Abstract
Breast cancer is the most frequent malignancy diagnosed in women. Approximately 70% of breast tumors express the estrogen receptor (ER). Tamoxifen and aromatase inhibitors (AIs) are the most common and effective therapies for patients with ERα-positive breast cancer. Alone or combined with chemotherapy, tamoxifen significantly reduces disease progression and is associated with more favorable impact on survival in patients. Unfortunately, endocrine resistance occurs, either de novo or acquired during the course of the treatment. The mechanisms that contribute to hormonal resistance include loss or modification in the ERα expression, regulation of signal transduction pathways, altered expression of specific microRNAs, balance of co-regulatory proteins, and genetic polymorphisms involved in tamoxifen metabolic activity. Because of the clinical consequences of endocrine resistance, new treatment strategies are arising to make the cells sensitive to tamoxifen. Here, we will review the current knowledge on mechanisms of endocrine resistance in breast cancer cells. In addition, we will discuss novel therapeutic strategies to overcome such resistance. Undoubtedly, circumventing endocrine resistance should help to improve therapy for the benefit of breast cancer patients.
Keywords: endocrine therapy, breast cancer, estrogen receptor, hormonal resistance
1. Introduction
Estrogens play an important role in the pathogenesis of breast cancer through the estrogen receptor alpha (ERα). Approximately 70% of breast tumors are ER-positive (+) [1]. Consequently, therapies aim to either reduce estrogen levels or to block signaling through ERα. Three classes of antihormone endocrine agents have been used: selective estrogen receptor modulators (SERMs, e.g., tamoxifen) that block the activity ER; estrogen synthesis inhibitors (e.g., aromatase inhibitors (AIs) such as anastrozole, letrozole, and exemestane); and selective estrogen receptor downregulators (SERD, e.g., ICI 182,780 (Fulvestrant) and ICI 164,384) that induce destabilization and degradation of ER. These approaches have proven clinical efficacy in women with ER(+) breast cancer [2].
For more than three decades, tamoxifen has been the basis of hormonal therapy in both early and advanced breast cancer patients [3]. However, almost 50% of patients with advanced disease do not respond to first line treatment with tamoxifen (de novo resistance); furthermore, a significant percentage of patients experience tamoxifen relapse, despite an initial positive drug response (acquired resistance) [4,5]. This differential response might reside in the expression (de novo or acquired) of specific molecules involved in different signaling pathways, which eventually could be used as predictive biomarkers of resistance. Moreover, these markers may be used to select patients that might benefit from additional targeted treatments aside from ER [6,7].
Tamoxifen resistance occurs in breast cancer patients and is the main problem limiting the efficacy of the treatment. AIs therapy (either as initial treatment or sequentially after tamoxifen) seems to produce more benefits than the use of tamoxifen alone and might be effective in tamoxifen-resistant patients. Nevertheless, the response rate to these compounds is only slightly higher as compared with tamoxifen in patients with advanced breast cancer, and both de novo and acquired resistance to AIs also occur [8–10]. Recently, fulvestrant has demonstrated clinical efficacy among patients who relapsed for a second time after responding to tamoxifen and AIs; more investigations are being conducted to explore the clinical potential of this approach [11,12].
However, despite the incorporation of more potent endocrine agents, resistance to all forms of endocrine therapy remains a major problem. A better understanding of the molecular mechanisms of endocrine resistance might enable the use of novel strategies for therapeutic intervention. The aim of this review is to summarize some of the key novel findings on the mechanisms of endocrine resistance and its therapeutic implications. First, we will provide a general overview of ERs. Then, we will focus on the different mechanisms proposed in hormonal resistance and discuss several examples of combined therapy as a potential approach to overwhelm such resistance. Finally, we will provide some conclusions and remarks on the strategies and potential future directions in this cancer field.
2. ER Action and Function
ER belongs to a superfamily of nuclear receptors that serve as transcription factors [13]. ERα and ERβ are produced by distinct genes located on chromosome 6 and 14, respectively [14–16] Both receptors are present in normal breast tissue, but only ERα is associated with breast cancer initiation and progression, while ERβ function in breast cancer is still unclear [17]. However, several studies have described that ERβ exerts an opposite effect to ERα, inhibiting the ability of estrogens to stimulate proliferation. Actually, ERβ impaired expression contributes to tumor progression [18]. Accordingly, high ERβ expression has been correlated with better survival [19].
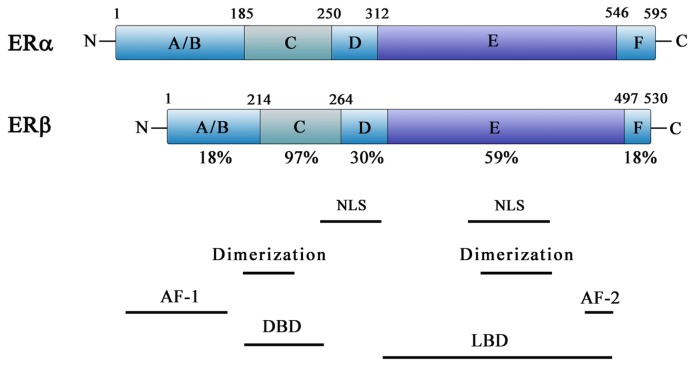
Both receptors share a common structural architecture; they are composed of six domains, designated A–F (Figure 1) [20,21]. The N-terminal or A/B domain contains the activation function 1 (AF1), a region responsible for the transcriptional activity of ER in the absence of ligand [17,22]. The C domain, known as the DNA-binding domain (DBD), is composed by a two zinc-finger structure that plays an important role in receptor dimerization and binding of receptors to specific DNA sequences [14,23–26]. The D domain is a hinge region between the C and E domains and includes a nuclear localization signal [27,28]. The E domain, referred to as ligand-binding domain (LBD), consists of a second nuclear localization signal, a dimerization site and a twelve-helix region involved in ligand binding [29]. The E domain also harbors the activation function 2 (AF2), responsible for the ligand-dependent activation of ER [30,31]. The F domain located at the C-terminal is a small region that modulates both AF1 and AF2, although it is unnecessary for transcriptional activation [17,22,31]. ERα and ERβ have a high degree of homology in their DBD (97% amino acid identity) and LBD (59% amino acid identity) [32,33].
Figure 1.
Schematic representation of functional domains of human ERα and ERβ. The A/B domain at the N-terminal contains AF-1 site. The C domain includes the DNA-binding domain (DBD) and a dimerization site. The D domain contains a nuclear localization signal. The E/F domain is located at the C-terminal and comprises the ligand binding, as well as the AF-2 domain, a second nuclear localization signal, and another dimerization site.
ERα is associated to cell proliferation and survival through two different mechanisms: genomic and non-genomic signaling pathway. The classical genomic pathway is the most characterized ER signaling pathway and is initiated by the ligand binding to its receptor. The binding induces a conformational change and dissociation of their chaperon proteins, leading to receptor dimerization and translocation to the nucleus [17]. This ligand-ER complex binds to the consensus sequence, known as estrogen-response element (ERE), directly or indirectly through protein-protein interactions with activator protein 1 (AP1) or SP 1 sites in the promoter region of target genes, resulting in recruitment of coregulatory proteins (coactivators or corepressors), which can either enhance or repress ER transcriptional activity depending on the specificity of the ligand [34,35]. Microarray analysis showed that approximately 70% of estrogen-responsive genes were downregulated by estradiol in MCF-7 cells, those genes were transcriptional repressors or anti-proliferative and pro-apoptotic genes, while genes involved in the induction of cell proliferation were upregulated [36].
In addition to their classic genomic actions mediated through activation of nuclear ERs, estrogens can also elicit rapid responses that are often non-genomic. These responses are mediated via ERs localized either near or at the plasma membrane within caveolar rafts, as well as in other membrane domains [37–39]. The plasma membrane ER may interact with many other proteins including adaptor proteins, G-proteins, Src, growth factor receptors (EGFR, IGFR1, HER2), cytoplasmic kinases (MAPKs, PI3K, AKT), and signaling enzymes (adenyl cyclase, nitric-oxide synthase), mediating mechanisms independent of gene transcription [40–44]. Some of these non-genomic estrogen actions have been attributed to either ERs or its truncated forms. However, the orphan GPCR-like protein, GPR30 (G protein coupled receptor 30) [45,46], recently renamed GPER [47], has also been suggested to be involved. Several data support a role for GPER as an intermediary element in the rapid, non-genomic actions of estrogens [48].
Both genomic and non-genomic pathways interact with each other in crosstalk. Whereas nuclear ER induces the expression of transforming growth factor (TGF)α and amphiregulin (AR), both TGFα and AR are able to bind and stimulate EGFR or EGFR/HER2 and consequently activate MAPK and AKT [34–36]. On the other hand, membrane ER can bind to caveolin 1 and activate specific G proteins and src, which activates matrix metalloproteinases that cleave transmembrane precursors of heparin binding-EGF (HB-EGF), an EGFR ligand [27]. In addition, several cytokines, growth factors, EGFR ligands, IGF1R and pathways such as MAPK/ERK, PI3K/AKT, p90rsk and p38 MAPK phosphorylate ER at key positions in the AF-1 (serines 118 and 167, and threonine 311) and other domains; this process is defined as ligand-independent activation and results in the activation of the ER genomic pathway [49–53]. It has been demonstrated that estrogen acts through human GPER to activate a stimulatory G protein, resulting in the stimulation of adenylyl cyclase activity and increased cAMP production by plasma membranes of ER-negative (−) cells and GPER-transfected cells. In turn, the estrogen/GPER complex can also release epidermal growth factor, EGF-related ligands, and transactivate the EGF receptor (EGFR) [54,55]. To date, GPER has been implicated in the development and progression of breast cancer [56,57]. Thus, it is important to consider both genomic and non-genomic signaling pathways of ER in the regulation of intracellular signaling cascades that ultimately contribute to breast cancer progression.
3. Mechanisms of Endocrine Resistance
Several mechanisms of resistance to endocrine therapy have been hypothesized and a include loss of ERs, different signaling pathways, altered expression of microRNAs, as well as tamoxifen metabolism. In addition, both genomic and non-genomic crosstalk and the complex interrelation between the estrogen receptors subtypes and growth factors contribute to endocrine resistance developing [58]. In the following, we will describe the main proposed mechanisms responsible for endocrine resistance.
3.1. Loss or Modification in the ER Expression
ER expression is the main predictor of response to endocrine therapy, and lack of expression of ER is the principal mechanism of de novo resistance to hormonal therapy. Several mechanisms have been proposed to explain the absence of ER expression. These mechanisms involve epigenetic changes such as aberrant methylation CpG islands of the ER promoter and histone deacetylation, resulting in a compact nucleosome structure that limits transcription [59–62]. The co-treatment with inhibitors of DNA methyltransferase-1 (DNMT-1, such as 5-aza-2-deoxycytidine (AZA)) and histone deacetylase (HDAC, such as Trichostatin A (TSA) and suberoylanilide hydroxamic acid (SAHA)) induce ER gene expression in ER(−) breast cancer cells and restore sensitivity to antiestrogen [59,63–66]. In ER(−) MDA-MB-231 cells, which overexpress EGFR, SAHA may not only reactivate silenced ER, but also simultaneously deplete EGFR expression and abolish EGF-initiated signaling pathways including phosphorylated PAK1, p38MAPK and AKT [67]. In vitro and in vivo studies showed that the treatment with the histone deacetylase inhibitor entinostat (ENT) increased the expression of ERα and aromatase. Notably, ERα and aromatase upregulation resulted in sensitization of breast cancer cells to estrogen and letrozole [68]. Moreover, Scriptaid (a novel HDAC inhibitor) has also shown to cause re-expression of functional ER, induce inhibition of tumor growth, and sensitize hormone-resistant breast cancer cells to tamoxifen [69,70].
It has been also hypothesized that loss of ER expression might be responsible for acquired resistance to endocrine therapy. However, only 17%–28% patients with acquired resistance do not express ER [71–73], and ER(+) patients responding initially to tamoxifen, usually do not lose expression of ER after developing resistance to therapy. Approximately 20% of patients respond to second-line treatment with either AIs or fulvestrant, which may mean that patients with acquired resistance to tamoxifen still express ER [74,75].
Other mechanisms proposed in the loss of ER expression are hypoxia, overexpression of EGFR or HER2, MAPKs hyperactivation, and involvement of p53 and pRb2/p130. Hypoxia induces proteasome-dependent degradation of ER in ZR-75 breast cancer cells, leading to decreased protein levels [76]. High expression of EGFR and HER2 in ER(−) breast cancers were found, suggesting that activation of growth factor signaling and consequently, the activation of MAPK might contribute to transcriptional repression of ER gene, resulting in endocrine resistance [77,78]. Recent results showed that p53 upregulates ER gene expression through elements located upstream of the ER promoter. Interestingly, a high percentage of breast tumors with p53 mutations are ER(−). These studies suggest that specific p53 mutations in breast tumors may contribute not only to oncogenesis but also to hormonal resistance. Furthermore, the treatment of MCF-7 cells with paclitaxel resulted in the induction of ER gene transcription, which may be mediated through the induction of p53 [79,80]. On the other hand, pRb2/p130 also plays an important role in the transcriptional regulation of the ER promoter by recruiting multi-molecular transcriptional complexes, and may be considered as a promising target in breast cancer treatment, especially for ER(−) tumors [81].
Modifications in ER expression such as mutations might affect the response to anti-estrogens. For example, substitution of aspartate by tyrosine at position 537 in the ligand-binding domain causes ER activation in the absence of the ligand [82]. The change of aspartate to tyrosine at amino acid 351 in the ER has been identified in a tamoxifen-stimulated cell line [83], and modification of lysine 303 to arginine results in increased ER sensitivity to estrogens [84]. This type of mutation might alter ER function, however, only 1% of primary breast cancer patients carry these mutations [85] and endocrine resistance also occurs even in the absence of these mutations.
Another mechanism that is associated with SERM resistance is the altered expression of ERβ. High levels of ERβ are found in pre-invasive mammary tumors of tamoxifen-resistant patients [86]. However, other studies have reported that ERβ has a negative effect on ERα promoted transcription and that low levels of ERβ may contribute to endocrine resistant [87,88]. Then, the role of ERβ in the resistance to therapy is still controversial.
3.2. Epigenetics Mechanisms Regulating ER Expression
Several epigenetic mechanisms that regulate the expression of many genes also regulate ER expression. Nowadays, epigenetic studies could provide new biomarkers to predict and diagnose acquired resistance in response to treatment [89].
Analysis of ERα methylation status offers an alternative to determine whether endocrine therapy will be effective in breast cancer patients. Loss of ERα expression is often associated with promoter hypermethylation of ERα gene [90,91]. Similarly, PR methylation has been observed in endocrine resistant tumors [92]. Several studies have reported that a high expression ratio of Homeobox protein (HOXB13)/Interleukin-17B receptor (IL17BR) predicts tumor recurrence in node-negative, ER(+) breast cancer patients treated with tamoxifen. While the details of deregulation of HOXB13 and IL17BR are still under investigation, there is evidence that expression of HOXB13 is under epigenetic control [93]. Patients with ER(+) breast tumors with low levels of CDK10 (Cyclin-dependent kinase 10) relapsed early under tamoxifen treatment. Importantly, this downregulated CDK10 expression is associated with methylation of the CDK10 promoter, suggesting CDK10 gene methylation as a novel marker for determining response to endocrine therapy [94,95]. At least three studies have used high-throughput DNA methylation profiling to associate DNA methylation of candidate genes with outcome of breast cancer patients after tamoxifen therapy [96].
Several inhibitors of enzymes controlling epigenetic modifications, specifically DNMT and HDACs, have been developed and shown promising anti-tumorigenic effects [89,91,96]. Epigenetic changes are frequent events, and unlike genetic mutations, epigenetic modifications are reversible events; thus, the inhibition of these mechanisms could be a potential therapeutic strategy for the treatment of endocrine resistant breast cancer, either through direct effects on epigenetic changes, or by modulating known targets of other therapies.
3.3. Regulation of Signal Transduction Pathways
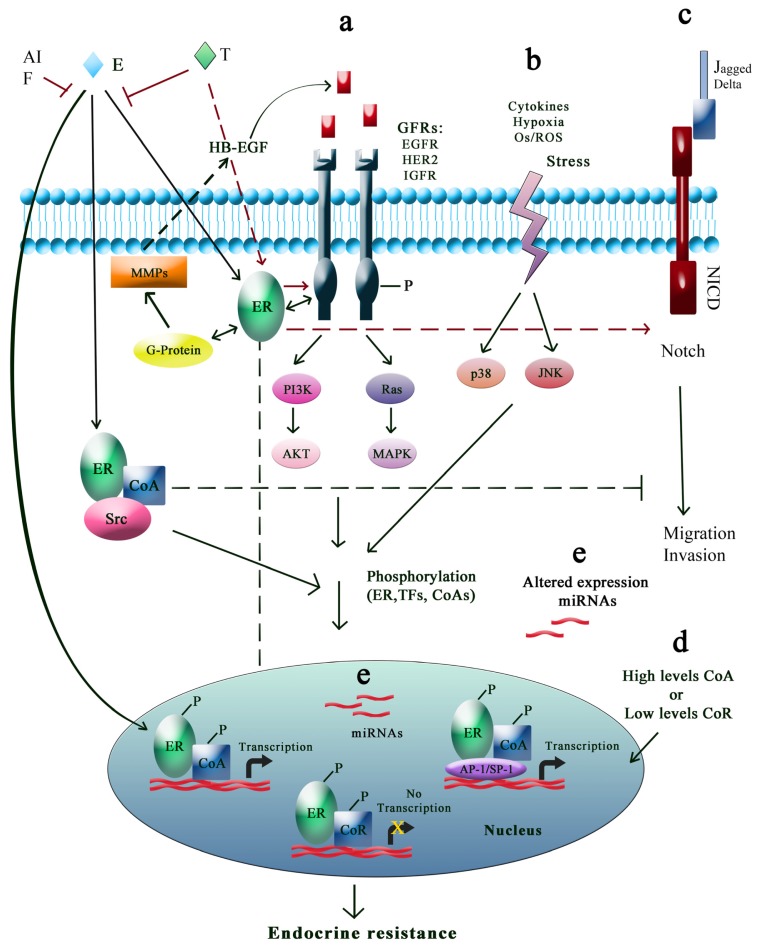
Crosstalk between ER and different signaling pathways, such as growth factor receptor, cell survival (PI3K/AKT), stress and/or cytokine signaling pathway, have been implicated in acquired and intrinsic resistance to endocrine agents, and are represented in Figure 2.
Figure 2.
Pathways involved in endocrine resistance. (a) While tamoxifen (T), aromatase inhibitors (AIs), or fulvestrant (F) inhibit estrogen (E) signalization, GFR pathways promote ER phosphorylation, transcription factors (TFs), and their coactivators (CoA) in a ligand-independent manner. E-ER complex outside the nucleus can interact with GFRs, Src, CoA and matrix metalloproteinases that release heparin-binding-EGF; (b) Stress may trigger signalization leading to ER and its coregulators phosphorylation; (c) Notch regulates the migration and invasion of breast cancer cells. E inhibits this pathway while T activates it; (d) High levels of CoA, low levels of corepressors (CoR) and altered expression of miRs (e) have been implicated in endocrine resistance development.
3.3.1. Growth Factor Receptor Signaling Pathways
The growth factor receptor signaling pathways can stimulate cancer growth either in concert with ER signaling or bypassing it. Preclinical and clinical evidence suggests that growth factor receptor signaling contributes to endocrine resistance. Both the ER and growth factor receptor pathway act in crosstalk, where the membrane ER can activate growth factor receptor signaling, in turn, this can phosphorylate ER and its coregulator proteins [97]. Phosphorylation of key serine residues within the activator function-1 (AF-1) domain of ER, in particular serine 118 and 167, can promote re-activation of ER function in a ligand-independent manner and contribute to transcription of estrogen-sensitive genes in the presence of antiestrogen agents [50,51,98]. Reporter gene construct studies in tamoxifen resistant cells indicate that the EGFR/MAPK-promoted ER AF-1 phosphorylation enhances the agonistic behavior of tamoxifen, resulting in the expression of estrogen regulated genes [99]. It has also been shown that growth factor signaling pathways promote phosphorylation of ER coactivators [100]. Indeed, overexpression of the co-activator AIB1 correlates with resistance to tamoxifen in breast cancer patients and in the EGFR/HER2/MAPK-dependent phosphorylation, and it has been proposed to mediate tamoxifen resistance in HER2 overexpressing MCF-7 cells [101].
Overexpression and activation of growth factor receptors, such as EGFR, HER2 and IGF1R, drive the proliferation and survival through activation of MAPK and PI3K/AKT signaling pathways in endocrine-resistant breast cancer [102]. One of the first indications of the crosstalk between ER and growth factors receptors was the transfection of ER(+) breast cancer cells with HER2, resulting in downregulation of ER and resistance to tamoxifen [103,104]. Subsequently, several works have supported a role for HER2 in intrinsic resistance to endocrine therapy. In MCF-7/HER2-18 cells manipulated to overexpress HER2 as well as ER, tamoxifen behaves as an estrogen agonist, stimulating their growth. Phosphorylation and activation of both ER and EGFR/HER2 receptors as well as MAPK and AKT signaling pathways, and recruitment of AIB1 coactivator were increased in these cells compared with wild-type MCF-7 cells, this observation was also supported in xenograft tumors [97]. The potential involvement of this crosstalk has been also observed in a meta-analysis of clinical trials in which patients with metastatic ER(+)/HER2(+) breast cancer were treated with tamoxifen after a shorter time of treatment failure in comparison to HER2(−) cancers [105]. In tumors that acquired resistance to fulvestrant, there was also a marked upregulation of HER-2 and the downstream signaling molecule MAPK, suggesting that this pathway may mediate the resistant phenotype [106]. Notably, breast cancer cells resistant to exemestane (AIs) induce amphiregulin expression in an ER-dependent manner. A possible mechanism of resistance to exemestane is that amphiregulin activates the EGFR pathway and leads to the activation of the MAPK pathway that drives cell proliferation [107]. Interestingly, in a xenograft model of ER(+)/HER2 overexpressing breast cancer, the loss of the ER protein and deregulation of the progesterone receptor with increased mucins, especially MUC4, which has been associated with endocrine resistance in breast cancer, has been observed [108].
Therefore, the use of either specific inhibitors or blockers of growth factor receptors has been one of the most promising therapeutic approaches in breast cancer. Gefitinib, a selective inhibitor of EGFR, restores the effects of tamoxifen in HER2-overexpressing tamoxifen-resistant MCF-7 cells, while trastuzumab, a monoclonal antibody that blocks HER2, can inhibit proliferation of endocrine resistant ZR-75-1 cells [97]. Furthermore, the HER2 tyrosine kinase inhibitor AG1478 and trastuzumab have also proved efficacy in MCF-7 models of de novo tamoxifen resistance and in BT-474 cells that overexpress HER2. The addition of either trastuzumab or lapatinib (a tyrosine kinase inhibitor which interrupts the HER2 growth receptor pathway) to aromatase inhibitor therapy has improved survival in patients with ER(+)/HER2(+) metastatic breast cancer [109]. Moreover, AEE788 (a combined inhibitor of EGFR, HER2 and VEGFR) plus tamoxifen or letrozole in breast cancer overexpressing HER2, may provide superior anti-tumour activity, compared with single agents [110].
As previously mentioned, the IGF1R pathway has a close bidirectional crosstalk with ER. Some studies have established that elevated IGF1R-promoted kinases contribute to the phosphorylation of ER. In turn, estrogens enhance expression of several IGF1R pathway components, like insulin-like growth factor II (IGF2), to reinforce IGF1R signaling [111]. The EGFR/IGF1R crosstalk in EGFR(+) tamoxifen-resistant variants of MCF-7 cells was studied. Although tamoxifen-resistant cells expressed reduced IGF1R protein levels compared with the wild-type MCF-7 cells, phosphorylated IGF1R protein levels were similar in the two cell lines under basal growth conditions, possibly as a consequence of increased IGF2 expression, which activated both IGF1R and EGFR. IGF2 promotes direct association of c-SRC with IGF1R, phosphorylated c-SRC, and increases EGFR phosphorylation at tyrosine 845, a c-SRC-dependent phosphorylation site [112]. A further mechanism observed in different tumor cell types is the ability of IGF2 to transactivate EGFR via metalloprotease-dependent release of the EGFR-ligands amphiregulin or HB-EGF [113]. ER(+) MCF7 cells with ectopic expression of IGF1R, are highly resistant to tamoxifen and fulvestrant, but in response to IGF1 ligand stimulation, displayed enhanced downstream activation of MAPK and PI3K signaling pathways, this was independent of ER signaling. Intriguingly, an agonistic behavior of tamoxifen at low doses was triggered in the presence of IGF1 [114]. In patients with tamoxifen-resistant tumors, the tumors with higher IGF1 ligand and ERα expression took longer to develop tamoxifen resistance, while tamoxifen-resistant tumors had lower IGF1 and ERα expression compared to tamoxifen-sensitive tumors [115]. However, while inhibitors of this pathway are currently in different trials, early reports have shown that IGF1R-specific inhibitors (like AG1024 and AEW541) or an IGF2 neutralizing antibody inhibited basal IGF-IR, c-SRC, AKT and EGFR phosphorylation, and significantly reduced tamoxifen-resistant basal cell growth. The c-SRC inhibitor SU6656 also inhibited growth, reduced basal and IGF2-induced c-SRC and EGFR phosphorylation, and blocked EGFR activation by TGFα. Similarly, AG1024 and SU6656 inhibited basal and IGF2-induced phosphorylation of c-SRC and EGFR, and SU6656 reduced TGFα-induced EGFR activity in tamoxifen-resistant T47D cells. Interestingly, AEW541 also inhibited insulin- and IGF2-stimulated effects in tamoxifen-resistant cells [112,116].
Recently, the estrogen receptor coactivator MED1 proved to be a novel crosstalk point for the HER2 and ERα pathways in breast cancer, showing a key role for MED1 in HER2-mediated tamoxifen resistance [117]. Indeed, MED1 expression positively correlated with HER2 status of the tumors, being highly phosphorylated in a HER2-dependent manner. Interestingly, downregulation of MED1 sensitized HER2-overexpressing cells to tamoxifen treatment, probably as a result of less MED1 recruitment to HER2 promoter, an event that is required for its expression. This is in accordance with previously mentioned gefitinib-dependent restoration of tamoxifen response in HER2-overexpressing tamoxifen-resistant MCF-7 cells.
3.3.2. PI3K Cell Survival Pathways
ER activity is also associated to the PI3K pathway, which is activated by tyrosine kinase receptors in response to growth factors. The PI3K/AKT signaling pathway has been extensively investigated for its role in oncogenic transformation. It is very important in regulating proteins that control cellular proliferation such as cyclins, cyclin-dependent kinases and cyclin-dependent kinase inhibitors [118]. AKT is one of the downstream targets of PI3K, promotes cellular proliferation and anti-apoptotic responses [119]. Recent studies have indicated that estrogen stimulates association of ERα with IGF-1 receptor and p85 regulatory subunit of PI3K in the plasma membrane [120,121], which leads to AKT activation and its subsequent downstream effects. In turn, AKT phosphorylates nuclear ERα at serine 167, resulting in ligand-independent activation [52,122].
In various experimental models it has been demonstrated that PI3K pathway activation confers antiestrogen resistance. Knockdown of the suppressor phosphatase and tensin homolog (PTEN, a negative regulator of AKT), increased PI3K and AKT phosphorylation in ER(+) breast cancer cell lines, resulting in hormone-independent growth and resistance to tamoxifen and fulvestrant [123,124]. Endocrine-resistant tumor cells display upregulation of IGF-1R, HER2, and EGFR levels, as well as increased PI3K/AKT/mTOR activation. Clinical evidence suggests that activation of PI3K via either overexpression of HER2 or fibroblast growth factor receptor (FGFR)1, or loss of inositol polyphosphate-4-phosphatase type II (INPP4B), also confers antiestrogen resistance to patients with ER(+) breast cancer [125]. Interestingly, mutations in the PI3K alpha catalytic subunit (PI3KCA) is the most common genetic abnormality identified in ER(+) breast cancer (~30%), whereas PTEN loss is more associated with ER(−) disease. Such mutations in PI3KCA correlate with good long-term outcome in the patients. However, conflicting data exists in the literature regarding the prognostic implications of PI3KCA mutation in breast cancer and it will have to be further analyzed [126]. The addition of PI3K pathway inhibitors increases the pro-apoptotic effects of tamoxifen, primarily in the cell line with the highest endogenous levels of AKT activity, supporting the notion that either high expression of AKT or altered activity of the PI3K/AKT pathway could be associated with endocrine resistance [52,121]. Similar results in four different cell lines tested showed that the combination of BEZ235 (PI3K/mTOR inhibitor) and tamoxifen inhibited growth more than either tamoxifen or BEZ235 alone [127]. In another study, patients with ER(+) tumors were randomly assigned to neoadjuvant letrozole with or without everolimus inhibitor for four months before surgery. The combination therapy induced a higher clinical response and greater suppression of tumor cell proliferation compared with letrozole alone. Patients with advanced breast cancer who had progressed while receiving an AI were randomly assigned to tamoxifen with or without everolimus. They showed a significantly improved rate of clinical benefit, time to progression and disease-free survival with the combination, in comparison with tamoxifen alone [128–130].
Studies in human breast cancer cells subjected to long-term estrogen deprivation (LTED) have demonstrated that these cells first develop hypersensitivity to low-dose estrogens and then become estrogen independent. This phenomenon seems to contribute to resistance to endocrine therapy, which may result in part by increased levels of ER and upregulation of the MAPK, PI-3-kinase and mTOR growth factor pathways and phosphorylation of ERα [131–136]. Studies in MCF-7 cells treated long-term with tamoxifen showed increased MAPK and aromatase activity during the acquisition of tamoxifen resistance. This may occur either as response to estrogen deprivation or interruption of the process of estrogen signaling [135,137]. In fact, three stages have been suggested that lead to tamoxifen resistance in cells with long-term exposure to tamoxifen: in stage 1, tamoxifen behaves as an estrogen antagonist; in stage 2, the tumors increasingly become sensitive to the agonistic effects of tamoxifen; and, in stage 3, the tumor has increased sensitivity to estrogen (hypersensitivity).
The PI3K/mTOR inhibitor BEZ235 and the TORC1 inhibitor everolimus (mTOR inhibitor, also known as RAD001) inhibit growth of LTED cell lines [138]. A recent study in ER(+) breast cancer cell lines before and after LTED or short-term estrogen deprivation (STED) showed that the treatment with the PI3K catalytic subunit inhibitor BKM120, the RAD001 inhibitor and the dual PI3K/mTOR inhibitor BGT226 induced apoptosis. Additionally, fulvestrant alone did not promote apoptosis in STED cells or LTED cells. However, when fulvestrant was used in combination with BGT226, BKM120 and RAD001, it potentiated apoptosis of these inhibitors in MCF7 LTED cells. In contrast, treatment with fulvestrant did not promote apoptosis in the ER-negative T47D LTED cells with any of the three agents tested. These data suggest that fulvestrant may sensitize cells to the therapeutic effects of PI3K inhibitors under circumstances where resistance to estrogen deprivation is associated with ligand-independent ER activity [139]. The crosstalk between ER and PI3K/AKT pathways shows a new strategy in the combat against endocrine resistance.
3.3.3. Stress-Activated Protein Kinase/c-junNH2 Terminal Kinase Pathway
Stress-activated protein kinase/c-junNH2 terminal kinase pathway may interact with ER either by binding with the AP-1 transcription complex or by direct p38 MAPK activation. AP-1 is a complex of proteins composed of Jun and Fos family members of nuclear phosphoproteins, which dimerize and bind to DNA consensus sequences TGAC/GTCA (AP-1 sites) and modulates expression of the target genes [140]. AP-1 transcriptional activity is increased either by their phosphorylation through the JUN NH2-terminal kinases (JNKs) or stress-activated protein kinases (SAPKs) or by increased abundance of any of its components [141]. One study reported that the development of tamoxifen resistance in MCF-7 cells is accompanied by enhanced AP-1 DNA binding [142]. It was also observed in a panel of 30 ER(+) primary human breast tumors with acquired tamoxifen resistance, as compared to a matched panel of 27 untreated control tumors [143]. Similarly, a study in a xenograft mouse model showed that tamoxifen-resistant tumors increased AP-1 dependent transcription and phosphorylated c-Jun and JNK levels, compared with estrogen treated tumors. Apparently, the tamoxifen resistant phenotype is associated with enhanced oxidative stress, leading to activation of JNK and increased AP-1 activity by an agonistic effect of tamoxifen at AP-1 sites [144–146].
On the other hand, activation of the p38 MAPK pathway has been reported to occur in response to environmental stress including ionizing radiation, heat, oxidative stress, inflammatory cytokines, growth factors, and tissue ischemia [147,148]. In clinical breast cancer specimens and in xenograft tumors resistant to tamoxifen high levels of p38 MAPK expression have been found [72]. The increase in p38 MAPK levels produced by tamoxifen, may account for the partial agonist activity of this drug. In endometrial cancer cells, p38 can phosphorylate and activate ER, inhibit ER nuclear export, enhance interaction of ER with coactivators, and increase the agonistic activity of tamoxifen [49,149], perhaps these mechanisms may well be important for tamoxifen resistance in clinical breast cancer. Moreover, inhibition of p38 by SB202190 reduces the proliferation of breast cancer cell lines. Studies in vitro and in vivo have shown that pharmacological inhibition of p38 by RWJ67657 (other p38 inhibitor) resulted in inhibition of the downstream p38 targets hsp27 and MAPK. Furthermore, decreased biological effects of p38, including ER-mediated gene expression and clonogenic survival in a dose-dependent manner were also observed [150]. Then, targeting this signaling pathway may be beneficial to overcome tamoxifen resistance [151].
3.3.4. Other Signal Transduction Pathways
Other signal transduction pathways related to the endocrine resistance are nuclear factor-κB (NFκB), Notch, keratinocyte growth factor (KGF), and platelet-derived growth factor (PDGF)/Abl signaling pathway [152–154].
NFkB stimulates cell proliferation and invasion by induced genes such as cyclin D1 and urokinase-type plasminogen activator (uPA). NFkB activation has been associated with progression of hormone-independent breast cancers. Low NFkB activation was found in ER(+) breast cancers cells but constitutively elevated in ER(−) breast cancers cells [155,156]. Moreover, in ER(+) breast cancers has been demonstrated that tamoxifen can activate NFκB, stimulate cell growth and survival, and thereby contribute to endocrine resistance [157]. Crosstalk between ER and NFκB pathways that contributes to the development of endocrine resistance was reviewed in Sas et al. [158].
KGF pathway can stimulate the growth of mammary epithelium by inducing aromatase activity, thereby promoting conversion of androgens to estrogens in primary cultured human breast cells. KGF might increase the endocrine resistance via decreasing ER, progesterone receptor (PR) and protein tyrosine phosphatases gamma (PTPγ). The presence of ER and PR is a good prognostic factor and indicator of benefit from endocrine therapy. Low levels of ER and PR in human breast cancers have been associated with resistance to tamoxifen and increased risk of breast cancer. On the other hand, PTPγ has been proposed as a candidate tumor suppressor gene in kidney, ovarian and lung tumors and may play an important role in neoplastic processes of human breast. This pathway suggests novel strategies for breast cancer treatment via interference with KGF signaling [159].
It has also been found that the Notch pathway is implicated in both cell fate in the normal human mammary gland and regulation of cancer stem cells (CCs) in both ductal carcinoma in situ and invasive carcinoma of the breast [152,160,161]. Binding of Notch ligand (Jagged or Delta) to the Notch receptor cleaves its intracellular domain (NICD), which translocates to the nucleus where it binds with co-activators to induce transcription of its target genes and regulates migration and invasion of breast cancer cells. Estradiol inhibits Notch activity, and affects Notch receptor cellular distribution. Tamoxifen and raloxifene block this effect, reactivating the Notch pathway. Pharmacologic inhibition of Notch activation with γ-secretase inhibitors was more effective in combination with tamoxifen that tamoxifen alone [162]. These data indicate that γ-secretase inhibitors block the proliferative effect of tamoxifen through Notch pathway, and at the same time allow tamoxifen to exert its antagonistic effect.
PDGF/Abl canonical signaling pathway has been also associated to resistance [153]. PDGF receptor (PDGFR) is classified as a tyrosine kinase receptor whose activation is dependent on the binding of PDGF resulting in stimulation of several intracellular pathways. PDGF can promote tumor growth via autocrine stimulation of malignant cells, overexpression or overactivation of PDGFRs, or by stimulating tumor angiogenesis. Abl is a Src-like nonreceptor protein kinase that acts downstream of the PDGFR [163]. Abl is involved in the regulation of cell proliferation, apoptosis, adhesion, cell migration and stress response [164,165]. In vitro and in vivo studies have demonstrated a novel interaction between ER and the PDGF/Abl signal transduction pathway during adaptation to LTED and which appears partly responsible for the resistant phenotype. These data suggest that this pathway may provide a novel biomarker of early resistance to AI therapy [153]. Despite the success of AIs in treating ER(+) breast cancer, approximately 15%–20% of patients that receive this treatment relapse within 5–10 years of therapy initiation because of either selection of cells insensitive to estrogen or activation of signaling by non-endocrine pathways [132,166].
3.4. Altered Expression of Specific microRNAs
MicroRNAs (miRs) are small regulatory and non-coding RNA molecules of 19–24 nucleotides in length that modulate the expression level of specific proteins based on sequence complementarities with their target mRNA molecules. This process occurs by either degrading the target messenger RNA (mRNA) or suppressing the protein synthesis [167]. miRs have been shown to regulate a variety of cellular processes such as differentiation, cell growth, and cell death [168]. miR genes are frequently located at cancer-associated genomic regions and the deregulation of its expression has been found to contribute in the tumorigenic process. Recent research suggests miRs as cancer prognostic biomarkers and therapeutic targets [169–171].
Altered expression of specific miRs has been implicated in tamoxifen resistance development and predicts outcome and therapeutic response in breast cancer [172–174]. Different miR expression profiles have been identified in tamoxifen resistant and sensitive breast cancer cell lines by microarray analysis (Table 1). By using this technique, 97 miRs differentially expressed in MCF-7 endocrine-sensitive versus resistant LY2 breast cancer cells were identified. Opposite expression of miR-10a, miR-21, miR-22, miR-29a, miR-93, miR-125b, miR-181, miR-200a, miR-200b, miR-200c, miR-205, and miR-222 in MCF-7 versus LY2 cells was confirmed by quantitative real-time PCR [175].
Table 1.
miRs expression profiles in tamoxifen-resistant breast cancer cells. miR overexpression (at least >1.5 fold higher), and underexpression (50% lower), in comparison with tamoxifen-sensitive breast cancer cells.
As previously mentioned, increased growth factor signaling (in particular the EGFR/HER2 pathway), the estrogen agonist activity of tamoxifen in breast cancer cells that express high levels of HER2, aberrant expression cell cycle regulators and lack of expression of ERα in breast cancer are factors that contributes to tamoxifen resistance [97,180–182]. In this regard, altered expression of miR-221 and miR-222 (miR-221/222) has an important role. The miR-221/222 expression levels were significantly elevated in HER2(+) breast cancer cells compared with cells with low or negative HER2 expression. Ectopic expression of miR-221/222 in tamoxifen sensitive cells resulted in downregulation of the cell cycle inhibitor p27kip1, and conferred resistance to tamoxifen [176]. p27kip1 is a target of miR-221/222 and its overexpression induced enhanced cytotoxicity in tamoxifen resistant breast tumor cells [176]. Also, aberrant expression of miR-221/222 has been associated with fulvestrant resistance by activating β-catenin and modulating TGF-β and p53 signaling [183]. In addition, miR-221/222 are highly expressed in ERα negative breast cancer cells. Knockdown of these two miRNAs partially restored ERα protein expression and tamoxifen induced cell growth arrest and apoptosis. In contrast, overexpression of miR-221/222 in ERα(+) cells reduced levels of this receptor and caused resistance to tamoxifen [184]. Indeed, several miRNAs were reported to be associated with the regulation of ERα expression [185–188]. These results indicate that miR-221/222 play an important role in the regulation of signaling cascades, control of cell proliferation and, regulation of ERα expression; which altogether impact anti-estrogen resistance.
HER2Δ16 is the oncogenic splice isoform of HER2, commonly coexpressed with HER2 in ER(+) breast tumors; its expression promotes estrogen-independent growth and tamoxifen resistance [189,190]. The mechanism of endocrine-resistance in HER2Δ16-expressing cells involves the upregulation of anti-apoptotic BCL-2 protein induced by tamoxifen and suppression of miR-15a and miR-16 (miR-15a/16) levels. Overexpression of miR-15a/16 reduced tamoxifen-induced BCL-2 expression and sensitized HER2Δ16-expressing cells to tamoxifen response. Inversely, miR-15a/16 inhibition in tamoxifen-sensitive cells increased BCL-2 expression and promoted tamoxifen resistance [190].
Altered expression of miR-342 has been identified in tamoxifen-resistant breast tumor cells. miRNA expression profiles of tamoxifen sensitive- and resistant-MCF-7 variants, including those expressing HER2Δ16, showed significant suppressed levels of miR-342 in tamoxifen-resistant cells. Moreover, miR-342 reduced expression was associated with tamoxifen failure in primary human breast tumors and downregulation of miR-342 in tamoxifen sensitive cells conferred tamoxifen resistance. In contrast, overexpression of miR-342 sensitized resistant breast tumor cells to tamoxifen induced apoptosis and inhibition of cell proliferation [177].
14-3-3ζ acts as a suppressor of apoptosis and has an important role in tumorgenesis in multiple types of cancer. Its overexpression has been found in 42% of breast tumors [191]. Previous studies demonstrated that 14-3-3ζ gene expression was upregulated by tamoxifen in ER(+) breast cancer cells and it was associated with poor outcome on endocrine therapy [192]. The mechanism by which tamoxifen regulated 14-3-3ζ levels, involves the downregulation of miR-451 that specifically targets 14-3-3ζ. The regulation of 14-3-3ζ and miR-451 was selective for tamoxifene because neither raloxifene nor fulvestrant had an effect on miR-451. Overexpression of miR-451 and downregulation of 14-3-3ζ expression in endocrine-resistant cells restored the effectiveness of tamoxifen to decrease cell proliferation, increase apoptosis and reduce activation of EGFR/HER2 signaling. The loss of miR-451 and upregulation of 14-3-3ζ induced by tamoxifen seems to be an additional mechanism by which ER(+) breast cancer cells develop resistance to tamoxifen therapy [179,193].
miR-210 is involved in different biological processes such as cell proliferation, migration and invasion, High miR-210 expression was associated with higher risk of recurrence in ER(+), tamoxifen treated breast cancer patients [194]. Other miRs related to therapeutic response are miR-301 and miR-101, which overexpression was associated with tumor growth and resistance to tamoxifen [195,196]. In MCF-7 cells, miR-101 promoted estrogen-independent growth and caused upregulation of phosphorylated Akt. MiR-101 activated Akt through targeting membrane-associated guanylate kinase (MAGI-2), an essential protein in the activity of the tumor suppressor PTEN. Upregulated miR-101 suppressed PTEN activity and facilitated Akt activation, which promoted the estrogen independent growth of breast cancer [196]. Also, miR-301 overexpression was associated with resistance to tamoxifen and breast cancer progression. miR-301 acts through multiple pathways and oncogenic targets, including PTEN, the transcription factor FoxF2, the pro-apoptotic protein BBC3, and the collagen, type II, alpha 1 (CoL2A1). Furthermore, miR-301 works in cooperation with SKA2 (essential for proper chromosomal segregation) contributing to breast cancer progression [195].
Some evidence has suggested that the effect of antiestrogens in breast cancer is mediated in part by regulation of TGF-β signaling pathway [197–200]. Yoo et al. demonstrated that the enhanced activation of Akt results in loss of TGF-β response induced by tamoxifen, and this is associated with the development of tamoxifen resistance in breast cancer [201]. In contrast, overexpression of TGF-β has been reported to mediate tamoxifen resistance in vivo by abrogating natural killer (NK) cell function involved in the antitumor effect of tamoxifen [202]. Human miR-128a has been associated with breast cancer aggressiveness [203] and was predicted to target the TGF-β signaling pathways members (TGF-β receptor I (TGFβRI) and the transcription factor SMAD2) [204]. Particularly, miR-128a targets the 3′UTR region of the TGFβRI and negatively regulates TGFβRI protein expression. The miR-128a expression levels were found significantly elevated in letrozole-resistant breast cancer cells compared with other resistant cell lines, suggesting functional specificity of this miR in letrozole-resistance. Moreover, downregulation of endogenous miR-128a resulted in re-sensitization of the aromatase inhibitor-resistant breast cancer cell line to the growth inhibitory effect of TGFβ [204].
3.5. Coregulatory Proteins
Tamoxifen acts as an ER antagonist in breast cancer but as an agonist in other tissues such as uterus, cardiovascular system, and bone. These differences in tamoxifen activity could be explained by several mechanisms [205]. One of these mechanisms involves changes in the level of expression of coregulatory proteins (coactivators and corepressors) that can influence regulation of ER transcriptional activity [206,207].
The ER coactivator AIB1 (also known as SRC-3) is considered to be a proto-oncogene, which is overexpressed in more than 30%, and genetically amplified in 5%–10%, of breast tumors [208–211]. High levels of ER coactivators may enhance the estrogen-agonist activity of tamoxifen and contribute to tamoxifen resistance [31,206,212]. High SRC-3 expression has been associated with poor overall breast cancer patient survival [213]. Moreover, overexpression of SRC-3 along with HER2 converts tamoxifen into an agonist with increase in the molecular crosstalk between the ER and HER2 pathway [97]. Further, it has been shown that tamoxifen induces ERα-SRC-3 interaction in HER2(+) human breast cancer [214]. In contrast, dissociation of SRC-3 from ER has been shown to restore sensitivity in tamoxifen resistant cells [215]. In a clinical study, patients who had received adjuvant tamoxifen with tumor overexpressing SRC-3 along with HER2 displayed worse disease-free survival. Better prognosis and longer disease-free survival was observed in patients not receiving adjuvant tamoxifen therapy whose tumors expressed high levels SRC-3 [101]. Other study confirmed that high SRC3 expression was a marker of tamoxifen resistance in HER2 overexpressing. Moreover, patients who had received adjuvant tamoxifen with high SRC-3 expression along with one or more of EGFR- or HER3-overexpressing tumors was associated with an increased risk of relapse [216]. In surgical specimens from ER(+) breast cancer patients who received neoadjuvant tamoxifen therapy, tamoxifen significantly increased the expression of SRC-1, SRC-2 and SRC-3 [217]. SRC-1 and SRC-3 protein levels were higher in an endocrine resistant cell lines in comparison with endocrine sensitive cells. Knockdown of SRC-1 and SRC-3 resensitized endocrine resistant cells to tamoxifen treatment. Also, colocalization of SRC-1 and SRC-3 with ERα was increased in endocrine resistant cells following treatment with tamoxifen in comparison with endocrine sensitive cells. These data implicate the involvement of inappropriate interactions between ER and its coactivator in the endocrine resistance [218]. Recent results showed that the expression SRCs, HER2 and HER3 is stimulated by tamoxifen treatment in DMBA induced breast cancer [219]. Knockdown of SRC in endocrine-resistant, HER2(+) cells restored the effectiveness of tamoxifen to decrease cell proliferation [214].
HER2 could be repressed by both estrogen and tamoxifen, thus the anti-proliferative effects of tamoxifen could be in part due to repression of HER2 [220–222]. Paired Box 2 (Pax2) was found to be an important mediator of repression of HER2 in ER-positive breast cancer cells. After tamoxifen treatment, Pax2 is recruited to the ER functioning as an ER-associated transcriptional repressor. Knockdown of Pax2 resulted in elevated HER2 transcription and protein levels in the presence of both estrogen and tamoxifen and reversed the growth inhibitory effects of tamoxifen. Overexpression of SRC-3 blocked Pax2 binding to ER, resulting in increased HER2 transcription and cell proliferation in the presence of tamoxifen. Furthermore, tamoxifen resistant cells with elevated HER2 levels showed low Pax2 protein levels and tamoxifen treatment increased SRC-3 recruitment. In contrast, when overexpressing Pax2 in these cells, tamoxifen decreased SRC-3 binding to the ER and repressed HER2 mRNA and protein levels, the growth inhibitory effects of tamoxifen were also restored. This suggests that SRC-3 competes with Pax2 for binding and regulating the HER2 gene. [222]. Interestingly, patients receiving tamoxifen with ER positive tumors with Pax2 positive and SRC-3 negative had the best prognosis compared with patients with Pax2 positive and SRC-3 positive tumors or patients with Pax2 negative tumors. The lowest percentage of HER2 positivity was observed in patients with Pax2 positive and SRC-3 negative tumors [222]. These data confirm an essential role for SRC-3 and HER2 in tamoxifen resistance in ER positive breast cancer cells.
Increased SRC-1-ERα interactions are observed in patients who are resistant to treatment [218]. As the tumor progresses, however, increasing evidence suggests that SRC-1 engages in transcriptional interactions independently of ER. A new ER-independent target of SRC-1, the ADAM22 protein involved in cell adhesion, spreading and migration, was identified [223,224]. Evidence from ChIP-seq, bioinformatics and molecular studies suggested that SRC-1 can regulate ADAM22 independently of ER in endocrine-resistant cells [223].
The role of coactivators in resistance to AI therapy has also been documented. Flageng et al. demonstrated that co-activators and HER2 are upregulated in the tumors from breast cancer patients during neo-adjuvant treatment with aromatase inhibitors [225]. Moreover, expression of AIB1 was associated with disease recurrence and reduced disease-free survival time in patients treated with an AI as first-line therapy [226]. In AI resistant cells, increased cell migration and loss of differentiation were found in comparison with endocrine sensitive cells. SRC-1 was an important modulator of this aggressive phenotype. AI treatment interacts with MAPK-activated Ets2 transcription factor regulating expression of Myc and MMP9 [227].
The nuclear receptor corepressor (NCoR) has been considered as an important predictor of the tamoxifen response [228,229]. In a xenograft model for human breast cancer, NCoR levels decreased in the tumors that acquired resistance to tamoxifen [230]. In a clinical study, patients with the best prognosis had high NCoR expression levels and normal HER2 expression [231]. Another study in ERα(+) breast tumors from postmenopausal patients treated with tamoxifen after surgery demonstrated that low NCoR expression was associated with significantly shorter relapse-free survival [229]. Higher NCoR expression levels in tumors from patients without recurrence compared with patients with recurrence supporting its role as a tamoxifen resistance mechanism [232].
The Tab2 protein is a facultative component of the NCoR complex [233] and was found as an important mediator of resistance to endocrine therapy [234]. Interaction between Tab2 and ERα was observed in tamoxifen resistance cells and interfering with this binding restored tamoxifen response. Furthermore, downregulation of Tab2 increased the antiproliferative response to tamoxifen [234]. Tab2 interacts with ERα/NCoR and causes dismissal of NCoR, leading to loss of the antiproliferative response in prostate cancer cells [235]. This issue has not yet been addressed in breast cancer cells and deserves further investigation.
Tamoxifen-sensitive cells showed high NCoR binding to the ER in comparison with tamoxifen-resistant cells. Combination treatment with either tamoxifen and IKK inhibitor parthenoide (PA), or tamoxifen and proteasome inhibitor bortezomib (PS341), which were capable of suppressing NFkB and AP-1, regulates gene expression. These combinations were able to restore the levels of NCoR binding to tamoxifen-liganded ER in the tamoxifen-resistant cells [154].
Taken together, these data raise the possibility that progressive reductions in corepressor activity during tamoxifen therapy may enhance the agonist effects of tamoxifen on the ER contributing to tamoxifen resistance.
3.6. Tamoxifen Metabolism and Genetically Based Resistance
The body converts drugs to metabolites that are more water soluble and thus more easily excreted. Nevertheless, during phase I reactions, some drugs may turn either into toxic metabolites or into more active compounds with significant therapeutic benefit [236]. Tamoxifen is metabolized in the liver mainly by two P450 cytochromes: CYP3A4 and CYP2D6, which produce N-desmethyltamoxifen and 4-hydroxytamoxifen, respectively. Afterwards, further oxidation of these metabolites results in the formation of a very active metabolite: 4-hydroxy-N-desmethyltamoxifen, also known as endoxifen, which production is mostly catalyzed by CYP2D6 [237,238]. Endoxifen and 4-hydroxytamoxifen have greater binding affinities for ERs and suppress cell proliferation more effectively than tamoxifen itself. Nevertheless, endoxifen plasma concentrations are 5–10-fold higher than those of 4-hydroxytamoxifen and are about 100 times more potent than tamoxifen in anti-estrogen activity [239,240]. Therefore, endoxifen is thought to be the responsible metabolite for the pharmacological activity of tamoxifen in vivo. Genetic variants of the cytochromes involved in tamoxifen metabolism may affect the final therapeutic response. Indeed, polymorphism in the gene coding for CYP2D6 significantly affects enzymatic activity, providing a potential mechanism for drug resistance. More than 75 CYP2D6 alleles have now been described and classified depending on their effects on CYP2D6 enzyme activity [241]. In this manner, extensive, intermediate or poor metabolizer alleles have been defined depending on how effectively tamoxifen is metabolized, which finally results in differential therapeutic responses to standard doses of the drug [236,241]. Therefore, CYP2D6 genotyping is advised in order to guide the correct selection and dosing of tamoxifen in a tailored manner. In this respect, a tool to simplify genotype interpretation has been designed and is known as “CYP2D6 activity score system” [242], which is useful to personalize drug therapy. Indeed, the use of pharmacogenomics in oncology is paramount to predict drug response or toxicity [243].
On the other hand, individual variations in the activity of phase II enzymes may partially explain final endoxifen bioavailability. For instance, women with high activity of UDP-glucuronosyltransferase-2B15 (UGT2B15) genotype had a worse recurrence-free survival than those with wild type alleles [244,245]. Inasmuch, an association between the rs9282861 homozygous variant AA genotype of sulfotransferase 1A1 (SULT1A1, involved in detoxification of 4-hydroxytamoxifen), and overall long-term survival of breast cancer patients treated with adjuvant tamoxifen, has been described [246]. Therefore, these findings suggest that polymorphism in the genes coding for phase II enzymes alter the long-term clinical outcome of patients receiving tamoxifen, and might explain some cases of resistance to therapy.
While tamoxifen is mainly metabolized in the liver, tumor cells may also contribute to this process by expressing functional P450 species. Indeed, the impaired ability of a tumor to efficiently bioactivate tamoxifen is another mechanism to explain resistance to therapy. In this respect, gene therapy holds promise as a new approach to achieve differential P450 cytochromes expression in order to bypass drug resistance [247,248]. The main purpose of this strategy is to selectively increase tumor cell exposure to the active metabolites generated locally by an exogenous P450 gene. This approach has been previously tested with cyclophosphamide [249], which is a prodrug bioactivated by P450 enzymes, as in the case of tamoxifen. Moreover, the efficacy of gene therapy can be enhanced by further increasing intra-tumor metabolism by means of co-expressing the P450 cytochrome together with the flavoenzyme NADPH-P450 reductase, which increases P450 metabolic activity [247]. Prominent among the benefits of this strategy are: enhanced therapeutic effect without increasing host toxicity and the possibility to use well-established and clinically effective anticancer prodrugs [248]. It is noteworthy to mention that even if localized CYP3A4 and CYP2D6 overexpression and activity might benefit patients under tamoxifen treatment, other anti-neoplastic drugs might easily be inactivated by these cytochromes, limiting the success of combined chemotherapy. For instance, low CYP3A4 expression in breast tumors resulted in a better response to docetaxel [250,251], while high CYP3A expression in osteosarcoma tumors predicted metastasis and poor prognosis [252]. This is explained by the fact that CYP3A4 catalyzes the oxidation of drugs frequently used as chemotherapeutic agents, such as doxorubicin and many others, whereas tamoxifen, as a prodrug, needs to be activated to achieve therapeutic results.
Another factor that plays a role in the response of patients to drug treatment is the concomitant administration of other drugs. Indeed, medications prescribed to patients on tamoxifen therapy can also block endoxifen production by inhibiting CYP2D6, as in the case of some antidepressants [240]. Indeed, selective serotonin and noradrenaline reuptake inhibitors are potent CYP2D6 inhibitors. For instance, fluoxetine and paroxetine may confer a poor metabolizer phenotype. This is important since these drugs are usually prescribed to patients under tamoxifen treatment, in order to alleviate some of its undesirable effects such as hot flashes [240], but might also be the cause of endocrine resistance, and, therefore, its concomitant prescription with tamoxifen should be carefully evaluated.
In summation, the probability of genetically based resistance to tamoxifen should be taken into consideration when designating a therapeutic approach. Although multiple mechanisms might be involved in hormonal resistance in patients, an interesting approach to overcome is the combined therapy, which is discussed next.
4. Combination Therapy
Combination regimens significantly improve the clinical outcome—especially in metastatic breast cancer. Therefore, drug combination regimens appear to be a good approach to delay cancer adaptation to treatment and secondary resistance. A common strategy designed to clinically address de novo and acquired endocrine therapy resistance is the use of combined hormonal treatment with targeted anti-HER2, anti-EGFR or multikinase inhibitor therapy [253]. Regarding hormonal treatment, several factors are being used in order to interfere with the estrogen signaling pathway, specifically: pure ER antagonists such as fulvestrant, and SERMs such as tamoxifen. Additionally, estrogen production might be blocked by using aromatase inhibitors (AI) such as anastrozole, exemestane and letrozole [254]. On the other hand, drugs that interfere with growth factor signalization include trastuzumab, gefitinib, lapatinib, inhibitors of farnesyltrasferase, inhibitors of the mammalian target of rapamycin and multitargeted tyrosine inhibitors [253]. Endocrine therapy is also being clinically tested in combination with antiangiogenic factors such as bevacizumab (a humanized monoclonal antibody that inhibits VEGF-A) and motesanib (an oral inhibitor of VEGF receptors) [253,255,256].
In general, interfering with both estrogen and growth factor signaling pathways by dual pharmacological inhibition seems to be more effective than monotherapy to inhibit tumor growth, especially for ER(+)/HER2(+) tumors. For example, combination of trastuzumab plus letrozole provides superior benefit over each drug alone in a MCF-7 xenograft model once acquisition of resistance is established by increased HER2 expression [257]. Moreover, lapatinib, an oral dual selective inhibitor of the tyrosine kinase domain of EGFR and HER2, increased the sensitivity of breast cancer cells to 4-hydroxy-tamoxifen, restoring endocrine sensitivity [258]. Similarly, synergistic effects of lapatinib in combination with fulvestrant have been reported in breast cancer cell lines expressing differential amounts of ER, HER2 and EGFR [259]. Clinically, the combined treatment with HER2 blockade and hormonal/AI therapy offers clinical advantages beyond those provided by endocrine or AI therapy alone in ER(+)/HER2(+) breast tumors. In these particular cases, it is known that gefitinib may eliminate the crosstalk between HER2 and ER signaling cascades. Indeed, tamoxifen behaves as an estrogen agonist in breast cancer cells that express high levels of HER2, resulting in de novo resistance. Gefitinib treatment reestablishes corepressor complexes with tamoxifen-bound ER on target gene promoters, eliminating tamoxifen agonistic effects, and restoring its antitumor activity both in vitro and in vivo [97]. The combination of trastuzumab plus an AI significantly improved progression-free survival, response rates, and clinical benefits when compared with AI monotherapy in postmenopausal women [260]. This might be explained by the fact that unlike tamoxifen, AIs have no ER agonistic activity, since their mechanism of action consists in blocking the final enzymatic step in the biosynthesis of estrogens and thus inhibit estrogen production.
5. Conclusions
The resistance to endocrine therapy involves multiple mechanisms including, at least in part, a switch from steroid signaling to growth factor signaling leading to steroid-independent tumors. Because breast cancer is a highly heterogeneous pathology both at the clinical and molecular level, the pharmacological prescription must be as tailored as possible. Therefore, the molecular signature of each tumor should be determined in order to accurately select the therapeutic approach, predict prognosis and response to therapies. The combination of endocrine therapy with other drugs targeting key molecules involved in hormone resistance is the most promising approach to prevent and/or overcome endocrine resistance and benefit these breast cancer patients.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Clark G.M., Osborne C.K., McGuire W.L. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J. Clin. Oncol. 1984;2:1102–1109. doi: 10.1200/JCO.1984.2.10.1102. [DOI] [PubMed] [Google Scholar]
- 2.Miller W.R., Bartlett J.M., Canney P., Verrill M. Hormonal therapy for postmenopausal breast cancer: the science of sequencing. Breast Cancer Res. Treat. 2007;103:149–160. doi: 10.1007/s10549-006-9369-7. [DOI] [PubMed] [Google Scholar]
- 3.Osborne C.K. Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 4.Ring A., Dowsett M. Mechanisms of tamoxifen resistance. Endocr. Relat. Cancer. 2004;11:643–658. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- 5.Gradishar W.J. Tamoxifen—What next? Oncologist. 2004;9:378–384. doi: 10.1634/theoncologist.9-4-378. [DOI] [PubMed] [Google Scholar]
- 6.Beelen K., Zwart W., Linn S.C. Can predictive biomarkers in breast cancer guide adjuvant endocrine therapy? Nat. Rev. Clin. Oncol. 2012;9:529–541. doi: 10.1038/nrclinonc.2012.121. [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi G.N. Toward individualized breast cancer therapy: Translating biological concepts to the bedside. Oncologist. 2012;17:577–584. doi: 10.1634/theoncologist.2012-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodie A., Sabnis G. Adaptive changes result in activation of alternate signaling pathways and acquisition of resistance to aromatase inhibitors. Clin. Cancer Res. 2011;17:4208–4213. doi: 10.1158/1078-0432.CCR-10-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strasser-Weippl K., Goss P.E. Advances in adjuvant hormonal therapy for postmenopausal women. J. Clin. Oncol. 2005;23:1751–1759. doi: 10.1200/JCO.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 10.Forbes J.F., Cuzick J., Buzdar A., Howell A., Tobias J.S., Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-Month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 11.Howell A., Robertson J.F., Abram P., Lichinitser M.R., Elledge R., Bajetta E., Watanabe T., Morris C., Webster A., Dimery I., et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: A multinational, double-blind, randomized trial. J. Clin. Oncol. 2004;22:1605–1613. doi: 10.1200/JCO.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 12.Robertson J.F., Osborne C.K., Howell A., Jones S.E., Mauriac L., Ellis M., Kleeberg U.R., Come S.E., Vergote I., Gertler S., et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women: A prospective combined analysis of two multicenter trials. Cancer. 2003;98:229–238. doi: 10.1002/cncr.11468. [DOI] [PubMed] [Google Scholar]
- 13.Weatherman R.V., Fletterick R.J., Scanlan T.S. Nuclear-receptor ligands and ligand-binding domains. Annu. Rev. Biochem. 1999;68:559–581. doi: 10.1146/annurev.biochem.68.1.559. [DOI] [PubMed] [Google Scholar]
- 14.Green S., Walter P., Greene G., Krust A., Goffin C., Jensen E., Scrace G., Waterfield M., Chambon P. Cloning of the human oestrogen receptor cDNA. J. Steroid. Biochem. 1986;24:77–83. doi: 10.1016/0022-4731(86)90035-x. [DOI] [PubMed] [Google Scholar]
- 15.Kuiper G.G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osborne C.K., Schiff R. Estrogen-receptor biology: Continuing progress and therapeutic implications. J. Clin. Oncol. 2005;23:1616–1622. doi: 10.1200/JCO.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Osborne C.K., Schiff R., Fuqua S.A., Shou J. Estrogen receptor: Current understanding of its activation and modulation. Clin. Cancer Res. 2001;7:4338s–4342s. discussion 4411s–4412s. [PubMed] [Google Scholar]
- 18.Roger P., Sahla M.E., Makela S., Gustafsson J.A., Baldet P., Rochefort H. Decreased expression of estrogen receptor β protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61:2537–2541. [PubMed] [Google Scholar]
- 19.Mann S., Laucirica R., Carlson N., Younes P.S., Ali N., Younes A., Li Y., Younes M. Estrogen receptor beta expression in invasive breast cancer. Hum. Pathol. 2001;32:113–118. doi: 10.1053/hupa.2001.21506. [DOI] [PubMed] [Google Scholar]
- 20.Evans R.M. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao W., Brown M. Advances in estrogen receptor biology: Prospects for improvements in targeted breast cancer therapy. Breast Cancer Res. 2004;6:39–52. doi: 10.1186/bcr742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- 23.Green S., Walter P., Kumar V., Krust A., Bornert J.M., Argos P., Chambon P. Human oestrogen receptor cDNA: Sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 24.Mader S., Chambon P., White J.H. Defining a minimal estrogen receptor DNA binding domain. Nucleic Acids Res. 1993;21:1125–1132. doi: 10.1093/nar/21.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar V., Green S., Staub A., Chambon P. Localisation of the oestradiol-binding and putative DNA-binding domains of the human oestrogen receptor. EMBO J. 1986;5:2231–2236. doi: 10.1002/j.1460-2075.1986.tb04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar V., Green S., Stack G., Berry M., Jin J.R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987;51:941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- 27.Norris J.D., Fan D., Kerner S.A., McDonnell D.P. Identification of a third autonomous activation domain within the human estrogen receptor. Mol. Endocrinol. 1997;11:747–754. doi: 10.1210/mend.11.6.0008. [DOI] [PubMed] [Google Scholar]
- 28.Picard D., Kumar V., Chambon P., Yamamoto K.R. Signal transduction by steroid hormones: Nuclear localization is differentially regulated in estrogen and glucocorticoid receptors. Cell Regul. 1990;1:291–299. doi: 10.1091/mbc.1.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webster N.J., Green S., Tasset D., Ponglikitmongkol M., Chambon P. The transcriptional activation function located in the hormone-binding domain of the human oestrogen receptor is not encoded in a single exon. EMBO J. 1989;8:1441–1446. doi: 10.1002/j.1460-2075.1989.tb03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry M., Metzger D., Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990;9:2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzukerman M.T., Esty A., Santiso-Mere D., Danielian P., Parker M.G., Stein R.B., Pike J.W., McDonnell D.P. Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol. Endocrinol. 1994;8:21–30. doi: 10.1210/mend.8.1.8152428. [DOI] [PubMed] [Google Scholar]
- 32.Enmark E., Gustafsson J.A. Oestrogen receptors—An overview. J. Intern. Med. 1999;246:133–138. doi: 10.1046/j.1365-2796.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 33.Klinge C.M. Estrogen receptor interaction with co-activators and co-repressors. Steroids. 2000;65:227–251. doi: 10.1016/s0039-128x(99)00107-5. [DOI] [PubMed] [Google Scholar]
- 34.McKenna N.J., Lanz R.B., O’Malley B.W. Nuclear receptor coregulators: Cellular and molecular biology. Endocr. Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 35.Deroo B.J., Korach K.S. Estrogen receptors and human disease. J. Clin. Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frasor J., Danes J.M., Komm B., Chang K.C., Lyttle C.R., Katzenellenbogen B.S. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: Insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144:4562–4574. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 37.Razandi M., Oh P., Pedram A., Schnitzer J., Levin E.R. ERs associate with and regulate the production of caveolin: Implications for signaling and cellular actions. Mol. Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 38.Razandi M., Pedram A., Park S.T., Levin E.R. Proximal events in signaling by plasma membrane estrogen receptors. J. Biol. Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 39.Marino M., Ascenzi P. Membrane association of estrogen receptor α and β influences 17β-estradiol-mediated cancer cell proliferation. Steroids. 2008;73:853–858. doi: 10.1016/j.steroids.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Migliaccio A., di Domenico M., Castoria G., de Falco A., Bontempo P., Nola E., Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 41.Migliaccio A., Piccolo D., Castoria G., Di Domenico M., Bilancio A., Lombardi M., Gong W., Beato M., Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kahlert S., Nuedling S., van Eickels M., Vetter H., Meyer R., Grohe C. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J. Biol. Chem. 2000;275:18447–18453. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- 43.Wong C.W., McNally C., Nickbarg E., Komm B.S., Cheskis B.J. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc. Natl. Acad. Sci. USA. 2002;99:14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Schiff R., Massarweh S.A., Shou J., Bharwani L., Mohsin S.K., Osborne C.K. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin. Cancer Res. 2004;10:S331–S336. doi: 10.1158/1078-0432.ccr-031212. [DOI] [PubMed] [Google Scholar]
- 45.Filardo E.J., Quinn J.A., Bland K.I., Frackelton A.R., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 46.Thomas P., Alyea R., Pang Y., Peyton C., Dong J., Berg A.H. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1 (GPER) in mammals and fish. Steroids. 2010;75:595–602. doi: 10.1016/j.steroids.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alexander S.P., Mathie A., Peters J.A. Guide to Receptors and Channels (GRAC), 5th edition. Br. J. Pharmacol. 2011;164:S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barton M. Position paper: The membrane estrogen receptor GPER—Clues and questions. Steroids. 2012;77:935–942. doi: 10.1016/j.steroids.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Kato S., Endoh H., Masuhiro Y., Kitamoto T., Uchiyama S., Sasaki H., Masushige S., Gotoh Y., Nishida E., Kawashima H., et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 50.Bunone G., Briand P.A., Miksicek R.J., Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 51.Joel P.B., Smith J., Sturgill T.W., Fisher T.L., Blenis J., Lannigan D.A. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol. Cell. Biol. 1998;18:1978–1984. doi: 10.1128/mcb.18.4.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell R.A., Bhat-Nakshatri P., Patel N.M., Constantinidou D., Ali S., Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: A new model for anti-estrogen resistance. J. Biol. Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 53.Sun M., Paciga J.E., Feldman R.I., Yuan Z., Coppola D., Lu Y.Y., Shelley S.A., Nicosia S.V., Cheng J.Q. Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor α (ERα) via interaction between ERα and PI3K. Cancer Res. 2001;61:5985–5991. [PubMed] [Google Scholar]
- 54.Thomas P., Pang Y., Filardo E.J., Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 55.Filardo E.J., Quinn J.A., Frackelton A.R., Jr, Bland K.I. Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 56.Carmeci C., Thompson D.A., Ring H.Z., Francke U., Weigel R.J. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- 57.Filardo E.J., Graeber C.T., Quinn J.A., Resnick M.B., Giri D., DeLellis R.A., Steinhoff M.M., Sabo E. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin. Cancer Res. 2006;12:6359–6366. doi: 10.1158/1078-0432.CCR-06-0860. [DOI] [PubMed] [Google Scholar]
- 58.Chang J., Fan W. Endocrine therapy resistance: Current status, possible mechanisms and overcoming strategies. Anticancer Agents Med. Chem. 2012 [Epub ahead of print] [PubMed] [Google Scholar]
- 59.Yang X., Phillips D.L., Ferguson A.T., Nelson W.G., Herman J.G., Davidson N.E. Synergistic activation of functional estrogen receptor (ER)-α by DNA methyltransferase and histone deacetylase inhibition in human ER-α-negative breast cancer cells. Cancer Res. 2001;61:7025–7029. [PubMed] [Google Scholar]
- 60.Parl F.F. Multiple mechanisms of estrogen receptor gene repression contribute to ER-negative breast cancer. Pharmacogenomics J. 2003;3:251–253. doi: 10.1038/sj.tpj.6500201. [DOI] [PubMed] [Google Scholar]
- 61.Weigel R.J., deConinck E.C. Transcriptional control of estrogen receptor in estrogen receptor-negative breast carcinoma. Cancer Res. 1993;53:3472–3474. [PubMed] [Google Scholar]
- 62.Ottaviano Y.L., Issa J.P., Parl F.F., Smith H.S., Baylin S.B., Davidson N.E. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 1994;54:2552–2555. [PubMed] [Google Scholar]
- 63.Robertson K.D., Ait-Si-Ali S., Yokochi T., Wade P.A., Jones P.L., Wolffe A.P. DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat. Genet. 2000;25:338–342. doi: 10.1038/77124. [DOI] [PubMed] [Google Scholar]
- 64.Rountree M.R., Bachman K.E., Baylin S.B. DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nat. Genet. 2000;25:269–277. doi: 10.1038/77023. [DOI] [PubMed] [Google Scholar]
- 65.Fan J., Yin W.J., Lu J.S., Wang L., Wu J., Wu F.Y., Di G.H., Shen Z.Z., Shao Z.M. ER alpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J. Cancer Res. Clin. Oncol. 2008;134:883–890. doi: 10.1007/s00432-008-0354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marks P.A. The mechanism of the anti-tumor activity of the histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA) Cell Cycle. 2004;3:534–535. doi: 10.4161/cc.3.5.827. [DOI] [PubMed] [Google Scholar]
- 67.Zhou Q., Shaw P.G., Davidson N.E. Inhibition of histone deacetylase suppresses EGF signaling pathways by destabilizing EGFR mRNA in ER-negative human breast cancer cells. Breast Cancer Res. Treat. 2009;117:443–451. doi: 10.1007/s10549-008-0148-5. [DOI] [PubMed] [Google Scholar]
- 68.Sabnis G.J., Goloubeva O., Chumsri S., Nguyen N., Sukumar S., Brodie A.M. Functional activation of the estrogen receptor-α and aromatase by the HDAC inhibitor entinostat sensitizes ER-negative tumors to letrozole. Cancer Res. 2011;71:1893–1903. doi: 10.1158/0008-5472.CAN-10-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keen J.C., Yan L., Mack K.M., Pettit C., Smith D., Sharma D., Davidson N.E. A novel histone deacetylase inhibitor, scriptaid, enhances expression of functional estrogen receptor alpha (ER) in ER negative human breast cancer cells in combination with 5-aza 2′-deoxycytidine. Breast Cancer Res. Treat. 2003;81:177–186. doi: 10.1023/A:1026146524737. [DOI] [PubMed] [Google Scholar]
- 70.Giacinti L., Giacinti C., Gabellini C., Rizzuto E., Lopez M., Giordano A. Scriptaid effects on breast cancer cell lines. J. Cell Physiol. 2012;227:3426–3433. doi: 10.1002/jcp.24043. [DOI] [PubMed] [Google Scholar]
- 71.Johnston S.R., Saccani-Jotti G., Smith I.E., Salter J., Newby J., Coppen M., Ebbs S.R., Dowsett M. Changes in estrogen receptor, progesterone receptor, and pS2 expression in tamoxifen-resistant human breast cancer. Cancer Res. 1995;55:3331–3338. [PubMed] [Google Scholar]
- 72.Gutierrez M.C., Detre S., Johnston S., Mohsin S.K., Shou J., Allred D.C., Schiff R., Osborne C.K., Dowsett M. Molecular changes in tamoxifen-resistant breast cancer: Relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J. Clin. Oncol. 2005;23:2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 73.Dowsett M. Overexpression of HER-2 as a resistance mechanism to hormonal therapy for breast cancer. Endocr. Relat. Cancer. 2001;8:191–195. doi: 10.1677/erc.0.0080191. [DOI] [PubMed] [Google Scholar]
- 74.Osborne C.K., Pippen J., Jones S.E., Parker L.M., Ellis M., Come S., Gertler S.Z., May J.T., Burton G., Dimery I., et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: Results of a North American trial. J. Clin. Oncol. 2002;20:3386–3395. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 75.Howell A., Pippen J., Elledge R.M., Mauriac L., Vergote I., Jones S.E., Come S.E., Osborne C.K., Robertson J.F. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma: A prospectively planned combined survival analysis of two multicenter trials. Cancer. 2005;104:236–239. doi: 10.1002/cncr.21163. [DOI] [PubMed] [Google Scholar]
- 76.Stoner M., Saville B., Wormke M., Dean D., Burghardt R., Safe S. Hypoxia induces proteasome-dependent degradation of estrogen receptor alpha in ZR-75 breast cancer cells. Mol. Endocrinol. 2002;16:2231–2242. doi: 10.1210/me.2001-0347. [DOI] [PubMed] [Google Scholar]
- 77.Creighton C.J., Hilger A.M., Murthy S., Rae J.M., Chinnaiyan A.M., El-Ashry D. Activation of mitogen-activated protein kinase in estrogen receptor α-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor α-negative human breast tumors. Cancer Res. 2006;66:3903–3911. doi: 10.1158/0008-5472.CAN-05-4363. [DOI] [PubMed] [Google Scholar]
- 78.Stoica A., Saceda M., Doraiswamy V.L., Coleman C., Martin M.B. Regulation of estrogen receptor-α gene expression by epidermal growth factor. J. Endocrinol. 2000;165:371–378. doi: 10.1677/joe.0.1650371. [DOI] [PubMed] [Google Scholar]
- 79.Angeloni S.V., Martin M.B., Garcia-Morales P., Castro-Galache M.D., Ferragut J.A., Saceda M. Regulation of estrogen receptor-α expression by the tumor suppressor gene p53 in MCF-7 cells. J. Endocrinol. 2004;180:497–504. doi: 10.1677/joe.0.1800497. [DOI] [PubMed] [Google Scholar]
- 80.Martin M.B., Angeloni S.V., Garcia-Morales P., Sholler P.F., Castro-Galache M.D., Ferragut J.A., Saceda M. Regulation of estrogen receptor-α expression in MCF-7 cells by taxol. J. Endocrinol. 2004;180:487–496. doi: 10.1677/joe.0.1800487. [DOI] [PubMed] [Google Scholar]
- 81.Macaluso M., Cinti C., Russo G., Russo A., Giordano A. pRb2/p130-E2F4/5-HDAC1- SUV39H1-p300 and pRb2/p130-E2F4/5-HDAC1-SUV39H1-DNMT1 multimolecular complexes mediate the transcription of estrogen receptor-alpha in breast cancer. Oncogene. 2003;22:3511–3517. doi: 10.1038/sj.onc.1206578. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Q.X., Borg A., Wolf D.M., Oesterreich S., Fuqua S.A. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 1997;57:1244–1249. [PubMed] [Google Scholar]
- 83.Wolf D.M., Jordan V.C. The estrogen receptor from a tamoxifen stimulated MCF-7 tumor variant contains a point mutation in the ligand binding domain. Breast Cancer Res. Treat. 1994;31:129–138. doi: 10.1007/BF00689683. [DOI] [PubMed] [Google Scholar]
- 84.Fuqua S.A., Wiltschke C., Zhang Q.X., Borg A., Castles C.G., Friedrichs W.E., Hopp T., Hilsenbeck S., Mohsin S., O’Connell P., et al. A hypersensitive estrogen receptor-α mutation in premalignant breast lesions. Cancer Res. 2000;60:4026–4029. [PubMed] [Google Scholar]
- 85.Herynk M.H., Fuqua S.A. Estrogen receptor mutations in human disease. Endocr Rev. 2004;25:869–898. doi: 10.1210/er.2003-0010. [DOI] [PubMed] [Google Scholar]
- 86.Speirs V., Parkes A.T., Kerin M.J., Walton D.S., Carleton P.J., Fox J.N., Atkin S.L. Coexpression of estrogen receptor α and β: Poor prognostic factors in human breast cancer? Cancer Res. 1999;59:525–528. [PubMed] [Google Scholar]
- 87.Hopp T.A., Weiss H.L., Parra I.S., Cui Y., Osborne C.K., Fuqua S.A. Low levels of estrogen receptor β protein predict resistance to tamoxifen therapy in breast cancer. Clin. Cancer Res. 2004;10:7490–7499. doi: 10.1158/1078-0432.CCR-04-1114. [DOI] [PubMed] [Google Scholar]
- 88.Borgquist S., Holm C., Stendahl M., Anagnostaki L., Landberg G., Jirstrom K. Oestrogen receptors α and β show different associations to clinicopathological parameters and their co-expression might predict a better response to endocrine treatment in breast cancer. J. Clin. Pathol. 2008;61:197–203. doi: 10.1136/jcp.2006.040378. [DOI] [PubMed] [Google Scholar]
- 89.Trimarchi M.P., Mouangsavanh M., Huang T.H. Cancer epigenetics: A perspective on the role of DNA methylation in acquired endocrine resistance. Chin. J. Cancer. 2011;30:749–756. doi: 10.5732/cjc.011.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Widschwendter M., Siegmund K.D., Muller H.M., Fiegl H., Marth C., Muller-Holzner E., Jones P.A., Laird P.W. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res. 2004;64:3807–3813. doi: 10.1158/0008-5472.CAN-03-3852. [DOI] [PubMed] [Google Scholar]
- 91.Lo P.K., Sukumar S. Epigenomics and breast cancer. Pharmacogenomics. 2008;9:1879–1902. doi: 10.2217/14622416.9.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Badia E., Duchesne M.J., Semlali A., Fuentes M., Giamarchi C., Richard-Foy H., Nicolas J.C., Pons M. Long-term hydroxytamoxifen treatment of an MCF-7-derived breast cancer cell line irreversibly inhibits the expression of estrogenic genes through chromatin remodeling. Cancer Res. 2000;60:4130–4138. [PubMed] [Google Scholar]
- 93.Jansen M.P., Sieuwerts A.M., Look M.P., Ritstier K., Meijer-van Gelder M.E., van Staveren I.L., Klijn J.G., Foekens J.A., Berns E.M. HOXB13-to-IL17BR expression ratio is related with tumor aggressiveness and response to tamoxifen of recurrent breast cancer: A retrospective study. J. Clin. Oncol. 2007;25:662–668. doi: 10.1200/JCO.2006.07.3676. [DOI] [PubMed] [Google Scholar]
- 94.Iorns E., Turner N.C., Elliott R., Syed N., Garrone O., Gasco M., Tutt A.N., Crook T., Lord C.J., Ashworth A. Identification of CDK10 as an important determinant of resistance to endocrine therapy for breast cancer. Cancer Cell. 2008;13:91–104. doi: 10.1016/j.ccr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 95.Heller G., Ziegler B., Brandstetter A., Novak S., Rudas M., Hennig G., Gehrmann M., Acht T., Zochbauer-Muller S., Filipits M. CDK10 is not a target for aberrant DNA methylation in breast cancer. Anticancer Res. 2009;29:3939–3944. [PubMed] [Google Scholar]
- 96.Pathiraja T.N., Stearns V., Oesterreich S. Epigenetic regulation in estrogen receptor positive breast cancer—Role in treatment response. J. Mammary Gland Biol. Neoplasia. 2010;15:35–47. doi: 10.1007/s10911-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shou J., Massarweh S., Osborne C.K., Wakeling A.E., Ali S., Weiss H., Schiff R. Mechanisms of tamoxifen resistance: Increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J. Natl. Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 98.Joel P.B., Traish A.M., Lannigan D.A. Estradiol-induced phosphorylation of serine 118 in the estrogen receptor is independent of p42/p44 mitogen-activated protein kinase. J. Biol. Chem. 1998;273:13317–13323. doi: 10.1074/jbc.273.21.13317. [DOI] [PubMed] [Google Scholar]
- 99.Britton D.J., Hutcheson I.R., Knowlden J.M., Barrow D., Giles M., McClelland R.A., Gee J.M., Nicholson R.I. Bidirectional cross talk between ERα and EGFR signalling pathways regulates tamoxifen-resistant growth. Breast Cancer Res. Treat. 2006;96:131–146. doi: 10.1007/s10549-005-9070-2. [DOI] [PubMed] [Google Scholar]
- 100.De Mora J.F., Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol. Cell. Biol. 2000;20:5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Osborne C.K., Bardou V., Hopp T.A., Chamness G.C., Hilsenbeck S.G., Fuqua S.A., Wong J., Allred D.C., Clark G.M., Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J. Natl. Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 102.Nicholson R.I., Hutcheson I.R., Jones H.E., Hiscox S.E., Giles M., Taylor K.M., Gee J.M. Growth factor signalling in endocrine and anti-growth factor resistant breast cancer. Rev. Endocr. Metab. Disord. 2007;8:241–253. doi: 10.1007/s11154-007-9033-5. [DOI] [PubMed] [Google Scholar]
- 103.Benz C.C., Scott G.K., Sarup J.C., Johnson R.M., Tripathy D., Coronado E., Shepard H.M., Osborne C.K. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res. Treat. 1992;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 104.Chung Y.L., Sheu M.L., Yang S.C., Lin C.H., Yen S.H. Resistance to tamoxifen-induced apoptosis is associated with direct interaction between Her2/neu and cell membrane estrogen receptor in breast cancer. Int. J. Cancer. 2002;97:306–312. doi: 10.1002/ijc.1614. [DOI] [PubMed] [Google Scholar]
- 105.Kaufman B., Mackey J.R., Clemens M.R., Bapsy P.P., Vaid A., Wardley A., Tjulandin S., Jahn M., Lehle M., Feyereislova A., et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J. Clin. Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 106.Massarweh S., Osborne C.K., Jiang S., Wakeling A.E., Rimawi M., Mohsin S.K., Hilsenbeck S., Schiff R. Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive, HER-2/neu-positive breast cancer. Cancer Res. 2006;66:8266–8273. doi: 10.1158/0008-5472.CAN-05-4045. [DOI] [PubMed] [Google Scholar]
- 107.Wang X., Masri S., Phung S., Chen S. The role of amphiregulin in exemestane-resistant breast cancer cells: Evidence of an autocrine loop. Cancer Res. 2008;68:2259–2265. doi: 10.1158/0008-5472.CAN-07-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen A.C., Migliaccio I., Rimawi M., Lopez-Tarruella S., Creighton C.J., Massarweh S., Huang C., Wang Y.C., Batra S.K., Gutierrez M.C., et al. Upregulation of mucin4 in ER-positive/HER2-overexpressing breast cancer xenografts with acquired resistance to endocrine and HER2-targeted therapies. Breast Cancer Res. Treat. 2012;134:583–593. doi: 10.1007/s10549-012-2082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Johnston S., Pippen J., Jr, Pivot X., Lichinitser M., Sadeghi S., Dieras V., Gomez H.L., Romieu G., Manikhas A., Kennedy M.J., et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 110.Evans A.H., Pancholi S., Farmer I., Thornhill A., Evans D.B., Johnston S.R., Dowsett M., Martin L.A. EGFR/HER2 inhibitor AEE788 increases ER-mediated transcription in HER2/ER-positive breast cancer cells but functions synergistically with endocrine therapy. Br. J. Cancer. 2010;102:1235–1243. doi: 10.1038/sj.bjc.6605641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Surmacz E. Function of the IGF-I receptor in breast cancer. J. Mammary Gland Biol. Neoplasia. 2000;5:95–105. doi: 10.1023/a:1009523501499. [DOI] [PubMed] [Google Scholar]
- 112.Knowlden J.M., Hutcheson I.R., Barrow D., Gee J.M., Nicholson R.I. Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: A supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146:4609–4618. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- 113.Hurbin A., Dubrez L., Coll J.L., Favrot M.C. Inhibition of apoptosis by amphiregulin via an insulin-like growth factor-1 receptor-dependent pathway in non-small cell lung cancer cell lines. J. Biol. Chem. 2002;277:49127–49133. doi: 10.1074/jbc.M207584200. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y., Moerkens M., Ramaiahgari S., de Bont H., Price L., Meerman J., van de Water B. Elevated insulin-like growth factor 1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routes. Breast Cancer Res. 2011;13:R52. doi: 10.1186/bcr2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chong K., Subramanian A., Sharma A., Mokbel K. Measuring IGF-1, ER-α and EGFR expression can predict tamoxifen-resistance in ER-positive breast cancer. Anticancer Res. 2011;31:23–32. [PubMed] [Google Scholar]
- 116.Fagan D.H., Uselman R.R., Sachdev D., Yee D. Acquired resistance to tamoxifen is associated with loss of the type I insulin-like growth factor receptor: Implications for breast cancer treatment. Cancer Res. 2012;72:3372–3380. doi: 10.1158/0008-5472.CAN-12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cui J., Germer K., Wu T., Wang J., Luo J., Wang S.C., Wang Q., Zhang X. Cross-talk between HER2 and MED1 regulates tamoxifen resistance of human breast cancer cells. Cancer Res. 2012;72:5625–5634. doi: 10.1158/0008-5472.CAN-12-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chang F., Lee J.T., Navolanic P.M., Steelman L.S., Shelton J.G., Blalock W.L., Franklin R.A., McCubrey J.A. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 119.Datta S.R., Brunet A., Greenberg M.E. Cellular survival: A play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 120.Simoncini T., Hafezi-Moghadam A., Brazil D.P., Ley K., Chin W.W., Liao J.K. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clark A.S., West K., Streicher S., Dennis P.A. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol. Cancer Ther. 2002;1:707–717. [PubMed] [Google Scholar]
- 122.Martin M.B., Franke T.F., Stoica G.E., Chambon P., Katzenellenbogen B.S., Stoica B.A., McLemore M.S., Olivo S.E., Stoica A. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 2000;141:4503–4511. doi: 10.1210/endo.141.12.7836. [DOI] [PubMed] [Google Scholar]
- 123.Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S.I., Puc J., Miliaresis C., Rodgers L., McCombie R., et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 124.Miller T.W., Perez-Torres M., Narasanna A., Guix M., Stal O., Perez-Tenorio G., Gonzalez-Angulo A.M., Hennessy B.T., Mills G.B., Kennedy J.P., et al. Loss of Phosphatase and Tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res. 2009;69:4192–4201. doi: 10.1158/0008-5472.CAN-09-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miller T.W., Rexer B.N., Garrett J.T., Arteaga C.L. Mutations in the phosphatidylinositol 3-kinase pathway: Role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13:224. doi: 10.1186/bcr3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ma C.X., Crowder R.J., Ellis M.J. Importance of PI3-kinase pathway in response/resistance to aromatase inhibitors. Steroids. 2011;76:750–752. doi: 10.1016/j.steroids.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 127.Creighton C.J., Fu X., Hennessy B.T., Casa A.J., Zhang Y., Gonzalez-Angulo A.M., Lluch A., Gray J.W., Brown P.H., Hilsenbeck S.G., et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010;12:R40. doi: 10.1186/bcr2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miller T.W., Balko J.M., Arteaga C.L. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J. Clin. Oncol. 2011;29:4452–4461. doi: 10.1200/JCO.2010.34.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barnadas A., Estevez L.G., Lluch-Hernandez A., Rodriguez-Lescure A., Rodriguez-Sanchez C., Sanchez-Rovira P. An overview of letrozole in postmenopausal women with hormone-responsive breast cancer. Adv. Ther. 2011;28:1045–1058. doi: 10.1007/s12325-011-0075-4. [DOI] [PubMed] [Google Scholar]
- 130.Cavazzoni A., Bonelli M.A., Fumarola C., la Monica S., Airoud K., Bertoni R., Alfieri R.R., Galetti M., Tramonti S., Galvani E., et al. Overcoming acquired resistance to letrozole by targeting the PI3K/AKT/mTOR pathway in breast cancer cell clones. Cancer Lett. 2012;323:77–87. doi: 10.1016/j.canlet.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 131.Chan C.M., Martin L.A., Johnston S.R., Ali S., Dowsett M. Molecular changes associated with the acquisition of oestrogen hypersensitivity in MCF-7 breast cancer cells on long-term oestrogen deprivation. J. Steroid Biochem. Mol. Biol. 2002;81:333–341. doi: 10.1016/s0960-0760(02)00074-2. [DOI] [PubMed] [Google Scholar]
- 132.Santen R.J., Song R.X., Masamura S., Yue W., Fan P., Sogon T., Hayashi S., Nakachi K., Eguchi H. Adaptation to estradiol deprivation causes up-regulation of growth factor pathways and hypersensitivity to estradiol in breast cancer cells. Adv. Exp. Med. Biol. 2008;630:19–34. doi: 10.1007/978-0-387-78818-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Santen R.J., Song R.X., Zhang Z., Kumar R., Jeng M.H., Masamura A., Lawrence J., Jr, Berstein L., Yue W. Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity. Endocr Relat Cancer. 2005;12:S61–S73. doi: 10.1677/erc.1.01018. [DOI] [PubMed] [Google Scholar]
- 134.Masamura S., Santner S.J., Heitjan D.F., Santen R.J. Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J. Clin. Endocrinol. Metab. 1995;80:2918–2925. doi: 10.1210/jcem.80.10.7559875. [DOI] [PubMed] [Google Scholar]
- 135.Hurvitz S.A., Pietras R.J. Rational management of endocrine resistance in breast cancer: A comprehensive review of estrogen receptor biology, treatment options, and future directions. Cancer. 2008;113:2385–2397. doi: 10.1002/cncr.23875. [DOI] [PubMed] [Google Scholar]
- 136.Masri S., Phung S., Wang X., Wu X., Yuan Y.C., Wagman L., Chen S. Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer Res. 2008;68:4910–4918. doi: 10.1158/0008-5472.CAN-08-0303. [DOI] [PubMed] [Google Scholar]
- 137.Berstein L.M., Zheng H., Yue W., Wang J.P., Lykkesfeldt A.E., Naftolin F., Harada H., Shanabrough M., Santen R.J. New approaches to the understanding of tamoxifen action and resistance. Endocr. Relat. Cancer. 2003;10:267–277. doi: 10.1677/erc.0.0100267. [DOI] [PubMed] [Google Scholar]
- 138.Miller T.W., Hennessy B.T., Gonzalez-Angulo A.M., Fox E.M., Mills G.B., Chen H., Higham C., Garcia-Echeverria C., Shyr Y., Arteaga C.L. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J. Clin. Invest. 2010;120:2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sanchez C.G., Ma C.X., Crowder R.J., Guintoli T., Phommaly C., Gao F., Lin L., Ellis M.J. Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Res. 2011;13:R21. doi: 10.1186/bcr2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 141.Kyriakis J.M., Banerjee P., Nikolakaki E., Dai T., Rubie E.A., Ahmad M.F., Avruch J., Woodgett J.R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 142.Dumont J.A., Bitonti A.J., Wallace C.D., Baumann R.J., Cashman E.A., Cross-Doersen D.E. Progression of MCF-7 breast cancer cells to antiestrogen-resistant phenotype is accompanied by elevated levels of AP-1 DNA-binding activity. Cell Growth Differ. 1996;7:351–359. [PubMed] [Google Scholar]
- 143.Johnston S.R., Lu B., Scott G.K., Kushner P.J., Smith I.E., Dowsett M., Benz C.C. Increased activator protein-1 DNA binding and c-Jun NH2-terminal kinase activity in human breast tumors with acquired tamoxifen resistance. Clin. Cancer Res. 1999;5:251–256. [PubMed] [Google Scholar]
- 144.Schiff R., Reddy P., Ahotupa M., Coronado-Heinsohn E., Grim M., Hilsenbeck S.G., Lawrence R., Deneke S., Herrera R., Chamness G.C., et al. Oxidative stress and AP-1 activity in tamoxifen-resistant breast tumors in vivo. J. Natl. Cancer Inst. 2000;92:1926–1934. doi: 10.1093/jnci/92.23.1926. [DOI] [PubMed] [Google Scholar]
- 145.Clarke R., Skaar T.C., Bouker K.B., Davis N., Lee Y.R., Welch J.N., Leonessa F. Molecular and pharmacological aspects of antiestrogen resistance. J. Steroid Biochem. Mol. Biol. 2001;76:71–84. doi: 10.1016/s0960-0760(00)00193-x. [DOI] [PubMed] [Google Scholar]
- 146.Webb P., Lopez G.N., Uht R.M., Kushner P.J. Tamoxifen activation of the estrogen receptor/AP-1 pathway: Potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol. Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- 147.Jiang Y., Chen C., Li Z., Guo W., Gegner J.A., Lin S., Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β) J. Biol. Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- 148.New L., Han J. The p38 MAP kinase pathway and its biological function. Trends Cardiovasc. Med. 1998;8:220–228. doi: 10.1016/s1050-1738(98)00012-7. [DOI] [PubMed] [Google Scholar]
- 149.Lee H., Bai W. Regulation of estrogen receptor nuclear export by ligand-induced and p38-mediated receptor phosphorylation. Mol. Cell. Biol. 2002;22:5835–5845. doi: 10.1128/MCB.22.16.5835-5845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Antoon J.W., Bratton M.R., Guillot L.M., Wadsworth S., Salvo V.A., Elliott S., McLachlan J.A., Burow M.E. Pharmacology and anti-tumor activity of RWJ67657, a novel inhibitor of p38 mitogen activated protein kinase. Am. J. Cancer Res. 2012;2:446–458. [PMC free article] [PubMed] [Google Scholar]
- 151.Aesoy R., Sanchez B.C., Norum J.H., Lewensohn R., Viktorsson K., Linderholm B. An autocrine VEGF/VEGFR2 and p38 signaling loop confers resistance to 4-hydroxytamoxifen in MCF-7 breast cancer cells. Mol. Cancer Res. 2008;6:1630–1638. doi: 10.1158/1541-7786.MCR-07-2172. [DOI] [PubMed] [Google Scholar]
- 152.Al Saleh S., Sharaf L.H., Luqmani Y.A. Signalling pathways involved in endocrine resistance in breast cancer and associations with epithelial to mesenchymal transition (Review) Int. J. Oncol. 2011;38:1197–1217. doi: 10.3892/ijo.2011.942. [DOI] [PubMed] [Google Scholar]
- 153.Weigel M.T., Ghazoui Z., Dunbier A., Pancholi S., Dowsett M., Martin L.A. Preclinical and clinical studies of estrogen deprivation support the PDGF/Abl pathway as a novel therapeutic target for overcoming endocrine resistance in breast cancer. Breast Cancer Res. 2012;14:R78. doi: 10.1186/bcr3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhou Y., Yau C., Gray J.W., Chew K., Dairkee S.H., Moore D.H., Eppenberger U., Eppenberger-Castori S., Benz C.C. Enhanced NF κB and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007;7:59. doi: 10.1186/1471-2407-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nakshatri H., Bhat-Nakshatri P., Martin D.A., Goulet R.J., Jr, Sledge G.W., Jr Constitutive activation of NF-κB during progression of breast cancer to hormone-independent growth. Mol. Cell. Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sovak M.A., Bellas R.E., Kim D.W., Zanieski G.J., Rogers A.E., Traish A.M., Sonenshein G.E. Aberrant nuclear factor-κB/Rel expression and the pathogenesis of breast cancer. J. Clin. Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Biswas D.K., Singh S., Shi Q., Pardee A.B., Iglehart J.D. Crossroads of estrogen receptor and NF-κB signaling. Sci STKE. 2005;2005:e27. doi: 10.1126/stke.2882005pe27. [DOI] [PubMed] [Google Scholar]
- 158.Sas L., Lardon F., Vermeulen P.B., Hauspy J., van dam P., Pauwels P., Dirix L.Y., van Laere S.J. The interaction between ER and NFκB in resistance to endocrine therapy. Breast Cancer Res. 2012;14:212. doi: 10.1186/bcr3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chang H.L., Sugimoto Y., Liu S., Ye W., Wang L.S., Huang Y.W., Lin Y.C. Keratinocyte growth factor (KGF) induces tamoxifen (Tam) resistance in human breast cancer MCF-7 cells. Anticancer Res. 2006;26:1773–1784. [PubMed] [Google Scholar]
- 160.Dontu G., Jackson K.W., McNicholas E., Kawamura M.J., Abdallah W.M., Wicha M.S. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stylianou S., Clarke R.B., Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66:1517–1525. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- 162.Rizzo P., Miao H., D’Souza G., Osipo C., Song L.L., Yun J., Zhao H., Mascarenhas J., Wyatt D., Antico G., et al. Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res. 2008;68:5226–5235. doi: 10.1158/0008-5472.CAN-07-5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dibb N.J., Dilworth S.M., Mol C.D. Switching on kinases: Oncogenic activation of BRAF and the PDGFR family. Nat. Rev. Cancer. 2004;4:718–727. doi: 10.1038/nrc1434. [DOI] [PubMed] [Google Scholar]
- 164.Yuan Z.M., Huang Y., Whang Y., Sawyers C., Weichselbaum R., Kharbanda S., Kufe D. Role for c-Abl tyrosine kinase in growth arrest response to DNA damage. Nature. 1996;382:272–274. doi: 10.1038/382272a0. [DOI] [PubMed] [Google Scholar]
- 165.Brasher B.B., van Etten R.A. c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J. Biol. Chem. 2000;275:35631–35637. doi: 10.1074/jbc.M005401200. [DOI] [PubMed] [Google Scholar]
- 166.Sikora M.J., Strumba V., Lippman M.E., Johnson M.D., Rae J.M. Mechanisms of estrogen-independent breast cancer growth driven by low estrogen concentrations are unique versus complete estrogen deprivation. Breast Cancer Res. Treat. 2012;134:1027–1039. doi: 10.1007/s10549-012-2032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li M., Li J., Ding X., He M., Cheng S.Y. microRNA and cancer. AAPS J. 2010;12:309–317. doi: 10.1208/s12248-010-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 169.Calin G.A., Sevignani C., Dumitru C.D., Hyslop T., Noch E., Yendamuri S., Shimizu M., Rattan S., Bullrich F., Negrini M., et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kosaka N., Iguchi H., Ochiya T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101:2087–2092. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cho W.C. MicroRNAs as therapeutic targets and their potential applications in cancer therapy. Expert Opin. Ther. Targets. 2012;16:747–759. doi: 10.1517/14728222.2012.696102. [DOI] [PubMed] [Google Scholar]
- 172.Iorio M.V., Casalini P., Piovan C., Braccioli L., Tagliabue E. Breast cancer and microRNAs: Therapeutic impact. Breast. 2011;20:S63–S70. doi: 10.1016/S0960-9776(11)70297-1. [DOI] [PubMed] [Google Scholar]
- 173.Xin F., Li M., Balch C., Thomson M., Fan M., Liu Y., Hammond S.M., Kim S., Nephew K.P. Computational analysis of microRNA profiles and their target genes suggests significant involvement in breast cancer antiestrogen resistance. Bioinformatics. 2009;25:430–434. doi: 10.1093/bioinformatics/btn646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lyng M.B., Laenkholm A.V., Sokilde R., Gravgaard K.H., Litman T., Ditzel H.J. Global microRNA expression profiling of high-risk ER+ breast cancers from patients receiving adjuvant tamoxifen mono-therapy: A DBCG study. PLoS One. 2012;7:e36170. doi: 10.1371/journal.pone.0036170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Manavalan T.T., Teng Y., Appana S.N., Datta S., Kalbfleisch T.S., Li Y., Klinge C.M. Differential expression of microRNA expression in tamoxifen-sensitive MCF-7 versus tamoxifen-resistant LY2 human breast cancer cells. Cancer Lett. 2011;313:26–43. doi: 10.1016/j.canlet.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Miller T.E., Ghoshal K., Ramaswamy B., Roy S., Datta J., Shapiro C.L., Jacob S., Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cittelly D.M., Das P.M., Spoelstra N.S., Edgerton S.M., Richer J.K., Thor A.D., Jones F.E. Downregulation of miR-342 is associated with tamoxifen resistant breast tumors. Mol. Cancer. 2010;9:317. doi: 10.1186/1476-4598-9-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Klinge C.M., Riggs K.A., Wickramasinghe N.S., Emberts C.G., McConda D.B., Barry P.N., Magnusen J.E. Estrogen receptor α 46 is reduced in tamoxifen resistant breast cancer cells and re-expression inhibits cell proliferation and estrogen receptor α 66-regulated target gene transcription. Mol. Cell. Endocrinol. 2010;323:268–276. doi: 10.1016/j.mce.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bergamaschi A., Katzenellenbogen B.S. Tamoxifen downregulation of miR-451 increases 14-3-3ζ and promotes breast cancer cell survival and endocrine resistance. Oncogene. 2012;31:39–47. doi: 10.1038/onc.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Carlomagno C., Perrone F., Gallo C., de Laurentiis M., Lauria R., Morabito A., Pettinato G., Panico L., D’Antonio A., Bianco A.R., et al. c-erb B2 overexpression decreases the benefit of adjuvant tamoxifen in early-stage breast cancer without axillary lymph node metastases. J. Clin. Oncol. 1996;14:2702–2708. doi: 10.1200/JCO.1996.14.10.2702. [DOI] [PubMed] [Google Scholar]
- 181.Giacinti L., Claudio P.P., Lopez M., Giordano A. Epigenetic information and estrogen receptor alpha expression in breast cancer. Oncologist. 2006;11:1–8. doi: 10.1634/theoncologist.11-1-1. [DOI] [PubMed] [Google Scholar]
- 182.Musgrove E.A., Sutherland R.L. Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 183.Rao X., di Leva G., Li M., Fang F., Devlin C., Hartman-Frey C., Burow M.E., Ivan M., Croce C.M., Nephew K.P. MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene. 2011;30:1082–1097. doi: 10.1038/onc.2010.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhao J.J., Lin J., Yang H., Kong W., He L., Ma X., Coppola D., Cheng J.Q. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J. Biol. Chem. 2008;283:31079–31086. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 185.Adams B.D., Furneaux H., White B.A. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-α (ERα) and represses ERα messenger RNA and protein expression in breast cancer cell lines. Mol. Endocrinol. 2007;21:1132–1147. doi: 10.1210/me.2007-0022. [DOI] [PubMed] [Google Scholar]
- 186.Kondo N., Toyama T., Sugiura H., Fujii Y., Yamashita H. miR-206 Expression is down-regulated in estrogen receptor α-positive human breast cancer. Cancer Res. 2008;68:5004–5008. doi: 10.1158/0008-5472.CAN-08-0180. [DOI] [PubMed] [Google Scholar]
- 187.Tessel M.A., Krett N.L., Rosen S.T. Steroid receptor and microRNA regulation in cancer. Curr. Opin. Oncol. 2010;22:592–597. doi: 10.1097/CCO.0b013e32833ea80c. [DOI] [PubMed] [Google Scholar]
- 188.Xiong J., Yu D., Wei N., Fu H., Cai T., Huang Y., Wu C., Zheng X., Du Q., Lin D., Liang Z. An estrogen receptor α suppressor, microRNA-22, is downregulated in estrogen receptor α-positive human breast cancer cell lines and clinical samples. FEBS J. 2010;277:1684–1694. doi: 10.1111/j.1742-4658.2010.07594.x. [DOI] [PubMed] [Google Scholar]
- 189.Mitra D., Brumlik M.J., Okamgba S.U., Zhu Y., Duplessis T.T., Parvani J.G., Lesko S.M., Brogi E., Jones F.E. An oncogenic isoform of HER2 associated with locally disseminated breast cancer and trastuzumab resistance. Mol. Cancer Ther. 2009;8:2152–2162. doi: 10.1158/1535-7163.MCT-09-0295. [DOI] [PubMed] [Google Scholar]
- 190.Cittelly D.M., Das P.M., Salvo V.A., Fonseca J.P., Burow M.E., Jones F.E. Oncogenic HER2δ16 suppresses miR-15a/16 and deregulates BCL-2 to promote endocrine resistance of breast tumors. Carcinogenesis. 2010;31:2049–2057. doi: 10.1093/carcin/bgq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Neal C.L., Yao J., Yang W., Zhou X., Nguyen N.T., Lu J., Danes C.G., Guo H., Lan K.H., Ensor J., Hittelman W., Hung M.C., Yu D. 14-3-3ζ overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res. 2009;69:3425–3432. doi: 10.1158/0008-5472.CAN-08-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Frasor J., Chang E.C., Komm B., Lin C.Y., Vega V.B., Liu E.T., Miller L.D., Smeds J., Bergh J., Katzenellenbogen B.S. Gene expression preferentially regulated by tamoxifen in breast cancer cells and correlations with clinical outcome. Cancer Res. 2006;66:7334–7340. doi: 10.1158/0008-5472.CAN-05-4269. [DOI] [PubMed] [Google Scholar]
- 193.Bergamaschi A., Christensen B.L., Katzenellenbogen B.S. Reversal of endocrine resistance in breast cancer: Interrelationships among 14-3-3ζ, FOXM1, and a gene signature associated with mitosis. Breast Cancer Res. 2011;13:R70. doi: 10.1186/bcr2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rothe F., Ignatiadis M., Chaboteaux C., Haibe-Kains B., Kheddoumi N., Majjaj S., Badran B., Fayyad-Kazan H., Desmedt C., Harris A.L., et al. Global microRNA expression profiling identifies MiR-210 associated with tumor proliferation, invasion and poor clinical outcome in breast cancer. PLoS One. 2011;6:e20980. doi: 10.1371/journal.pone.0020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shi W., Gerster K., Alajez N.M., Tsang J., Waldron L., Pintilie M., Hui A.B., Sykes J., P’ng C., Miller N., et al. MicroRNA-301 mediates proliferation and invasion in human breast cancer. Cancer Res. 2011;71:2926–2937. doi: 10.1158/0008-5472.CAN-10-3369. [DOI] [PubMed] [Google Scholar]
- 196.Sachdeva M., Wu H., Ru P., Hwang L., Trieu V., Mo Y.Y. MicroRNA-101-mediated Akt activation and estrogen-independent growth. Oncogene. 2011;30:822–831. doi: 10.1038/onc.2010.463. [DOI] [PubMed] [Google Scholar]
- 197.Knabbe C., Zugmaier G., Schmahl M., Dietel M., Lippman M.E., Dickson R.B. Induction of transforming growth factor β by the antiestrogens droloxifene, tamoxifen, and toremifene in MCF-7 cells. Am. J. Clin. Oncol. 1991;14:S15–S20. doi: 10.1097/00000421-199112002-00005. [DOI] [PubMed] [Google Scholar]
- 198.Knabbe C., Kopp A., Hilgers W., Lang D., Muller V., Zugmaier G., Jonat W. Regulation and role of TGF β production in breast cancer. Ann. N. Y. Acad. Sci. 1996;784:263–276. doi: 10.1111/j.1749-6632.1996.tb16241.x. [DOI] [PubMed] [Google Scholar]
- 199.Buck M.B., Pfizenmaier K., Knabbe C. Antiestrogens induce growth inhibition by sequential activation of p38 mitogen-activated protein kinase and transforming growth factor-β pathways in human breast cancer cells. Mol. Endocrinol. 2004;18:1643–1657. doi: 10.1210/me.2003-0278. [DOI] [PubMed] [Google Scholar]
- 200.Perry R.R., Kang Y., Greaves B.R. Relationship between tamoxifen-induced transforming growth factor β 1 expression, cytostasis and apoptosis in human breast cancer cells. Br. J. Cancer. 1995;72:1441–1446. doi: 10.1038/bjc.1995.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yoo Y.A., Kim Y.H., Kim J.S., Seo J.H. The functional implications of Akt activity and TGF-β signaling in tamoxifen-resistant breast cancer. Biochim. Biophys. Acta. 2008;1783:438–447. doi: 10.1016/j.bbamcr.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 202.Arteaga C.L., Koli K.M., Dugger T.C., Clarke R. Reversal of tamoxifen resistance of human breast carcinomas in vivo by neutralizing antibodies to transforming growth factor-β. J. Natl. Cancer Inst. 1999;91:46–53. doi: 10.1093/jnci/91.1.46. [DOI] [PubMed] [Google Scholar]
- 203.Foekens J.A., Sieuwerts A.M., Smid M., Look M.P., de Weerd V., Boersma A.W., Klijn J.G., Wiemer E.A., Martens J.W. Four miRNAs associated with aggressiveness of lymph node-negative, estrogen receptor-positive human breast cancer. Proc. Natl. Acad. Sci. USA. 2008;105:13021–13026. doi: 10.1073/pnas.0803304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Masri S., Liu Z., Phung S., Wang E., Yuan Y.C., Chen S. The role of microRNA-128a in regulating TGFβ signaling in letrozole-resistant breast cancer cells. Breast Cancer Res. Treat. 2010;124:89–99. doi: 10.1007/s10549-009-0716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sommer S., Fuqua S.A. Estrogen receptor and breast cancer. Semin. Cancer Biol. 2001;11:339–352. doi: 10.1006/scbi.2001.0389. [DOI] [PubMed] [Google Scholar]
- 206.Smith C.L., Nawaz Z., O’Malley B.W. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol. Endocrinol. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- 207.Jordan V.C., O’Malley B.W. Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J. Clin. Oncol. 2007;25:5815–5824. doi: 10.1200/JCO.2007.11.3886. [DOI] [PubMed] [Google Scholar]
- 208.Lydon J.P., O’Malley B.W. Minireview: Steroid receptor coactivator-3: A multifarious coregulator in mammary gland metastasis. Endocrinology. 2011;152:19–25. doi: 10.1210/en.2010-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Anzick S.L., Kononen J., Walker R.L., Azorsa D.O., Tanner M.M., Guan X.Y., Sauter G., Kallioniemi O.P., Trent J.M., Meltzer P.S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 210.Murphy L.C., Simon S.L., Parkes A., Leygue E., Dotzlaw H., Snell L., Troup S., Adeyinka A., Watson P.H. Altered expression of estrogen receptor coregulators during human breast tumorigenesis. Cancer Res. 2000;60:6266–6271. [PubMed] [Google Scholar]
- 211.List H.J., Reiter R., Singh B., Wellstein A., Riegel A.T. Expression of the nuclear coactivator AIB1 in normal and malignant breast tissue. Breast Cancer Res. Treat. 2001;68:21–28. doi: 10.1023/a:1017910924390. [DOI] [PubMed] [Google Scholar]
- 212.Kressler D., Hock M.B., Kralli A. Coactivators PGC-1β and SRC-1 interact functionally to promote the agonist activity of the selective estrogen receptor modulator tamoxifen. J. Biol. Chem. 2007;282:26897–26907. doi: 10.1074/jbc.M705596200. [DOI] [PubMed] [Google Scholar]
- 213.Harigopal M., Heymann J., Ghosh S., Anagnostou V., Camp R.L., Rimm D.L. Estrogen receptor co-activator (AIB1) protein expression by automated quantitative analysis (AQUA) in a breast cancer tissue microarray and association with patient outcome. Breast Cancer Res. Treat. 2009;115:77–85. doi: 10.1007/s10549-008-0063-9. [DOI] [PubMed] [Google Scholar]
- 214.McIlroy M., Fleming F.J., Buggy Y., Hill A.D., Young L.S. Tamoxifen-induced ER-α-SRC-3 interaction in HER2 positive human breast cancer; a possible mechanism for ER isoform specific recurrence. Endocr. Relat. Cancer. 2006;13:1135–1145. doi: 10.1677/erc.1.01222. [DOI] [PubMed] [Google Scholar]
- 215.Wang L.H., Yang X.Y., Zhang X., An P., Kim H.J., Huang J., Clarke R., Osborne C.K., Inman J.K., Appella E., et al. Disruption of estrogen receptor DNA-binding domain and related intramolecular communication restores tamoxifen sensitivity in resistant breast cancer. Cancer Cell. 2006;10:487–499. doi: 10.1016/j.ccr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 216.Kirkegaard T., McGlynn L.M., Campbell F.M., Muller S., Tovey S.M., Dunne B., Nielsen K.V., Cooke T.G., Bartlett J.M. Amplified in breast cancer 1 in human epidermal growth factor receptor-positive tumors of tamoxifen-treated breast cancer patients. Clin. Cancer Res. 2007;13:1405–1411. doi: 10.1158/1078-0432.CCR-06-1933. [DOI] [PubMed] [Google Scholar]
- 217.Haugan Moi L.L., Hauglid Flageng M., Gandini S., Guerrieri-Gonzaga A., Bonanni B., Lazzeroni M., Gjerde J., Lien E.A., DeCensi A., Mellgren G. Effect of low-dose tamoxifen on steroid receptor coactivator 3/amplified in breast cancer 1 in normal and malignant human breast tissue. Clin. Cancer Res. 2010;16:2176–2186. doi: 10.1158/1078-0432.CCR-09-1859. [DOI] [PubMed] [Google Scholar]
- 218.Redmond A.M., Bane F.T., Stafford A.T., McIlroy M., Dillon M.F., Crotty T.B., Hill A.D., Young L.S. Coassociation of estrogen receptor and p160 proteins predicts resistance to endocrine treatment; SRC-1 is an independent predictor of breast cancer recurrence. Clin. Cancer Res. 2009;15:2098–2106. doi: 10.1158/1078-0432.CCR-08-1649. [DOI] [PubMed] [Google Scholar]
- 219.Haugan Moi L.L., Flageng M.H., Gjerde J., Madsen A., Rost T.H., Gudbrandsen O.A., Lien E.A., Mellgren G. Steroid receptor coactivators, HER-2 and HER-3 expression is stimulated by tamoxifen treatment in DMBA-induced breast cancer. BMC Cancer. 2012;12:247. doi: 10.1186/1471-2407-12-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bates N.P., Hurst H.C. An intron 1 enhancer element mediates oestrogen-induced suppression of ERBB2 expression. Oncogene. 1997;15:473–481. doi: 10.1038/sj.onc.1201368. [DOI] [PubMed] [Google Scholar]
- 221.Frasor J., Stossi F., Danes J.M., Komm B., Lyttle C.R., Katzenellenbogen B.S. Selective estrogen receptor modulators: Discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res. 2004;64:1522–1533. doi: 10.1158/0008-5472.can-03-3326. [DOI] [PubMed] [Google Scholar]
- 222.Hurtado A., Holmes K.A., Geistlinger T.R., Hutcheson I.R., Nicholson R.I., Brown M., Jiang J., Howat W.J., Ali S., Carroll J.S. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature. 2008;456:663–666. doi: 10.1038/nature07483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.McCartan D., Bolger J.C., Fagan A., Byrne C., Hao Y., Qin L., McIlroy M., Xu J., Hill A.D., Gaora P.O., et al. Global characterization of the SRC-1 transcriptome identifies ADAM22 as an ER-independent mediator of endocrine-resistant breast cancer. Cancer Res. 2012;72:220–229. doi: 10.1158/0008-5472.CAN-11-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Duffy M.J., McKiernan E., O’Donovan N., McGowan P.M. Role of ADAMs in cancer formation and progression. Clin. Cancer Res. 2009;15:1140–1144. doi: 10.1158/1078-0432.CCR-08-1585. [DOI] [PubMed] [Google Scholar]
- 225.Flageng M.H., Moi L.L., Dixon J.M., Geisler J., Lien E.A., Miller W.R., Lonning P.E., Mellgren G. Nuclear receptor co-activators and HER-2/neu are upregulated in breast cancer patients during neo-adjuvant treatment with aromatase inhibitors. Br. J. Cancer. 2009;101:1253–1260. doi: 10.1038/sj.bjc.6605324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.O’Hara J., Vareslija D., McBryan J., Bane F., Tibbitts P., Byrne C., Conroy R.M., Hao Y., Gaora P.O., Hill A.D., et al. AIB1:ERα transcriptional activity is selectively enhanced in aromatase inhibitor-resistant breast cancer cells. Clin. Cancer Res. 2012;18:3305–3315. doi: 10.1158/1078-0432.CCR-11-3300. [DOI] [PubMed] [Google Scholar]
- 227.McBryan J., Theissen S.M., Byrne C., Hughes E., Cocchiglia S., Sande S., O’Hara J., Tibbitts P., Hill A.D., Young L.S. Metastatic progression with resistance to aromatase inhibitors is driven by the steroid receptor coactivator SRC-1. Cancer Res. 2012;72:548–559. doi: 10.1158/0008-5472.CAN-11-2073. [DOI] [PubMed] [Google Scholar]
- 228.Cottone E., Orso F., Biglia N., Sismondi P., de Bortoli M. Role of coactivators and corepressors in steroid and nuclear receptor signaling: Potential markers of tumor growth and drug sensitivity. Int. J. Biol. Markers. 2001;16:151–166. doi: 10.1177/172460080101600301. [DOI] [PubMed] [Google Scholar]
- 229.Girault I., Lerebours F., Amarir S., Tozlu S., Tubiana-Hulin M., Lidereau R., Bieche I. Expression analysis of estrogen receptor α coregulators in breast carcinoma: Evidence that NCOR1 expression is predictive of the response to tamoxifen. Clin. Cancer Res. 2003;9:1259–1266. [PubMed] [Google Scholar]
- 230.Lavinsky R.M., Jepsen K., Heinzel T., Torchia J., Mullen T.M., Schiff R., Del-Rio A.L., Ricote M., Ngo S., Gemsch J., et al. Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc. Natl. Acad. Sci. USA. 1998;95:2920–2925. doi: 10.1073/pnas.95.6.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kurokawa H., Lenferink A.E., Simpson J.F., Pisacane P.I., Sliwkowski M.X., Forbes J.T., Arteaga C.L. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60:5887–5894. [PubMed] [Google Scholar]
- 232.Kurebayashi J., Otsuki T., Kunisue H., Tanaka K., Yamamoto S., Sonoo H. Expression levels of estrogen receptor-α, estrogen receptor-β, coactivators, and corepressors in breast cancer. Clin. Cancer Res. 2000;6:512–518. [PubMed] [Google Scholar]
- 233.Baek S.H., Ohgi K.A., Rose D.W., Koo E.H., Glass C.K., Rosenfeld M.G. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-κB and β-amyloid precursor protein. Cell. 2002;110:55–67. doi: 10.1016/s0092-8674(02)00809-7. [DOI] [PubMed] [Google Scholar]
- 234.Cutrupi S., Reineri S., Panetto A., Grosso E., Caizzi L., Ricci L., Friard O., Agati S., Scatolini M., Chiorino G., et al. Targeting of the adaptor protein Tab2 as a novel approach to revert tamoxifen resistance in breast cancer cells. Oncogene. 2012;31:4353–4361. doi: 10.1038/onc.2011.627. [DOI] [PubMed] [Google Scholar]
- 235.Zhu P., Baek S.H., Bourk E.M., Ohgi K.A., Garcia-Bassets I., Sanjo H., Akira S., Kotol P.F., Glass C.K., Rosenfeld M.G., et al. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell. 2006;124:615–629. doi: 10.1016/j.cell.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 236.Weinshilboum R. Inheritance and drug response. N. Engl. J. Med. 2003;348:529–537. doi: 10.1056/NEJMra020021. [DOI] [PubMed] [Google Scholar]
- 237.Desta Z., Ward B.A., Soukhova N.V., Flockhart D.A. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: Prominent roles for CYP3A and CYP2D6. J. Pharmacol. Exp. Ther. 2004;310:1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 238.Kiyotani K., Mushiroda T., Nakamura Y., Zembutsu H. Pharmacogenomics of tamoxifen: roles of drug metabolizing enzymes and transporters. Drug Metab. Pharmacokinet. 2012;27:122–131. doi: 10.2133/dmpk.dmpk-11-rv-084. [DOI] [PubMed] [Google Scholar]
- 239.Rodriguez-Antona C., Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25:1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- 240.Jin Y., Desta Z., Stearns V., Ward B., Ho H., Lee K.H., Skaar T., Storniolo A.M., Li L., Araba A., et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J. Natl. Cancer Inst. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 241.Hoskins J.M., Carey L.A., McLeod H.L. CYP2D6 and tamoxifen: DNA matters in breast cancer. Nat. Rev. Cancer. 2009;9:576–586. doi: 10.1038/nrc2683. [DOI] [PubMed] [Google Scholar]
- 242.Gaedigk A., Simon S.D., Pearce R.E., Bradford L.D., Kennedy M.J., Leeder J.S. The CYP2D6 activity score: Translating genotype information into a qualitative measure of phenotype. Clin. Pharmacol. Ther. 2008;83:234–242. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]
- 243.O’Donnell P.H., Ratain M.J. Germline pharmacogenomics in oncology: Decoding the patient for targeting therapy. Mol. Oncol. 2012;6:251–259. doi: 10.1016/j.molonc.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nowell S., Sweeney C., Winters M., Stone A., Lang N.P., Hutchins L.F., Kadlubar F.F., Ambrosone C.B. Association between sulfotransferase 1A1 genotype and survival of breast cancer patients receiving tamoxifen therapy. J. Natl. Cancer Inst. 2002;94:1635–1640. doi: 10.1093/jnci/94.21.1635. [DOI] [PubMed] [Google Scholar]
- 245.Nowell S.A., Ahn J., Rae J.M., Scheys J.O., Trovato A., Sweeney C., MacLeod S.L., Kadlubar F.F., Ambrosone C.B. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res. Treat. 2005;91:249–258. doi: 10.1007/s10549-004-7751-x. [DOI] [PubMed] [Google Scholar]
- 246.Tengstrom M., Mannermaa A., Kosma V.M., Hirvonen A., Kataja V. SULT1A1 rs9282861 polymorphism-a potential modifier of efficacy of the systemic adjuvant therapy in breast cancer? BMC Cancer. 2012;12:257. doi: 10.1186/1471-2407-12-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chen L., Waxman D.J. Cytochrome P450 gene-directed enzyme prodrug therapy (GDEPT) for cancer. Curr. Pharm. Des. 2002;8:1405–1416. doi: 10.2174/1381612023394566. [DOI] [PubMed] [Google Scholar]
- 248.Jounaidi Y. Cytochrome P450-based gene therapy for cancer treatment: From concept to the clinic. Curr. Drug Metab. 2002;3:609–622. doi: 10.2174/1389200023337027. [DOI] [PubMed] [Google Scholar]
- 249.Braybrooke J.P., Slade A., Deplanque G., Harrop R., Madhusudan S., Forster M.D., Gibson R., Makris A., Talbot D.C., Steiner J., et al. Phase I study of MetXia-P450 gene therapy and oral cyclophosphamide for patients with advanced breast cancer or melanoma. Clin. Cancer Res. 2005;11:1512–1520. doi: 10.1158/1078-0432.CCR-04-0155. [DOI] [PubMed] [Google Scholar]
- 250.Miyoshi Y., Ando A., Takamura Y., Taguchi T., Tamaki Y., Noguchi S. Prediction of response to docetaxel by CYP3A4 mRNA expression in breast cancer tissues. Int. J. Cancer. 2002;97:129–132. doi: 10.1002/ijc.1568. [DOI] [PubMed] [Google Scholar]
- 251.Miyoshi Y., Taguchi T., Kim S.J., Tamaki Y., Noguchi S. Prediction of response to docetaxel by immunohistochemical analysis of CYP3A4 expression in human breast cancers. Breast Cancer. 2005;12:11–15. doi: 10.2325/jbcs.12.11. [DOI] [PubMed] [Google Scholar]
- 252.Dhaini H.R., Thomas D.G., Giordano T.J., Johnson T.D., Biermann J.S., Leu K., Hollenberg P.F., Baker L.H. Cytochrome P450 CYP3A4/5 expression as a biomarker of outcome in osteosarcoma. J. Clin. Oncol. 2003;21:2481–2485. doi: 10.1200/JCO.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 253.Schiavon G., Tonini G. Hormone-biological therapy in breast cancer: Preclinical evidences, clinical studies and future directions. Curr. Cancer Drug Targets. 2010;10:3–18. doi: 10.2174/156800910790980278. [DOI] [PubMed] [Google Scholar]
- 254.Mokbel K. The evolving role of aromatase inhibitors in breast cancer. Int. J. Clin. Oncol. 2002;7:279–283. doi: 10.1007/s101470200040. [DOI] [PubMed] [Google Scholar]
- 255.Bareschino M.A., Schettino C., Colantuoni G., Rossi E., Rossi A., Maione P., Ciardiello F., Gridelli C. The role of antiangiogenetic agents in the treatment of breast cancer. Curr. Med. Chem. 18:5022–5032. doi: 10.2174/092986711797636072. [DOI] [PubMed] [Google Scholar]
- 256.Coxon A., Bush T., Saffran D., Kaufman S., Belmontes B., Rex K., Hughes P., Caenepeel S., Rottman J.B., Tasker A., et al. Broad antitumor activity in breast cancer xenografts by motesanib, a highly selective, oral inhibitor of vascular endothelial growth factor, platelet-derived growth factor, and Kit receptors. Clin. Cancer Res. 2009;15:110–118. doi: 10.1158/1078-0432.CCR-08-1155. [DOI] [PubMed] [Google Scholar]
- 257.Sabnis G., Schayowitz A., Goloubeva O., Macedo L., Brodie A. Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res. 2009;69:1416–1428. doi: 10.1158/0008-5472.CAN-08-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Leary A.F., Drury S., Detre S., Pancholi S., Lykkesfeldt A.E., Martin L.A., Dowsett M., Johnston S.R. Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin. Cancer Res. 2010;16:1486–1497. doi: 10.1158/1078-0432.CCR-09-1764. [DOI] [PubMed] [Google Scholar]
- 259.Emde A., Mahlknecht G., Maslak K., Ribba B., Sela M., Possinger K., Yarden Y. Simultaneous inhibition of estrogen receptor and the HER2 pathway in breast cancer: Effects of HER2 abundance. Transl. Oncol. 2011;4:293–300. doi: 10.1593/tlo.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Buzdar A.U. Role of biologic therapy and chemotherapy in hormone receptor- and HER2-positive breast cancer. Ann. Oncol. 2009;20:993–999. doi: 10.1093/annonc/mdn739. [DOI] [PubMed] [Google Scholar]