Hollway, and Rayner (2010) fitted the ex-Gaussian distribution to individual participants’ first fixation duration and gaze duration distributions on both HF and LF target words. Based on this analysis,Staub et al. (2010) reported that the LF distribution was significantly shifted to the right of the HF distribution, and that the LF distribution also exhibited greater positive skew (right skew) as compared to the HF distribution. The finding that word frequency caused a shift in the distributions across conditions clearly indicates that this lexical variable had an impact on both short and long fixations (see also, Rayner, 1995), as predicted by the direct lexical control view. In addition, the greater positive skew for the LF distribution indicated that long fixations were differentially lengthened over and above the global shift across distributions. In the present study we contrasted the magnitude of these shift and skew effects across the valid and invalid preview conditions. If, as we hypothesized, a large part of the influence of word frequency on first fixation durations is due to parafoveal processing, then the shift effect should be reduced or even eliminated in the invalid preview condition. In contrast, a skew effect is still predicted in the invalid preview condition because longer fixations might still allow enough time for the influence of word frequency to emerge even in the absence of parafoveal processing.
In addition to analyzing ex-Gaussian distribution parameters as described above, we also plotted and contrasted survival curves for the HF and LF first fixation duration distributions in both the valid and invalid preview conditions. Although survival curves have previously been used to analyze aspects of fixation times primarily in the study of visual expertise in medicine (for a review, see Reingold & Sheridan, 2011; see also Feng, Miller, Shu, and Zhang (2001) for another application of survival curves) the current methodology constitutes a novel application of this technique. Specifically, for a given time t, the percentage of first fixations with a duration greater than t are referred to as the percent survival at time t. Thus, when t equals zero, survival is at one hundred percent, but then declines as t increases and approaches zero percent as t approaches the duration of the longest observed first fixation. We argue that the earliest point in time at which the LF and HF survival curves begin to significantly diverge (henceforth referred to as the divergence point) might provide a promising and unique estimate for the earliest significant influence of word frequency on first fixation durations. The present use of survival curves is also similar to the use of hazard functions by McConkie and colleagues (McConkie & Dyre, 2000; McConkie, Kerr, & Dyre, 1994; Yang & McConkie, 2001; see Feng (2009) for a review) to study the distribution of fixation duration in reading. Specifically, the hazard rate for a given time interval is determined by the number of fixations terminating during that interval divided by the total number of fixations with durations greater than the start of the interval. However, unlike our survival analysis which allowed for a very high temporal resolution in determining the divergence point between the HF and LF curves (e.g., 1 ms in our analyses), the requirement of hazard functions for data to be aggregated into wider “bins” (e.g., 25-ms intervals used by Yang and McConkie (2001)) would not have allowed for a similar precision.
Finally, the effect of the word frequency variable was compared to the influence on fixation times of the location of the first fixation on HF and LF target words (henceforth, location effect). It is well documented that first fixation duration is longer when initial landing position is near the center of the word (central location) than when landing position is near the beginning or the end of the word (outer location) (e.g., Kliegl, Nuthmann, & Engbert, 2006; McDonald et al., 2005; Nuthmann, Engbert, & Kliegl, 2005, 2007; Vitu, Lancelin, & d’Unienville, 2007; Vitu, McConkie, Kerr, & O’Regan, 2001). Although there is no general consensus concerning the origin of the location effect (for a review see Vitu et al. (2007)), it is widely considered to represent an extremely rapid non-lexical influence on first fixation duration (i.e., a form of direct non-lexical control). Consequently, the location effect might provide an invaluable temporal “benchmark” against which one can directly compare the time course of the word-frequency effect (see Vitu et al. (2001) for a related comparison). Thus, the present study was designed to provide a strong test for the direct lexical control hypothesis as well as to explore the potential role of parafoveal processing in enabling direct control. Finally, we explore the implications of the present findings for eye-movement control theories, in general, and the triggering and interference mechanisms of direct lexical control, in particular.
2. Method
2.1. Participants
Sixty undergraduate students at the University of Toronto participated in the experiment. The participants were all native English speakers and were given either one course credit, or $10.00 (Canadian) per hour. All participants had normal or corrected to normal vision.
2.2. Materials and design
A total of 120 high-frequency (HF) nouns (M = 112.1 words per million; Brysbaert & New, 2009) and 120 low-frequency (LF) nouns (M = 2.5 words per million) were used as target words. High- and low-frequency words ranged between 5 and 10 characters in length (M = 6.5). 120 pairs of high- and low-frequency words were then created (matched on word length), and two low-constraint sentence frames were composed for each word pair so that either word could plausibly fit into the sentences. For example, sentences 1a and 1b below were created for the pair of words, table and banjo:
1a. John decided to sell the table/banjo in the garage sale.
1b. I was told that the table/banjo was made out of expensive wood.
Target word predictability in these sentence frames was assessed by providing an additional group of 10 participants with the beginning of each sentence frame and asking them to write a word that could fit as the next word in the sentence. Average predictability was extremely low, amounting to 1.3% for the high-frequency target words and 0.1% for low-frequency target words.
In addition to the word frequency manipulation, on half of the trials (valid preview trials), the sentences appeared normally with one of the target words in the target location. On the other half of the trials (invalid preview trials), a pronounceable non-word (e.g., purty for the table and banjo target pair and sentence frames shown above) equal in length to the target was initially displayed in the target location. Each of the letters in the non-word previews were different from the corresponding letter in both the HF and LF targets.
Thus, four experimental conditions resulted from crossing frequency (high vs. low) and preview (valid vs. invalid). Each participant read any given target word or sentence frame only once and the assignment of target words to sentence frames and preview conditions was counterbalanced across participants. Participants read five practice sentences followed by 280 sentences (240 experimental and 40 filler) that were presented in a random order.
2.3. Apparatus and procedure
Eye movements were measured with an SR Research EyeLink 1000 system with high spatial resolution and a sampling rate of 1000 Hz. The experiment was programmed and analyzed using SR Research Experiment Builder and Data Viewer software and the survival analysis was programmed using MATLAB software. In addition, the online saccade detector of the eye tracker was set to detect saccades using an acceleration threshold of 9500°/s2 and a velocity threshold of 30°/s. Viewing was binocular, but only the right eye was monitored. A chin rest and forehead rest were used to minimize head movements. Following calibration, gaze-position error was less than 0.5°. The sentences were displayed on a 21 in. ViewSonic monitor with a refresh rate of 150 Hz and a screen resolution of 1024 × 768 pixels. All letters were lowercase (except when capitals were appropriate) and in a mono-spaced Courier font. The text was presented in black (4.7 cd/m2) on a white background (56 cd/m2). Participants were seated 60 cm from the monitor, and 2.4 characters equaled approximately 1 degree of visual angle.
During invalid preview trials, an invisible boundary was defined in the middle of the space between the final letter of the pre-target word and the first letter of the target word. Following the first eye-movement sample with a gaze position to the right of this boundary, a display change was initiated replacing the non-word occupying the target position with either the HF or LF target word. This change was accomplished within 6.7 ms. No display change occurred in valid preview trials (i.e., the target word was presented in its sentence frame for the entire duration of the trial) and valid and invalid preview trials were randomly intermixed. Participants were not informed of the occurrence of the display changes and were instructed to read the sentences for comprehension. After reading each sentence, they pressed a button to end the trial and proceed to the next sentence. To ensure that participants were reading for comprehension, about 15% of the sentences (all were filler sentences) were followed by multiple-choice comprehension questions. The average accuracy rate was 96%.
3. Results and discussion
Our main focus involved examining the distributions of first fixation duration as a function of the frequency of target words (frequency effects), their availability for parafoveal processing during fixations on the pre-target word (preview effects), as well as the initial landing position on these words (location effects). We accomplished that by fitting fixation time data using the ex-Gaussian distribution, as well as by employing a survival analysis technique. However, in order to facilitate comparison with prior studies, we first explored the influence of word frequency and location on commonly used eye movements measures. Accordingly, in this section of the paper we begin by discussing the results from the analyses of means followed by a discussion of the findings from the distributional analyses.
3.1. Analysis of means: Word frequency effects
We examined the influence of the frequency by preview manipulation on the following measures: (a) first fixation duration (i.e., the duration of the first forward fixation on the target, regardless of the number of subsequent fixations on the target); (b) single-fixation duration (i.e., the first fixation value for the subset of trials in which there was only one first-pass fixation on the target); (c) gaze duration (i.e., the sum of all the consecutive first-pass fixations on the target, prior to a saccade to another word); (d) first of multiple first-pass fixations (i.e., the first fixation duration for the subset of trials in which there was more than one first-pass fixation on the target); (e) the probability of skipping (i.e., trials in which there was no first-pass fixation on the target regardless of whether or not the target was fixated later in the trial); and (f) the probability of a single first-pass fixation. For all of these dependent measures, 2 × 2 analyses of variance (ANOVAs) were carried out on the data via both participants (F1) and items (F2), and with frequency (HF vs. LF) and preview (valid vs. invalid) as independent variables. Table 2 summarizes the results of the frequency by preview ANOVAs and the means and standard errors for the different measures. In addition, this table displays the results from two types of planned comparisons that were carried out on the data via both participants (t1) and items (t2) in order to compute the magnitude and significance of: (1) the word frequency effects in the valid and invalid preview conditions, and (2) the preview benefits for HF and LF targets.
Table 2.
First fixation, gaze duration, single fixation and first in multiple first-pass fixations (ms), and the probability (proportion) of skipping and single fixation by preview condition and word frequency. Standard errors are shown in parentheses.
| Variable | Valid preview |
Invalid preview |
Preview benefit |
Significance |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HF | LF | Freq. | HF | LF | Freq. | HF | LF | Frequency | Preview | Frequency × Preview | |
| First fixation | 214 | 234 | 20*** | 251 | 260 | 9** | 37*** | 26*** | F1 = 75.58, p < .001 | F1 = 60.86, p < .001 | F1 = 21.21, p < .001 |
| (all trials) | (3.8) | (4.6) | (5.9) | (6.9) | F2 = 56.89, p < .001 | F2 = 279.75, p < .001 | F2 = 14.74, p < .001 | ||||
| Gaze duration | 247 | 305 | 58*** | 309 | 356 | 47*** | 62*** | 51*** | F1 = 67.33, p < .001 | F1 = 73.18, p < .001 | F1 = 4.99, p < .05 |
| (all trials) | (5.8) | (11.1) | (8.7) | (13.3) | F2 = 150.29, p < .001 | F2 = 422.81, p < .001 | F2 = 4.60, p < .05 | ||||
| Single fixation | 216 | 239 | 23*** | 267 | 276 | 9* | 51*** | 37*** | F1 = 52.19, p < .001 | F1 = 73.68, p < .001 | F1 = 15.73, p < .001 |
| (4.0) | (5.1) | (7.3) | (8.3) | F2 = 51.40, p < .001 | F2 = 340.54, p < .001 | F2 = 14.10, p < .001 | |||||
| First fixation | 211 | 226 | 15* | 218 | 238 | 20*** | 7 | 12* | F1 = 16.60, p < .001 | F1 = 5.25, p < .05 | F1 < 1 |
| (multiple) | (5.2) | (6.3) | (5.2) | (6.5) | (n.s.) | F2 = 29.44, p < .001 | F2 = 12.27, p < .001 | F2 < 1 | |||
| Prob. of | 0.10 | 0.06 | 0.04*** | 0.07 | 0.06 | 0.01 | 0.03*** | 0.00 | F1 = 30.17, p < .001 | F1 = 15.35, p < .001 | F1 = 18.50, p < .001 |
| skipping | (.01) | (.01) | (.01) | (.01) | (n.s.) | (n.s.) | F2 = 26.52, p < .001 | F2 = 8.45, p < .01 | F2 < 16.27, p < .001 | ||
| Prob. of | 0.74 | 0.66 | 0.08*** | 0.67 | 0.58 | 0.09*** | 0.07*** | 0.08*** | F1 = 81.18, p < .001 | F1 = 37.08, p < .001 | F1 < 1 |
| single fixation | (.01) | .02) | (.02) | (.02) | F2 = 73.39, p < .001 | F2 = 60.76, p < .001 | F2 < 1 | ||||
Note For F tests, df for F1 = (1, 59), and df for F2 = (1,119). For t tests df for t1 = 59, and df for t2 = 119; For t tests the p-value is given for t1 if t1 < t2 or for t2 if t2 < t1, and these p-values are denoted as follows:
p < .05,
p < .01,
p < .001, n.s. p > .1; HF = high frequency, LF = low frequency, Freq. = word frequency effect; For the fixation time variables Freq. = LF–HF and preview benefit = invalid–valid. For the probability variables Freq. = HF–LF and preview benefit = valid–invalid.
Trials were excluded from the analyses described below due to track losses (0.04% of all trials). In the invalid preview condition, trials in which the invisible boundary was crossed during a fixation were also excluded (17.6% of invalid preview trials). In addition, trials in which the target word was skipped were excluded. As shown in Table 2, in the valid but not in the invalid preview condition there was a significant word frequency effect on the probability of skipping (with HF targets skipped more often than LF targets). This is consistent with the idea that parafoveal lexical processing occasionally resulted in the skipping of HF targets (seeRayner et al. (1996) for a similar finding). Furthermore, both the valid and invalid preview trials produced longer first fixation and gaze durations on LF than HF targets. Importantly, the word frequency effects on these measures were significantly larger in the valid than invalid preview condition. These significant interactions were due to larger parafoveal preview benefits for HF than LF targets and this pattern was consistent with the findings from previous studies (Inhoff & Rayner, 1986; Kennison & Clifton, 1995; Schroyens, Vitu, Brysbaert, & d’Ydewalle, 1999; Sereno & Rayner, 2000). In the majority of trials only a single first-pass fixation occurred, and the probability of single fixation was greater for HF than LF targets and for valid than invalid preview (see Table 2). In addition, the single-fixation duration measure displayed the pattern of frequency by preview interaction that was described above for the overall first fixation and gaze duration measures. Thus, for the standard first-pass fixation time measures (i.e., first fixation, single-fixation, and gaze duration), our findings provided support for the hypothesis that, given the faster lexical access for HF than LF targets, more lexical information is encoded from HF than LF parafoveal targets during the preview period, and this in turn results in larger parafoveal preview benefits for HF than LF targets once these words are fixated.
However, an interesting dissociation occurred when comparing the single-fixation duration data with the pattern of findings that was observed for first fixation duration in trials with multiple first-pass fixations. Specifically, as can be seen in Table 2, in contrast to single-fixation trials in which word frequency effects were larger for the valid than the invalid preview condition, in multiple first-pass fixation trials the magnitude of the word frequency effect did not significantly vary as a function of preview validity. This difference in the influence of word frequency and preview validity on first fixation duration as a function of the number of first-pass fixations (single vs. multiple) was reflected in a significant 3-way interaction between these factors [F1(1,59) = 6.20, p < .05; F2(1,119) = 5.80, p < .05]. If as discussed earlier larger preview benefits for HF than LF targets are due to greater parafoveal lexical processing of the former as compared to the latter, then the absence of such a difference in trials with multiple first-pass fixations might suggest that such trials reflect processing episodes in which limited or no lexical information was obtained during the parafoveal preview. This interpretation is also consistent with the hypothesis that immediate refixation on the target word is at least sometimes due to incomplete lexical processing of that word (see Pollatsek & Rayner, 1990; Pynte, 1996; Reingold et al., 2010) and consequently, lexical processing is more often incomplete at the termination of the first in multiple first-pass fixations than at the end of single first-pass fixation.
3.2. Analysis of means: Location effects
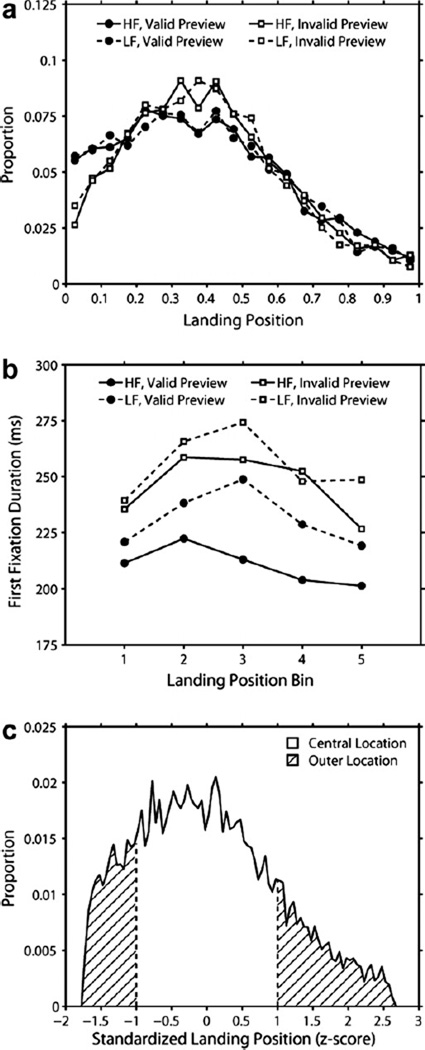
The above analyses examined eye movements measures regardless of the location of the first fixation in the word (i.e., initial landing position).3 In this section, we explored the impact of location and any interactions between this variable and word frequency and/or preview validity. In order to examine the effect of location, for each target word we defined a target region from the middle of the space prior to this word to the middle of the space following this word. Location was then quantified as the proportion of the target region to the left of the position of the first fixation on the target word. As shown in Fig. 3 (Panel a), the distribution of locations was extremely similar across the frequency by preview conditions. Replicating prior findings (e.g., Dunn-Rankin, 1978; McConkie et al., 1988; Rayner, 1979; Vitu, O’Regan, & Mittau, 1990), the mean location was slightly left of center, and did not significantly differ as a function of preview, F(1,59) = 1.09, p > 0.3, frequency, F < 1, or their interaction F < 1. Next, we divided the target region into five equal landing position bins and computed the average first fixation duration for each bin in each frequency by preview condition. As can be seen by an inspection of Fig. 3 (Panel b), first fixation duration was longer in central locations than in outer location. In order to analyze the influence of location on fixation times as a function of the frequency by preview manipulation, we devised a more formal definition of a central location as encompassing all fixations in each condition within a standardized location z, such that −1 < z < 1. All other fixations (z ≥ 1 or z ≤ −1) were considered as landing on an outer location (see Fig. 3, Panel c).
Fig. 3.
Proportion of the fixated word to the left of the initial landing position on this word by condition (Panel a), first fixation duration by landing position bin and condition (Panel b), and an illustration of our definition of central location to include landing positions within one standard deviation from mean landing position in either direction and outer location as including landing positions outside this area (Panel c).
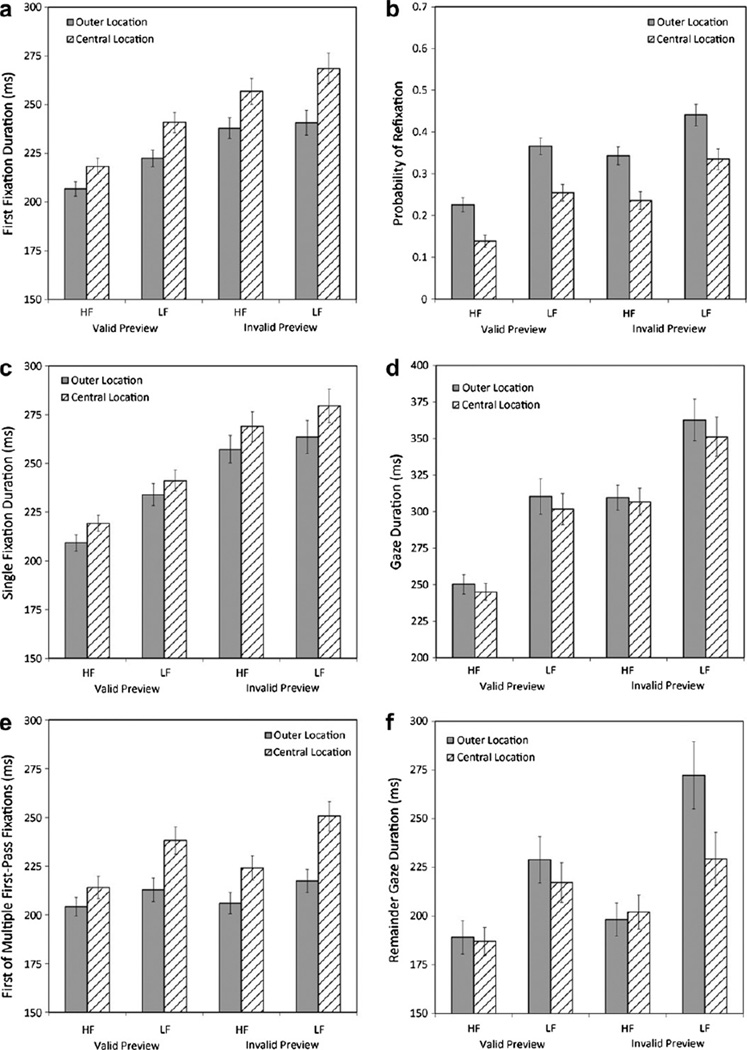
As summarized in Fig. 4 and Table 3, we examined the influence of location on eye movements measures by conducting 2 × 2 × 2 ANOVAs via both participants (F1) and items (F2), and with location (central vs. outer), frequency (HF vs. LF), and preview (valid vs. invalid) as independent variables. Central locations resulted in longer first fixation duration than outer locations (Fig. 4, Panel a), and this was true for both trials with a single first-pass fixation (Fig. 4, Panel c) and trials with multiple first-pass fixations (Fig. 4, Panel e). However, unlike first fixation duration, gaze duration was longer in outer than central locations (Fig. 4, Panel d), and this was due to a higher probability of multiple first-pass fixations for outer than central locations (Fig. 4, Panel b), and longer remainder gaze duration (i.e., gaze duration minus first fixation duration in trials with multiple first-pass fixations) in outer than central locations (Fig. 4, Panel f). The above main effects of location successfully replicated prior findings in the literature (e.g., Kliegl et al., 2006; McDonald et al., 2005; Nuthmann et al., 2005, 2007; Vitu et al., 2001; for a review see Vitu et al., 2007), thereby helping to establish the validity of our operational definition of location.
Fig. 4.
First fixation duration (Panel a), probability of refixation (Panel b), single fixation duration (Panel c), gaze duration (Panel d), first of multiple first-pass fixations (Panel e), and remainder gaze duration (Panel f) by location, word frequency and preview validity.
Table 3.
Summary of the results of by participant (F1) and by item (F2) ANOVAs examining the main effects and interactions of location on first fixation, single fixation, first in multiple first-pass fixations, gaze duration, remainder gaze duration, and the probability of refixation.
| Variable | Location | Location × Preview | Location × Frequency | Location × Frequency × Preview |
|---|---|---|---|---|
| First fixation | F1 = 51.2, p < .001 | F1 = 5.2, p < .05 | F1 = 4.9, p < .05 | F1 < 1 |
| (all trials) | F2 = 116.3, p < .001 | F2 = 10.7, p < .001 | F2 = 4.5, p < .05 | F2 <1 |
| Single fixation | F1 = 12.4, p < .001 | F1 = 1.3, p > .25 | F1 < 1 | F1 < 1 |
| F2 = 28.6, p < .001 | F2 = 3.2, p = .076 | F2 < 1 | F2 < 1 | |
| First fixation | F1 = 42.9, p < .001 | F1 = 1.8, p > .179 | F1 = 5.1, p < .05 | F1 < 1 |
| (multiple) | F2 = 48.6, p < .001 | F2 = 2.4, p > .126 | F2 = 6.7, p < .05 | F2 = 1.0, p > .313 |
| Gaze duration | F1 = 3.2, p = .079 | F1 < 1 | F1 = 1.7, p > .200 | F1 < 1 |
| F2 = 11.1, p < .001 | F2 < 1 | F2 = 1.5, p > .222 | F2 < 1 | |
| Remainder | F1 = 5.1, p < .05 | F1 = 1.5, p > .222 | F1 = 5.2, p < .05 | F1 = 2.3, p > .136 |
| gaze duration | F2 = 6.0, p < .05 | F2 = 3.1, p = .079 | F2 = 4.2, p < .05 | F2 < 1 |
| Probability of | F1 = 62.9, p < .001 | F1 < 1 | F1 < 1 | F1 < 1 |
| refixation | F2 = 153.8, p < .001 | F2 < 1 | F2 < 1 | F2 < 1 |
Note: For F tests, df for F1 = (1, 59), and df for F2 = (1, 119).
In addition, our design allowed for an examination of variations in the size of location effects as a function of word frequency and preview validity. As shown in Table 3 and Fig. 4, location effects on first fixation duration were larger in the invalid than valid preview condition. However, this location by preview interaction was not significant when trials with a single first-pass fixation on the target word and trials with multiple first-pass fixations were analyzed separately. Location effects on first fixation duration were also larger for LF than HF target words, and this effect was entirely due to trials with multiple first-pass fixations on the target word (i.e., no hint of such an interaction on single fixation duration). Furthermore, location effects on remainder gaze duration were larger for LF than HF target words. Finally, for all of the dependent variables, the 3-way interaction (location × preview × frequency) was not significant. Thus, the present findings documented an exception to the commonly assumed independence between the word frequency and location effects on first fixation duration (e.g., Nuthmann et al., 2005; Rayner et al., 1996; Vitu et al., 2001). Determining the theoretical importance of the location by preview and location by frequency interactions that emerged in the present study clearly require further investigation. Nevertheless, the present methodology appears to have promise for exploring the interrelatedness of these variables.
Next we report on the findings obtained from the analyses of the distribution of first fixation durations by fitting fixation time data using the ex-Gaussian distribution, as well as by employing a survival analysis technique. Distributional analysis were conducted to explore the time course of: (1) frequency effects in valid and invalid preview, (2) preview effects for HF and LF targets, (3) location effects in valid and invalid preview, and (4) location effects for HF and LF targets.
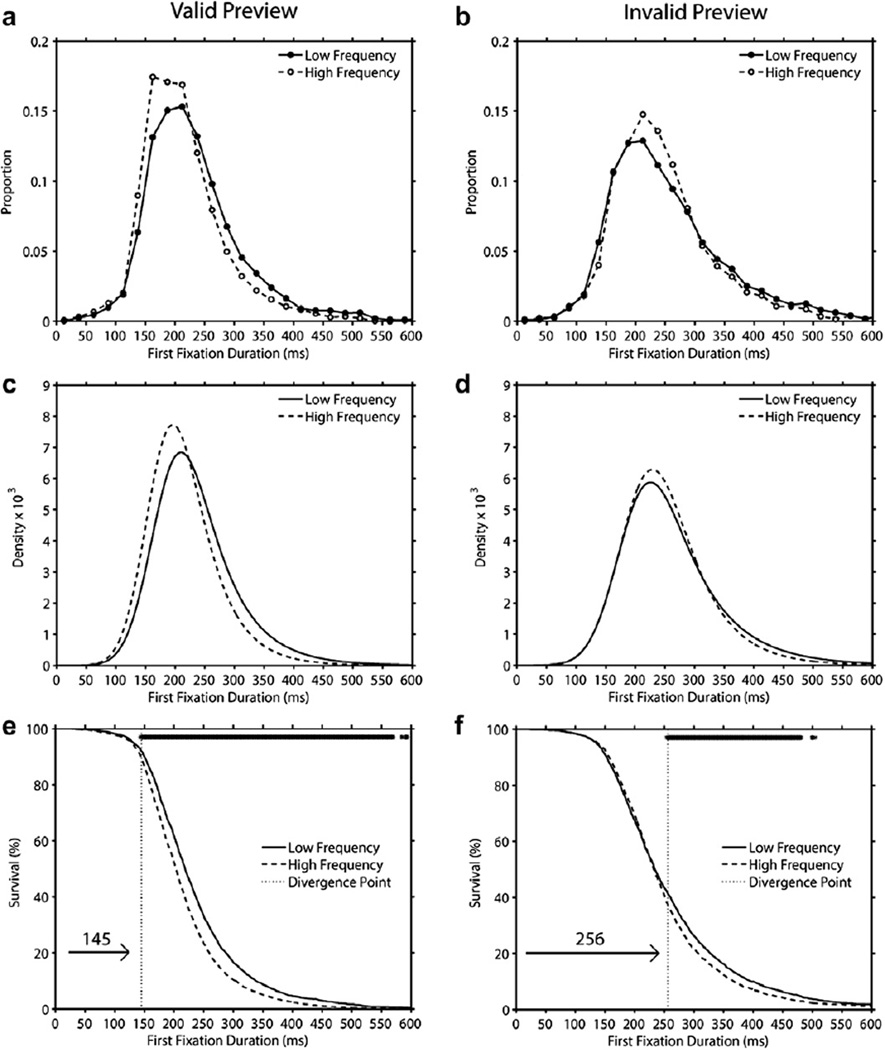
3.3. Analysis of distributions: Frequency effects in valid and invalid preview
For each participant and each condition we computed the proportion of first fixation durations that fell within each successive 25-ms bin over the range from 0 to 600 ms. These values were averaged across participants to produce the distributions that are shown in Fig. 5 (Panels a–b). As can be seen in this figure, each distribution appears approximately normal yet presents a clear rightward skew. This shape is characteristic of the first fixation duration distribution in reading and has been successfully modeled by Staub et al. (2010) using the ex-Gaussian distribution. The ex-Gaussian distribution is the convolution of the Gaussian normal distribution and an exponential distribution, and can be specified with the following three parameters: µ (the mean of the Gaussian normal distribution), σ (the standard deviation of the Gaussian normal distribution) and τ (the slope of the exponential function). Following Staub et al. (2010), we fitted the ex-Gaussian distribution to our first fixation duration data using an algorithm known as quantile maximum likelihood estimation (QMPE; Cousineau, Brown, & Heathcote, 2004; Heathcote, Brown, & Mewhort, 2002). First fixation duration data for each participant in each condition were fitted separately.
Fig. 5.
Distributions of first fixation duration on high-frequency and low-frequency targets in valid preview trials (Panel a) and invalid preview trials (Panel b), and ex-Gaussian density functions (Panels c and d), and survival curves (Panels e and f) that were generated from these distributions (the row of asterisks at the top of Panels e and f indicate time bins with a significant difference between the LF and HF curves). See text for details.
The mean number of usable observations per cell, the parameter estimates, and the magnitude and significance of the word frequency effects are presented in Table 4. The present study was designed to provide sufficient power for modeling the first fixation distribution for each participant and condition. Accordingly, 60 target words per condition were used, resulting on average in 55 and 46 usable observations per cell in the valid and invalid preview conditions, respectively (see Table 4). Importantly, for each participant and condition, the QMPE converged onto stable estimates of the µ, σ, and τ parameters. Fig. 5 (Panels c–d) displays the density functions generated from the best-fitting ex-Gaussian parameters averaged across participants.
Table 4.
Number of observation per cell and ex-Gaussian parameters by condition. Standard errors are shown in parentheses.
| n | Mu (µ) | Sigma (σ) | Tau (τ) | |
|---|---|---|---|---|
| Valid preview | ||||
| Low frequency | 56 (0.6) | 175 (4.0) | 39 (2.3) | 60 (4.3) |
| High frequency | 54 (0.7) | 166 (3.1) | 37 (2.0) | 49 (3.2) |
| Frequency effect | 2*** | 9** | 2 | 11** |
| Invalid preview | ||||
| Low frequency | 46 (0.9) | 184 (5.9) | 43 (3.0) | 76 (5.6) |
| High frequency | 46 (1.0) | 192 (4.6) | 45 (2.8) | 60 (4.9) |
| Frequency effect | 0 | −8 | −2 | 16*** |
| High frequency | ||||
| Invalid preview | 46 (1.0) | 191 (4.6) | 43 (2.7) | 60 (5.2) |
| Valid preview | 54 (0.7) | 167 (3.4) | 37 (2.0) | 47 (3.3) |
| Preview effect | −8*** | 24*** | 6* | 13* |
| Low frequency | ||||
| Invalid preview | 46 (0.9) | 186 (6.1) | 42 (3.1) | 73 (5.7) |
| Valid preview | 56 (0.6) | 176 (4.1) | 39 (2.4) | 59 (4.3) |
| Preview effect | 10*** | 10* | 3 | 14** |
| Valid preview | ||||
| Central location | 71 (1.0) | 174 (3.4) | 40 (2.0) | 55 (3.8) |
| Outer location | 39 (0.8) | 163 (3.4) | 34 (2.3) | 53 (3.9) |
| Location effect | 32*** | 11*** | 6** | 2 |
| Invalid preview | ||||
| Central location | 61 (1.4) | 193 (5.4) | 44 (2.9) | 70 (5.2) |
| Outer location | 31 (0.8) | 173 (4.8) | 36 (3.0) | 68 (5.6) |
| Location effect | 30*** | 20*** | 8* | 2 |
| High frequency | ||||
| Central location | 65 (1.1) | 179 (4.0) | 41 (2.3) | 58 (4.2) |
| Outer location | 34 (0.7) | 165 (3.8) | 36 (2.7) | 55 (4.1) |
| Location effect | 31*** | 14*** | 5* | 3 |
| Low frequency | ||||
| Central location | 67 (1.1) | 186 (5.1) | 44 (2.6) | 69 (5.2) |
| Outer location | 35 (0.7) | 165 (4.5) | 32 (2.8) | 65 (4.4) |
| Location effect | 32*** | 21*** | 12*** | 4 |
Note: For the t test results shown above, df = 59.
p < .05.
p < .01.
p < .001.
An examination of the data summarized in Table 4 and Fig. 5, indicates that the results from the valid preview condition in the present experiment replicated the findings reported by Staub et al. (2010). Specifically, the LF distribution was shifted to the right of the HF distribution resulting in a significant word frequency effect on µ. In addition, the LF distribution also exhibited greater rightward skew as compared to the HF distribution as reflected in a significant word frequency effect on τ. There was no word frequency effect on σ. A different pattern of results emerged in the invalid preview condition. Most notably, unlike in the valid preview condition, in the invalid preview condition the LF distribution was not shifted to the right of the HF distribution (no significant word frequency effect on µ). In addition, the influence of word frequency on the skew of the distributions (i.e., the word frequency effect on τ) did not vary as a function of preview validity. To confirm the impact of preview validity on the frequency effects that were observed for the ex-Gaussian parameters, we conducted 2 × 2 ANOVAs with frequency (HF vs. LF) and preview (valid vs. invalid) as independent variables. Larger µ values were obtained in invalid than valid preview [F(1,59) = 23.87, p < .001], and more importantly a significant frequency × preview interaction occurred [F(1,59) = 7.88, p < .01] reflecting the absence of a frequency effect in the invalid preview condition coupled with the presence of such an effect in the valid preview condition. As can be seen in Table 4, the values of τ were significantly larger for invalid than valid preview [F(1,59) = 9.96, p < .01] and for LF than HF targets [F(1,59) = 15.81, p < .001], and there was no frequency × preview interaction (F < 1).
Thus, consistent with the interpretation of the differences in mean first-pass fixation times across the word frequency by preview conditions, the results of the ex-Gaussian fitting provided strong convergent evidence for the importance of parafoveal lexical processing of the target in determining first-pass fixation time on this word once it is later fixated. Specifically, consistent with the direct lexical control hypothesis, the shift of the LF distribution to the right of the HF distribution in the valid preview condition is a clear demonstration of a lexical effect impacting most first fixations regardless of their duration (see Staub et al., 2010). Consequently, the present demonstration that this effect is absent in the invalid preview condition strongly reinforces the critical role of parafoveal processing in enabling direct lexical control of first fixation duration.
Next, we computed survival curves for first fixation durations in each frequency by preview condition. For each 1-ms time bin t (t was varied from 0 to 600 ms), the percentage of first fixations with a duration greater than t constituted the percent survival at time t. The survival curve for each preview by frequency condition was computed separately for each participant, and then averaged across participants. As can be seen by an inspection of Fig. 5 (Panels e–f), in both the valid and the invalid preview conditions, the HF and LF survival curves appear to diverge. Importantly, this divergence point corresponds by definition to the shortest first fixation duration value at which word frequency had a significant impact. In order to estimate the divergence point between the HF and LF survival curves, we employed a bootstrap re-sampling procedure (Efron & Tibshirani, 1994). On each iteration of this procedure, the set of observations (first fixation durations) for each participant in each condition was randomly re-sampled with replacement. For each iteration of the bootstrap procedure, individual participant’s survival curves were then computed and averaged. Next, the value for each 1-ms bin in the HF survival curve was subtracted from the corresponding value in the LF survival curve. This procedure was repeated 10,000 times, and the obtained differences for each bin were then sorted in order of magnitude. The range between the 5th and the 9,995th value was then defined as the confidence interval of the difference for each bin (given the multiple comparisons we performed, we used this conservative confidence interval in order to protect against making a Type I error). To compute the divergence point between the LF and HF survival curves, we identified the time bins for which LF survival rate was significantly greater than HF survival rate (i.e., for which the lower bound of the confidence interval of the difference between the LF and HF curves was greater than zero). The divergence point was then defined as the earliest significant difference point that was part of a run of five consecutive significant difference points (significant differences between the LF and HF curves are shown in Panels e–f of Fig. 5 as a row of asterisks above the survival curves).
As can be seen in Fig. 5 (Panel e), in the valid preview condition the HF and LF survival curves significantly diverged at a duration of 145 ms. In contrast, in the invalid preview condition (Fig. 5, Panel f), the HF and LF survival curves significantly diverged at 256 ms. Furthermore, these divergence points also define the percentage of first fixations with durations that were too short to exhibit an influence of word frequency. In the valid preview condition only approximately 9% of first fixations had durations that were shorter than the divergence point. In contrast, the corresponding percentage in the invalid preview condition was approximately 60%. Thus, a comparison of the divergence points across preview conditions indicates a dramatic 111 ms difference in the earliest discernable influence of word frequency on first fixation duration as a function of preview validity. Most importantly, we would argue that the temporal estimates that were derived from the survival analysis conclusively demonstrate a fast acting direct lexical control of first-pass fixation times in normal reading (i.e., with valid preview).
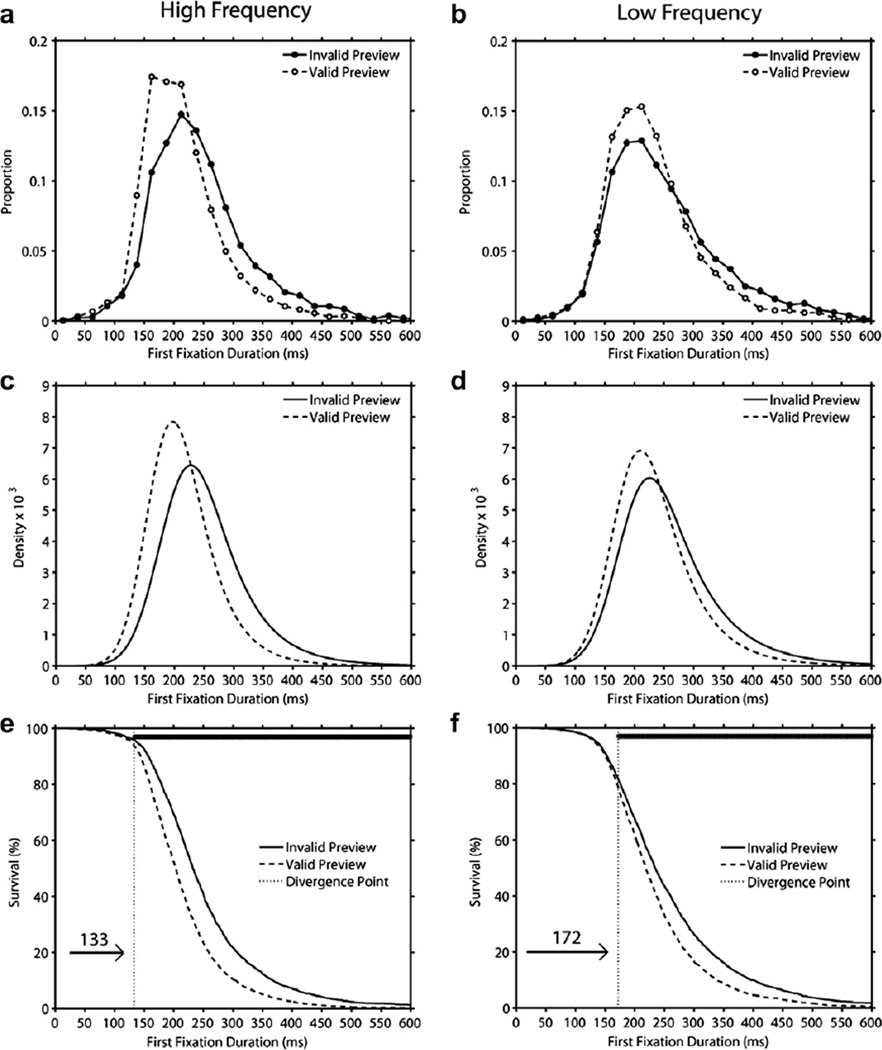
3.4. Analysis of distributions: Preview effects for HF and LF targets
The mean number of usable observations per cell, the ex-Gaussian parameter estimates, and the magnitude and significance of the preview effects for HF and LF targets are presented in Table 4. In addition, Fig. 6 displays histograms of first fixation duration (Panels a–b), density functions generated from the best-fitting ex-Gaussian parameters (Panels c–d), and survival curves (Panels e–f). As can be seen in Fig. 6 and Table 4, for both HF and LF targets, the invalid preview distribution was shifted to the right of the valid preview distribution resulting in a significant preview effect on µ (the mean of the Gaussian normal distribution). In addition, there was also a preview effect on the skew of the distributions (i.e., on τ) reflecting greater skew in invalid than valid preview condition for both HF and LF targets. Finally, the analysis of σ (the standard deviation of the Gaussian normal distribution) indicated a significant effect of preview reflecting greater variance in the invalid than valid preview condition [F(1,59) = 5.22, p < .05].
Fig. 6.
Distributions of first fixation duration by preview validity in high-frequency trials (Panel a) and low-frequency trials (Panel b), and ex-Gaussian density functions (Panels c and d), and survival curves (Panels e and f) that were generated from these distributions (the row of asterisks at the top of Panels e and f indicate time bins with a significant difference between the invalid and valid preview curves). See text for details.
Consistent with the present and prior findings of larger preview benefits for HF than LF targets, our survival analysis of preview effects determined divergence points of 133 ms and 172 ms in the HF and LF condition respectively. These divergence points also indicated that approximately 5% of first fixations on HF targets and 20% of first fixations on LF targets were shorter than the divergence point.
Importantly, the results of the survival analysis provide strong support for the conclusion that more lexical information is encoded from HF than LF parafoveal targets during the preview period resulting in larger parafoveal preview benefits in the former than the latter.
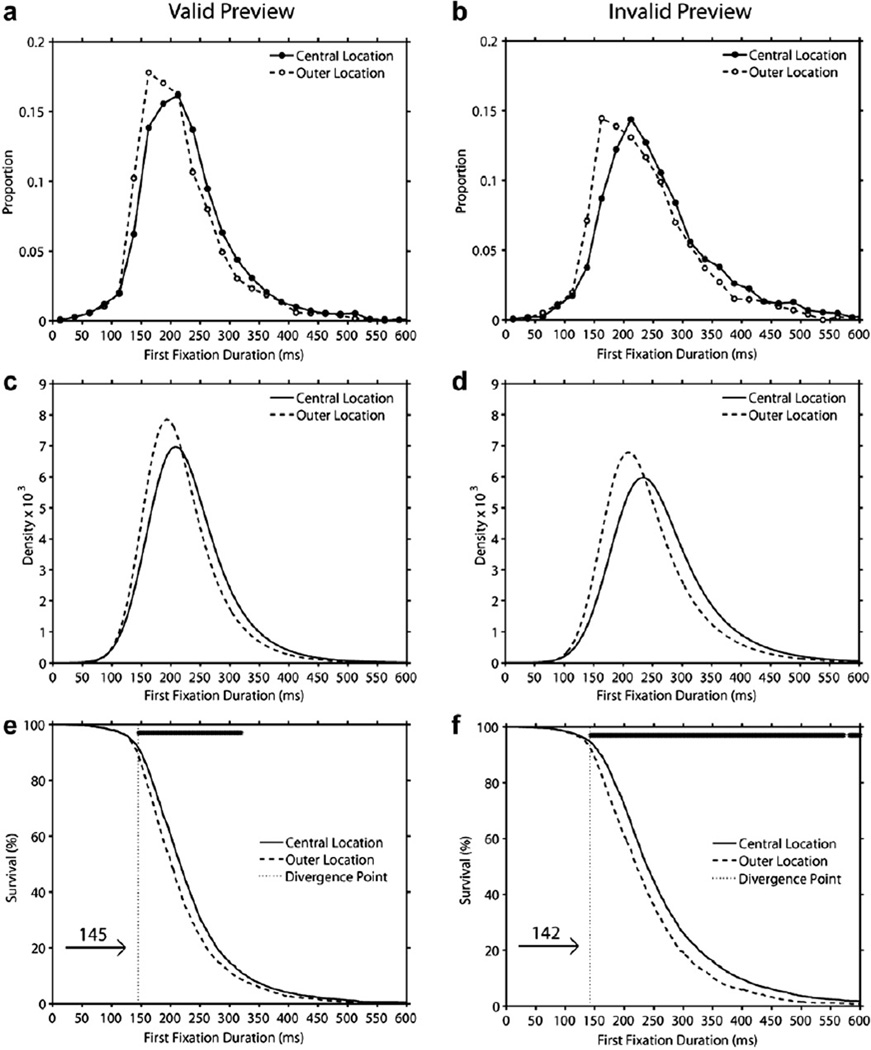
3.5. Analysis of distributions: Location effects in valid and invalid preview
The mean number of usable observations per cell, the ex-Gaussian parameter estimates, and the magnitude and significance of the location effects for valid and invalid preview are presented in Table 4. In addition, Fig. 7 displays histograms of first fixation duration (Panels a–b), density functions generated from the best-fitting ex-Gaussian parameters (Panels c–d), and survival curves (Panels e–f). As can be seen in Fig. 7 and Table 4, for both valid and invalid preview, the central location distribution was shifted to the right of the outer location distribution resulting in a significant location effect on µ (the mean of the Gaussian normal distribution). Although the magnitude of this shift was numerically larger in the invalid than valid preview condition, the location by preview interaction on µ was only marginally significant [F(1,59) = 3.14, p = .082]. In addition, there were no location effects on the skew of the distributions (i.e., on τ) in either the valid or invalid preview condition. Finally, the analysis of σ (the standard deviation of the Gaussian normal distribution) indicated a significant effect of location reflecting greater variance in the central than outer locations [F(1,59) = 218.40, p < .001], but no interaction with preview [F < 1].
Fig. 7.
Distributions of first fixation duration on central-location and outer-location in valid preview trials (Panel a) and invalid preview trials (Panel b), and ex-Gaussian density functions (Panels c and d), and survival curves (Panels e and f) that were generated from these distributions (the row of asterisks at the top of Panels e and f indicate time bins with a significant difference between the central and outer location curves). See text for details.
Thus, consistent with the interpretation of the location effect as a very rapid effect the influence of location was manifested as a shift in the distributions of first fixation duration, indicating that most first fixations were impacted by this variable (see Staub et al., 2010). This was confirmed by our survival analysis which determined divergence points of 145 ms and 142 ms in the valid and the invalid preview condition respectively. These early divergence points also indicated that only approximately 9.5% of first fixations in the valid preview condition and 6.5% in the invalid condition had durations that were shorter than the divergence point.
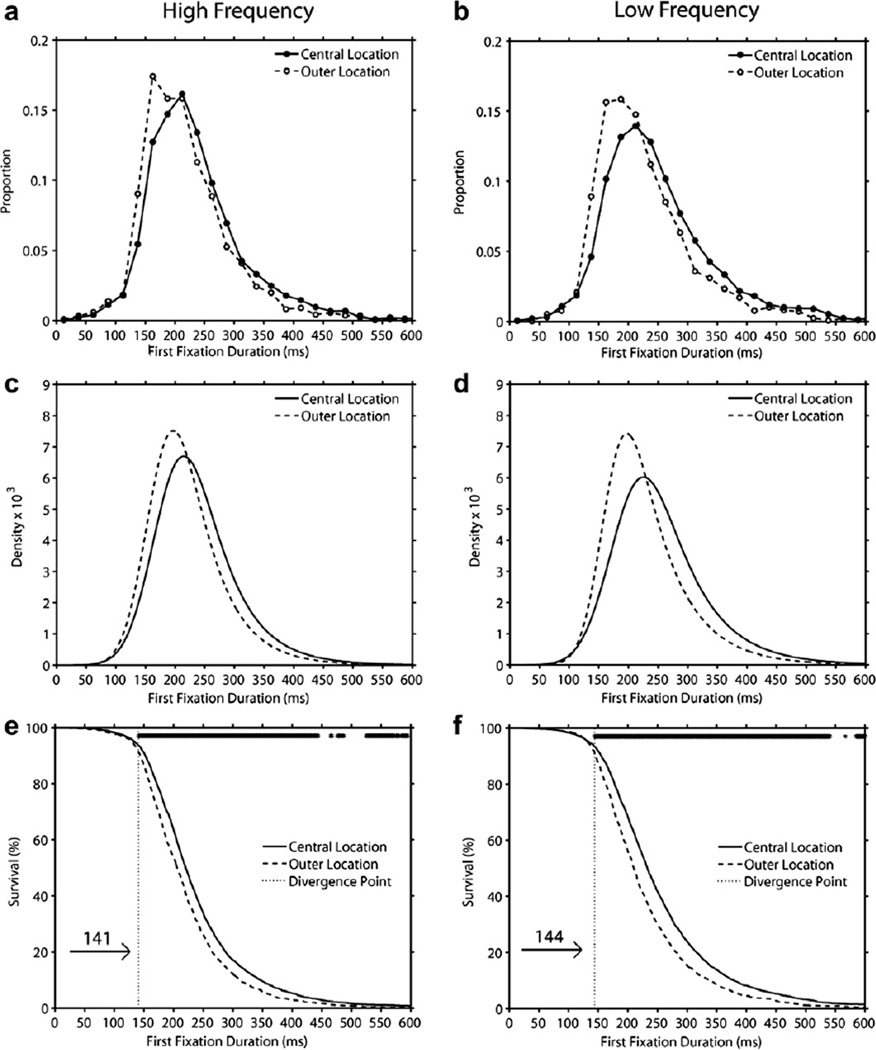
3.6. Analysis of distributions: Location effects for HF and LF targets
The mean number of usable observations per cell, the ex-Gaussian parameter estimates, and the magnitude and significance of the location effects for HF and LF targets are presented in Table 4. In addition, Fig. 8 displays histograms of first fixation duration (Panels a–b), density functions generated from the best-fitting ex-Gaussian parameters (Panels c–d), and survival curves (Panels e–f). As can be seen in Fig. 8 and Table 4, for both HF and LF targets, the central location distribution was shifted to the right of the outer location distribution resulting in a significant location effect on µ. Although the magnitude of this shift was numerically larger for LF than HF targets, the location by frequency interaction was not significant [F(1,59) = 1.99, p = .164]. In addition, regardless of word frequency, there were no location effects on skew. Furthermore, there was a significant effect of location on σ reflecting greater variance in the central than outer locations [F(1,59) = 218.40, p < .001], and this effect was stronger for LF than HF targets resulting in a significant interaction with frequency [F(1,59) = 4.08, p < .05]. Finally, the location × frequency survival analysis produced divergence points of 141 ms and 144 ms in the HF and LF condition respectively, and only approximately 7.5% of first fixations in either condition had durations that were shorter than the divergence point.
Fig. 8.
Distributions of first fixation duration on central-location and outer-location in high-frequency trials (Panel a) and low-frequency trials (Panel b), and ex-Gaussian density functions (Panels c–d), and survival curves (Panels e–f) that were generated from these distributions (the row of asterisks at the top of Panels e–f indicate time bins with a significant difference between the central and outer location curves). See text for details.
4. General discussion
The main finding to emerge from the present study was strong support for the direct lexical control hypothesis, and for the critical role of parafoveal processing in enabling direct control of fixation times in reading despite the tight temporal constraints caused by neural delays in the perceptual and oculomotor systems. The present methodology was similar to the procedure employed in several prior studies that manipulated both word frequency and preview validity (e.g., Inhoff & Rayner, 1986; Kennison & Clifton, 1995; Rayner et al., 2006; Schroyens, Vitu, Brysbaert, & d’Ydewalle, 1999; Sereno & Rayner, 2000; Vitu, 1991). However, while these prior studies primarily focused on a comparison of mean fixation times across conditions, the present investigation introduced a survival analysis as a method for deriving quantitative estimates of the earliest significant influence of word frequency on first fixation durations. Furthermore, replicatingStaub et al. (2010), we fitted the ex-Gaussian distribution to the distribution of first fixation durations on HF and LF targets under a valid preview condition. Extending the findings reported by Staub and colleagues, we documented the influence of preview validity on the values of the best-fitting ex-Gaussian parameters.
We would argue that the present results provided decisive convergent evidence in favor of direct lexical control of fixation times in reading. In particular, it is instructive to integrate the results from the present survival analysis with the findings from the electrophysiological studies that were discussed earlier (see Fig. 2). Collectively, these studies demonstrate rapid differential brain responses for HF vs. LF words as early as 120 ms post-stimulus. This estimate also suggests that following a 50–60 ms neural delay of visual input (i.e., the retina-brain lag; Clark et al., 1995; Foxe & Simpson, 2002; Mouchetant-Rostaing et al., 2000; Van Rullen & Thorpe, 2001), a minimum of 60–70 ms of lexical processing time is required prior to cortical differentiation between HF and LF words. Given that electrophysiological studies involved the presentation of single letter strings, no preview was available and consequently it is most appropriate to consider their timing estimates in conjunction with our divergence point estimate of 256 ms in the invalid preview condition (see Fig. 5, Panel f). Accordingly, if the first discernable effect of word frequency on first fixation duration occurred approximately 256 ms after the onset of the fixation on wordn, and the minimum latency for cortical discrimination between HF and LF words is 120 ms, then it follows that the minimum latency for available word frequency information to impact first fixation duration is 136 ms (i.e., the former minus the latter estimate). It is important to note that this 136 ms interval must include the transmission delay from the lexical processing system to the ocolomotor system, as well as any neural delays within the oculomotor system.
We can now turn to the consideration of the timing constraints that operate in the valid preview condition (i.e., in normal reading). As indicated by our survival analysis (see Fig. 5, Panel e), an effect of word frequency on first fixation duration in the valid preview condition is evident as early as 145 ms after the onset of the fixation on wordn. Given that in the absence of parafoveal preview (i.e., invalid preview condition) no effects of word frequency were observed for first fixations with duration of less than 256 ms, our results strongly suggest that any word frequency effects that were observed for first fixations with durations between 145 and 256 ms (69% of HF fixations and 60% of LF fixations) were solely due to lexical processing that was initiated parafoveally.
Furthermore, the interpretation of the findings from our survival analysis is also consistent with the results we obtained by fitting the distributions of first fixation duration with the ex-Gaussian distribution. Recall that when Staub et al. (2010) first applied this analysis method, they reported that in normal reading (i.e., with valid preview) the LF distribution was significantly shifted to the right of the HF distribution. Staub and colleagues interpreted this finding as strong evidence that word frequency effects on first-pass fixations constitute the rule rather than the exception. They also convincingly argued that the fact that the vast majority of first fixations were impacted by word frequency is consistent with direct lexical control of fixation times, and is clearly inconsistent with reading models which suggest that lexical effects are limited to a small subset of long fixations (e.g., Deubel, O’Regan, & Radach, 2000; Feng, 2006; McConkie & Yang, 2003; Yang, 2006; Yang & McConkie, 2001). In the present study, we replicatedStaub et al. (2010) in the valid preview condition. In addition, we demonstrated that the rightward shift effect was eliminated in the invalid preview condition. This finding strongly suggests that the rightward shift effect seen in normal reading is almost entirely due to parafoveal lexical processing.
Our evidence for direct lexical control is also consistent with the results of several “disappearing text” experiments, which in our estimation provide some of the most compelling evidence that first fixation durations are influenced by early lexical processing (Blythe, Liversedge, Joseph, White, & Rayner, 2009; Ishida & Ikeda, 1989; Liversedge et al., 2004; Rayner, Inhoff, Morrison, Slowiaczek, & Bertera, 1981; Rayner et al., 2003). In these experiments, HF and LF target words either disappeared or were replaced by a pattern mask 50–60 ms after being fixated. Remarkably, this seemingly disruptive manipulation had little or no effect on the magnitude of the influence of word frequency. These findings therefore indicate that the word frequency effect solely depends on the availability of normal parafoveal processing of target words coupled with visual information that is presented during the first 50 ms or so of visual input when these words are subsequently fixated. As such, the disappearing text paradigm as well as the present methodology dramatically underscore the importance of parafoveal processing in enabling direct lexical control during reading.
More generally, the present findings have several important implications for models of eye-movement control of fixation times in reading. It seems abundantly clear that in order to adequately account for the present findings as well as prior results (e.g., Rayner et al., 2003; Reingold et al., 2010; Sereno, Brewer, & O’Donnell, 2003; Sereno et al., 1998; Staub et al., 2010), a model of eye-movement control in reading must incorporate some form of a direct lexical control mechanism. This is the case because there is now very strong evidence that in normal reading the majority of first fixations on wordn are impacted by the lexical properties of that word. Furthermore, a unique contribution of the survival analysis that was introduced in the present study is that it provided a method for deriving precise quantitative estimates of the time-course of such an influence. Consequently, a promising direction for future research would involve applying the present paradigm to study the time-course of other lexical and non-lexical variables that are known to impact reading fixation times. Such estimates would be especially valuable given that in recent years the theoretical focus in the study of eye-movement control in reading has shifted away from qualitative models and toward quantitative implemented models (Rayner, 2009b)).
Indeed, we would argue that our comparison of the time-course of the word frequency and location variables provides an excellent illustration of the promise of the present approach. Specifically, in normal reading (i.e., with valid preview) word frequency and location appear to produce equally rapid effects on first fixation duration. However, the word frequency effect, but not the location effect, critically depends on parafoveally initiated lexical processing and consequently the time-course of the former but not the latter was strongly impacted by preview validity. Thus, the present findings strongly support the inference that the location effect is mediated by a direct non-lexical eye-movement control mechanism (see Table 1), which likely involves the processing of the efference copy of the landing saccade (Nuthmann et al., 2005, 2007; see also Engbert et al., 2005; McDonald et al., 2005; Reichle et al., 2009), and/or the processing of visual cues that are extracted very early during the first fixation (e.g., Vitu et al., 2007, 2001). More generally, our results as well as prior findings (e.g., Kliegl et al., 2006; McDonald et al., 2005; Nuthmann et al., 2005, 2007; Vitu et al., 2001; for a review see Vitu et al., 2007), have demonstrated that non-lexical variables, such as initial fixation location constitute important determinants of when the eyes move during reading. The importance of non-lexical variables is evident in the fact that most, if not all current eye-movement control models make provisions for them. For example, the E-Z Reader model (e.g., Reichle et al., 1998, 2009). Which might be described as being primarily a direct lexical control model allows visual acuity limitations to modulate the time course of saccade triggering. This model also assumes that the initial fixation location on a word directly modulates the propensity to refixate and hence the duration of that initial fixation. Accordingly, our evidence that fixation durations are rapidly affected by local lexical processing does not diminish the importance of visual and/or oculomotor variables, but instead indicates that models of eye-movement control during reading will have to incorporate a mixture of control mechanisms to adequately explain when and where readers move their eyes.
The present results also have more specific implications for the triggering and interference mechanisms of direct control. Specifically, the present results as well as prior studies impose very strict temporal constraints on the feasibility of any specific implementation of a direct control mechanism. For example, consistent with the assumptions adopted in more recent models incorporating a triggering mechanism (e.g., Reichle et al., 1998, 2009), complete lexical processing of wordn prior to the triggering of the terminating saccade is clearly not feasible given the very limited duration that is available for such processing. This restriction would obviously rule out models in which the triggering mechanism is the full identification of wordn (e.g., Just & Carpenter, 1980) because this event would occur too slowly. In addition, the findings from the present experiment suggest that once the triggering signal is initiated by the lexical processing system it takes a minimum of approximately 136 ms for the saccade to begin. The latter estimate is consistent with previous estimates of the minimum oculomotor latency associated with saccadic programming (e.g., Becker & Jurgens, 1979).
Although some of the timing constraints might differ between the triggering and the interference mechanism, the present findings provide the overall temporal constraints that must be satisfied by an interference mechanism. Specifically, the finding of a divergence point of 256 ms in the invalid preview condition suggests that, excluding the retina-brain lag (50–60 ms), there remains approximately 200 ms for the lexical processing system to establish the difficulty associated with the lexical processing of LF words and initiate the interference signal, which following a yet to be determined interval would act to delay, inhibit, or cancel a saccade (Engbert et al., 2002; Reilly & Radach, 2006; Yang, 2006). However, this interference mechanism would have to be rapid enough to allow lexical properties of a word to influence when the eyes move from that word; an interference mechanism in which the processing difficulty associated with wordn most often manifests itself as an inflated fixation on wordn+1 (e.g., Engbert et al., 2005) might be too slow to explain our results or the other evidence for direct lexical control (e.g., Rayner et al., 2003).
Thus, at the present, based on both experimental data and computational modeling, both the triggering and interference mechanisms of direct lexical control appear equally plausible. Furthermore, as mentioned above, these two types of mechanisms are not mutually exclusive. In order to illustrate this, in the remainder of this section we briefly propose a hybrid model that combines both a triggering and an interference mechanism. Imagine that the process of identifying a printed word can be described by a network of interconnected orthographic, phonological, and semantic features that converges towards states that correspond to stable configuration of those features—states that represent words (e.g., Perry, Ziegler, & Zorzi, 2007; Plaut, McClelland, Seidenberg, & Patterson, 1996; Seidenberg & McClelland, 1990; Van Orden & Goldinger, 1994). The rate of convergence will be influenced by a variety of factors, such as the frequency with which particular states (i.e., words) have been experienced, the degree of consistency in the grapheme-to-phoneme mappings, etc. This convergence rate would therefore be informative if it could somehow be monitored; for example, in the context of eye-movement control, this rate of convergence is predictive of when the word’s meaning will become available, so that a saccadic program can be initiated accordingly.
This intuition can be made more concrete using the E-Z Reader model (Reichle et al., 1998, 2009) as a framework for thinking about how the monitoring of lexical processing might be “harnessed” to make decisions about when to move the eyes. Remember that, in the E-Z Reader model, lexical processing is completed in two stages: The completion of the first stage, L1, causes the oculomotor system to start programming an eye movement to the next word, and the completion of the second stage, L2, causes attention to shift to the next word. A very natural way to use the rate of lexical processing (i.e., rate of convergence in a word-identification network) to implement direct lexical control of eye movements is to simply assume that there is another system that monitors the rate of convergence, and that generates two signals in response to how that rate changes. The first signal occurs whenever the rate of convergence reaches its maximum but then begins to slow (which would presumably occur prior to full convergence and thus be predictive of convergence); when this happens, the monitoring system signals the oculomotor system to start programming a saccade to the next word. The second signal occurs whenever the rate of converges falls below some minimum threshold; when this happens, the monitoring system sends a signal to the attention system so that it can start moving attention to the next word.
Although this hypothetical description of how the rate of lexical processing might be used to decide when to move can explain everything that the E-Z Reader model can explain, our new assumptions add a considerable amount of complexity (e.g., we have posited a system that monitors the rate of convergence in the word-identification network) and we have gained no additional explanatory power. In essence, we have simply provided a more precise description of the L1–L2 distinction and of how the cognitive-triggering mechanism is implemented in the E-Z Reader model. Our “model” becomes much more interesting, however, if we also consider how the lexical-convergence monitor might seamlessly be used to instantiate an interference mechanism. For example, the convergence monitoring system might also monitor aspects of convergence that are correlated with lexical-processing difficulty. One possibility is that the monitor is sensitive to irregularities in the rate of convergence because these might be predictive of problems (e.g., as might occur when a word is misidentified). When such an activation pattern is detected, we might assume that the monitor sends signals to both the oculomotor system and the attention system, so that both the eyes and attention can be directed towards the source of processing difficulty (wordn). In some cases, a saccadic program to move the eyes to wordn+1 will have already been initiated but will be labile; in such cases, the program will be delayed or canceled, resulting in a pause on wordn. In other cases, a saccadic program to move the eyes to wordn+1 will have been initiated and will be non-labile; in such cases, the saccade will be executed, resulting in a short fixation on wordn+1 followed by an inter-word regression back to wordn. In the latter cases, these short, rapid regressions would reflect lexical-processing difficulty rather than problems with higher-level language processing (e.g., syntactic parsing; Frazier & Rayner, 1982).
We presented this hybrid model in the present context simply to illustrate the possible intricacy that likely underlies eye-movement control of fixation times in complex visual tasks such as reading. The investigation of models of eye-movement control in reading constitutes an area of research in which considerable progress has been made over the past three decades. We hope that in the coming years the quest for an existence proof for direct lexical control would give way to the investigation of the possible mechanisms that might mediate this effect. The proposal we outlined here is based on our strong intuition that features from several existing models might be profitably combined in order to better approximate the true nature of eye-movement control in reading.
Acknowledgments
This research was supported by grant by an NSERC Grant to Eyal Reingold and by an NIH R01 Grant (HD053639) to Erik Reichle.
Footnotes
Given that direct control is defined in part based on an immediate adjustment of fixation duration, the fact that SWIFT assumes a delay in the inhibition due to lexical difficulty appears to contradict our classification of this mechanism as an example of direct lexical control. Admittedly, what constitutes an immediate influence is ill defined. However, given that the mechanism proposed by SWIFT is based on local processing with an occasional influence on wordn fixation duration, we elected to classify this mechanism as direct control. Note, that the original version of SWIFT did not include such a delay and consequently that version was certainly an instantiation of a direct control mechanism.
There have also been a few recent attempts to co-register eye movements and standard ERP measures (e.g., P200 and N400) to better understand the time course of lexical processing and saccadic programming (Dambacher & Kliegl, 2007; Dambacher, Kliegl, Hofmann, & Jacobs, 2006; Kliegl, Dambacher, Dimigen, Jacobs, & Sommer, 2012). Although this work was not intended to determine the minimal latencies at which lexical variables can have their effects, Dambacher et al. (2006, p. 101) do indicate that “… an epoch between 100 and 200 ms post-stimulus revealed differences between frequency classes…peaking at 170 ms…,” which is roughly consistent with our proposed timeline.
All of our preview by frequency analyses of means and distributions were also performed separately on the subset of trials in which initial landing position (i.e., location) was central. The qualitative pattern of results from these analyses was identical to the overall results.
References
- Assadollahi R, Pulvermüller F. Neuromagnetic evidence for early access to cognitive representations. NeuroReport. 2001;12:207–213. doi: 10.1097/00001756-200102120-00007. [DOI] [PubMed] [Google Scholar]
- Assadollahi R, Pulvermüller F. Early evidence of word length and frequency: A group study using MEG. NeuroReport. 2003;14:1183–1187. doi: 10.1097/00001756-200306110-00016. [DOI] [PubMed] [Google Scholar]
- Becker W, Jürgens R. An analysis of the saccadic system by means of double step stimuli. Vision Research. 1979;19:967–983. doi: 10.1016/0042-6989(79)90222-0. [DOI] [PubMed] [Google Scholar]
- Blythe HI, Liversedge SP, Joseph HSSL, White SJ, Rayner K. Visual information capture during fixations in reading for children and adults. Vision Research. 2009;49:1583–1591. doi: 10.1016/j.visres.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma H, DeVoogd AH. On the control of eye saccades in reading. Vision Research. 1974;14:273–284. [Google Scholar]
- Brysbaert M, New B. Moving beyond Kučera and Francis: A critical evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Behavior Research Methods, Instruments & Computers. 2009;41:977–990. doi: 10.3758/BRM.41.4.977. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fan S, Hillyard SA. Identification of early visual evoked potential generators by retinotopic and topographic analyses. Human Brain Mapping. 1995;2:170–187. [Google Scholar]
- Cousineau D, Brown S, Heathcote A. Fitting distributions using maximum likelihood: Methods and packages. Behavior Research Methods, Instruments, & Computers. 2004;36:742–756. doi: 10.3758/bf03206555. [DOI] [PubMed] [Google Scholar]
- Dambacher M, Kliegl R. Synchronizing timelines: Relations between fixation durations and N400 amplitudes during sentence reading. Brain Research. 2007;1155:147–162. doi: 10.1016/j.brainres.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Dambacher M, Kliegl R, Hofmann M, Jacobs AM. Frequency and predictability effects on event-related potentials during reading. Brain Research. 2006;1084:89–103. doi: 10.1016/j.brainres.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Deubel H, O’Regan K, Radach R. Attention, information processing and eye movement control. In: Kennedy A, Radach R, Heller D, Pynte J, editors. Reading as a perceptual process. Oxford: Elsevier; 2000. pp. 355–376. [Google Scholar]
- Duffy SA, Kambe G, Rayner K. The effect of prior disambiguating context on the comprehension of ambiguous words: Evidence from eye movements. In: Gorfein D, editor. On the consequences of meaning selection: Perspectives on resolving lexical ambiguity. Washington, DC: American Psychological Association; 2001. pp. 27–43. [Google Scholar]
- Duffy SA, Morris RK, Rayner K. Lexical ambiguity and fixation times in reading. Journal of Memory & Language. 1988;27:429–446. [Google Scholar]
- Dunn-Rankin P. The visual characteristics of words. Scientific American. 1978;238:122–130. doi: 10.1038/scientificamerican0178-122. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. Boca Raton, FL: Chapman & Hall; 1994. [Google Scholar]
- Ehrlich SE, Rayner K. Contextual effects on word perception and eye movements during reading. Journal of Verbal Learning and Verbal Behavior. 1981;20:641–655. [Google Scholar]
- Engbert R, Longtin A, Kliegl R. A dynamical model of saccade generation in reading based on spatially distributed lexical processing. Vision Research. 2002;42:621–636. doi: 10.1016/s0042-6989(01)00301-7. [DOI] [PubMed] [Google Scholar]
- Engbert R, Nuthmann A, Richter ED, Kliegl R. SWIFT: A dynamical model of saccade generation during reading. Psychological Review. 2005;112:777–813. doi: 10.1037/0033-295X.112.4.777. [DOI] [PubMed] [Google Scholar]
- Feng G. Eye movements as time-series random variables: A stochastic model of eye movement control in reading. Cognitive Systems Research. 2006;7(1):70–95. [Google Scholar]
- Feng G. Time course and hazard function: A distributional analysis of fixation duration in reading. Journal of Eye Movement Research. 2009;3:1–23. [Google Scholar]
- Feng G, Miller K, Shu H, Zhang H. Rowed to recovery: The use of phonological and orthographic information in reading Chinese and English. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:1079–1101. doi: 10.1037//0278-7393.27.4.1079. [DOI] [PubMed] [Google Scholar]
- Findlay JM, Walker R. A model of saccade generation based on parallel processing and competitive inhibition. Behavioral and Brain Sciences. 1999;22:661–675. doi: 10.1017/s0140525x99002150. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans: A framework for defining “early” visual processing. Experimental Brain Research. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Frazier L, Rayner K. Making and correcting errors during sentence comprehension: Eye movements in the analysis of structurally ambiguous sentences. Cognitive Psychology. 1982;14:178–210. [Google Scholar]
- Heathcote A, Brown S, Mewhort DJK. Quantile maximum likelihood estimation of response time distributions. Psychonomic Bulletin & Review. 2002;9:394–401. doi: 10.3758/bf03196299. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Ferreira F. Effects of foveal processing difficulty on the perceptual span in reading: Implications for attention and eye movement control. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:417–429. doi: 10.1037//0278-7393.16.3.417. [DOI] [PubMed] [Google Scholar]
- Inhoff AW, Rayner K. Parafoveal word processing during eye fixations in reading: Effects of word frequency. Perception & Psychophysics. 1986;40:431–439. doi: 10.3758/bf03208203. [DOI] [PubMed] [Google Scholar]
- Ishida T, Ikeda M. Temporal properties of information extraction during reading studied by a text-mask replacement technique. Journal of Optical Society A: Optics and Image Science. 1989;6:1624–1632. doi: 10.1364/josaa.6.001624. [DOI] [PubMed] [Google Scholar]
- Juhasz BJ, Rayner K. The role of age of acquisition and word frequency in reading: Evidence from eye fixation durations. Visual Cognition. 2006;13:846–863. [Google Scholar]
- Just MA, Carpenter PA. A theory of reading: From eye fixations to comprehension. Psychological Review. 1980;87:329–354. [PubMed] [Google Scholar]
- Just MA, Carpenter PA. The psychology of reading and language comprehension. Newton, MA: Allyn and Bacon; 1987. [Google Scholar]
- Kennison SM, Clifton C. Determinants of parafoveal preview benefit in high and low working memory capacity readers: Implications for eye movement control. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:68–81. doi: 10.1037//0278-7393.21.1.68. [DOI] [PubMed] [Google Scholar]
- Kliegl R, Dambacher M, Dimigen O, Jacobs AM, Sommer W. Eye movements and brain electric potentials during reading. Psychological Research. 2012;76(2):145–158. doi: 10.1007/s00426-011-0376-x. [DOI] [PubMed] [Google Scholar]
- Kliegl R, Engbert R. How tight is the link between lexical processing and saccade programs? Behavioral and Brain Sciences. 2003;26:491–492. [Google Scholar]
- Kliegl R, Nuthmann A, Engbert R. Tracking the mind during reading: The influence of past, present, and future words on fixation durations. Journal of Experimental Psychology: General. 2006;135:12–35. doi: 10.1037/0096-3445.135.1.12. [DOI] [PubMed] [Google Scholar]
- Liversedge SP, Rayner K, White SJ, Vergilino-Perez D, Findlay JM, Kentridge RW. Eye movements when reading disappearing text: Is there a gap effect in reading? Vision Research. 2004;44:1013–1024. doi: 10.1016/j.visres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Matin E. Saccadic suppression: A review. Psychological Bulletin. 1974;81:899–917. doi: 10.1037/h0037368. [DOI] [PubMed] [Google Scholar]
- McConkie GW, Dyre BP. Eye fixation durations in reading: Models of frequency distributions. In: Kennedy A, Radach R, Heller D, Pynte J, editors. Reading as a perceptual process. Amsterdam, Holland: North-Holland; 2000. pp. 683–700. [Google Scholar]
- McConkie GW, Kerr PW, Dyre BP. What are “normal” eye movements during reading: Toward a mathematical description. In: Ygge J, Lennerstand G, editors. Eye movements in reading. New York, NY: Pergamon Press; 1994. pp. 315–327. [Google Scholar]
- McConkie GW, Kerr PW, Reddix MD, Zola D. Eye movement control during reading: I. The location of initial eye fixations in words. Vision Research. 1988;28:1107–1118. doi: 10.1016/0042-6989(88)90137-x. [DOI] [PubMed] [Google Scholar]
- McConkie GW, Kerr PW, Reddix MD, Zola D, Jacobs AM. Eye movement control during reading: II. Frequency of refixating a word. Perception & Psychophysics. 1989;46:245–253. doi: 10.3758/bf03208086. [DOI] [PubMed] [Google Scholar]
- McConkie GW, Yang S-N. How cognition affects eye movements during reading. In: Hyönä J, Radach R, Deubel H, editors. The mind’s eye: Cognitive and applied aspects of eye movement research. Oxford, UK: Elsevier; 2003. pp. 413–427. [Google Scholar]
- McDonald SA, Carpenter RHS, Shillcock RC. An anatomically-constrained, stochastic model of eye movement control in reading. Psychological Review. 2005;112:814–840. doi: 10.1037/0033-295X.112.4.814. [DOI] [PubMed] [Google Scholar]
- Morrison RE. Manipulation of stimulus onset delay in reading: Evidence for parallel programming of saccades. Journal of Experimental Psychology: Human Perception and Performance. 1984;10:667–682. doi: 10.1037//0096-1523.10.5.667. [DOI] [PubMed] [Google Scholar]
- Mouchetant-Rostaing Y, Giard M-H, Bentin S, Aguera P-E, Pernier J. Neurophysiological correlates of face gender processing in humans. European Journal of Neuroscience. 2000;12:303–310. doi: 10.1046/j.1460-9568.2000.00888.x. [DOI] [PubMed] [Google Scholar]
- Nuthmann A, Engbert R, Kliegl R. Mislocated fixations during reading and the inverted optimal viewing position effect. Vision Research. 2005;45:2201–2217. doi: 10.1016/j.visres.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Nuthmann A, Engbert R, Kliegl R. The IOVP effect in mindless reading: Experiment and modelling. Vision Research. 2007;47:990–1002. doi: 10.1016/j.visres.2006.11.005. [DOI] [PubMed] [Google Scholar]
- O’Regan JK. Eye movements and reading. In: Kowler E, editor. Eye movements and their role in visual and cognitive processes. Amsterdam: Elsevier; 1990. pp. 395–453. [Google Scholar]
- O’Regan JK. Optimal viewing position in words and the strategy-tactics theory of eye movements in reading. In: Rayner K, editor. Eye movements and visual cognition: Scene perception and reading. New York: Springer-Verlag; 1992. pp. 333–354. [Google Scholar]
- Penolazzi B, Hauk O, Pulvermuller F. Early semantic context integration and lexical access as revealed by event-related brain potentials. Biological Psychology. 2007;74:374–388. doi: 10.1016/j.biopsycho.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Perry C, Ziegler JC, Zorzi M. Nested incremental modelling in the development of computational theories: The CDP t model or reading aloud. Psychological Review. 2007;114:273–315. doi: 10.1037/0033-295X.114.2.273. http://dx.doi.org/10.1037/0033-295X.114.2.273. [DOI] [PubMed] [Google Scholar]
- Plaut D, McClelland JL, Seidenberg MS, Patterson KE. Understanding normal and impaired word reading: Computational principles in quasi-regular domains. Psychological Review. 1996;103:56–115. doi: 10.1037/0033-295x.103.1.56. http://dx.doi.org/10.1037//0033-295X.103.1.56. [DOI] [PubMed] [Google Scholar]
- Pollatsek A, Rayner K. Eye movements and lexical access in reading. In: Balota DA, Flores d’Arcais GB, Rayner K, editors. Comprehension processes in reading. Hillsdale, NJ: Erlbaum; 1990. pp. 143–164. [Google Scholar]
- Pollatsek A, Reichle ED, Rayner K. Tests of the E-Z Reader model: Exploring the interface between cognition and eye-movement control. Cognitive Psychology. 2006;52:1–56. doi: 10.1016/j.cogpsych.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Pynte J. Lexical control of within-word eye movements. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:958–969. doi: 10.1037//0096-1523.22.4.958. [DOI] [PubMed] [Google Scholar]
- Rayner K. The perceptual span and peripheral cues in reading. Cognitive Psychology. 1975;7:65–81. [Google Scholar]
- Rayner K. Eye guidance in reading: Fixation location within words. Perception. 1979;8:21–30. doi: 10.1068/p080021. [DOI] [PubMed] [Google Scholar]
- Rayner K. Eye movements in reading and information processing: 20 years of research. Psychological Bulletin. 1998;124(3):372–422. doi: 10.1037/0033-2909.124.3.372. [DOI] [PubMed] [Google Scholar]
- Rayner K. The Thirty-Fifth Sir Frederick Bartlett Lecture: Eye movements and attention in reading, scene perception, and visual search. The Quarterly Journal of Experimental Psychology. 2009a;62:1457–1506. doi: 10.1080/17470210902816461. [DOI] [PubMed] [Google Scholar]
- Rayner K. Eye movements in reading: Models and data. Journal of Eye Movement Research. 2009b;2:1–10. [PMC free article] [PubMed] [Google Scholar]
- Rayner K, Ashby J, Pollatsek A, Reichle ED. The effects of frequency and predictability on eye fixations in reading: Implications for the E-Z Reader model. Journal of Experimental Psychology: Human Perception and Performance. 2004;30:720–732. doi: 10.1037/0096-1523.30.4.720. [DOI] [PubMed] [Google Scholar]
- Rayner K, Duffy SA. Lexical complexity and fixation times in reading: Effects of word frequency, verb complexity, and lexical ambiguity. Memory & Cognition. 1986;14:191–201. doi: 10.3758/bf03197692. [DOI] [PubMed] [Google Scholar]
- Rayner K. Eye movements and cognitive processes in reading, visual search, and scene perception. In: Findlay JM, Walker R, Kentridge PW, editors. Eye movement research: Mechanisms, processes and applications. Amsterdam: North Holland; 1995. pp. 3–22. [Google Scholar]
- Rayner K, Inhoff AW, Morrison RE, Slowiaczek ML, Bertera JH. Masking of foveal and parafoveal vision during eye fixations in reading. Journal of Experimental Psychology: Human Perception and Performance. 1981;7:167–179. doi: 10.1037//0096-1523.7.1.167. [DOI] [PubMed] [Google Scholar]
- Rayner K, Liversedge SP, White SJ. Eye movements when reading disappearing text: The importance of the word to the right of fixation. Vision Research. 2006;46:310–323. doi: 10.1016/j.visres.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Rayner K, Liversedge SP, White SJ, Vergilino-Perez D. Reading disappearing text: Cognitive control of eye movements. Psychological Science. 2003;14:385–389. doi: 10.1111/1467-9280.24483. [DOI] [PubMed] [Google Scholar]
- Rayner K, Pollatsek A. Eye movement control during reading: Evidence for direct control. Quarterly Journal of Experimental Psychology. 1981;33A:351–373. doi: 10.1080/14640748108400798. [DOI] [PubMed] [Google Scholar]
- Rayner K, Pollatsek A. The psychology of reading. Englewood Cliffs, NJ: Prentice Hall; 1989. [Google Scholar]
- Rayner K, Sereno SC, Raney GE. Eye movement control in reading: A comparison of two types of models. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:1188–1200. doi: 10.1037//0096-1523.22.5.1188. [DOI] [PubMed] [Google Scholar]
- Rayner K, Well AD. Effects of contextual constraint on eye movements in reading: A further examination. Psychonomic Bulletin & Review. 1996;3:504–509. doi: 10.3758/BF03214555. [DOI] [PubMed] [Google Scholar]
- Reichle ED. Serial attention models of reading. In: Liversedge SP, Gilchrist ID, Everling S, editors. Oxford handbook on eye movements. Oxford, UK: Oxford University Press; 2011. pp. 767–786. [Google Scholar]
- Reichle ED, Liversedge SP, Pollatsek A, Rayner K. Encoding multiple words simultaneously in reading is implausible. Trends in Cognitive Sciences. 2009;13:115–119. doi: 10.1016/j.tics.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Reichle ED, Perfetti CA. Morphology in word identification: A word experience model that accounts for morpheme frequency effects. Scientific Studies of Reading. 2003;7:219–237. [Google Scholar]
- Reichle ED, Pollatsek A, Fisher DL, Rayner K. Toward a model of eye movement control in reading. Psychological Review. 1998;105:125–157. doi: 10.1037/0033-295x.105.1.125. [DOI] [PubMed] [Google Scholar]
- Reichle ED, Pollatsek A, Rayner K. E-Z Reader: A cognitive-control, serial-attention model of eye-movement behavior during reading. Cognitive Systems Research. 2006;7:4–22. [Google Scholar]
- Reichle ED, Rayner K, Pollatsek A. Eye movement control in reading: Accounting for initial fixation locations and refixations within the E-Z reader model. Vision Research. 1999;39:4403–4411. doi: 10.1016/s0042-6989(99)00152-2. [DOI] [PubMed] [Google Scholar]
- Reichle ED, Rayner K, Pollatsek A. The E-Z Reader model of eye movement control in reading: Comparison to other models. Behavioral and Brain Sciences. 2003;26:445–476. doi: 10.1017/s0140525x03000104. [DOI] [PubMed] [Google Scholar]
- Reichle ED, Tokowicz N, Liu Y, Perfetti CA. Testing an assumption of the E-Z Reader model of eye-movement control during reading: Using event-related potentials to examine the familiarity check. Psychophysiology. 2011;48:993–1003. doi: 10.1111/j.1469-8986.2011.01169.x. [DOI] [PubMed] [Google Scholar]
- Reilly R. A connectionist framework for modeling eye movement control in reading. In: d’Ydewalle G, Van Rensbergen J, editors. Perception and cognition: Advances in eye movement research. Amsterdam, Holland: North-Holland; 1993. pp. 193–212. [Google Scholar]
- Reilly R, Radach R. Some empirical tests of an interactive activation model of eye movement control in reading. Cognitive Systems Research. 2006;7:34–55. [Google Scholar]
- Reilly R, Radach R. Foundations of an interactive activation model of eye movement control in reading. In: Hyona J, Radach R, Deubel H, editors. The mind’s eye: Cognitive and applied aspects of eye movement research. Amsterdam: North-Holland; 2003. pp. 429–456. [Google Scholar]
- Reingold EM, Rayner K. Examining the word identification stages hypothesized by the E-Z Reader model. Psychological Science. 2006;17:742–746. doi: 10.1111/j.1467-9280.2006.01775.x. [DOI] [PubMed] [Google Scholar]
- Reingold EM, Sheridan H. Eye movements and visual expertise in chess and medicine. In: Liversedge SP, Gilchrist ID, Everling S, editors. Oxford handbook on eye movements. Oxford, UK: Oxford University Press; 2011. pp. 767–786. [Google Scholar]
- Reingold EM, Stampe DM. Saccadic inhibition in voluntary and reflexive saccades. Journal of Cognitive Neuroscience. 2002;14:371–388. doi: 10.1162/089892902317361903. [DOI] [PubMed] [Google Scholar]
- Reingold EM, Stampe DM. Saccadic inhibition in reading. Journal of Experimental Psychology: Human Perception and Performance. 2004;30:194–211. doi: 10.1037/0096-1523.30.1.194. [DOI] [PubMed] [Google Scholar]
- Reingold EM, Stampe DM. Saccadic inhibition in complex visual tasks. In: Becker W, Deubel H, Mergner T, editors. Current oculomotor research: Physiological and psychological aspects. London: Plenum; 1999. pp. 249–255. [Google Scholar]
- Reingold EM, Stampe DM. Using the saccadic inhibition paradigm to investigate saccadic control in reading. In: Hyona J, Radach R, Deubel H, editors. The mind’s eye: Cognitive and applied aspects of eye movements. Amsterdam: Elsevier Science Publishers; 2003. pp. 347–360. [Google Scholar]
- Reingold EM, Stampe DM. Saccadic inhibition and gaze contingent research paradigms. In: Kennedy A, Radach R, Heller D, Pynte J, editors. Reading as a perceptual process. Amsterdam: Elsevier; 2000. pp. 119–145. [Google Scholar]
- Reingold EM, Yang J, Rayner K. The time course of word frequency and case alternation effects on fixation times in reading: Evidence for lexical control of eye movements. Journal of Experimental Psychology: Human Perception and Performance. 2010;36:1677–1683. doi: 10.1037/a0019959. [DOI] [PubMed] [Google Scholar]
- Richter EM, Engbert R, Kliegl R. Current advances in SWIFT. Cognitive Systems Research. 2006;7:23–33. [Google Scholar]
- Salvucci DD. An integrated model of eye movements and visual encoding. Cognitive Systems Research. 2001;1:201–220. [Google Scholar]
- Schroyens W, Vitu F, Brysbaert M, d’Ydewalle G. Eye movement control during reading: Foveal load and parafoveal processing. Quarterly Journal of Experimental Psychology. 1999;52A(4):1021–1046. doi: 10.1080/713755859. [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. More words but still no lexicon: A reply to Besner et al. (1990) Psychological Review. 1990;97:447–452. http://dx.doi.org/10.1037//0033-295X.97.3.447. [Google Scholar]
- Sereno SC, Brewer CC, O’Donnell PJ. Context effects in word recognition: Evidence for early interactive processing. Psychological Science. 2003;14:328–333. doi: 10.1111/1467-9280.14471. [DOI] [PubMed] [Google Scholar]
- Sereno SC, Rayner K. Spelling-sound regularity effects on eye fixations in reading. Perception & Psychophysics. 2000;62:402–409. doi: 10.3758/bf03205559. [DOI] [PubMed] [Google Scholar]
- Sereno SC, Rayner K. Measuring word recognition in reading: Eye movements and event-related potentials. Trends in Cognitive Science. 2003;7:489–493. doi: 10.1016/j.tics.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Sereno SC, Rayner K, Posner MI. Establishing a time-line of word recognition: Evidence from eye movements and event-related potentials. NeuroReport. 1998;9:2195–2200. doi: 10.1097/00001756-199807130-00009. [DOI] [PubMed] [Google Scholar]
- Sheridan H, Reingold EM, Daneman M. Using puns to study contextual influences on lexical ambiguity resolution: Evidence from eye movements. Psychonomic Bulletin & Review. 2009;16(5):875–881. doi: 10.3758/PBR.16.5.875. [DOI] [PubMed] [Google Scholar]
- Starr MS, Rayner K. Eye movements during reading: Some current controversies. Trends in Cognitive Sciences. 2001;5:156–163. doi: 10.1016/s1364-6613(00)01619-3. [DOI] [PubMed] [Google Scholar]
- Staub A, White SJ, Drieghe D, Hollway EC, Rayner K. Distributional effects of word frequency on eye fixation durations. Journal of Experimental Psychology: Human Perception and Performance. 2010;36:1280–1293. doi: 10.1037/a0016896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibadeau R, Just MA, Carpenter PA. A model of the time course and content of reading. Cognitive Science. 1982;6:157–203. [Google Scholar]
- Van Orden GC, Goldinger SD. Interdependence of form and function in cognitive systems explains perception of printed words. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:1269–1291. http://dx.doi.org/10.1037//0096-1523.20.6.1269. [PubMed] [Google Scholar]
- Van Rullen R, Thorpe S. The time course of visual processing: From early perception to decision-making. Journal of Cognitive Neuroscience. 2001;13:454–461. doi: 10.1162/08989290152001880. [DOI] [PubMed] [Google Scholar]
- Vitu F. The influence of parafoveal preprocessing and linguistic context on the optimal landing position effect. Perception and Psychophysics. 1991;50:58–75. doi: 10.3758/bf03212205. [DOI] [PubMed] [Google Scholar]
- Vitu F, Lancelin D, d’Unienville VM. A perceptual-economy account for the inverted-optimal viewing position effect. Journal of Experimental Psychology: Human Perception and Performance. 2007;33(5):1220–1249. doi: 10.1037/0096-1523.33.5.1220. [DOI] [PubMed] [Google Scholar]
- Vitu F, McConkie GW, Kerr P, O’Regan JK. Fixation location effects on fixation durations during reading: An inverted optimal viewing position effect. Vision Research. 2001;41:3511–3531. doi: 10.1016/s0042-6989(01)00166-3. [DOI] [PubMed] [Google Scholar]
- Vitu F, O’Regan JK, Mittau M. Optimal landing position in reading isolated words and continuous text. Perception & Psychophysics. 1990;47:583–600. doi: 10.3758/bf03203111. [DOI] [PubMed] [Google Scholar]
- White SJ. Eye movement control during reading: Effects of word frequency and orthographic familiarity. Journal of Experimental Psychology: Human Perception and Performance. 2008;24:205–223. doi: 10.1037/0096-1523.34.1.205. [DOI] [PubMed] [Google Scholar]
- Yang S-N. An oculomotor-based model of eye movements in reading: The competition/interaction model. Cognitive Systems Research. 2006;7:56–59. [Google Scholar]
- Yang S-N, McConkie GW. Eye movements during reading: A theory of saccade initiation times. Vision Research. 2001;41:3567–3585. doi: 10.1016/s0042-6989(01)00025-6. [DOI] [PubMed] [Google Scholar]