Abstract
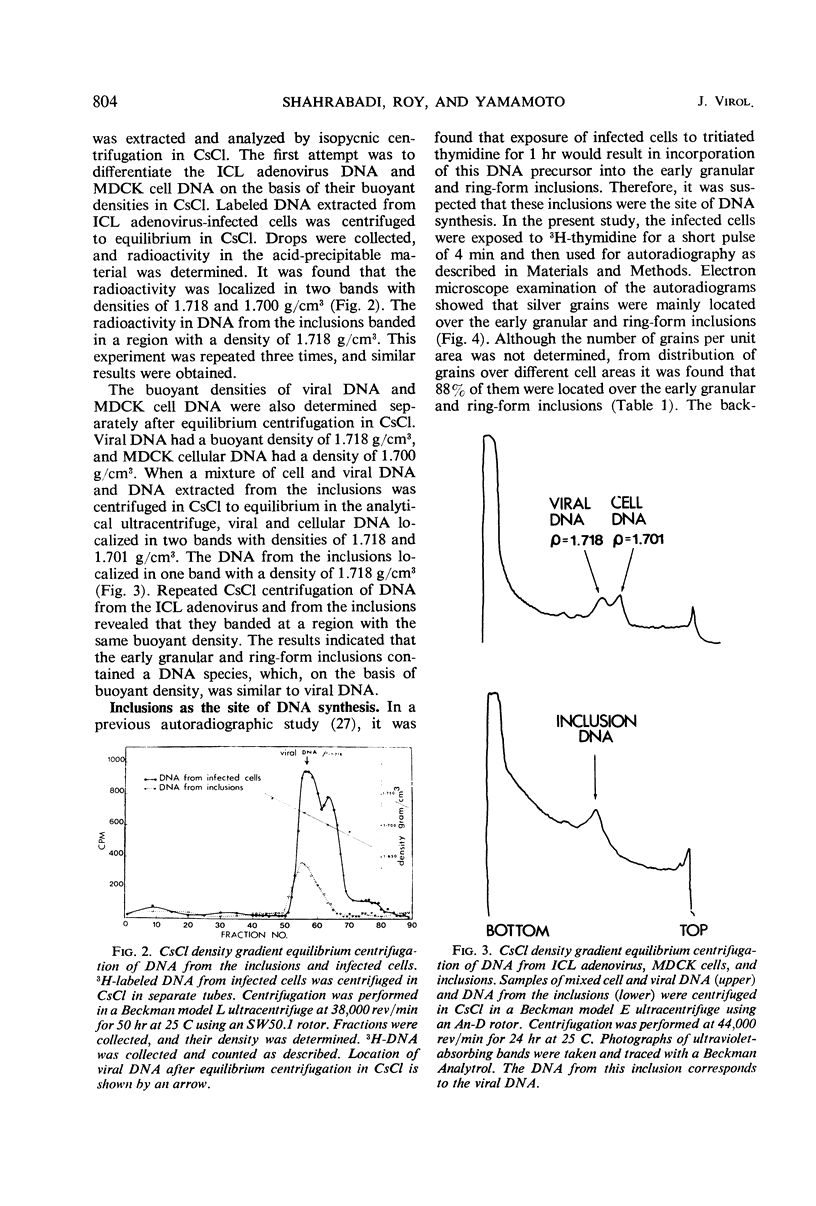
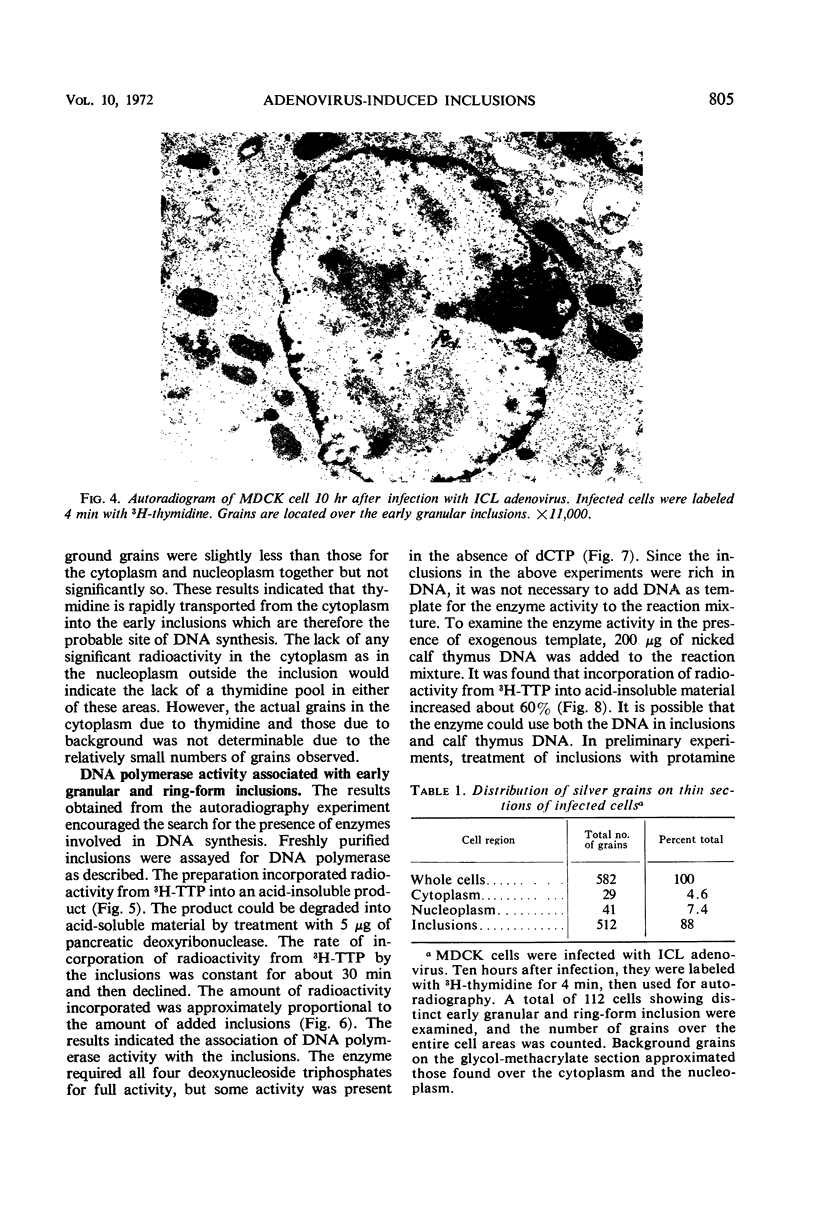
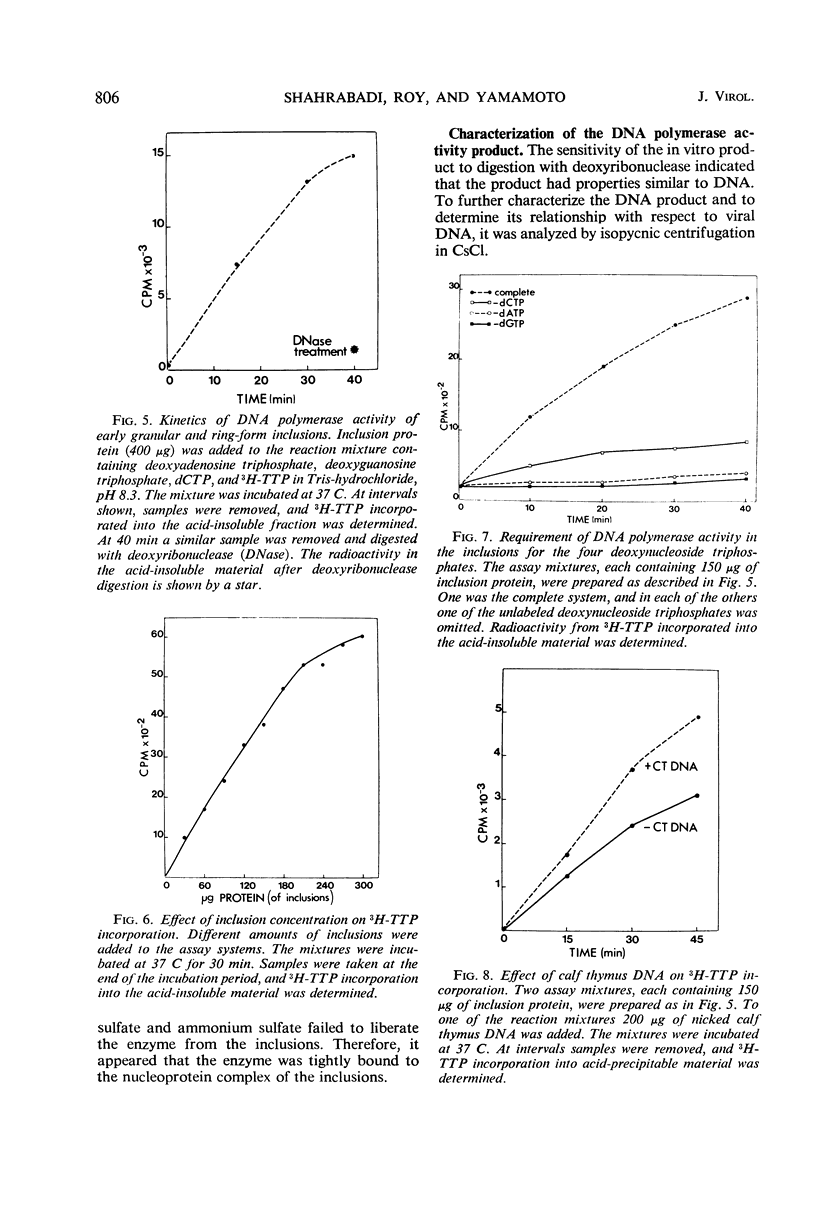
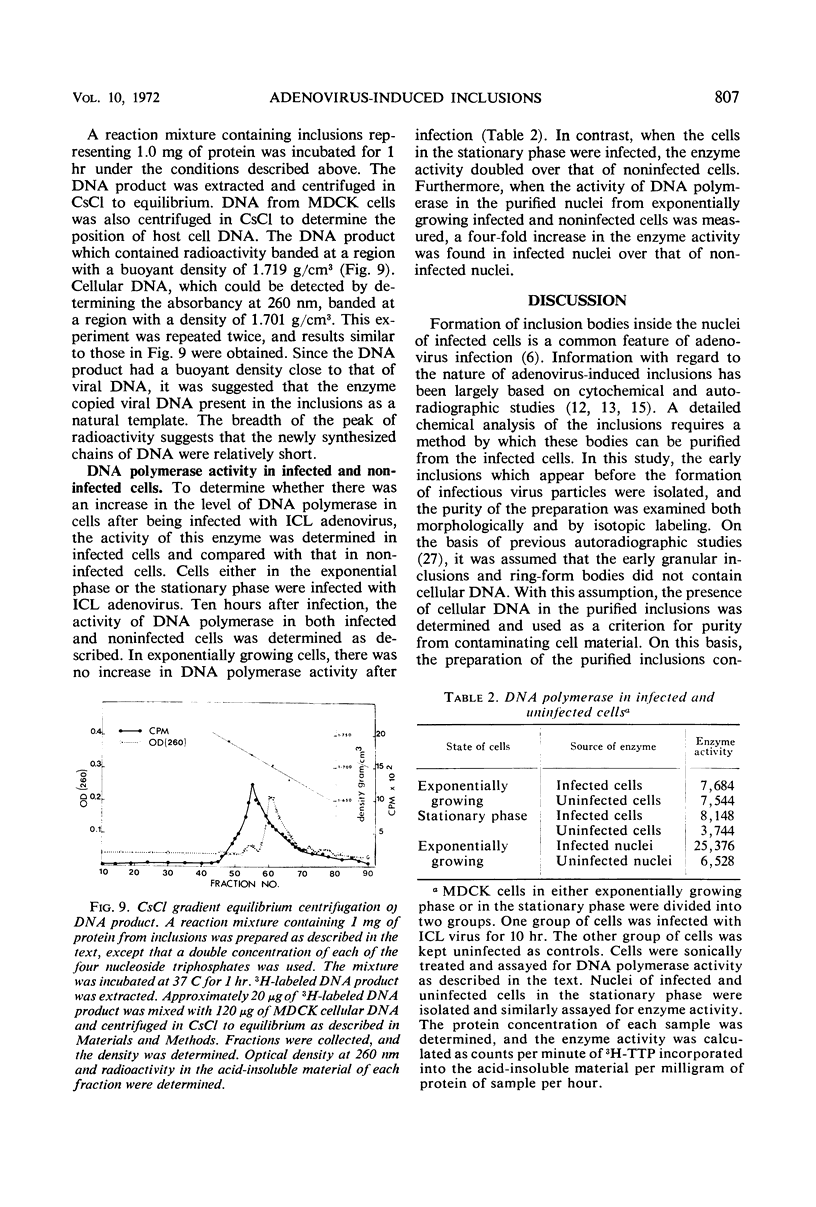
Early inclusions induced by a canine adenovirus in a canine cell line, appearing before the formation of infectious virus particles, were purified by differential centrifugation in sucrose followed by CsCl density gradient centrifugation. Chemical analysis of these inclusions revealed that they contained deoxyribonucleic acid (DNA), ribonucleic acid, and protein. On the basis of density gradient centrifugation, the DNA extracted from the inclusions was found to be viral DNA. Electron microscope autoradiography showed that these inclusions were the sites of DNA synthesis. In addition, association of DNA polymerase activity with the inclusions was detected by incorporation of radioactivity from 3H-thymidine triphosphate into a DNA product. The in vitro product of the enzyme had a density equal to that of viral DNA rather than host DNA. The level of DNA polymerase activity in exponentially growing infected and uninfected whole cells was similar, but in cells in stationary phase the enzyme activity of infected cells was twice that in noninfected cells. Furthermore, nuclei isolated from infected cells showed a fourfold increase in DNA polymerase activity over the noninfected cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEHKI R. M., SCHNEIDER W. C. Incorporation of tritiated thymidine into deoxyribonucleic acid by isolated nuclei. Biochim Biophys Acta. 1963 Jan 29;68:34–44. doi: 10.1016/0006-3002(63)90108-2. [DOI] [PubMed] [Google Scholar]
- BOYER G. S., DENNY F. W., Jr, GINSBERG H. S. Sequential cellular changes produced by types 5 and 7 adenoviruses in HeLa cells and in human amniotic cells; cytological studies aided by fluorescein-labelled antibody. J Exp Med. 1959 Nov 1;110:827–844. doi: 10.1084/jem.110.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Hirsch-Kauffmann M. Intracellular forms of adenovirus deoxyribonucleic acid. I. Evidence for a deoxyribonucleic acid-protein complex in baby hamster kidney cells infected with adenovirus type 12. J Virol. 1972 Feb;9(2):297–308. doi: 10.1128/jvi.9.2.297-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION, VI. PROPERTIES OF HIGHLY PURIFIED TUMORIGENIC HUMAN ADENOVIRUSES AND THEIR DNA. Proc Natl Acad Sci U S A. 1964 Jun;51:1251–1259. doi: 10.1073/pnas.51.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ledinko N. Enhanced deoxyribonucleic acid polymerase activity in human embryonic kidney cultures infected with adenovirus 2 or 12. J Virol. 1968 Feb;2(2):89–98. doi: 10.1128/jvi.2.2.89-98.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., GODMAN G. C., ROSE H. M., HOWE C., HUANG J. S. Electron microscopic and histochemical studies of an unusual crystalline protein occurring in cells infected by type 5 adenovirus. J Biophys Biochem Cytol. 1957 May 25;3(3):505–508. doi: 10.1083/jcb.3.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Palomo A., Granboulan N. Electron microscopy of adenovirus 12 replication. II. High-resolution autoradiography of infected KB cells labeled with tritiated thymidine. J Virol. 1967 Oct;1(5):1010–1018. doi: 10.1128/jvi.1.5.1010-1018.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Palomo A., Le Buis J., Bernhard W. Electron microscopy of adenovirus 12 replication. 1. Fine structural changes in the nucleus of infected KB cells. J Virol. 1967 Aug;1(4):817–829. doi: 10.1128/jvi.1.4.817-829.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk R. G., Norrby E., Lundqvist U. Biophysical comparison of two canine adenoviruses. J Virol. 1970 Apr;5(4):507–512. doi: 10.1128/jvi.5.4.507-512.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. D., Hanawalt P. C. Isolation of DNA replication complexes from uninfected and adenovirus-infected HeLa cells. J Mol Biol. 1971 Nov 28;62(1):65–80. doi: 10.1016/0022-2836(71)90131-8. [DOI] [PubMed] [Google Scholar]
- Penman S., Smith I., Holtzman E., Greenberg H. RNA metabolism in the HeLa cell nucleus and nucleolus. Natl Cancer Inst Monogr. 1966 Dec;23:489–512. [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. XIV. Macromolecule and enzyme synthesis in cells replicating oncogenic and nononcogenic human adenovirus. Virology. 1969 Aug;38(4):573–586. doi: 10.1016/0042-6822(69)90178-0. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Brodaty E., Armstrong J. A. A cytochemical study of basic proteins in adenovirus-infected cells. J Gen Virol. 1971 May;11(2):87–93. doi: 10.1099/0022-1317-11-2-87. [DOI] [PubMed] [Google Scholar]
- Shahrabadi M. S., Yamamoto T. Localization of canine adenovirus capsid antigens in a MDCK cell line by immunoferritin and immunofluorescent techniques. Can J Microbiol. 1972 Aug;18(8):1299–1305. doi: 10.1139/m72-200. [DOI] [PubMed] [Google Scholar]
- Sheek M. R. DNA polymerase in mammalian cells. Influence of adenovirus type 2 on the distribution in nuclear and cytoplasmic extracts. Tex Rep Biol Med. 1966 Fall;24(3):479–488. [PubMed] [Google Scholar]
- Sister M J SMITH, KEIR H. M. DNA nucleotidyltransferase in nuclei and cytoplasm prepared from thymus tissue in nonaqueous media. Biochim Biophys Acta. 1963 Apr 30;68:578–588. doi: 10.1016/0006-3002(63)90187-2. [DOI] [PubMed] [Google Scholar]
- Wallace R. D., Kates J. State of adenovirus 2 deoxyribonucleic acid in the nucleus and its mode of transcription: studies with isolated viral deoxyribonucleic acid-protein complexes and isolated nuclei. J Virol. 1972 Apr;9(4):627–635. doi: 10.1128/jvi.9.4.627-635.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Maruysk R. G. Morphological studies of a canine adenovirus. J Gen Virol. 1968 Jan;2(1):191–194. doi: 10.1099/0022-1317-2-1-191. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Shahrabadi M. S. Enzyme cytochemistry and autoradiography of adenovirus-infected cells as determined with the electron microscope. Can J Microbiol. 1971 Feb;17(2):249–256. doi: 10.1139/m71-042. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Some physical and growth characteristics of a canine adenovirus isolated from dogs with laryngotracheitis. Can J Microbiol. 1966 Apr;12(2):303–311. doi: 10.1139/m66-041. [DOI] [PubMed] [Google Scholar]