Abstract
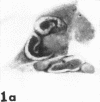
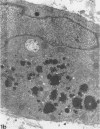
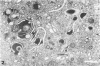
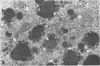
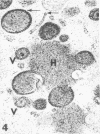
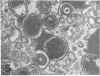
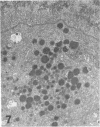
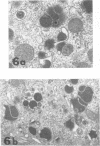
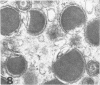
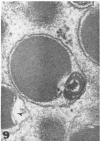
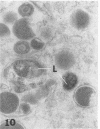
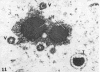
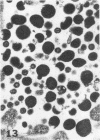
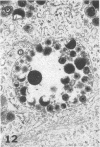
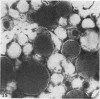
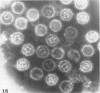
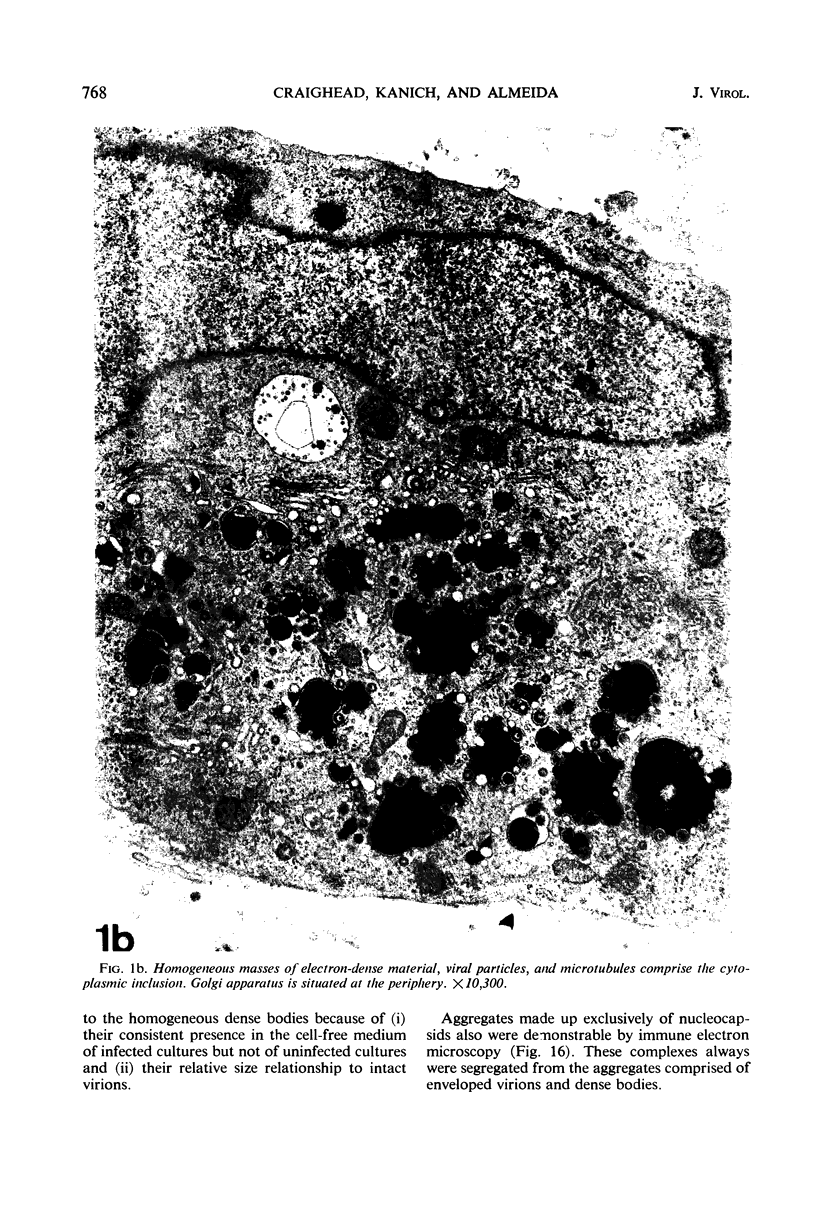
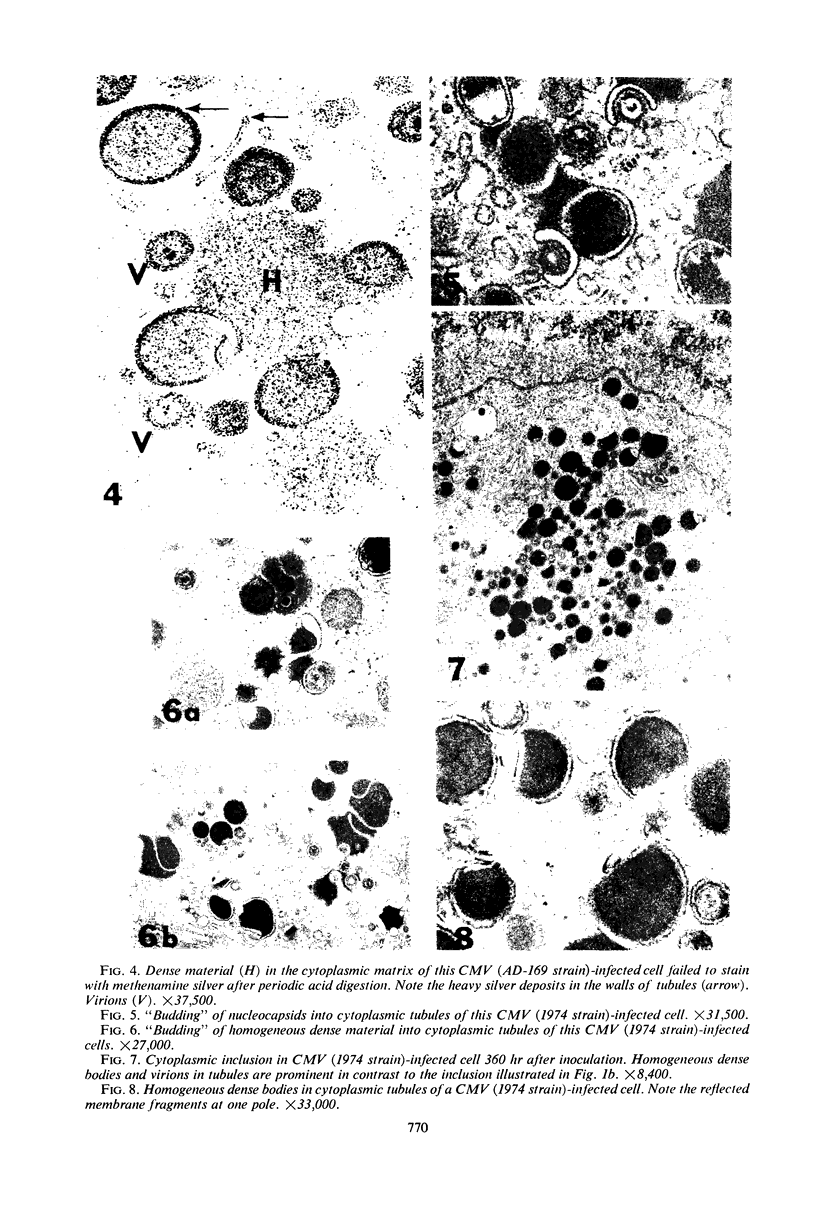
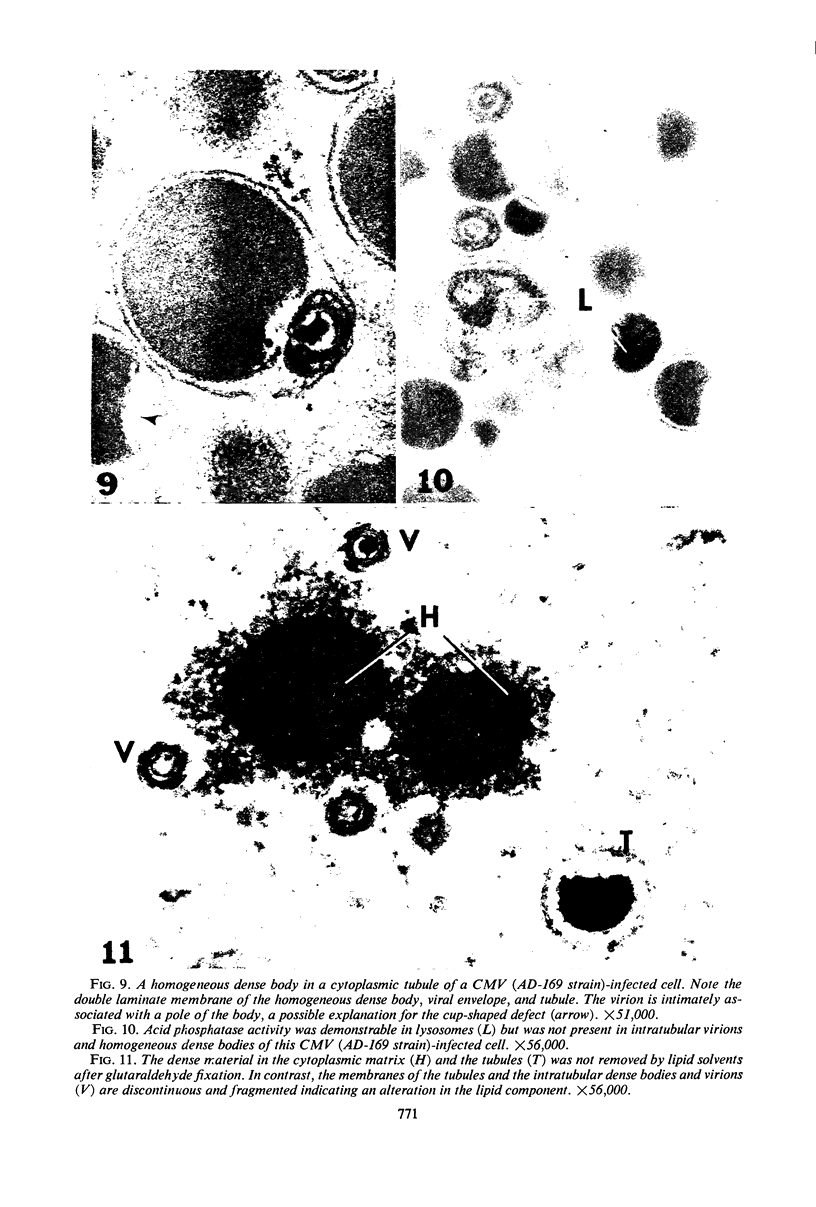
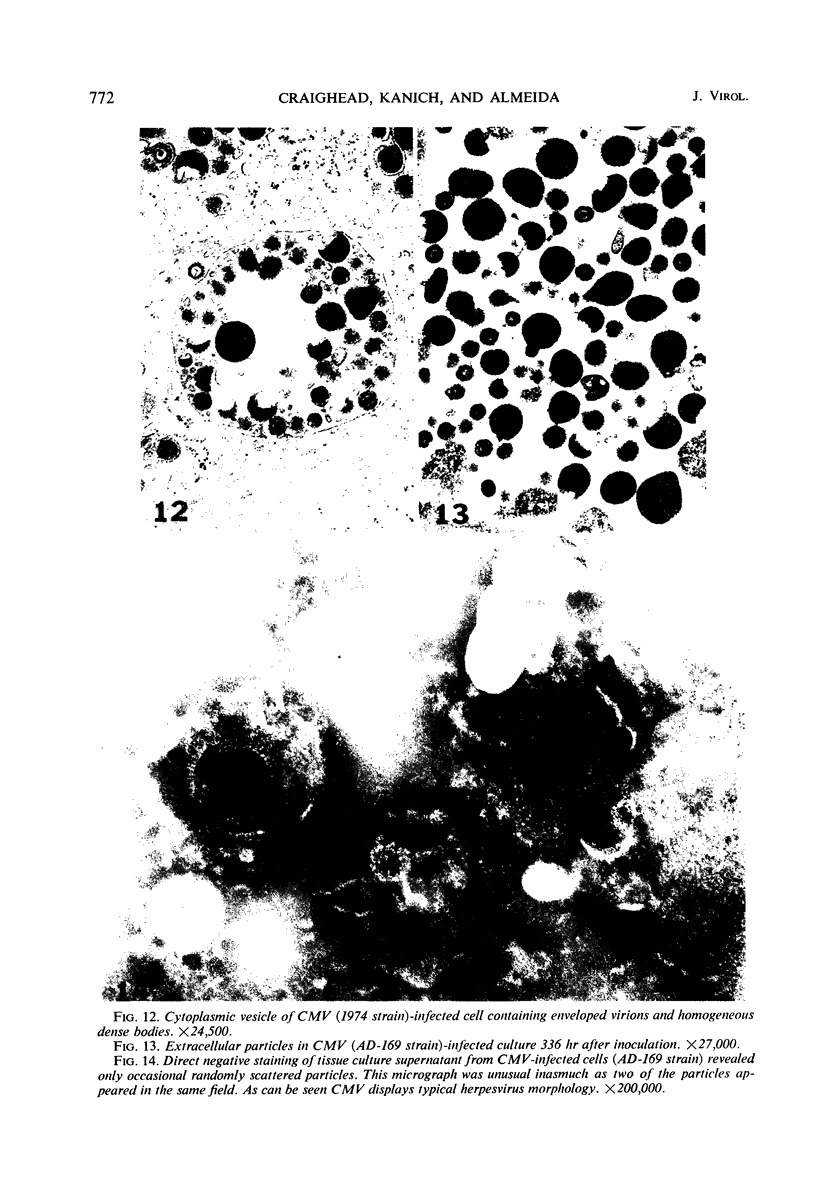
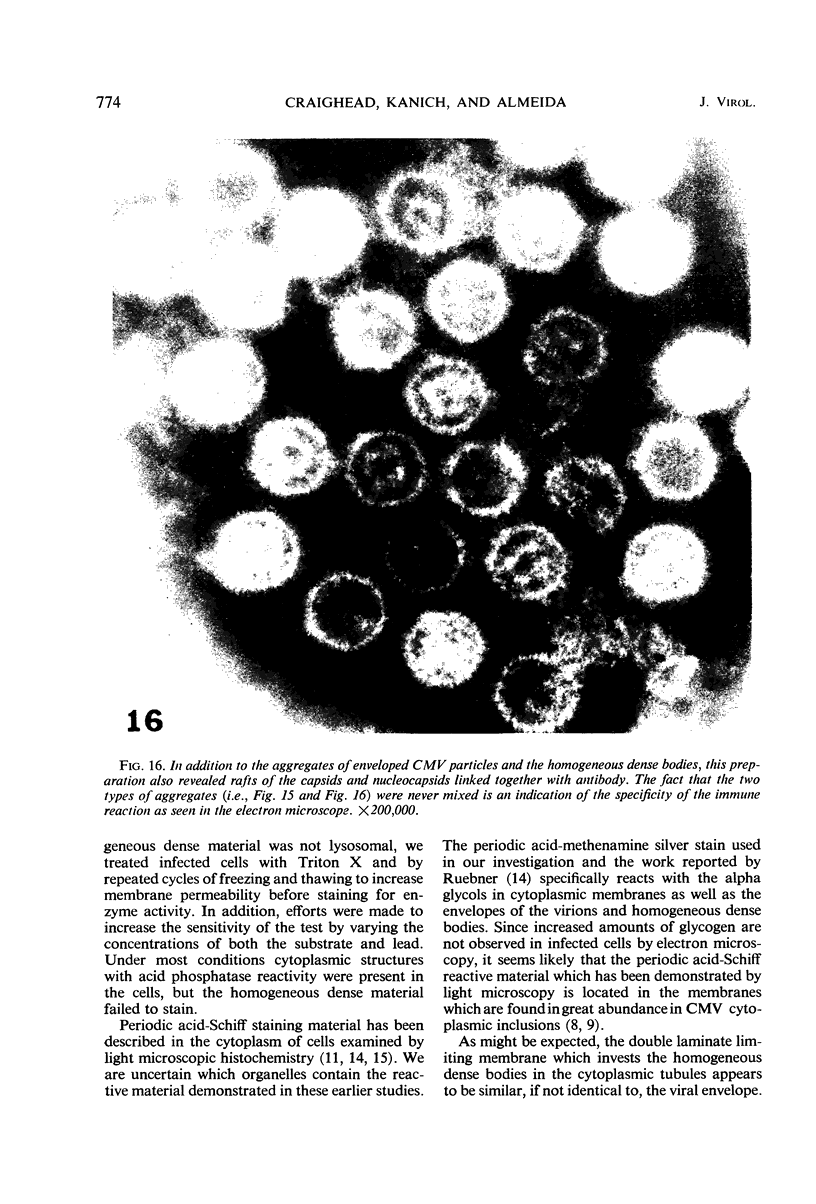
Masses of homogeneous electron-dense material accumulate in the cytoplasmic inclusions of cultured fibroblasts which have been infected with “wild” and “adapted” strains of human cytomegalovirus. The substance appears to be produced by microtubular membranes and the Golgi apparatus; ultrastructural histochemistry suggests that it is not lysosomal in nature nor is it comprised of lipids or polysaccharides. The dense material “buds” into cytoplasmic tubules forming circumscribed bodies having an investing membrane similar to the viral envelope. After transport to the extracellular milieu in cytoplasmic tubules and vesicles, virions and dense bodies can be demonstrated by immune electron microscopy. The homogeneous dense body appears to be a unique product of the cytomegalovirus-infected cell which possesses a limiting membrane having antigenic determinants common with the viral envelope.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D., Waterson A. P. The morphology of virus-antibody interaction. Adv Virus Res. 1969;15:307–338. doi: 10.1016/S0065-3527(08)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER P., MELNICK J. L., MAYOR H. D. A MORPHOLOGIC COMPARISON BETWEEN THE DEVELOPMENTAL STAGES OF HERPES ZOSTER AND HUMAN CYTOMEGALOVIRUS. Exp Mol Pathol. 1965 Feb;76:11–23. doi: 10.1016/0014-4800(65)90020-1. [DOI] [PubMed] [Google Scholar]
- Cok M. L., Stevens J. G. Replication of varicella-zoste virus in cell culture: an ultrastructural study. J Ultrastruct Res. 1970 Aug;32(3):334–350. doi: 10.1016/s0022-5320(70)80014-4. [DOI] [PubMed] [Google Scholar]
- Craighead J. E. Immunologic response to cytomegalovirus infection in renal allograft recipients. Am J Epidemiol. 1969 Dec;90(6):506–513. doi: 10.1093/oxfordjournals.aje.a121096. [DOI] [PubMed] [Google Scholar]
- Craighead J. E. Pulmonary cytomegalovirus infection in the adult. Am J Pathol. 1971 Jun;63(3):487–504. [PMC free article] [PubMed] [Google Scholar]
- ERICSSON J. L., TRUMP B. F. ELECTRON MICROSCOPIC STUDIES OF THE EPITHELIUM OF THE PROXIMAL TUBULE OF THE RAT KIDNEY. I. THE INTRACELLULAR LOCALIZATION OF ACID PHOSPHATASE. Lab Invest. 1964 Nov;13:1427–1456. [PubMed] [Google Scholar]
- JONES D. B. Nephrotic glomerulonephritis. Am J Pathol. 1957 Mar-Apr;33(2):313–329. [PMC free article] [PubMed] [Google Scholar]
- LUSE S. A., SMITH M. G. Electron microscopy of salivary gland viruses. J Exp Med. 1958 May 1;107(5):623–632. doi: 10.1084/jem.107.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGAVRAN M. H., SMITH M. G. ULTRASTRUCTURAL, CYTOCHEMICAL, AND MICROCHEMICAL OBSERVATIONS ON CYTOMEGALOVIRUS (SALIVARY GLAND VIRUS) INFECTION OF HUMAN CELLS IN TISSUE CULTURE. Exp Mol Pathol. 1965 Feb;76:1–10. doi: 10.1016/0014-4800(65)90019-5. [DOI] [PubMed] [Google Scholar]
- PATRIZI G., MIDDELKAMP J. N., HERWEG J. C., THORNTON H. K. HUMAN CYTOMEGALOVIRUS: ELECTRON MICROSCOPY OF A PRIMARY VIRAL ISOLATE. J Lab Clin Med. 1965 May;65:825–838. [PubMed] [Google Scholar]
- RUEBNER B. H., HIRANO T., SLUSSER R. J., MEDEARIS D. N., Jr HUMAN CYTOMEGALOVIRUS INFECTION. ELECTRON MICROSCOPIC AND HISTOCHEMICAL CHANGES IN CULTURES OF HUMAN FIBROBLASTS. Am J Pathol. 1965 Mar;46:477–496. [PMC free article] [PubMed] [Google Scholar]
- RUEBNER B. H., MIYAI K., SLUSSER R. J., WEDEMEYER P., MEDEARIS D. N., Jr MOUSE CYTOMEGALOVIRUS INFECTION. AN ELECTRON MICROSCOPIC STUDY OF HEPATIC PARENCHYMAL CELLS. Am J Pathol. 1964 May;44:799–821. [PMC free article] [PubMed] [Google Scholar]
- Ruebner B. H., Hirano T., Slusser R., Osborn J., Medearis D. N., Jr Cytomegalovirus infection. Viral ultrastructure with particular reference to the relationship of lysosomes to cytoplasmic inclusions. Am J Pathol. 1966 Jun;48(6):971–989. [PMC free article] [PubMed] [Google Scholar]
- Stackpole C. W. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J Virol. 1969 Jul;4(1):75–93. doi: 10.1128/jvi.4.1.75-93.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]