Abstract
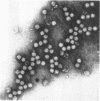
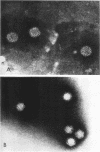
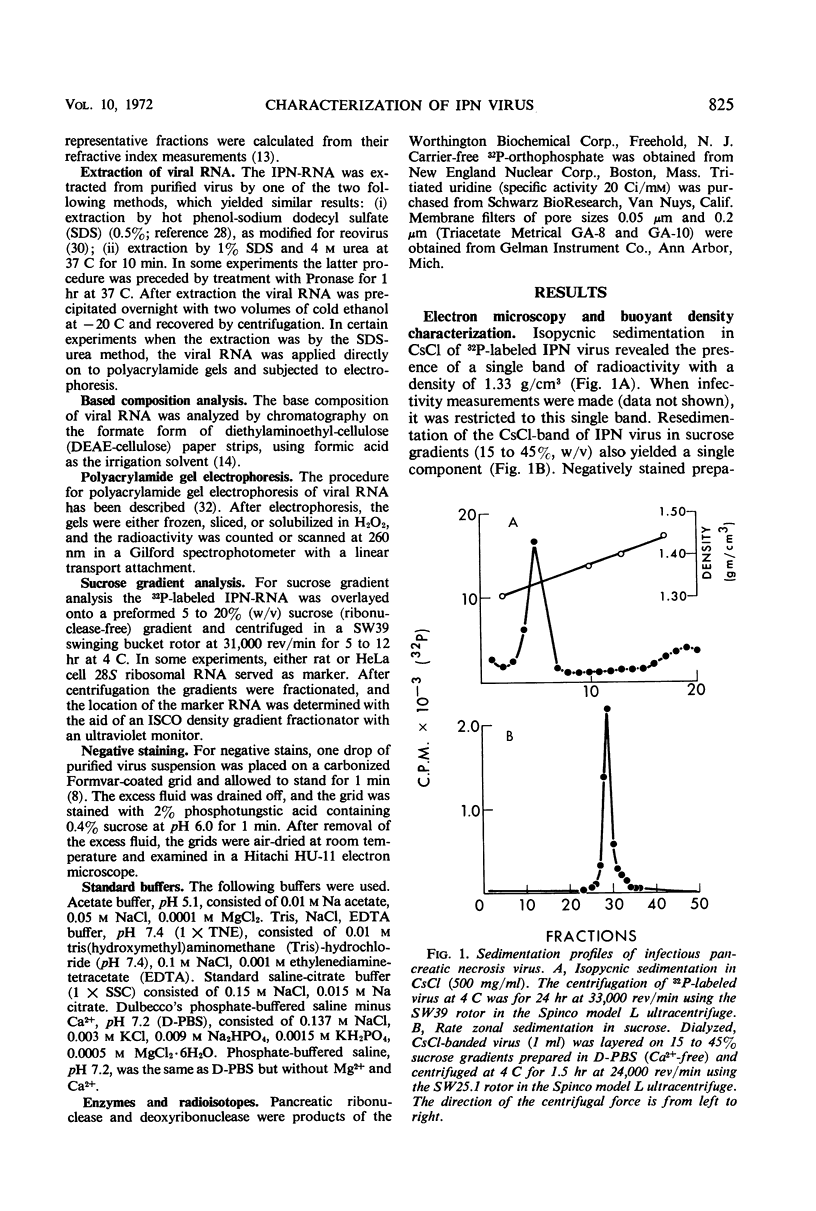
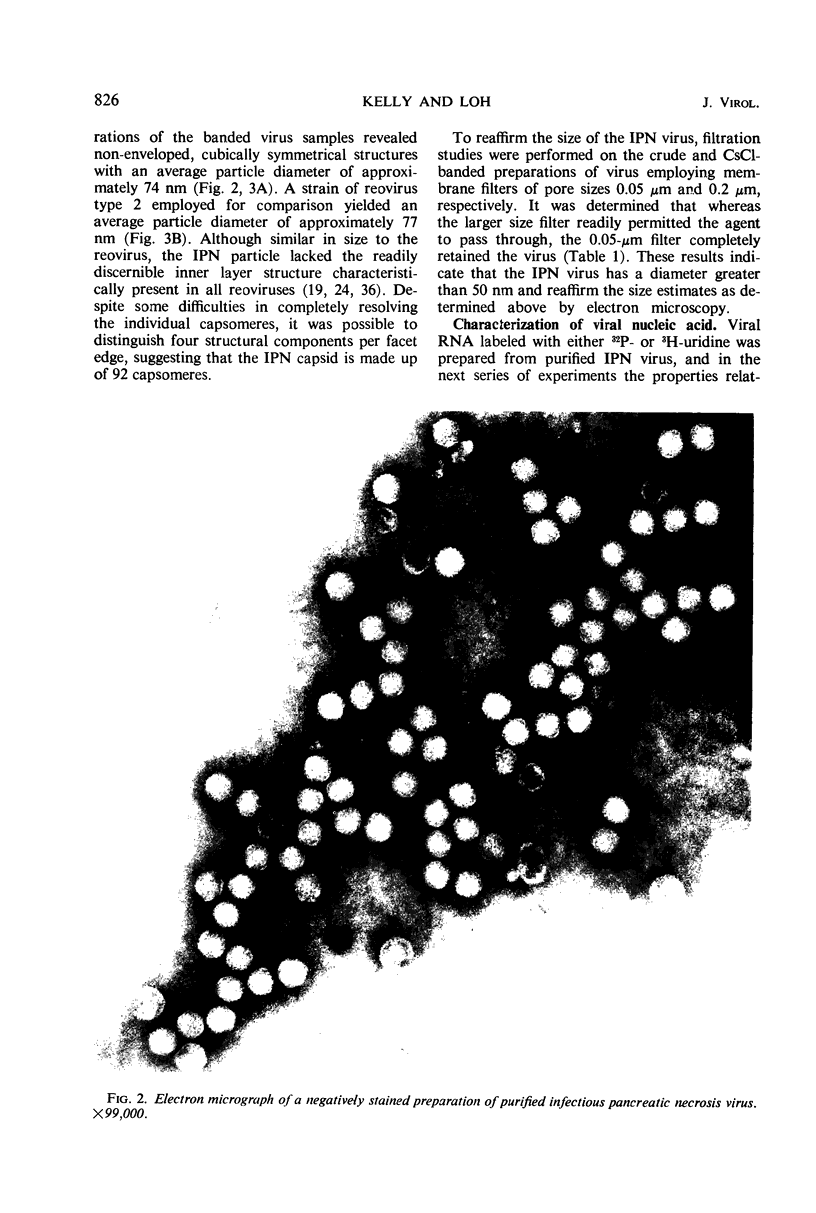
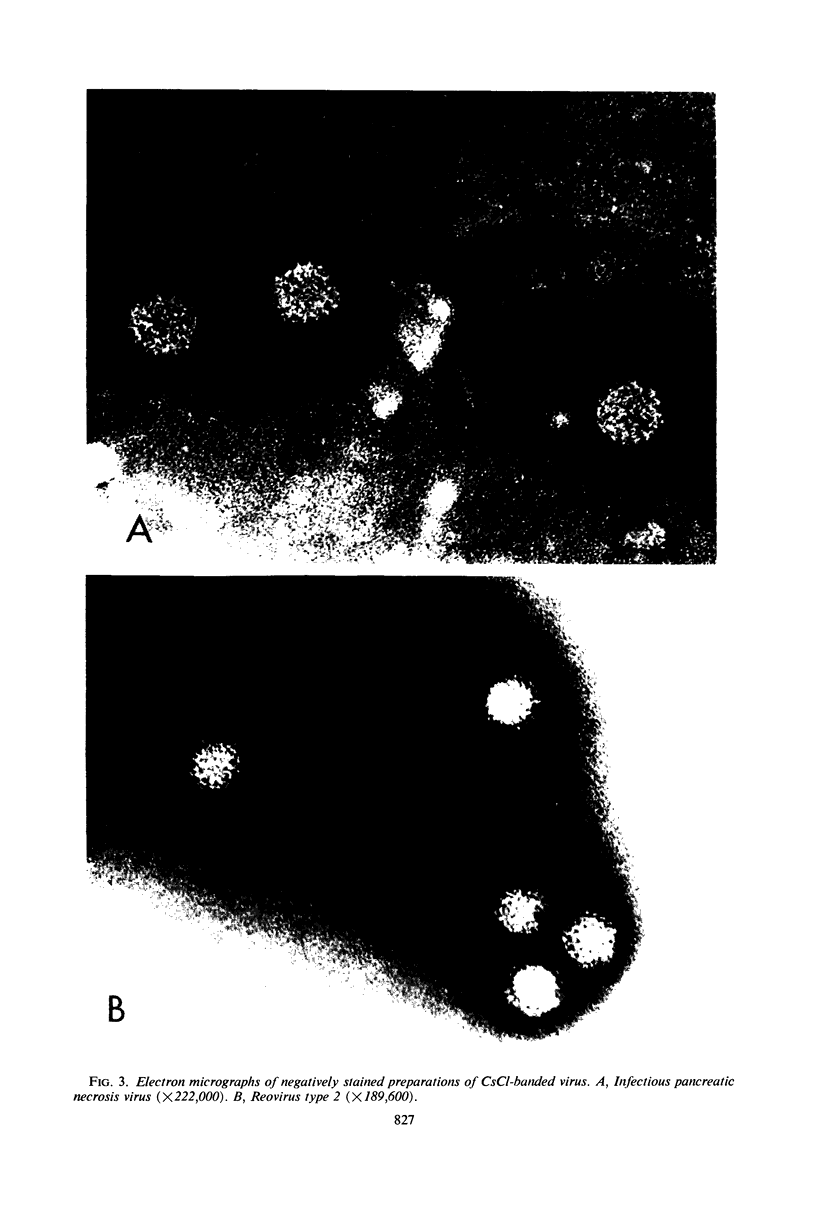
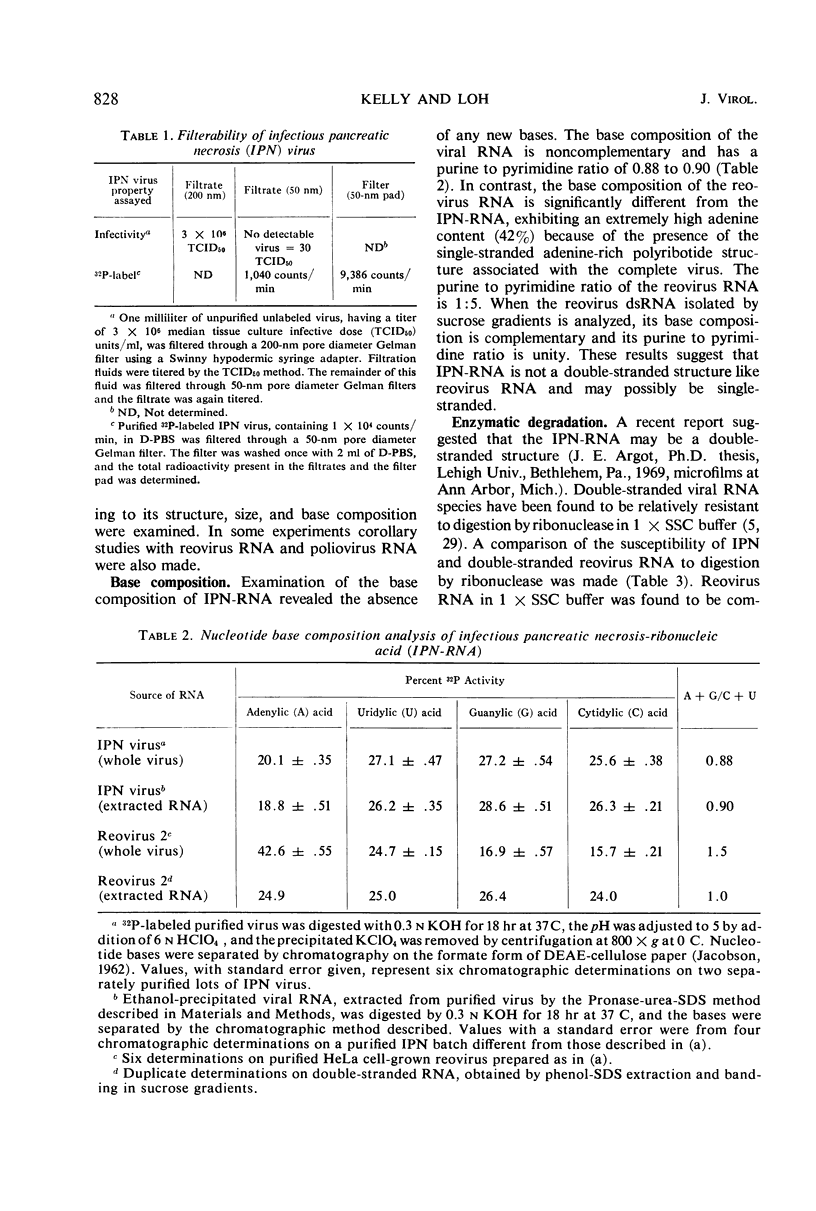
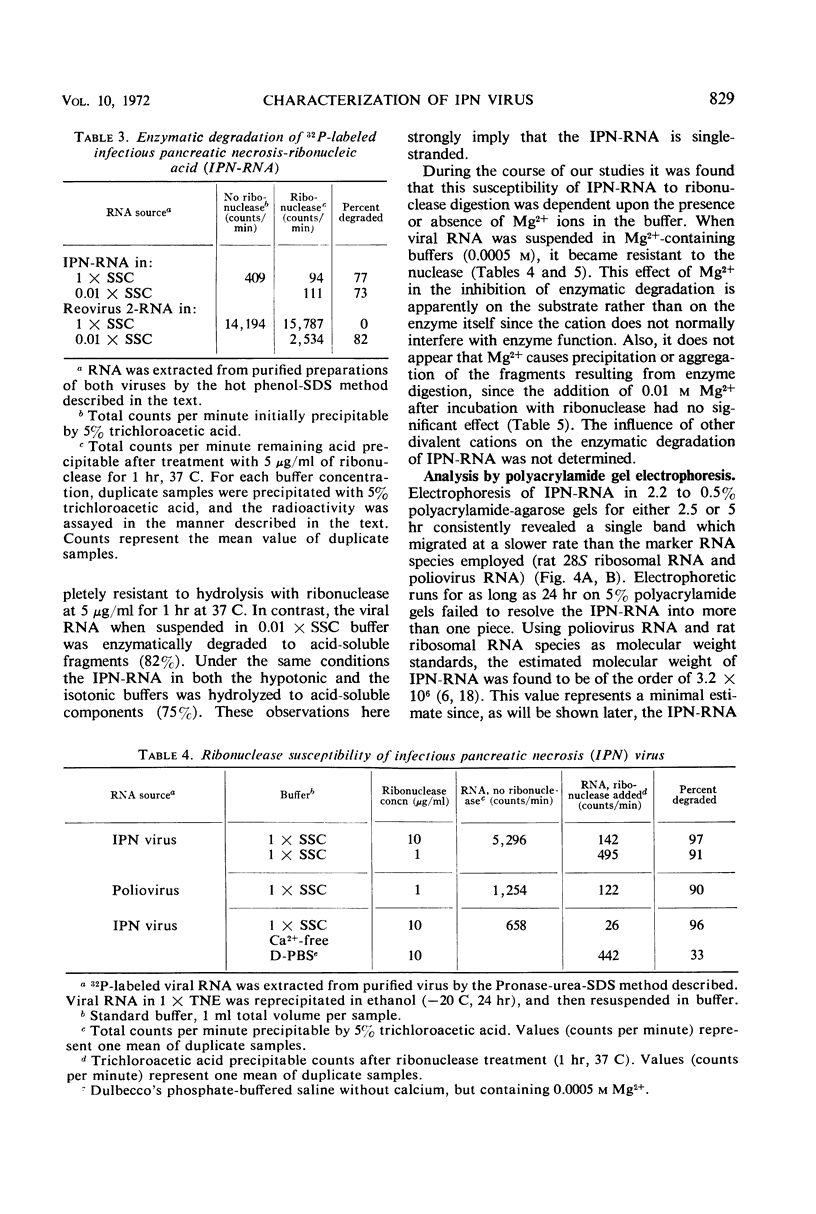
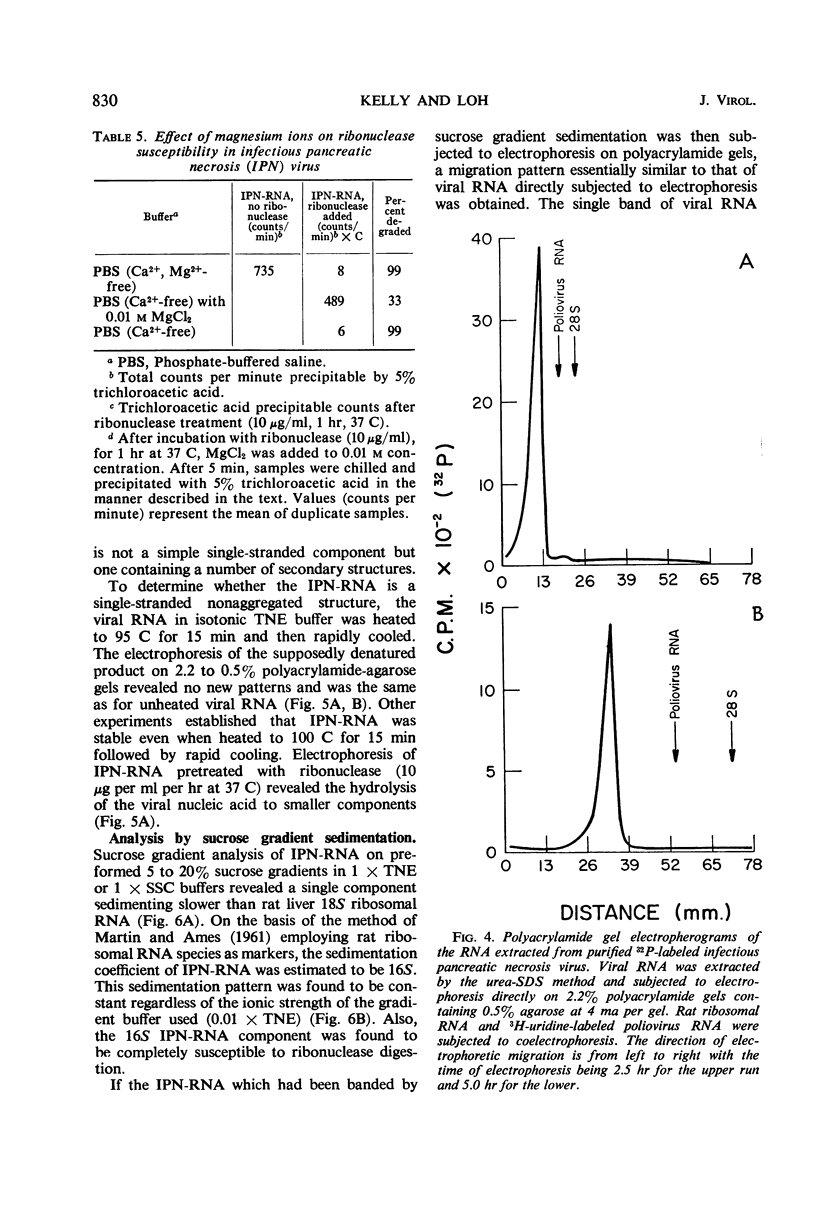
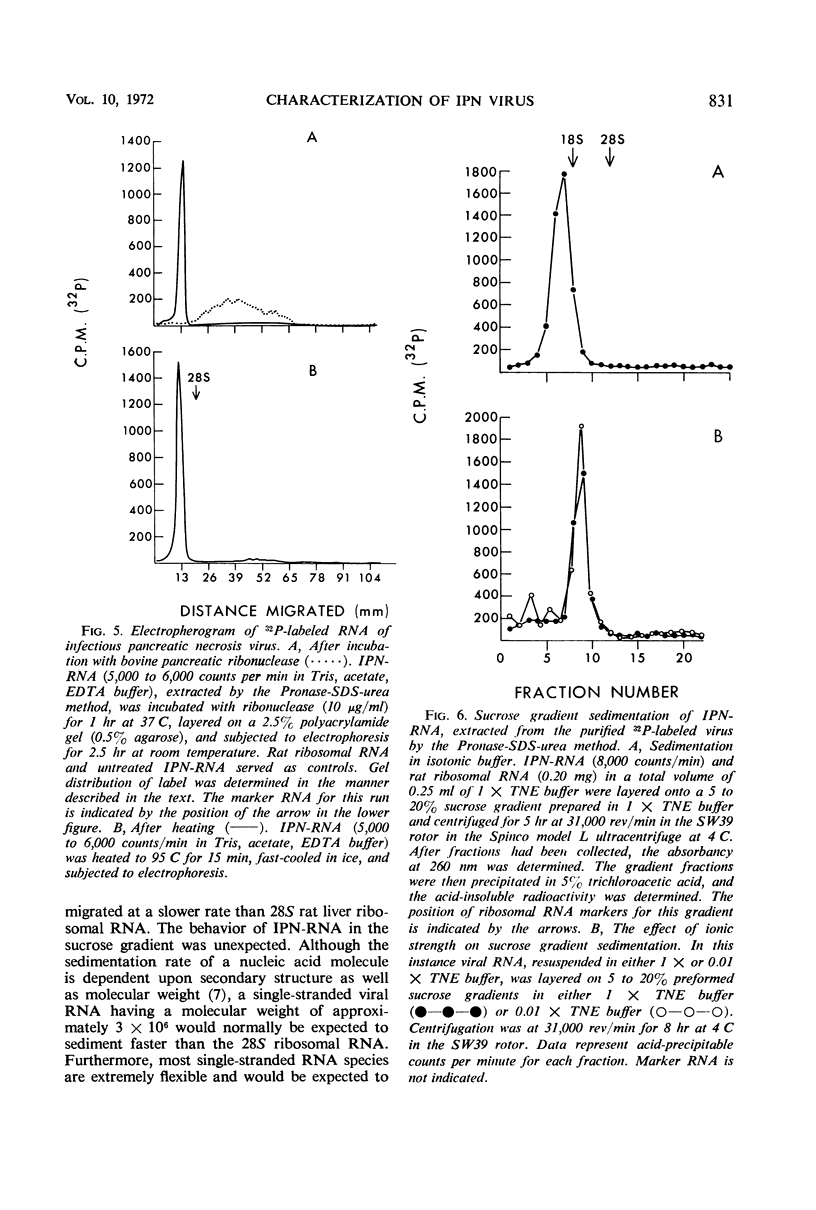
An electron microscopical and biochemical examination of the properties of infectious pancreatic necrosis virus (IPN) and of its ribonucleic acid (RNA) was made. The buoyant density of IPN in CsCl was found to be 1.33 g/cm3. Electron microscopical examination of the banded virus revealed structures similar in size (74 nm) and shape to reoviruses but lacking a characteristic inner capsid structure. Polyacrylamide gel electrophoretic analysis of IPN-RNA revealed a single non-segmented component of molecular weight 3.2 × 106. Its susceptibility to ribonuclease, base composition, and resistance to thermal denaturation indicated a single-stranded RNA structure. However, its sedimentation behavior (16S) independent of ionic strength in sucrose gradients, partial solubility in 2 m LiCl, and ribonuclease resistance in the presence of Mg2+ suggest an unusual secondary structure of unknown nature. The accumulated data indicate that IPN virus does not belong to either the picornavirus or reovirus groups and may represent a new group of viruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Hutchinson F., Spencer M., Wilkins M. H., Fuller W., Langridge R. X-ray diffraction studies of double helical ribonucleic acid. Nature. 1966 Jul 16;211(5046):227–232. doi: 10.1038/211227a0. [DOI] [PubMed] [Google Scholar]
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Purification and properties of poliovirus double-stranded ribonucleic acid. J Mol Biol. 1966 Jul;18(3):421–428. doi: 10.1016/s0022-2836(66)80034-7. [DOI] [PubMed] [Google Scholar]
- Bellamy A. R., Joklik W. K. Studies on the A-rich RNA of reovirus. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1389–1395. doi: 10.1073/pnas.58.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Cerini C. P., Malsberger R. G. Morphology of infectious pancreatic necrosis virus. Ann N Y Acad Sci. 1965 Aug 10;126(1):315–319. doi: 10.1111/j.1749-6632.1965.tb14282.x. [DOI] [PubMed] [Google Scholar]
- Cramer F. Three-dimensional structure of tRNA. Prog Nucleic Acid Res Mol Biol. 1971;11:391–421. doi: 10.1016/s0079-6603(08)60333-5. [DOI] [PubMed] [Google Scholar]
- Gomatos P. J., Tamm I. THE SECONDARY STRUCTURE OF REOVIRUS RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):707–714. doi: 10.1073/pnas.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON K. B. Ribonucleotides of RNA: separation by chromatography on sheets of diethylaminoethylcellulose. Science. 1962 Oct 26;138(3539):515–516. doi: 10.1126/science.138.3539.515. [DOI] [PubMed] [Google Scholar]
- KISSELEV N. A., GAVRILOVA L. P., SPIRIN A. S. On configurations of high-polymer ribonucleic acid macromolecules as revealed by electron microscopy. J Mol Biol. 1961 Dec;3:778–783. doi: 10.1016/s0022-2836(61)80083-1. [DOI] [PubMed] [Google Scholar]
- Kalmakoff J., Lewandowski L. J., Black D. R. Comparison of the ribonucleic Acid subunits of reovirus, cytoplasmic polyhedrosis virus, and wound tumor virus. J Virol. 1969 Dec;4(6):851–856. doi: 10.1128/jvi.4.6.851-856.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A., Darlington R. W. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970 Dec;42(4):857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- LOH P. C., HOHL H. R., SOERGEL M. FINE STRUCTURE OF REOVIRUS TYPE 2. J Bacteriol. 1965 Apr;89:1140–1144. doi: 10.1128/jb.89.4.1140-1144.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh P. C., Oie H. K. Role of lysine in the replication of reovirus: I. Synthesis of complete and empty virions. J Virol. 1969 Dec;4(6):890–895. doi: 10.1128/jvi.4.6.890-895.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALSBERGER R. G., CERINI C. P. CHARACTERISTICS OF INFECTIOUS PANCREATIC NECROSIS VIRUS. J Bacteriol. 1963 Dec;86:1283–1287. doi: 10.1128/jb.86.6.1283-1287.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MAYOR H. D., JAMISON R. M., JORDAN L. E., VANMITCHELL M. REOVIRUSES. II. STRUCTURE AND COMPOSITION OF THE VIRION. J Bacteriol. 1965 Jun;89:1548–1556. doi: 10.1128/jb.89.6.1548-1556.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malsberger R. G., Cerini C. P. Multiplication of infectious pancreatic necrosis virus. Ann N Y Acad Sci. 1965 Aug 10;126(1):320–327. doi: 10.1111/j.1749-6632.1965.tb14283.x. [DOI] [PubMed] [Google Scholar]
- Moss L. H., 3rd, Gravell M. Ultrastructure and sequential development of infectious pancreatic necrosis virus. J Virol. 1969 Jan;3(1):52–58. doi: 10.1128/jvi.3.1.52-58.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson B. L. Macromolecule synthesis in RTG-2 cells following infection with infectious pancreatic necrosis (IPN) virus. J Gen Virol. 1971 Nov;13(2):369–372. doi: 10.1099/0022-1317-13-2-369. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- STRAUSS J. H., Jr, SINSHEIMER R. L. Purification and properties of bacteriophage MS2 and of its ribonucleic acid. J Mol Biol. 1963 Jul;7:43–54. doi: 10.1016/s0022-2836(63)80017-0. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Inactivity of purified reovirus RNA as a template for E. coli polymerases in vitro. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1721–1728. doi: 10.1073/pnas.54.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Rada B. Reovirus-directed ribonucleic acid synthesis in infected L cells. J Virol. 1967 Feb;1(1):24–35. doi: 10.1128/jvi.1.1.24-35.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D., Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J Virol. 1968 Oct;2(10):986–991. doi: 10.1128/jvi.2.10.986-991.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. Single-stranded, adenine-rich RNA from purified reoviruses. Proc Natl Acad Sci U S A. 1968 Jan;59(1):246–253. doi: 10.1073/pnas.59.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VASQUEZ C., TOURNIER P. The morphology of reovirus. Virology. 1962 Aug;17:503–510. doi: 10.1016/0042-6822(62)90149-6. [DOI] [PubMed] [Google Scholar]
- Vasquez C., Kleinschmidt A. K. Electron microscopy of RNA strands released from individual Reovirus particles. J Mol Biol. 1968 May 28;34(1):137–147. doi: 10.1016/0022-2836(68)90240-4. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Millward S., Graham A. F. Regulation of transcription of the Reovirus genome. J Mol Biol. 1968 Aug 28;36(1):107–123. doi: 10.1016/0022-2836(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Wolf K. The fish viruses. Adv Virus Res. 1966;12:35–101. doi: 10.1016/s0065-3527(08)60846-5. [DOI] [PubMed] [Google Scholar]