Abstract
MS (multiple sclerosis) is the most prevalent autoimmune disease of the CNS (central nervous system) historically characterized as an inflammatory and demyelinating disease. More recently, extensive neuronal pathology has lead to its classification as a neurodegenerative disease as well. While the immune system initiates the autoimmune response it remains unclear how it orchestrates neuronal damage. In our previous studies, using in vitro cultured embryonic neurons, we demonstrated that MBP (myelin basic protein)-specific encephalitogenic CD4 T-cells induce early neuronal damage. In an extension of those studies, here we show that polarized CD4 Th1 and Th17 cells as wells as CD8 T-cells and NK (natural killer) cells induce microtubule destabilization within neurites in a contact-independent manner. Owing to the cytotoxic potential of these immune cells, we isolated the luminal components of lytic granules and determined that they were sufficient to drive microtubule destabilization. Since lytic granules contain cytolytic proteins, we determined that the induction of microtubule destabilization occurred prior to signs of apoptosis. Furthermore, we determined that microtubule destabilization was largely restricted to axons, sparing dendrites. This study demonstrated that lymphocytes with cytolytic activity have the capacity to directly drive MAD (microtubule axonal destabilization) in a bystander manner that is independent of neuronal death.
Keywords: axonopathies, CD4 T-cell, CD8 T-cell, multiple sclerosis, neurodegeneration, NK cells
Abbreviations: APC, antigen presenting cells; BBB, blood–brain barrier; CA, conalbumin; CNS, central nervous system; DAPI, 4′,6-diamidino-2-phenylindole; EAE, experimental autoimmune encephalomyelitis; FBS, foetal bovine serum; IFNγ, interferon γ; IL, interleukin; MAD, microtubule axonal destabilization; MAP2, microtubule associated protein; MBP, myelin basic protein; MEM, minimal essential medium; MS, multiple sclerosis; NK, natural killer; OVA, ovalbumin; PFA, paraformaldehyde; TCR, T-cell receptor; TGFβ, transforming growth factor β; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick-end labelling; YFP, yellow fluorescent protein
INTRODUCTION
MS (multiple sclerosis) affects an estimated 400000 people in the US and 2.5 million worldwide (Sospedra and Martin, 2005). Although MS is considered a demyelinating disease, it is now well recognized that neuronal damage and loss is a major component of the disease (Nylander and Hafler, 2012). The prevailing view is that CD4 T-cells of the Th1 and/or Th17 phenotype with specificity to myelin antigens drive lesion formation in the CNS (central nervous system), resulting in immune cell infiltration, demyelination and neuronal damage/loss leading to a variety of neurological deficits (Edwards et al., 2010; Hedegaard et al., 2008; Herz et al., 2009; Kebir et al., 2007; Kornek et al., 2000; Lovett-Racke et al., 2011; Montes et al., 2009; Nylander and Hafler, 2012; Tzartos et al., 2008).
In newly diagnosed MS patients, brain biopsies exhibited evidence of axonal injury in demyelinated plaques and normal appearing white matter, suggesting that neurodegeneration occurs simultaneously with the establishment of lesions by immune cells (Bjartmar et al., 2003; Ferguson et al., 1997; Kuhlmann et al., 2002). In primary progressive MS, accumulating neuronal damage/loss is thought to be the major contributor to the decline in health status of the patients (Bjartmar et al., 2003; Sepulcre et al., 2006; Tallantyre et al., 2009). While the correlation between the presence of immune cell infiltrates in the CNS and neuronal damage is strong, the exact mechanisms whereby the immune system induces such damage are unknown. Potential immune-mediated mechanisms include direct cytotoxicity or indirect mechanisms such as glutamate excitotoxicity, vulnerability due to demyelination or oxidative stress (Lassmann, 2010; Miller, 2012; Nikic et al., 2011; Siffrin et al., 2010b).
Relapsing and remitting MS is not consistent with wide-spread neuronal loss, but rather reversible neuronal damage. To this end, using the animal model of MS, EAE (experimental autoimmune encephalomyelitis), we previously demonstrated that neuronal damage evident in areas of immune cell infiltration was reversed when the mice underwent spontaneous recovery (Shriver and Dittel, 2006). Interestingly, this neuronal damage occurred prior to overt demyelination. Further support of this concept is evidence of immune-mediated neuronal damage in the absence of co-localized demyelinating processes in MS (Aboul-Enein et al., 2006; Bitsch et al., 2000; DeLuca et al., 2006). Thus, even though oligodendrocytes have recently been shown to provide metabolic support for neurons (Funfschilling et al., 2012; Lee et al., 2012), their loss is not the sole predictor of vulnerability to neuronal injury in MS.
Previously, we reported that encephalitogenic CD4 T-cells induced neuronal damage (Shriver and Dittel, 2006). Here, we extended these findings and demonstrate that CD4 Th1, CD4 Th17, CD8 as well as NK (natural killer) cells induce neuronal damage via the induction of MAD (microtubule axonal destabilization). Furthermore, we provide evidence that neuronal damage is mediated by a protein expressed within the luminal contents of lytic granules expressed by immune cells with cytolytic activity. We also found that MAD occurred rapidly after exposure to lytic granules and prior to signs of neuronal cell death. In addition, the restriction of MAD to the axon indicates that immune cells have the potential to contribute to axonopathies in diseases with a strong immune cell component such as autoimmunity and infectious disease.
MATERIALS AND METHODS
Rodents
FVB/NJ (FVB), B10.PL-H-2u H2-T18a/(73NS)SnJ (B10.PL), C57BL/6J, B10.BR-H-2k H2-T18a/SgSnJJrep (B10.BR), C57BL/6J-Lyst bg-J/J (Beige), C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-1), CByJ.B6-Prf1tm1Sdz/J (perforin−/−) and 129×1/SvJ-Gzmatm1Ley/Gzmbtm2.1Ley/J (granzyme A/B−/−) mice were purchased from The Jackson Laboratory. B6.Cg-Tg [Thy1-YFP (yellow fluorescent protein)]16Jrs/J (Thy1-YFP) B10.PL mice were bred with FVB mice and (Thy1-YFPxFVB)F1 embryos were collected on day 15 as described (Shriver and Dittel, 2006). MBP–TCR (myelin basic protein–T-cell receptor) transgenic mice specific for the acetylated NH2-terminal peptide MBP (Ac1–11) have been previously described (Dittel et al., 1999). Animals were housed in the Translation Biomedical Research Center of the Medical College of Wisconsin, Milwaukee, WI. All animal protocols were approved by the Medical College of Wisconsin's Institutional Animal Care and Use Committee.
Peptides, antibodies, cytokines and YT NK cell line
The MBP Ac1-11 (Ac- ASQKRPSQRSK) and the OVA (ovalbumin) peptide (SIINFEKL) were generated by the Protein Chemistry Core of the Blood Research Institute (BloodCenter of Wisconsin, Milwaukee, WI). The CA (conalbumin)134–146 peptide (HRGAIEWEGIESG) was generated by the W.M. Keck Foundation Biotechnology Resource Laboratory at Yale University (Dittel et al., 1997). A monoclonal antibody to β3-tubulin (IgG3) antibody was generated locally by immunization with the ESESQGPK peptide coupled to KLH [keyhole-limpet (Diodora aspera) haemocyanin] (IgG3) and purified with HiTrap Protein G HP columns (GE Healthcare). Mouse anti-ankyrin G (IgG1) was purchased from Santa Cruz Biotechnology, Inc. Anti-MAP2 (microtubule associated protein 2) chicken polyclonal was purchased from Abcam. Goat anti-mouse Alexa fluor 488 IgG3, goat anti-mouse Alexa fluor 546 IgG1 and goat anti-chicken Alexa fluor 633 IgG (H+L) were purchased from Invitrogen. Mouse anti-human β-tubulin was purchased from Millipore. Anti-IFNγ (interferon γ) clone XMG1.2 was purchased from eBioscience and anti-IL-4 (interleukin 4) clone 11B1 was generated locally. TGFβ (transforming growth factor β), IL-6, IL-23 and IL-1 were purchased from R&D Systems. Mouse IL-2 was either generated locally or human recombinant IL-2 was a gift from Dr. J. Verbsky (Medical College of Wisconsin, Milwaukee, WI). YT cells, a human ‘NK cell-like’ cell line, were obtained from Dr. Carol Clayberger (National Cancer Institute, Bethesda, MD) (Yodoi et al., 1985).
NK cell cultures
Splenocytes were isolated from C57BL/6J, Beige, granzyme AB−/− or perforin−/− mice and passed over prehydrated nylon fibre (ZeptoMetrix Corporation) columns and incubated for 1 h at 37°C to eliminate B cells and macrophages. Cells were eluted from the column with RPMI 1640 and 5% (v/v) FBS (foetal bovine serum) and pelleted by centrifugation. The cells were resuspended in RPMI 1640 and 10% (v/v) FBS supplemented with 2 mM l-glutamine, 10000 international units penicillin and 10000 mg/ml streptomycin, 1 M Hepes, BME (2-mercaptoethanol) and 1000 units/ml IL-2 and cultured for 4 days at 37°C, 5% (v/v) CO2 (Regunathan et al., 2005). After 4 days, non-adherent cells were transferred to a new flask and the adherent (NK cells) and non-adherent cells were provided fresh RPMI 1640 and 10% (v/v) FBS with 1000 units/ml of IL-2 and cultured for an additional 2–3 days. Adherent and floating cells were combined for subsequent procedures.
Generation of Th1 and Th17 MBP-specific T cell lines and the Th2 D10 clone
To generate Th1 and Th17 CD4 T-cell lines, total splenocytes from MBP-TCR transgenic mice (H-2u) specific for the MBP Ac1-11 peptide in the context of I–Au were cultured with 5 μg/ml of the peptide in Th1 and Th17 skewing conditions (Dittel et al., 1999). For Th1 skewing the splenocytes were cultured with 8 μg/ml IL-12 and 5 ng/ml anti-IL-4 and for Th17 skewing with 5 ng/ml TGFβ, 10 ng/ml IL-6, 0.1 ng/ml IL-23, 1 ng/ml IL-1, 5 ng/ml anti-IL-4 (clone 11B11) and 125 ng/ml anti-IFNγ. The cultures were maintained by the addition of fresh medium along with skewing antibodies and cytokines on day 3. The T-cell lines were restimulated on day 7 with Ac1-11 and irradiated splenocytes as APC (antigen-presenting cells) and provided with fresh medium on day 10, which for the Th17 cultures contained skewing cytokines and antibodies. For Th2 cells, the D10 clone specific for the CA137–146 peptide in the context of I-Ak was cultured with irradiated B10.BR (H-2k) splenocytes with 5 μg/ml CA137–146 as described (Dittel et al., 1997). D10 cultures were restimulated on days 11 and 22 with irradiated B10.BR splenocytes and 5 μg/ml of CA137–146 peptide and allowed to expand for 10 days. Intracellular expression of IFNγ, IL-17 and IL-4 was detected as described (Ray et al., 2012).
OT-1 (CD8) cell lines
CD8+ splenic T-cells were isolated from OT-1 TCR transgenic mice specific for OVA peptide in the context of H-2Kb by positive selection using anti-CD8-biotin (eBioscience), anti-biotin microbeads and MACS separation columns (Miltenyi Biotec). Purified CD8+ cells (3×106) were cultured in the presence of OVA peptide (1 μg/ml) and irradiated T-cell-depleted syngeneic splenocytes (12×106) for 5 days.
Preparation of cell supernatants and lysates
For isolation of supernatants, 6–10×106 cultured NK cells were treated with 20 ng/ml PMA and 500 ng/ml ionomycin for 4–6 h after which the supernatants were collected, filtered (0.2 μm) and frozen at −20°C. For cell lysates, NK or T-cells were activated with 20 ng/ml PMA and 500 ng/ml ionomycin for 4–6 h after which the cell pellet was resuspended in neurobasal medium (Invitrogen) supplemented with 2 mM l-glutamine, 100 μg/ml of gentamicin, 0.05 μg/ml fungizone, 6 mg/ml glucose and 1.5 mg/ml sodium bicarbonate at 1×106 cells/ml and incubated at 37°C, 5% (v/v) CO2 overnight. The cell-culture suspensions were collected, frozen at −80°C for 15 min and then thawed at 37°C. The ruptured cells were pelleted by centrifugation and the supernatants were filtered, aliquoted and frozen at −20°C.
Isolation of lytic granules
Lytic granules were isolated using an adapted protocol (Borregaard et al., 1983; Thiery et al., 2010). Briefly, mouse NK (150–300×106) or YT (0.09–1.5×109) cells were harvested by centrifugation and washed twice with PBS and resuspended in 5 ml of relaxation buffer (1×) containing 10 mM KCl, 3.5 mM MgCl2, 10 mM PIPES pH 6.8, 1.25 mM EGTA and 1 mM ATP. Plasma membranes were disrupted by N2 cavitation at 450 psi (1 psi = 6.9 kPa) on ice and the nuclei and unbroken cells were removed by centrifugation at 4°C for 5 min. The supernatant was collected and the pellet was washed once with 4 ml relaxation buffer (1×) and pooled with the first washed supernatant. The disrupted cell supernatant was overlayed onto a discontinuous percoll gradient with a density of 1.120, 1.090 and 1.050 g/ml and centrifuged at 37000 g for 30 min at 4°C with a Beckman JA 20 rotor (Masson and Tschopp, 1985). Then 3 ml fractions were collected from the top to the bottom of the gradient and centrifuged at 110000 g at 4°C for 3 h with a TLA 100.3 Beckman Coulter rotor, causing the subcellular organelles to form a white cotton-like consistency lying above a hard-packed percoll pellet (Podack and Konigsberg, 1984). For the disruption of the granular membranes, the supernatant was collected and a final concentration of 2 M NaCl was added that was then subjected to four freeze/thaw cycles and centrifuged at 110000 g at 4°C for 1 h with a TLA 100.3 rotor. The supernatant was collected and dialysed against one change of PBS overnight at 4°C. The protein concentration of the final suspension was measured by an ND 1000 spectrophotometer and frozen at −20°C.
Embryonic neuronal cultures
Embryonic neurons were cultured using an adapted protocol from Ransom et al. (Ransom et al., 1977; Shriver and Dittel, 2006). For Figure 1, brains and spinal cords were isolated on embryonic day 15 and triturated in DISGH buffer containing 135 mM NaCl, 5 mM KCl, 0.3 mM Na2HPO4, 0.2 mM KH2PO4, 16.5 mM glucose, 22 mM sucrose and 9.86 mM Hepes followed by digestion in 0.67 mg/ml papain at 37°C for 30 min. The tissue was titrated in 40 μg/ml DNase diluted in MEM (minimal essential medium) and were pelleted by centrifugation and resuspended in MEM containing 10% (v/v) horse serum and 10% (v/v) FBS. Neurons were plated on polyethylenimine coated plates and after 8 h the medium was replaced with neurobasal medium containing N2 supplement (Invitrogen), 2 mM glutamine, 100 μg/ml gentamicin and 0.05 μg/ml fungizone. Three days later, the cultures were treated with 0.054 mM fluorodeoxyuridine (Sigma-Aldrich) and 0.014 mM uridine (Sigma-Aldrich) to inhibit proliferation of astrocytes. Fresh medium was added to the culture every 2 days and cultures were used approximately 10 days after seeding. For Figures 2–7, brains and spinal cords on embryonic day 15 were dissected out and dissociated with 0.67 mg/ml papain (Fisher) in HBSS (Hanks balanced salt solution) (Mediatech) supplemented with glucose (6 mg/ml) and sodium bicarbonate (1.5 mg/ml) at 37°C. The tissue was then mechanically tritruated with a fire-polished Pasteur pipette and 40 μg/ml of DNaseI (Sigma-Aldrich) diluted in MEM (Mediatech). The non-dissociated tissue was allowed to settle out and the clear cell suspension was collected and pelleted by centrifugation. The neuronal cells were resuspended in MEM supplemented with 10% (v/v) FBS, 10% (v/v) horse serum, sodium bicarbonate and glucose. The neuronal cells were seeded on German glass coverslips pretreated with poly-d-lysine (20 μg/ml) and laminin (10 μg/ml) (Sigma-Aldrich) at 0.1–0.2×106/ml and incubated at 37°C, 10% (v/v) CO2. After 4 h, the medium was removed and replaced with neurobasal medium (Invitrogen) supplemented with 2% B-27 (Invitrogen), 2 mM l-glutamine, 100 μg/ml gentamicin sulphate, 0.05 μg/ml fungizone, 6 mg/ml glucose and 1.5 mg/ml sodium bicarbonate. After 3 days, neurons were treated with 1–5 μM Ara-C (Cytosine B-D-arabinofuranoside) (Sigma-Aldrich) to inhibit proliferation of glial cells. Neuronal cultures were maintained with the addition of fresh neurobasal medium every 3–4 days. Neuronal cultures on days 10–12 were used for experimentation. Human embryonic neuronal cultures were generated as described (Jana and Pahan, 2004, 2010). The experimental protocols were reviewed and approved by the Institutional Review Boards of the Rush University Medical Center. Briefly, foetal brains (11-17 week) were obtained from the Human Embryology Laboratory (University of Washington, Seattle, WA). The tissue was triturated and then trypsinized (0.25%) for 15 min at 37°C followed by inactivation. The dissociated tissue was then filtered through 380 and 140 μm meshes (Sigma-Aldrich) and centrifuged. The cellular pellet was washed once with PBS followed by a wash in neurobasal medium supplemented with 2% B27 with antibiotics. Following adhesion to poly-d-lysine-coated plates for 5 min the non-adherent cells were removed and the remaining cells were treated with 10 μm Ara-C and were cultured for 10 days. Purity of the neurons (98%) was determined by MAP2 staining.
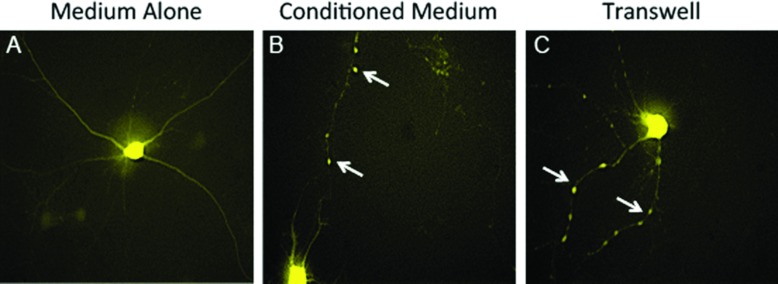
Figure 1. Encephalitogenic T-cell-driven destabilization of the neuronal network does not require cell contact.
Embryonic neurons were cultured from (Thy1-YFPxFVB)F1 mice and were treated with medium alone (A), conditioned medium collected from encephalitogenic CD4 T-cell cultures (B) or 10×106 MBP-specific encephalitogenic CD4 T-cells added to the top well of a transwell (C) and live cell imaging was performed after 4 h. Original magnifications: ×400 (A–C). Arrows indicate neurites exhibiting a change in the YFP fluorescence pattern.
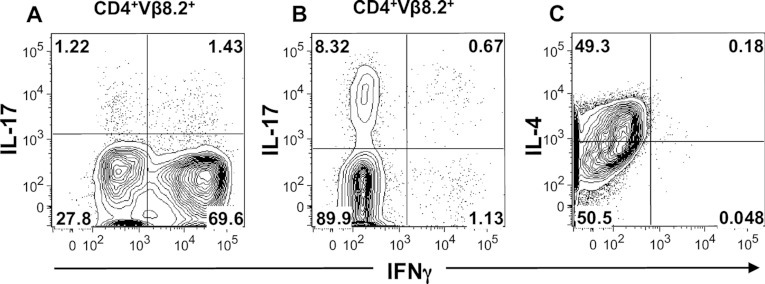
Figure 2. Cytokine production by polarized Th1, Th17 and Th2 cells.
Th1 (A) and Th17 (B) T-cell lines generated from MBP–TCR transgenic mice and D10 cells (C) were stimulated with PMA (50 ng/ml) and ionomycin (750 ng/ml) for 4–5 h. Th1 and Th17 cells were stained with CD4 and Vβ8.2 prior to intracellular staining for IFNγ and IL-17. D10 cells were intracellularly stained for IFNγ and IL-4. Representative two-colour contour plots are shown gating on CD4+Vβ8.2+ Th1 and Th17 cells with IFNγ on the x-axis and IL-17 (A, B) on the y-axis. For D10 cells, a representative contour plot shows IFNγ on the x-axis and IL-4 on the y-axis (C).
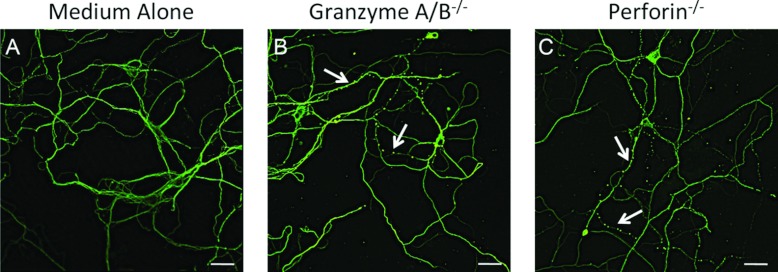
Figure 7. Destabilization of β3-tubulin is independent of granzyme A/B and perforin.
Embryonic neuronal cultures were treated with medium alone (A) or with cell lysates (1:2) from NK cells from granzyme A/B double knockout (B) or perforin knockout (C) mice for 2 h. The neurons were then fixed and stained for β3-tubulin and visualized by confocal microscopy. Arrows indicate neurites exhibiting microtubule destabilization. Scale bar: 30 μm.
Immunocytochemistry
Embryonic neurons were treated with 0.5 ml activated NK, Beige, CD8, Th1, Th2, Th17, granzyme A/B−/− or perforin−/− cell supernatants or lysates or 75–300 μg/ml Beige or YT lytic granules for 1–2 h at 37°C, 10% (v/v) CO2. The cells were then washed with PBS and fixed and permeabilized with 4% (v/v) PFA (paraformaldehyde) and ice-cold methanol. The fixed cells were washed with 0.1% (v/v) Tween in PBS for five cycles at 5 min per cycle at room temperature prior to 1 h of blocking with 3% (w/v) BSA in 0.1% (v/v) Tween. Subsequently, cells were incubated with combinations of primary antibodies: anti-mouse β3-tubulin (IgG3), anti-chicken polyclonal MAP2 (IgY) and/or anti-mouse anykrin G (IgG1) overnight at 4°C. The secondary antibodies goat anti-mouse IgG3 Alexa fluor 488, goat anti-mouse IgG1 Alexa fluor 546 and goat anti-chicken IgG (H+L) Alexa fluor 633 were applied to neuronal cells and incubated for 1 h at room temperature. Neuronal coverslips were mounted in aqueous mounting media (Electron Microscopy Sciences). Controls consisting of the secondary antibodies alone exhibited minimal background staining (results not shown). In addition, all possible primary and secondary antibody combinations were examined to confirm specificity of the desired staining (results not shown).
Transwell and live cell imaging
Transwells with a 0.4 μm filter (Corning Inc) were placed above Thy1-YFP neuronal cultures on day 10 and 10×106 activated CD4+ T-cells or conditioned medium from T-cells were collected and added to the top well. After 4 h, the transwells were removed and the neurons were imaged using a Nikon inverted microscope and MetaMorph software.
TUNEL (terminal deoxynucleotidyltransferase-based nick-end labelling)
The DeadEndTM Fluorometric TUNEL System (Promega) kit was used for the detection of apoptotic cells. Neuronal cultures were treated with 150–300 μg/ml Beige or YT lytic granules for 1 h at 37°C, 10% (v/v) CO2. Neuronal cells were washed with PBS, fixed with 4% (v/v) PFA and permeabilized with 0.2% (v/v) Trition X-100 (Sigma-Aldrich) or ice-cold methanol. Neuronal cells were either stained with TUNEL and DAPI (4′,6-diamidino-2-phenylindole) for quantification purposes or with β3-tubulin and TUNEL for qualitative purposes. Positive control samples for TUNEL were treated with 10 units/ml DNase I (Sigma-Aldrich) at room temperature and the negative control sample was incubated without the recombinant terminal deoxynucleotidyl transferase enzyme.
Confocal microscopy and imaging
Laser scanning confocal microscopy was performed using the Olympus Fluoview FV1000 MPE Multiphoton Laser Scanning Microscope. The objective used was a ×40, 1.30 oil N.A. For triple labelling experiments sequential scanning was selected to allow for separation of signals from each channel. Fluoview FV10-ASW v3.1c software (Olympus) was used for the application of images.
RESULTS
Encephalitogenic T-cell driven destabilization of the neuronal microtubule network does not require cell contact
We previously showed that when embryonic neurons from Thy1-YFP mice were cocultured with MBP-specific encephalitogenic CD4 T capable of inducing EAE (Dittel et al., 1999) YFP fluorescence was redistributed from a continuous pattern in the cell body and neuritic processes (Figure 1A) to that of aggregated domains contained within the neurites (Shriver and Dittel, 2006). We next asked whether cell–cell contact was required and found that it was not since the continuous YFP fluorescence in unmanipulated embryonic neurons (Figure 1A) was disrupted by both supernatants from encephalitogenic T-cell cultures (Figure 1B) and their presence in the top of chamber of a transwell (Figure 1C).
Microtubule destabilization is mediated by CD4 Th1, CD4 Th17, CD8 and NK cells, but not CD4 Th2 cells
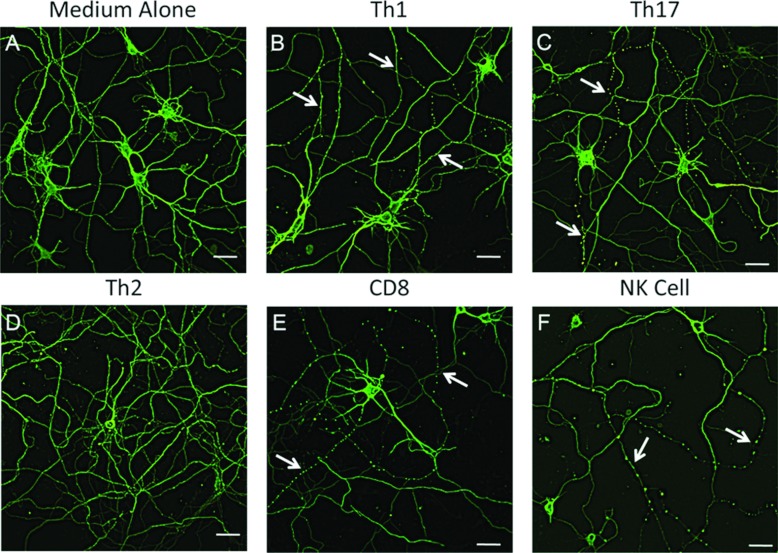
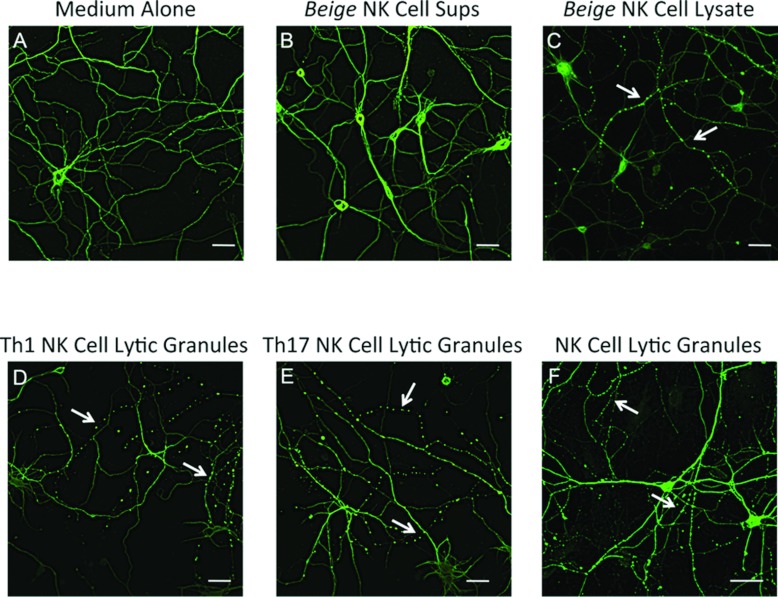
Since EAE is induced by CD4 T-cells of the Th1 and Th17 subtypes (El-behi et al., 2010), we next asked whether one or both would drive microtubule destabilization. In our previous studies, we determined that alterations in YFP fluorescence was associated with destabilization of the microtubule network as visualized by staining with anti-β3-tubulin (Shriver and Dittel, 2006). Because the detection of microtubule destabilization by immunofluorescence proved more robust than alterations in YFP fluorescence, all subsequent studies were performed using the former method. When we stained unmanipulated neuronal cultures with anti-β3-tubulin, we found that the microtubule network was uniformly expressed in all neuritis, which we used as a general indicator of the health of the cultures (Figure 2A). Owing to differences in granular composition and secretion profiles of lymphocyte subsets, we choose to use cellular lysates instead of supernatants to normalize the potential of each cell type to drive microtubule destabilization. Using MBP–TCR transgenic mice, we generated Th1 (Figure 3A) and Th17 (Figure 3B) cell lines and confirmed their differentiated phenotype by intracellular cytokine staining. We found that both Th1 (Figure 3B) and Th17 (Figure 3C) CD4 T-cells drove microtubule destabilization resulting in a β3-tubulin punctate ‘beads-on-a-string’ staining pattern in a subset of neuritis. Next, we reasoned that, due to their inability to induce EAE, CD4 Th2 cells would not drive microtubule destabilization (Khoruts et al., 1995). Using the well-characterized D10 Th2 clone (Dittel et al., 1997) (Figure 2C), we confirmed that it lacked the ability to drive microtubule destabilization (Figure 3D). We then examined other immune cell types and found that CD8 (Figure 3E) and NK cells (Figure 3F), but not splenocytes, B cells, macrophages nor microglial cells, were able to induce neuronal damage (results not shown). The use of cell lysates in this study also demonstrated that intracellular proteins such as calpains, lysosomal enzymes and cell-signalling molecules that are common to all T-cells do not play a role in microtubule destabilization.
Figure 3. Microtubule destabilization is induced by Th1, Th17, CD8 and NK cells.
Embryonic neurons from FVB mice were cultured for 10 days and then treated with medium alone (A) or activated Th1 (B), Th17 (C), Th2 (D), CD8 (E) or NK (F) cell lysates diluted 1:2 in neurobasal media with supplements for 2 h. The neurons were then fixed and stained for β3-tubulin and visualized by confocal microscopy. Arrows indicate neurites exhibiting microtubule destabilization. Scale bar: 30 μm.
Microtubule destabilization is driven by a component of lytic granules
One common feature of CD4 Th1, CD8 and NK cells is their cytotoxic potential via the secretion of lytic granules following activation. To determine whether the ability to induce microtubule destabilization was due to a component of lytic granules, we used several approaches. First, we treated encephaltiogenic CD4 T-cells with nocodazole to disrupt the polymerization of microtubules required for the release of lytic granules and found that the treated supernatants failed to mediate neuronal damage (results not shown). Secondly, we generated NK cell cultures from Beige mice, which harbour a frame-shift mutation in the Lyst gene leading to defects in vesicle trafficking resulting in defective cytolytic functions (Barbosa et al., 1996; Kaplan et al., 2008; Roder et al., 1979). When we compared the staining pattern of β3-tubulin in control neurons (Figure 4A), we found that supernatants from Beige NK cells (Figure 4B) did not alter microtubule stability. In contrast, cellular lysates from the same NK cells did exhibit the activity (Figure 4C). We then isolated the luminal contents of lytic granules from CD4 Th1 (Figure 4D), CD4 Th17 (Figure 4E), NK cells (Figure 4F) and CD8 T-cells (results not shown) and found that they drove microtubule destabilization. These data demonstrate that T-cells with cytotoxic potential and NK cells are similar in their ability to drive microtubule destabilization. In addition, the component of lytic granules that drives microtubule destabilization is probably a protein because the methods used to isolate the luminal components of lytic granules enriches for proteins and eliminates small molecules such a peptides, nucleotides or amino acids.
Figure 4. Microtubule destabilization is induced by the luminal contents of lytic granules.
Embryonic neuronal cultures were generated as for Figure 2 (A–F). Neuronal cultures were treated with medium alone (A) or with cell supernatants (B) or with cell lysates from activated NK cells from Beige mice diluted 1:2 in neurobasal media with supplements for 2 h (C). Lytic granules were isolated from in vitro skewed CD4 Th1 (33.5×106) (D) or Th17 cells (33.5×106) (E) or from mouse NK cells (150–300×106) (F) and diluted 1:2 in neurobasal media with supplements and added to neuronal cultures for 1 h. Neurons were then fixed and stained for β3-tubulin and imaged by confocal microscopy. Arrows indicate neurites exhibiting microtubule destabilization. Scale bar: 30 μm.
The ability to mediate microtubule destabilization is conserved among species
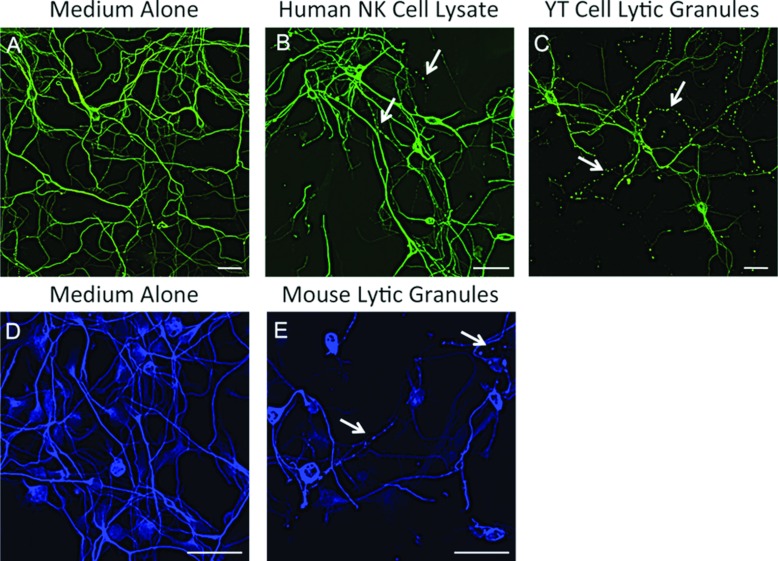
To determine the applicability of our current findings to humans, we asked whether human NK cells were able to induce microtubule destabilization. Using mouse neuronal cultures (Figure 5A), we found that cell lysates generated from human peripheral blood NK cells were able to induce microtubule destabilization (Figure 5B). Likewise, lytic granules from the human YT ‘NK-like’ cell line (Figure 5C) were also able to induce microtubule destabilization. In addition, splenic rat NK cell lytic granules exhibited similar activity (results not shown). Furthermore, we found that human embryonic neurons (Figure 5D) were susceptible to neuronal damage when treated with mouse NK cell lytic granules (Figure 5E). These data indicate that microtubule neuronal damage is conserved across species and that studies in mice are directly applicable to humans.
Figure 5. Microtubule destabilization is conserved among species.
Mouse embryonic neuronal cultures (A–C) were treated with medium alone (A), human NK cell lysate diluted 1:2 in neurobasal media with supplements for 2 h (B), or with YT cell lytic granules (C) for 1 h. Human embryonic neuronal cultures (D, E) were treated with medium alone (D) or with mouse NK cell lytic granules for 1.5 h. The neurons were then fixed and stained for β3-tubulin and imaged by confocal microscopy. Arrows indicate neurites exhibiting microtubule destabilization. Scale bar: 30 μm.
Microtubule destabilization is independent of neuronal apoptosis
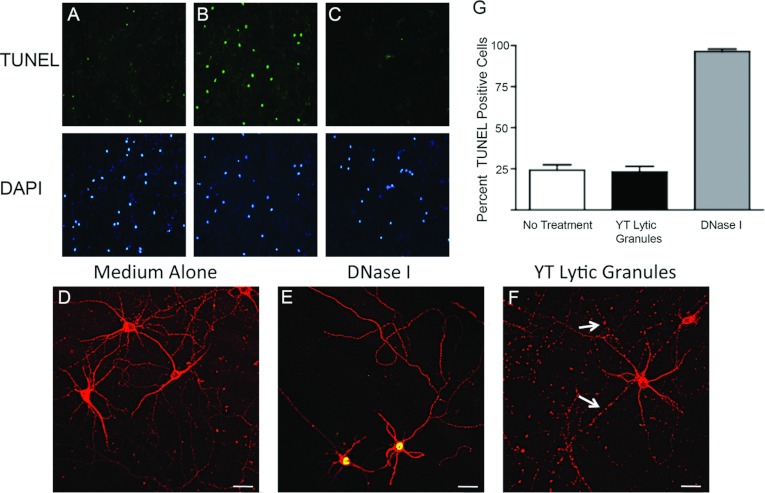
Previously, it has been shown that cytotoxic lymphocytes as well as NK cells can induce death of neurons (Backstrom et al., 2003; Giuliani et al., 2003; Suvas et al., 2006). In our previous study, we demonstrated that membrane integrity remained intact in neurons co-cultured with CD4+ encephalitogenic T-cells, indicating that the viability of the neurons was not compromised (Shriver and Dittel, 2006). Because the treated neurons could have been undergoing stages of apoptosis prior to loss of membrane integrity, we next examined that possibility using TUNEL to measure DNA fragmentation, a hallmark of apoptosis. We found that about 25% of mouse embryonic neurons in the medium alone were undergoing apoptosis as determined by computing the percent TUNEL+ cells among the DAPI+ nuclei (Figures 6A and 6G). DNase I treatment was used as a positive control for DNA fragmentation and was about 100% (Figures 6B and 6G). The addition of lytic granules from YT cells had no effect on basal levels of apoptosis (Figures 6C and 6G). To confirm that microtubule destabilization is independent of apoptosis, we stained for both TUNEL and β3-tubulin. Control neurons exhibited a continuous β3-tubulin staining pattern and did not exhibit signs of apoptosis (Figure 6D). DNase I treated neurons were positive for TUNEL staining, but did not exhibit microtubule destabilization (Figure 6E). In contrast, treatment with YT lytic granules resulted in microtubule destabilization but not apoptosis (TUNEL−) (Figure 6F).
Figure 6. Microtubule destabilization is an early even that occurs in the absence of apoptosis.
(A–C, D–F) DNA fragmentation was detected by TUNEL in neuronal cultures generated as for Figure 2. Neuronal cultures were treated with medium alone (A, D), 10 units/ml of DNase I (B, E) or with 300 μg of YT lytic granules (C, F) for 1 h. Cultures were stained for TUNEL (green) (top panels) and counterstained with DAPI (blue) (bottom panels) (A–C) or for β3-tubulin (red) and TUNEL (yellow) (D–F) and imaged by confocal microscopy. The percentage of TUNEL-positive cells were quantified (G). The arrow indicates neurites exhibiting microtubule destabilization. Scale bar: 30 μm.
The lack of apoptosis induction in our model of neuronal damage is important since the lytic granule components perforin and granzyme A/B have been implicated in neuronal death by apoptosis (Gobel et al., 2010; Haile et al., 2011). To confirm that these cell death-inducing proteins are not mediating microtubule destabilization, we compared β3-tubulin staining in control neurons (Figure 7A) with neurons exposed to NK cell lysates from granzyme A/B double (Figure 7B) or perforin (Figure 7C) knockout mice and found that both knockouts retained activity. These data indicate that the early microtubule destabilization that we observe is mediated by a mechanism independent of neuronal death.
Axons, but not dendrites, are susceptible to MAD
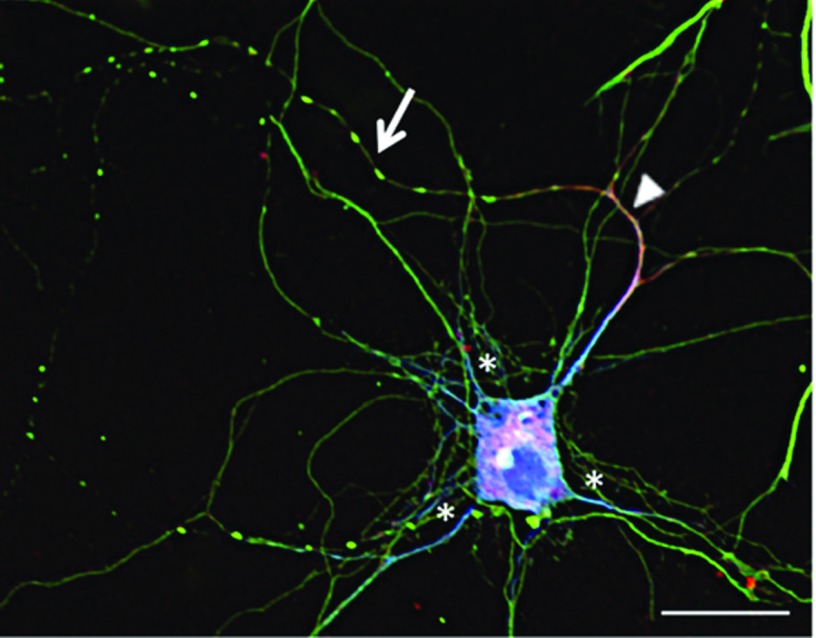
As evident in Figures 3–6, microtubule destabilization only occurs in a subset of neurites, suggesting that dendrites and axons are differentially affected. To investigate this question, we stained neurons that had been exposed to lytic granules with anti-β3-tubulin (green) to stain microtubules in both axons and dendrites in combination with anti-MAP2 (blue) to mark dendrites and anti-ankyrin G (red) to mark axons (Figure 8) (Boiko et al., 2007; Kaech and Banker, 2006; Kanai and Hirokawa, 1995; Kordeli et al., 1995; Kosik and Finch, 1987). We found that microtubule destabilization was largely localized to axons (asterisk). The ankyrin G+ axon also still expressed MAP2 within the axonal initial segment, which is consistent with early stages of axonogenesis (Boiko et al., 2007; Kosik and Finch, 1987). These cumulative data indicate that MAD is a unique mechanism whereby cells of the immune system with cytotoxic potential have the capacity to induce axonopathies.
Figure 8. Microtubule destabilization is restricted to axons and not dendrites.
Embryonic neuronal cultures were treated with 75 μg of lytic granules from Beige mice for 1 h. The neurons were then fixed and stained for β3-tubulin (green), ankyrin G (red) and MAP2 (blue) and visualized by confocal microscopy. The arrow indicates microtubule destabilization. The arrowhead marks the axon and the asterisks mark dendrites. Scale bar: 20 μm.
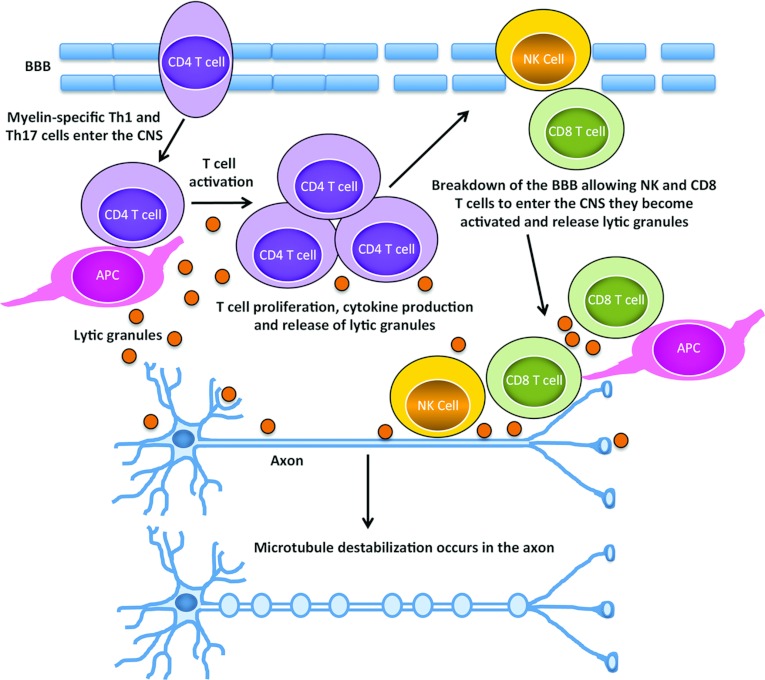
Figure 9 depicts our model of MAD. We propose that myelin-specific CD4 T-cells enter the CNS, and at or near the BBB (blood–brain barrier) become activated via interaction with APC via MHC II: self-peptide, resulting in their cell proliferation, cytokine production and the release of lytic granular contents. The inflammation driven by the CD4 T-cells would lead to a breakdown of the BBB allowing other immune cells including CD8 T and NK cells to enter the CNS. CD8 T-cells interacting with either APC, oligodendrocytes or neurons via MHC I: self-peptide interactions would become activated and release lytic granular contents. NK cells, which do not require a specific antigen have the potential to interact with any cell type within the CNS and would also release lytic granular contents upon activation. Most likely via a bystander mechanism, some soluble protein component of the lytic granules would induce a signalling cascade in neurons, ultimately resulting in the destabilization of microtubules in axons.
Figure 9. Model of MAD.
Myelin-specific Th1 and/or Th17 cells drive inflammation within the CNS that results in the breakdown of the BBB and the release of their lytic granular contents. In addition, CD8 T and NK cells enter the CNS and if they become activated also release the contents of their lytic granules. The destabilization of microtubules within axons occurs via a bystander mechanism as a consequence of the presence of lytic granular contents within the CNS microenvironment.
DISCUSSION
Neuronal damage is a prominent feature in MS and has been well documented in EAE (Kornek et al., 2000; Nylander and Hafler, 2012). In this study, we first investigated the immune cell types that could contribute to neuronal damage and found that both CD4 Th1 and Th17 cells (Figure 3), thought to be the drivers of MS and EAE, induced MAD. Furthermore, we found that CD8 and NK cells also induced microtubule destabilization. Using a variety of strategies, we next found that MAD activity resided within the lumen of lytic granules and did not require direct cell contact between the immune cells and neurons. Although lytic granules contain cytolytic proteins, we found that microtubule destabilization occurred early in the absence of evidence of cell death. Finally, since axonal injury is thought to play a key role in the progression of MS, we determined that microtubule destabilization was restricted to axons, largely sparing the dendrites. Together, these data provide strong evidence that the immune system has the potential to directly cause neuronal damage resulting in axonopathy in both autoimmunity and in infectious disease.
Using the EAE model, it is well established that both CD4 Th1 and Th17 cells contribute to disease. Although the exact mechanisms whereby CD4 T-cells drive CNS autoimmunity are not well understood, both cell types produce GM-CSF (granulocyte/macrophage colony-stimulating factor), a key cytokine required for the initiation of disease (El-Behi et al. 2011; McQualter et al., 2001; Ponomarev et al., 2007). They both also produce chemokines, which draw other inflammatory cells into the CNS. In addition, IL-17 is a potent inducer of chemokine production and has been shown to induce chemokine production by astrocytes (Awane et al., 1999; Kang et al., 2010; Meares et al., 2012). Here, we now provide evidence that both CD4 Th1 and Th17 cells also directly drive neuronal damage (Figure 3). Consistent with our observations, a recent study used intravital two-photon imaging to demonstrate that CD4 Th17 cells induced neuronal signalling in the brain stem of mice with EAE (Siffrin et al., 2010a). However, this study did not determine the nature of the T-cell:neuronal interactions and thus it is not known if direct cell–cell contact was required. Since neurons do not express MHC II, it is highly unlikely that cognate antigen was required in this study. Our data demonstrate that, at least for MAD, the direct interactions between T-cells and neurons are not required (Figure 1).
Although CD8 T-cells are highly prevalent in MS lesions (Babbe et al., 2000; Traugott et al., 1983), their role remains controversial. In mouse EAE, the studies demonstrating both protection and pathogenesis have been documented using a variety of different models (Huseby et al., 2001; Ji and Goverman, 2007; York et al., 2010). In terms of protection, CD8 T-cells are thought to kill autoreactive CD4 T-cells (Hu et al., 2004). In pathogenesis, it is possible that CD8 T-cells kill CNS resident cells via cytotoxic mechanisms that include Fas/FasL, TRAIL (tumour-necrosis-factor-related apoptosis-inducing ligand) and granzyme/perforin (Aktas et al., 2005; Giuliani et al., 2003; Haile et al., 2011; Medana et al., 2000; Murray et al., 1998; Shrestha and Diamond, 2007; Vogt et al., 2009). In addition, CD8 T-cells have been shown to transect neurites in vitro (Medana et al., 2001). Also, in vitro, CD8 cytolytic mechanisms requiring cognate antigen were recently demonstrated in virally infected cortical neurons (Chevalier et al., 2011). However, a similar mechanism has never been definitively demonstrated for self-antigens such as in MS/EAE. Of interest to our observations is a recent study, which demonstrated that collateral neuronal apoptosis following the antigen-specific attack by OT-1 CD8 T-cells of OVA expressing oligodendrocytes in coronal brain slices was probably due to spillover of perforin and granzymes from the T-cell itself (Gobel et al., 2010). These data provide additional evidence that T-cell:neuronal contacts are not required for the induction of neuronal damage by proteins contained within the lytic granule.
NK cells also kill via lytic granules and have been shown to be present in MS lesions (Kaur et al., 2012). While their presence within the CNS may suggest a pathogenic role, in EAE most studies point towards a protective role for NK cells (Kaur et al., 2012). The antibody-mediated depletion of NK cells prior to the induction of EAE led to increased disease severity (Hao et al., 2010; Matsumoto et al., 1998; Zhang et al., 1997), whereas NK cell activation had the opposite effect of greatly reducing disease (Jahng et al., 2001; Singh et al., 2001). Potential protective mechanisms include direct lysis of pathogenic CD4 T-cells or antigen presenting dendritic cells and neuroprotection by the production of neurotrophic factors (Al-Falahi et al., 2009; Hammarberg et al., 2000; Kaur et al., 2012; Lu et al., 2007; Zhang et al., 1997). Consistent with a protective role, the trend is for reduced numbers and function of NK cells in the peripheral blood of patients with active MS disease (Kaur et al., 2012). In contrast, a recent study using a similar NK cell antibody depletion strategy resulted in less severe EAE that was accompanied by a reduction in IFNγ and TNF-α production by draining lymph node cells following antigen-specific activation (Winkler-Pickett et al., 2008), suggesting a role for NK cells in tuning the extent of adaptive immune responses. NK cells migrate into the CNS via CX3CR1 and when deficient mice exhibited more severe EAE, indicating that the site of NK cell regulation is within the CNS (Huang et al., 2006). However, once in the CNS, NK cells could contribute to pathology by directly lysing CNS resident cells including oligodendrocytes and neurons (Backstrom et al., 2003; Saikali et al., 2007), or as our data suggest, induce MAD via a bystander mechanism.
The majority of MS patients exhibit a relapsing and remitting form of disease (Compston and Coles, 2008), which is consistent with reversible neuronal damage. To model reversible neuronal damage, we used an acute model of EAE and previously demonstrated that accumulating neuronal injury paralleled the clinical signs of EAE and was reversed when the animals underwent spontaneous recovery (Shriver and Dittel, 2006). Here, we extend these observations and provide evidence that the damage is probably due to a loss in the integrity of the microtubule structure in axons, the consequence of which would be a defect in axonal transport. Interestingly, transport defects are a common feature of many neurodegenerative diseases, including MS (Morfini et al., 2009; Neumann, 2003). A defect in transport would affect both neuronal function and survival. Neuronal transmission is dependent upon the anterograde delivery of synaptic vesicles containing neurotransmitters and proteins synthesized in the cell body to the axon terminal. Similarly, neuronal survival is dependent upon the retrograde transport of neurotrophic growth factors towards the cell body (Guzik and Goldstein, 2004). Intracellular trafficking largely occurs upon microtubules composed of α- and β-tubulin subunits (Conde and Caceres, 2009) on which the motor proteins dynein and kinesin carry cargo consisting of vesicles and intracellular organelles, including mitochondria that generate the ATP required to drive the motors (Hirokawa et al., 2010). Microtubule stability is maintained by the binding of MAP, the best studied of which is tau (Johnson and Stoothoff, 2004). Tau is a substrate for numerous protein kinases and its hyperphosphorylation negatively regulates the stabilization of neuronal microtubules (Johnson and Stoothoff, 2004). Destabilization of microtubule integrity has been associated with transport defects resulting in the accumulation of cytosolic proteins in discrete domains along the neuronal processes, termed ‘beads-on-a-string’ (Coleman et al., 2005; Trushina et al., 2004). While highly studied in AD (Alzheimer's disease), hyperphosphorylation of tau has been reported in primary progressive MS and EAE (Anderson et al., 2008, 2010; Schneider et al., 2004). In addition, changes in mitochondrial function altering neuronal metabolism, consistent with a transport defect, is becoming increasingly reported in MS (Campbell et al., 2011; Ciccarelli et al., 2010; Nikic et al., 2011). While a sustained transport defect puts the neuron at risk of death, our data demonstrate that MAD is an early event that occurs prior to signs of neuronal cell death. What is not clear is the contribution MAD makes to the ultimate death of in vitro cultured neurons exposed to granzyme and perforin that are present in the cell supernatants, cell lysates and lytic granular protein preparations. The sensitivity of neurons in situ to granzyme and perforin-induced cell death is not clear, but such bystander cell death would be occurring in the presence of MAD as well. Thus, our observations are of clinical importance and indicate that if neuronal damage could be reversed prior to the induction of irreversible cell death widespread neurodegeneration could be circumvented and may extend the time for patients to enter the progressive phase of MS.
Our data indicate that cell–cell contact is not required for lymphocytes to induce MAD and thus this pathology is probably due to bystander collateral damage due to the release of granules following their activation within the CNS. It is unclear why lymphocytes evolved the ability to disrupt axonal transport, but we speculate that it is to prevent access of pathogenic agents to the CNS. Viruses, toxins and even prions gain access to the CNS from the periphery via retrograde axonal transport. Given that cognate antigen is not required in our model of neuronal damage, bystander effects on neuronal function probably follow the activation of CD4, CD8 or NK cells in the tissues at the site of infection. For instance, immune cells responding to a dirty stab wound that are reactivated at the site of inflammation causing the release of lytic granules could induce microtubule destabilization causing a transport defect thereby preventing the spread of tetanus toxin into the CNS from the peripheral site. Thus, we propose that a least some axonal damage associated with MS is potentially a consequence of immune protective mechanisms that are inadvertently triggered during a CNS autoimmune response.
ACKNOWLEDGEMENTS
We thank Shelley Morris for assistance with the animal colony, Ashley Conrad for the blind analysis of β3-tubulin slides, the Hybridoma Core of BloodCenter of Wisconsin for the generation of the MBC 363.1 β3-tubulin antibody, Dr. Subramaniam Malarkannan and his personnel for technical support with the NK cell cultures, Dr. Laurent Malherbe for B10.BR mice and Katherine Schulz and Cynthia Opansky for assistance with confocal microscopy.
FUNDING
This work was supported by the National Institutes of Health [grant number NS062235], the National Multiple Sclerosis Society [grant number RG 3550A3/1] and the BloodCenter of Wisconsin Research Foundation.
References
- Aboul-Enein F, Weiser P, Hoftberger R, Lassmann H, Bradl M. Transient axonal injury in the absence of demyelination: a correlate of clinical disease in acute experimental autoimmune encephalomyelitis. Acta Neuropathol. 2006;111:539–547. doi: 10.1007/s00401-006-0047-y. [DOI] [PubMed] [Google Scholar]
- Aktas O, Smorodchenko A, Brocke S, Infante-Duarte C, Topphoff US, Vogt J, Prozorovski T, Meier S, Osmanova V, Pohl E, Bechmann I, Nitsch R, Zipp F. Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL. Neuron. 2005;46:421–432. doi: 10.1016/j.neuron.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Al-Falahi Y, Sand KL, Knudsen E, Damaj BB, Rolin J, Maghazachi AA. Splenic natural killer cell activity in two models of experimental neurodegenerative diseases. J Cell Mol Med. 2009;13:2693–2703. doi: 10.1111/j.1582-4934.2008.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Hampton DW, Patani R, Pryce G, Crowther RA, Reynolds R, Franklin RJ, Giovannoni G, Compston DA, Baker D, Spillantini MG, Chandran S. Abnormally phosphorylated tau is associated with neuronal and axonal loss in experimental autoimmune encephalomyelitis and multiple sclerosis. Brain. 2008;131:1736–1748. doi: 10.1093/brain/awn119. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Patani R, Reynolds R, Nicholas R, Compston A, Spillantini MG, Chandran S. Abnormal tau phosphorylation in primary progressive multiple sclerosis. Acta Neuropathol. 2010;119:591–600. doi: 10.1007/s00401-010-0671-4. [DOI] [PubMed] [Google Scholar]
- Awane M, Andres PG, Li DJ, Reinecker HC. NF-κB-inducing kinase is a common mediator of IL-17-, TNF-α-, and IL-1β-induced chemokine promoter activation in intestinal epithelial cells. J Immunol. 1999;162:5337–5344. [PubMed] [Google Scholar]
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, Ravid R, Rajewsky K. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom E, Chambers BJ, Ho EL, Naidenko OV, Mariotti R, Fremont DH, Yokoyama WM, Kristensson K, Ljunggren HG. Natural killer cell-mediated lysis of dorsal root ganglia neurons via RAE1/NKG2D interactions. Eur J Immunol. 2003;33:92–100. doi: 10.1002/immu.200390012. [DOI] [PubMed] [Google Scholar]
- Barbosa MD, Nguyen QA, Tchernev VT, Ashley JA, Detter JC, Blaydes SM, Brandt SJ, Chotai D, Hodgman C, Solari RC, Lovett M, Kingsmore SF. Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature. 1996;382:262–265. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 2000;123:1174–1183. doi: 10.1093/brain/123.6.1174. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Wujek JR, Trapp BD. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003;206:165–171. doi: 10.1016/s0022-510x(02)00069-2. [DOI] [PubMed] [Google Scholar]
- Boiko T, Vakulenko M, Ewers H, Yap CC, Norden C, Winckler B. Ankyrin-dependent and -independent mechanisms orchestrate axonal compartmentalization of L1 family members neurofascin and L1/neuron-glia cell adhesion molecule. J Neurosci. 2007;27:590–603. doi: 10.1523/JNEUROSCI.4302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N, Heiple JM, Simons ER, Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Bio. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GR, Ziabreva I, Reeve AK, Krishnan KJ, Reynolds R, Howell O, Lassmann H, Turnbull DM, Mahad DJ. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann Neurol. 2011;69:481–492. doi: 10.1002/ana.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier G, Suberbielle E, Monnet C, Duplan V, Martin-Blondel G, Farrugia F, Le Masson G, Liblau R, Gonzalez-Dunia D. Neurons are MHC class I-dependent targets for CD8 T cells upon neurotropic viral infection. PLoS Pathog. 2011;7:e1002393. doi: 10.1371/journal.ppat.1002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli O, Toosy AT, De Stefano N, Wheeler-Kingshott CA, Miller DH, Thompson AJ. Assessing neuronal metabolism in vivo by modeling imaging measures. J Neurosci. 2010;30:15030–15033. doi: 10.1523/JNEUROSCI.3330-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Adalbert R, Beirowski B. Neuroprotective strategies in MS: lessons from C57BL/Wld(S) mice. J Neurol Sci. 2005;233:133–138. doi: 10.1016/j.jns.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- DeLuca GC, Williams K, Evangelou N, Ebers GC, Esiri MM. The contribution of demyelination to axonal loss in multiple sclerosis. Brain. 2006;129:1507–1516. doi: 10.1093/brain/awl074. [DOI] [PubMed] [Google Scholar]
- Dittel BN, Merchant RM, Janeway CA., Jr. Evidence for Fas-dependent and Fas-independent mechanisms in the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 1999;162:6392–6400. [PubMed] [Google Scholar]
- Dittel BN, Sant’Angelo DB, Janeway CA., Jr. Peptide antagonists inhibit proliferation and the production of IL-4 and/or IFN-γ in T helper 1, T helper 2, and T helper 0 clones bearing the same TCR. J Immunol. 1997;158:4065–4073. [PubMed] [Google Scholar]
- Edwards LJ, Robins RA, Constantinescu CS. Th17/Th1 phenotype in demyelinating disease. Cytokine. 2010;50:19–23. doi: 10.1016/j.cyto.2009.12.003. [DOI] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Behi M, Rostami A, Ciric B. Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2010;5:189–197. doi: 10.1007/s11481-009-9188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani F, Goodyer CG, Antel JP, Yong VW. Vulnerability of human neurons to T cell-mediated cytotoxicity. J Immunol. 2003;171:368–379. doi: 10.4049/jimmunol.171.1.368. [DOI] [PubMed] [Google Scholar]
- Gobel K, Melzer N, Herrmann AM, Schuhmann MK, Bittner S, Ip CW, Hunig T, Meuth SG, Wiendl H. Collateral neuronal apoptosis in CNS gray matter during an oligodendrocyte-directed CD8+ T cell attack. Glia. 2010;58:469–480. doi: 10.1002/glia.20938. [DOI] [PubMed] [Google Scholar]
- Guzik BW, Goldstein LS. Microtubule-dependent transport in neurons: steps towards an understanding of regulation, function and dysfunction. Curr Opin Cell Biol. 2004;16:443–450. doi: 10.1016/j.ceb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Haile Y, Simmen KC, Pasichnyk D, Touret N, Simmen T, Lu JQ, Bleackley RC, Giuliani F. Granule-derived granzyme B mediates the vulnerability of human neurons to T cell-induced neurotoxicity. J Immunol. 2011;187:4861–4872. doi: 10.4049/jimmunol.1100943. [DOI] [PubMed] [Google Scholar]
- Hammarberg H, Lidman O, Lundberg C, Eltayeb SY, Gielen AW, Muhallab S, Svenningsson A, Linda H, van Der Meide PH, Cullheim S, Olsson T, Piehl F. Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J Neurosci. 2000;20:5283–5291. doi: 10.1523/JNEUROSCI.20-14-05283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Liu R, Piao W, Zhou Q, Vollmer TL, Campagnolo DI, Xiang R, La Cava A, Van Kaer L, Shi FD. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med. 2010;207:1907–1921. doi: 10.1084/jem.20092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology. 2008;125:161–169. doi: 10.1111/j.1365-2567.2008.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Zipp F, Siffrin V. Neurodegeneration in autoimmune CNS inflammation. Exp Neurol. 2009;225:9–17. doi: 10.1016/j.expneurol.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- Huang D, Shi FD, Jung S, Pien GC, Wang J, Salazar-Mather TP, He TT, Weaver JT, Ljunggren HG, Biron CA, Littman DR, Ransohoff RM. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific CD8+ T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K. Human immunodeficiency virus type 1 gp120 induces apoptosis in human primary neurons through redox-regulated activation of neutral sphingomyelinase. J Neurosci. 2004;24:9531–9540. doi: 10.1523/JNEUROSCI.3085-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K. Fibrillar amyloid-beta-activated human astroglia kill primary human neurons via neutral sphingomyelinase: implications for Alzheimer's disease. J Neurosci. 2010;30:12676–12689. doi: 10.1523/JNEUROSCI.1243-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q, Goverman J. Experimental autoimmune encephalomyelitis mediated by CD8+ T cells. Ann N Y Acad Sci. 2007;1103:157–166. doi: 10.1196/annals.1394.017. [DOI] [PubMed] [Google Scholar]
- Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117:5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hirokawa N. Sorting mechanisms of tau and MAP2 in neurons: suppressed axonal transit of MAP2 and locally regulated microtubule binding. Neuron. 1995;14:421–432. doi: 10.1016/0896-6273(95)90298-8. [DOI] [PubMed] [Google Scholar]
- Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C, Stohlman S, Tuohy VK, Li X. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414–425. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J, De Domenico I, Ward DM. Chediak-Higashi syndrome. Cur Opin Hematol. 2008;15:22–29. doi: 10.1097/MOH.0b013e3282f2bcce. [DOI] [PubMed] [Google Scholar]
- Kaur G, Trowsdale J, Fugger L. Natural killer cells and their receptors in multiple sclerosis. Brain. 2012 doi: 10.1093/brain/aws159. doi: 10.1093/brain/aws159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoruts A, Miller SD, Jenkins MK. Neuroantigen-specific Th2 cells are inefficient suppressors of experimental autoimmune encephalomyelitis induced by effector Th1 cells. J Immunol. 1995;155:5011–5017. [PubMed] [Google Scholar]
- Kordeli E, Lambert S, Bennett V. Ankyrin G. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, Linington C, Schmidbauer M, Lassmann H. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Finch EA. MAP2 and tau segregate into dendritic and axonal domains after the elaboration of morphologically distinct neurites: an immunocytochemical study of cultured rat cerebrum. J Neurosci. 1987;7:3142–3153. doi: 10.1523/JNEUROSCI.07-10-03142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Bruck W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125:2202–2212. doi: 10.1093/brain/awf235. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Axonal and neuronal pathology in multiple sclerosis: what have we learnt from animal models. Exp Neurol. 2010;225:2–8. doi: 10.1016/j.expneurol.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Racke AE, Yang Y, Racke MK. Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis? Biochim Biophys Acta. 2011;1812:246–251. doi: 10.1016/j.bbadis.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Ikizawa K, Hu D, Werneck MB, Wucherpfennig KW, Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D, Tschopp J. Isolation of a lytic, pore-forming protein (perforin) from cytolytic T-lymphocytes. J Biol Chem. 1985;260:9069–9072. [PubMed] [Google Scholar]
- Matsumoto Y, Kohyama K, Aikawa Y, Shin T, Kawazoe Y, Suzuki Y, Tanuma N. Role of natural killer cells and TCRγδ T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1681–1688. doi: 10.1002/(SICI)1521-4141(199805)28:05<1681::AID-IMMU1681>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CC. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194:873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meares GP, Ma X, Qin H, Benveniste EN. Regulation of CCL20 expression in astrocytes by IL-6 and IL-17. Glia. 2012;60:771–781. doi: 10.1002/glia.22307. [DOI] [PubMed] [Google Scholar]
- Medana I, Martinic MA, Wekerle H, Neumann H. Transection of major histocompatibility complex class I-induced neurites by cytotoxic T lymphocytes. Am J Pathol. 2001;159:809–815. doi: 10.1016/S0002-9440(10)61755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medana IM, Gallimore A, Oxenius A, Martinic MM, Wekerle H, Neumann H. MHC class I-restricted killing of neurons by virus-specific CD8+ T lymphocytes is effected through the Fas/FasL, but not the perforin pathway. Eur J Immunol. 2000;30:3623–3633. doi: 10.1002/1521-4141(200012)30:12<3623::AID-IMMU3623>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Miller E. Multiple sclerosis. Adv Exp Med Biol. 2012;724:222–238. doi: 10.1007/978-1-4614-0653-2_17. [DOI] [PubMed] [Google Scholar]
- Montes M, Zhang X, Berthelot L, Laplaud DA, Brouard S, Jin J, Rogan S, Armao D, Jewells V, Soulillou JP, Markovic-Plese S. Oligoclonal myelin-reactive T-cell infiltrates derived from multiple sclerosis lesions are enriched in Th17 cells. Clin Immunol. 2009;130:133–144. doi: 10.1016/j.clim.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, Brown RH, Jr, Brown H, Tiwari A, Hayward L, Edgar J, Nave KA, Garberrn J, Atagi Y, Song Y, Pigino G, Brady ST. Axonal transport defects in neurodegenerative diseases. J Neurosci. 2009;29:12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PD, McGavern DB, Lin X, Njenga MK, Leibowitz J, Pease LR, Rodriguez M. Perforin-dependent neurologic injury in a viral model of multiple sclerosis. J Neurosci. 1998;18:7306–7314. doi: 10.1523/JNEUROSCI.18-18-07306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H. Molecular mechanisms of axonal damage in inflammatory central nervous system diseases. Curr Opin Neurol. 2003;16:267–273. doi: 10.1097/01.wco.0000073926.19076.29. [DOI] [PubMed] [Google Scholar]
- Nikic I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, Bruck W, Bishop D, Misgeld T, Kerschensteiner M. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2011;17:495–499. doi: 10.1038/nm.2324. [DOI] [PubMed] [Google Scholar]
- Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122:1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack ER, Konigsberg PJ. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984;160:695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- Ransom BR, Neale E, Henkart M, Bullock PN, Nelson PG. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977;40:1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol. 2012;188:3188–3198. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regunathan J, Chen Y, Wang D, Malarkannan S. NKG2D receptor-mediated NK cell function is regulated by inhibitory Ly49 receptors. Blood. 2005;105:233–240. doi: 10.1182/blood-2004-03-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder JC, Lohmann-Matthes ML, Domzig W, Wigzell H. The beige mutation in the mouse. II. Selectivity of the natural killer (NK) cell defect. J Immunol. 1979;123:2174–2181. [PubMed] [Google Scholar]
- Saikali P, Antel JP, Newcombe J, Chen Z, Freedman M, Blain M, Cayrol R, Prat A, Hall JA, Arbour N. NKG2D-mediated cytotoxicity toward oligodendrocytes suggests a mechanism for tissue injury in multiple sclerosis. J Neurosci. 2007;27:1220–1228. doi: 10.1523/JNEUROSCI.4402-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Araujo GW, Trajkovic K, Herrmann MM, Merkler D, Mandelkow EM, Weissert R, Simons M. Hyperphosphorylation and aggregation of tau in experimental autoimmune encephalomyelitis. J Biol Chem. 2004;279:55833–55839. doi: 10.1074/jbc.M409954200. [DOI] [PubMed] [Google Scholar]
- Sepulcre J, Sastre-Garriga J, Cercignani M, Ingle GT, Miller DH, Thompson AJ. Regional gray matter atrophy in early primary progressive multiple sclerosis: a voxel-based morphometry study. Arch Neurol. 2006;63:1175–1180. doi: 10.1001/archneur.63.8.1175. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Diamond MS. Fas ligand interactions contribute to CD8+ T-cell-mediated control of West Nile virus infection in the central nervous system. J Virol. 2007;81:11749–11757. doi: 10.1128/JVI.01136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriver LP, Dittel BN. T-cell-mediated disruption of the neuronal microtubule network: correlation with early reversible axonal dysfunction in acute experimental autoimmune encephalomyelitis. Am J Pathol. 2006;169:999–1011. doi: 10.2353/ajpath.2006.050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, Leuenberger T, Lehmann SM, Luenstedt S, Rinnenthal JL, Laube G, Luche H, Lehnardt S, Fehling HJ, Griesbeck O, Zipp F. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. 2010a;33:424–436. doi: 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Siffrin V, Vogt J, Radbruch H, Nitsch R, Zipp F. Multiple sclerosis – candidate mechanisms underlying CNS atrophy. Trends Neurosci. 2010b;33:202–210. doi: 10.1016/j.tins.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Singh AK, Wilson MT, Hong S, Olivares-Villagomez D, Du C, Stanic AK, Joyce S, Sriram S, Koezuka Y, Van Kaer L. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Suvas S, Azkur AK, Rouse BT. Qa-1b and CD94-NKG2a interaction regulate cytolytic activity of herpes simplex virus-specific memory CD8+ T cells in the latently infected trigeminal ganglia. J Immunol. 2006;176:1703–1711. doi: 10.4049/jimmunol.176.3.1703. [DOI] [PubMed] [Google Scholar]
- Tallantyre EC, Bo L, Al-Rawashdeh O, Owens T, Polman CH, Lowe J, Evangelou N. Greater loss of axons in primary progressive multiple sclerosis plaques compared to secondary progressive disease. Brain. 2009;132:1190–1199. doi: 10.1093/brain/awp106. [DOI] [PubMed] [Google Scholar]
- Thiery J, Walch M, Jensen DK, Martinvalet D, Lieberman J. Isolation of cytotoxic T cell and NK granules and purification of their effector proteins. Curr Protoc Cell Biol. 2010:43:3.37.1–3.37.29. doi: 10.1002/0471143030.cb0337s47. [DOI] [PubMed] [Google Scholar]
- Traugott U, Reinherz EL, Raine CS. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983;219:308–310. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- Trushina E, Dyer RB, Badger JD, II, Ure D, Eide L, Tran DD, Vrieze BT, Legendre-Guillemin V, McPherson PS, Mandavilli BS, Van Houten B, Zeitlin S, McNiven M, Aebersold R, Hayden M, Parisi JE, Seeberg E, Dragatsis I, Doyle K, Bender A, Chacko C, McMurray CT. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol Cell Biol. 2004;24:8195–8209. doi: 10.1128/MCB.24.18.8195-8209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt J, Paul F, Aktas O, Muller-Wielsch K, Dorr J, Dorr S, Bharathi BS, Glumm R, Schmitz C, Steinbusch H, Raine CS, Tsokos M, Nitsch R, Zipp F. Lower motor neuron loss in multiple sclerosis and experimental autoimmune encephalomyelitis. Ann Neurol. 2009;66:310–322. doi: 10.1002/ana.21719. [DOI] [PubMed] [Google Scholar]
- Winkler-Pickett R, Young HA, Cherry JM, Diehl J, Wine J, Back T, Bere WE, Mason AT, Ortaldo JR. In vivo regulation of experimental autoimmune encephalomyelitis by NK cells: alteration of primary adaptive responses. J Immunol. 2008;180:4495–4506. doi: 10.4049/jimmunol.180.7.4495. [DOI] [PubMed] [Google Scholar]
- Yodoi J, Teshigawara K, Nikaido T, Fukui K, Noma T, Honjo T, Takigawa M, Sasaki M, Minato N, Tsudo M, Uchiyama T, Maeda M. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells) J Immunol. 1985;134:1623–1630. [PubMed] [Google Scholar]
- York NR, Mendoza JP, Ortega SB, Benagh A, Tyler AF, Firan M, Karandikar NJ. Immune regulatory CNS-reactive CD8+ T cells in experimental autoimmune encephalomyelitis. J Autoimmun. 2010;35:33–44. doi: 10.1016/j.jaut.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]