Abstract
MicroRNAs (miRNAs) are potent post-transcriptional regulators of gene expression. In mammalian cells, miRNAs typically suppress mRNA stability and/or translation through partial complementarity with target mRNAs. Each miRNA can regulate a wide range of mRNAs, and a single mRNA can be regulated by multiple miRNAs. Through these complex regulatory interactions, miRNAs participate in many cellular processes, including carcinogenesis. By altering gene expression patterns, cancer cells can develop specific phenotypes that allow them to proliferate, survive, secure oxygen and nutrients, evade immune recognition, invade other tissues and metastasize. At the same time, cancer cells acquire miRNA signature patterns distinct from those of normal cells; the differentially expressed miRNAs contribute to enabling the cancer traits. Over the past decade, several miRNAs have been identified, which functioned as oncogenic miRNAs (oncomiRs) or tumor-suppressive miRNAs (TS-miRNAs). In this review, we focus specifically on TS-miRNAs and their effects on well-established cancer traits. We also discuss the rising interest in TS-miRNAs in cancer therapy.
Keywords: post-transcriptional gene regulation, oncomiR, tumor suppressor microRNA, senescence, carcinogenesis
1. Introduction
MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression post-transcriptionally. In mammalian cells, miRNAs suppress the expression of proteins encoded by target mRNAs with which miRNAs interact with incomplete complementarity; typically occurring with the 3′-untranslated region (UTR) of the target mRNA and leading to its degradation and/or its translational suppression [1,2]. The target mRNAs of a given miRNA can be predicted with a relatively high degree of success using sequence algorithms that identify homologies between the “seed” region of the miRNA and the complementary site on the target mRNA. These interactions typically occur between the miRNA seed region and a complementary sequence in the 3′-untranslated region (UTR) of the target mRNA, but they may also take place with the mRNA coding region or 5′UTR, as well as with seedless miRNAs [3,4]. Through their influence on subsets of target mRNAs, miRNAs have emerged as critical regulators of numerous cellular processes, including cell division, differentiation, senescence and apoptosis [5–7]. Thus, dysregulated miRNA expression can have a profound impact upon the cell fate and can lead to the development of pathologies like cancer [8]. Cancer cells typically display special miRNA signatures distinct from those of normal cells; accordingly, some miRNAs are currently used as cancer biomarkers [9–13]. There is strong interest in studying the patterns of miRNAs in cancer, since they can directly influence the production of tumor suppressor proteins and oncoproteins and hence affect tumor development and response to therapy. Some miRNAs are selectively increased in cancer cells, but more often, miRNAs show decreased expression in cancer cells [14–16]. Considering their influence on the cancer cell phenotype, some miRNAs are considered to be oncogenic (oncomiRs), and other miRNAs are considered to be tumor-suppressive (TS-miRNAs).
As proposed by Hanahan and Weinberg, normal cells acquire a number of characteristics as they transform into cancerous cells [17]. For cancer cells to thrive, they must remain in a proliferative state, survive despite adverse surrounding conditions, elicit local angiogenesis, invade other tissues, metastasize and evade recognition by the body’s immune system. Numerous TS-miRNAs are downregulated in cancer tissues; upon re-expression, they suppress various processes relevant to tumorigenesis, including proliferation, apoptosis and migration. In this review, we focus on the influence of TS-miRNAs on the hallmark traits acquired by cancer cells. We consider TS-miRNAs broadly as miRNAs targeting mRNAs that encode proteins, which enable cancer traits. We also discuss the growing efforts to exploit TS-miRNAs in cancer therapy.
2. TS-miRNAs that Suppress Cell Growth and Proliferation
For cells to become a tumor, they must proliferate in order to augment the size of the transformed cell population. Sustained cancer cell proliferation normally requires a continuous supply of proliferative signals. For example, the levels and/or function of proteins that promote cell cycle progression, such as cyclins and cyclin-dependent kinases (cdks), are commonly elevated in cancer cells and result in shorter division periods [18]. In addition, there is increased expression of several other proteins that promote cancer cell growth and proliferation, including those required to sustain proliferative signals, like B cell CLL/lymphoma 2 (Bcl-2), B-cell lymphoma-extra large (Bcl-xL), sirtuin 1 (SirT1), high-motility group AT-hook gene 1 (HMGA1) and other factors discussed below. Factors that stimulate cancer cell growth include the epidermal growth factor (EGF), vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) [19]. In this section, we review examples of TS-miRNAs that block or reduce cancer cell proliferation and growth.
Several TS-miRNAs suppress the expression of one or more of these proliferation enhancers and thus block the growth of different cancers. For example, miR-34a represses the production of Bcl-2 and SirT1, two proteins displaying high levels of expression in cancer cells and implicated in promoting proliferation and cell survival [20–22], although miR-34a does not affect Bcl-2 expression levels in all cell systems [23]. In agreement with the finding that transgenic mice overexpressing AIB1 (Amplified In Breast Cancer 1) developed mammary epithelial cell proliferation, hyperplasia and tumorigenesis, low levels of miR-17-5p in breast cancer correlated with increased expression of the mRNA encoding AIB1, and conversely, miR-17-5p overexpression suppressed cell proliferation [24–26]. Also in breast cancer cells, miR-125a was shown to target the RNA-binding protein HuR, which is essential for proliferation and broadly enhances cancer traits [27,28]. Another miRNA targeting HuR, miR-519 suppresses cell proliferation and inhibits tumorigenesis [29,30]. The effect of miR-519 on cell proliferation was extended to ovarian, lung and kidney tumors, where the low abundance of miR-519 correlated inversely with HuR protein levels [30]. In breast cancer, the promoter of miR-125b was methylated and silenced, which allowed the levels of the miR-125b target oncoprotein (Ets1) to rise. In agreement with the finding that restoring miR-125b expression inhibited breast cancer cell proliferation by blocking Ets1, high levels of Ets1 correlated with poor patient prognosis [31]. miR-125b was also able to block cell proliferation in hepatocellular carcinoma (HCC) by targeting the anti-apoptotic protein Bcl-2 [32]. The influence of miR-125b on tumorigenesis may be cell- and tissue-specific, since it promoted malignant transformation of different hematopoietic lineages in mice and was upregulated in acute megakaryoblastic leukemia [33,34]. By contrast, the levels of miR-1 were drastically low in thyroid adenomas and carcinomas, and in these cells, miR-1 levels were inversely correlated with those of cyclin D1, required for G1/S transition [35].
miR-1 is epigenetically silenced in primary and distant metastatic human prostate tumors. Overexpression of miR-1 disrupted cell cycle progression, which subsequently led to growth inhibition and suppression of prostate cancer xenograft growth [36]. miR-1 expression levels are also reduced in primary human HCC compared with normal liver tissues, and ectopic expression of miR-1 in HCC cells inhibited cell growth and reduced replication potential and clonogenic survival. These effects were associated with inhibition of cell cycle progression and induction of apoptosis [37]. In addition, miR-1 is downregulated in human primary lung cancer tissues and cell lines, and its overexpression in lung carcinoma cell lines A549 and H1299 reduced cell growth and tumor formation in nude mice. These effects were associated with reduced expression of oncogenes, including the receptor tyrosine kinase MET and serine/threonine-protein kinase Pim-1, which are often upregulated in lung cancer and are involved in cell growth and proliferation [38].
miR-28 expression was lower in colorectal cancer than in normal colon, and its restoration led to an inhibition of cell proliferation [39]. Expression of miR-205 was silenced in prostate cancer; since miR-205 transcriptionally activated the expression of tumor suppressors interleukin (IL)-24 and IL-32, miR-205 reintroduction led to the re-expression of IL-24 and IL-32, triggering apoptosis and growth arrest [40]. miR-296 downregulated HMGA1 expression in prostate cancer cells, in turn reducing cell proliferation [41]. In gastric cancer, miR-148b inhibited cell proliferation in vitro and in vivo by lowering the levels of cholecystokinin-B receptor (CCKBR), a protein that promotes tumorigenesis [42].
miR-135a suppressed gastric cell proliferation at least partly by reducing the production of the cytoplasmic tyrosine kinase JAK2 (Janus kinase 2), which influences cell proliferation through its downstream signaling effectors STAT3, cyclin D1 and Bcl-XL [43]. miR-146a suppressed cell proliferation in extranodal NK/T cell lymphoma (NKTL) and was proposed to function as a general TS-miRNA by targeting genes involved in cell proliferation (reviewed in [44,45]). In colon and breast cancer cells, miR-145 suppressed tumor growth indirectly by targeting p70S6K1 (required for expression of VEGF and hypoxia-inducible factor 1 (HIF-1)) and directly by targeting VEGF-A [46,47]. In glioma cells, miR-128 suppressed tumor growth by targeting EGF and PDGF receptors, thus inhibiting mitogenic growth signals [48].
Other proposed TS-miRNAs include miR-101, miR-143, miR-24, miR-133a, miR-133b, miR-138, miR-216b, miR-155, miR-138, miR-508-3p and miR-509-3p, since they suppressed cell proliferation and growth in different cancers, such as bladder transitional cell carcinoma (TCC), laryngeal squamous cell carcinoma (LSCC), esophageal squamous cell carcinoma (ESCC) and melanoma [49–54]. In sum, altered miRNA levels in cancer cells can help to promote cancer progression by increasing cell proliferation. In some instances, the restoration or overexpression of these miRNAs was effective in suppressing cancer proliferation both in vitro and in vivo.
3. TS-miRNAs that Enhance Cell Death
The survival of cancer cells can be enhanced through the accumulation of genetic mutations that lower the levels of some tumor suppressors, like p53 (which triggers growth arrest, senescence and apoptosis), or by altering the expression of tumor suppressor-regulatory proteins, like Mdm2, which enhances p53 degradation [55–58]. In addition, cancer cell survival can be improved by the expression of anti-apoptotic proteins, such as members of the Bcl-2 family. Thus, TS-miRNAs can enhance cancer cell death by regulating anti-apoptotic factors. For example, p53 is induced in response to DNA-damaging agents, such as ionizing radiation, in turn transcriptionally enhancing miR-34a expression. Indeed, irradiated chronic lymphocytic leukemia (CLL) showed higher levels of miR-34a, leading to the induction of Bax and p21, but not Puma. These findings suggest that functional p53 increases miR-34a expression upon DNA damage and that, in turn, miR-34a may block cancer cell growth by triggering cell cycle arrest or apoptosis through the suppression of target proteins, like SirT1, Bcl-2 or cyclin D1 [59]. Since miR-34a targets include the anti-apoptotic protein Bcl-2, baculoviral IAP repeat-containing 3 (BIRC3) and decoy receptor 3 (DcR3), the combined effects of upregulating p53 and downregulating miR-34a targets strongly enhance cancer cell death [20,60–62]. While Bcl-2 inhibits the formation of the apoptosome by blocking the release of cytochrome c from mitochondria, BIRC3 inhibits apoptosis by interacting with the tumor necrosis factor (TNF) receptor-associated factors TRAF1 and TRAF2 [63]. DcR3 is highly expressed in many tumors and suppresses apoptosis by acting as a decoy receptor for ligands that would otherwise trigger cell death by binding to proteins, such as Fas receptor [64]. In cultured brain tumor (glioma) stem cells, miR-34a induced apoptosis through the inhibition of the oncogenic, pro-survival factors c-Met, Notch-1 and Notch-2, which are important for cell survival [65]. miR-34a also targets mRNAs encoding cell cycle regulators necessary for cell division, such as Cyclin D1, Cyclin E2, Cdk4, Cdk6 and E2F [66,67], indicating that cell cycle arrest is also a powerful mechanism through which miR-34a suppresses tumorigenesis. In HCC, miR-125b inhibits cell proliferation, as explained above, and promotes apoptosis by lowering Bcl-2 expression [32], while miR-1 promotes apoptosis, as mentioned above [37]. In glioma cell lines, miR-181d suppressed tumor growth by lowering the levels of both K-Ras and Bcl-2, while in glioblastoma cells, miR-451 reduced the levels of Cyclin D1 and Bcl-2, causing growth arrest and enhancing cell death [68,69]. In cervical cancer cells, miR-519 increased the levels of p53 and p21, causing cell cycle arrest and cellular senescence, and it induced DNA damage by lowering the abundance of several DNA repair enzymes [70,71]. In conclusion, reducing TS-miRNA activity in cancer cells can enable cancer progression by inducing cell survival and reducing apoptosis. Conversely, overexpression of these TS-miRNAs can restore the sensitivity of cancer cells to death signals; in some cases, it can enhance cellular senescence.
4. TS-miRNAs that Suppress Angiogenesis
Cancer cells need to secure a constant supply of nutrients in order to thrive and expand. Growth of the tumor requires enhanced angiogenesis, a process that generates new blood vessels to deliver nutrients and oxygen. This cancer trait is elicited by major angiogenesis factors: vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF-2) and platelet-derived growth factors (PDGF)-B and C [72,73]. The transcription factor HIF-1α is highly expressed in hypoxic conditions and transcriptionally increases VEGF expression in adaptive and neoplastic angiogenesis [74]. In addition, matrix metalloproteinases (MMPs) facilitate angiogenesis by assisting in the degradation of the extracellular matrix (ECM), a process that releases pro-angiogenic growth factors like VEGF and FGF-2 [75,76].
Several TS-microRNAs inhibit this tumor trait. miR-145 suppresses tumor angiogenesis, growth and invasion by lowering the production of the oncogene VEGF-A and by suppressing the expression of the serine/threonine kinase p70S6K1, which promotes HIF-1α and VEGF activities in colon cancer cells [46,47]. Similarly, suppression of p70S6K1 by miR-128 in glioma cell lines led to the downregulation of HIF-1α and VEGF and to the subsequent inhibition of angiogenesis in vivo [77]. In glioma cell lines and tumors, miR-205 was further identified as a direct repressor of VEGF-A and, hence, a suppressor of angiogenesis [78]. miR-519c reduced HIF-1α levels, suppressing tumor formation and angiogenesis in lung and breast cancer cell lines, while it also inhibited the biosynthesis of HuR, which regulates numerous cancer traits, including angiogenesis [27,29,30,70,71,79,80]. In breast cancer cells, miR-340 suppressed tumor cell migration and invasion by lowering the abundance of c-Met, a factor that promotes expression of MMP-2 and MMP-9; MMP-2 was additionally repressed by miR-29b in HCC cells, attenuating HCC cell invasiveness and angiogenic activity [81,82]. Finally, miR-9 lowered MMP-14 in neuroblastoma cells, causing an attenuation of tumor growth, metastasis and angiogenesis in vivo [83]. In sum, downregulation of several TS-miRNAs in cancer cells can augment angiogenesis by promoting the expression of pro-angiogenic factors and enzymes that degrade the ECM. Together, they facilitate the generation of new blood vessels that support the cancer tissue with nutrients and oxygen. Restoration of some of these miRNAs has been shown to suppress the expression of these factors and to attenuate angiogenesis.
5. TS-miRNAs that Enhance Immune Recognition
The immune system is a vital barrier against tumor formation and progression. Constant surveillance by the immune cells leads to eradication of virus-induced and some forms of non-virus-induced tumors. Immunodeficient mice, particularly mice lacking natural killer cells, CD4+ Th1 helper T-cells or CD8+ cytotoxic T-lymphocytes, developed tumors more frequently and faster than control animals [84,85]. A number of tumor-suppressive mechanisms have been identified that enable cancer cells to escape immune system surveillance and render them refractory to immune attack. Tumors can evade immune recognition by overproducing inhibitors of T-cell responses, such as galectins (small proteins involved in immune response, inflammation and tumorigenesis [86]) and indoleamine 2,3-dioxygenase (IDO) [87,88], as well as by increasing the production of immune suppressive cytokines, like transforming growth factor beta (TGF-β) and interleukin 10 (IL-10) [89,90]. Tumors can also suppress proinflammatory signals by activating the signal transducer and activator of the transcription 3 (STAT3) pathway, thereby blocking tumor-specific T-cell responses [91]. Other immune-suppressive mechanisms involve the downregulation of natural killer cell receptor protein G2D (NKG2D) to reduce lymphocyte-mediated cytotoxicity and the generation of active immune-suppressive cells, such as myeloid-derived suppressor cells (MDSCs) [92,93].
TS-miRNAs can inhibit this tumor trait by facilitating the immune response, diminishing immune-suppressive mechanisms and/or suppress the STAT3 pathway. In this regard, miR-322 suppressed the expression of galectin-3 [94], while miR-181a blocked the biosynthesis of TGF-β receptor 1 (TGFBR1) and TGF-β receptor associated protein 1 (TGFBRAP1), suggesting that miR-181a may enhance immune recognition by attenuating the immunosuppressive TGF-β pathway [95]. In neuroblastoma cells, miR-335 inhibited the non-canonical TGF-β pathway by targeting mitogen-activated protein kinase 1 (MAPK1) and Rho-associated coiled-coil-containing protein (ROCK1), leading to the suppression of cell migration and invasion [96]. miR-148a also reduced ROCK1 expression in gastric cancer and suppressed tumor cell invasion and metastasis [97]. Whether these TS-miRNAs increase immune recognition of cancer cells in vivo awaits further study. IL-10 expression is regulated by miR-106a in the T-cell leukemia Jurkat cell line [98]. Although it has not been tested in vivo if miR-106a enhances immune recognition, miR-106a suppressed the growth of glioma cells lines U87 and SHG44 and correlated inversely with glioma tumor grade [99]. Several additional lines of evidence support the notion that TS-miRNAs can target the STAT3 pathway to alleviate immune suppression: (i) let-7a overexpression in hepatoma cells suppressed cell proliferation through STAT3, (ii) miR-17-5p and miR-20a abrogated the immune-suppressive effects of MDSCs in vivo through the regulation of STAT3 and (iii) miR-93 inhibited tumor development and metastasis in mouse xenografts, at least in part by attenuating the TGF-β and/or STAT3 pathways [100–102]. Collectively, these findings indicate that altered miRNA expression is associated with failure of the immune system to recognize and eradicate cancer cells at an early time. Accordingly, increased expression of TS-miRNAs that enhance immune-suppressive mechanisms could strengthen the immune system to recognize cancer cells and eliminate them.
6. TS-miRNAs that Suppress Invasion and Metastasis
Metastasis is often associated with changes in cell morphology and adherence to other cells in the ECM. For instance, during epithelial-to-mesenchymal transition (EMT) or transformation, cancer cells can lose E-cadherin, a protein that strongly represses transformation [103]. This transition is accompanied by increased expression of N-, P- and T-cadherins in cancer cells, which can promote tumor cell invasion, even if E-cadherin function is unaltered [104]. Elevated levels of transcriptional repressors of E-cadherin, such as Snail, Twist and zinc finger E-box binding homeobox (ZEB1/2), are also responsible for EMT [105]. MMPs also facilitate or enhance tumor invasion and metastasis by degrading the extracellular matrix [75,106]. Concurrent inhibition of c-Met and VEGF signaling was recently shown to suppress tumor invasion and metastasis [107]. In addition, factors, such as HMGA1, Bcl-2, SirT1, N-Ras, K-RAS, Ezrin, Mucin 4, E2F and metastasis-associated gene (MTA1), are also involved in tumor invasion and metastasis [108–116]. These factors enhance invasion and metastasis, although they can also play roles in other steps of tumorigenesis.
Several TS-miRNAs have been identified as having an anti-metastatic and anti-invasion influence. miR-9 upregulated E-cadherin and downregulated Snail through its direct effect on NF-κB, leading to inhibition of melanoma growth and metastasis in vivo [117]. miR-200c inhibited EMT and cancer cell migration by suppressing ZEB1 and ZEB2, two suppressors of E-cadherin, as explained above [118]. In breast cancer and melanoma cells, miR-340 and miR-34a lowered c-Met (an inducer of MMP-2 and MMP-9) and, thus, suppressed tumor cell migration and invasion [81,119]. miR-29b inhibited MMP-2 expression, leading to an attenuation of the invasive capacity of HCC cells in vitro [73], while in glioma cell lines and tumors, miR-205 was found to inhibit invasion by targeting VEGF-A [78,82]. In breast cancer cells, miR-145 suppressed growth and invasion through VEGF and N-Ras, while overexpression of miR-183 resulted in reduced migration and invasion, an effect that was attributed in part to the downregulation of villin 2 (Ezrin) [46,120]. Repression of HMGA1 by miR-296 suppressed invasion of prostate cancer [41], while expression of K-Ras, a promoter of migration and invasion, was blocked by miR-96 in pancreatic cancer cells and by miR-216 in nasopharyngeal carcinoma [52,121]. In glioma cells, overexpression of miR-195, a repressor of E2F3 and Cyclin D3, induced cell cycle arrest and inhibited cell invasion, while in endometrial cancer cells, miR-30c repressed MTA1 production and blocked cell proliferation, migration and invasion [122,123]. In pancreatic cancer, miR-150 was identified as a potential tumor suppressor and correlated inversely with the levels of Mucin 4, a factor that promotes growth, invasion and metastasis of cancer cells [124]. miR-1 was found to target the oncogene purine nucleoside phosphorylase (PNP) in prostate cancer, thus suppressing cell migration and invasion, in agreement with the effects of PNP silencing in prostate cancer lines PC3 and DU145 [125].
Taken together, suppression of TS-miRNAs in cancer cells promotes the expression of many pro-invasion and pro-metastatic proteins. Overexpression of some of these miRNAs in cancer cells has shown that they are capable of suppressing tumor invasion and metastasis in vivo.
7. TS-miRNAs in Cancer Therapeutics
As highlighted above and listed in Table 1, several miRNAs were identified as tumor suppressors, because they inhibit one or more cancer traits. These miRNAs are often dysregulated in cancers, and several studies demonstrated that their restoration attenuates tumorigenesis both in cultured cells and in vivo. The discovery of TS-miRNAs has prompted researchers, clinicians and pharmaceutical companies to consider TS-miRNA-based approaches in cancer therapy. Although interventions to modulate a single miRNA or sets of miRNAs alone are unlikely to cure cancer, they are being actively considered in combination with other treatment regimens, including chemotherapy and radiotherapy. Just as miRNA antagonists are being developed to target oncomiRs elevated in cancer, the development of mimics for the downregulated TS-miRNAs is also underway [126,127]. Moreover, interventions to restore lost TS-miRNA activity may hold greater promise than efforts to antagonize endogenous miRNAs, since mimics resemble the native molecules and can control the same range of genes and pathways as those depleted in cancer cells. Mimics of the widely known TS-miRNA miR-34a were investigated for their therapeutic potential in multiple myeloma; the study concluded that miR-34a has therapeutic activity in preclinical models and prompted the development of miR-34a-based treatment in multiple myeloma patients [128]. Another study found that low expression of miR-148a was associated with poor survival rates in patients with advanced colorectal cancer, suggesting that miR-148a may be used to improve colorectal cancer therapy [129]. Pharmaceutical companies, such as Rosetta Genomics, Mirna Therapeutics, Alnylam and Santaris Pharma, have programs dedicated to the development and improvement of miRNA-based cancer diagnosis and therapy [130]. Future clinical and pharmaceutical studies will likely expand miRNA-based therapy to include many more miRNA candidates, not only for cancer treatments, but for other diseases as well.
Table 1.
Tumor-suppressive miRNAs (TS-miRNAs) involved in oncogenic traits. The table lists the TS-miRNAs discussed in this review, categorized by cancer trait (column 1), the oncogenic proteins encoded by TS-miRNA target mRNAs (column 2), the tumors in which TS-miRNAs have been characterized (column 3) and representatively referenced (column 4).
| TS-miRNAs | Cancer-related proteins encoded by mRNAs that are TS-miRNA targets | Cancer models implicating TS-miRNAs | References |
|---|---|---|---|
| Cell growth and proliferation | |||
| miR-34a | Bcl-2, SirT1 | breast cancer, glioma stem cells (GSCs) | [20] |
| miR-17-5p | AIB1 | breast cancer | [24] |
| miR-125a | HuR | breast carcinoma cell lines | [27,28] |
| miR-519 | HuR | cervical, colon, ovarian, lung, kidney cancer | [29,30] |
| miR-125b | Ets1, Bcl-2 | breast cancer, hepatocellular carcinoma | [31,32] |
| miR-28 | Cyclin D1 | colorectal cancer | [39] |
| miR-296 | HMGA1 | prostate cancer | [41] |
| miR-148b | CCKBR | gastric cancer | [42] |
| miR-135a | JAK2 | gastric cancer | [43] |
| miR-146a | FADD, EGFR, ROCK1, NOTCH1, CXCR4, TRAF6 | glioblastoma and breast, pancreatic, gastric, prostate cancer glioblastoma | [44,45] |
| miR-145 | VEGF-A, N-Ras, 70S6K1, FSCN1 | Kaposi’s sarcoma, T lymphocyte Jurkat cells, leukemia, extranodal NK/T cell lymphoma | [46,47] |
| miR-128 | EGFR, PDGFRα | colon and breast cancer, esophageal squamous cell carcinoma | [48] |
| miR-101 | EZH2 | glioma | [49] |
| miR-143, miR-145 | Bcl-2, Top2A, PRC1, Plk1 | bladder transitional cell carcinoma | [50] |
| miR-24 | S100A8 | liposarcoma | [51] |
| miR-216b | K-Ras | laryngeal squamous cell carcinoma | [52] |
| Cell survival | |||
| miR-34a | Bcl2, SirT1, BIRC3, DcR3, c-Met, Notch-1, Notch-2, Cyclin D1, Cyclin E2, Cdk4, Cdk6, E2F | brain tumors, glioma stem cell lines, breast, colon, pancreatic cancer | [20,61,62, 65–67] |
| miR-181d | K-Ras, Bcl-2 | glioma | [68] |
| miR-451 | Cyclin D1, Bcl-2, Akt1, MMP-2, MMP-9 | glioblastoma | [69] |
| Angiogenesis | |||
| miR-145 | VEGF-A, N-Ras, p70S6K1 | colon and breast cancer | [46,47] |
| miR-128 | p70S6K1 | glioma | [77] |
| miR-205 | VEGF-A | glioma | [78] |
| miR-519c | HIF-1α, HuR | lung, breast, cervical, colon, ovarian cancer | [27,29,30, 70,71,80] |
| miR-340 | c-Met | breast cancer | [81] |
| miR-29b | MMP-2 | hepatocellular carcinoma | [82] |
| miR-9 | MMP-14 | neuroblastoma | [83] |
| Suppressors of immune recognition | |||
| miR-322 | galectin-3 | breast, lung, prostate, kidney cancer | [94] |
| miR-181a | TGFBR1, TGFBRAP1 | mesenchymal stem cells | [95] |
| miR-335 | MAPK1, ROCK1 | neuroblastoma | [96] |
| miR-148a | ROCK1 | gastric cancer | [97] |
| miR-106a | IL-10, E2F1 | T lymphocyte Jurkat cells, glioma | [98,99] |
| let-7a | STAT3 | hepatocellular carcinoma | [100] |
| miR-17-5p | STAT3 | MDSCs | [101] |
| miR-20a | STAT3 | MDSCs | [101] |
| miR-93 | Genes of the TGF-β and/or STAT3 pathway | breast cancer | [102] |
| Invasion and metastasis | |||
| miR-9 | PNP | Melanoma | [117,125] |
| miR-1 | NF-κB | prostate cancer | |
| miR-200 | ZEB1 | colorectal cancer | [118] |
| miR-340 | c-Met | breast cancer | [81] |
| miR-34a | c-Met | breast cancer, melanoma | [119] |
| miR-29b | MMP-2 | hepatocellular carcinoma | [82] |
| miR-145 | VEGF, N-Ras | breast cancer | [46] |
| miR-205 | VEGF-A | glioblastoma | [78] |
| miR-183 | villin 2 (Ezrin), | breast cancer | [120] |
| miR-296 | HMGA1 | prostate cancer | [41] |
| miR-96 | KRAS | pancreatic cancer | [121] |
| miR-216b | KRAS | nasopharyngeal carcinoma | [52] |
| miR-195 | E2F3, CCND3 | glioblastoma | [122] |
| miR-150 | MUC4 | pancreatic cancer | [124] |
| miR-30c | MTA1 | endometrial cancer | [123] |
8. Concluding Remarks and Perspectives
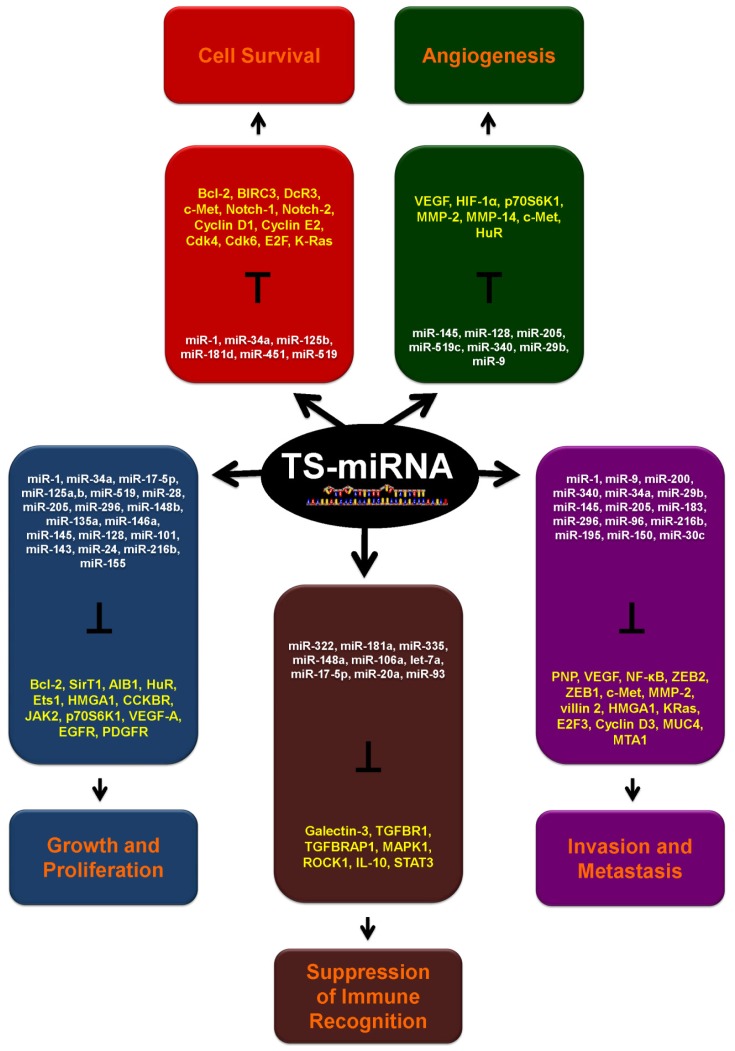
As shown in Figure 1, TS-miRNAs are capable of regulating multiple cancer traits. A few miRNAs have entered preclinical and clinical studies, but many more in vivo studies are needed in order to determine which TS-miRNAs are effective for inclusion in specific cancer treatments. The miRNAs studied thus far only comprise a small fraction of the miRNAs discovered by high-throughput approaches, such as RNA sequencing [131–133]. Studies that consider TS-miRNAs together with radiotherapy or chemotherapeutic drugs may also be beneficial in cancer treatments. In this regard, altered miRNA expression in cancer cells can also influence the mechanisms of drug resistance, as low miRNA levels in cancer cells reduced the sensitivity to chemotherapeutic agents, while miRNA restoration or overexpression increased it (as shown for let-7a, miR-34a and miR-200c, miR-128 and miR-125b, reviewed in reference [134]). In addition, studying miRNA signatures (oncomiRs and TS-miRs) linked to drug responses can enhance drug efficacy and minimize toxicity. Circulating cancer-related miRNAs, including TS-miRNAs, are gaining preclinical and clinical attention, and many of them originate from circulating tumor cells [135–138]. Circulating miRNAs can correlate with tumor progression, as shown in prostate cancer patients [139], and can help with the design of therapeutic regimes. A number of clinical studies are already underway ( ClinicalTrials.gov) [140]. As our knowledge of TS-miRNAs continues to expand, we anticipate an escalation in interest in this class of microRNAs for cancer diagnosis and therapy.
Figure 1.
TS-miRNAs and their target mRNAs encoding proteins that enable cancer traits. Boxes depict five major cancer traits: Growth and Proliferation (blue), Cell Survival (red), Angiogenesis (green), Suppression of Immune Recognition (brown) and Invasion and Metastasis (purple). For each cancer trait, TS-miRNAs are indicated in white, the pro-oncogenic proteins encoded by mRNAs that are targets of TS-miRNAs are indicated in yellow and the cancer traits in orange.
Acknowledgments
IG, KA and MG were supported by the NIA-IRP, NIH. Our apologies to those whose work could not be included due to space constraints.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Bartel D.P. MicroRNAs: Target recognition and regulatory functions. Cell. 2004;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabian M.R., Sonenberg N., Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 3.Lal A., Navarro F., Maher C.A., Maliszewski L.E., Yan N., O’Day E., Chowdhury D., Dykxhoorn D.M., Tsai P., Hofmann O., et al. MiR-24 inhibits cell proliferation by targeting E2F2, MYC and other cell-cycle genes via binding to “seedless” 3′UTR microRNA recognition elements. Mol. Cell. 2009;35:610–625. doi: 10.1016/j.molcel.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas M., Lieberman J., Lal A. Desperately seeking microRNA targets. Nat. Struct. Mol. Biol. 2010;17:1169–1174. doi: 10.1038/nsmb.1921. [DOI] [PubMed] [Google Scholar]
- 5.Kloosterman W.P., Plasterk R.H.A. The diverse functions of MicroRNAs in animal development and disease. Dev. Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Bushati N., Cohen S.M. MicroRNA functions. Annu. Rev. Cell. Dev. Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 7.Braun T., Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell. Bio. 2011;12:349–361. doi: 10.1038/nrm3118. [DOI] [PubMed] [Google Scholar]
- 8.Lima R.T., Busacca S., Almeida G.M., Gaudino G., Fennell D.A., Vasconcelos M.H. MicroRNA regulation of core apoptosis pathways in cancer. Eur. J. Cancer. 2011;47:163–174. doi: 10.1016/j.ejca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Daoud M.S.A., Nuin P., Chen J., Zhang X., Feilloter H., Tron V.A. MicroRNAs as prognostic biomarkers in malignant melanoma. Mod. Pathol. 2012;25:113a. [Google Scholar]
- 10.Wang Q.M., Wang S., Wang H.J., Li P., Ma Z.Y. MicroRNAs: Novel biomarkers for lung cancer diagnosis, prediction and treatment. Exp. Biol. Med. 2012;237:227–235. doi: 10.1258/ebm.2011.011192. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z.H., Zhang G.L., Li H.R., Luo J.D., Li Z.X., Chen G.M., Yang J. A panel of five circulating microRNAs as potential biomarkers for prostate cancer. Prostate. 2012;72:1443–1452. doi: 10.1002/pros.22495. [DOI] [PubMed] [Google Scholar]
- 12.Chen X., Hu Z.B., Wang W.J., Ba Y., Ma L.J., Zhang C.N., Wang C., Ren Z.J., Zhao Y., Wu S.J., et al. Identification of ten serum microRNAs from a genome-wide serum microRNA expression profile as novel noninvasive biomarkers for nonsmall cell lung cancer diagnosis. Inter. J. Cancer. 2012;130:1620–1628. doi: 10.1002/ijc.26177. [DOI] [PubMed] [Google Scholar]
- 13.Corsini L.R., Bronte G., Terrasi M., Amodeo V., Fanale D., Fiorentino E., Cicero G., Bazan V., Russo A. The role of microRNAs in cancer: Diagnostic and prognostic biomarkers and targets of therapies. Expert Opin. Ther. Targets. 2012;16:S103–S109. doi: 10.1517/14728222.2011.650632. [DOI] [PubMed] [Google Scholar]
- 14.Thomson J.M., Newman M., Parker J.S., Morin-Kensicki E.M., Wright T., Hammond S.M. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006;20:2202–2207. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faggad A., Budczies J., Tchernitsa O., Darb-Esfahani S., Sehouli J., Muller B.M., Wirtz R., Chekerov R., Weichert W., Sinn B., et al. Prognostic significance of Dicer expression in ovarian cancer—Link to global microRNA changes and oestrogen receptor expression. J. Pathol. 2010;220:382–391. doi: 10.1002/path.2658. [DOI] [PubMed] [Google Scholar]
- 16.Dedes K.J., Natrajan R., Lambros M.B., Geyer F.C., Lopez-Garcia M.A., Savage K., Jones R.L., Reis-Filho J.S. Down-regulation of the miRNA master regulators Drosha and Dicer is associated with specific subgroups of breast cancer. Eur. J. Cancer. 2011;47:138–150. doi: 10.1016/j.ejca.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Malumbres M., Barbacid M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 19.Witsch E., Sela M., Yarden Y. Roles for growth factors in cancer progression. Physiology. 2010;25:85–101. doi: 10.1152/physiol.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L., Yuan L., Luo J., Gao J., Guo J., Xie X. MiR-34a inhibits proliferation and migration of breast cancer through down-regulation of Bcl-2 and SIRT1. Clin. Exp. Med. 2012 doi: 10.1007/s10238-012-0186-5. [DOI] [PubMed] [Google Scholar]
- 21.Sun L., Wu Z., Shao Y., Pu Y., Miu W., Yao J., Wu Y., Yang Z.X. MicroRNA-34a suppresses cell proliferation and induces apoptosis in U87 glioma stem cells. Technol. Cancer Res. Treat. 2012;11:483–490. doi: 10.7785/tcrt.2012.500264. [DOI] [PubMed] [Google Scholar]
- 22.Yang J., Liu X., Bhalla K., Kim C.N., Ibrado A.M., Cai J., Peng T.I., Jones D.P., Wang X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 23.Concepcion C.P., Han Y.C., Mu P., Bonetti C., Yao E., D’Andrea A., Vidigal J.A., Maughan W.P., Ogrodowski P., Ventura A. Intact p53-dependent responses in miR-34-deficient mice. PLoS Genet. 2012;8:e1002797. doi: 10.1371/journal.pgen.1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hossain A., Kuo M.T., Saunders G.F. Mir-17-5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol. Cell. Biol. 2006;26:8191–8201. doi: 10.1128/MCB.00242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilli M.T., Reiter R., Oh A.S., Henke R.T., McDonnell K., Gallicano G.I., Furth P.A., Riegel A.T. Overexpression of an N-terminally truncated isoform of the nuclear receptor coactivator amplified in breast cancer 1 leads to altered proliferation of mammary epithelial cells in transgenic mice. Mol. Endocrinol. 2005;19:644–656. doi: 10.1210/me.2004-0106. [DOI] [PubMed] [Google Scholar]
- 26.Fereshteh M.P., Tilli M.T., Kim S.E., Xu J., O’Malley B.W., Wellstein A., Furth P.A., Riegel A.T. The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling and mammary tumorigenesis in mice. Cancer Res. 2008;68:3697–3706. doi: 10.1158/0008-5472.CAN-07-6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdelmohsen K., Gorospe M. Posttranscriptional regulation of cancer traits by HuR. Wiley Interdiscip. Rev. RNA. 2010;1:214–229. doi: 10.1002/wrna.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo X., Wu Y., Hartley R.S. MicroRNA-125a represses cell growth by targeting HuR in breast cancer. RNA Biol. 2009;6:575–583. doi: 10.4161/rna.6.5.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdelmohsen K., Srikantan S., Kuwano Y., Gorospe M. miR-519 reduces cell proliferation by lowering RNA-binding protein HuR levels. Proc. Natl. Acad. Sci. USA. 2008;105:20297–20302. doi: 10.1073/pnas.0809376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdelmohsen K., Kim M.M., Srikantan S., Mercken E.M., Brennan S.E., Wilson G.M., Cabo R., Gorospe M. MiR-519 suppresses tumor growth by reducing HuR levels. Cell Cycle. 2010;9:1354–1359. doi: 10.4161/cc.9.7.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y., Yan L.X., Wu Q.N., Du Z.M., Chen J., Liao D.Z., Huang M.Y., Hou J.H., Wu Q.L., Zeng M.S., et al. MiR-125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto-oncogene in human invasive breast cancer. Cancer Res. 2011;71:3552–3562. doi: 10.1158/0008-5472.CAN-10-2435. [DOI] [PubMed] [Google Scholar]
- 32.Zhao A.H., Zeng Q., Xie X.Y., Zhou J.N., Yue W., Li Y.L., Pei X.T. MicroRNA-125b induces cancer cell apoptosis through suppression of Bcl-2 expression. J. Genet. Genomics. 2012;39:29–35. doi: 10.1016/j.jgg.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Bousquet M., Harris M.H., Zhou B., Lodish H.F. MicroRNA miR-125b causes leukemia. Proc. Natl. Acad. Sci. USA. 2012;107:21558–21563. doi: 10.1073/pnas.1016611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klusmann J.H., Li Z., Böhmer K., Maroz A., Koch M.L., Emmrich S., Godinho F.J., Orkin S.H., Reinhardt D. MiR-125b-2 is a potential oncomiR on human chromosome 21 in megakaryoblastic leukemia. Genes Dev. 2010;24:478–490. doi: 10.1101/gad.1856210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leone V., D’Angelo D., Rubio I., de Freitas P.M., Federico A., Colamaio M., Pallante P., Medeiros-Neto G., Fusco A. MiR-1 is a tumor suppressor in thyroid carcinogenesis targeting CCND2, CXCR4 and SDF-1alpha. J. Clin. Endocrinol. MeTable. 2011;96:E1388–E1398. doi: 10.1210/jc.2011-0345. [DOI] [PubMed] [Google Scholar]
- 36.Hudson R.S., Yi M., Esposito D., Watkins S.K., Hurwitz A.A., Yfantis H.G., Lee D.H., Borin J.F., Naslund M.J., Alexander R.B., et al. MicroRNA-1 is a candidate tumor suppressor and prognostic marker in human prostate cancer. Nucleic Acids Res. 2012;40:3689–3703. doi: 10.1093/nar/gkr1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Datta J., Kutay H., Nasser M.W., Nuovo G.J., Wang B., Majumder S., Liu C.G., Volinia S., Croce C.M., Schmittgen T.D., et al. Methylation mediated silencing of MicroRNA-1 gene and its role in hepatocellular carcinogenesis. Cancer Res. 2008;68:5049–5058. doi: 10.1158/0008-5472.CAN-07-6655. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Nasser M.W., Datta J., Nuovo G., Kutay H., Motiwala T., Majumder S., Wang B., Suster S., Jacob S.T., Ghoshal K. Down-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression of tumorigenic property of lung cancer cells and their sensitization to doxorubicin-induced apoptosis by miR-1. J. Biol. Chem. 2008;283:33394–33405. doi: 10.1074/jbc.M804788200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Almeida M.I., Nicoloso M.S., Zeng L.Z., Ivan C., Spizzo R., Gafa R., Xiao L.C., Zhang X.N., Vannini I., Fanini F., et al. Strand-specific miR-28-5p and miR-28-3p have distinct effects in colorectal cancer cells. Gastroenterology. 2012;142:886–896. e9. doi: 10.1053/j.gastro.2011.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majid S., Dar A.A., Saini S., Yamamura S., Hirata H., Tanaka Y., Deng G.R., Dahiya R. MicroRNA-205-directed transcriptional activation of tumor suppressor egnes in prostate cancer. Cancer. 2010;116:5637–5649. doi: 10.1002/cncr.25488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei J.J., Wu X.Y., Peng Y., Shi G.Z., Olca B., Yang X.M., Daniels G., Osman I., Ouyang J.Y., Hernando E., et al. Regulation of HMGA1 expression by microRNA-296 affects prostate cancer growth and invasion. Clin. Cancer Res. 2011;17:1297–1305. doi: 10.1158/1078-0432.CCR-10-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song Y.X., Yue Z.Y., Wang Z.N., Xu Y.Y., Luo Y., Xu H.M., Zhang X., Jiang L., Xing C.Z., Zhang Y. MicroRNA-148b is frequently down-regulated in gastric cancer and acts as a tumor suppressor by inhibiting cell proliferation. Mol. Cancer. 2011;10:1. doi: 10.1186/1476-4598-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu H., Huang M., Cao P., Wang T.S., Shu Y.Q., Liu P. MiR-135a targets JAK2 and inhibits gastric cancer cell proliferation. Cancer Biol. Ther. 2012;13:281–288. doi: 10.4161/cbt.18943. [DOI] [PubMed] [Google Scholar]
- 44.Labbaye C., Testa U. The emerging role of MIR-146A in the control of hematopoiesis, immune function and cancer. J. Hematol. Oncol. 2012;5:13. doi: 10.1186/1756-8722-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paik J.H., Jang J.Y., Jeon Y.K., Kim W.Y., Kim T.M., Heo D.S., Kim C.W. MicroRNA-146a downregulates NF kappa B Activity via targeting TRAF6 and functions as a tumor suppressor having strong prognostic implications in NK/T cell lymphoma. Clin. Cancer Res. 2011;17:4761–4771. doi: 10.1158/1078-0432.CCR-11-0494. [DOI] [PubMed] [Google Scholar]
- 46.Zou C., Xu Q., Mao F., Li D., Bian C.X., Liu L.Z., Jiang Y., Chen X.N., Qi Y.T., Zhang X.L., et al. MiR-145 inhibits tumor angiogenesis and growth by N-RAS and VEGF. Cell Cycle. 2012;11:2137–2145. doi: 10.4161/cc.20598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Q., Liu L.Z., Qian X., Chen Q., Jiang Y., Li D., Lai L.H., Jiang B.H. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012;40:761–774. doi: 10.1093/nar/gkr730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papagiannakopoulos T., Friedmann-Morvinski D., Neveu P., Dugas J.C., Gill R.M., Huillard E., Liu C., Zong H., Rowitch D.H., Barres B.A., et al. Pro-neural miR-128 is a glioma tumor suppressor that targets mitogenic kinases. Oncogene. 2012;31:1884–1895. doi: 10.1038/onc.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedman J.M., Liang G.N., Liu C.C., Wolff E.M., Tsai Y.C., Ye W., Zhou X.H., Jones P.A. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009;69:2623–2629. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- 50.Ugras S., Brill E., Jacobsen A., Hafner M., Socci N.D., DeCarolis P.L., Khanin R., O’Connor R., Mihailovic A., Taylor B.S., et al. Small RNA sequencing and functional characterization reveals microRNA-143 tumor suppressor activity in Liposarcoma. Cancer Res. 2011;71:5659–5669. doi: 10.1158/0008-5472.CAN-11-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo Y., Fu W.N., Chen H., Shang C., Zhong M. MiR-24 functions as a tumor suppressor in Hep2 laryngeal carcinoma cells partly through down-regulation of the S100A8 protein. Oncol. Rep. 2012;27:1097–1103. doi: 10.3892/or.2011.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng M., Tang H.L., Zhou Y.H., Zhou M., Xiong W., Zheng Y., Ye Q.R., Zeng X., Liao Q.J., Guo X.F., et al. MiR-216b suppresses tumor growth and invasion by targeting KRAS in nasopharyngeal carcinoma. J. Cell Sci. 2011;124:2997–3005. doi: 10.1242/jcs.085050. [DOI] [PubMed] [Google Scholar]
- 53.Levati L., Alvino E., Pagani E., Arcelli D., Caporaso P., Bondanza S., di Leva G., Ferracin M., Volinia S., Bonmassar E., et al. Altered expression of selected microRNAs in melanoma: Antiproliferative and proapoptotic activity of miRNA-155. Int. J. Oncol. 2009;35:393–400. [PubMed] [Google Scholar]
- 54.Zhai Q.N., Zhou L., Zhao C.J., Wan J., Yu Z.D., Guo X., Qin J., Chen J., Lu R.J. Identification of miR-508-3p and miR-509-3p that are associated with cell invasion and migration and involved in the apoptosis of renal cell carcinoma. Biochem. Biophys. Res. Commun. 2012;419:621–626. doi: 10.1016/j.bbrc.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 55.Sigal A., Rotter V. Oncogenic mutations of the p53 tumor suppressor: The demons of the guardian of the genome. Cancer Res. 2000;60:6788–6793. [PubMed] [Google Scholar]
- 56.Oren M., Vousden K.H. Hot papers—Cancer research—Mdm2 promotes the rapid degradation of p53 by Y. Haupt, R. Maya, A. Kazaz, M. Oren and Regulation of p53 stability by Mdm2 by M.H.G. Kubbutat, S.N. Jones, K.H. Vousden—Comments. The Scientist. 1999;13:11–11. [Google Scholar]
- 57.Levine A.J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 58.Lukashchuk N., Vousden K.H. Ubiquitination and degradation of mutant p53. Mol. Cell. Biol. 2007;27:8284–8295. doi: 10.1128/MCB.00050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zenz T., Mohr J., Eldering E., Kater A.P., Bühler A., Kienle D., Winkler D., Dürig J., van Oers M.H., Mertens D., et al. miR-34a as part of the resistance network in chronic lymphocytic leukemia. Blood. 2009;113:3801–3808. doi: 10.1182/blood-2008-08-172254. [DOI] [PubMed] [Google Scholar]
- 60.Chang T.C., Wentzel E.A., Kent O.A., Ramachandran K., Mullendore M., Lee K.H., Feldmann G., Yamakuchi M., Ferlito M., Lowenstein C.J., et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liston P., Fong W.G., Korneluk R.G. The inhibitors of apoptosis: There is more to life than Bcl2. Oncogene. 2003;22:8568–8580. doi: 10.1038/sj.onc.1207101. [DOI] [PubMed] [Google Scholar]
- 62.Sheikh M.S., Fornace A.J. Death and decoy receptors and p53-mediated apoptosis. Leukemia. 2000;14:1509–1513. doi: 10.1038/sj.leu.2401865. [DOI] [PubMed] [Google Scholar]
- 63.Uren A.G., Pakusch M., Hawkins C.J., Puls K.L., Vaux D.L. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc. Natl. Acad. Sci. USA. 1996;93:4974–4978. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ashkenazi A., Dixit V.M. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 65.Guessous F., Zhang Y., Kofman A., Catania A., Li Y., Schiff D., Purow B., Abounader R. MicroRNA-34a is tumor suppressive in brain tumors and glioma stem cells. Cell Cycle. 2010;9:1031–1036. doi: 10.4161/cc.9.6.10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen F., Hu S.J. Effect of microRNA-34a in cell cycle, differentiation and apoptosis: A review. J. Biochem. Mol. Toxicol. 2012;26:79–86. doi: 10.1002/jbt.20412. [DOI] [PubMed] [Google Scholar]
- 67.Tazawa H., Tsuchiya N., Izumiya M., Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc. Natl. Acad. Sci. USA. 2007;104:15472–15477. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X.F., Shi Z.M., Wang X.R., Cao L., Wang Y.Y., Zhang J.X., Yin Y., Luo H., Kang C.S., Liu N., et al. MiR-181d acts as a tumor suppressor in glioma by targeting K-ras and Bcl-2. J. Cancer Res. Clin. 2012;138:573–584. doi: 10.1007/s00432-011-1114-x. [DOI] [PubMed] [Google Scholar]
- 69.Nan Y., Han L., Zhang A.L., Wang G.X., Jia Z.F., Yang Y., Yue X.A., Pu P.Y., Zhong Y., Kang C.S. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Res. 2010;1359:14–21. doi: 10.1016/j.brainres.2010.08.074. [DOI] [PubMed] [Google Scholar]
- 70.Abdelmohsen K., Srikantan S., Tominaga K., Kang M.J., Yaniv Y., Martindale J.L., Yang X., Park S.S., Becker K.G., Subramanian M., et al. Growth inhibition by miR-519 via multiple p21-inducing pathways. Mol. Cell. Biol. 2012;32:2530–2548. doi: 10.1128/MCB.00510-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marasa B.S., Srikantan S., Martindale J.L., Kim M.M., Lee E.K., Gorospe M., Abdelmohsen K. MicroRNA profiling in human diploid fibroblasts uncovers miR-519 role in replicative senescence. Aging. 2010;2:333–343. doi: 10.18632/aging.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69:4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 73.Baeriswyl V., Christofori G. The angiogenic switch in carcinogenesis. Semin. Cancer Biol. 2009;19:329–337. doi: 10.1016/j.semcancer.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 74.Pugh C.W., Ratcliffe P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 75.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 76.Rundhaug J.E. Matrix metalloproteinases, angiogenesis and cancer—Commentary re: A.C. Lockhart et al., reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin. Cancer Res. 2003;9:551–554. [PubMed] [Google Scholar]
- 77.Shi Z.M., Wang J., Yan Z.P., You Y.P., Li C.Y., Qian X., Yin Y., Zhao P., Wang Y.Y., Wang X.F., et al. MiR-128 inhibits tumor growth and angiogenesis by targeting p70S6K1. PLoS One. 2012;7:e32709. doi: 10.1371/journal.pone.0032709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yue X., Wang P.G., Xu J., Zhu Y.F., Sun G., Pang Q., Tao R.J. MicroRNA-205 functions as a tumor suppressor in human glioblastoma cells by targeting VEGF-A. Oncol. Rep. 2012;27:1200–1206. doi: 10.3892/or.2011.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cha S.T., Chen P.S., Johansson G., Chu C.Y., Wang M.Y., Jeng Y.M., Yu S.L., Chen J.S., Chang K.J., Jee S.H., et al. MicroRNA-519c suppresses hypoxia-inducible factor-1 alpha expression and tumor angiogenesis. Cancer Res. 2010;70:2675–2685. doi: 10.1158/0008-5472.CAN-09-2448. [DOI] [PubMed] [Google Scholar]
- 80.Ristimaki A. Tumor suppressor effect of the microRNA miR-519 is mediated via the mRNA-binding protein HuR. Cell Cycle. 2010;9:1234–1234. [PubMed] [Google Scholar]
- 81.Wu Z.S., Wu Q., Wang C.Q., Wang X.N., Huang J., Zhao J.J., Mao S.S., Zhang G.H., Xu X.C., Zhang N. MiR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met. Cancer. 2011;117:2842–2852. doi: 10.1002/cncr.25860. [DOI] [PubMed] [Google Scholar]
- 82.Fang J.H., Zhou H.C., Zeng C.X., Yang J., Liu Y.L., Huang X.Z., Zhang J.P., Guan X.Y., Zhuang S.M. MicroRNA-29b suppresses tumor angiogenesis, invasion and metastasis by regulating matrix metalloproteinase 2 expression. Hepatology. 2011;54:1729–1740. doi: 10.1002/hep.24577. [DOI] [PubMed] [Google Scholar]
- 83.Zhang H.Y., Qi M., Li S.W., Qi T., Mei H., Huang K., Zheng L.D., Tong Q.S. MicroRNA-9 targets matrix metalloproteinase 14 to inhibit invasion, metastasis and angiogenesis of neuroblastoma cells. Mol. Cancer Ther. 2012;11:1454–1466. doi: 10.1158/1535-7163.MCT-12-0001. [DOI] [PubMed] [Google Scholar]
- 84.Teng M.W.L., Swann J.B., Koebel C.M., Schreiber R.D., Smyth M.J. Immune-mediated dormancy: An equilibrium with cancer. J. Leukocyte Biol. 2008;84:988–993. doi: 10.1189/jlb.1107774. [DOI] [PubMed] [Google Scholar]
- 85.Kim R., Emi M., Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang R.Y., Rabinovich G.A., Liu F.T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 87.Rubinstein N., Ilarregui J.M., Toscano M.A., Rabinovich G.A. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens. 2004;64:1–12. doi: 10.1111/j.0001-2815.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- 88.Uyttenhove C., Pilotte L., Theate I., Stroobant V., Colau D., Parmentier N., Boon T., van den Eynde B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 89.Teicher B.A. Transforming growth factor-beta and the immune response to malignant disease. Clin. Cancer Res. 2007;13:6247–6251. doi: 10.1158/1078-0432.CCR-07-1654. [DOI] [PubMed] [Google Scholar]
- 90.Khong H.T., Restifo N.P. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat. Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang T., Niu G.L., Kortylewski M., Burdelya L., Shain K., Zhang S.M., Bhattacharya R., Gabrilovich D., Heller R., Coppola D., et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat. Med. 2004;10:209. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 92.Groh V., Rhinehart R., Randolph-Habecker J., Topp M.S., Riddell S.R., Spies T. Costimulation of CD8αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 93.Kusmartsev S., Nagaraj S., Gabrilovich D.I. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J. Immunol. 2005;175:4583–4592. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramasamy S., Duraisamy S., Barbashov S., Kawano T., Kharbanda S., Kufe D. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol. Cell. 2007;27:992–1004. doi: 10.1016/j.molcel.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu L., Wang Y., Fan H., Zhao X., Liu D., Hu Y., Kidd A.R., III, Bao J., Hou Y. MicroRNA-181a regulates local immune balance by inhibiting proliferation and immunosuppressive properties of mesenchymal stem cells. Stem cells. 2012;30:1756–1770. doi: 10.1002/stem.1156. [DOI] [PubMed] [Google Scholar]
- 96.Lynch J., Fay J., Meehan M., Bryan K., Watters K.M., Murphy D.M., Stallings R.L. MiRNA-335 suppresses neuroblastoma cell invasiveness by direct targeting of multiple genes from the non-canonical TGF-β signalling pathway. Carcinogenesis. 2012;33:976–985. doi: 10.1093/carcin/bgs114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng B., Liang L., Wang C., Huang S., Cao X., Zha R., Liu L., Jia D., Tian Q., Wu J., et al. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin. Cancer Res. 2011;17:7574–7583. doi: 10.1158/1078-0432.CCR-11-1714. [DOI] [PubMed] [Google Scholar]
- 98.Sharma A., Kumar M., Aich J., Hariharan M., Brahmachari S.K., Agrawal A., Ghosh B. Posttranscriptional regulation of interleukin-10 expression by hsa-miR-106a. Proc. Natl. Acad. Sci. USA. 2009;106:5761–5766. doi: 10.1073/pnas.0808743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang G., Zhang R., Chen X., Mu Y., Ai J., Shi C., Liu Y., Shi C., Sun L., Rainov N.G., et al. MiR-106a inhibits glioma cell growth by targeting E2F1 independent of p53 status. J. Mol. Med. 2011;89:1037–1050. doi: 10.1007/s00109-011-0775-x. [DOI] [PubMed] [Google Scholar]
- 100.Wang Y., Lu Y., Toh S.T., Sung W.K., Tan P., Chow P., Chung A.Y., Jooi L.L., Lee C.G. Lethal-7 is down-regulated by the hepatitis B virus x protein and targets signal transducer and activator of transcription 3. J. Hepatol. 2010;53:57–66. doi: 10.1016/j.jhep.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 101.Zhang M., Liu Q., Mi S., Liang X., Zhang Z., Su X., Liu J., Chen Y., Wang M., Zhang Y., et al. Both miR-17–5p and miR-20a alleviate suppressive potential of myeloid-derived suppressor cells by modulating STAT3 expression. J. Immunol. 2011;186:4716–4724. doi: 10.4049/jimmunol.1002989. [DOI] [PubMed] [Google Scholar]
- 102.Liu S., Patel S.H., Ginestier C., Ibarra I., Martin-Trevino R., Bai S., McDermott S.P., Shang L., Ke J., Ou S.J., et al. MicroRNA93 regulates proliferation and differentiation of normal and malignant breast stem cells. PLoS Genet. 2012;8:e1002751. doi: 10.1371/journal.pgen.1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berx G., van Roy F. Involvement of members of the cadherin superfamily in cancer. CSH Perspect. Biol. 2009;1:a003129. doi: 10.1101/cshperspect.a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cavallaro U., Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 105.Micalizzi D.S., Farabaugh S.M., Ford H.L. Epithelial-mesenchymal transition in cancer: Parallels between normal development and tumor progression. J. Mammary Gland Biol. Neopl. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kerrigan J.J., Mansell J.P., Sandy J.R. Matrix turnover. J. Orthodont. 2000;27:227–233. doi: 10.1179/ortho.27.3.227. [DOI] [PubMed] [Google Scholar]
- 107.Sennino B., Ishiguro-Oonuma T., Wei Y., Naylor R.M., Williamson C.W., Bhagwandin V., Tabruyn S.P., You W.K., Chapman H.A., Christensen J.G., et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012;2:270–287. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Belton A., Gabrovsky A., Bae Y.K., Reeves R., Iacobuzio-Donahue C., Huso D.L., Resar L.M. HMGA1 induces intestinal polyposis in transgenic mice and drives tumor progression and stem cell properties in colon cancer cells. PLoS One. 2012;7:e30034. doi: 10.1371/journal.pone.0030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Choi J., Choi K., Benveniste E.N., Rho S.B., Hong Y.S., Lee J.H., Kim J., Park K. Bcl-2 promotes invasion and lung metastasis by inducing matrix metalloproteinase-2. Cancer Res. 2005;65:5554–5560. doi: 10.1158/0008-5472.CAN-04-4570. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y., Zhang M., Dong H., Yong S., Li X., Olashaw N., Kruk P.A., Cheng J.Q., Bai W., Chen J., et al. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28:445–460. doi: 10.1038/onc.2008.388. [DOI] [PubMed] [Google Scholar]
- 111.Moon A., Kim M.S., Kim T.G., Kim S.H., Kim H.E., Chen Y.Q., Kim H.R. H-ras, but not N-ras, induces an invasive phenotype in human breast epithelial cells: A role for MMP-2 in the H-ras-induced invasive phenotype. Int. Cancer J. 2000;85:176–181. doi: 10.1002/(sici)1097-0215(20000115)85:2<176::aid-ijc5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 112.Meng Y., Lu Z., Yu S., Zhang Q., Ma Y., Chen J. Ezrin promotes invasion and metastasis of pancreatic cancer cells. J. Transl. Med. 2010;8:61. doi: 10.1186/1479-5876-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Campbell P.M., Groehler A.L., Lee K.M., Ouellette M.M., Khazak V., Der C.J. K-Ras promotes growth transformation and invasion of immortalized human pancreatic cells by Raf and phosphatidylinositol 3-kinase signaling. Cancer Res. 2007;67:2098–2106. doi: 10.1158/0008-5472.CAN-06-3752. [DOI] [PubMed] [Google Scholar]
- 114.Singh A.P., Chaturvedi P., Batra S.K. Emerging roles of MUC4 in cancer: A novel target for diagnosis and therapy. Cancer Res. 2007;67:433–436. doi: 10.1158/0008-5472.CAN-06-3114. [DOI] [PubMed] [Google Scholar]
- 115.Yoon S.O., Shin S., Mercurio A.M. Ras stimulation of E2F activity and a consequent E2F regulation of integrin alpha6beta4 promote the invasion of breast carcinoma cells. Cancer Res. 2006;66:6288–6295. doi: 10.1158/0008-5472.CAN-06-0826. [DOI] [PubMed] [Google Scholar]
- 116.Mahoney M.G., Simpson A., Jost M., Noe M., Kari C., Pepe D., Choi Y.W., Uitto J., Rodeck U. Metastasis-associated protein (MTA)1 enhances migration, invasion and anchorage-independent survival of immortalized human keratinocytes. Oncogene. 2002;21:2161–2170. doi: 10.1038/sj.onc.1205277. [DOI] [PubMed] [Google Scholar]
- 117.Liu S.J., Kumar S.M., Lu H.Z., Liu A.H., Yang R.F., Pushparajan A., Guo W., Xu X.W. MicroRNA-9 up-regulates E-cadherin through inhibition of NF-κB1-Snail1 pathway in melanoma. J. Pathol. 2012;226:61–72. doi: 10.1002/path.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schmalhofer O., Brabletz S., Brabletz T. E-cadherin, beta-catenin and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 119.Yan D.S., Zhou X.T., Chen X.Y., Hu D.N., Da Dong X., Wang J., Lu F., Tu L.L., Qu J. MicroRNA-34a inhibits uveal melanoma cell proliferation and migration through downregulation of c-Met. Invest. Ophth. Vis. Sci. 2009;50:1559–1565. doi: 10.1167/iovs.08-2681. [DOI] [PubMed] [Google Scholar]
- 120.Lowery A.J., Miller N., Dwyer R.M., Kerin M.J. Dysregulated miR-183 inhibits migration in breast cancer cells. BMC Cancer. 2010;10:502. doi: 10.1186/1471-2407-10-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu S.N., Lu Z.H., Liu C.Z., Meng Y.X., Ma Y.H., Zhao W.G., Liu J.P., Yu J., Chen J. MiRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 2010;70:6015–6025. doi: 10.1158/0008-5472.CAN-09-4531. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Q.Q., Xu H., Huang M.B., Ma L.M., Huang Q.J., Yao Q., Zhou H., Qu L.H. MicroRNA-195 plays a tumor-suppressor role in human glioblastoma cells by targeting signaling pathways involved in cellular proliferation and invasion. Neuro-Oncology. 2012;14:278–287. doi: 10.1093/neuonc/nor216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou H.J., Xu X.F., Xun Q.Y., Yu D.Q., Ling J.X., Guo F.F., Yan Y.H., Shi J.Y., Hu Y.L. MicroRNA-30c negatively regulates endometrial cancer cells by targeting metastasis-associated gene-1. Oncol. Rep. 2012;27:807–812. doi: 10.3892/or.2011.1574. [DOI] [PubMed] [Google Scholar]
- 124.Srivastava S.K., Bhardwaj A., Singh S., Arora S., Wang B., Grizzle W.E., Singh A.P. MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis. 2011;32:1832–1839. doi: 10.1093/carcin/bgr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kojima S., Chiyomaru T., Kawakami K., Yoshino H., Enokida H., Nohata N., Fuse M., Ichikawa T., Naya Y., Nakagawa M., Seki N. Tumour suppressors miR-1 and miR-133a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Br. J. Cancer. 2012;106:405–413. doi: 10.1038/bjc.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bader A.G., Brown D., Stoudemire J., Lammers P. Developing therapeutic microRNAs for cancer. Gene Ther. 2011;18:1121–1126. doi: 10.1038/gt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bader A.G., Brown D., Winkler M. The promise of microRNA replacement therapy. Cancer Res. 2010;70:7027–7030. doi: 10.1158/0008-5472.CAN-10-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Di Martino M.T., Leone E., Amodio N., Foresta U., Lionetti M., Pitari M.R., Cantafio M.E.G., Gulla A., Conforti F., Morelli E., et al. Synthetic miR-34a mimics as a novel therapeutic agent for Multiple Myeloma: In vitro and in vivo evidence. Clin. Cancer Res. 2012;18:6260–6270. doi: 10.1158/1078-0432.CCR-12-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Takahashi M., Cuatrecasas M., Balaguer F., Hur K., Toiyama Y., Castells A., Boland C.R., Goel A. The clinical significance of miR-148a as a predictive biomarker in patients with advanced colorectal cancer. PLoS One. 2012;7:e46684. doi: 10.1371/journal.pone.0046684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wahid F., Shehzad A., Khan T., Kim Y.Y. MicroRNAs: Synthesis, mechanism, function and recent clinical trials. Biochim. Biophys. Acta. 2010;1803:1231–1243. doi: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 131.Ryu S., Joshi N., McDonnell K., Woo J., Choi H., Gao D., McCombie W.R., Mittal V. Discovery of novel human breast cancer microRNAs from deep sequencing data by analysis of pri-microRNA secondary structures. PLoS One. 2011;6:e16403. doi: 10.1371/journal.pone.0016403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Friedlander M.R., Mackowiak S.D., Li N., Chen W., Rajewsky N. MiRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012;40:37–52. doi: 10.1093/nar/gkr688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dhahbi J.M., Atamna H., Boffelli D., Magis W., Spindler S.R., Martin D.I.K. Deep sequencing reveals novel microRNAs and regulation of microRNA expression during cell senescence. PLoS One. 2011;6:e20509. doi: 10.1371/journal.pone.0020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Giovannetti E., Erozenci A., Smit J., Danesi R., Peters G.J. Molecular mechanisms underlying the role of microRNAs (miRNAs) in anticancer drug resistance and implications for clinical practice. Crit. Rev. Oncol. Hematol. 2012;81:103–122. doi: 10.1016/j.critrevonc.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 135.Duttagupta R., Jiang R., Gollub J., Getts R.C., Jones K.W. Impact of Cellular miRNAs on Circulating miRNA Biomarker Signatures. PLoS One. 2011;6:e20769. doi: 10.1371/journal.pone.0020769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kosaka N., Iguchi H., Ochiya T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101:2087–2092. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou H., Xiao B.X., Zhou F., Deng H.X., Zhang X.J., Lou Y.R., Gong Z.H., Du C.G., Guo J.M. MiR-421 is a functional marker of circulating tumor cells in gastric cancer patients. Biomarkers. 2012;17:104–110. doi: 10.3109/1354750X.2011.614961. [DOI] [PubMed] [Google Scholar]
- 138.Wang Y., Zheng D.L., Tan Q.L., Wang M.X., Gu L.Q. Nanopore-based detection of circulating microRNAs in lung cancer patients. Nat. Nanotechnol. 2011;6:668–674. doi: 10.1038/nnano.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brase J.C., Johannes M., Schlomm T., Falth M., Haese A., Steuber T., Beissbarth T., Kuner R., Sultmann H. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int. J. Cancer. 2011;128:608–616. doi: 10.1002/ijc.25376. [DOI] [PubMed] [Google Scholar]
- 140. [accessed on 28 December 2012];ClinicalTrials.gov. Available online: http://www.clinicaltrials.gov/ [Google Scholar]