Abstract
The increased incidence of opportunistic fungal infections, associated with greater resistance to the antifungal drugs currently in use has highlighted the need for new solutions. In this study twenty four coumarin derivatives were screened in vitro for antifungal activity against strains of Aspergillus. Some of the compounds exhibited significant antifungal activity with MICs values ranging between 16 and 32 μg/mL. The structure-activity relationships (SAR) study demonstrated that O-substitutions are essential for antifungal activity. It also showed that the presence of a short aliphatic chain and/or electron withdrawing groups (NO2 and/or acetate) favor activity. These findings were confirmed using density functional theory (DFT), when calculating the LUMO density. In Principal Component Analysis (PCA), two significant principal components (PCs) explained more than 60% of the total variance. The best Partial Least Squares Regression (PLS) model showed an r2 of 0.86 and q2cv of 0.64 corroborating the SAR observations as well as demonstrating a greater probe N1 interaction for active compounds. Descriptors generated by TIP correlogram demonstrated the importance of the molecular shape for antifungal activity.
Keywords: coumarin derivatives, antifungal activity, Aspergillus sp., structure-activity relationships (SAR), molecular modeling, principal component analysis (PCA), partial least squares regression (PLS), density functional theory (DFT)
1. Introduction
The increased incidence of fungal infections, especially dangerous hospital-acquired infections and infections in immunocompromised patients has highlighted the need for new antifungal treatments. Drug-resistant fungal isolates have been reported for all known classes of antifungal drugs. As a result, mortality, morbidity, and the associated cost of medical care for fungal infections are all steadily rising. In addition, because many of the currently available drugs are toxic and have other drawbacks involving spectrum, tissue distribution, central nervous system penetration, and high cost, the number of efficacious anti-mycotic drugs is limited [1]. Many of these drugs actually produce infection recurrence, for being fungistatic and not fungicidal.
In particular, Aspergillus infections have been increasing, and while the most frequent pathogen to cause aspergillosis is Aspergillus fumigatus, A. terreus, A. flavus and A. niger are becoming increasingly common [2]. A. fumigatus is an opportunistic pathogen which incites disease in hosts whose local or systemic immune response has been impaired, damaged, or is innately dysfunctional. After Aspergillus fumigatus, A. flavus is the second leading cause of invasive aspergillosis, and it is the most common cause of superficial infections. Common clinical syndromes associated with A. flavus infections include chronic granulomatous sinusitis, keratitis, cutaneous aspergillosis, wound infections, and osteomyelitis following trauma and inoculation. In addition, A. flavus produces aflatoxins, the most toxic natural hepatic-carcinogens ever characterized [3].
There is an urgent need for discovery and testing of new compounds with antifungal properties. Natural products are inexhaustible as a source of chemical structures and for more than a century have been used as scaffolds for the synthesis of new drugs. The coumarins, phenolic compounds which possess a benzopyranone nucleus [4] are one of the major classes of secondary metabolites, which has been highlighted in biological studies as being (anti-HIV [5], hepato-protective [6], anti-inflammatory [7], antimicrobial [8], antimitotic [9] and antitumor [10]), and in part, due to their antifungal properties [11–13] they are believed to exert a role in plant protection against herbivorous, fungal, and bacterial infections [14].
It was recently reported that coumarin derivatives may be used effectively as antifungal agents. Johann et al. [11] demonstrated that coumarins isolated from plant extracts exhibit activity against certain strains of Sporothrix schenckii (MICs of 125–250 μg/mL), and Cryptococcus gattii (MIC of 250 μg/mL). Daoubi et al. [13] showed their antifungal activity against Botrytis cinerea in concentrations ranging from 25 to 200 mg/L with IC50 values of 33.3–157.5 mg/L. Creaven et al. [15] demonstrated that coumarin derivatives are active against Candida albicans (MIC80 of 4.5–246.6 μM). Jurd et al. [16,17] studied several coumarin derivatives showing inhibitory activity against varied strains including Candida (Candida albicans, C. tropicalis, C. chalmersi), Saccharomyces cerevisiae, Aspergillus flavus, A. niger, A. oryzae, A. glaucus, and Penicillium chrysogenum.
Pursuing our research in the field [18–20], we herewith describe the synthesis and in vitro antifungal evaluation of coumarin derivatives against Aspergillus species. After antifungal evaluation, the derivatives were subjected to structure-activity relationship (SAR) analysis, electronic surface analyses, molecular modelling, and chemometrics (Principal Component Analysis (PCA), and Partial Least Square Regression (PLS)), all to extract information on structure and its relation to antifungal properties [21,22].
2. Results and Discussion
2.1. Synthesis
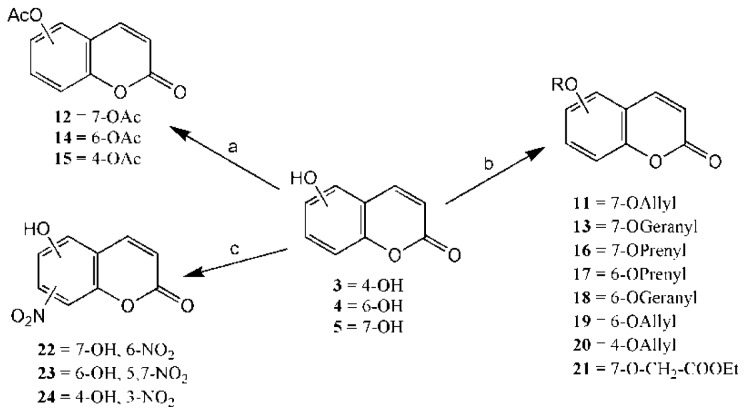
As shown in Scheme 1, the coumarin derivatives (11–24) were synthesized by alkylation, acetylation, and nitration of commercial coumarins: 4-hydroxy- (3), 6-hydroxy- (4) and 7-hydroxy-coumarin (5). Alkylation reactions were done according to procedures previously described with small modifications [23,24], using different alkyl halides; (allyl bromide, geranyl bromide, prenyl bromide and ethyl chloro-acetate) which gave coumarin derivatives: 11 [25], 13 [26], 16 [26], 17 [27], 18 [28], 19 [29], 20 [30] and 21 [31] in satisfactory yields (45.5%–98%, except 18). Acetylation was carried out under ultrasonic irradiation (which increases the rate, speed and yield of chemical reactions, by liquids emulsification [32]), using an acetic anhydride and pyridine mixture afforded derivatives 15 [33], 14 [17], and 12 [34], in good yields (77%–90%). Nitro-coumarins 22 [35], 23, and 24 [36] were synthesized by standard nitration procedures, using a mixture of nitric and acetic acids in an ice bath [37].
Scheme 1.
Synthesis of alkyl-, acetyl- and nitro-coumarin derivatives. Reagents and Conditions: (a) Acetic Anhydride, Pyridine, rt., ultrasound irradiation; (b) Allyl Bromide, Geranyl Bromide, Prenyl Bromide, or Ethyl Chloroacetate, K2CO3, Acetonitrile, reflux; (c) HNO3/AcOH, 0–5 °C for 30 min, then 90 min at rt.
The chemical structures of 11–22 and 24 were confirmed by comparing their physical and spectral data with the respective literatures. The purity of all synthesized compounds were >98% determined by HPLC.
Structural confirmation of 23 was based on its NMR, mass spectra and elemental analysis. In the 1H NMR we observed only three signals related to three hydrogens which indicated a probable bi-nitration. There were two doublets coupled together (J = 10.0 Hz) at 6.75 and 7.77 ppm, which were attributed to H-3 and H-4, and one singlet at 8.23 ppm (related to H-8). These signals provide good evidence that a bi-nitration occurred on the aromatic ring at the C-5 and C-7 positions, both were ortho-positioned with respect to the 6-hydroxyl group, as also observed in previous works [38]. The unequivocal determination of the carbons was done by interpretation of their 13C NMR, 13C–1H HMQC and 13C–1H HMBC spectra.
In the 13C–1H HMQC was possible to determine unambiguously the three primary carbons at 116.3 (C-8), 124.0 (C-3) and 136.7 ppm (C-4). Signals from quaternary carbons were determined by analysis of the correlations observed in the 13C–1H HMBC, which are described in Table 1.
Table 1.
13C–1H HMBC correlations for 23.
| Hydrogen | Carbon | C-2 | C-3 | C-4 | C-4′ | C-5 | C-6 | C-7 | C-8 | C-8′ |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| (ppm) | 159.5 | 124.0 | 136.7 | 118.8 | 139.6 | 138.9 | 143.1 | 116.3 | 146.3 | |
| H-3 | 6.75 | α | linked | α | β | - | - | - | - | - |
| H-4 | 7.77 | β | α | linked | α | β | - | - | - | β |
| H-8 | 8.23 | - | - | - | β | - | β | α | linked | α |
The mass spectrum also confirmed the proposed structure, where it is observed as the base peak, the fragment [M-1]+• at 250.9, characteristic for phenolic compounds.
2.2. In Vitro Antifungal Evaluation and SAR Study
From Table 2, the in vitro antifungal activity of the commercial (1–10) and synthesized coumarins (11–24) was investigated using two Aspergillus species, including four Aspergillus fumigatus strains (ATCC 16913, ATCC 40640, ATCC 46913, and IPP 210) and four Aspergillus flavus strains (ATCC 16013, LM-247, LM-210, and LM-26). The chemical structure, and Minimum Inhibitory Concentration (MIC) values, expressed in micrograms per milliliter compared with amphotericin B (AmpB) are presented.
Table 2.
Chemical structures and in vitro antifungal activity of compounds 1–24 against A. fumigatus and A. flavus.
| A. fumigatus | A. flavus | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Compound | Chemical Structures | ATCC 16913 (log1/cMIC) | IPP 210 | ATCC 46913 | ATCC 40640 | LM 247 | ATCC 16013 | LM 210 | LM 26 |
| 1 |
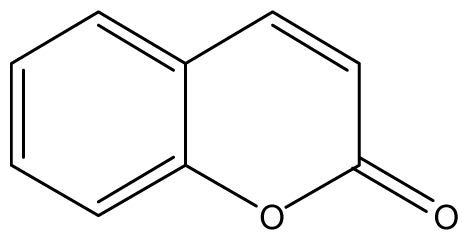
|
1024 (2.15) | 1024 | 1024 | 1024 | 1024 | 1024 | 1024 | 1024 |
| 2 |
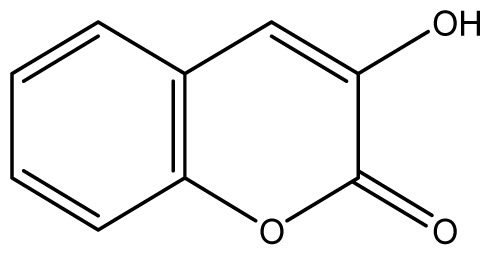
|
64 (3.40) | 64 | 64 | 64 | 128 | 128 | 128 | 128 |
| 3 |
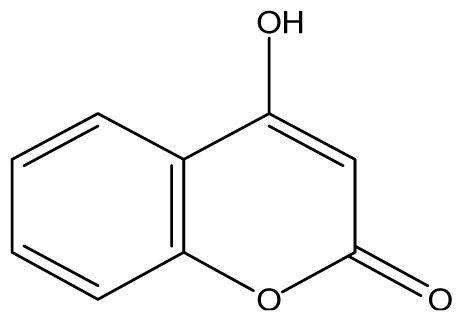
|
≥2048 (1.89) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 4 |
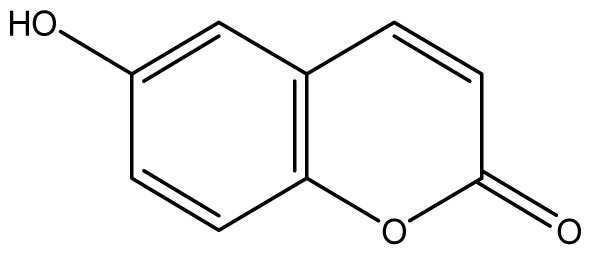
|
≥2048 (1.89) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 5 |
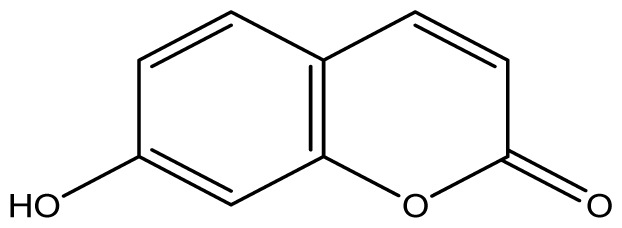
|
≥2048 (1.89) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 6 |
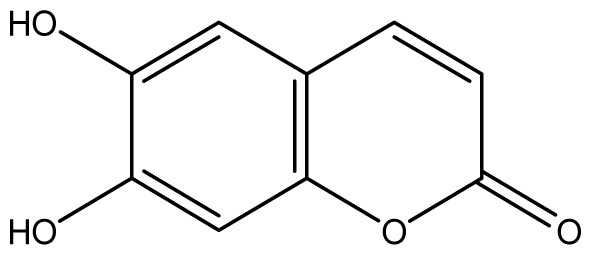
|
≥2048 (1.93) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 7 |
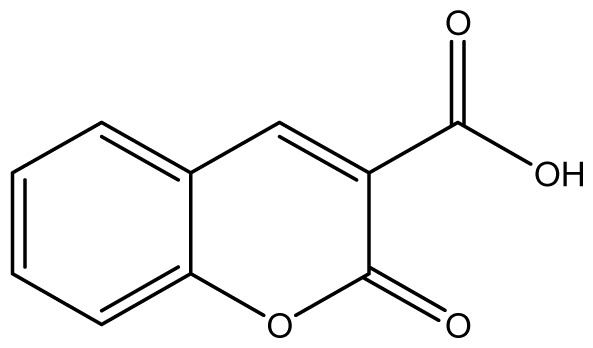
|
1024 (2.26) | 1024 | 1024 | 1024 | ≥2048 | 1024 | 1024 | 1024 |
| 8 |
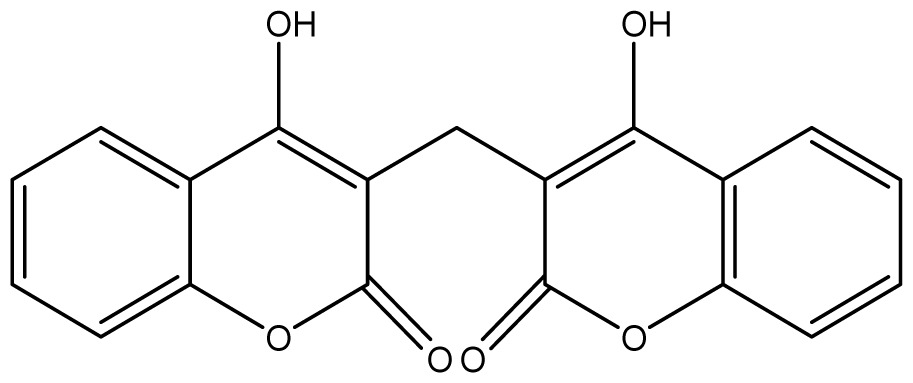
|
≥2048 (2.21) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 9 |
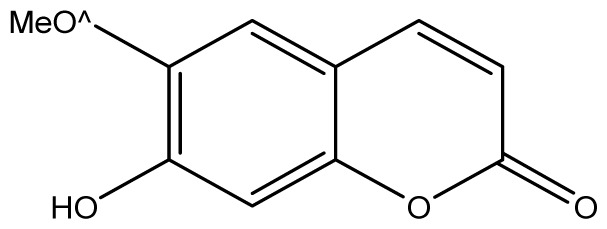
|
≥2048 (1.97) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 10 |
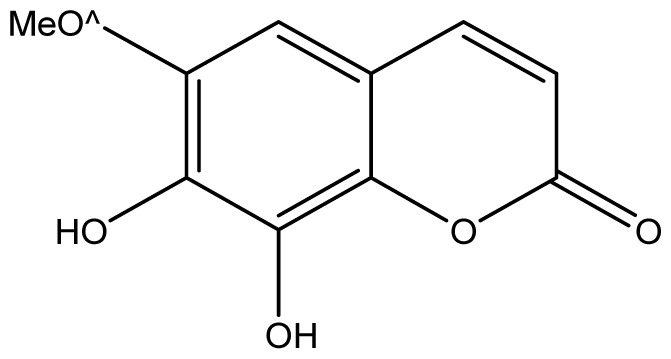
|
≥2048 (2.00) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 11 |
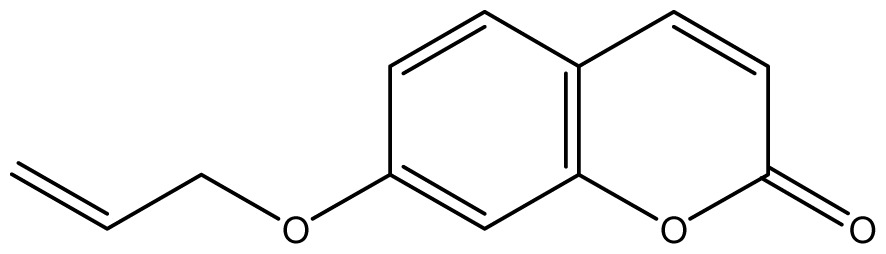
|
64 (3.49) | 64 | 64 | 64 | 256 | 64 | 64 | 64 |
| 12 |
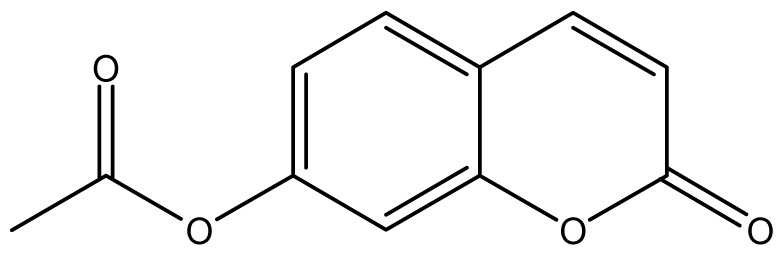
|
64 (3.50) | 64 | 64 | 64 | 128 | 64 | 64 | 64 |
| 13 |
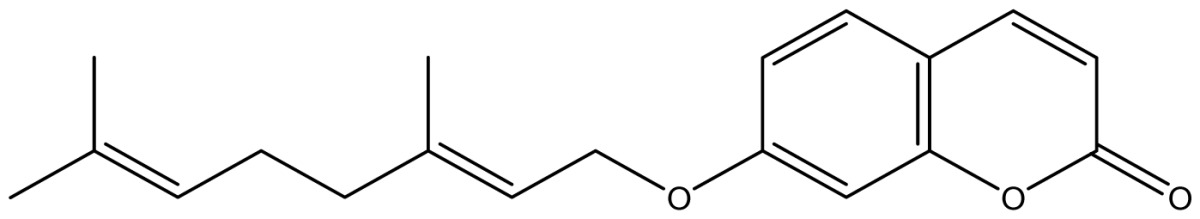
|
≥2048 (2.16) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 14 |
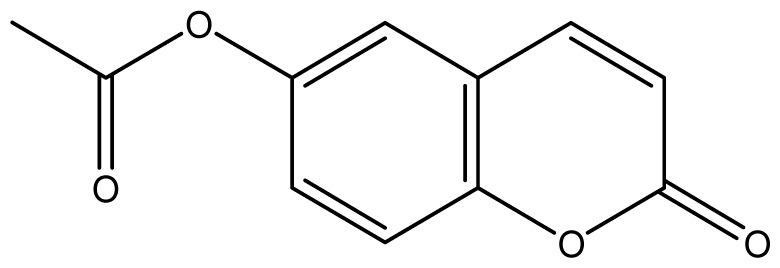
|
256 (2.89) | 256 | 256 | 256 | 256 | 256 | 256 | 256 |
| 15 |
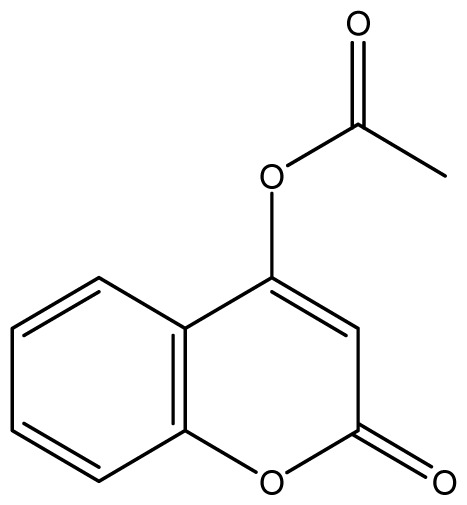
|
16 (4.10) | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| 16 |
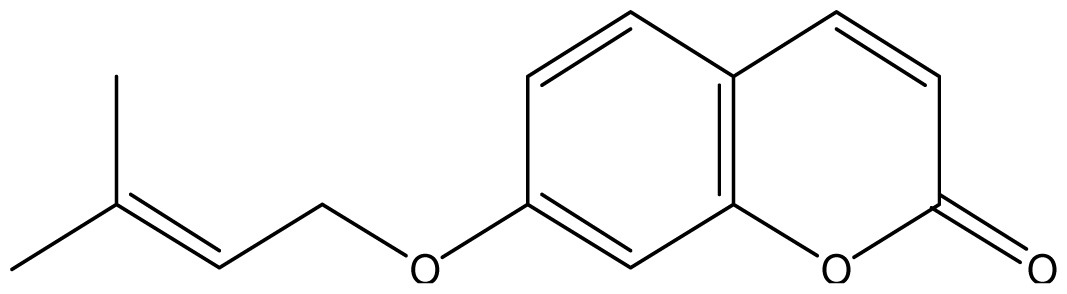
|
128 (3.25) | 128 | 128 | 128 | 1024 | 1024 | 1024 | 1024 |
| 17 |
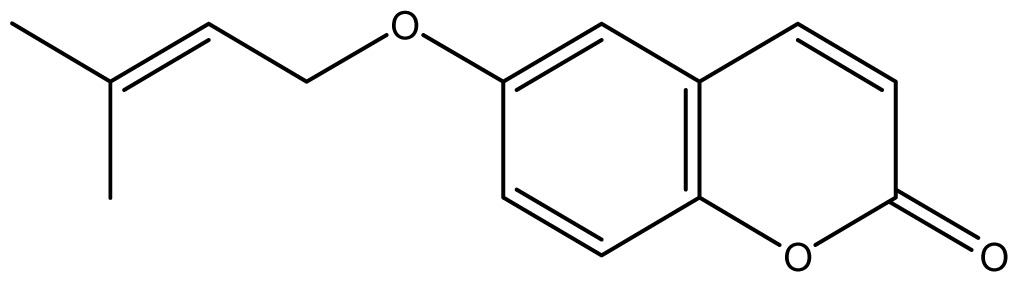
|
≥2048 (2.05) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 18 |
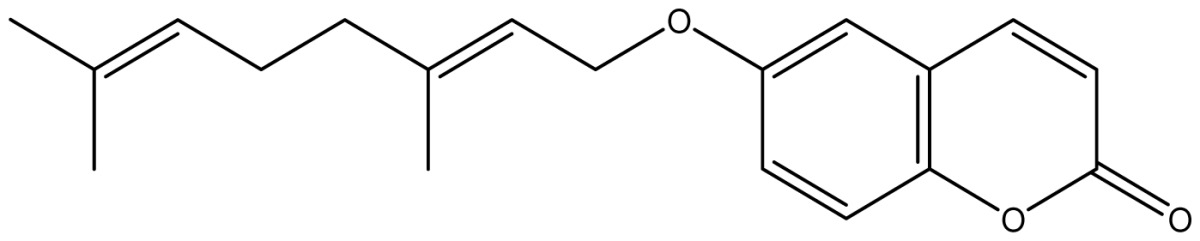
|
≥2048 (2.16) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 19 |
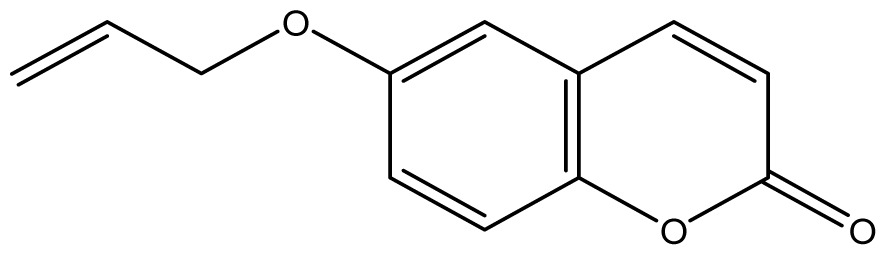
|
≥2048 (1.99) | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 | ≥2048 |
| 20 |
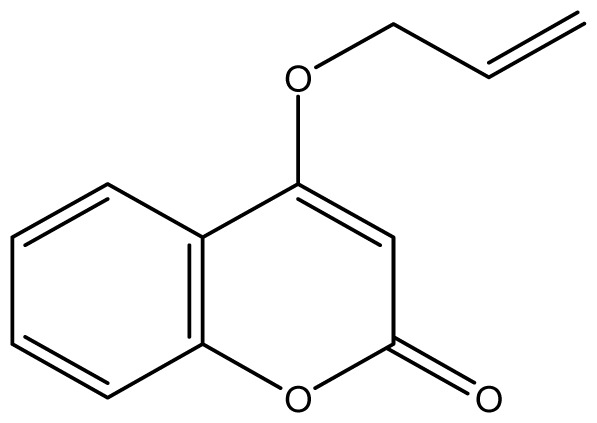
|
32 (3.80) | 64 | 32 | 32 | 1024 | 32 | 32 | 64 |
| 21 |
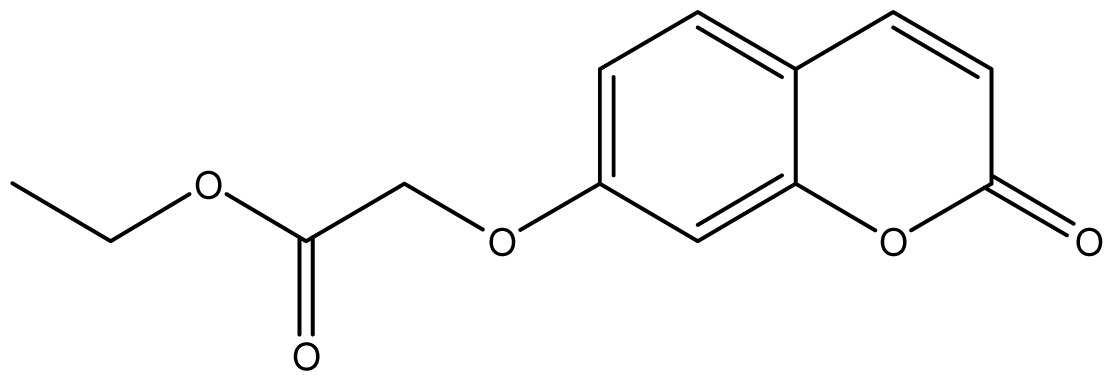
|
512 (2.68) | 512 | 512 | 512 | 512 | 512 | 512 | 512 |
| 22 |
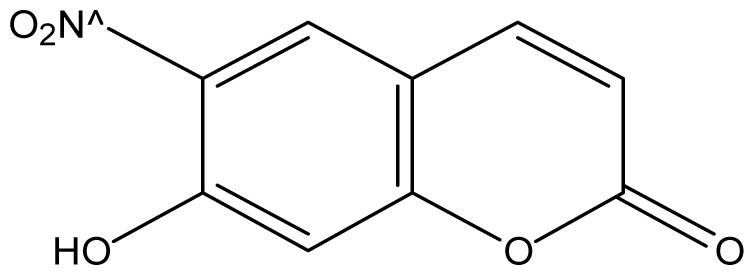
|
16 (4.11) | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| 23 |
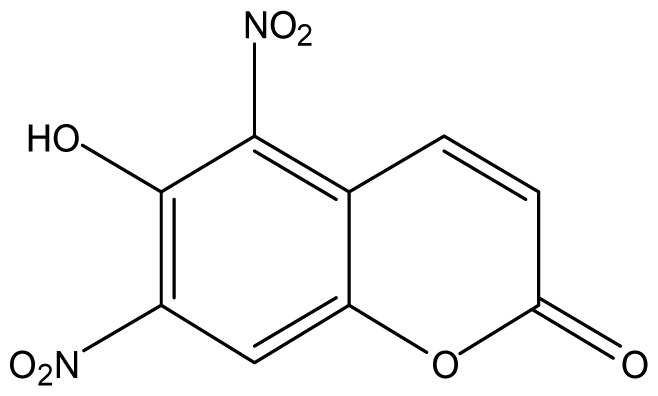
|
512 (2.69) | 512 | 512 | 512 | 512 | 512 | 512 | 512 |
| 24 |
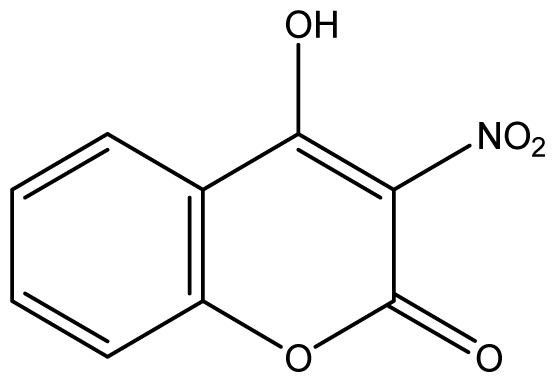
|
1024 (2.30) | 1024 | 1024 | 1024 | 1024 | 1024 | 1024 | 1024 |
| AmpB* | 2 | 2 | 2 | 2 | 8 | 2 | 2 | 512 | |
AmpB, Amphotericin B.
The data presented in Table 2 show that commercial coumarins (1–10) are generally ineffective against the tested strains (MIC ≥ 1024 μg/mL), with the exception of coumarin 2 which presented moderately strong activity (MIC = 64 μg/mL for A. fumigatus, and 128 μg/mL for A. flavus). This difference in sensitivity between the two species was also observed for derivative 16 (MIC = 128 μg/mL for A. fumigatus, and 1024 μg/mL for A. flavus), and for the reference drug Amphotericin B (AmpB) with an MIC of 2 μg/mL for A. fumigatus, and between 2 and 512 μg/mL for A. flavus).
Despite the apparent similar sensitivity profile, it is observed that for one sixth of compounds (7, 11, 12 and 20) the Aspergillus flavus strain LM-247 proved to be more resistant than the other A. flavus strains, with MIC values of 2 to 32 times higher.
Most derivatives did not compare well to the antifungal activity of AmpB, however nine compounds showed MIC values that were either lower (2, 11, 12, 14, 15, 20, 22), or equal (21, 23) to the reference drug (AmpB) for the strain LM-26 which is AmpB-resistant).
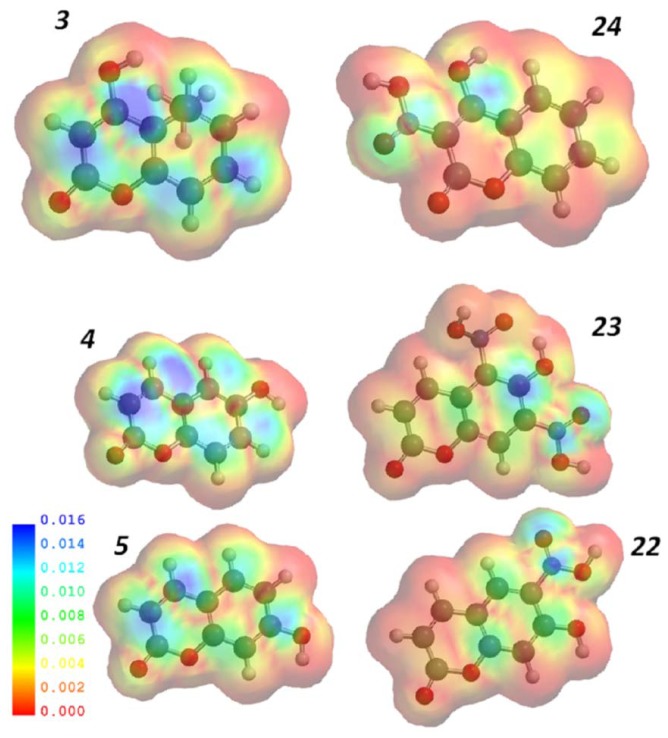
The introduction of electron withdrawing groups to the coumarin skeleton appears to contribute positively to the fungicidal activity, as seen with all the nitro-derivatives evaluated. Nitration of the inactive compounds (3, 4 and 5) resulted respectively in derivatives: 24 (MIC = 1024 μg/mL), 23 (MIC = 512 μg/mL), and 22 the most active compound (MIC = 16 μg/mL) each derivative being (respectively) 2, 4, and 128 times more active than its precursor. DFT confirmed these findings. In the LUMO density surfaces we highlight the differences between active (nitro-coumarins) and inactive compounds. The active compounds have stronger red regions (lower electronic concentration). Although the electrostatic potential map also represents the electronic concentration, LUMO density better demonstrates the ability of one molecule to receive electrons (Figure 1).
Figure 1.
Representation of LUMO density surfaces for active (22–24) and inactive (3–5) compounds. Red regions represent lower electronic concentrations.
The positive influence of electron withdrawing groups can also be observed for the three series (4-, 6- and 7-hydroxycoumarins) of synthesized compounds. In every case it was observed that the compounds resulting from acetylation (respectively 15, 14, and 12), are the most active from each series. Compound 15 was as active as compound 22 (the most active compound). The hypothesis that electron withdrawing groups favor antifungal activity corroborates the observations found with PLS analysis (Section 2.3.), where it was found that the interaction of compounds with the probe N1, increases with antifungal activity.
Analyzing the other replacements performed (introduction of geranyl, prenyl and allyl groups), we observed differing patterns of activity for the 6-hydroxy- and 7-hydroxy-coumarin derivatives. With the 6-hydroxy-coumarin derivatives, substitutions did not result in increased fungicidal activity, all compounds showed to be inactive (17, 18 and 19). This was also observed in previous studies [17]. However for the 7-hydroxy-coumarin series, there is a clear relationship between the size of the introduced group and the fungicidal activity. Reduction of the alkenyl side-chain length increases the activity proportionately, so that the compound having the largest radical (geranyl) is inactive (13 MIC ≥ 2048 μg/mL), the compound with an intermediate side-chain (prenyl) showed strong to moderate activity (16 MIC = 1024 μg/mL for A. fumigatus and 128 μg/mL for A. flavus), and the compound with the smallest aliphatic chain displayed the best activity profile (11 MIC = 64 μg/mL). These results are in agreement with studies observed by Jurd et al. [16] who observed that the antifungal activity of umbelliferone (7-hydroxy-coumarin) may be increased by O-alkylation, and O-acylation with shorter alkyl and acyl groups.
2.3. Computational Studies
For performing chemometric analysis (Principal Component Analysis (PCA)), and Partial Least Square Regression (PLS) the MIC values for Aspergillus fumigatus ATCC-16913 were used [21,39,40]. Inhibitory activity data of the investigated compounds determined as micrograms per milliliter were converted to the negative logarithms of molar MICs (log1/cMIC) (Table 2) which was used as a dependent variables set in this study. Principal component analysis (PCA) and partial least squares (PLS) are chemometric tools for extracting and rationalizing the information from any multivariate description of a biological system. Complexity reduction and data simplification are two of the most important features of such tools. These chemometric tools were developed in the Pentacle software [41]. The Pentacle software is a computational tool for computing alignment-free molecular descriptors, also called GRid-INdependent descriptors or GRIND. The software is based on Molecular Interaction Fields, describe the ability of the molecules to interact with other molecules and do not require to superimpose the compounds. GRIND descriptors are highly relevant for describing biological properties of compounds [42] (see item 3.4. Molecular modeling and electronic surfaces).
Principal Component Analysis (PCA) and Partial Lest Squares Regression (PLS)
The PCA is a technique used to reduce the principal matrix information, splitting into two smaller matrices called loading and score: the matrix loading (P) contains information about the variables and is composed of vectors (principal components, PCs) which are obtained from the original variables; the matrix score (T) contains information about the objects. Each object is described in terms of the projections from the PC instead of the original variables.
Preliminary exploratory analysis was developed using PCA and considering 405 independent variables or descriptors. Two significant principal components (PCs) explain more than 60% of the total variance (Table 3).
Table 3.
Explained variance using PCA.
| PC | % explained variance from original data |
|---|---|
| 1 | 50.04 |
| 2 | 10.62 |
| 3 | 7.96 |
| 4 | 5.80 |
| 5 | 3.54 |
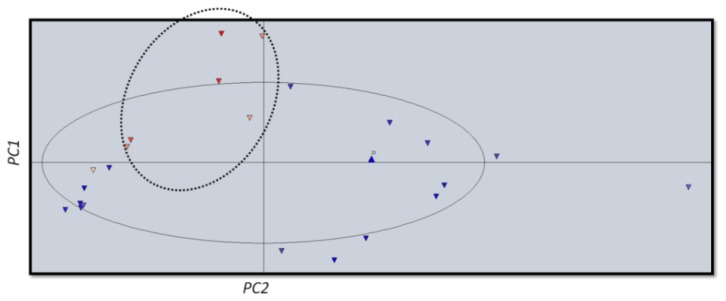
Score and loading plots are interconnected until any descriptor change in the loading plot is reflected by changes in the position of compounds in the score plot [43]. The score plot exhibits satisfactory discrimination between more potent (blue) and less potent (red) compounds. The less active compounds are concentrated in the upper left quadrand (Figure 2).
Figure 2.
Scores plot from PCA. Red triangle represents less active and blue triangle represents more active compounds.
The best model from PLS was obtained with three LVs and 72 descriptors selected from 405. The variable selection via fractional factorial design (FFD), which evaluates the effect on the model standard derivation of error of prediction (SDEP) of every single variable and variable combination. The GRIND descriptors condensed represent a small number of principal properties (GRIND-PP), requiring a biologically relevant description of the molecular similarity. Two significant latent variables emerged from PLS model and leave-one-out [44]. The best model showed an r2 of 0.86, and a q2cv of 0.64. Figure 3 represent the score plot obtained with PLS.
Figure 3.
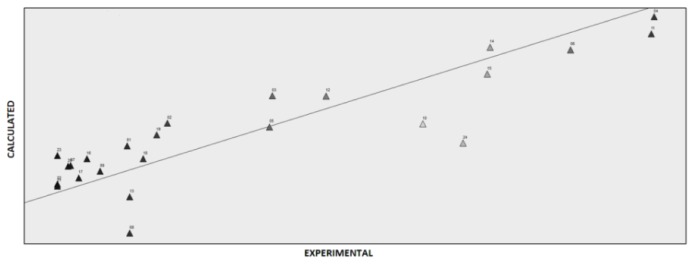
Best fit model obtained in PLS.
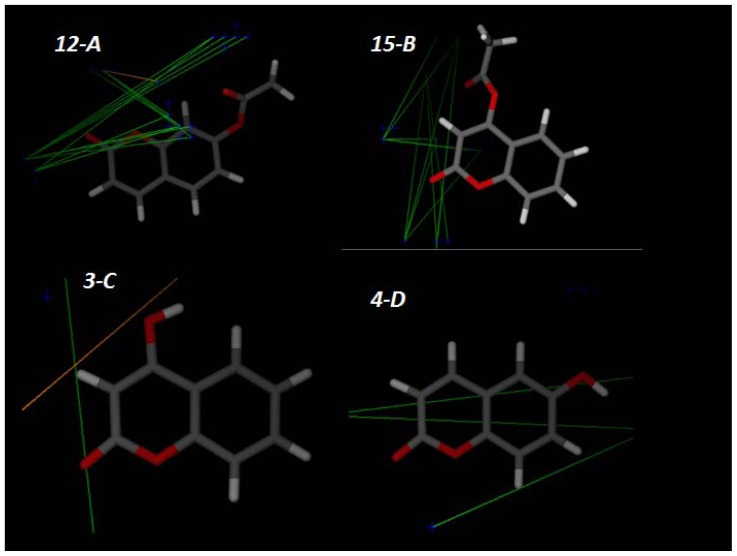
The principal descriptors highlighted in the PLS model were: 350 (5.6–6 A), 531 (10.8–11.2 A), 527 (7.2–7.6 A), and 201 (13.2–13.6 A) were generated through interactions with the probe TIP (shape), the molecular contour also appears to be important. The molecular descriptors obtained can be observed in graphical diagrams called “correlograms”. We observed the largest number of interactions of active compounds with the probe N1. This may indicate that coumarins devoid of electrons have greater attraction with electronegative atom of this probe. Otherwise occurs in inactive compounds. The Figure 4 shows the active compounds 12 and 15 and their interactions with N1. Comparatively, we can see the inactive compounds (3 and 4). Inactive interactions are smaller than active ones, corroborating the fact that electron withdrawing groups increases the antifungal activity.
Figure 4.
Examples of important structural features for antifungal activity, highlighted by the PLS analysis: (A) and (B) 12 and 15, respectively—active compounds; (C) and (D) 3 and 4—inactive compounds, respectively. N1–TIP interactions (orange), N1–N1 (green) interactions.
3. Material and Methods
3.1. General Methods
All synthetic coumarins (1,2-Benzopyrone 1; 3-Hydroxycoumarin 2; 4-Hydroxycoumarin 3; 6-Hydroxycoumarin 4; 7-Hydroxycoumarin 5; 6,7-Dihydroxycoumarin 6; Coumarin-3-carboxylic acid 7; 3,3′-Methylene-bis-(4-hydroxycoumarin) 8; 6-Methoxy-7-hydroxycoumarin 9 and 7,8-Dihydroxy-6-methoxycoumarin 10), reagents and solvents were purchase from Sigma-Aldrich (Seelze, Germany), and used without further purification. All reactions were monitored by thin-layer chromatography (TLC) on pre-coated silica gel GF254 plates (Fluka, St. Gallen, Switzerland). Melting points were determined on a Fisaton 430 apparatus (Fisaton, São Paulo, Brazil) using open capillaries, and the reports values are uncorrected. Acetylation reactions were performed with an ultrasound USC-1400A (40 kHz, Unique, Indaiatuba, Brazil). Infrared (IR) spectra were recorded using potassium bromide pellets on a Bruker IFS-66 IR spectrometer (Bruker, San Francisco, CA, USA), with the frequencies expressed in cm−1. NMR were recorded on a Varian Unity Plus 300, 400 and 500 MHz spectrometer (Varian, Palo Alto, CA, USA), using TMS as an internal standard. Peak assignment in 13C spectra are based on 2D HSQC and HMBC spectra. Chemical shifts were reported in ppm (δ), and coupling constants (J) were reported in Hertz. Signals were designated as follows: s, singlet; d, doublet and bs, broad. HRMS was recorded on a MicroTOF mass spectrometer (ESI) (Bruker). Low-resolution ESI mass spectra was recorded on a Amazon X (Bruker). Elemental analysis was performed using an EA 1110 CHNS-O elemental analyzer (CE instruments, Wigan, UK).
3.2. Synthesis
3.2.1. General Alkylation Procedure
A mixture of commercial coumarins (3, 4 or 5) (3 mmol), K2CO3 (3.9 mmol), and an alkyl halides (3.9 mmol) in anhydrous acetonitrile (20 mL) was stirred in reflux for 22–46 h, and monitored by TLC. The reaction was filtered and washed two times with ethyl acetate (10 mL). The organic phase was washed with water (50 mL), dried over Na2SO4, and then evaporated in vacuum. The residue obtained was recrystallized using Methanol/THF mixtures to give the compounds (by % yield/time reaction): 11 [25] (56.7/37 h); 13 [26] (45.5/27 h); 16 [26] (90/22 h); 17 [27] (86/22 h); 18 [28] (13.5/46 h); 19 [29] (66.7/35 h); 20 [30] (78/44 h), and 21 [31] (98/38 h) as white solids.
3.2.2. General Acetylation Procedure
Coumarins (3, 4 and 5) (5 mmol), Ac2O (50 mmol), and pyridine (5.5 mmol) were mixed in a Schlenk in an ultrasound bath, at room temperature for the appropriate time (monitored by TLC). After completion, the reaction was quenched with cold water (20 mL). The mixtures were filtered, washed three times with water (20 mL), and dried in a desiccator giving pure solid products (% yield/time reaction): 12 [34] (90/15 min); 14 [17] (76.7/15 min) and 15 [33] (86/30 min).
3.2.3. General Nitration Procedure
Nitro-coumarins were synthesized by standard nitration procedures [37], using a mixture of nitric/acetic acids in an ice bath for 30 min., and then at room temperature for an additional 90 min. affording: 22 [35] (84%), 23 (70%) and 24 [36] (53.3%).
5,7-Dinitro-6-hydroxycoumarin (23): Yield 70%; mp: 155–157 °C; 1H NMR [500 MHz, δ (ppm), cd3od]: 6.75 (d, J = 10.0 Hz, 1H, H-3), 7.77 (d, J = 10 Hz, 1H, H-4), 8.23 (s, 1H, H-8), 10.65 (bs, OH); 13C NMR [100MHz, δ (ppm), cd3od]: 116.3 (C-8), 118.8 (C-4′), 124.0 (C-3), 136.7 (C-4), 138.9 (C-6), 139.6 (C-5), 143.1 (C-7), 146.3 (C-8′), 159.5 (C-2); MS (EI) m/z (relative intensity) 250.9 ([M-1]+•, 100), 220.9 (40), 190.9 (10). HRMS [ESI (m/z)] calcd for C9H4N2O7 = 252.0019, found for [M-1]+• = 250.9517; Anal. Calcd for C9H4N2O7: C, 42.87; H, 1.60; N, 11.11; O, 44.42; Found: C, 42.87; H, 1.61; N, 11.14; O, 44.38.
3.3. Antifungal Activity
The in vitro antifungal activity of the studied compounds was investigated using eight Aspergillus strains, including four strains of Aspergillus fumigatus (ATCC 16913, ATCC 40640, ATCC 46913 and IPP 210), and four strains of Aspergillus flavus (ATCC 16013, LM-247, LM-210 and LM-26). These strains were supplied by the URM Culture Collection of the Department of Mycology, Department of Pharmaceutical Sciences of the Federal University of Paraiba, Brazil. The fungi were maintained on potato dextrose agar (PDA)—Difco®—at 28 °C and 4 °C until testing procedures.
Stock cultures were kept on sterile Sabouraud Dextrose Agar (SDA) slants under 7 °C (±1 °C). For preparing the inoculum of the anti-mold assays, we used 7 day-old cultures grown on sterile SDA at 25–28 °C. After the incubation period, the mold conidia were removed by adding sterile NaCl 0.85% to the growth media followed by gentle shaking for 30 s. Mold conidia were counted using a hemocytometer. The conidial suspension inoculum was adjusted using sterile NaCl 0.85%, for approximately 5 × 106 conidia/mL.
MIC values were determined by the microdilution broth method using 96-wells microplates [45,46]. Conidial suspension from 7-day-old A. flavus culture was prepared and standardized by hemocytometer in sterile NaCl 0.85% for susceptibility testing as described previously. To a 96-well plate Sabouraud broth and coumarins were added at concentrations of 2048 to 8 μg/mL. The MIC determination was conducted with an inoculum of approximately 2.5 × 105 conidia/mL microorganism in each well. The plates were incubated at 25–28 °C for 72 h. Within 72 h (and confirmed at 7 days) there was visible fungal growth. The MIC was defined as the lowest concentration of antifungal agent that completely inhibited the growth of the fungi, as detected by the unaided eye [47]. Amphotericin B was used as the reference fungicide.
3.4. Molecular Modeling and Electronic Surfaces
Using the program Hyperchem version 8.0 [48], the chemical structures of the compounds of interest were drawn and their geometry was optimized using MM+ force field [49]. Afterwards, we performed a new geometry optimization using the semi-empiric method RM1 (Recife Model 1) [50]. The RHF (Restricted Hartree-Fock) level was used. The optimised structures were subjected to conformational analyses using the random search method [22,51] with 1000 interactions, 100 cycles of optimisation, and 10 lowest minimum energy conformers. The selected dihedrals were evaluated by rotation in accordance with the standard (default) conditions of the program, in which the number of simultaneous variations was 1 to 8, and acyclic chains were submitted to rotations from 60 to 180°, while torsion rings were in the range of 30° to 120°.
The lowest energy conformers selected were saved in. sdf format with the Spartan for Windows 8.0 program [52], and then exportedto the Pentacle 1.5 program [41], PCA and PLS methodologies were carried out. The Pentacle software is a computational tool for computing alignment-free molecular descriptors, also called GRid-Independent descriptors or GRIND. The software is based on Molecular Interaction Fields and describes the ability of the molecules to interact with other molecules without requiring compound superimposition. Aims to find the underlying relationship between the structure of a molecule and its binding affinity (or other biological properties) using information extracted from molecular descriptors. The calculation of descriptors includes the hydrophobic probe (DRY), the hydrogen bond acceptor probe (O), the shape probe (TIP), and the hydrogen bond donor probe (N1) [42].
The electronic surfaces were calculated in Spartan for Windows 8.0. The compared gradient was 1.02893 ×10−7 (red) 0.0161204 ×10−7 (blue).
4. Conclusions
Synthesis and antifungal evaluation of twenty-four coumarin derivatives against Aspergillus fumigatus and A. flavus are described. Some derivatives showed significant antifungal activities with MICs values ranging between 16 and 32 μg/mL, including seven compounds (2, 11, 12, 14, 15, 20 and 22) which were more active than the reference drug Amphotericin B for the LM-26 strain (A. flavus).
SAR study permitted two conclusions: O-substitution is essential for antifungal activity and the presence of a short aliphatic chain (geranyl<prenyl<allyl), and/or electron withdrawing groups (NO2 and/or acetate) favors activity.
In parallel, the compounds were submitted to the chemometric tools: PCA and PLS, using the program Pentacle. The results were satisfactory and corroborated the SAR analysis. The most active compounds showed greater interaction with the probe N1, which shows greater electronic concentration. These findings were also confirmed by density functional theory (DFT), calculating the LUMO density. Descriptors generated by the probe TIP were also highlighted, indicating that the molecular contour is indeed important for the antifungal activity, as observed in the SAR analysis.
These results suggest derivatives of coumarin as promising compounds for the development of new anti-Aspergillus agents.
Acknowledgments
We are indebted to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), and to UEPB, through the Programa de Incentivo à Pós-Graduação e Pesquisa (PROPESQ) for financial support. The authors also thank both the CNPQ/FAPESQ and INCT, if for scholarships; Rodrigo Santos Aquino de Araújo and Luciana Scotti.
Footnotes
Sample Availability: Samples of the compounds 11–24 are available from the authors.
References
- 1.Mendonça F.J.B., Junior, Lima-Neto R.G., Oliveira T.B., Lima M.C.A., Pitta I.R., Galdino S.L., Cruz R.M.D., Araújo R.S.A., Neves R.P. Synthesis and evaluation of the antifungal activity of 2-(substituted-amino)-4,5-dialkyl-thiophene-3-carbonitrile derivatives. Lat. Am. J. Pharm. 2011;30:1492–1499. [Google Scholar]
- 2.Krappmann S. Tools to study molecular mechanisms of Aspergillus pathogenicity. Trends Microbiol. 2006;14:356–364. doi: 10.1016/j.tim.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Hedayati M.T., Pasqualotto A.C., Warn P.A., Bowyer P., Denning D.W. Aspergillus flavus: Human pathogen, aleergen and mycotoxin producer. Microbiology. 2007;153:1677–1692. doi: 10.1099/mic.0.2007/007641-0. [DOI] [PubMed] [Google Scholar]
- 4.Maggi F., Barboni L., Caprioli G., Papa F., Ricciutelli M., Sagratini G., Vittori S. HPLC quantification of coumarin in bastard balm (Melittis melissophyllum L., Lamiaceae) Fitoterapia. 2011;82:1215–1221. doi: 10.1016/j.fitote.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Olmedo D., Sancho R., Bedoya L.M., López-Pérez J.L., Olmo E., Muñoz E., Alcamí J., Gupta M.P., Feliciano A.S. 3-Phenylcoumarins as inhibitors of HIV-1 replication. Molecules. 2012;17:9245–9257. doi: 10.3390/molecules17089245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahebkar A. Citrus auraptene: A potential multifunctional therapeutic agent for nonalcoholic fatty liver disease. Ann. Hepatol. 2011;10:575–577. [PubMed] [Google Scholar]
- 7.Onuma K., Suenaga Y., Sakaki R., Yoshitome S., Sato Y., Ogawara S., Suzuki S., Kuramitsu Y., Yokoyama H., Murakami A., et al. Development of a quantitative bioassay to assess preventive compounds against inflammation-based carcinogenesis. Nitric Oxide. 2011;25:183–194. doi: 10.1016/j.niox.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Hamdi N., Al-Ayed A.S., Said R.B., Fabienne A. Synthesis and characterization of new thiazolidinones containing coumarin moieties and their antibacterial and antioxidant activities. Molecules. 2012;17:9321–9334. doi: 10.3390/molecules17089321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim N.M. The behavior of certain coumarins and furanocoumarins toward sulfur reagents. Phosphorus Sulfur. 2006;181:1773–1784. [Google Scholar]
- 10.Benci K., Mandić L., Suhina T., Sedić M., Klobučar M., Pavelić S.K., Pavelić K., Wittine K., Mintas M. Novel coumarin derivatives containing 1,2,4-triazole, 4,5-dicyanoimidazole and purine moieties: Synthesis and evaluation of their cytostatic activity. Molecules. 2012;17:11010–11025. doi: 10.3390/molecules170911010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mladenović M., Vuković N., Sukdolak S., Solujić S. Design of novel 4-hydroxy-chromene-2-one derivatives as antimicrobial agents. Molecules. 2010;15:4294–4308. doi: 10.3390/molecules15064294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johann S., Mendes B.G., Missau F.C., Resende M.A., Pizzolatti M.G. Antifungal activity of five species of Polygala. Braz. J. Microbiol. 2011;42:1065–1075. doi: 10.1590/S1517-838220110003000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daoubi M., Durán-Patrón R., Hmamouchi M., Hernández-Galán R., Benharref A., Collado I.G. Screening study for potential lead compounds for natural product-based fungicides: I. Synthesis and in vitro evaluation of coumarins against Botrytis cinerea. Pest Manag. Sci. 2004;60:927–932. doi: 10.1002/ps.891. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu B.-I., Miyagawa H., Ueno T., Sakata K., Watanabe K., Ogawa K. Morning glory systematically accumulates scopoletin and scopolin after interaction with Fusariumoxysporum. Z. Naturforsch. 2005;60:83–90. doi: 10.1515/znc-2005-1-216. [DOI] [PubMed] [Google Scholar]
- 15.Creaven B.S., Egan D.A., Kavanagh K., McCann M., Mahon M., Noble A., Thati B., Walsh M. Synthesis and antimicrobial activity of copper(II) and silver(I) complexes of hydroxyl-nitrocoumarins: X-ray crystal structures of [Cu(hnc)2(H2O)2]·2H2O and [Ag(hnc)] (hnc = 4-hydroxy-3-nitro-2H-chromen-2-one) Polyhedron. 2005;24:949–957. [Google Scholar]
- 16.Jurd L., King A.D., Mihara K., Jr Antimicrobial properties of umbelliferone derivatives. Phytochemistry. 1971;10:2965–2970. [Google Scholar]
- 17.Jurd L., Corse J., King A.D., Bayne H., Jr, Mihara K., Jr Antimicrobial properties of 6,7-dihydroxy-, 7,8-dihydroxy-, 6-hydroxy- and 8-hydroxycoumarins. Phytochemistry. 1971;10:2971–2974. [Google Scholar]
- 18.Scotti L., Scotti M.T., Lima E.O., Silva M.S., Lima M.C.A., Pitta I.R., Moura R.O., Oliveira J.G.B., Cruz R.M.D., Mendonça-Junior F.J.B. Experimental methodologies and evaluations of computer-aided drug design methodologies applied to a series of 2-aminothiophene derivatives with antifungal activities. Molecules. 2012;17:2298–2315. doi: 10.3390/molecules17032298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima-Neto R.G., Cavalcante N.N.M., Srivastava R.M., Mendonça-Júnior F.J.B., Wanderley A.G., Neves R.P., Dos Anjos J.V. Synthesis of 1,2,3-triazole derivatives and in vitro antifungal evaluation on Candida strains. Molecules. 2012;17:5882–5892. doi: 10.3390/molecules17055882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmo E.S., Lima E.O., Souza E.L., Sousa F.B. Effect of Cinnamomum zeylanicum Blume essential oil on the grow and morphogenesis of some potentially pathogenic Aspergillus species. Braz. J. Microbiol. 2008;39:91–97. doi: 10.1590/S1517-838220080001000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beebe K.R., Pell R.J., Seasholtz M.B. Chemometrics: A Practical Guide. Wiley; New York, NY, USA: 1998. [Google Scholar]
- 22.Cohen N.C. Guidebook on Molecular Modeling in Drug Design. Academic Press; San Diego, CA, USA: 1996. [Google Scholar]
- 23.Chung J.W., Lee K., Neikirk C., Nelson C.M., Priestley R.D. Photoresponsive coumarin-stabilized polymeric nanoparticles as a detectable drug carrier. Small. 2012;8:1693–1700. doi: 10.1002/smll.201102263. [DOI] [PubMed] [Google Scholar]
- 24.Nechifor M. Synthesis and properties of some aromatic polyamides with coumarin chromophores. React. Funct. Polym. 2009;69:27–35. [Google Scholar]
- 25.Magolan J., Coster M.J. Total synthesis of (+)-angelmarin. J. Org. Chem. 2009;74:5083–5086. doi: 10.1021/jo900613u. [DOI] [PubMed] [Google Scholar]
- 26.Epifano F., Genovese S., Squires E.J., Gray M.A. Nelumal A, the active principle from Ligulariane lumbifolia, is a novel farnesoid X receptor agonist. Bioorg. Med. Chem. Lett. 2012;22:3130–3135. doi: 10.1016/j.bmcl.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y., Wang T., Tzeng C., Chang N. Geiparvarin analogues: Synthesis and anticancer evaluation of α-methylidene-γ-butyrolactone-bearing coumarins. Helv. Chim. Acta. 1999;82:191–197. [Google Scholar]
- 28.Josudong K. Trifoliate orange extract with anti-inflammatory activity, with potential anti-inflammatory activity of 7—including Zera Neil oxy coumarin induced Bodies and to include them for the anti-inflammatory drug. 10-2010-0023690. Korean Patent. 2010 Mar 4;
- 29.Adfa M., Hattori Y., Yoshimura T., Komura R., Koketsu M. Antifeedant and termiticidal activities of 6-alkoxycoumarins and related analogs against Coptotermes formosanus Shiraki. J. Chem. Ecol. 2011;37:598–606. doi: 10.1007/s10886-011-9968-6. [DOI] [PubMed] [Google Scholar]
- 30.Avetisyan A.A., Alvandzhyan A.G. Synthesis on the basis of 2H-chromen-2-one and 2H-chromene-2-thione. Russ. J. Org. Chem. 2006;42:1063–1067. [Google Scholar]
- 31.Al-Amiery A.A., Musa A.Y., Kadhum A.A.H., Mohamad A.B. The use of umbelliferone in the synthesis of new heterocyclic compounds. Molecules. 2011;16:6833–6843. doi: 10.3390/molecules16086833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martines M.A.U., Davolos M.R., Jafelicci M., Júnior O efeito do ultra-som em reações químicas. Quim. Nova. 2000;23:251–256. [Google Scholar]
- 33.Woods L.L., Fooladi M. 5-Aroyl- (or -Acyl-) 4-hydroxycoumarins. J. Org. Chem. 1968;33:2966–2968. doi: 10.1021/jo01271a081. [DOI] [PubMed] [Google Scholar]
- 34.He W., Zhang B., Zhou S., Sun X., Zhang S. Facile total synthesis of xanthoxol. Synth. Commun. 2007;37:361–367. [Google Scholar]
- 35.Selvam J.J.P., Suresh V., Rajesh K., Reddy S.R., Venkateswarlu Y. Highly efficient nitration of phenolic compounds by zirconyl nitrate. Tetrahedron Lett. 2006;47:2507–2509. [Google Scholar]
- 36.Brady I., Leane D., Hughes H.P., Forster R.J., Keyes T.E. Electronic properties of Ru(II) complexes bound to a bisphenolate bridge with low lying π* orbitals. Dalton Trans. 2004;2:334–341. doi: 10.1039/b312641b. [DOI] [PubMed] [Google Scholar]
- 37.Leite A.C.L., Silva K.P., Souza I.A., Araújo J.M., Brondani D.J. Synthesis, antitumor and antimicrobial activities of new peptidyl derivatives containing the 1,3-benzodioxole system. Eur. J. Med. Chem. 2004;39:1059–1065. doi: 10.1016/j.ejmech.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Lei L., Yang D., Liu Z., Wu L. Mono-nitration of coumarin by nitric oxide. Synth. Commun. 2004;34:985–992. [Google Scholar]
- 39.Sharaf M.A., Illman D.L., Kowalski B.R. Chemometrics. John Wiley & Sons; New York, NY, USA: 1986. [Google Scholar]
- 40.Souza B.B.C., De Oliveira T.B., Aquino T.M., De Lima M.C.A., Pitta I.R., Galdino S.L., Lima E.O., Gonçalves-Silva T., Militão G.C.G., Scotti L., et al. Preliminary antifungal and citotoxic evaluation of synthetic cycloalkyl[b]thiophene derivatives with PLS-DA analysis. Acta Pharm. 2012;62:221–236. doi: 10.2478/v10007-012-0017-y. [DOI] [PubMed] [Google Scholar]
- 41.Pentacle, Version 1.5. Molecular Discovery Ltd.; Pinner, Middlesex, UK: [accessed on 26 September 2012]. Available online: http://www.moldiscovery.com/soft_pentacle.php. [Google Scholar]
- 42.Pastor M., Mclay I., Pickett S., Clementi S. Grid-Independent descriptors (GRIND): A novel class of alignment-independent three-dimensional molecular descriptors. Med. Chem. 2000;43:3233–3243. doi: 10.1021/jm000941m. [DOI] [PubMed] [Google Scholar]
- 43.Crivori P., Cruciani G., Carrupt P.-A., Testa B. Predicting blood-brain barrier permeation from three-dimensional molecular structure. J. Med. Chem. 2000;43:2204–2216. doi: 10.1021/jm990968+. [DOI] [PubMed] [Google Scholar]
- 44.Durán Á., Zamora I., Pastor M. Suitability of GRIND-based principal properties for the description of molecular similarity and ligand-based virtual screening. J. Chem. Inf. Model. 2009;49:2129–2138. doi: 10.1021/ci900228x. [DOI] [PubMed] [Google Scholar]
- 45.Sahin F., Gulluce M., Daferera D., Sokman A., Polissiou M., Agar G., Sharma N., Tripathi A. Effects of Citrus sinensis (L.) Osbeckepicarp essential oil on growth and morphogenesis of Aspergillusniger (L.) Van Tieghem. Microbiol. Res. 2006;163:337–344. doi: 10.1016/j.micres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Moreira A.C.P., Lima E.O., Wanderley P.A., Carmo E.S., Souza E.S. Chemical composition and antifungal activity of Hyptissuaveolens (L.) poit leaves essential oil against Aspergillusspecies. Braz. J. Microbiol. 2010;41:28–33. doi: 10.1590/S1517-83822010000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pereira F.O. M.Sc. Thesis. Post-Graduate Program in Natural and Synthetic Bioactive Produts, Federal University of Paraiba; João Pessoa, Paraíba, Brasil: 2009. Atividade antifúngica do óleo essencial de Cymbopogonwinterianus JowittexBor sobre dermatófitos do gênero Trichophyton. [Google Scholar]
- 48.HyperChem, Version 8.0. Hybercube Inc.; Gainesville, FL, USA: 2009. [Google Scholar]
- 49.Allinger N.L. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977;99:8127–8134. [Google Scholar]
- 50.Rocha G.B., Freire R.O., Simas A.M., Stewart J.J.P. RM1: A reparameterization of AM1 for H, C, N, O, P, S, F, Cl, Br and I. J. Comput. Chem. 2006;27:1101–1111. doi: 10.1002/jcc.20425. [DOI] [PubMed] [Google Scholar]
- 51.Leach A.R. Molecular Modeling: Principles and Applications. Prentice Hall; London, UK: 2001. [Google Scholar]
- 52.Spartan model homepage for windows, Version 8.0. Wavefunction, Inc.; Irvine, CA, USA: 2008. [accessed on 10 September 2012]. Available online: http://www.wavefun.com/products/windows/SpartanModel/win_model.html. [Google Scholar]