Abstract
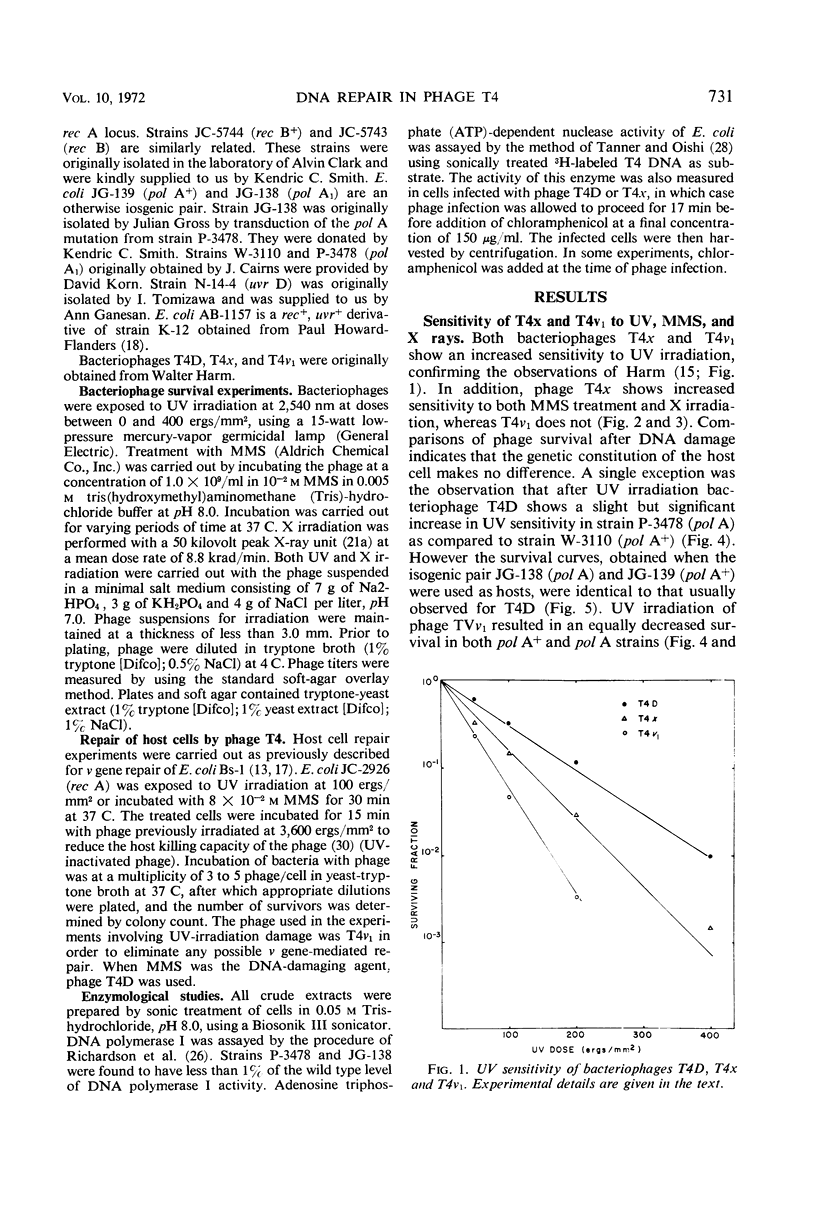
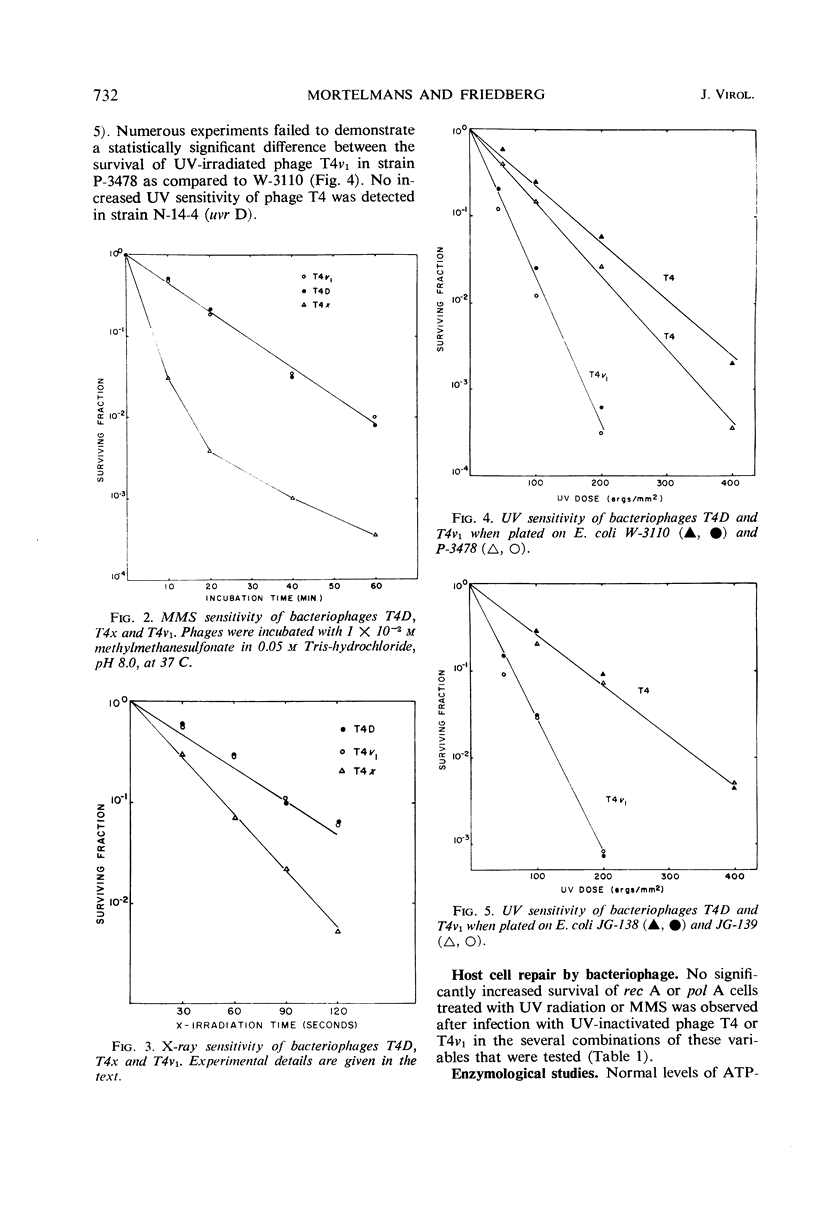
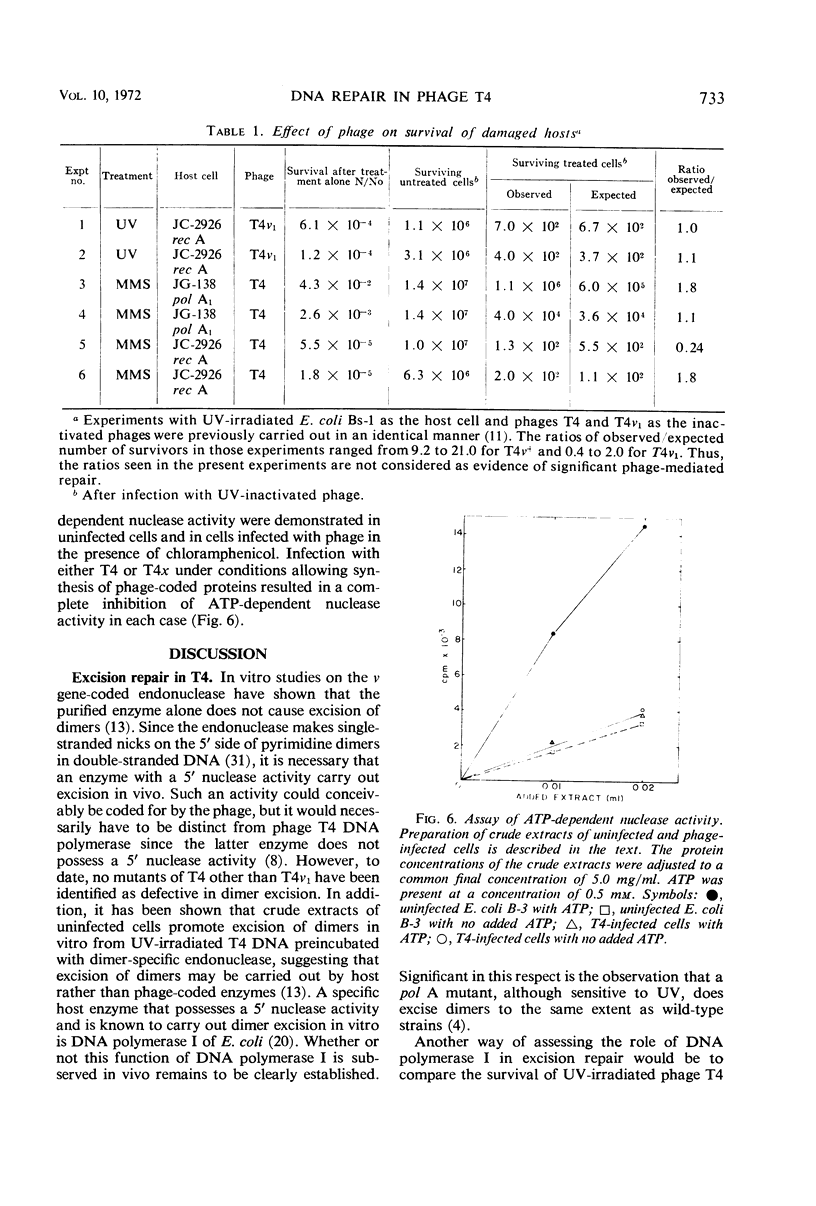
Studies were carried out to determine the effect of mutation in the host pol I gene on survival of ultraviolet (UV)-irradiated bacteriophage T4. Whereas a slightly reduced survival was observed in Escherichia coli strain P-3478 (pol A1) compared to strain W-3110 (pol A+), no such difference was observed in two strains isogenic except for the pol A gene. It was also shown that, whereas bacteriophage T4x is sensitive to UV irradiation, X irradiation, and treatment with methyl-methanesulfonate (MMS), phage T4v1 is sensitive only to UV irradiation. The survival of damaged phage T4x is neither affected by the presence of the rec A, rec B, or pol A mutations in the host, nor is there evidence that phage T4 effects repair of rec A or pol A mutants previously treated with either UV or MMS.
Full text
PDF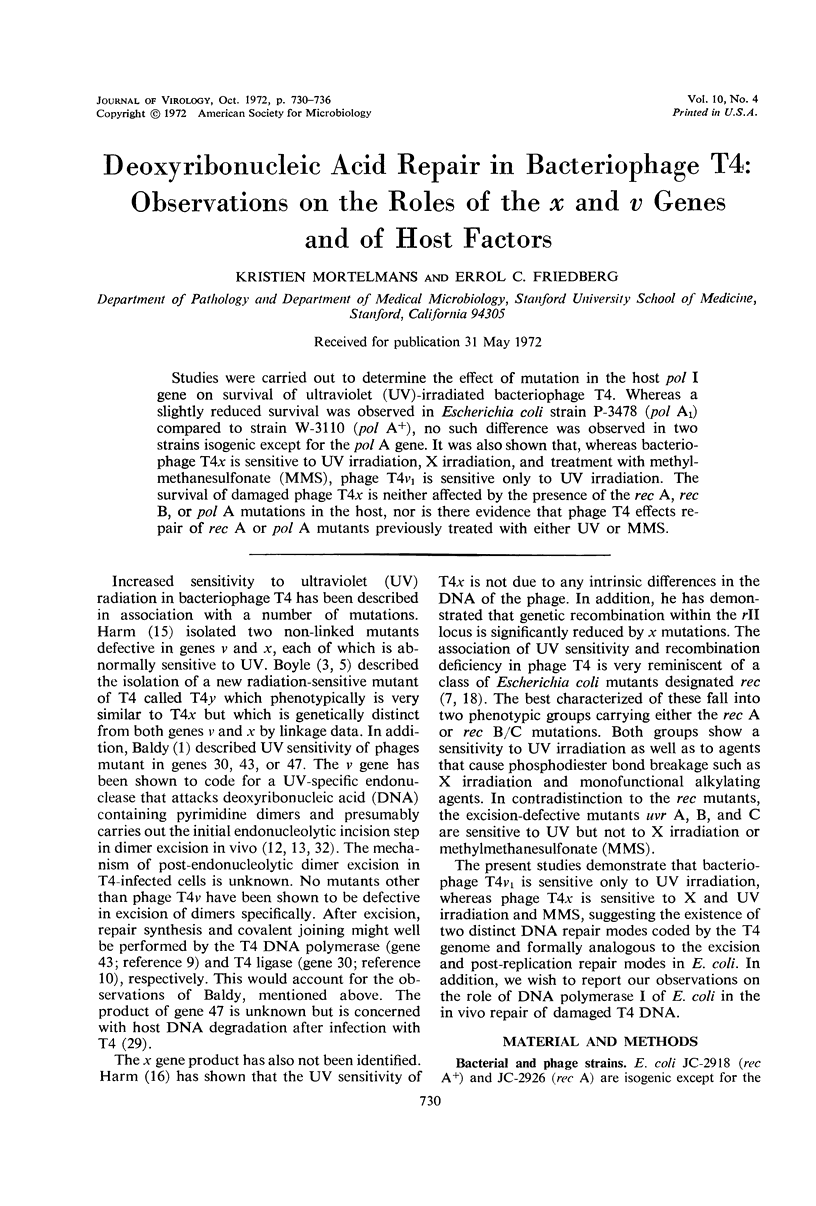


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldy M. W. Repair and recombination in phage T4. II. Genes affecting UV sensitivity. Cold Spring Harb Symp Quant Biol. 1968;33:333–338. doi: 10.1101/sqb.1968.033.01.038. [DOI] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. M., Paterson M. C., Setlow R. B. Excision-repair properties of an Escherichia coli mutant deficient in DNA polymerase. Nature. 1970 May 23;226(5247):708–710. doi: 10.1038/226708a0. [DOI] [PubMed] [Google Scholar]
- Boyle J. M. Radiation-sensitive mutants of T4D. II. T4y: genetic characterization. Mutat Res. 1969 Nov-Dec;8(3):441–449. doi: 10.1016/0027-5107(69)90061-x. [DOI] [PubMed] [Google Scholar]
- Boyle J. M., Symonds N. Radiation-sensitive mutants of T4D. I. T4y: a new radiation-sensitive mutant; effect of the mutation on radiation survival, growth and recombination. Mutat Res. 1969 Nov-Dec;8(3):431–439. doi: 10.1016/0027-5107(69)90060-8. [DOI] [PubMed] [Google Scholar]
- Buttin G., Wright M. Enzymatic DNA degradation in E. coli: its relationship to synthetic processes at the chromosome level. Cold Spring Harb Symp Quant Biol. 1968;33:259–269. doi: 10.1101/sqb.1968.033.01.030. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Toward a metabolic interpretation of genetic recombination of E. coli and its phages. Annu Rev Microbiol. 1971;25:437–464. doi: 10.1146/annurev.mi.25.100171.002253. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. Enzymic synthesis of DNA. 33. Hydrolysis of a 5'-triphosphate-terminated polynucleotide in the active center of DNA polymerase. J Mol Biol. 1969 Nov 14;45(3):513–531. doi: 10.1016/0022-2836(69)90309-x. [DOI] [PubMed] [Google Scholar]
- De Waard A., Paul A. V., Lehman I. R. The structural gene for deoxyribonucleic acid polymerase in bacteriophages T4 and T5. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1241–1248. doi: 10.1073/pnas.54.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. II. The structural gene for polynucleotide ligase in bacteriophage T4. Proc Natl Acad Sci U S A. 1967 Aug;58(2):665–672. doi: 10.1073/pnas.58.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Endonucleolytic cleavage of UV-irradiated DNA controlled by the V+ gene in phage T4. Biochem Biophys Res Commun. 1969 Nov 6;37(4):646–651. doi: 10.1016/0006-291x(69)90859-6. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C. Studies on the substrate specificity of the T 4 excision repair endonuclease. Mutat Res. 1972 Jun;15(2):113–123. doi: 10.1016/0027-5107(72)90024-3. [DOI] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- HARM W. Mutants of phage T4 with increased sensitivity to ultraviolet. Virology. 1963 Jan;19:66–71. doi: 10.1016/0042-6822(63)90025-4. [DOI] [PubMed] [Google Scholar]
- Harm W. Recovery of UV-inactivated E. coli cells by the v-gene action of phage T4. Mutat Res. 1968 Jul-Aug;6(1):175–179. doi: 10.1016/0027-5107(68)90115-2. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Kaplan J. C., Kushner S. R., Grossman L. Enzymatic repair of DNA. 3. Properties of the UV-endonuclease and UV-exonuclease. Biochemistry. 1971 Aug 31;10(18):3315–3324. doi: 10.1021/bi00794a001. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Kaplan J. C., Ono H., Grossman L. Enzymatic repair of deoxyribonucleic acid. IV. Mechanism of photoproduct excision. Biochemistry. 1971 Aug 31;10(18):3325–3334. doi: 10.1021/bi00794a002. [DOI] [PubMed] [Google Scholar]
- LOEVINGER R., HUISMAN P. A TWIN-TUBE 50-KVP BERYLLIUM-WINDOW X-RAY UNIT FOR MICROBIAL RADIOBIOLOGY. Radiat Res. 1965 Feb;24:357–367. [PubMed] [Google Scholar]
- Ogawa H. Genetic locations of uvrD and pol genes of E. coli. Mol Gen Genet. 1970;108(4):378–381. doi: 10.1007/BF00267777. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- Ray U., Bartenstein L., Drake J. W. Inactivation of bacteriophage T4 by ethyl methanesulfonate: influence of host and viral genotypes. J Virol. 1972 Mar;9(3):440–447. doi: 10.1128/jvi.9.3.440-447.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithsm, Ymonds N., White P. The Kornberg polymerase and the repair of irradiated T4 bacteriophage. J Mol Biol. 1970 Dec 14;54(2):391–393. doi: 10.1016/0022-2836(70)90438-9. [DOI] [PubMed] [Google Scholar]
- Tanner D., Oishi M. The effect of bacteriophage T4 infection on an ATP-dependent deoxyribonuclease in Escherichia coli. Biochim Biophys Acta. 1971 Feb 11;228(3):767–769. doi: 10.1016/0005-2787(71)90747-7. [DOI] [PubMed] [Google Scholar]
- WINKLER U., JOHNS H. E., KELLENBERGER E. Comparative study of some properties of bacteriophage T4D irradiated with monochromatic ultraviolet light. Virology. 1962 Nov;18:343–358. doi: 10.1016/0042-6822(62)90026-0. [DOI] [PubMed] [Google Scholar]
- Wiberg J. S. Mutants of bacteriophage T4 unable to cause breakdown of host DNA. Proc Natl Acad Sci U S A. 1966 Mar;55(3):614–621. doi: 10.1073/pnas.55.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. T4 endonuclease involved in repair of DNA. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1839–1845. doi: 10.1073/pnas.67.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]



