Abstract
This study deals with mitochondrial phylogenetic information of Japanese flounder in the Pacific coast of Tohoku Japan to estimate the genetic population subdivision that was undetectable by conventional population statistics. We determined complete sequences of mitochondrial NADH dehydrogenase subunit-2 (ND2) and subunit-5 (ND5) genes for 151 individuals from northern (Aomori and Iwate prefectures, 40–41°N) and southern (Miyagi and Fukushima prefectures, 37–38°N) waters. Samples from both waters showed high genetic diversity, including 126 haplotypes. These haplotypes were located at mixed and nested positions on an inferred phylogenetic tree, and traditional F-statistics indicated no significant population divergence (ϕST = −0.00335, p > 0.05), corroborating our previous study. Three variable sites, however, showed significant base composition heterogeneity between samples from the northern and southern waters (Fisher’s exact-test, p < 0.01). Nucleotide substitutions at the three sites converged on an apical clade, which consisted of the five southern individuals, whereas its sister clade consisted only of the three northern individuals. This phylogenetic information corroborates previous ecological studies indicating the presence of separate stocks in the northern and southern waters.
Keywords: marine fish, Pleuronectiformes, gene flow, haplotype, fixation index, phylogenetic information
1. Introduction
Many marine organisms spawn pelagic eggs, and their larvae are capable of dispersal over long distances [1]. Significant gene flow in such species has been found among populations over wide geographic regions [2–8]. These would reflect the assumption that many marine populations operate as genetically open systems [9,10]. Such characteristics of marine organisms would be an impediment to population genetic studies with traditional F-statics based mainly on frequency data [11]. A growing number of studies, however, indicated that pelagic larvae are capable of recruiting back to their source population in marine species [12]. Oceanographic conditions where there are sharp discontinuities of physical and biochemical variables could assist in increasing their tendency to self-recruitment and increasing their vulnerability [13]. Thus, it has been assumed that the relationship between dispersal potential and realized gene flow among marine populations is more complex than previously thought.
Japanese flounder Paralichthys olivaceus is widely distributed along the coastal waters off Japan, Korea and China [14,15]. This temperate marine fish inhabiting littoral sandy bottom (shallower than 200 m in depth) broadcasts floating eggs, and the larvae spend pelagic stages for more than a month [16,17]. Ecological and morphological evidence suggested the existence of a subdivision among flounder populations along the coastal waters off Japan [17–24]. These studies are, however, not supported by population genetic works. Population genetic studies detected geographical heterogeneity in some regions, but inferred genetic population structure is inconsistent with ecological and morphological research [25–27]. Elevated genetic diversity within the flounder population and substantial gene flow among stocks [26,27], including ancient admixture between major mitochondrial lineages [27], obscure the population subdivision of the flounder along the Japanese coast.
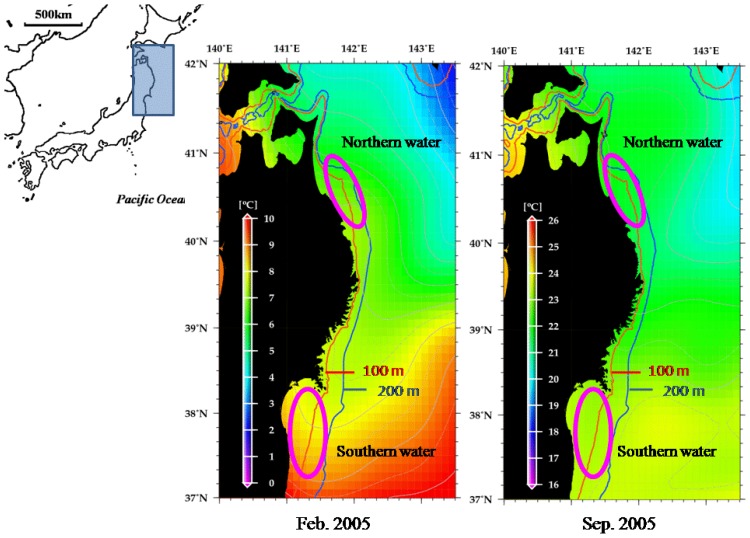
In the Pacific coast off northern Japan, Tohoku district, previous tagging data and life history research suggested two diverged populations, each comprising northern (Iwate and Aomori prefectures, 40–41°N) or southern (Fukushima and Miyagi prefectures, 37–38°N) waters [22–24]. There are limited littoral areas distributed in the intermediate region between the two waters. The oceanographic conditions also differ between the two waters. The northern and southern waters are influenced by Tsushima and Kuroshio warm currents throughout the year, respectively, while in the intermediate region, Oyashio cold current occurs (Figure 1). It would be assumed that such geological and oceanographic conditions in these waters may represent potential barriers to flounder exchange.
Figure 1.
Sampling locations of Japanese flounder used in this study, and sea surface temperatures along the Pacific coast of Tohoku Japan for February and September 2005. The sea surface temperature data set was provided by NCDC, USA [28].
The mitochondrial genetic difference between samples from the northern and southern waters, if any, would also be small or undetectable by conventional population statistics, e.g., FST or even ϕST that was suggested to be a better index for population diversification [29]. In an incipient state of population subdivision, haplotypes from phylogenetic clades with various degrees of sequence divergence distribute to each subpopulation in a nested state in which members of clades appear almost evenly in both subpopulations (ϕST ≈ 0). However, if migration between subpopulations is limited for a while during which new clades appear by additional nucleotide substitutions, migrants do not carry members of some of these clades by chance. The limited migration thus leaves genetic footprints, i.e., biased distribution of some clades and base composition heterogeneity at sites that define these clades.
In this study, to explore the possibility of genetic divergence of Japanese flounder in the Pacific coast of Tohoku Japan, we tried to reveal genetic footprints of the incipient state of population subdivision with mitochondrial nucleotide variation in relation to the character state along phylogenetic tree.
2. Results and Discussion
2.1. Genetic Variability of Japanese Flounder
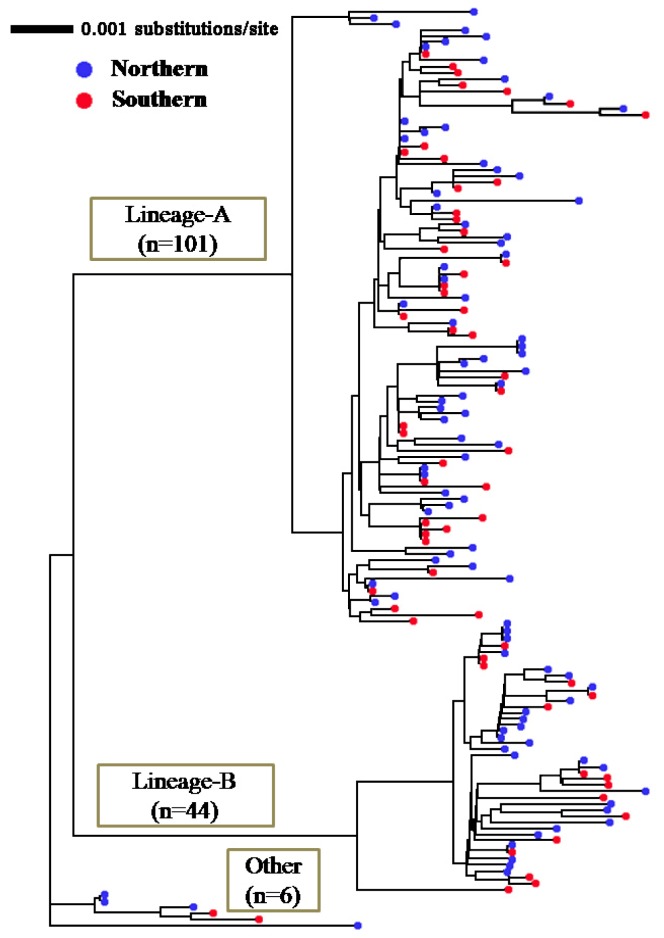
We determined the whole sequences of NADH dehydrogenase subunit-2 (ND2: 1046 bp) and subunit-5 (ND5: 1839 bp) genes for all 151 individuals. There was no gap in these regions among the material fish examined. The haplotype diversity values (h) of samples from the northern and southern waters were 0.9973 ± 0.0022 and 0.9966 ± 0.0040, respectively. The nucleotide diversity values (π) of the samples from northern and southern waters were 0.0079 ± 0.0039 and 0.0075 ± 0.0037, respectively. The neighbor-joining tree showed the two major mitochondrial clades (lineage-A and -B) with deep branching (Figure 2), as in our previous study [27]. Haplotypes of both mitochondrial lineages coexisted within samples from the northern and southern waters, with several cases of common haplotype between them.
Figure 2.
Neighbor-joining tree for 151 individuals of Japanese flounder derived from the concatenated NADH dehydrogenase subunit-2 (ND2) and subunit-5 ND5 sequences. Genetic distances among the individuals were estimated by Tamura-Nei + I + G distance model.
2.2. Segregation Sites, Character Mapping and F(ϕ)-Statistics
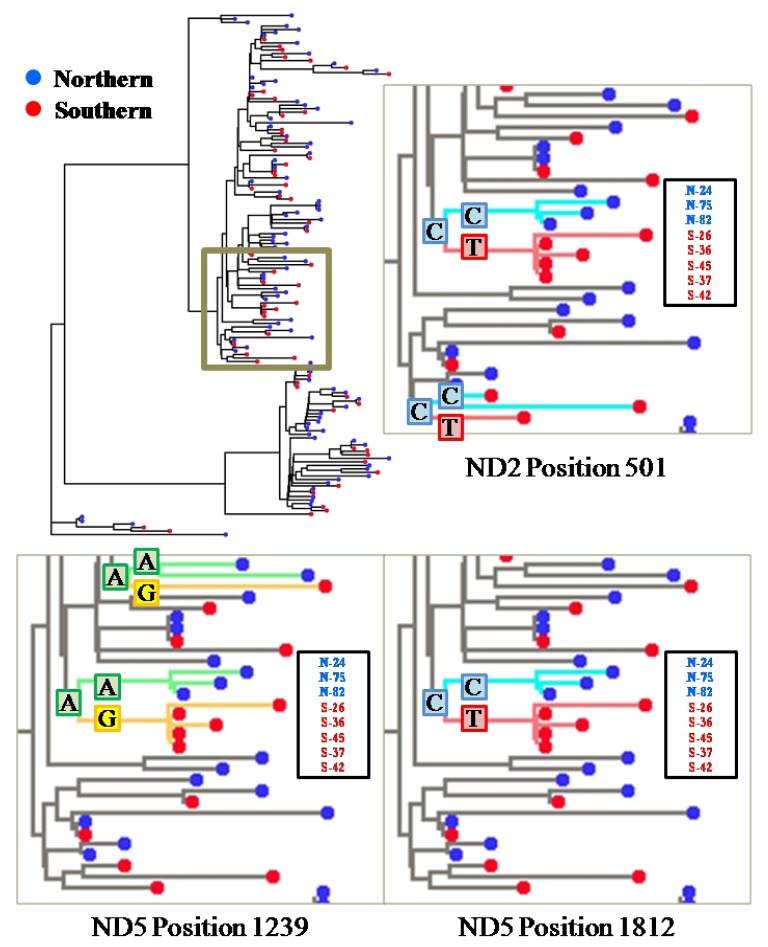
We found three nucleotide sites (ND2 position 501, ND5 position 1239, ND5 position 1812), which show base composition heterogeneity between samples from the northern and southern waters (Fisher’s exact-test, p < 0.01) (Table 1). All of these sites were located at third codon positions and contained synonymous transitional base substitutions, whereas other variable sites did not exhibit base composition heterogeneity between samples from the northern and southern waters regardless of being synonymous or nonsynonymous. Character mapping along the phylogenetic tree revealed that the three sites converged on a shallow clade, which consisted of five individuals from the southern water (S-26, S-36, S-37, S-42 and S-45), whereas its sister clade consisted of three from the northern water (N-24, N-75 and N-82) (Figure 3). A “C” to “T” substitution at the ND5 position 1812 was solely mapped at a clade and apomorphic to the five individuals from southern water. The other substitutions (“C” to “T” at the ND2 position 501 and “A” to “G” at the ND5 position 1239) were also mapped at this clade in the same way, though these two substitutions were multiply mapped on other shallow clades separately. On the other hand, to the base composition heterogeneity, there was no significant genetic difference between samples from the northern and southern waters based on the value of fixation index (ϕST = −0.00335, p > 0.05).
Table 1.
The numbers of each nucleotide at the three synonymous substitution sites for which the base composition was statistically different between the northern and southern samples.
| Northern samples | Southern samples | p-value of Fisher’s exact-test | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| A | G | C | T | A | G | C | T | ||
| ND2 Position 501 | 91 | 54 | 6 | 0.0034 | |||||
| ND5 Position 1239 | 91 | 54 | 6 | 0.0034 | |||||
| ND5 Position 1812 | 91 | 55 | 5 | 0.0089 | |||||
Figure 3.
Character assignment for the three synonymous substitution sites (ND2 position 501, ND5 position 1239 and ND5 position 1812) under the accelerated character state optimization. Colored lines indicate the genetic footprint of each variable site. S-26, 36, 37, 42 and 45 are individuals of southern water.
2.3. Discussion
Wild Japanese flounder show high mitochondrial haplotype diversity with two major lineages sympatrically distributed along the coast of Japan [27]. Samples from the northern and southern waters of the Pacific coast of Tohoku Japan also show the same genetic property (Figure 2). Flounders harboring these mitochondrial lineages comprise a single panmictic population at each locality, because of Hardy-Weinberg equilibria at microsatellite loci in all the flounder populations from the Japanese coast examined [26]. Co-existence of these deep branched lineages would be of a hybridizing swarm of populations in old geological times. Genetic admixture of previously isolated populations can increase genetic variation [30]. Raised genetic variability, both in haplotype and nucleotide diversity, within population is empirically known to drive F- or related statistics lower [11,29]. Accordingly, such a historical background of Japanese flounder might be a factor of high genetic variability and difficulty in detecting population subdivision.
A value of fixation index (ϕST = −0.00335, p > 0.05) that is better to display divergence between highly variable populations than FST [29] suggested that there has been gene flow between the northern and southern waters. Existence of common haplotypes between samples from the northern and southern waters also indicates migration has taken place until recently (mutation has not yet hit the sequenced regions) or even presently.
If the northern and southern waters contain a single panmictic population, every mitochondrial lineage could be detected from both samples evenly. However, we confirmed a sample-specific lineage defined by the three synapomorphic sites (Figure 2). This phylogenetic information corroborates previous ecological studies indicating separate stocks in these waters [18,19,22–24]. Even in the highly connective marine environments, oceanographic fronts could impede admixture among fish populations [13]. Cold water front and littoral bottle-neck between the northern and southern waters indicate that a corridor connecting these two waters for the temperate littoral fish is narrow (Figure 1). Limited migration between the northern and southern waters may thus be responsible for the biased distribution of some clades between the two samples.
Under limited migration between populations, migrants do not or hardly carry haplotypes of some clades by chance. Such limited migration thus leaves genetic footprints on heterogeneity in haplotype distribution and base composition between populations.
Recently, several nuclear DNA markers, which were suggested to be under adaptive evolution, provided valuable results for population genetic studies [31]. Population specific lineages are conspicuous in adaptable environments, even if gene flow among geographic waters is frequent. On the other hand, the three nucleotide substitutions found in the genetic marker employed in this study are at third codon positions and synonymous, and they are most likely neutral or under very week adaptive selection, if any. The present study showed that upon phylogenetic and base composition analyses, even neutral or near neutral genetic markers could indicate small and incipient population subdivision, which is undetectable with conventional population statistics.
3. Materials and Methods
3.1. Samples
We purchased 91 wild individuals at wholesale fish markets adjunct to fishing ports in Iwate and Aomori Prefectures (northern water, 40–41°N) and 60 in Fukushima and Miyagi Prefectures (southern water, 37–38°N) in 2005 (Figure 1). Because usage of gears for the flounder fishery is regionally regulated, fishermen bring their catch to the nearest fishing port where they belong. We could thus obtain geographically structured samples from these fish markets. We excluded hatchery-reared Japanese flounder, which had abnormal pigmentation [32,33].
3.2. Population Genetic Analysis
Extractions of genomic DNAs from muscle tissues followed [34]. We sequenced the mitochondrial ND2 and ND5 genes, using a two-step polymerase chain reaction (PCR) direct sequencing technique. PCR amplification and sequencing strategy followed [27]. The concatenated sequences of ND2 and ND5 genes worked for subsequent analyses.
Calculations of haplotype and nucleotide diversities for each samples followed [35]. We counted the number of variable nucleotide sites by eyes and tested base composition heterogeneity between the two samples at each variable site by Fisher’s exact-test. Phylogenetic relationships among haplotypes were inferred from the neighbor-joining method based on Tamura-Nei + I + G distance, whose substitution model was selected by AIC comparison, using MEGA5 [36]. Then, the genetic footprints at the variable sites, which show base composition heterogeneity between the two samples, were traced along branches of the neighbor-joining tree by PAUP [37] under the accelerated transition optimization. Fixation index (ϕST) between the two samples was calculated using Alrequin version 3.11 [38].
4. Conclusions
The present study showed that upon phylogenetic and base composition analyses, even neutral or near neutral genetic markers could indicate small and incipient population subdivision, which is undetectable with conventional population statistics. The phylogenetic information of Japanese flounder corroborates previous ecological studies indicating the presence of separate stocks in the northern and southern waters of the Pacific coast of Tohoku Japan.
Acknowledgments
This work was partially supported by a Grant-in-Aid for the Promotion Program for Fisheries Resources Survey in Waters around Japan from the Fisheries Agency of Japan. We are grateful to Takuma Sugaya and Motoshige Yasuike (National Research Institute of Fisheries Science) for providing variable comments.
Conflict of Interest
Yutaka Kurita takes part in the Promotion Program for International Resources Survey from the Fisheries Agency of Japan in which fisheries stocks as management units are designated.
References
- 1.Thorrold S.M., Jones G.P., Hellberg M.E., Burton R.S., Swearer S.E., Neoge J.E., Morgan S.G., Warner R.R. Quantifying larval retention and connectivity in marine populations with artificial and natural markers. Bull. Mar. Sci. 2002;70:291–308. [Google Scholar]
- 2.Ishikawa S., Kimura Y., Tokai T., Tsukamoto K., Nishida M. Genetic variation in the mitochondrial and nuclear DNA of Japanese conger Conger myriaster. Fish. Sci. 2001;67:1081–1087. [Google Scholar]
- 3.Schrey A.W., Heist E.J. Microsatellite analysis of population structure in the shortfin mako (Isurusoxyrinchus) Can. J. Fish. Aquat. Sci. 2003;60:670–675. [Google Scholar]
- 4.De Innoceniis S., Lesti A., Livi S., Rossi A.R., Crosetti D., Sola L. Microsatellite markers reveal population structure in gilthead sea bream Sparus auratus from the Atlantic Ocean and Mediterranean Sea. Fish. Sci. 2004;70:852–859. [Google Scholar]
- 5.Karaiskou N., Triantafyllidis A., Triantaphyllidis C. Shallow genetic structure of three species of the genus Trachurus in European waters. Mar. Ecol. Prog. Ser. 2004;281:193–205. [Google Scholar]
- 6.Jeffrey I.B., Hale P., Degmam B.M., Degnan S.M. Pleistocene isolation and recent gene flow in Haliotis asinine, an Indo-Pacific vetigastropod with limited dispersal capacity. Mol. Ecol. 2007;16:289–304. doi: 10.1111/j.1365-294X.2006.03141.x. [DOI] [PubMed] [Google Scholar]
- 7.Han Z.Q., Gao T.X., Yanagimoto T., Sakurai Y. Genetic population structure of Nibea albiflora in Yellow Sea and East China Sea. Fish. Sci. 2008;74:544–552. [Google Scholar]
- 8.Shui B.N., Han Z.Q., Gao T.X., Miao Z.Q., Yanagimoto T. Mitochondrial DNA variation in the East China Sea and Yellow Sea populations of Japanese Spanish Mackerel Scomberomorus niphonius. Fish. Sci. 2009;75:593–600. [Google Scholar]
- 9.Ward R.D., Woodwark M., Skibinski D.O.F. A comparison of genetic diversity levels in marine, freshwater, and anadromous fishes. J. Fish Biol. 1994;44:213–227. [Google Scholar]
- 10.Avise J.C. Phylogeography: The History and Formation of Species. Harvard University Press; Cambridge, MA, USA: 2000. [Google Scholar]
- 11.Helberg M.E. Gene flow and isolation among populations of marine animals. Ann. Rev. Ecol. Evol. Syst. 2009;40:291–310. [Google Scholar]
- 12.Swearer S.E., Shima J.S., Hellberg M.E., Thorrold S.R., Jones G.P., Robertson D.R., Morgan S.G., Selkoe K.A., Ruiz G.M., Warner R.R. Evidence of self-recruitment in demersal marine populations. Bull. Mar. Sci. 2002;70:251–271. [Google Scholar]
- 13.Galarza J.A., Carreras-Carbonell J., Macpherson E., Pascual M., Roques S., Turner G.F., Rico C. The influence of oceanographic fronts and early-life-history traits on connectivity among littoral fish species. Proc. Natl. Acad. Sci. USA. 2009;106:1473–1478. doi: 10.1073/pnas.0806804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashima K., Shindo S. On the grouping of fishery grounds based on the species composition of the demersal fishes in the East China Sea and the Yellow Sea. Bull. Seikai. Reg. Fish. Res. Lab. 1974;44:1–26. [Google Scholar]
- 15.Minami T. Life History. In: Minami T., Tanaka M., editors. Biology and Stock Enhancement of Japanese Flounder. Koseisha-Koseikaku; Tokyo, Japan: 1997. pp. 9–24. [Google Scholar]
- 16.Seikai T., Tanangonan J.B., Tanaka M. Temperature influence on larval growth and metamorphosis of the Japanese flounder Paralichthys olivaceus in the laboratory. Bull. Japan. Soc. Sci. Fish. 1986;52:977–982. [Google Scholar]
- 17.Kinoshita I., Seikai T., Tanaka M., Kuwamura K. Geographic variations in dorsal and anal ray counts of juvenile Japanese flounder, Paralichthys olivaceus, in the Japan Sea. Environ. Biol. Fish. 2000;57:305–313. [Google Scholar]
- 18.Nihira A., Takase H., Betsui K., Ishikawa K. Results of mark-recapture experiments of flounder Paralichthys olivaceus (Temminck et Schlegel) on the coastal region of Ibaraki Prefecture. Bull. Ibaraki. Pref. Fish. Exp. Sta. 1988;26:137–159. [Google Scholar]
- 19.Ishito Y. Distribution and migration of the young Japanese flounder (Paralichthys olivaceus) along the northeastern coast of Japan. Bull. Tohoku Natl. Fish. Res. Inst. 1990;52:33–43. [Google Scholar]
- 20.Tanaka M., Ohkawa T., Maeda T., Kinoshita I., Seikai T., Nishida M. Ecological diversities and stock structure of the flounder in the Sea of Japan in relation to stock enhancement. Bull. Natl. Res. Inst. Aquacult. 1997;3:77–85. [Google Scholar]
- 21.Nakamura R., Watanabe M., Satoh K. On the structure of subpopulation of Paralichthys olivaceus in the coastal area around Kantoh district, Japan. Bull. Kanagawa Pref. Fish. Res. Inst. 2001;6:113–121. [Google Scholar]
- 22.Yoneda M., Kurita Y., Kitagawa D., Ito M. Spatial variation in the relationship between growth and maturation rate in male Japanese flounder Paralichthys olivaceus off the Pacific coast of northern Japan. J. Sea Res. 2007;57:171–179. [Google Scholar]
- 23.Yoneda M., Kurita Y., Kitagawa D., Ito M., Tomiyama T., Goto T., Takahashi K. Age validation and growth variability of Japanese flounder Paralichthys olivaceus off the Pacific coast of northern Japan. Fish. Sci. 2007;73:585–592. [Google Scholar]
- 24.Kurita Y., Tamate T., Ito M. Marine Fisheries Stock Assessment and Evaluation for Japanese Waters (Fiscal Year 2010/2011) Fisheries Agency and Fisheries Research Agency of Japan; Tokyo, Japan: 2011. Stock Assessment and Evaluation for Japanese Flounder (Fiscal Year 2010) pp. 1358–1384. [Google Scholar]
- 25.Fujii T., Nishida M. High sequence variability in the mitochondrial DNA control region of the Japanese flounder Paralichthys olivaceus. Fish. Sci. 1997;63:906–910. [Google Scholar]
- 26.Sekino M., Hara M. Application of microsatellite markers to population genetic studies of Japanese flounder Paralichthys olivaceus. Mar. Biotechnol. 2001;3:572–589. doi: 10.1007/s10126-001-0064-8. [DOI] [PubMed] [Google Scholar]
- 27.Shigenobu Y., Hayashizaki K., Asahida T., Ida H., Saitoh K. Stock structure of Japanese flounder inferred from morphological and genetic analyses. Fish. Sci. 2007;73:1104–1112. [Google Scholar]
- 28.Reynolds R.W., Smith T.M., Liu C., Chelton D.B., Casey K.S., Schlax M.G. Daily high-resolution-blended analyses for sea surface temperature. J. Climate. 2007;20:5473–5496. [Google Scholar]
- 29.Meirmans P.G., Hedrick P.W. Assessing population structure: Fst and related measures. Mol. Ecol. Resourc. 2011;11:5–18. doi: 10.1111/j.1755-0998.2010.02927.x. [DOI] [PubMed] [Google Scholar]
- 30.Kolbe J.J., Larson A., Losos J.B., de Queiroz K. Admixture determines genetic diversity and population differentiation in the biological invasion of a lizard species. Biol. Lett. 2008;4:434–437. doi: 10.1098/rsbl.2008.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen E.E., Hemmer-Hansen J., Larsen P.F., Bekkevold D. Population genomics of marine fishes: identifying adaptive variation in space and time. Mol. Ecol. 2009;18:3128–3150. doi: 10.1111/j.1365-294X.2009.04272.x. [DOI] [PubMed] [Google Scholar]
- 32.Seikai T. Mechanism of Abnormal Pigmentation. In: Minami T., Tanaka M., editors. Biology and Stock Enhancement of Japanese Flounder. Koseisha-Koseikaku; Tokyo, Japan: 1997. pp. 63–73. [Google Scholar]
- 33.Tomiyama T., Mizuno T., Watanabe M., Fujita T., Kawata G. Patterns and frequency of hypermelanosis on the blind side in wild Japanese flounder. Nippon Suisan Gakkaishi. 2008;74:171–176. [Google Scholar]
- 34.Asahida T., Kobayashi T., Saitoh K., Nakayama I. Tissue preservation and total DNA extraction from fish stored at ambient temperature using buffers containing high concentration of urea. Fish. Sci. 1996;62:727–730. [Google Scholar]
- 35.Nei M. Molecular Evolutionary Genetics. Columbia University Press; New York, NY, USA: 1987. [Google Scholar]
- 36.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swofford D.L. Paup*: Phylogenetic Analysis Using Parsimony (and Other Methods), version 4. Sinauer Associates; Sunderland, MA, USA: 1998. [Google Scholar]
- 38.Excoffier L., Laval G., Schneider S. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evol. Bioinform. Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]