Abstract
A conjugated-bisimidazolylporphyrin bridged by bis(ethynylfluorene) was synthesized and organized into linear polymer through self-coordination having mean molecular weights, Mw and Mn, of ~2.1 × 105 Da and ~1.6 × 105 Da, respectively. A large two-photon absorption cross section value of 3.4 × 105 GM (per dimer unit) was observed. This value was comparable to that of the previously reported self-assembled linear polymer consisting of butadiyne-bridged imidazolylporphyrins. The two-photon absorption properties could be controlled by tuning the wavelength and absorption intensity of the one-photon absorption.
Keywords: porphyrin, self-assembly, two-photon absorption, fluorene, imidazole, Z-scan
1. Introduction
Recently, much attention has been focused on organic dye molecules in view of optical applications such as organic solar cell [1], electronic luminescence [2], photon-mode optical memory using photochromic molecules [3], and nonlinear optics including two-photon absorption (2PA) materials [4–11]. Novel functions would be expected upon conjugation of two or more chromophores, even when a monomeric dye itself exhibits insignificant function. We developed two-photon absorption materials [12–22] using self-assembled multi-porphyrin arrays and found that the extension of the π-conjugation between the porphyrins with triple bonds was the most significant for 2PA enhancement. Complementary coordination of imidazolyl to zinc, in which the stability constant of the complementary dimer exceeded 1011 M−1 in CHCl3 [23–25], also contributed to the enhancement. However, an additional expansion of π-conjugation gave rise to a large red-shift of the S0→S1 one-photon absorption (1PA) to the two-photon resonance range (800 nm to 900 nm) [22]. To prevent such an overlap of 2PA and 1PA, it is necessary to control the position of the S0→S1 one-photon absorption by tuning the interaction between chromophores. Fluorene is a well-known π-spacer for two-photon absorption materials [26–33] and provides an angle of 160° between two ethynylimidazolylporphyrins when these are connected at the 2- and 7-positions of fluorene (1). Therien and Anderson reported bisporphyrins bridged by a π-spacer comprising an acetylenic connection [5,34]. Compound 1 gave the linear polymer 1P by zinc-imidazolyl complementary coordination. Polymer 1P could easily be dissociated into the unit 1M by ligand coordination, such as with pyridine and imidazole. The slightly bent angle of 160° was expected to reduce the exciton interaction between the two porphyrins and shift the 1PA bands to shorter wavelengths than those of the butadiyne-bridged bisimidazolylporphyrin array 2P without fluorene, allowing the measurement of the hidden 2PA bands in the shorter wavelength range. Similar fluorene-conjugated bisimidazolylporphyrins having two allyl groups at meso-positions have been synthesized to construct large macrocyclic arrays. The self-coordinated structures in the macrocycles were fixed by the ring-closing metathesis reaction of the allyl groups to investigate the effect of cyclization on 2PA by a femtosecond fluorescence method in the range between 800 and 875 nm [20]. Here, we report the synthesis of linear polymers self-organized by fluorene-conjugated bisimidazolylporphyrins without allyl groups, and their 2PA properties in the range from 740 to 930 nm.
2. Results and Discussion
2.1. Synthesis
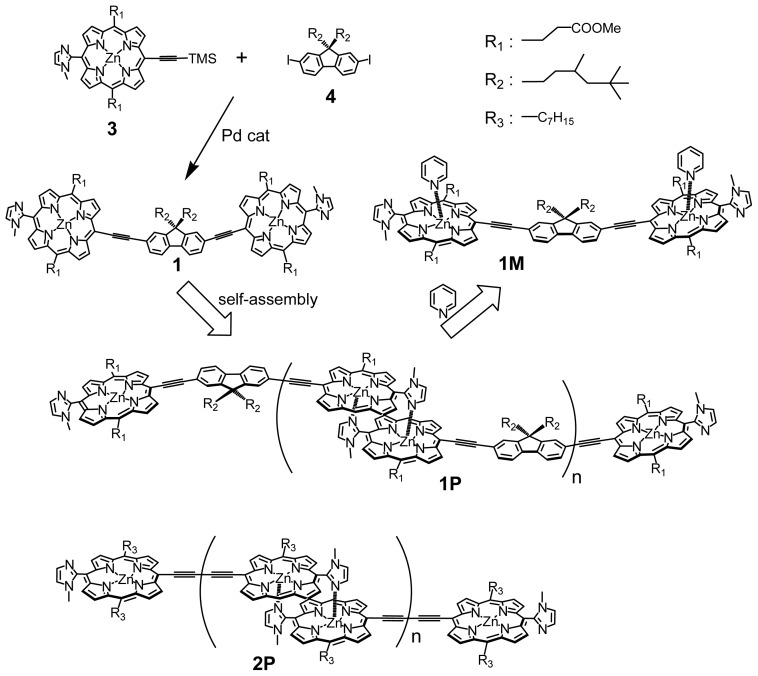
Bisporphyrin 1 was synthesized from 5,15-bis(2-methoxycarbonylethyl)-10- (trimethylsilylpropargyl)-20-(1-methyl-2-imidazolyl)porphyrinatozinc 3 [15] and 2,7-diiodo-9,9- bis(3,5,5-trimethylhexyl)fluorene 4 [35,36] using a Pd2(dba)3/AsPh3 catalytic system, where deprotection of the TMS group and the coupling reaction were conducted in a one-pot procedure because of the low solubility of the deprotected ethynylimidazolylporphyrin (Scheme 1). Purification by preparative gel permeation chromatography (GPC) with pyridine elution allowed for the isolation of 1 from the crude mixture in 33% yield. Both the MALDI-TOF mass spectral and analytical GPC measurements showed a single species corresponding to the molecular weight of 1. However, broad signals were obtained in the NMR spectrum of 1, even in a coordinating solvent such as pyridine-d5. Accordingly, the free base 5 was prepared by treatment with HCl to obtain a clearer NMR spectrum (Figure 1). Bisporphyrin 1 could not be obtained when 2,7-dibromofluorene was used as a starting material, due to its low reactivity.
Scheme 1.
Self-assembled imidazolylporphyrins connected by triple bond.
Figure 1.
600 MHz 1H NMR spectrum of free base 5 in CDCl3.
2.2. Formation of Self-Assembled Polymer
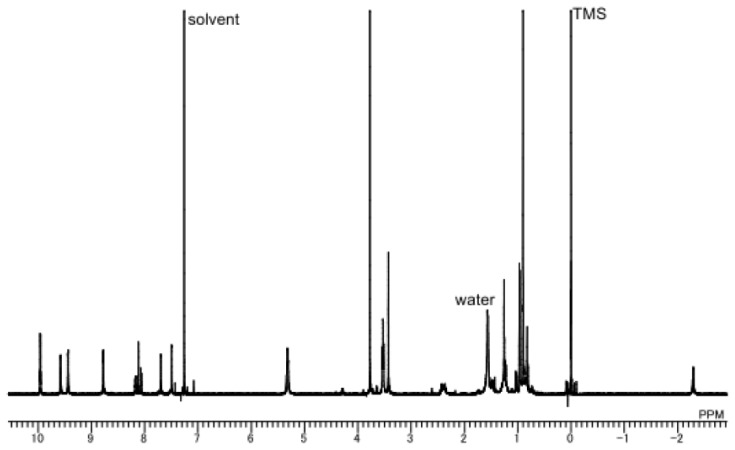
When 1 was dissolved in a nonpolar solvent such as chloroform or 1,1,2,2,-tetrachloroethane after the evaporation of pyridine, the long linear polymer 1P was formed by self-coordination. Polymer 1P changed gradually to cyclic arrays of various sizes in chloroform above 45 °C or in chloroform/methanol (7:3, v/v%) at room temperature. Such cyclic arrays have been reported elsewhere [20]. 1P retains its linear polymer structure in chloroform or in tetrachloroethane without methanol at room temperature sufficiently long for 2PA measurements. The molecular weight of 1P was analyzed by GPC with chloroform elution on a column with an exclusion limit of 5 × 105 Da. Figure 2 shows the GPC chart of 1P monitored at 420 nm by an UV-vis photodiode array. The arrows indicate the peak positions of polystyrene standards. The absorption spectra of 1P at different retention times between 7 and 10 min were identical. The distribution maximum of the elution curve of 1P appeared around Mw 1.5 × 105 Da. The mean molecular weights, Mw and Mn, estimated by comparing the data with those of polystyrene standards [37], were ~2.1 × 105 Da and ~1.6 × 105 Da, respectively, which results in a polydispersity index of Mw/Mn = 1.4. The mean molecular weight of Mn = 1.6 × 105 corresponded to ca. 94 units of 1, and these values were comparable with linear self-assembled bisporphyrin arrays [14].
Figure 2.
GPC chart of 1P along with the peak positions of polystyrene standards.
2.3. UV-Vis Absorption Spectra
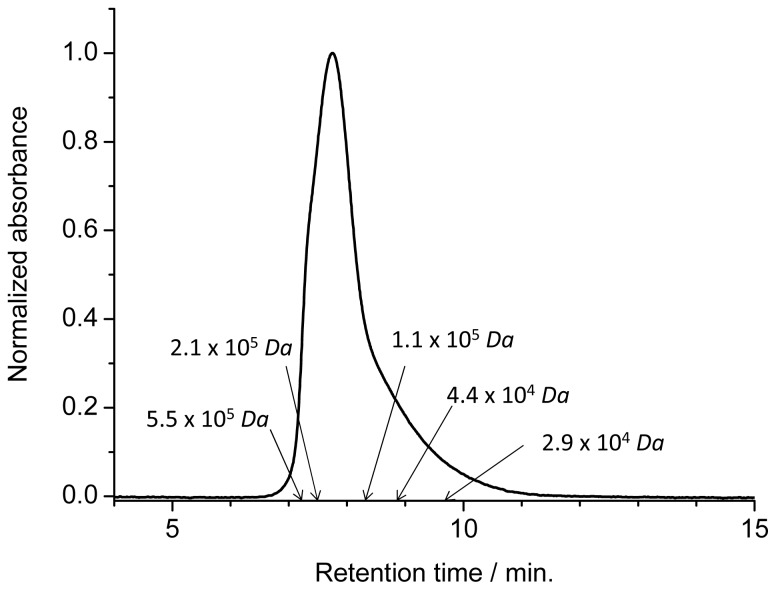
The UV-vis absorption spectra of 1P in tetrachloroethane (solid line), 1M in tetrachloroethane/pyridine (7:3, v/v%), and 2P in chloroform (dashed line) are shown in Figure 3. The Soret and Q-bands of 1P were observed at around 485 and 670 nm, respectively. These were blue-shifted by 15 and 70 nm compared with those of 2P (500 nm and 740 nm), suggesting a decrease in interaction due to the long distance and torsional angle of 160° between the two porphyrins. However, the absorption intensity of the Q-band in 1P was still strong. On the other hand, the Q-band of unit 1M was blue-shifted to 655 nm and its intensity was considerably decreased because of the disappearance of excitonic interactions through the zinc-imidazolyl complementary coordination.
Figure 3.
One-photon absorption spectra of 1P (bold line) in tetrachloroethane, 1M (thin line) in tetrachloroethane/pyridine (7:3, v/v), and 2P (dashed line) in CHCl3.
2.4. Nonlinear Absorption
The 2PA cross sections of 1P and 1M were measured with an open-aperture Z-scan technique at wavelengths from 740 to 920 nm, using a Nd:YAG nanosecond pulse laser system with optical parametric oscillator. The nanosecond measurement system and analysis method were previously reported [14]. In general, the effective cross section value, effσ(2), measured using nanosecond pulses, is 2–3 orders of magnitude larger than the pure 2PA cross section, σ (2), obtained with femtosecond pulses, because the excited state absorption (ESA) contributes to the data. In our porphyrin systems, the effσ (2) values were 30 to 50 times larger than the σ (2) values, and the 2PA spectra measured with nanosecond and femtosecond pulses were similar. Therefore, effσ (2) values obtained by nanosecond measurements were a good measure for the 2PA properties. Moreover, in some 2PA applications, such as 2PA-PDT and 3-D optical memory, nanosecond pulses will be more appropriate because of the ease of operation and availability.
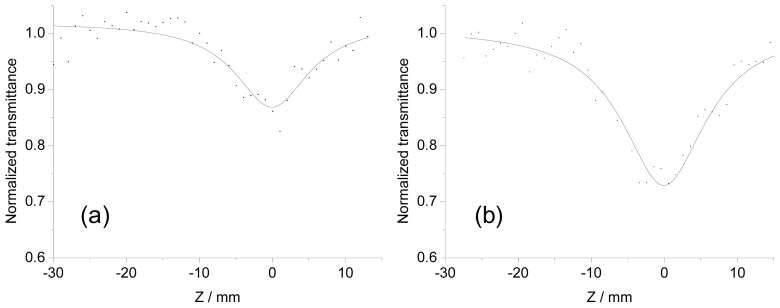
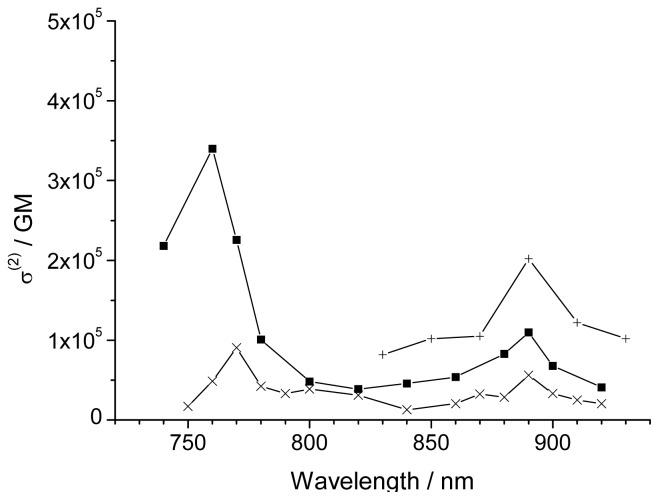
Figure 4 shows typical Z-scan traces for 1P in tetrachloroethane (dotted trace) and theoretically fitted curves (solid line) at 890 nm ((a) at a concentration of 7.66 × 10−5 M with a pulse energy of 1.6 mJ) and 760 nm ((b) at a concentration of 6.87 × 10−5 M with a pulse energy of 1.45 mJ). The effσ (2) values were obtained from the fitted curves and theoretical equations [14,38]. Figure 5 shows 2PA spectra of 1P (square). Two peaks were observed at 760 and 890 nm with large effσ (2) values of 3.4 × 105 and 1.1 × 105 GM (per dimer unit), respectively. 2PA spectra of 1M (Figure 5, cross) also showed two peaks at 770 and 890 nm, with values of 9.1 × 104 and 5.6 × 104 GM, respectively. The values obtained for 1M were almost one-third to one-half of those of 1P. As described for the absorption spectra, 1P showed strong one-photon absorption of the Q-band at 670 nm. On the other hand, that of 1M was blue-shifted to 655 nm and the oscillator strength was almost half that of 1P, indicating that the resonance enhancement for 1M, which comes from the one-photon absorption near the two-photon resonance [38,39], would be expected to be poorer than that for 1P. This may clearly explain the difference in the effσ (2) values between 1P and 1M, and we can confirm again that self-organization through complementary coordination is effective for the enhancement [13,14]. The shapes of the 2PA spectra of 1P and 1M were similar, but did not correspond to those of the one-photon spectra in the wavelength range of half of 2PA, i.e., 370 nm to 500 nm, suggesting that the final state by 2PA is different from the one-photon excited state [5,39]. The effσ (2) values per dimer unit of 1P in the wavelength range between 800 and 860 nm ranged from 3.9 × 104 to 5.4 × 104 GM. These values were similar to those of macrocycles measured by femtosecond pulses [20], indicating negligible ESA contributions in this wavelength range, considering nanosecond measurement.
Figure 4.
Typical open-aperture Z-scan traces for 1P (dotted trace) and theoretically fitted curves (solid line) at 890 nm (a at a concentration of 7.66 × 10−5 M in 1,1,2,2,-tetrachloroethane with a pulse energy of 1.6 mJ) and 760 nm (b at a concentration of 6.87 × 10−5 M in 1,1,2,2,-tetrachloroethane with a pulse energy of 1.45 mJ).
Figure 5.
2PA spectra of 1P (■), 1M (×), and 2P (+).
In the previous report, the linear polymer 2P exhibited a large effσ (2) value of 2.0 × 105 GM (per dimer unit) at 890 nm [14], which was twice of that of 1P at the same wavelength. 2P had a strong one-photon absorption of the Q-band at 740 nm that was longer than 1P by 70 nm, suggesting that 2P undergoes a stronger resonance enhancement effect than 1P. However, the shift of the strong Q-band to the shorter wavelength of 670 nm for 1P allows the Z-scan measurements in the wavelength range of 720 to 820 nm, which is prohibited by one-photon absorption in the case of 2P. Then, we could find another strong 2PA band at around 760 nm with the largest effσ(2) value of 3.4 × 105 GM. The cross sections increased with the shortening of the incident light wavelength below around 800 nm. Similar behavior was observed in simple porphyrins such as tetraphenylporphyrin, Zn octaethylporphyrin, and a self-assembled dimer measured using a femtosecond fluorescence method [39] or nanosecond Z-scan method [16]. This has been explained as a resonance enhancement near the one-photon absorption of the Q-band.
3. Experimental Section
General
1H NMR spectra were obtained in CDCl3 with Me4Si as the internal standard (δ 0 ppm) and recorded on either a JEOL JNM EX270 or JEOL ECP600. UV-vis spectra were obtained on either a Shimadzu UV-1650PC or UV-3100PC. MALDI-TOF mass spectra were obtained on Perseptive Biosystems Voyager DE-STR and Shimadzu/KRATOS Axima-CFR Kompact MALDI with dithranol (Aldrich, St. Louis, MO, USA) or 2-[3-(4-tert-butylphenyl)-2-methylprop-2-enylidene]-malononitrile (DCTB, Aldrich) as the matrix. Reactions were monitored on silica gel 60 F254 TLC plates (Merck, Darmstadt, Germany). The silica gel utilized for column chromatography was purchased from Kanto Chemical Co. Inc.: Silica Gel 60N (Spherical, Neutral) 60–210 mm and 40–50 mm (Flash). The alumina used for column chromatography was purchased from Merck: Aluminum oxide 90 active basic. Analytical GPC measurements were performed on a HEWLETT PACKARD 1100 SERIES with a JAIGEL 3H-A or 4H-A column for chloroform eluent. The nanosecond Z-scan measurement system and analysis method were previously reported [14].
Synthesis of 1: Under argon atmosphere, 3 [15] (95 mg, 132 μmol) and 4 [35,36] (35.4 mg, 52.6 μmol) were dissolved in dry THF (45 mL). Then, tetrabutylammonium fluoride in THF (264 μL, 264 μmol) was added. After 30 min, triethylamine (9 mL), Pd2(dba)3 (27.2 mg, 26.3 μmol), and AsPh3 (32.2 mg, 105 μmol) were added. After stirring 4 h, the reaction solvents were evaporated. The residue was dissolved in chloroform and washed with water. The crude residue after concentration was purified by preparative GPC (Tosoh G2500HHR, pyridine elution) to give 1 (29 mg, 33%). MALDI-TOF mass (dithranol) m/z 1711.66 (M + H)+, calcd for C99H98N12O8Zn2, 1710.62; UV-vis λmax (nm, in CHCl3) 449, 484, 583, 667; fluorescence λmax (nm, λex = 484 nm, in CHCl3) 670, 731.
Synthesis of 5: Under an Ar atmosphere, 1 (4.5 mg, 2.63 μmol) in chloroform (30 mL) was added into a mixture of 12 N hydrochloric acid (8 mL) and methanol (40 mL). After stirring for 30 min at room temperature, the reaction solution was diluted with water and extracted with chloroform. The chloroform layer was washed with saturated NaHCO3 aqueous solution. The crude residue after concentration was purified with silica gel column chromatography (CHCl3/MeOH = 20/1) (yield: 53%). 1H NMR (600 MHz, CDCl3) δ 9.96 (q, 4H, J = 4.8 Hz, β), 9.58 (q, 4H, J = 4.8 Hz, β), 9.44 (d, 4H, J = 4.8 Hz, β), 8.78 (d, 4H, J = 4.8 Hz, β), 8.17(m, 2H, Ph), 8.12 (s, 2H, Ph), 8.07 (d, 2H, J = 7.8 Hz, Ph), 7.70 (d, 1H, J = 1.8 Hz, im), 7.50 (d, 1H, J = 1.8 Hz, im), 5.32 (t, 8H, J = 7.8 Hz, CH2), 3.77 (s, 12H, J = 7.8 Hz, OMe), 3.53 (t, 8H, J = 7.8 Hz, CH2), 3.43 (s, 6H, NMe), 2.36–0.81 (m, 38H, alkyl groups in fluorene), −2.29 (s, 4H, NH); MALDI-TOF mass (dithranol), m/z 1587.75 (M + H)+, calcd for C99H102N12O8, 1586.79.
4. Conclusions
In conclusion, a porphyrin-fluorene conjugate connected by triple bonds 1 was successfully synthesized through a Pd-catalyzed coupling reaction. Polymer 1P, self-assembled through zinc-imidazolyl complementary coordination, was successfully formed. The 2PA properties could be controlled by tuning the wavelength and absorption intensity of 1PA bands, which were altered by changing the interactions between the porphyrins by the introduction of fluorene or complementary coordination.
Acknowledgments
We gratefully acknowledge financial support through Grants-in-Aid for Scientific Research (A) (No. 15205020) and Young Scientists (B) (No. 18750118) (Kazuya Ogawa) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (Monbu Kagakusho).
References
- 1.Gunes S., Neugebauer H., Sariciftci N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007;107:1324–1338. doi: 10.1021/cr050149z. [DOI] [PubMed] [Google Scholar]
- 2.Grimsdale A.C., Chan K.L., Martin R.E., Jokisz P.G., Holmes A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009;109:897–1091. doi: 10.1021/cr000013v. [DOI] [PubMed] [Google Scholar]
- 3.Irie M. Photochromism: Memories and switches—Introduction. Chem. Rev. 2000;100:1683–1683. doi: 10.1021/cr980068l. [DOI] [PubMed] [Google Scholar]
- 4.Drobizhev M., Stepanenko Y., Rebane A., Wilson C.J., Screen T.E.O., Anderson H.L. Strong cooperative enhancement of two-photon absorption in double-strand conjugated porphyrin ladder arrays. J. Am. Chem. Soc. 2006;128:12432–12433. doi: 10.1021/ja0649623. [DOI] [PubMed] [Google Scholar]
- 5.Drobizhev M., Stepanenko Y., Dzenis Y., Karotki A., Rebane A., Taylor P.N., Anderson H.L. Understanding strong two-photon absorption in pi-conjugated porphyrin dimers via double-resonance enhancement in a three-level model. J. Am. Chem. Soc. 2004;126:15352–15353. doi: 10.1021/ja0445847. [DOI] [PubMed] [Google Scholar]
- 6.Hisaki I., Hiroto S., Kim K.S., Noh S.B., Kim D., Shinokubo H., Osuka A. Synthesis of doubly beta-to-beta 1,3-butadiyne-bridged diporphyrins: Enforced planar structures and large two-photon absorption cross sections. Angew. Chem. Int. Ed. 2007;46:5125–5128. doi: 10.1002/anie.200700550. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda C., Yoon Z.S., Park M., Inoue H., Kim D., Osuka A. Helicity induction and two-photon absorbance enhancement in zinc(ii) meso-meso linked porphyrin oligomers via intermolecular hydrogen bonding interactions. J. Am. Chem. Soc. 2005;127:534–535. doi: 10.1021/ja043428x. [DOI] [PubMed] [Google Scholar]
- 8.Bhaskar A., Guda R., Haley M.M., Goodson T. Building symmetric two-dimensional two-photon materials. J. Am. Chem. Soc. 2006;128:13972–13973. doi: 10.1021/ja062709x. [DOI] [PubMed] [Google Scholar]
- 9.Bhaskar A., Ramakrishna G., Lu Z.K., Twieg R., Hales J.M., Hagan D.J., van Stryland E., Goodson T. Investigation of two-photon absorption properties in branched alkene and alkyne chromophores. J. Am. Chem. Soc. 2006;128:11840–11849. doi: 10.1021/ja060630m. [DOI] [PubMed] [Google Scholar]
- 10.Arnbjerg J., Jimenez-Banzo A., Paterson M.J., Nonell S., Borrell J.I., Christiansen O., Ogilby P.R. Two-photon absorption in tetraphenylporphycenes: Are porphycenes better candidates than porphyrins for providing optimal optical properties for two-photon photodynamic therapy. J. Am. Chem. Soc. 2007;129:5188–5199. doi: 10.1021/ja0688777. [DOI] [PubMed] [Google Scholar]
- 11.Kamada K., Ohta K., Kubo T., Shimizu A., Morita Y., Nakasuji K., Kishi R., Ohta S., Furukawa S., Takahashi H., et al. Strong two-photon absorption of singlet diradical hydrocarbons. Angew. Chem. Int. Ed. 2007;46:3544–3546. doi: 10.1002/anie.200605061. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa K., Zhang T.Q., Yoshihara K., Kobuke Y. Large third-order optical nonlinearity of self-assembled porphyrin oligomers. J. Am. Chem. Soc. 2002;124:22–23. doi: 10.1021/ja0169015. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa K., Ohashi A., Kobuke Y., Kamada K., Ohta K. Strong two-photon absorption of self-assembled butadiyne-linked bisporphyrin. J. Am. Chem. Soc. 2003;125:13356–13357. doi: 10.1021/ja035056i. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa K., Ohashi A., Kobuke Y., Kamada K., Ohta K. Two-photon absorption properties of self-assemblies of butadiyne-linked bis(imidazolylporphyrin) J. Phys. Chem. B. 2005;109:22003–22012. doi: 10.1021/jp054444z. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa K., Hasegawa H., Inaba Y., Kobuke Y., Inouye H., Kanemitsu Y., Kohno E., Hirano T., Ogura S., Okura I. Water-soluble bis(imidazolylporphyrin) self-assemblies with large two-photon absorption cross sections as potential agents for photodynamic therapy. J. Med. Chem. 2006;49:2276–2283. doi: 10.1021/jm051072+. [DOI] [PubMed] [Google Scholar]
- 16.Tanihara J., Ogawa K., Kobuke Y. Two-photon absorption properties of conjugated supramolecular porphyrins with electron donor and acceptor. J. Photochem. Photobiol. A. 2006;178:140–149. [Google Scholar]
- 17.Dy J.T., Ogawa K., Satake A., Ishizumi A., Kobuke Y. Water-soluble self-assembled butadiyne-bridged bisporphyrin: A potential two-photon-absorbing photosensitizer for photodynamic therapy. Chem. Eur. J. 2007;13:3491–3500. doi: 10.1002/chem.200601205. [DOI] [PubMed] [Google Scholar]
- 18.Dy J.T., Maeda R., Nagatsuka Y., Ogawa K., Kamada K., Ohta K., Kobuke Y. A photochromic porphyrin-perinaphthothioindigo conjugate and its two-photon absorption properties. Chem. Commun. 2007:5170–5172. doi: 10.1039/b713520c. [DOI] [PubMed] [Google Scholar]
- 19.Dy J., Ogawa K., Kamada K., Ohta K., Kobuke Y. Stepwise elongation effect on the two-photon absorption of self-assembled butadiyne porphyrins. Chem. Commun. 2008:3411–3413. doi: 10.1039/b804056g. [DOI] [PubMed] [Google Scholar]
- 20.Raymond J.E., Bhaskar A., Goodson T., Makiuchi N., Ogawa K., Kobuke Y. Synthesis and two-photon absorption enhancement of porphyrin macrocycles. J. Am. Chem. Soc. 2008;130:17212–17213. doi: 10.1021/ja804415b. [DOI] [PubMed] [Google Scholar]
- 21.Morisue M., Ogawa K., Kamada K., Ohta K., Kobuke Y. Strong two-photon and three-photon absorptions in the antiparallel dimer of a porphyrin-phthalocyanine tandem. Chem. Commun. 2010;46:2121–2123. doi: 10.1039/b921278g. [DOI] [PubMed] [Google Scholar]
- 22.Kamada K., Hara C., Ogawa K., Ohta K., Kobuke Y. Strong two-photon absorption and its saturation of a self-organized dimer of an ethynylene-linked porphyrin tandem. Chem. Commun. 2012;48:7988–7990. doi: 10.1039/c2cc30879g. [DOI] [PubMed] [Google Scholar]
- 23.Kobuke Y., Miyaji H. Supramolecular organization of imidazolyl-porphyrin to a slipped cofacial dimer. J. Am. Chem. Soc. 1994;116:4111–4112. [Google Scholar]
- 24.Kobuke Y., Ogawa K. Porphyrin supramolecules for artificial photosynthesis and molecular photonic/electronic materials. Bull. Chem. Soc. Jpn. 2003;76:689–708. [Google Scholar]
- 25.Ogawa K., Kobuke Y. Formation of a giant supramolecular porphyrin array by self-coordination. Angew. Chem. Int. Ed. 2000;39:4070–4073. doi: 10.1002/1521-3773(20001117)39:22<4070::aid-anie4070>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 26.Rogers J.E., Slagle J.E., McLean D.G., Sutherland R.L., Brant M.C., Heinrichs J., Jakubiak R., Kannan R., Tan L.S., Fleitz P.A. Insight into the nonlinear absorbance of two related series of two-photon absorbing chromophores. J. Phys. Chem. A. 2007;111:1899–1906. doi: 10.1021/jp070050r. [DOI] [PubMed] [Google Scholar]
- 27.Morales A.R., Belfield K.D., Hales J.M., Van Stryland E.W., Hagan D.J. Synthesis of two-photon absorbing unsymmetrical fluorenyl-based chromophores. Chem. Mater. 2006;18:4972–4980. [Google Scholar]
- 28.Padmawar P.A., Rogers J.E., He G.S., Chiang L.Y., Tan L.S., Canteenwala T., Zheng Q.D., Slagle J.E., McLean D.G., Fleitz P.A., et al. Large cross-section enhancement and intramolecular energy transfer upon multiphoton absorption of hindered diphenylaminofluorene-c-60 dyads and triads. Chem. Mater. 2006;18:4065–4074. [Google Scholar]
- 29.Zheng Q.D., He G.S., Prasad P.N. Pi-conjugated dendritic nanosized chromophore with enhanced two-photon absorption. Chem. Mater. 2005;17:6004–6011. [Google Scholar]
- 30.Belfield K.D., Morales A.R., Kang B.S., Hales J.M., Hagan D.J., van Stryland E.W., Chapela V.M., Percino J. Synthesis, characterization, and optical properties of new two-photon-absorbing fluorene derivatives. Chem. Mater. 2004;16:4634–4641. [Google Scholar]
- 31.Belfield K.D., Hagan D.J., van Stryland E.W., Schafer K.J., Negres R.A. New two-photon absorbing fluorene derivatives: Synthesis and nonlinear optical characterization. Org. Lett. 1999;1:1575–1578. [Google Scholar]
- 32.Reinhardt B.A., Brott L.L., Clarson S.J., Dillard A.G., Bhatt J.C., Kannan R., Yuan L.X., He G.S., Prasad P.N. Highly active two-photon dyes: Design, synthesis, and characterization toward application. Chem. Mater. 1998;10:1863–1874. [Google Scholar]
- 33.Yao S., Belfield K.D. Synthesis of two-photon absorbing unsymmetrical branched chromophores through direct tris(bromomethylation) of fluorene. J. Org. Chem. 2005;70:5126–5132. doi: 10.1021/jo0503512. [DOI] [PubMed] [Google Scholar]
- 34.Susumu K., Duncan T.V., Therien M.J. Potentiometric, electronic structural, and ground- and excited-state optical properties of conjugated bis[(porphinato)zinc(ii)] compounds featuring proquinoidal spacer units. J. Am. Chem. Soc. 2005;127:5186–5195. doi: 10.1021/ja040243h. [DOI] [PubMed] [Google Scholar]
- 35.Lee S.H., Nakamura T., Tsutsui T. Synthesis and characterization of oligo(9,9-dihexyl-2,7-fluorene ethynylene)s: For application as blue light-emitting diode. Org. Lett. 2001;3:2005–2007. doi: 10.1021/ol010069r. [DOI] [PubMed] [Google Scholar]
- 36.Kannan R., He G.S., Lin T.C., Prasad P.N., Vaia R.A., Tan L.S. Toward highly active two-photon absorbing liquids. Synthesis and characterization of 1,3,5-triazine-based octupolar molecules. Chem. Mater. 2004;16:185–194. [Google Scholar]
- 37.Balke S.T., Hamielec A.E., LeClair B.P., Pearce S.L. Gel permeation chromatography. Ind. Eng. Chem. Prod. Res. Dev. 1969;8:54–57. [Google Scholar]
- 38.Kamada K., Ohta K., Yoichiro I., Kondo K. Two-photon absorption properties of symmetric substituted diacetylene: Drastic enhancement of the cross section near the one-photon absorption peak. Chem. Phys. Lett. 2003;372:386–393. [Google Scholar]
- 39.Drobizhev M., Karotki A., Kruk M., Rebane A. Resonance enhancement of two-photon absorption in porphyrins. Chem. Phys. Lett. 2002;355:175–182. [Google Scholar]