Abstract
Recruitment and trafficking of autoreactive leucocytes across the BNB (blood–nerve barrier) is an early pathological insult in GBS (Guillain-Barré syndrome), an aggressive autoimmune disorder of the PNS (peripheral nervous system). Whereas the aetiology and pathogenesis of GBS remain unclear, pro-inflammatory cytokines, including TNFα (tumour necrosis factor α), are reported to be elevated early in the course of GBS and may initiate nerve injury by activating the BNB. Previously, we reported that disrupting leucocyte trafficking in vivo therapeutically attenuates the course of an established animal model of GBS. Here, PNMECs (peripheral nerve microvascular endothelial cells) that form the BNB were harvested from rat sciatic nerves, immortalized by SV40 (simian virus 40) large T antigen transduction and subsequently challenged with TNFα. Relative changes in CCL2 (chemokine ligand 2) and ICAM-1 (intercellular adhesion molecule 1) expression were determined. We report that TNFα elicits marked dose- and time-dependent increases in CCL2 and ICAM-1 mRNA and protein content and promotes secretion of functional CCL2 from immortalized and primary PNMEC cultures. TNFα-mediated secretion of CCL2 promotes, in vitro, the transendothelial migration of CCR2-expressing THP-1 monocytes. Increased CCL2 and ICAM-1 expression in response to TNFα may facilitate recruitment and trafficking of autoreactive leucocytes across the BNB in autoimmune disorders, including GBS.
Keywords: cytokine, trafficking, endothelial cell, peripheral nerve
Abbreviations: AIDP, acute inflammatory demyelinating polyneuropathy; BNB, blood–nerve barrier; CAM, cell adhesion molecule; CCL2, CC chemokine ligand 2; CSF, cerebrospinal fluid; DAPI, 4′,6-diamidino-2-phenylindole; EAN, experimental autoimmune neuritis; ECL, enhanced chemiluminescence; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GBS, Guillain-Barré syndrome; HRP, horseradish peroxidase; ICAM-1, intercellular adhesion molecule 1; JAM1, junctional adhesional molecule 1; MEC, microvascular endothelial cell; NF-κB, nuclear factor κB; PECAM-1, platelet endothelial cell adhesion molecule 1; PFA, paraformaldehyde; PNMEC, peripheral nerve microvascular endothelial cell; PNS, peripheral nervous system; RT–PCR, reverse transcription–PCR; SV40, simian virus 40; TEER, transendothelial electrical resistance; TNFα, tumour necrosis factor α; vWF, von Willebrand factor; ZO-1, zona occludens 1
INTRODUCTION
AIDP (acute inflammatory demyelinating polyneuropathy), the most prevalent North American and European variant of GBS (Guillain-Barré syndrome), is an aggressively debilitating autoimmune disorder that affects the PNS (peripheral nervous system) and remains a leading cause of autoimmune neuromuscular paralysis (Zhu et al., 1998). Although the aetiology of GBS remains unknown, the pathogenesis of GBS probably involves both humoral and cell-mediated immune responses (Quarles and Weiss, 1999; Hartung et al., 2001; Kiefer et al., 2001; Maurer et al., 2002; Ariga and Yu, 2005). Enhanced cytokine-mediated recruitment and trafficking of autoreactive leucocytes across the BNB (blood–nerve barrier) is an established early pathological hallmark of GBS (Hartung et al., 1995; Hughes and Cornblath, 2005) and EAN (experimental autoimmune neuritis), a well-characterized animal model that shares many pathological characteristics with AIDP (Spies et al., 1995; Hahn, 1996; Sarkey et al., 2007).
Circulating autoreactive leucocytes may initially home to peripheral nerves by a mechanism that involves enhanced local gradients of chemokine expression (Zou et al., 1999; Kieseier et al., 2000). At these sites, proinflammatory cytokines, in particular TNFα (tumour necrosis factor α), play a pivotal role in the pathogenesis of both GBS and EAN, as recently reviewed by Lu and Zhu (Lu and Zhu, 2011). An increased presence of TNFα has been detected within affected peripheral nerves of GBS patients (Putzu et al., 2000). Others have demonstrated a strong correlation between elevated plasma concentrations of TNFα and clinical severity of GBS (Exley et al., 1994). Similarly, previous studies have demonstrated an enhanced presence of TNFα expressing macrophage infiltrates within nerve roots from rats with EAN (Stoll et al., 1993). Therapeutically, neutralizing TNFα or its homotrimeric cell surface receptor TNFR1 has been demonstrated to ameliorate the course of EAN (Stoll et al., 1993; Bao et al., 2003). Similarly, TNFα receptor knockout mice exhibit an attenuated course of EAN (Mao et al., 2010).
Chemotactic cytokines and immunoglobulin-like CAMs (cell adhesion molecules) are inflammatory mediators that are highly involved in leucocyte trafficking and transendothelial migration. In patients with GBS, disease severity correlates with enhanced expression of CCL2 (chemokine ligand 2) in CSF (cerebrospinal fluid), as well as by epineurial and endoneurial vascular endothelial cells (Orlikowski et al. 2003, Press et al. 2003). In EAN, marked enhancement of CCL2 expression is reported to preclude the onset of clinical deficits (Fujioka et al., 1999; Kieseier et al., 2000). Antibody-mediated neutralization of CCL2 in vivo ameliorates the course of EAN in rats (Zou et al., 1999). By comparison, expression of ICAM-1 (intercellular adhesion molecule 1), a CAM involved in the firm adhesion stage of leucocyte migration, is elevated in the CSF and by sural nerve endothelial cells in patients at the peak of GBS (Putzu et al., 2000; Sainaghi et al., 2010). In animal studies, similar increases in ICAM-1 mRNA and protein were detected in cauda equina harvested from Lewis rats with EAN (Stoll et al., 1993; Tran et al., 2010), and antibody-mediated neutralization of ICAM-1 attenuates the course of EAN (Archelos et al., 1993).
The mechanism by which inflammatory mediators contribute to peripheral nerve injury in GBS remains unclear. Given that (i) there are clear phenotypic and functional differences between vascular endothelium from different tissues (Yosef et al., 2010) and that (ii) recruitment and trafficking of leucocytes across the activated vascular endothelial barriers is most probably governed by changes in the expression of specific inflammatory mediators unique to the localized vascular bed (Springer, 1994; Harkness et al., 2003; Chui and Dorovini-Zis, 2010), we chose in this study to focus on TNFα-mediated activation of MECs (microvascular endothelial cells) harvested from the rat peripheral nerve. Although advancements have been made towards the purification and culture of MECs derived from peripheral nerve (Argall et al., 1994; Sano et al., 2007; Yosef et al., 2010), no studies to date have addressed how PNMEC (peripheral nerve microvascular endothelial cell) cultures respond to a localized inflammatory challenge such as that experienced during AIDP/EAN. To address this concern, we harvested PNMECs from sciatic nerves of naïve Lewis rats and immortalized them by SV40 (simian virus 40) large T antigen transduction.
Here, we report the stable immortalization of rat PNMEC cultures and demonstrate their retention of key phenotypic, morphologic and biochemical characteristics expected of MECs from the endoneurium of rat peripheral nerve. Activation of primary or immortalized PNMEC cultures with TNFα elicited marked dose- and time-dependent changes in CCL2 and ICAM-1 expression and CCL2 release. Enhanced TNFα-mediated CCL2 secretion also facilitated chemotaxis of CCR2-expressing human THP-1 monocytes in an in vitro migration assay. We argue that increased CCL2 and ICAM-1 expression in response to TNFα may facilitate recruitment and trafficking of autoreactive leucocytes across the BNB in autoimmune disorders, including GBS. Collectively, these findings support the application of PNMEC cultures as a novel in vitro resource for the functional assessment of pathological alterations in endoneurial homoeostasis during an inflammatory challenge, such as that encountered during GBS/EAN.
MATERIALS AND METHODS
PNMEC
This study was conducted using protocols approved by the Edward Hines Jr. VA Hospital Institutional Animal Care and Use Committee in accordance with the principles of laboratory animal care. Primary cultures of MECs were prepared from sciatic nerves of naïve Lewis rats as described previously (Argall et al., 1994; Sarkey et al., 2007). Briefly, harvested sciatic nerves were desheathed to remove the epineurium, perineurium and macrovascular endothelium. Remaining endoneurium was minced and digested with 0.05% (w/v) trypsin and 0.25% (w/v) collagenase at 37°C for 2 h. After incubation, cells were collected, filtered and sedimented at 700 g for 5 min. The cell pellet was resuspended in Endo media [Ham's F10 basal media (Life Technologies) supplemented with 10% (v/v) FBS (foetal bovine serum), 50 μg/ml ECGS (endothelial cell growth supplement; BD Biosciences), 0.4 μg/ml heparin, 5.6 μg/ml amphotericin B (Sigma-Aldrich), 100 unit/ml penicillin, and 100 μg/ml streptomycin (Life Technologies)] and plated on a collagen-coated (6.0 μg/cm2, rat tail collagen type I, Millipore) tissue culture flask under an atmosphere of 5% CO2/95% air. Initial cultures consisted of a mixed population of microvascular endoneurial endothelial cells, perineurial epithelioid myofibroblasts and Schwann cells (Yosef et al., 2010). Myofibroblasts were selectively eliminated by Thy1.1 antibody-mediated complement-dependent cell lysis (Argall et al., 1994). Cultures prepared in this manner were 95±7.5% (n=3) pure, 96±5% (n=12; Trypan Blue dye exclusion) viable (means ± S.D.), and consistently established contact-inhibited monolayers with a distinctive cobblestone-like morphology. Primary PNMEC cultures were restricted to six passages. Under these culture conditions, proliferation of microvascular endoneurial endothelial cells was optimal, whereas Schwann cells did not appreciably proliferate (Argall et al., 1994).
A stably immortalized PNMEC cell line was produced from purified rat primary PNMEC cultures using a replication-deficient SV40 retrovirus encoding a temperature-sensitive, non-SV40-origin binding mutant of the large T antigen and a selectable neomycin resistance gene (gift from Dr. P. Jat, University College of London, London, U.K.). Briefly, semi-confluent primary PNMEC cultures were incubated for 36 h at 37°C in the presence of 8 μg/ml polybrene (Sigma-Aldrich) with undiluted filtered viral supernatant collected from SVU19.5 producer cells (Jat et al., 1986; Jat and Sharp, 1986). Selection was achieved by passage into Endo media containing 200 μg/ml gentamycin (G418; Life Technologies). Antibiotic resistant clones were isolated by dilution into 96-well plates at a theoretical density of 0.33 cells/well. Several clones exhibiting phenotypic (contact-inhibition), morphologic (cobblestone-like appearance) and biochemical [expression of vWF (von Willebrand Factor)/Factor VIII and PECAM-1 (platelet endothelial cell adhesion molecule 1)] properties characteristic of primary MECs were expanded, and a single clone (clone 4.3) was arbitrarily selected for use throughout this study. Immortalized PNMEC cultures were maintained on collagen-coated flasks in Endo media at 37°C under an atmosphere of 5% CO2/95% air.
For migration experiments, a CCR2 expressing human acute monocytic leukaemia cell line (THP-1; a gift from Dr. E. Kovacs, Loyola University Chicago) was maintained in RPMI 1640 containing 10% FBS, 100 unit/ml penicillin, 100 μg/ml streptomycin and 5 mM 2-mercaptoethanol (Sigma-Aldrich).
TEER (transendothelial electrical resistance)
Immortalized PNMEC cultures (1×105 cells/insert) were passaged onto collagen-coated Transwell® microporous (5.0 μm pore size, 0.33 cm2 growth area) inserts in 24-well tissue culture plates. TEER was measured with a DT92 Advanced Series Digital Multimeter (World Precision Instruments) at 24 h intervals after passaging. Collagen coated inserts without cultured cells served as negative controls, and these resistance values were subtracted from experimental resistance values. Measurements were taken in triplicate for each insert and averaged to ensure uniformity.
Immunocytochemistry
Semi-confluent rat primary or immortalized PNMEC cultures grown on collagen-coated coverslips were fixed for 5 min at 23°C by immersion in phosphate-buffered (pH 7.4) 4% PFA (paraformaldehyde), washed and blocked with 1% (v/v) normal goat serum and 0.1% (v/v) Triton X-100. Coverslips were incubated overnight (4°C) in the presence of a 1:50 dilution of mouse anti-human Factor VIII/vWF (Dako) or a 1:50 dilution of rabbit anti-rat PECAM-1 (Serotec) monoclonal antibody. Washed coverslips were incubated with a 1:1000 dilution of either AlexaFluor488-conjugated goat anti-mouse IgG secondary antibody (Life Technologies) or FITC-conjugated goat anti-rabbit IgG secondary antibody (Jackson ImmunoResearch Labs, Inc.), respectively. Coverslips were mounted onto slides using Fluoroshield containing DAPI (4′,6-diamidino-2-phenylindole, Sigma-Aldrich) and visualized with a Leica DMLB upright fluorescent microscope. Images were captured using QCapture Pro software (Q Imaging, Inc.).
Immunoblot analysis
PNMEC cultures were harvested by gentle scraping and lysates were prepared in deionized water supplemented with a commercial cocktail of protease inhibitors (Roche Applied Science). Protein concentrations in cell lysates were quantified by the BCA method (ThermoScientific) using BSA as the standard. Proteins in cell lysates were resolved by SDS/PAGE and transferred onto nitrocellulose membranes as previously described (Von Zee and Stubbs, 2011). Washed membranes were blocked and incubated overnight at 4°C in the presence of primary antibody (1:10 dilution of mouse anti-rat large T antigen (provided by Dr. P. Jat), 1:4000 dilution of rabbit anti-rat CCL2 polyclonal, or 1:100 dilution of mouse anti-rat ICAM-1 monoclonal antibody (Serotec). Washed membranes were incubated for 1 h at 23°C in the presence of HRP (horseradish peroxidase)-conjugated goat anti-mouse IgG (1:2500 dilution) or goat anti-rabbit IgG (1:10000 dilution) secondary antibody (Jackson ImmunoResearch). Immunostained proteins were visualized by ECL (enhanced chemiluminescence; ThermoScientific). To confirm equal protein loading, immunoblots were separately incubated overnight at 4°C in the presence of a 1:10000 dilution of rabbit anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) polyclonal antibody (Trevigen) followed by washing, incubation with HRP-conjugated goat anti-rabbit IgG (1:10000 dilution) secondary antibody and ECL detection.
Real-time RT–PCR (reverse transcription–PCR)
Total RNA was extracted from rat primary and immortalized PNMEC cultures with TRIzol reagent, and 5 μg was reverse-transcribed using Super Script III First Strand Synthesis System (Life Technologies) as previously described (Von Zee et al., 2009). CCL2, ICAM-1, and tight junction associated specific cDNA sequences were amplified by real-time PCR on a Mini-Opticon PCR detection system using iQ SYBR Green Supermix (Bio-Rad) with the rat-specific primer pairs listed in Table 1. For each sample, GAPDH (Table 1) was used as a reference control. Optimized amplification steps of 94°C for 5 min; 94°C for 15 s, annealing at 55–60°C for 30 s, 72°C for 1 min were used. Reaction efficiencies were typically >90%. For each sample, the specificity of the real-time reaction product was determined by melting curve analysis. In some cases, amplified products were visualized by resolution on a 2% (w/v) agarose gel impregnated with ethidium bromide. The size of each amplified product was compared with a 0–500-bp ladder. In all cases, the amplified product corresponded with the predicted amplicon size. Endogenous expression of GAPDH was unaltered by TNFα treatment. Relative fold-changes in CCL2 and ICAM-1 mRNA content in each sample were therefore normalized to expressed levels of GAPDH.
Table 1. Rat primer pairs used in RT-PCR analysis.
| Molecule | Sense (5′→3′) | Antisense (5′→3′) | Amplicon size (bp) | Annealing temperature (°) |
|---|---|---|---|---|
| ICAM-1 | CTGCAGAGCACAAACAGCAGAG | AAGGCCGCAGAGCAAAAGAAGC | 332 | 60 |
| CCL2 | ATGCAGGTCTCTGTCACG | CTAGTTCTCTGTCATACT | 447 | 55 |
| Claudin-5 | GCAGAGCACCGGGCACATGC | TAGTTCTTCTTGTCGTAATCGCC | 483 | 55 |
| Occludin | GCCTTTTGCTTCATCGCTTCC | AACAATGATTAAAGCAAAAGCCAC | 351 | 55 |
| JAM-1 | ACAGCCATGAGGTCAGAGGCT | ACCTAGAAGACATTGAAGGCATC | 348 | 55 |
| ZO-1 | GCGAGGCATCGTTCCTAATAAG | TCGCCACCTGCTGTCTTTG | 81 | 60 |
| GAPDH | TCCCTCAAGATTGTCAGCAA | AGATCCACAACGGATACATT | 308 | 55 |
ELISA
Relative changes in intracellular CCL2 or ICAM-1 protein content were quantified using a cell-based ELISA. Briefly, confluent rat primary or immortalized PNMECs were cultured on collagen-coated 96-well plates and fixed with phosphate-buffered (pH 7.4) 4% PFA for 10 min at 23°C. Fixed cells were permeabilized with 0.1% (v/v) Triton X-100 and blocked for 1 h at 23°C with 1% (w/v) BSA. Fixed PNMEC cultures were incubated overnight at 4°C in the presence of either a 1:2000 dilution of rabbit anti-rat CCL2 polyclonal antibody or a 1:500 dilution of mouse anti-rat ICAM-1 monoclonal antibody (Serotec). Immunostained cells were washed and incubated for 1 h at 23°C in the presence of a 1:5000 dilution of HRP-conjugated goat anti-rabbit or goat anti-mouse secondary antibody (Jackson ImmunoResearch), respectively. Colour development was initiated with SigmaFast OPD substrate (Sigma-Aldrich) for 30 min at 23°C, and stopped with 3 M HCl. Samples were read at 492 nm. Non-specific binding (secondary only control) was subtracted and data reported as percentage increase over quiescent vehicle-treated cells.
The content of CCL2 released from treated PNMEC cultures was determined using a commercially available ELISA kit (ThermoScientific). The working range for this rat-specific CCL2 ELISA kit is 38–1500 pg/ml. Briefly, PNMECs were cultured at a density of 2×105 cells per well (24-well collagen-coated) and media from treated cultures were collected, clarified by centrifugation (700g), and stored at −80°C until use. Samples were diluted with Endo media (1:50 for primary PNMEC cultures, 1:200 for immortalized PNMEC cultures), and aliquots (50 μl) were assayed according to the manufacturer's instructions. Samples were read at 450 nm with a 550 nm correction and results expressed as ng/ml of undiluted CCL2 released.
Transendothelial migration assay
PNMEC cultures were grown to confluency on Transwell® microporous inserts as described above, creating a physiological barrier separating upper and lower compartments. An aliquot (1×105 cells) of CCR2-expressing human THP-1 monocytes was added to the upper compartment, while conditioned medium harvested from the separate vehicle- or TNFα-treated immortalized PNMEC cultures was simultaneously added to the bottom compartment. Vehicle- or TNFα-treated cultures were discarded after the collection of conditioned media. After 4 h at 37°C, cells that migrated into the lower chamber were collected and counted using a haemocytometer. To assess the specific role of CCL2 in THP-1 cell chemotaxis, conditioned medium was pre-treated (30 min, 37°C) with a neutralizing polyclonal antibody directed against rat CCL2 (5 μg/ml; Serotec). As an additional control, the neutralizing CCL2 antibody was denatured (95°C, 10 min) in some experiments prior to addition to conditioned medium.
Statistical analysis
Data are expressed as the mean ± SEM of n observations unless noted otherwise. Statistical significance between two groups was determined by unpaired Student's t-test. Significance between multiple experimental groups was determined by one-way or two-way ANOVA with a Bonferroni or Dunnett's post-hoc analysis. In each case, P<0.05 was considered statistically significant.
RESULTS
PNMEC
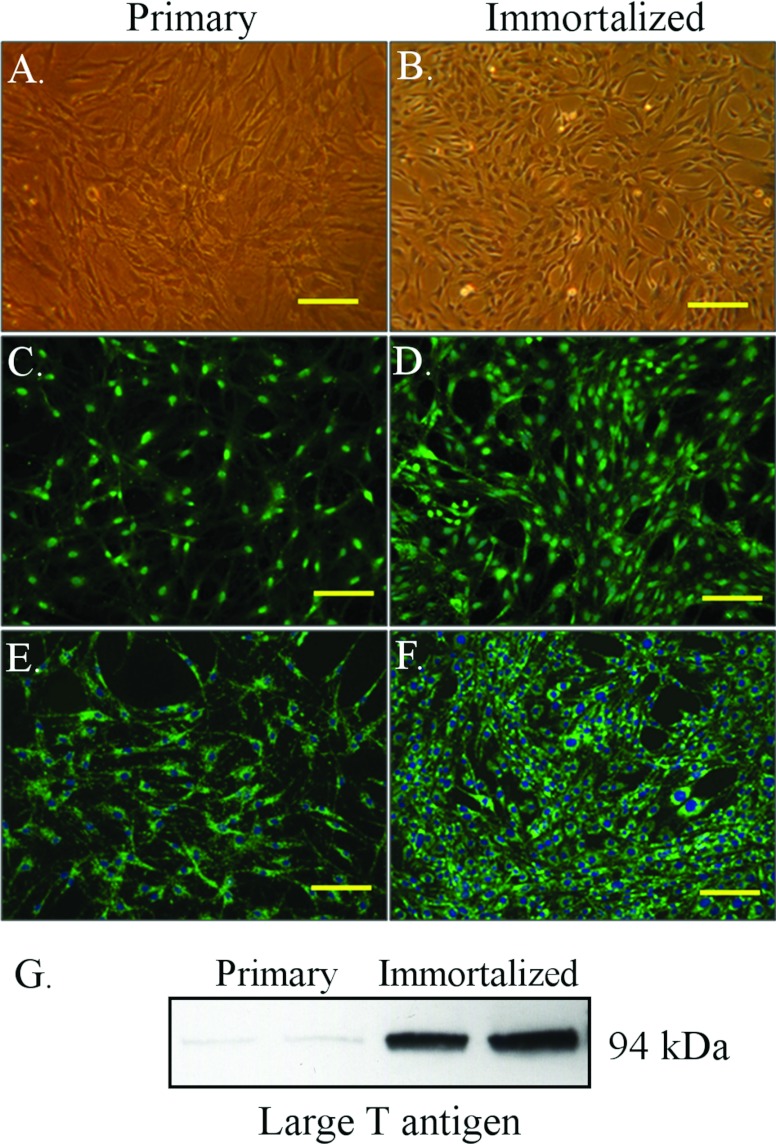
Primary MECs were freshly harvested from rat sciatic nerve using a modification of the procedure used by Argall et al. (1994). Epithelioid myofibroblasts, the major cell contami-nant (Yosef et al., 2010), were routinely eliminated from prepared cultures by Thy 1.1 antibody-mediated comple-ment-driven cell lysis (Argall et al., 1994). PNMEC cultures purified in this manner were found to (i) be >95±7.5% (n=3) free of Thy-1.1 expressing fibroblasts, (ii) consistently establish contact-inhibited monolayers with a distinctive cobblestone-like morphology (Figure 1A) and (iii) markedly express vWF/Factor VIII (Figure 1C) and PECAM-1 (CD31, Figure 1E), cell markers highly characteristic of MECs. Cell viability of prepared primary cultures was typically 96±5% (n=12), as routinely monitored by Trypan Blue dye exclusion. SV40 large T antigen transduction of rat primary PNMECs was found to yield stable immortalized clones that uniquely retain these phenotypic and morphologic characteristics of primary MECs (Figures 1B, 1D and 1F). Expression of large T antigen was specific to immortalized clones, as primary PNMEC cultures did not express measurable quantities of this protein (Figure 1G).
Figure 1. Immortalized PNMEC cultures retain their primary characteristics.
(A, B) Phase-contrast or (C–F) immunofluorescent photomicrographs of primary or immortalized PNMEC cultures, as indicated, immunostained for expression of (C, D) vWF or (E, F) PECAM-1 (CD31). Data shown are photomicrographs of stained cells representative of six separate cultures. Nuclei are counterstained with DAPI. Bar, 100 μm. (G) Western immunoblot of large T antigen protein expressed in lysates prepared from primary or immortalized PNMEC cultures, as indicated. Data shown are from a single experiment performed in duplicate.
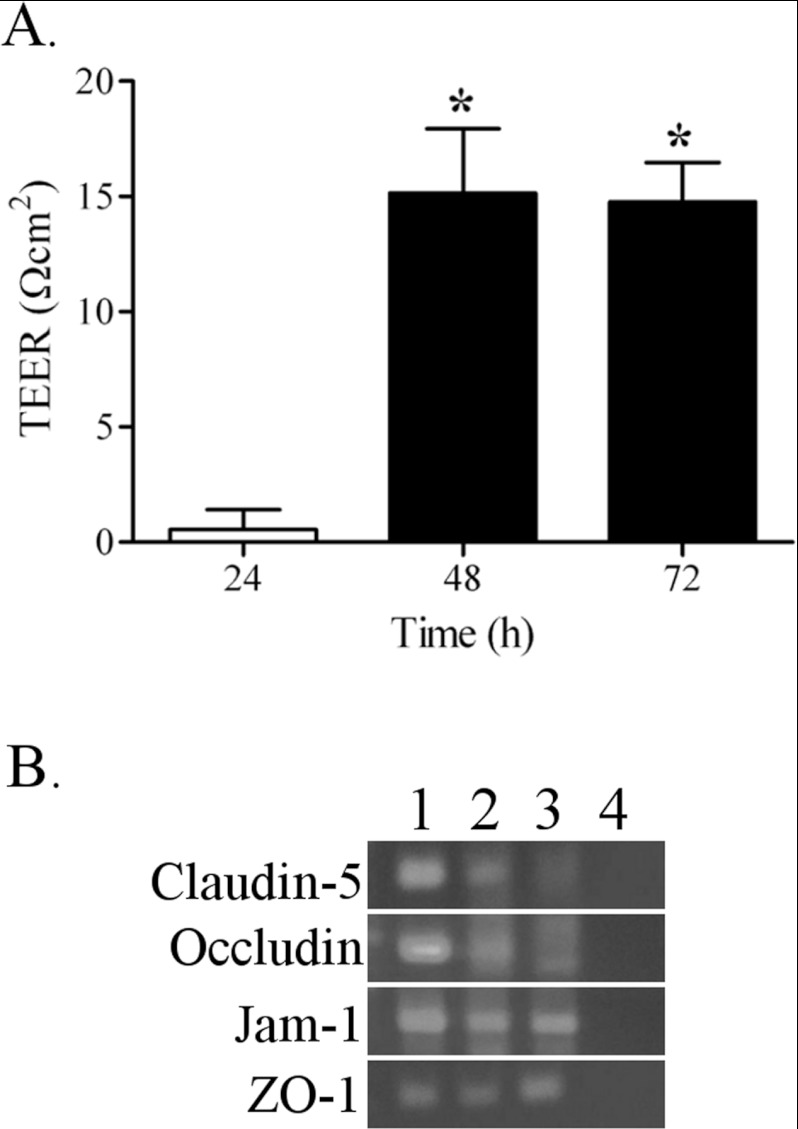
To confirm barrier formation and function in PNMEC cultures, we measured TEER and determined expression of tight junction-associated molecules. Twenty-four hours after passaging immortalized cells onto collagen-coated Transwell inserts, TEER values did not significantly differ from negative control (insert only) (Figure 2A). However, 48–72 h after passaging, an increase (15 Ω cm2) in TEER values was observed across monolayers of immortalized PNMEC cultures (Figure 2A), in good agreement with previously reported TEER values of endothelial cells that form the highly restrictive blood–brain barrier (Davidson et al., 2009).
Figure 2. Barrier properties of immortalized PNMEC cultures.
(A) TEER values of immortalized PNMECs cultured on collagen-coated inserts. Data shown are results from a single experiment (n=5). * P<0.01; one-way ANOVA with Dunnett's post-hoc analysis. (B) Qualitative comparison of tight junction associated molecules claudin-5, occludin, JAM1 and ZO-1 expressed in (1) primary PNMEC cultures, (2) immortalized PNMEC cultures and (3) rat brain as determined by RT-PCR. Lane (4), no template control.
Both primary and immortalized PNMECs express measurable levels of tight junction associated molecules including claudin-5, occludin, JAM1 (junctional adhesional molecule 1) and ZO-1 (zona occludens 1), as qualitatively demonstrated by RT-PCR (Figure 2B). In each case, the observed amplicon size of amplified products agreed well with predicted values. For comparison, we demonstrate that these molecules are also measurably expressed in brain, where they are known to participate in establishing tight junctions of the blood–brain barrier (Brown et al., 2007).
TNFα induces CCL2 mRNA and protein expression
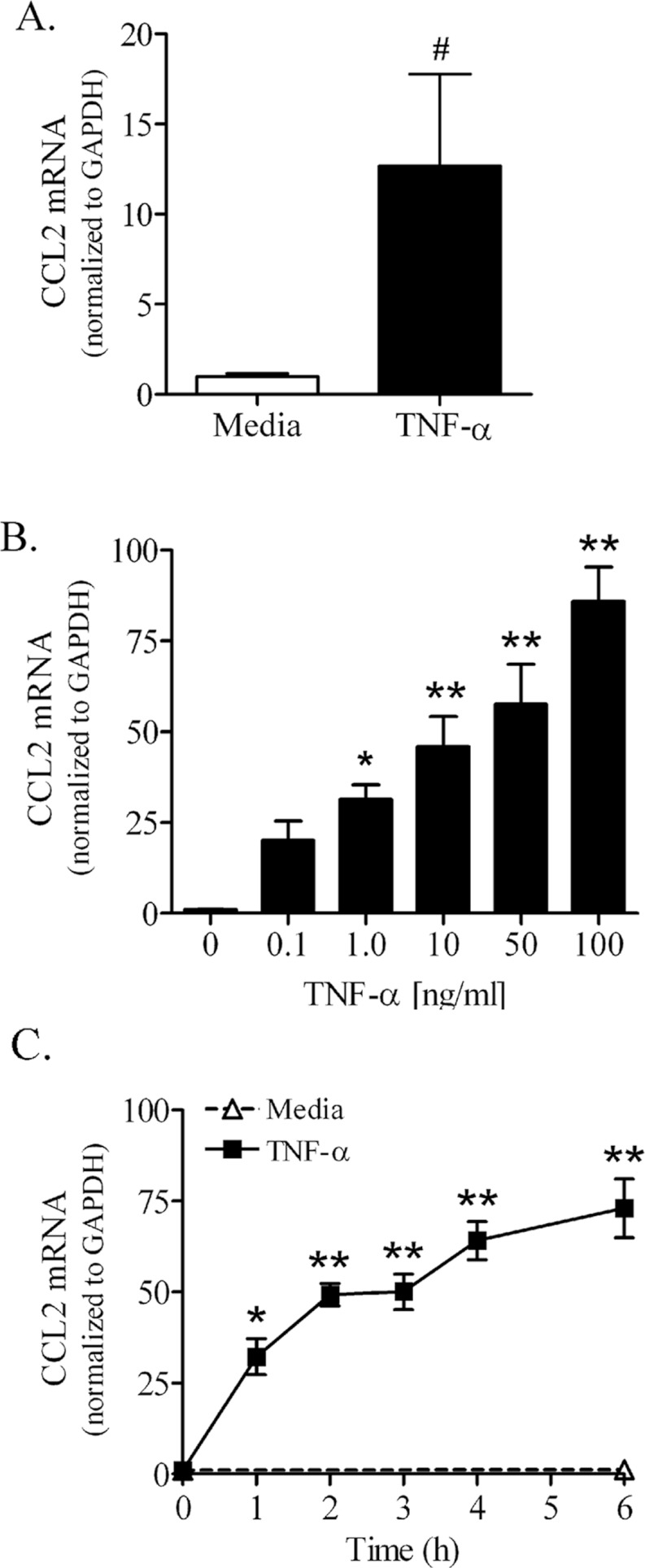
Confluent PNMEC cultures responded robustly to TNFα treatment by markedly increasing CCL2 mRNA. In primary PNMEC cultures, TNFα (10 ng/ml, 3 h) elicited a marked (>12-fold) increase in the content of CCL2 mRNA compared with quiescent (media-treated) cells (Figure 3A). Immortalized PNMEC cultures responded similarly to TNFα stimulation, with both a dose- (Figure 3B) and time- (Figure 3C) dependent increase (>75-fold) in CCL2 mRNA content. In contrast, the content of CCL2 mRNA in quiescent (media-treated) immortalized PNMEC cultures was below the level of detection (Figure 3C).
Figure 3. TNFα increases CCL2 mRNA content in primary and immortalized PNMEC cultures.
(A) Primary PNMEC cultures were incubated in the absence (media) or presence of TNFα (10 ng/ml) for 3 h. (B, C) Immortalized PNMEC cultures were treated with (B) 0–100 ng/ml TNFα for 2 h or (C) 0–6 h with 10 ng/ml TNFα, as indicated. Relative changes in CCL2 mRNA content were quantified by qRT-PCR (quantitative real-time PCR). Data shown are the GAPDH-normalized fold changes from two separate experiments (n=4–9 cultures) and expressed as means±S.E.M. #, P<0.05; unpaired Student's t-test. *, P<0.05; ** P<0.01; one-way ANOVA with Dunnett's post-hoc analysis.
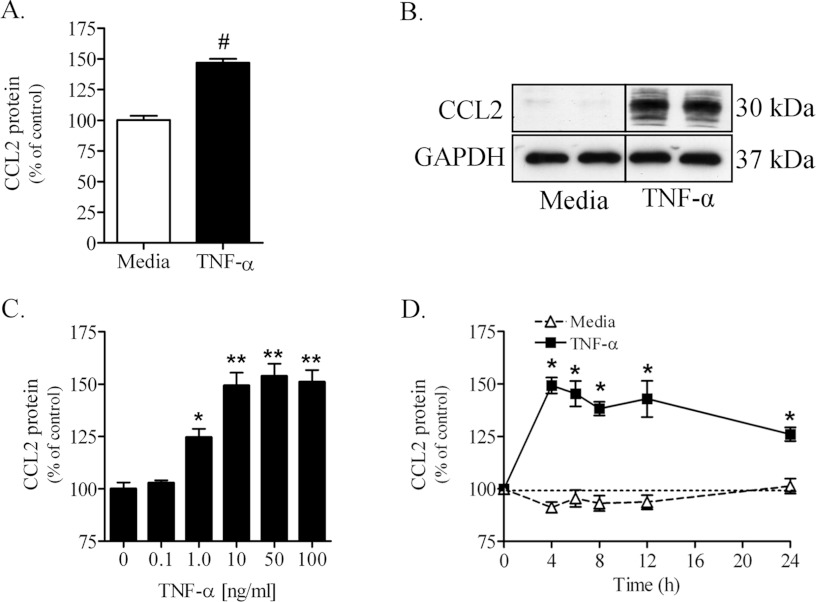
Treating primary PNMEC cultures with TNFα (10 ng/ml, 4 h) increased, by approximately 50%, the content of intracellular CCL2 protein as measured by cell-based ELISA (Figure 4A). Consistent with this observation was a robust TNFα (10 ng/ml, 4 h)-mediated increase of a 30 kDa protein identified by immunoblot as CCL2 in lysates of treated immortalized PNMEC cultures (Figure 4B). The higher molecular mass isoform of this chemokine is in agreement with the previous reports of N-terminal glycosylation of CCL2 in rodents (Liu et al., 1996; Ruggiero et al., 2003). By comparison, the content of CCL2 protein in lysates of quiescent (media-treated) PNMEC cultures was below the detectable levels (Fig-ure 4B). TNFα elicited both dose- (Figure 4C) and time- (Figure 4D) dependent increases in CCL2 protein content in immortalized PNMEC cultures. No significant changes in intracellular CCL2 protein content were observed over the course of 24 h in media-treated immortalized PNMEC cultures (Figure 4D), consistent with minimal constitutive expression of this chemokine.
Figure 4. TNFα increases CCL2 protein content in primary and immortalized PNMEC cultures.
Intracellular CCL2 content in (A) primary or (B) immortalized PNMEC cultures incubated in the absence (media) or presence of TNFα (10 ng/ml) for 4 h was quantified by cell-based ELISA or immunoblot, respectively. (C, D) Immortalized PNMEC cultures were cultured with (C) 0–100 ng/ml TNFα for 4 h or (D) 0–24 h with 10 ng/ml TNFα, as indicated, and intracellular CCL2 protein was quantified by cell-based ELISA. Data shown are results from 2–3 separate experiments (A, n=12–14 cultures; C, D, n=6–10 cultures). Immunoblot data shown in (B) are from a single experiment performed in duplicate. (A) #, P<0.0001 unpaired Student's t-test; (C) *, P<0.05; ** P<0.01; one-way ANOVA with Dunnett's post-hoc analysis. (D) *, P<0.001; two-way ANOVA with Bonferroni's post-hoc analysis.
TNFα induces ICAM-1 mRNA and protein expression
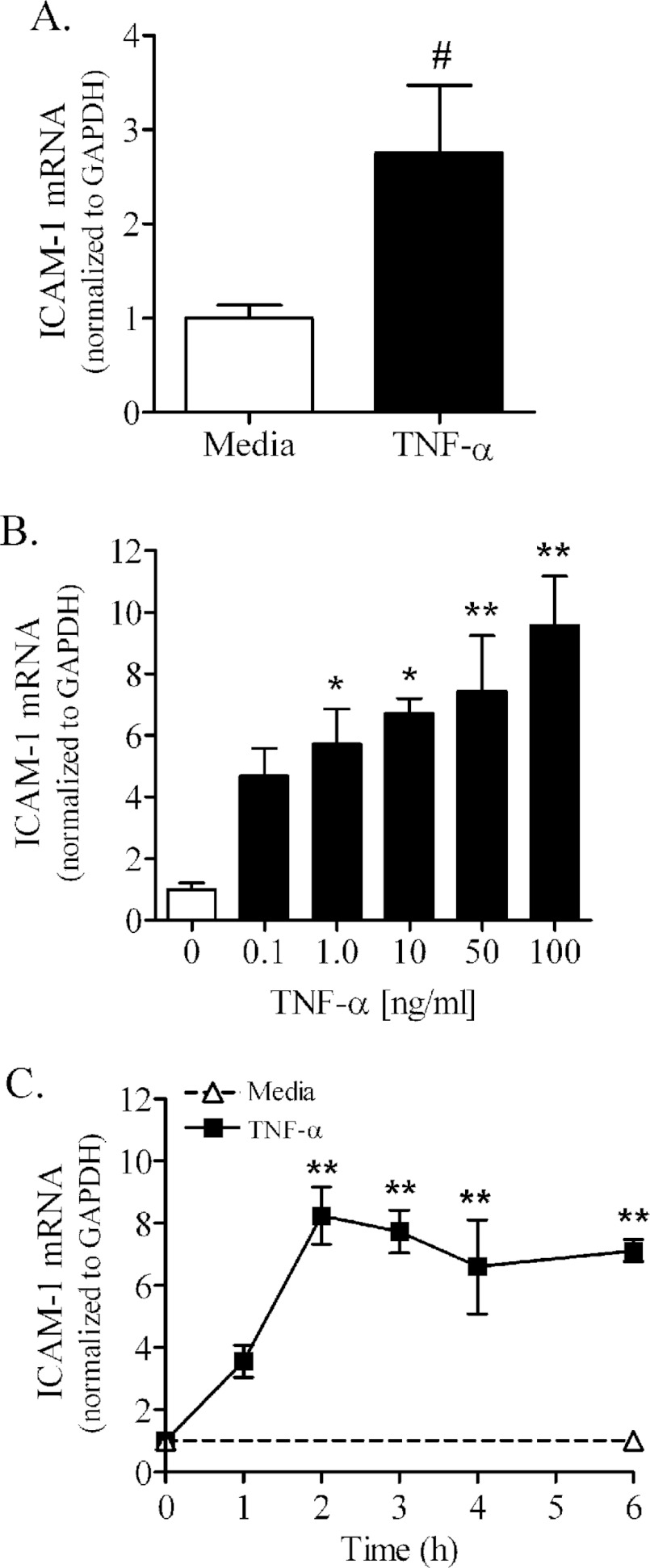
PNMEC cultures treated with TNFα exhibited marked increases in ICAM-1 mRNA and protein expression (Figures 5 and 6). TNFα (10 ng/ml, 3 h) elicited in primary PNMEC cultures a modest but significant (>2-fold) increase in ICAM-1 mRNA content (Figure 5A). Immortalized PNMEC cultures similarly responded to TNFα treatment by up-regulating ICAM-1 mRNA content nearly 10-fold in a manner that was both dose- and time-dependent (Figures 5B and 5C). By comparison, constitutive ICAM-1 mRNA expression was unchanged in quiescent (media-treated) PNMEC cultures over the course of the 6 h assay (Figure 5C).
Figure 5. TNFα increases ICAM-1 mRNA content in primary or immortalized PNMEC cultures.
(A) Primary PNMEC cultures were incubated in the absence (media) or presence of TNFα (10 ng/ml) for 3 h. (B, C) Immortalized PNMEC cultures were treated with (B) 0–100 ng/ml TNFα for 2 h or (C) 0–6 h with 10 ng/ml TNFα, as indicated. Relative changes in ICAM-1 mRNA content were quantified by qRT-PCR (quantitative RT-PCR). Data shown are the GAPDH-normalized fold changes from two separate experiments (n=4–9 cultures) and expressed as means ± S.E.M. #, P<0.05; unpaired Student's t-test. *, P<0.05; ** P<0.01; one-way ANOVA with Dunnett's post-hoc analysis.
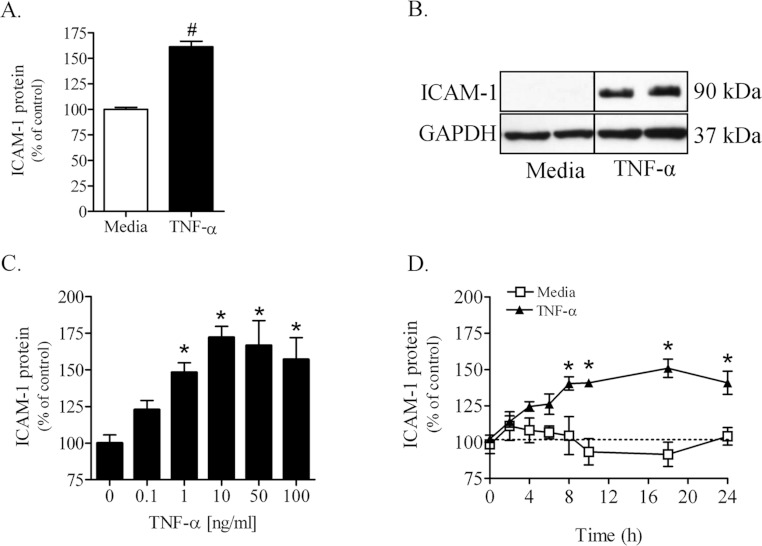
Figure 6. TNFα increases ICAM-1 protein content in primary and immortalized PNMEC cultures.
ICAM-1 content in (A) primary or (B) immortalized PNMEC cultures incubated in the absence (media) or presence of TNFα (10 ng/ml) for 16 h was quantified by cell-based ELISA or immunoblot, respectively. (C, D) Immortalized PNMEC cultures were cultured with (C) 0–100 ng/ml TNFα for 16 h or (D) 0–24 h with 10 ng/ml TNFα, as indicated, and ICAM-1 protein was quantified by cell-based ELISA. Data shown are results from two separate experiments (A, n=12–14 cultures; C, D, n=6 cultures). Immunoblot data shown in (B) are from a single experiment performed in duplicate, representative of three separate experiments. (A) #, P<0.0001 unpaired Student's t-test; (C) *, P<0.01; one-way ANOVA with Dunnett's post-hoc analysis. (D) *, P<0.01; two-way ANOVA with Bonferroni's post-hoc analysis.
Constitutive expression of ICAM-1 protein in quiescent primary or immortalized PNMEC cultures was below the level of detection (Figure 6). Primary PNMEC cultures treated with TNFα, however, exhibited a 50% increase in ICAM-1 protein content as quantified by cell-based ELISA (Figure 6A). Lysates of TNFα-treated immortalized PNMEC cultures contained a marked increase in a 90 kDa protein identified by immunoblot as ICAM-1 (Figure 6B). TNFα elicited increases in ICAM-1 protein content within immortalized PNMEC cultures were dose- (Figure 6C) and time- (Figure 6D) dependent. By comparison, constitutive ICAM-1 protein expression was unchanged in quiescent (media-treated) PNMEC cultures over the course of 24 h assayed (Figure 6D).
TNFα promotes CCL2-mediated chemotaxis
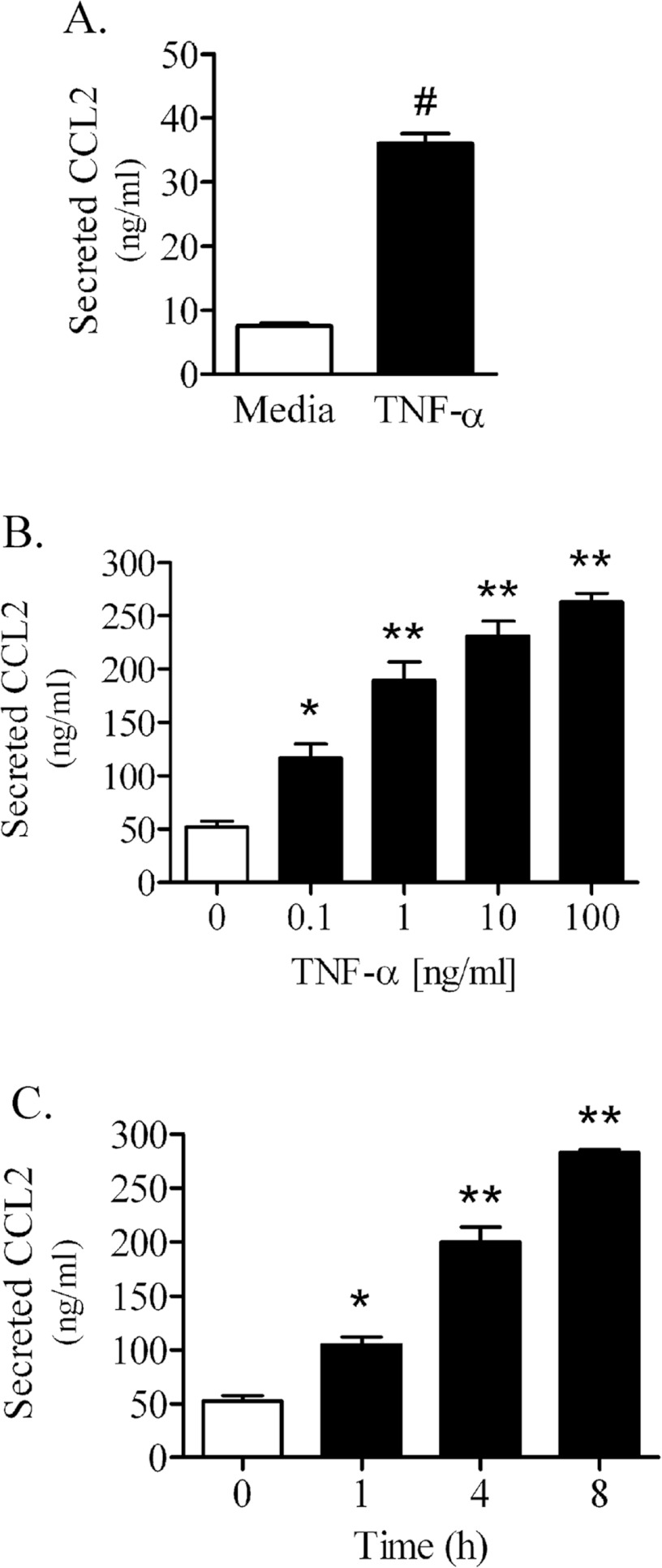
In addition to inducing quantifiable increases in CCL2 mRNA and protein expression, TNFα significantly enhanced the release of CCL2 protein into the culture media from primary and immortalized PNMEC cultures (Figure 7). The absolute content of CCL2 protein constitutively secreted from quiescent (media-treated) PNMEC cultures was within detectable limits (10–50 ng/ml), and was significantly enhanced by TNFα treatment (Figures 7A and 7B). TNFα (10 ng/ml, 4 h) enhanced CCL2 secretion >4-fold from primary PNMEC cultures (Figure 7A). By comparison, TNFα treatment elicited a dose- (Figure 7B) and time- (Figure 7C) dependent effect on CCL2 release from immortalized PNMEC cultures.
Figure 7. TNFα promotes secretion of CCL2 from primary and immortalized PNMEC cultures.
(A) Content of CCL2 secreted from primary PNMEC cultures incubated in the absence (media) or presence of TNFα (10 ng/ml) for 4 h. (B, C) Immortalized PNMEC cultures were treated with (B) 0–100 ng/ml TNFα for 4 h or (C) 0–8 h with 10 ng/ml TNFα, as indicated, and the content of CCL2 released into the cell culture media was quantified by ELISA. Data shown are the means±S.E.M. (n=3–6 cultures). (A) #, P<0.0001, Student's t-test. (B, C) *; P<0.05; **; P<0.01, one-way ANOVA with Dunnett's post-hoc analysis.
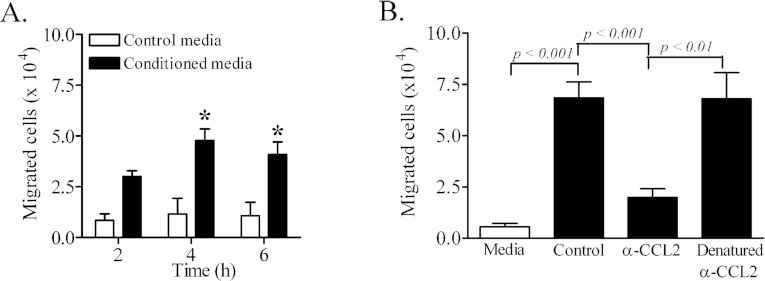
The functional consequence of TNFα-mediated enhancement of CCL2 secretion was determined in vitro using CCR2-expressing human THP-1 monocytes (Figure 8). Human CCR2 is reported to recognize rodent CCL2 (Sarafi et al., 1997). In addition, CCR2-expressing human THP-1 cells respond to rodent CCL2 (Neumark et al., 1999). Human THP-1 monocytes have been used in in vitro assays with mouse or rat CCL2 as a chemoattractant (Lynn et al., 2001; Matoba et al., 2010). Chemotaxis of THP-1 monocytes through monolayers of quiescent immortalized PNMEC cultures in response to control media was minimal (Figure 8). In contrast, addition of conditioned media harvested from TNFα (10 ng/ml, 4 h) treated immortalized PNMEC cultures significantly promoted THP-1 monocyte chemotaxis (Figure 8A). The presence of a CCL2-neutralizing antibody significantly attenuated THP-1 monocyte chemotaxis. Heat-denatured CCL2-neutralizing antibody was, however, ineffective at preventing THP-1 monocyte chemotaxis in response to TNFα-conditioned media (Figure 8B).
Figure 8. Secreted CCL2 promotes migration of THP-1 monocytes across PNMEC culture monolayers.
Immortalized PNMEC cultures were incubated in the absence (media) or presence of TNFα (10 ng/ml) for 4 h, and conditioned cell-culture medium was used to assay for THP-1 monocyte chemotaxis across PNMEC monolayers. (A) Total migrated cells in response to 2–6 h treatment with medium or TNFα-conditioned medium. (B) Total migrated cells in response to 4 h treatment with medium or TNFα-conditioned medium supplemented without (control) or with an CCL2 neutralizing antibody (α-CCL2) or with heat-denatured anti-CCL2 antibody. Data shown are the means±S.E.M. (n=3–7 cultures). (A) *, P<0.01, two-way ANOVA with Bonferroni's post-hoc analysis. (B) One-way ANOVA with Bonferroni's post-hoc analysis.
DISCUSSION
To our knowledge, this is the first report demonstrating stable SV40 large T antigen immortalization and partial characterization of purified microvascular endoneurial endothelial cells harvested from sciatic nerves of naïve Lewis rats. Immortalized PNMEC cultures are shown to retain key phenotypic, morphologic and biochemical properties highly characteristic of primary microvascular endoneurial endothelial cells. In addition to exhibiting contact-inhibited monolayers with a cobblestone-like morphology and marked expression of endothelial cell and barrier markers, immortalized PNMEC cultures responded robustly to a proinflammatory cytokine (TNFα) challenge by increasing the expression of inflammatory mediators CCL2 and ICAM-1. The functional consequence of a TNFα challenge on localized up-regulation of inflammatory mediators is underscored by a marked increase in release of CCL2 from the activated peripheral nerve microvascular endothelium. Here, we propose that increased CCL2 and ICAM-1 expression in response to TNFα may facilitate recruitment and trafficking of autoreactive leucocytes across the BNB in autoimmune disorders, including GBS.
Advancements have been made towards the purification and culture of MECs derived from peripheral nerve (Argall et al., 1994; Sano et al., 2007; Yosef et al., 2010). Vascular endothelial cell characteristics vary according to tissue origin and species (Bell and Weddell, 1984a, 1984b) and, to our knowledge, no studies to date have addressed how PNMECs respond to a localized inflammatory challenge such as that experienced during GBS/EAN. In this study, viable cultures of primary microvascular endoneurial endothelial cells from rat sciatic nerve were routinely prepared at >95% purity. We used a replication-deficient SV40 retrovirus encoding a temperature sensitive non-SV40-origin binding mutant of the large T antigen (Jat et al., 1986; Jat and Sharp, 1986; Greenwood et al., 1996) to produce from these primary PNMEC cultures a stably immortalized clone. Immortalized PNMECs were found to retain key phenotypic (contact-inhibited monolayers), morphologic (distinctive cobblestone-like appearance), biochemical (localized expression of vascular endothelial cell markers vWF/Factor VIII and PECAM-1) and barrier-related (expression of tight junction associated molecules) characteristics exhibited by primary rat PNMEC cultures. Immortalized PNMEC monolayers also exhibited increased TEER, an established measure of barrier function.
Recruitment and extravasation of autoreactive leucocytes into privileged tissue compartments, such as peripheral nerves, is thought to initially involve establishment of localized chemotactic cytokine gradients, including CCL2 (Yadav et al., 2010). Consistent with this thesis, we observed a rapid and marked increase in CCL2 mRNA and protein expression in PNMEC cultures responding to a TNFα challenge. The physiological concentration of TNFα in plasma of patients with GBS is reported to exceed 0.1 ng/ml (Reuben et al., 2002; Radhakrishnan et al., 2004). At this low concentration, TNFα elicited measurable increases in CCL2 mRNA content in immortalized PNMEC cultures. Higher concentrations of TNFα (10–100 fold) were found to elicit measurable changes in both CCL2 mRNA and protein expression within PNMECs. Localized concentrations of TNFα within peripheral nerves during an immune challenge remain to be determined, and may very well mimic or exceed concentrations used in this study. In vascular endothelial cells, chemokines (including CCL2) are reportedly stored in novel regulated secretory granules that are distinct from vWF-containing Weibel-Palade bodies (Knipe et al., 2010). Exposure of endothelial cells to an inflammatory mediator elicits rapid translocation to the cell surface of stored vesicles where they release their chemotactic contents into the extracellular space (Oynebraten et al., 2004, 2005; Deshmane et al., 2009; Knipe et al., 2010). Consistent with these observations, primary and immortalized PNMEC cultures used in this study similarly responded to a TNFα challenge by releasing into the culture medium measurable quantities of functionally active CCL2 protein. Antibody-mediated neutralization of released CCL2 protein attenuated migration of CCR2-expressing human THP-1 monocytes in an in vitro migration assay. A modest, although statistically insignificant, increase in migrated monocytes over baseline was observed with antibody neutralization. This discrepancy may be attributed to the presence of other C–C chemokines [MIP-1α (macrophage inflammatory protein 1α) and RANTES (regulated upon activation, normal T-cell expressed and secreted)] in the TNFα-conditioned media.
Responding leucocytes migrate through the endothelium into peripheral nerves, in part, by a process that first involves their engagement of temporally expressed endothelial CAMs precipitating tethering, slow rolling, firm adhesion and ultimate diapedesis (Springer, 1994; Raab et al., 2002). Cytokine-mediated activation of the endothelium facilitates migration of autoreactive leucocytes into peripheral nerves in EAN (Stoll et al., 1993). Given that ICAM-1 is directly involved in leucocyte trafficking (Greenwood et al., 2002), we determined the effects of a TNFα challenge on eliciting changes in ICAM-1 expression. Previous studies have reported constitutive ICAM-1 expression in vascular endothelial cells (Rahman and Fazal, 2009). However, endogenous expression of ICAM-1 by primary and immortalized rat PNMEC cultures was below the level of detection. Challenging immortalized PNMEC cultures with TNFα induced a dose- and time-dependent increase in ICAM-1 mRNA and protein expression, rendering these cells capable of firm adhesion with autoreactive leucocytes. Importantly, the effect of TNFα on ICAM-1 expression was similarly observed in primary PNMEC cultures, in strong support of a physiological response rather than an artefact of cell transformation. Compared with expression of CCL2, the time course by which TNFα elicited an increase in ICAM-1 expression was slightly delayed, reflecting differential temporal dynamics consistent with their respective physiological roles in leucocyte recruitment and extravasation (Springer, 1994; Raab et al., 2002).
The mechanism by which a TNFα challenge elicits increases in CCL2 and ICAM-1 mRNA content in PNMEC cultures may involve NF-κB (nuclear factor κB)-dependent transcription of the CCL2 and ICAM-1genes (Xing and Remick, 2007; Deshmane et al., 2009). Consistent with this thesis, TNFα was capable of inducing translocation of NF-κB p65 to the nucleus in immortalized PNMECs (results not shown). The precise mechanisms by which TNFα binding to its receptor leads to an increase in NF-κB activation remain unknown, but a role for Rho GTPase activation in this process has been previously suggested (Zhao and Pothoulakis, 2003; Williams et al., 2008).
Expression of CCL2 and ICAM-1 protein content by TNFα in PNMEC cultures may result from an increase in de novo synthesis, reduced proteasomal-, autophagosomal- or lysosomal-dependent degradation of existing protein or from decreased release (exocytosis) of stored chemokine (Knipe et al., 2010). While these various mechanisms have not yet been elucidated in these PNMEC cultures, the observed rapid increase in CCL2 protein release from TNFα-challenged PNMEC cultures suggests, to us, that exocytosis of CCL2 is not impaired in these cells. Additional studies are currently being conducted to determine the mechanism by which TNFα elicits changes in CCL2 and ICAM-1 gene and protein expression.
In conclusion, we report the stable immortalization of rat PNMECs, which retain key phenotypic, morphological, biochemical characteristics and barrier-forming properties expected of primary microvascular endoneurial endothelium. We demonstrate that TNFα elicits robust increases in CCL2 and ICAM-1 mRNA and protein content, while significantly promoting CCL2 release, in both primary and immortalized PNMEC cultures. TNFα-mediated enhancement of CCL2 secretion facilitated migration of human THP-1 monocytes in vitro. TNFα facilitated alterations in other inflammatory mediators in PNMEC cultures are anticipated and remain to be established. Increased TEER, coupled with the expression of tight junction associated molecules, represent unique characteristics of specialized barrier-forming endothelial cells. Collectively, these findings support the application of PNMEC cultures as a novel in vitro resource for the functional assessment of pathological alterations in endoneurial homoeostasis during an inflammatory challenge, such as that encountered during GBS/EAN.
FUNDING
This work was supported, in part, by the Department of Veterans Affairs [grant numbers B3413R and B3756F (to E.B.S.)], a Pre-Doctoral Associated Health Rehabilitation Research Fellowship (to K.A.L.) and the National Institutes of Health [grant number 1R03NS061033 (to E.B.S.)].
References
- Archelos JJ, Maurer M, Jung S, Toyka KV, Hartung HP. Suppression of experimental allergic neuritis by an antibody to the intracellular adhesion molecule ICAM-1. Brain. 1993;116:1043–1058. doi: 10.1093/brain/116.5.1043. [DOI] [PubMed] [Google Scholar]
- Argall KG, Armati PJ, Pollard JD. A method for the isolation and culture of rat peripheral nerve vascular endothelial cells. Mol Cell Neurosci. 1994;5:413–417. doi: 10.1006/mcne.1994.1051. [DOI] [PubMed] [Google Scholar]
- Ariga T, Yu RK. Antiglycolipid antibodies in Guillain-Barre syndrome and related diseases: review of clinical features and antibody specificities. J Neurosci Res. 2005;80:1–17. doi: 10.1002/jnr.20395. [DOI] [PubMed] [Google Scholar]
- Bao L, Lindgren JU, Zhu Y, Ljunggren HG, Zhu J. Exogenous soluble tumor necrosis factor receptor type I ameliorates murine experimental autoimmune neuritis. Neurobiol Dis. 2003;12:73–81. doi: 10.1016/s0969-9961(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Bell MA, Weddell AG. A descriptive study of the blood vessels of the sciatic nerve in the rat, man and other mammals. Brain. 1984a;107(Pt 3):871–898. doi: 10.1093/brain/107.3.871. [DOI] [PubMed] [Google Scholar]
- Bell MA, Weddell AG. A morphometric study of intrafascicular vessels of mammalian sciatic nerve. Muscle Nerve. 1984b;7:524–534. doi: 10.1002/mus.880070703. [DOI] [PubMed] [Google Scholar]
- Brown RC, Morris AP, O’Neil RG. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007;1130:17–30. doi: 10.1016/j.brainres.2006.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui R, Dorovini-Zis K. Regulation of CCL2 and CCL3 expression in human brain endothelial cells by cytokines and lipopolysaccharide. J Neuroinflammation. 2010;7:1. doi: 10.1186/1742-2094-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MM, Walker WF, Hernandez-Rosa E. The m.3243A>G mtDNA mutation is pathogenic in an in vitro model of the human blood brain barrier. Mitochondrion. 2009;9:463–470. doi: 10.1016/j.mito.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley AR, Smith N, Winer JB. Tumour necrosis factor-alpha and other cytokines in Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. 1994;57:1118–1120. doi: 10.1136/jnnp.57.9.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka T, Purev E, Rostami A. Chemokine mRNA expression in the cauda equina of Lewis rats with experimental allergic neuritis. J Neuroimmunol. 1999;97:51–59. doi: 10.1016/s0165-5728(99)00048-x. [DOI] [PubMed] [Google Scholar]
- Greenwood J, Etienne-Manneville S, Adamson P, Couraud PO. Lymphocyte migration into the central nervous system: implication of ICAM-1 signalling at the blood–brain barrier. Vascul Pharmacol. 2002;38:315–322. doi: 10.1016/s1537-1891(02)00199-4. [DOI] [PubMed] [Google Scholar]
- Greenwood J, Pryce G, Devine L, Male DK, dos Santos WL, Calder VL, Adamson P. SV40 large T immortalised cell lines of the rat blood–brain and blood–retinal barriers retain their phenotypic and immunological characteristics. J Neuroimmunol. 1996;71:51–63. doi: 10.1016/s0165-5728(96)00130-0. [DOI] [PubMed] [Google Scholar]
- Hahn AF. Experimental allergic neuritis (EAN) as a model for the immune-mediated demyelinating neuropathies. Rev Neurol (Paris) 1996;152:328–332. [PubMed] [Google Scholar]
- Harkness KA, Sussman JD, Davies-Jones GA, Greenwood J, Woodroofe MN. Cytokine regulation of MCP-1 expression in brain and retinal microvascular endothelial cells. J Neuroimmunol. 2003;142:1–9. doi: 10.1016/s0165-5728(03)00251-0. [DOI] [PubMed] [Google Scholar]
- Hartung HP, Kieseier BC, Kiefer R. Progress in Guillain-Barre syndrome. Curr Opin Neurol. 2001;14:597–604. doi: 10.1097/00019052-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Hartung HP, Pollard JD, Harvey GK, Toyka KV. Immunopathogenesis and treatment of the Guillain-Barre syndrome–Part I. Muscle Nerve. 1995;18:137–153. doi: 10.1002/mus.880180202. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Cornblath DR. Guillain-Barre syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- Jat PS, Sharp PA. Large T antigens of simian virus 40 and polyomavirus efficiently establish primary fibroblasts. J Virol. 1986;59:746–750. doi: 10.1128/jvi.59.3.746-750.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat PS, Cepko CL, Mulligan RC, Sharp PA. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol Cell Biol. 1986;6:1204–1217. doi: 10.1128/mcb.6.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer R, Kieseier BC, Stoll G, Hartung HP. The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog Neurobiol. 2001;64:109–127. doi: 10.1016/s0301-0082(00)00060-5. [DOI] [PubMed] [Google Scholar]
- Kieseier BC, Krivacic K, Jung S, Pischel H, Toyka KV, Ransohoff RM, Hartung HP. Sequential expression of chemokines in experimental autoimmune neuritis. J Neuroimmunol. 2000;110:121–129. doi: 10.1016/s0165-5728(00)00323-4. [DOI] [PubMed] [Google Scholar]
- Knipe L, Meli A, Hewlett L, Bierings R, Dempster J, Skehel P, Hannah MJ, Carter T. A revised model for the secretion of tPA and cytokines from cultured endothelial cells. Blood. 2010;116:2183–2191. doi: 10.1182/blood-2010-03-276170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZG, Haelens A, Wuyts A, Struyf S, Pang XW, Proost P, Chen WF, van Damme J. Isolation of a lymphocyte chemotactic factor produced by the murine thymic epithelial cell line MTEC1: identification as a 30 kDa glycosylated form of MCP-1. Eur Cytokine Netw. 1996;7:381–388. [PubMed] [Google Scholar]
- Lu MO, Zhu J. The role of cytokines in Guillain-Barre syndrome. J Neurol. 2011;258:533–548. doi: 10.1007/s00415-010-5836-5. [DOI] [PubMed] [Google Scholar]
- Lynn EG, Siow YL, Frohlich J, Cheung GT, O K. Lipoprotein-X stimulates monocyte chemoattractant protein-1 expression in mesangial cells via nuclear factor-kappa B. Kidney Int. 2001;60:520–532. doi: 10.1046/j.1523-1755.2001.060002520.x. [DOI] [PubMed] [Google Scholar]
- Mao XJ, Zhang XM, Zhang HL, Quezada HC, Mix E, Yang X, Winblad B, Adem A, Zhu J. TNF-alpha receptor 1 deficiency reduces antigen-presenting capacity of Schwann cells and ameliorates experimental autoimmune neuritis in mice. Neurosci Lett. 2010;470:19–23. doi: 10.1016/j.neulet.2009.12.045. [DOI] [PubMed] [Google Scholar]
- Matoba K, Kawanami D, Ishizawa S, Kanazawa Y, Yokota T, Utsunomiya K. Rho-kinase mediates TNF-alpha-induced MCP-1 expression via p38 MAPK signaling pathway in mesangial cells. Biochem Biophys Res Commun. 2010;402:725–730. doi: 10.1016/j.bbrc.2010.10.093. [DOI] [PubMed] [Google Scholar]
- Maurer M, Toyka KV, Gold R. Cellular immunity in inflammatory autoimmune neuropathies. Rev Neurol (Paris) 2002;158:S7–S15. [PubMed] [Google Scholar]
- Neumark E, Anavi R, Witz IP, Ben-Baruch A. MCP-1 expression as a potential contributor to the high malignancy phenotype of murine mammary adenocarcinoma cells. Immunol Lett. 1999;68:141–146. doi: 10.1016/s0165-2478(99)00043-7. [DOI] [PubMed] [Google Scholar]
- Orlikowski D, Chazaud B, Plonquet A, Poron F, Sharshar T, Maison P, Raphael JC, Gherardi RK, Creange A. Monocyte chemoattractant protein 1 and chemokine receptor CCR2 productions in Guillain-Barre syndrome and experimental autoimmune neuritis. J Neuroimmunol. 2003;134:118–127. doi: 10.1016/s0165-5728(02)00393-4. [DOI] [PubMed] [Google Scholar]
- Oynebraten I, Bakke O, Brandtzaeg P, Johansen FE, Haraldsen G. Rapid chemokine secretion from endothelial cells originates from 2 distinct compartments. Blood. 2004;104:314–320. doi: 10.1182/blood-2003-08-2891. [DOI] [PubMed] [Google Scholar]
- Oynebraten I, Barois N, Hagelsteen K, Johansen FE, Bakke O, Haraldsen G. Characterization of a novel chemokine-containing storage granule in endothelial cells: evidence for preferential exocytosis mediated by protein kinase A and diacylglycerol. J Immunol. 2005;175:5358–5369. doi: 10.4049/jimmunol.175.8.5358. [DOI] [PubMed] [Google Scholar]
- Press R, Pashenkov M, Jin JP, Link H. Aberrated levels of cerebrospinal fluid chemokines in Guillain-Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Clin lmmunol. 2003;23:259–267. doi: 10.1023/a:1024532715775. [DOI] [PubMed] [Google Scholar]
- Putzu GA, Figarella-Branger D, Bouvier-Labit C, Liprandi A, Bianco N, Pellissier JF. Immunohistochemical localization of cytokines, C5b-9 and ICAM-1 in peripheral nerve of Guillain-Barre syndrome. J Neurol Sci. 2000;174:16–21. doi: 10.1016/s0022-510x(99)00328-7. [DOI] [PubMed] [Google Scholar]
- Quarles RH, Weiss MD. Autoantibodies associated with peripheral neuropathy. Muscle Nerve. 1999;22:800–822. doi: 10.1002/(sici)1097-4598(199907)22:7<800::aid-mus2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Raab M, Daxecker H, Markovic S, Karimi A, Griesmacher A, Mueller MM. Variation of adhesion molecule expression on human umbilical vein endothelial cells upon multiple cytokine application. Clin Chim Acta. 2002;321:11–16. doi: 10.1016/s0009-8981(02)00048-7. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan VV, Sumi MG, Reuben S, Mathai A, Nair MD. Serum tumour necrosis factor-alpha and soluble tumour necrosis factor receptors levels in patients with Guillain-Barre syndrome. Acta Neurol Scand. 2004;109:71–74. doi: 10.1034/j.1600-0404.2003.00179.x. [DOI] [PubMed] [Google Scholar]
- Rahman A, Fazal F. Hug tightly and say goodbye: role of endothelial ICAM-1 in leukocyte transmigration. Antioxid Redox Signal. 2009;11:823–839. doi: 10.1089/ars.2008.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben S, Mathai A, George SM, Nair MD, Radhakrishnan VV. Serum tumor necrosis factor-alpha in Guillain-Barre syndrome and its relation to plasma exchange. Neurologist. 2002;8:47–50. doi: 10.1097/00127893-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Ruggiero P, Flati S, Di Cioccio V, Maurizi G, Macchia G, Facchin A, Anacardio R, Maras A, Lucarelli M, Boraschi D. Glycosylation enhances functional stability of the chemotactic cytokine CCL2. Eur Cytokine Netw. 2003;14:91–96. [PubMed] [Google Scholar]
- Sainaghi PP, Collimedaglia L, Alciato F, Leone MA, Naldi P, Molinari R, Monaco F, Avanzi GC. The expression pattern of inflammatory mediators in cerebrospinal fluid differentiates Guillain-Barre syndrome from chronic inflammatory demyelinating polyneuropathy. Cytokine. 2010;51:138–143. doi: 10.1016/j.cyto.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Sano Y, Shimizu F, Nakayama H, Abe M, Maeda T, Ohtsuki S, Terasaki T, Obinata M, Ueda M, Takahashi R, Kanda T. Endothelial cells constituting blood–nerve barrier have highly specialized characteristics as barrier-forming cells. Cell Struct Funct. 2007;32:139–147. doi: 10.1247/csf.07015. [DOI] [PubMed] [Google Scholar]
- Sarafi MN, Garcia-Zepeda EA, MacLean JA, Charo IF, Luster AD. Murine monocyte chemoattractant protein (MCP)-5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. J Exp Med. 1997;185:99–109. doi: 10.1084/jem.185.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkey JP, Richards MP, Stubbs EB., Jr. Lovastatin attenuates nerve injury in an animal model of Guillain-Barre syndrome. J Neurochem. 2007;100:1265–1277. doi: 10.1111/j.1471-4159.2006.04309.x. [DOI] [PubMed] [Google Scholar]
- Spies JM, Pollard JD, Bonner JG, Westland KW, McLeod JG. Synergy between antibody and P2-reactive T cells in experimental allergic neuritis. J Neuroimmunol. 1995;57:77–84. doi: 10.1016/0165-5728(94)00164-j. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jung S, Jander S, van der Meide P, Hartung HP. Tumor necrosis factor-alpha in immune-mediated demyelination and Wallerian degeneration of the rat peripheral nervous system. J Neuroimmunol. 1993;45:175–182. doi: 10.1016/0165-5728(93)90178-2. [DOI] [PubMed] [Google Scholar]
- Tran GT, Hodgkinson SJ, Carter NM, Killingsworth M, Nomura M, Verma ND, Plain KM, Boyd R, Hall BM. Membrane attack complex of complement is not essential for immune mediated demyelination in experimental autoimmune neuritis. J Neuroimmunol. 2010;229:98–106. doi: 10.1016/j.jneuroim.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Von Zee CL, Stubbs EB., Jr. Geranylgeranylation facilitates proteasomal degradation of rho G-proteins in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:1676–1683. doi: 10.1167/iovs.10-6171. [DOI] [PubMed] [Google Scholar]
- Von Zee CL, Richards MP, Bu P, Perlman JI, Stubbs EB., Jr. Increased RhoA and RhoB protein accumulation in cultured human trabecular meshwork cells by lovastatin. Invest Ophthalmol Vis Sci. 2009;50:2816–2823. doi: 10.1167/iovs.08-2466. [DOI] [PubMed] [Google Scholar]
- Williams LM, Lali F, Willetts K, Balague C, Godessart N, Brennan F, Feldmann M, Foxwell BM. Rac mediates TNF-induced cytokine production via modulation of NF-kappaB. Mol Immunol. 2008;45:2446–2454. doi: 10.1016/j.molimm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Xing L, Remick DG. Promoter elements responsible for antioxidant regulation of MCP-1 gene expression. Antioxid Redox Signal. 2007;9:1979–1989. doi: 10.1089/ars.2007.1921. [DOI] [PubMed] [Google Scholar]
- Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010;411:1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Yosef N, Xia RH, Ubogu EE. Development and characterization of a novel human in vitro blood–nerve barrier model using primary endoneurial endothelial cells. J Neuropathol Exp Neurol. 2010;69:82–97. doi: 10.1097/NEN.0b013e3181c84a9a. [DOI] [PubMed] [Google Scholar]
- Zhao D, Pothoulakis C. Rho GTPases as therapeutic targets for the treatment of inflammatory diseases. Expert Opin Ther Targets. 2003;7:583–592. doi: 10.1517/14728222.7.5.583. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mix E, Link H. Cytokine production and the pathogenesis of experimental autoimmune neuritis and Guillain-Barre syndrome. J Neuroimmunol. 1998;84:40–52. doi: 10.1016/s0165-5728(97)00238-5. [DOI] [PubMed] [Google Scholar]
- Zou LP, Pelidou SH, Abbas N, Deretzi G, Mix E, Schaltzbeerg M, Winblad B, Zhu J. Dynamics of production of MIP-lα, MCP-1 and MIP-2 and potential role of neutralization of these chemokines in the regulation of immune responses during experimental autoimmune neuritis in Lewis rats. J Neuroimmunol. 1999;98:168–175. doi: 10.1016/s0165-5728(99)00100-9. [DOI] [PubMed] [Google Scholar]